Adsorption of Cd(II) in water by graphene oxide/calcium alginate hydrogel composite membrane
-
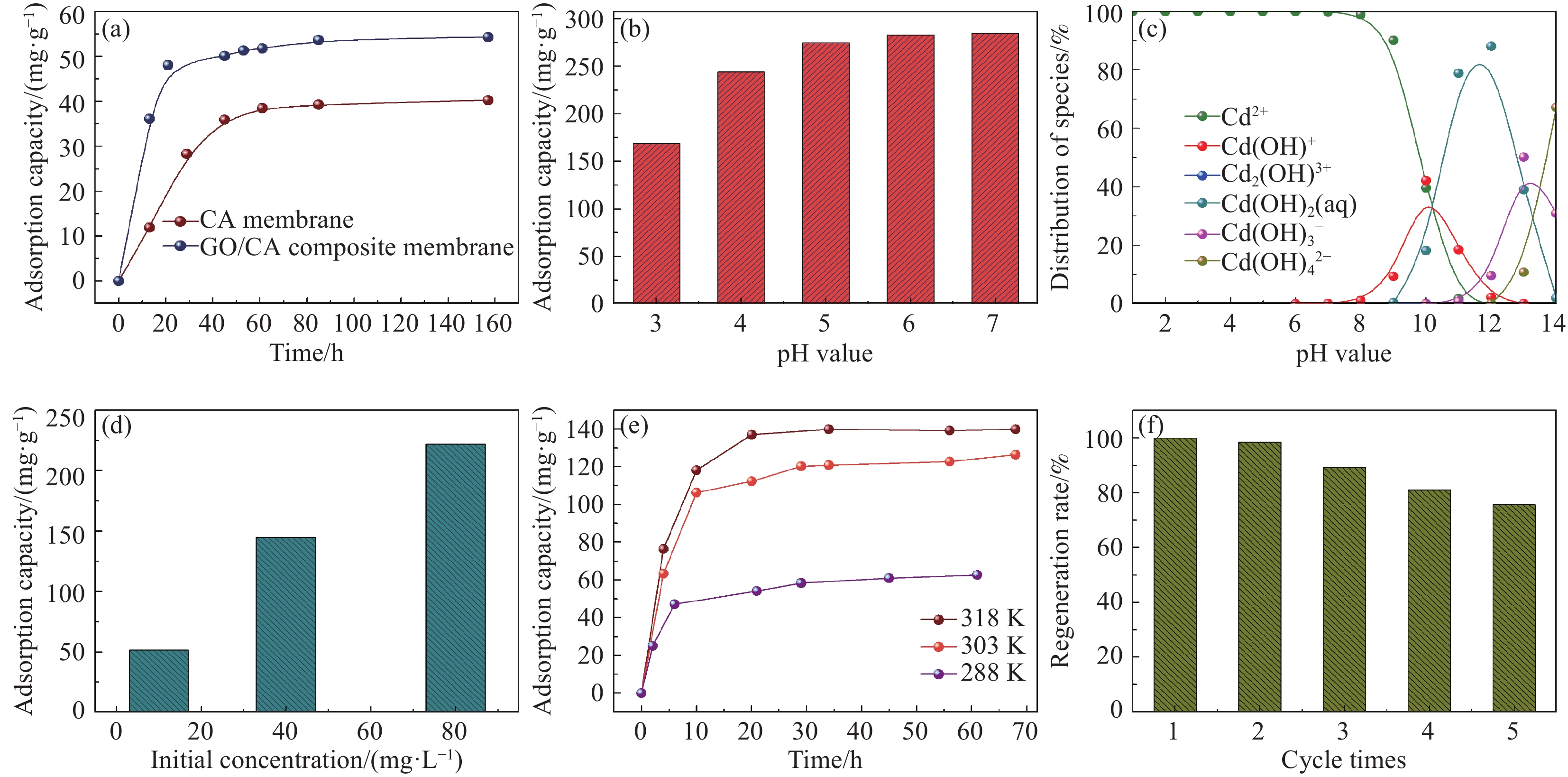
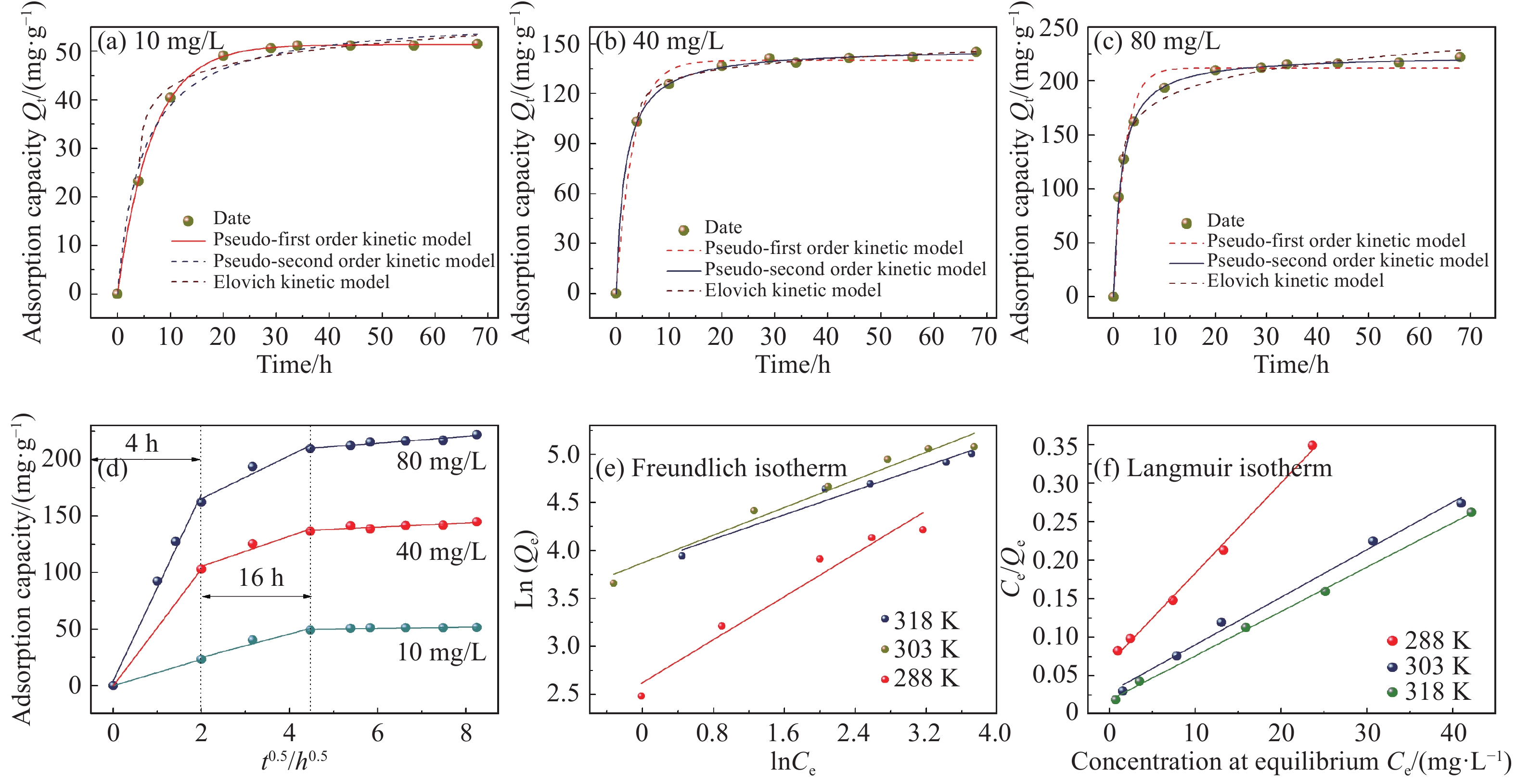
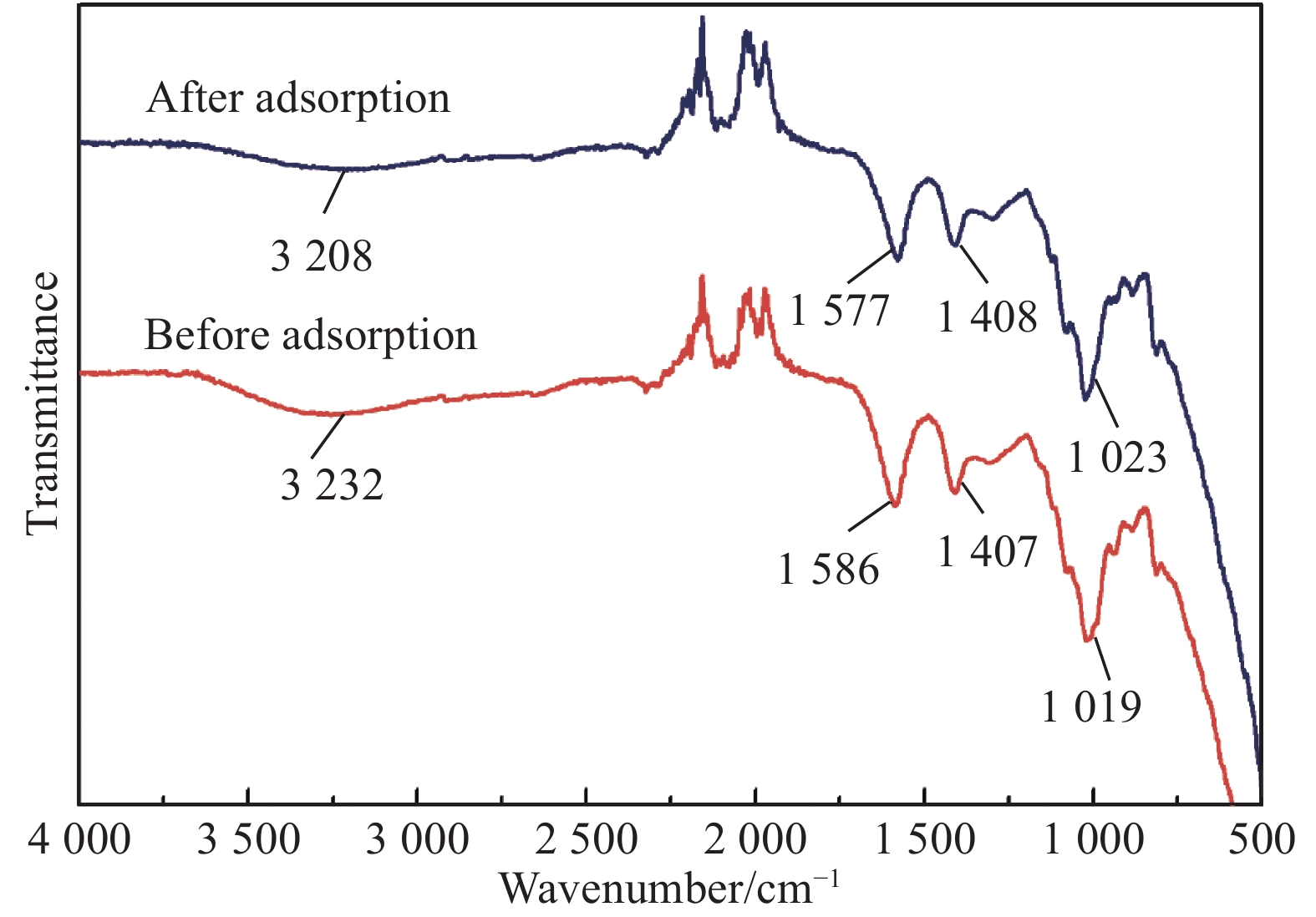
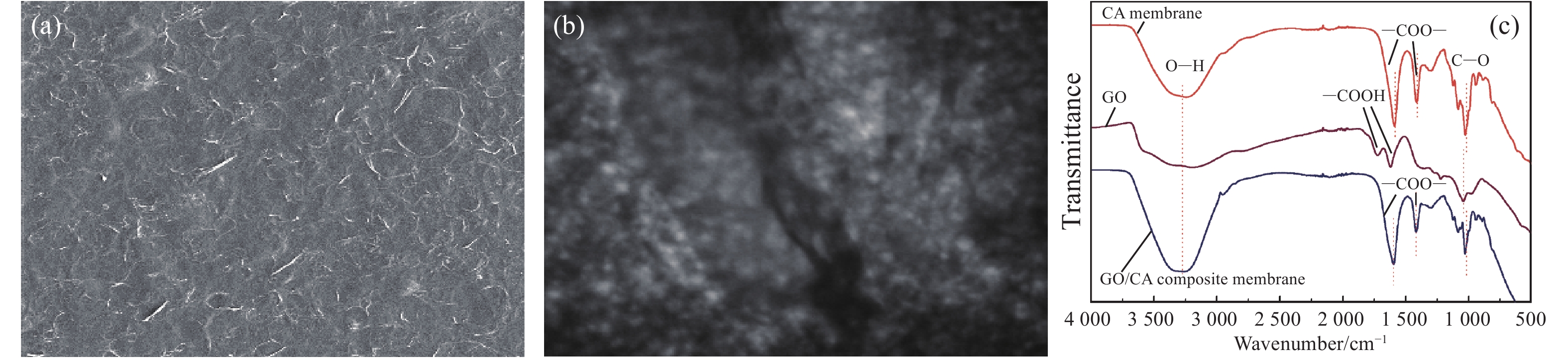
摘要: 将氧化石墨烯(GO)、致孔剂与海藻酸钠共混后与CaCl2交联制备的GO/海藻酸钙(CA)水凝胶复合膜作为含重金属废水的吸附材料。采用SEM和TEM表征了复合膜的表面形貌及透射性能,且分析了GO的加入对复合膜的力学性能、平均孔径、水通量及表面官能团的影响。为探究GO/CA水凝胶复合膜的吸附性能,考察了其吸附Cd(II)的影响因素:pH(6~7)值、初始离子浓度、接触时间、温度(三者均正相关)。用FTIR、XPS在吸附前后对复合膜进行了表征;引入了吸附动力学和等温线模型分析其吸附机制。