Adsorption of Cr(Ⅵ) on porous sodium alginate/polyethyleneimine hydrogel beads and its mechanistic study
-
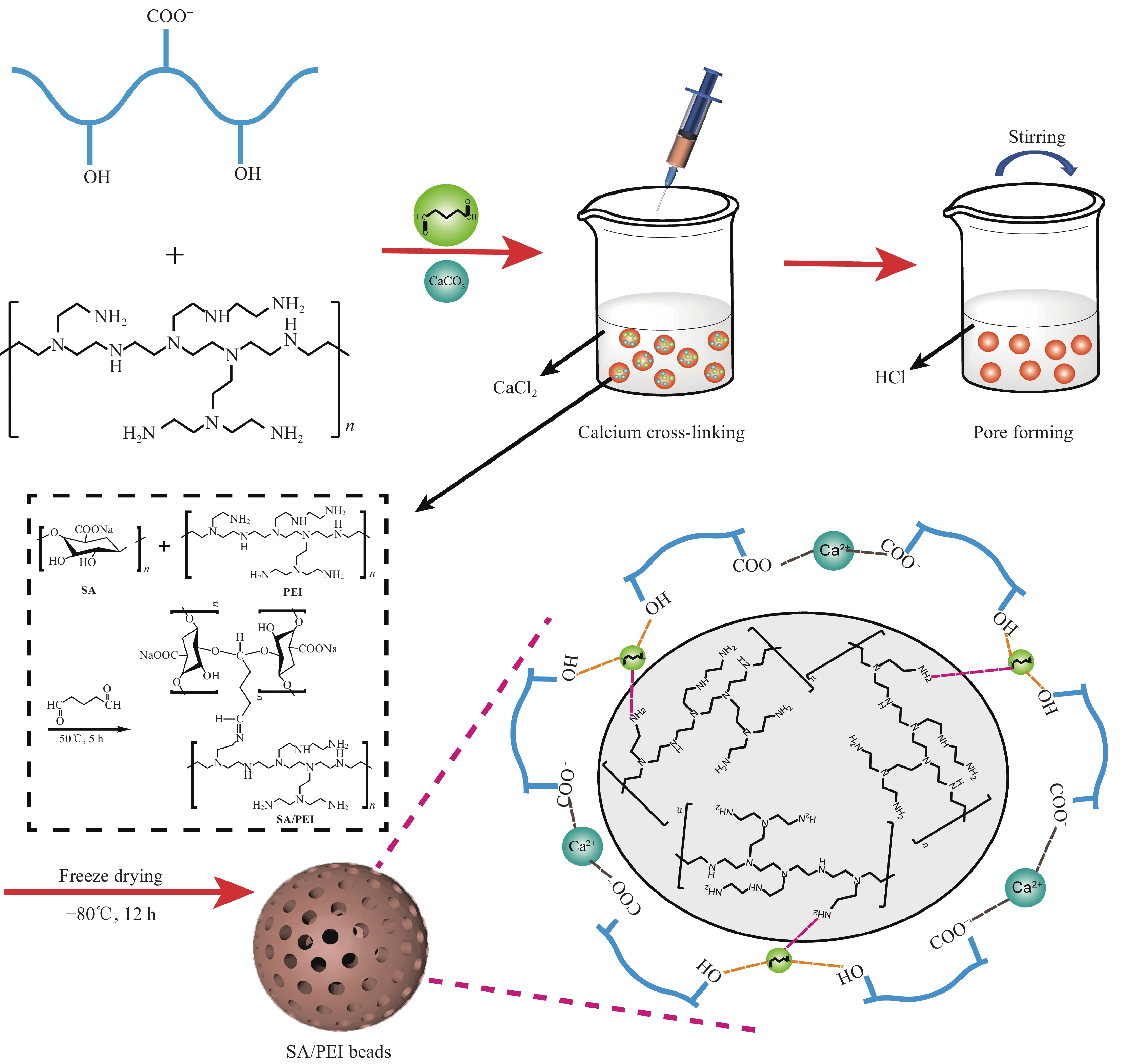
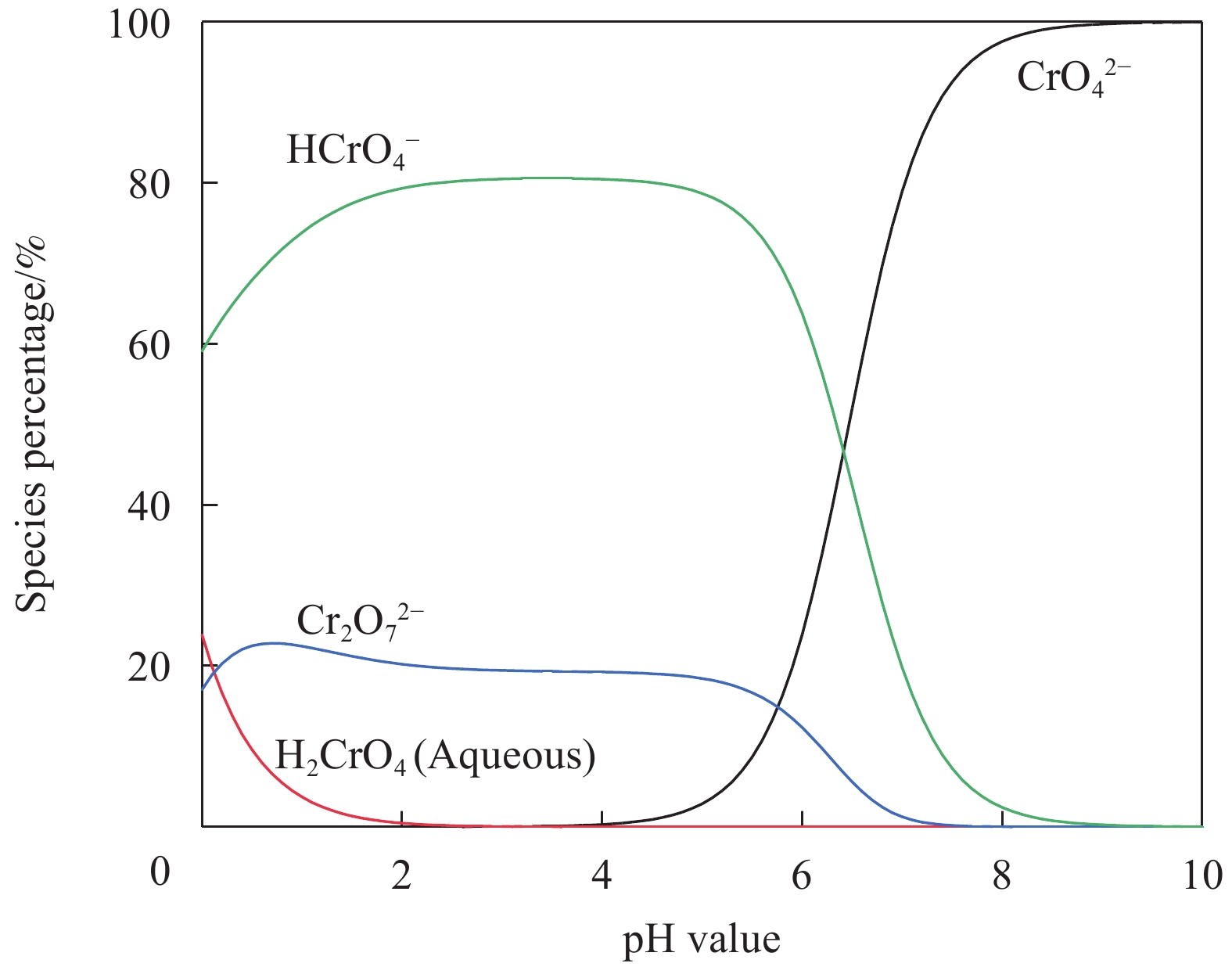
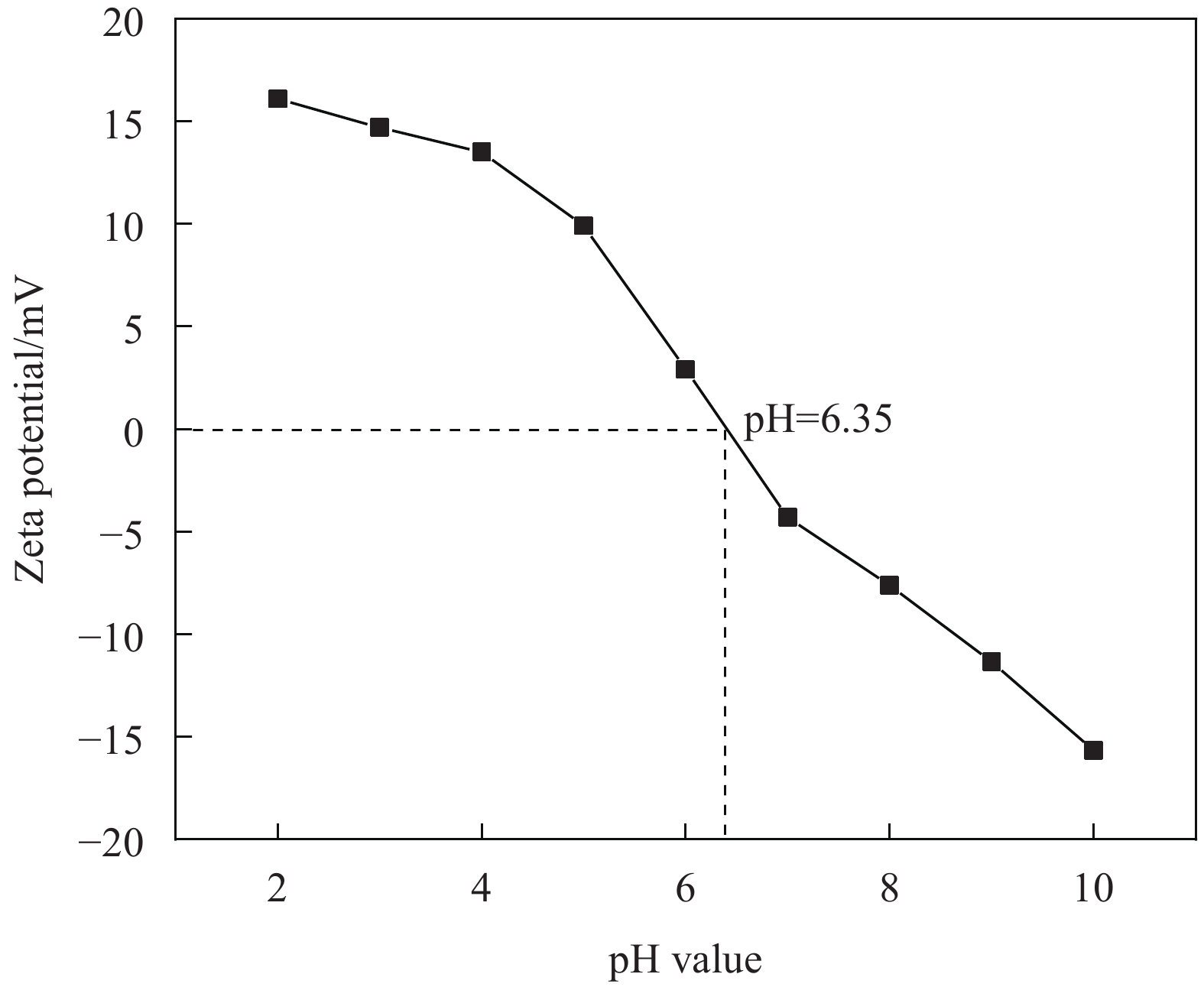
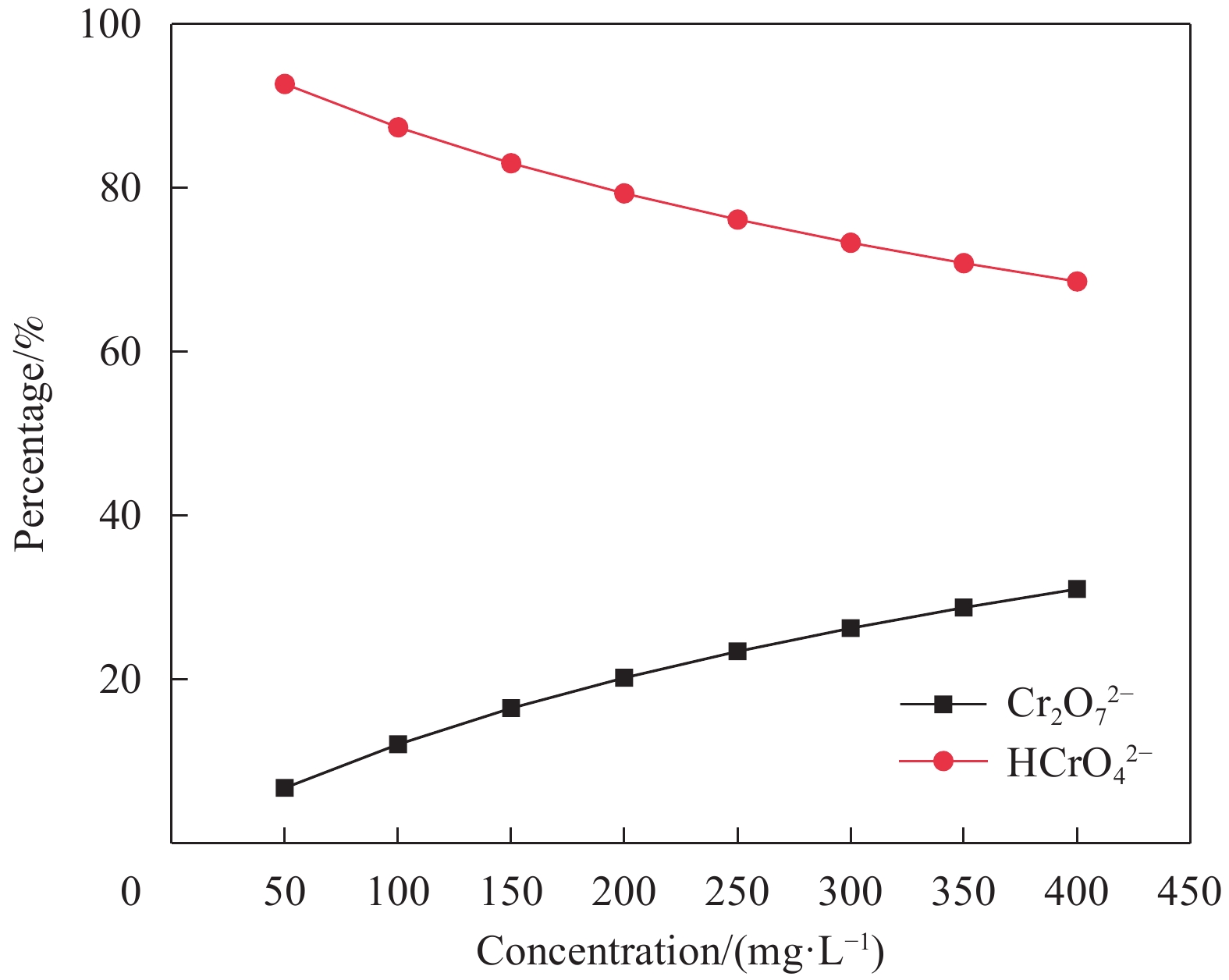
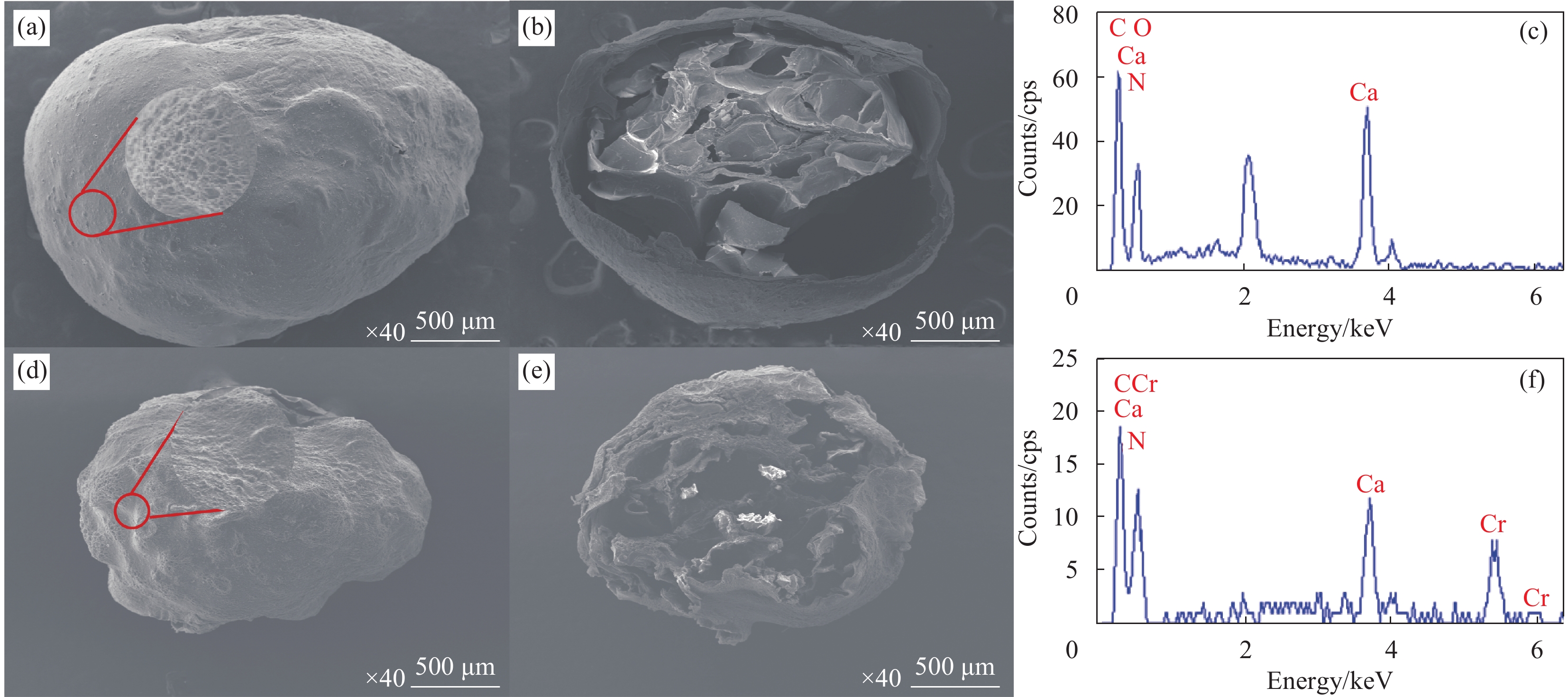
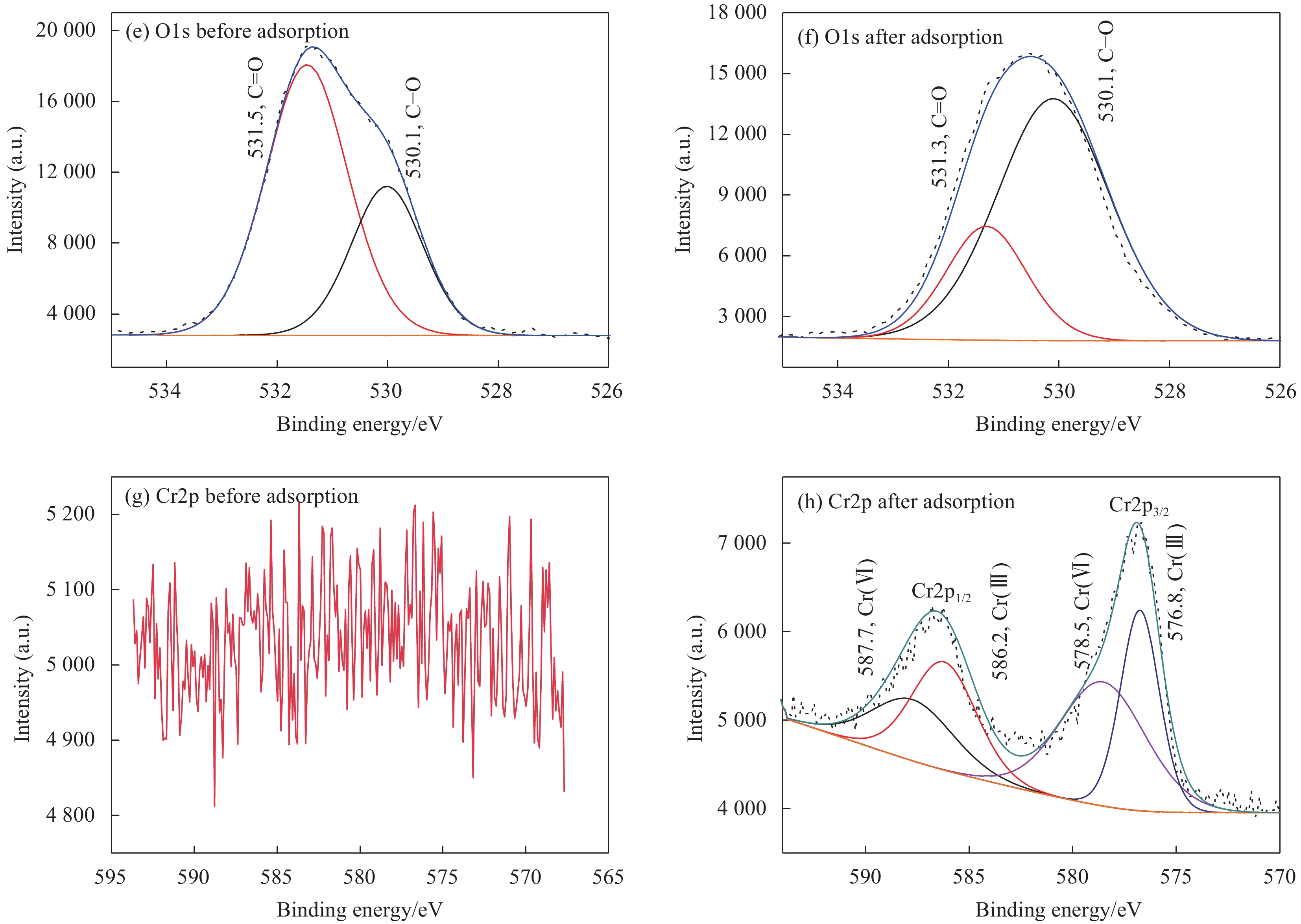
摘要: 海藻酸钠(SA)是一种生物质材料,具有来源广泛、价格低廉的特性,被众多科研人员用于实验室研究,制备成吸附剂去除水溶液中的金属离子。但目前制备的大多数SA基吸附材料是实心水凝胶状,具有比表面积较低、吸附速率慢、吸附容量小的缺点。本研究以SA为基体,向其中添加碳酸钙和聚乙烯亚胺(PEI),以戊二醛为交联剂,经冷冻干燥后制备出多孔的SA/PEI凝胶球,探究其对水溶液中Cr(Ⅵ)的吸附特性。通过改变实验条件,研究pH值、Cr(Ⅵ)初始浓度、吸附温度、吸附时间等对SA/PEI凝胶球吸附性能的影响;引入吸附动力学和热力学模型对吸附过程进行分析;采用FTIR、Zeta电位、SEM、XPS对SA/PEI凝胶球合成及吸附Cr(Ⅵ)机制进行综合分析。结果表明,SA/PEI凝胶球对Cr(Ⅵ)的去除率与初始浓度呈负相关;该吸附过程符合拟二级动力学和Langmuir等温吸附模型,且该吸附反应是自发的吸热过程,在温度为318.15 K、pH值为2时,Langmuir等温吸附拟合所得最大吸附量为262.83 mg/g。SA/PEI凝胶球对Cr(Ⅵ)的吸附机制主要为静电作用导致的物理吸附。