探究结果表明GO的加入提高了复合膜的力学性能、平均孔径及水通量;吸附过程遵循Langmuir等温线,属于单层吸附,拟合得到的最大吸附量为173.61 mg/g;伪一级和伪二级吸附动力学分别在低浓度和高浓度时能较好地描述吸附过程的动力学行为;吸附机制主要为物理作用力吸附和离子交换。经过5个连续的吸附-解吸循环证明了GO/CA水凝胶复合膜的可重复利用性。Abstract: The crosslinked graphene oxide (GO)/calcium alginate (CA) hydrogel composite membranes, prepared by blending GO, porogenas as well as Na alginate hydrogel (SA), cross-linking with CaCl2, were used as adsorbent for wastewater containing heavy metal ions. SEM and TEM were used to characterize the composite membranes. The influences of GO on the mechanical properties, average pore diameter, water flux and surface functional groups of the composite membrane were analyzed. To explore the adsorption performance of GO/CA hydrogel composite membrane, the influencing factors of Cd(II) adsorption including pH (6-7), initial ion concentration, contact time and temperature (all positively correlated) were investigated. The composite membranes before and after adsorption of Cd(II) were characterized by FTIR and XPS; The adsorption kinetics and adsorption isotherm model were introduced to analyze the adsorption mechanism. The results show that the introduction of GO improves the mechanical properties, average pore diameter and water flux of the composite membrane. The adsorption process follows the Langmuir isotherm, belonging to the monolayer adsorption. The maximum adsorption capacity is 173.61 mg/g. The pseudo-first and pseudo-second adsorption kinetics could describe the adsorption process at low and high concentration, respectively. The adsorption mechanism is mainly physical force adsorption and ion exchange. The recyclability of the GO/CA hydrogel composite membranes was demonstrated by five successive adsorption-desorption cycles.