Abstract: The sodium alginate (SA) is a biomass material which is abundant and can be easily acquired. It is currently used by many scientific researchers in laboratory research to prepare adsorbents to remove metal ions from aqueous solutions. However, SA based adsorbents generally exist as hydrogels, which are low in specific surface areas, slow in adsorption rates and have low adsorption capacities. In this study, calcium carbonate and polyethyleneimine (PEI) were added to SA matrix, and glutaraldehyde was used as a crosslinking agent to prepare porous SA/PEI beads via freeze-drying. The adsorption characteristics of synthesized adsorbent for Cr(Ⅵ) in aqueous solution were studied. The adsorption behaviors of Cr(Ⅵ) ions were evaluated by varying the experimental conditions including pH values, initial metal ion concentrations, adsorption temperature and adsorption time. Adsorption kinetics and thermodynamic models were applied to analyze the adsorption process. Characterization methods, including FTIR, Zeta potential, SEM, and XPS were comprehensively used to analyze the synthesis mechanism of SA/PEI beads and the mechanism of Cr(Ⅵ) adsorption. The results show that the removal rate of Cr(Ⅵ) by SA/PEI beads is negatively related to the initial concentration; the adsorption process conforms to the pseudo-second-order kinetics and Langmuir isotherm adsorption model, and the adsorption reaction is a spontaneous endothermic process. When the temperature is 318.15 K and the pH value is 2, the Langmuir isotherm adsorption fitting shows that the maximum adsorption capacity is 262.83 mg/g. The adsorption mechanism of SA/PEI beads on Cr(Ⅵ) is mainly physical adsorption dominated by electrostatic interactions.
-
Keywords:
- sodium alginate /
- hydrogel /
- adsorption mechanism /
- Cr(Ⅵ) /
- adsorbent /
- chemical modification
-
工程用水泥基复合材料(Engineered cementitious composites,ECC)是一种通过微观力学和断裂力学原理对材料体系进行系统设计和优化的短纤维乱向水泥基复合材料[1-2],国内外学者围绕ECC的受拉、受弯等基本力学性能开展了大量相关研究工作,并取得了众多优异成果[3-8]。然而ECC的抗拉强度仍然有限,用于结构加固时对构件的抗拉、抗弯承载力和抗裂性能的增强效果有限,仍需与性能优异的增强材料共同使用,为此,众多学者相继开展筋材、网材等用于增强ECC受力性能的研究。
Fischer等[9]、Mihashi等[10]进行了钢筋增强ECC单轴拉伸试验,结果表明,钢筋增强ECC试件裂缝分布更加均匀,减小了裂缝宽度,提高了变形能力。郑宇宙等[11]、朱忠锋等[12]对纤维增强ECC复合材料进行了单轴拉伸试验,结果表明,增强材料可以明显改善ECC的受拉性能,其轴向刚度、极限承载力也得到了显著提高。但是已研究的增强材料中,采用普通钢筋时,ECC的优越性能不能完全发挥,使用纤维编织网增强材料又降低了经济性。而高强不锈钢绞线抗拉强度高,具有良好的耐腐蚀性能和经济性。相关学者对高强不锈钢绞线加固混凝土结构的研究表明,其能有效提高加固构件的抗弯、抗剪承载力和刚度[13-17]。目前该加固技术已成功应用于实际工程,并取得了优异的效果[18-19]。
综上分析,高强不锈钢绞线网是由不锈钢绞线编制而成,本身具有很强的耐腐蚀、抗锈蚀能力;ECC具有良好的裂缝分散能力,正常使用状态裂缝宽度很小[1-10],二者结合后,可以使ECC开裂后钢绞线不发生锈蚀,耐久性好。因此,朱俊涛等[20]提出了新型高性能复合材料“高强不锈钢绞线网增强ECC”。已对高强不锈钢绞线网与ECC的粘结锚固性能[21-22]进行了试验和理论研究,结果表明,在试验所得的粘结滑移曲线中,平均粘结应力达到峰值开始微下降至峰值的85%左右之后,即进入延性强化段,该阶段平均粘结应力不再降低,仅滑移量呈水平线增长,说明高强不锈钢绞线网与ECC具有良好的粘结锚固性能。为了尽快使该复合材料在实际工程中得到应用,本文对高强不锈钢绞线网增强ECC的受拉性能进行试验和理论研究。
1. 试验概况
1.1 试件设计及材性试验
为了研究ECC抗拉强度、高强不锈钢绞线网受拉方向配筋率及高强不锈钢绞线网增强ECC试件宽度三个影响因素对高强不锈钢绞线增强ECC抗拉性能的影响,共设计了9组试件,每组3个共27个试件,各组试件参数见表1。为了避免试件在加载过程中在变截面处应力集中破坏,本试验受拉试件均采用哑铃型试件,试件变截面处为弧形,试件示意图如图1所示。试验中ECC的两种配合比见刘伟康[5]研究成果,试件标准养护28天。对预留的ECC受拉薄板试件(280 mm×40 mm×15 mm)进行拉伸试验,得到两种ECC配合比的抗拉性能,各组试件的ECC抗拉性能见表2。高强不锈钢绞线直径均为2.4 mm,对其进行拉伸试验(3根),得到高强不锈钢绞线应力-应变曲线如图2所示。
表 1 高强不锈钢绞线网增强工程水泥基复合材料(ECC)试件参数Table 1. Parameters of engineered cementitious composites(ECC) reinforced by high-strength stainless steel wire mesh specimensGroup number Test section width bc /mm Steel strand spacing/mm Reinforcement ratio of steel strand TC12 80 50 0.0028 TC22 70 40 0.0032 TC32 60 30 0.0037 TC42 50 20 0.0048 TC52 90 30 0.0037 TD12 80 50 0.0028 TD22 70 40 0.0032 TD32 60 30 0.0037 TD42 50 20 0.0048 表 2 ECC受拉试验结果Table 2. Tensile test results of ECCGroup number Tensile strength/MPa Ultimate tensile strain Cracking stress/MPa Cracking strain TC12 3.53 0.0179 2.45 0.000204 TC22 3.53 0.0179 2.45 0.000204 TC32 3.53 0.0179 2.45 0.000204 TC42 3.53 0.0179 2.45 0.000204 TC52 3.53 0.0179 2.45 0.000204 TD12 3.46 0.0297 2.39 0.000189 TD22 3.46 0.0297 2.39 0.000189 TD32 3.46 0.0297 2.39 0.000189 TD42 3.46 0.0297 2.39 0.000189 1.2 加载及测试方法
单轴拉伸试验采用100 kN电液伺服万能试验机。试验加载采用位移控制,加载速率为0.2 mm/min。试件正反面分别粘贴应变片,测试区段两侧面分别布置量程为30 mm的位移计。
1.3 试验现象及机制分析
高强不锈钢绞线网增强ECC试件受拉试验的加载装置如图3(a)所示。当荷载达到极限荷载的25%~30%时,在试件接近中间位置表面出现一条细微裂缝(如图3(b)所示),宽度仅为0.02 mm。随着荷载的增加,试件表面不断出现新的裂缝,已有的裂缝宽度缓慢增大;当荷载增加到极限荷载的85%~90%,裂缝数量达到饱和状态(如图3(c)所示),试件表面布满相互平行的细微裂缝,此时各试件的最大裂缝宽度为0.2~0.28 mm,该状态的荷载远远超过了规范GB 50010—2010[23]规定的正常使用极限状态。继续加载,试件表面不再出现新裂缝,已有裂缝变宽。达到极限荷载时,试件表面的某条裂缝发展成主裂缝,同时伴随着明显的纤维拔出和断裂声,随后主裂缝处的纵向高强不锈钢绞线拉断。试件破坏形态如图3(d)所示。
分析上述试验现象发现,高强不锈钢绞线的加入增加了ECC裂缝分散能力,其机制主要是钢绞线表面的螺旋进一步限制了ECC裂缝的开展,使受拉试件在达到极限承载力的85%~90%时,最大裂缝宽度依然满足规范GB 50010—2010[23]中正常使用极限状态裂缝要求。充分表明了本文研究的新型复合材料高强不锈钢绞线网增强ECC具有良好的抗裂性能;验证了高强不锈钢筋网和ECC具有很好的粘结性能。
2. 试验结果及分析
2.1 试验结果
各组高强不锈钢绞线网增强ECC试件拉伸试验结果见表3 (试验值均取同一组试件的平均值,应力为试验拉力除以受力面积)。