-
Keywords:
- graphene oxide /
- hydrogel /
- composite membrane /
- adsorption /
- Cd(II)
-
-
表 1 CA膜和GO/CA水凝复合膜的渗透性能
Table 1 Permeability of CA membrane and GO/CA hydrogel composite membrane
Membrane Mean pore size/nm Poriness/% Water flux/(L·m-2h-1) CA 10.6 86.5 14.7 GO/CA 12.6 90.1 18.1 表 2 CA膜和GO/CA水凝复合膜的力学性能
Table 2 Mechanical properties of the CA membrane and GO/CA hydrogel composite membrane
Membrane Elongation at break/% Fracture energy/(kJ·m−2) Stress/MPa CA 95 34 914 GO/CA 143 65 1 725 表 3 Cd(II)的初始浓度C0不同时GO/CA水凝复合膜吸附性能的动力学模型拟合参数
Table 3 Kinetic model parameters of GO/CA hydrogel composite membrane adsorption at different initial concentration C0 of Cd(II)
C0/(mg·L−1) Pseudo-first order kinetic model Pseudo-second order kinetic model Elovich model k1/min−1 Qe/(mg·g−1) R2 k2/min−1 Qe/(mg·g−1) R2 A B R2 10 0.1530 51.37 0.9999 0.0826 57.28 0.9874 34.53 −4.497 0.964 1 40 0.3131 140.0 0.9919 0.0116 147.5 0.9993 113.7 −7.579 0.9785 80 0.4497 211.9 0.9804 0.0066 224.1 0.9993 135.3 −22.17 0.9714 Notes: C0—Initial concentration of Cd(II); R2—Goodness; Qe—Adsorption capacity at adsorption equilibrium; k1, k2 and A—Constant of kinetic models, respectively; B—Coefficient of elovich kinetic models. 表 4 GO/CA水凝胶复合膜吸附Cd(II)的吸附等温线模型参数
Table 4 Isothermal adsorption model parameters of Cd(II) adsorbed by GO/CA hydrogel composite membrane
Temper-
ature/KFreundlich isotherm Langmuir isotherm kf N R2 kl Qm/(mg·g−1) R2 288 13.81 1.787 0.9445 0.1766 85.40 0.9914 303 47.99 2.770 0.9618 0.2168 161.8 0.9952 318 47.67 3.147 0.9589 0.3146 173.6 0.9981 Notes: kf—Capacity factor of Freundlich; N—Liquid phase adsorption isotherm index of Freundlich; k1—Langmuir constant of affinity point; Qm—Adsorption capacity of single layer. -
[1] SUN X F, HAO Y W, GAO Y Y, et al. Superadsor-bent hydrogel based on lignin and montmorillonite for Cu(II) ions removal from aqueous solution[J]. International Journal of Biological Macromolecules,2019,127:511-519. DOI: 10.1016/j.ijbiomac.2019.01.058
[2] YU Z Z, DANG Q F, LIU C S, et al. Preparation and characterization of poly(maleic acid)-grafted cross-linked chitosan microspheres for Cd(II) adsorption[J]. Carbohydrate Polymers,2017,172:28-39. DOI: 10.1016/j.carbpol.2017.05.039
[3] PENG H B, GAO P, CHU G, et al. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars[J]. Environmental Pollution,2017,229:846-853. DOI: 10.1016/j.envpol.2017.07.004
[4] SHARMA A, LEE B K. Cd(II) removal and recovery enhancement by using acrylamide-titanium nanocomposite as an adsorbent[J]. Applied Surface Science,2014,313(15):624-632.
[5] CUI L, WANG Y G, GAO L, et al. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property[J]. Chemical Engineering Journal,2015,281:1-10. DOI: 10.1016/j.cej.2015.06.043
[6] SASMAL D, KOLYA H, TRIPATHY T, et al. Amylopecting-poly(methylacrylate-co-sodium acrylate): An efficient Cd(II) binder[J]. International Journal of Biological Macromolecules,2016,91:934-945. DOI: 10.1016/j.ijbiomac.2016.06.022
[7] AHMAD Z, GAO B, MOSA A, et al. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass[J]. Journal of Cleaner Production,2017,180:437-449.
[8] SHAN Z B, ZAHNG L W, ZHAO X Y, et al. Removal of Pb(II), Cd(II) and Hg(II) from aqueous solution by mercapto modified coal gangue[J]. Journal of Environmental Management,2019,231:391-396.