表 3 高强不锈钢绞线网增强ECC试件拉伸试验结果Table 3. Tensile test results of ECC reinforced by high-strength stainless steel wire mesh specimensGroup number Cracking stress/MPa Cracking strain Ultimate tensile stress/MPa Ultimate tensile strain Elastic modulus/MPa TC12 2.20 0.000156 7.09 0.030874 14 471 TC22 2.25 0.000162 7.94 0.034056 14 122 TC32 2.41 0.000171 8.77 0.030581 14 492 TC42 2.56 0.000182 10.65 0.034815 14 578 TC52 2.54 0.000181 8.82 0.033683 14 642 TD12 1.97 0.000145 6.41 0.031929 14 216 TD22 1.99 0.000149 7.81 0.043660 14 727 TD32 2.07 0.000139 8.62 0.041116 14 460 TD42 2.19 0.000159 10.30 0.035051 14 496 2.1.1 试件宽度对高强不锈钢绞线网增强ECC受拉性能的影响
对比表3中TC32组和TC52组试件,结果表明,增大截面宽度对受拉试件的开裂应力和极限应力几乎没有影响。
2.1.2 高强不锈钢绞线配筋率对高强不锈钢绞线网增强ECC受拉性能的影响
对比表3中TC12~TC42及TD12~TD42试件的试验结果可知,随着高强不锈钢绞线配筋率的增大,受拉试件的开裂应力略有增大,极限应力明显增大。
2.1.3 ECC抗拉强度对高强不锈钢绞线网增强ECC受拉性能的影响
对比表3中TC12组与TD12组、TC22组与TD22组、TC32组与TD32组及TC42组与TD42组试件结果可知,随着ECC抗拉强度的增大,受拉试件的开裂应力和极限应力均明显增大。
2.2 高强不锈钢绞线网增强ECC受拉应力-应变曲线
高强不锈钢绞线网增强ECC试件在单轴荷载作用下的受拉应力-应变曲线如图4所示。可知,高强不锈钢绞线网增强ECC试件受拉应力-应变曲线呈现出明显的两阶段特征,故可将其分为两个阶段:(1)可表征为弹性阶段,从试件开始加载到试件开裂为止,为峰值的25%~30%。在该阶段ECC和不锈钢绞线均处于弹性工作状态;应力-应变曲线接近为直线;(2)可表征为弹塑性阶段,对应于试件ECC开裂至完全破坏(纵向高强不锈钢绞线拉断)。该阶段应力-应变曲线为非线性关系,试件表现出明显的弹塑性性质。此阶段主要是ECC裂缝出现和发展,由于裂缝处的聚乙烯醇(PVA)纤维尚未从水泥基中拔出或拉断,而高强不锈钢绞线表面的螺旋增大了与ECC的粘结力。因此,该阶段ECC和高强不锈钢绞线依然共同承担拉力,亦是高强不锈钢绞线网增强ECC受拉的主要阶段。由图4还可以看出,极限应力是开裂应力的3~5倍,极限应变为开裂应变的200倍以上,达到3%~5%,超过了本文采用的ECC和高强不锈钢绞线本身的极限拉应变,显示了该新型高性能复合材料优越的变形能力。
3. 高强不锈钢绞线网增强ECC受拉本构模型
3.1 模型提出
由图4可知,高强不锈钢绞线网增强ECC受拉应力-应变曲线可分为弹性阶段(OA段)和应变硬化阶段(AB段)。弹性阶段即认为应力-应变为线性关系;弹塑性(应变硬化)阶段应力由ECC和钢绞线共同承担,其本构模型由二者应力-应变关系组合而成。同时考虑该阶段高强不锈钢绞线网和ECC可能产生相对滑移,用γ表示钢绞线的应力发挥系数。本文提出了高强不锈钢绞线网增强ECC受拉本构模型,如图5所示。其表达式如下:
σse={Eseεse(εse⩽ (1) 式中:σse、σse,cr和σse,u分别为高强不锈钢绞线网增强ECC试件的受拉应力、开裂应力和极限拉应力;σe和σs分别为ECC和高强不锈钢绞线的应力;Ese为高强不锈钢绞线网增强ECC的弹性模量;εse、εse,cr和εse,u分别为高强不锈钢绞线网增强ECC试件的受拉应变、开裂应变和极限拉应变;γ为高强不锈钢绞线的应力发挥系数;ρs为高强不锈钢绞线的配筋率。
3.2 模型参数分析
3.2.1 弹性阶段
高强不锈钢绞线网增强ECC受拉试件开裂前,由高强不锈钢绞线和ECC共同承担外部荷载;该阶段高强不锈钢绞线网与ECC间无滑移。根据应变协调原理得出弹性阶段高强不锈钢绞线网增强ECC受拉试件的弹性模量Ese如下式:
{E_{\rm{se}}} = {E_{\rm{e}}} + {E_{\rm{s}}}\frac{{{A_{\rm{s}}}}}{{{A_{\rm{se}}}}} (2) 式中:Ee和Es分别为ECC和高强不锈钢绞线的弹性模量;Ase和As分别为高强不锈钢绞线网增强ECC和高强不锈钢绞线的截面面积。
3.2.2 弹塑性阶段
首先建立高强不锈钢绞线受拉本构模型。对本试验得到的高强不锈钢绞线应力-应变曲线(图2)采用三次多项式进行拟合,如下式:
\frac{{{\sigma _{\rm{s}}}}}{{{\sigma _{\rm{s,u}}}}} = a\frac{{{\varepsilon _{\rm{s}}}}}{{{\varepsilon _{\rm{s,u}}}}} + (3 - 2a){\left( {\frac{{{\varepsilon _{\rm{s}}}}}{{{\varepsilon _{\rm{s,u}}}}}} \right)^2} + (a - 2){\left( {\frac{{{\varepsilon _{\rm{s}}}}}{{{\varepsilon _{\rm{s,u}}}}}} \right)^3}\tag{3a} (3a) 式中:σs,u为高强不锈钢绞线的极限拉应力;εs和εs,u分别为高强不锈钢绞线应变和极限拉应变。
根据式(3a),基于最小二乘法对试验数据进行回归分析,得到待定常数a的值。对三组数据进行拟合,其值分别为3.37、3.26和3.36。由拟合结果可知,各组值较接近,故可取其平均值a=3.33。将a代入式(3a),则有:
\frac{{{\sigma _{\rm{s}}}}}{{{\sigma _{\rm{s,u}}}}} = 3.33\frac{{{\varepsilon _{\rm{s}}}}}{{{\varepsilon _{\rm{s,u}}}}} - 3.66{\left( {\frac{{{\varepsilon _{\rm{s}}}}}{{{\varepsilon _{\rm{s,u}}}}}} \right)^2} + 1.33{\left( {\frac{{{\varepsilon _{\rm{s}}}}}{{{\varepsilon _{\rm{s,u}}}}}} \right)^3}\tag{3b} (3b) ECC的受拉应力-应变关系采用刘伟康[5]的研究成果,如下式:
\!\!\!\!\!\!\quad{\sigma _{\rm{e}}} = \left\{ {\begin{array}{*{20}{l}} {\dfrac{{{\sigma _{\rm{e,cr}}}}}{{{\varepsilon _{\rm{e,cr}}}}}{\varepsilon _{\rm{e}}}}&{\;\;\left( {{\varepsilon _{\rm{e}}} \leqslant {\varepsilon _{\rm{e,cr}}}} \right)}\\ {\left( {0.31\dfrac{{{\varepsilon _{\rm{e}}}}}{{{\varepsilon _{\rm{e,u}}}}} + 0.69} \right){\sigma _{\rm{e,u}}}}&{\left( {{\varepsilon _{\rm{e,cr}}} \leqslant {\varepsilon _{\rm{e}}} \leqslant {\varepsilon _{\rm{e,u}}}} \right)} \end{array}} \right. (4) 式中:σe,cr和σe,u为ECC的开裂应力和极限拉应力;εe、εe,cr和εe,u为ECC的应变、开裂应变和极限拉应变。
3.2.3 高强不锈钢绞线应力发挥系数γ
将式(2)、式(3b)和式(4)代入式(1),则高强不锈钢绞线网增强ECC受拉本构关系可表示为
{\sigma _{\rm{se}}}{\rm{ = }}\left\{ {\begin{array}{*{20}{l}} {\left( {{E_{\rm{e}}} + {E_{\rm{s}}}\dfrac{{{A_{\rm{s}}}}}{{{A_{\rm{se}}}}}} \right){\varepsilon _{\rm{se}}}} \\ \begin{array}{l} \!\!\!\!\!\!\!\!\gamma \left[ {{\rm{3}}{\rm{.33}}\dfrac{{{\varepsilon _{\rm{se}}}}}{{{\varepsilon _{\rm{se,u}}}}}{\rm{ - 3}}{\rm{.66}}{{\left( {\dfrac{{{\varepsilon _{\rm{se}}}}}{{{\varepsilon _{\rm{se,u}}}}}} \right)}^2}{\rm{ + 1}}{\rm{.33}}{{\left( {\dfrac{{{\varepsilon _{\rm{se}}}}}{{{\varepsilon _{\rm{se,u}}}}}} \right)}^3}} \right]{\sigma _{\rm{s,u}}}\dfrac{{{A_{\rm{s}}}}}{{{A_{\rm{se}}}}} +\\ \!\! \left( {{\rm{0}}{\rm{.31}}\dfrac{{{\varepsilon _{\rm{se}}}}}{{{\varepsilon _{\rm{se,u}}}}} + 0.69} \right){\sigma _{\rm{e,u}}} \\ \end{array} \end{array}} \right. (5) 基于应变协调,高强不锈钢绞线网增强ECC的开裂应变与ECC相等,即εse,cr=εe,cr;同时高强不锈钢绞线网增强ECC受拉试件的破坏形态为纵向高强不锈钢绞线拉断破坏,其极限应变主要取决于高强不锈钢绞线的极限应变大小,即εse,u=εs,u。
分别将开裂状态和破坏状态的应变值代入式(5),利用MATLAB软件进行分析,得到式(5)中高强不锈钢绞线应力发挥系数γ的值,如表4所示。可知,各组值较接近,故取其平均值(0.725)作为式(5)中γ的值。
表 4 高强不锈钢绞线应力发挥系数γ取值Table 4. Value of stress development coefficient γ of high-strength stainless steel strandGroup number γ R2 TC12 0.73 0.9267 TC22 0.72 0.9459 TC32 0.76 0.9607 TD12 0.71 0.9157 TD22 0.72 0.9755 TD32 0.71 0.9325 3.2.4 开裂应力和抗拉强度公式
基于高强不锈钢绞线网增强ECC受拉试件的本构关系模型(式(5)),推导出高强不锈钢绞线网增强ECC受拉试件的开裂应力和极限拉应力即抗拉强度的计算公式:
\left\{ {\begin{array}{*{20}{l}} {{\sigma _{\rm{se,cr}}} = \left( {{E_{\rm{e}}} + {E_{\rm{s}}}\dfrac{{{A_{\rm{s}}}}}{{{A_{\rm{se}}}}}} \right){\varepsilon _{\rm{se,cr}}}} \\ {{f_{\rm{se,u}}} = 0.725{\sigma _{\rm{s,u}}}\dfrac{{{A_{\rm{s}}}}}{{{A_{\rm{se}}}}} + {\sigma _{\rm{e,u}}}} \end{array}} \right. (6) 式中,fse,u为高强不锈钢绞线网增强ECC受拉试件的极限应力,即抗拉强度。
3.3 模型验证
为验证本文提出的高强不锈钢绞线网增强ECC受拉本构模型,将试验得出的应力-应变曲线与式(5)计算的应力-应变曲线进行对比,如图6所示。将试验得出的开裂应力和极限拉应力(抗拉强度)与式(6)计算的结果进行对比,如表5所示。
表 5 试验与式(6)计算得到的高强不锈钢绞线网增强ECC试件开裂应力和极限应力的对比Table 5. Comparison of cracking stress and ultimate tensile stress of ECC reinforced by high-strength stainless steel wire mesh specimens obtained by test and equation (6)Group number Cracking stress/MPa Ultimate tensile stress/MPa T C R T C R TC12 2.20 2.26 0.97 7.09 6.79 1.04 TC22 2.25 2.27 0.99 7.94 7.25 1.10 TC32 2.41 2.28 1.06 8.77 7.87 1.11 TC42 2.56 2.30 1.11 10.65 9.19 1.16 TC52 2.54 2.28 1.11 8.82 7.87 1.12 TD12 1.97 1.94 1.01 6.41 6.58 0.97 TD22 1.99 1.95 1.02 7.81 7.03 1.11 TD32 2.07 1.96 1.06 8.62 7.63 1.13 TD42 2.19 1.98 1.11 10.30 8.90 1.16 Notes: T—Test value; C—Calculated value; R—T/C. 从图6可看出,本文提出的本构模型与试验应力-应变曲线整体趋势完全一致。其中,本文所提的受拉本构关系曲线的弹性阶段与试验值基本重合,符合良好;其弹塑性阶段与试验曲线弯曲度均吻合较好(TD42稍有差别),仅试验在ECC开裂后,荷载有微小波动,属于正常情况。因此,本文提出的高强不锈钢绞线网增强ECC受拉本构模型能较准确地预测受拉应力-应变关系全曲线。表5中的高强不锈钢绞线网增强ECC受拉试件的开裂应力试验值与计算值比值的平均值为1.05,标准差为0.05,变异系数为0.05;极限应力试验值与计算值比值的平均值为1.10,标准差为0.06,变异系数为0.05,说明本文提出的式(6)计算结果和试验值吻合良好。
综上所述,本文提出的高强不锈钢绞线网增强ECC受拉本构模型和开裂应力、抗拉强度计算公式可用于反映高强不锈钢绞线网增强ECC受拉状态的应力-应变关系和分析其受拉性能。
4. 结 论
对高强不锈钢绞线网增强工程水泥基复合材料(Engineered cementitious composites,EEC) 的受拉性能进行试验和理论研究,提出了其受拉本构关系模型。
(1)高强不锈钢绞线表面的螺旋进一步限制了ECC裂缝的开展,增加了ECC裂缝的分散能力,使受拉试件在达到极限承载力的85%时,最大裂缝宽度依然小于规范GB 50010—2010[23]中正常使用极限状态裂缝要求。充分显示了高强不锈钢绞线网增强ECC的良好抗裂性能。
(2)高强不锈钢绞线网增强ECC受拉应力-应变曲线分为弹性阶段和弹塑性阶段,弹塑性阶段是其主要受拉阶段,该阶段是ECC和钢绞线共同受拉至钢绞线拉断,极限应变可达到3%~5%,显示了该新型复合材料优越的变形能力。
(3)随着ECC抗拉强度提高和纵向钢绞线配筋率增大,高强不锈钢绞线网增强ECC的开裂应力和极限拉应力(抗拉强度)均增大。
(4)所建立的受拉本构模型及模型参数计算公式与试验结果吻合良好,能够较好地反映高强不锈钢绞线网增强ECC的受拉应力-应变关系,预测其受力状态;所提出的高强不锈钢绞线网增强ECC受拉开裂应力、抗拉强度的计算公式与试验结果吻合良好,可以用于分析该新型复合材料受拉性能。
-
表 1 各类试剂及其性能
Table 1 Various reagents and properties
Reagent Property Sodium alginate Viscosity: 200−500 mPa.s Polyethyleneimine Molecular weight Mw=600 Glutaraldehyde Purity 25%−28% CaCO3 Analytical reagent 1,5-Diphenylcarbohydrazide Analytical reagent K2Cr2O7 Analytical reagent CaCl2 Analytical reagent 表 2 SA/PEI凝胶球吸附Cr(Ⅵ)的热力学参数
Table 2 Thermodynamic parameters of Cr(Ⅵ) adsorbed by SA/PEI hydrogel beads
T/K \ln {K_{\rm{c}}} \Delta G/(kJ·mol−1) \Delta H/(kJ·mol−1) \Delta S/(J(mol·K)−1) 298.15 1.83 −4.54 17.52 74.58 308.15 1.98 −5.07 318.15 2.45 −6.48 Notes: T—Absolute temperature; Kc—Equilibrium constant; ΔG—Gibbs free energy; ΔH—Enthalpy change; ΔS—Entropy change. 表 3 SA/PEI凝胶球吸附Cr(Ⅵ)的动力学模型拟合参数
Table 3 Kinetic parameters of pseudo-first/second-order models for Cr(Ⅵ) adsorption by SA/PEI hydrogel beads
C0/(mg·L−1) Pseudo-first-order kinetic model Pseudo-second-order kinetic model K1/(mg(g·h−1)−1) qe/(mg·g−1) R2 K2/(mg(g·h−1)−1) qe/(mg·g−1) R2 100 0.5829 91.3769 0.9900 0.0101 99.0000 0.9473 200 0.5031 167.3778 0.9602 0.0036 191.4030 0.9433 Notes: C0—Initial concentration of Cr(Ⅵ); K1, K2—Rate constants for pseudo-first-order and pseudo-second-order equations, respectively; qe—Amounts of metal adsorbed at equilibrium; R2—Goodness-of-fitting. 表 4 SA/PEI凝胶球去除Cr(Ⅵ)的Langmuir和Freundlich等温线模型参数
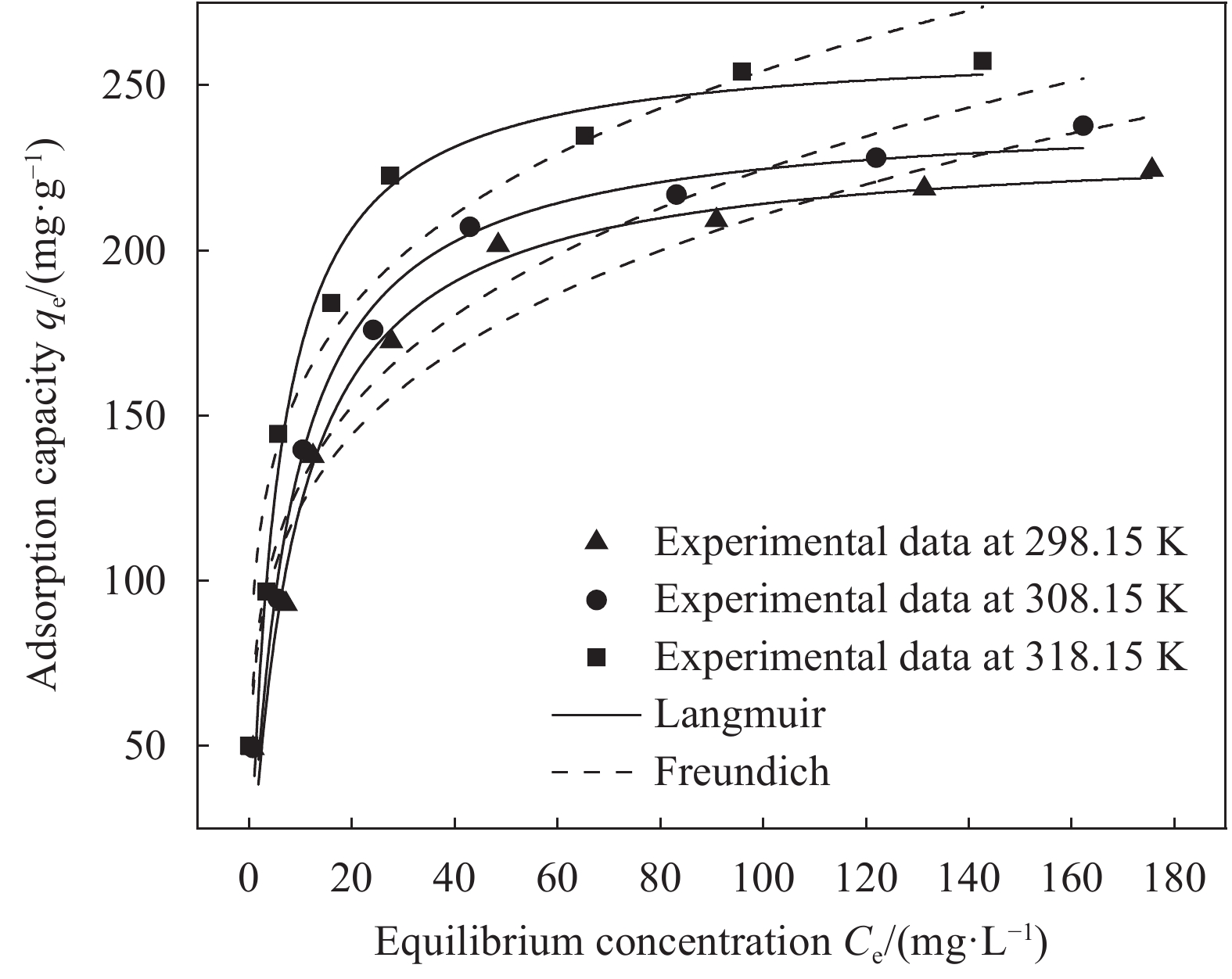
Table 4 Parameters of Langmuir and Freundlich isotherms on Cr(Ⅵ) removal by SA/PEI hydrogel beads
T/K Langmuir isotherm Freundlich isotherm KL/(L·mg−1) qm/(mg·g−1) R2 KF/(L·mg−1) n R2 298.15 0.1113 233.31 0.9587 71.24 4.2827 0.9337 308.15 0.1286 242.07 0.9679 74.67 4.1841 0.9438 318.15 0.1837 262.83 0.9826 99.09 4.8828 0.9077 Notes: KL—Langmuir isotherm constant; KF, n—Two Freundlich isotherm constants; qm—Maximum metal uptake capacity. 表 5 SA/PEI凝胶球与已报道的SA基吸附剂去除Cr(Ⅵ)性能比较
Table 5 Comparison of adsorption capacity of Cr(Ⅵ) removal by SA/PEI hydrogel beads and others reported SA based adsorbents
Adsorbent Adsorption capacity/(mg·g−1) Reference SA-polyaniline nanofibers 75.82 [16] TEPA functionalized alginate beads 77.00 [29] Nanoscale zerovalent iron/biochar/Ca-alginate beads 86.40 [30] Nano zero-valent iron/carbon/alginate composite gel 35.25 [31] Magnetic nano-hydroxyapatite encapsulated alginate beads 29.14 [32] Fe nanoparticles embedded graphene oxide alginate beads 33.90 [33] SA/PEI 262.83 This work -
[1] MARIA C G. Toxic and mutagenic effects of chromium(Ⅵ): A review[J]. Polyhedron,1996,15(21):3667-3689. DOI: 10.1016/0277-5387(96)00141-6
[2] CARLOS E B D, VIOLETA L L, BILYEU B. A review of chemical, electrochemical and biological methods for aqueous Cr(Ⅵ) reduction[J]. Journal of Hazardous Materials,2012,223-224:1-12.
[3] CESPONROMERO R M, YEBRABIURRUN M C, BERMEJOBARRERA M P. Preconcentration and speciation of chromium by the determination of total chromium and chromium(Ⅲ) in natural waters by flame atomic absorption spectrometry with a chelating ion-exchange flow injection system[J]. Analytica Chimica Acta,1996,327(1):37-45. DOI: 10.1016/0003-2670(96)00062-1
[4] JOBBY R, JHA P, YADAV A K, et al. Biosorption and biotransformation of hexavalent chromium [Cr(Ⅵ)]: A comprehensive review[J]. Chemosphere,2018,207:255-266. DOI: 10.1016/j.chemosphere.2018.05.050
[5] SARIN V, SINGH T S, PANT K K. Thermodynamic and breakthrough column studies for the selective sorption of chromium from industrial effluent on activated eucalyptus bark[J]. Bioresource Technology,2006,97(16):1986-1993. DOI: 10.1016/j.biortech.2005.10.001
[6] FAN S, ZHOU J, ZHANG Y, et al. Preparation of sugarcane bagasse succinate/alginate porous gel beads via a self-assembly strategy: Improving the structural stability and adsorption efficiency for heavy metal ions[J]. Bioresource Technology,2020,306:123128. DOI: 10.1016/j.biortech.2020.123128
[7] FU F, WANG Q. Removal of heavy metal ions from wastewaters: A review[J]. Journal of Environmental Management,2011,92(3):407-418. DOI: 10.1016/j.jenvman.2010.11.011
[8] GAO X, LIU J, LI M, et al. Mechanistic study of selective adsorption and reduction of Au(Ⅲ) to gold nanoparticles by ion-imprinted porous alginate microspheres[J]. Chemical Engineering Journal,2020,385:123897. DOI: 10.1016/j.cej.2019.123897
[9] ZENG H, WANG L, ZHANG D, et al. Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent[J]. Chemical Engineering Journal,2019,358:253-263. DOI: 10.1016/j.cej.2018.10.001
[10] HU Z H, OMER A M, OUYANG X K, et al. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(Ⅱ) from aqueous solution[J]. International Journal of Biological Macromolecules,2018,108:149-157. DOI: 10.1016/j.ijbiomac.2017.11.171
[11] LI Z, WANG Z, WANG C, et al. Preparation of magnetic resin microspheres M-P(MMA-DVB-GMA) and the adsorption property to heavy metal ions[J]. Applied Surface Science,2019,496:143708. DOI: 10.1016/j.apsusc.2019.143708
[12] YAN R, QIU Z, BIAN X, et al. Effective adsorption of antimony from aqueous solution by cerium hydroxide loaded on Y-tape molecular sieve adsorbent: Performance and mechanism[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2020,604:125317. DOI: 10.1016/j.colsurfa.2020.125317
[13] MOHAMMED A, RIVERS A, STUCKEY D C, et al. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology[J]. Carbohydrate Polymers,2020,245:116419. DOI: 10.1016/j.carbpol.2020.116419
[14] GAO X, LI M, ZHAO Y, et al. Mechanistic study of selective adsorption of Hg2+ ion by porous alginate beads[J]. Chemical Engineering Journal,2019,378:122096. DOI: 10.1016/j.cej.2019.122096
[15] GAO X, ZHANG Y, ZHAO Y. Zinc oxide templating of porous alginate beads for the recovery of gold ions[J]. Carbohydrate Polymers,2018,200:297-304. DOI: 10.1016/j.carbpol.2018.07.097
[16] KARTHIK R, MEENAKSHI S. Removal of Cr(Ⅵ) ions by adsorption onto sodium alginate-polyaniline nanofibers[J]. International Journal of Biological Macromolecules,2015,72:711-717. DOI: 10.1016/j.ijbiomac.2014.09.023
[17] LIU B, HUANG Y. Polyethyleneimine modified eggshell membrane as a novel biosorbent for adsorption and detoxification of Cr(Ⅵ) from water[J]. Journal of Materials Chemistry,2011,21(43):17413-17418. DOI: 10.1039/c1jm12329g
[18] MIRETZKY P, CIRELLI A F. Cr(Ⅵ) and Cr(Ⅲ) removal from aqueous solution by raw and modified lignocellulosic materials: A review[J]. Journal of Hazardous Materials,2010,180(1):1-19.
[19] YAN Y, AN Q, XIAO Z, et al. Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(Ⅵ)[J]. Chemical Engineering Journal,2017,313:475-486. DOI: 10.1016/j.cej.2016.12.099
[20] 国家环境保护局. 水质 六价铬的测定 二苯碳酰二肼分光光度法: GB 7467—1987[S]. 北京: 中国标准出版社, 1987. State Department of Environmental Conservation. Water quality: Determination of chromium(Ⅵ): 1,5-Diphenylcarbohydrazide spectrophotometric method: GB 7467—1987[S]. Beijing: China Standards Press, 1987(in Chinese).
[21] ZHANG W, WANG H, HU X, et al. Multicavity triethylenetetramine-chitosan/alginate composite beads for enhanced Cr(Ⅵ) removal[J]. Journal of Cleaner Production,2019,231:733-745. DOI: 10.1016/j.jclepro.2019.05.219
[22] ZHANG W, ZHANG S, WANG J, et al. Hybrid functionalized chitosan-Al2O3@SiO2 composite for enhanced Cr(Ⅵ) adsorption[J]. Chemosphere,2018,203:188-198. DOI: 10.1016/j.chemosphere.2018.03.188
[23] RAO R A K, KASHIFUDDIN M. Kinetics and isotherm studies of Cd(Ⅱ) adsorption from aqueous solution utilizing seeds of bottlebrush plant (Callistemon chisholmii)[J]. Applied Water Science,2014,4:371-383. DOI: 10.1007/s13201-014-0153-2
[24] LIAN L, GUO L, GUO C. Adsorption of Congo red from aqueous solutions onto Ca-bentonite[J]. Journal of Hazardous Materials,2009,161(1):126-131. DOI: 10.1016/j.jhazmat.2008.03.063
[25] XU L Q, WAN D, GONG H F, et al. One-pot preparation of ferrocene-functionalized polymer brushes on gold substrates by combined surface-initiated atom transfer radical polymerization and “click chemistry”[J]. Langmuir,2010,26(19):15376-15382. DOI: 10.1021/la102775y
[26] GAO X, ZHANG Y, ZHAO Y. Biosorption and reduction of Au(Ⅲ) to gold nanoparticles by thiourea modified alginate[J]. Carbohydrate Polymers,2017,159:108-115. DOI: 10.1016/j.carbpol.2016.11.095
[27] 王磊, 白成玲, 朱振亚. 氧化石墨烯/海藻酸钠复合膜对Pb(Ⅱ)的吸附性能和机制[J]. 复合材料学报, 2020, 37(3):681-689. WANG L, BAI C L, ZHU Z Y. Adsorption properties and mechanism of Pb(Ⅱ) adsorbed by graphene oxide/sodium alginate composite membrane[J]. Acta Materiae Compositae Sinica,2020,37(3):681-689(in Chinese).
[28] SUN Z, LIU Y, HUANG Y, et al. Fast adsorption of Cd2+ and Pb2+ by EGTA dianhydride (EGTAD) modified ramie fiber[J]. Journal of Colloid and Interface Science,2014,434:152-158. DOI: 10.1016/j.jcis.2014.07.036
[29] OMER A M, KHALIFA R E, HU Z, et al. Fabrication of tetraethylenepentamine functionalized alginate beads for adsorptive removal of Cr(Ⅵ) from aqueous solutions[J]. International Journal of Biological Macromolecules,2019,125:1221-1231. DOI: 10.1016/j.ijbiomac.2018.09.097
[30] WAN Z, CHO D W, TSANG D C W, et al. Concurrent adsorption and micro-electrolysis of Cr(Ⅵ) by nanoscale zerovalent iron/biochar/Ca-alginate compositen[J]. Environmental Pollution,2019,247:410-420. DOI: 10.1016/j.envpol.2019.01.047
[31] WEN R, TU B, GUO X, et al. An ion release controlled Cr(Ⅵ) treatment agent: Nano zero-valent iron/carbon/alginate composite gel[J]. International Journal of Biological Macromolecules,2020,146:692-704. DOI: 10.1016/j.ijbiomac.2019.12.168
[32] PERIYASAMY S, GOPALAKANNAN V, VISWANATHAN N. Hydrothermal assisted magnetic nano-hydroxyapatite encapsulated alginate beads for efficient Cr(Ⅵ) uptake from water[J]. Journal of Environmental Chemical Engineering,2018,6(1):1443-1454. DOI: 10.1016/j.jece.2018.01.007
[33] LV X, ZHANG Y, FU W, CAO J, et al. Zero-valent iron nanoparticles embedded into reduced graphene oxide-alginate beads for efficient chromium(Ⅵ) removal[J]. Journal of Colloid and Interface Science,2017,506:633-643. DOI: 10.1016/j.jcis.2017.07.024
[34] GAO X, GUO C, HAO J, et al. Selective adsorption of Pd(Ⅱ) by ion-imprinted porous alginate beads: Experimental and density functional theory study[J]. International Journal of Biological Macromolecules,2020,157:401-413. DOI: 10.1016/j.ijbiomac.2020.04.153
[35] 张继国, 王艳, 张琼, 等. 聚乙烯亚胺-羧甲基纤维素的合成及对金属离子的吸附性能[J]. 