[9] KARTHIK R, MEENAKSHI S. Removal of Cr(VI) ions by adsorption onto sodiumalginate-polyaniline nanofibers[J]. International Journal of Biological Macromolecules,2015,72:711-717. DOI: 10.1016/j.ijbiomac.2014.09.023
[10] LIM J Y, MUBARAK N M, ABDULLAH E C, et al. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals: A review[J]. Journal of Industrial and Engineering Chemistry,2018,66:29-44. DOI: 10.1016/j.jiec.2018.05.028
[11] CHEN J H, LIU Q L, HU S R, et al. Adsorption mechanism of Cu(II) ions from aqueous solution by glutaraldehydecrosslinkedhumic acid-immobilized sodium alginate porous membrane adsorbent[J]. Chemical Engineering Journal,2011,173:511-519. DOI: 10.1016/j.cej.2011.08.023
[12] ZHAO K Y, ZHANG X X, WEI J F, et al. Calcium alginate hydrogel filtration membrane with excellent anti-fouling property and controlled separation performance[J]. Journal of Membrane Science,2015,492:536-546. DOI: 10.1016/j.memsci.2015.05.075
[13] 朱振亚, 王磊, 姜家良, 等. 纳米SiO2-氧化石墨烯/聚偏氟乙烯杂化膜的制备及特性[J]. 复合材料学报, 2018, 35(4):785-792. ZHU Z Y, WANG L, JIANG J L, et al. Preparation and properties of nano SiO2-GO/polyvinylidene fluoride hybrid membrane[J]. Journal of Composite Materials,2018,35(4):785-792(in Chinese).
[14] LODEIRO P, FUENTES A, HERRERO R, et al. CrIII binding by surface polymers in natural biomass: The role of carboxylic groups[J]. Environmental Chemistry,2008,5(5):355-365. DOI: 10.1071/EN08035
[15] CHEN J H, LI G P, LIU Q L, et al. Cr(III) ionic imprinted polyvinyl alcohol/sodium alginate (PVA/SA) porous composite membranes for selective adsorption of Cr(III) ions[J]. Chemical Engneering Journal,2010,165(2):465-473. DOI: 10.1016/j.cej.2010.09.034
[16] NAG S, MONDAL A, ROY D N, et al. Sustainable bioremediation of Cd(II) from aqueous solution using natural waste materials: Kinetics, equilibrium, thermodynamics, toxicity studies and GA-ANN hybrid modeling[J]. Environmental Technology & Innovation,2018,11:83-104.
[17] 谢水波, 罗景阳, 刘清, 等. 羟乙基纤维素/海藻酸钠复合膜对六价铀的吸附性能及吸附机制[J]. 复合材料学报, 2015, 32(1):268-275. XIE S B, LUO J Y, LIU Q, et al. Adsorption characteristics and mechanism of hydroxyethyl cellulose/sodium alginate blend films for uranium(VI)[J]. Acta Materiae Compo-sitae Sinica,2015,32(1):268-275(in Chinese).
[18] DIVISEKARA T, NAVARATNE A N, ABEY-SEKARA A S K. Impact of a commercial glyphosate formulation on adsorption of Cd(II) and Pb(II) ions on paddy soil[J]. Chemosphere,2018,198:334-341. DOI: 10.1016/j.chemosphere.2018.01.155
[19] 于长江, 王苗, 董心雨, 等. 海藻酸钙@Fe3O4/生物碳磁性复合材料的制备及其对Co (II)的吸附性能和机制[J]. 复合材料学报, 2018, 35(6):1549-1557. YU C J, WANG M, DONG X Y, et al. Preparation and characterization of calcium alginate@Fe3O4/biochar magnetic microsphere and its adsorption characteristics and mechanism for Co (II)[J]. Acta Materiae Compositae Sinica,2018,35(6):1549-1557(in Chinese).
[20] ALGOTHMI W M, BANDARU N M, YU Y, et al. Alginate–graphene oxide hybrid gel beads: An efficient copper adsorbent material[J]. Journal of Colloid and Interface Science,2013,397:32-38. DOI: 10.1016/j.jcis.2013.01.051
-
期刊类型引用(16)
1. 薛丹,郭笑一,张浩田,李善建. 丙烯酰胺基互穿网络的制备及对Fe~(3+)的吸附. 工程塑料应用. 2025(01): 15-22 .  百度学术
百度学术
2. 张金梅,边亮,宋绵新,张琴,杨敬杰,聂嘉男,张娇,罗伟恢,解鑫,张鹏. 海藻酸钠-聚乙烯包覆贝得石复合材料(SA-Bd@PE)去除低浓度铀酰试验研究. 化工矿物与加工. 2024(03): 45-52 .  百度学术
百度学术
3. 代甜甜,刘晓南,张晓春,杨熙. 改性沸石/GA/CS膜的制备及Pb(Ⅱ)吸附性能研究. 现代塑料加工应用. 2024(04): 25-27+31 .  百度学术
百度学术
4. 李永宽,王曦,王醒,贾楠,李登新. UIO-66-NH_2/SA复合材料的制备及对含铅废水吸附性能的研究. 广东化工. 2023(15): 1-4 .  百度学术
百度学术
5. 李昕,杨早,钟欣茹,韩昊轩,庄绪宁,白建峰,董滨,徐祖信. 污泥超高温堆肥衍生胡敏酸对Pb~(2+)的结合机制. 化工进展. 2023(09): 4957-4966 .  百度学术
百度学术
6. 马培林,宋亚婷,李旭,刘志明. 复合水凝胶SA/AAm-Lgs的制备及其对Fe~(3+)的静/动态吸附. 生物质化学工程. 2023(05): 35-43 .  百度学术
百度学术
7. 刘锋,黄钦裕,陈燕舞. 大豆蛋白基多孔凝胶球对Pb(Ⅱ)的吸附研究. 黑龙江农业科学. 2023(11): 100-107 .  百度学术
百度学术
8. 邹成龙,徐志威,聂发辉,吴道,王佳琪. Fe_3O_4@SA/GO凝胶球的制备及对亚甲基蓝的吸附性能. 环境工程学报. 2022(01): 121-132 .  百度学术
百度学术
9. 郝军杰,郭成,高翔鹏,李明阳,龙红明. 硫脲/海藻酸钠对Cr(VI)的吸附和光催化还原协同去除机制. 复合材料学报. 2022(04): 1657-1666 .  本站查看
本站查看
10. 付秋平,张海润,石登红,娄杰,莫昌琍,罗军,严伟. ZIF-8@SA对Pb(Ⅱ)的吸附性能和机理研究. 化学试剂. 2022(05): 697-702 .  百度学术
百度学术
11. 付秋平,张海润,罗云,娄杰,石登红,莫昌琍,罗军,严伟. 海藻酸钠/聚乙烯亚胺复合材料对Pb(Ⅱ)的吸附性能研究. 化学试剂. 2022(10): 1457-1462 .  百度学术
百度学术
12. 狄婧,刘海霞,姜永强,郭金鑫,赵国虎. 聚吡咯/壳聚糖复合膜的制备及其对Cu(Ⅱ)和Cr(Ⅵ)吸附机制. 复合材料学报. 2021(01): 221-231 .  本站查看
本站查看
13. 郭成,高翔鹏,李明阳,郝军杰,龙红明,赵卓. 海藻酸钠基吸附材料去除水中重金属离子的研究进展. 过程工程学报. 2021(01): 3-17 .  百度学术
百度学术
14. 郭成,郝军杰,李明阳,龙红明,高翔鹏. 海藻酸钠/聚乙烯亚胺凝胶球的合成及对Cr(Ⅵ)的吸附性能和机制. 复合材料学报. 2021(07): 2140-2151 .  本站查看
本站查看
15. 张宏伟,谢鸿,肖欣荣,方志强. 不同氧化程度氧化石墨烯/聚乙烯醇气凝胶对亚甲基蓝的吸附. 复合材料学报. 2021(09): 2788-2795 .  本站查看
本站查看
16. 武鑫霞,曹占平,苏婷,李岚. Ce改性金属有机骨架材料对氟的吸附. 复合材料学报. 2020(10): 2636-2644 .  本站查看
本站查看
其他类型引用(9)
-




 下载:
下载: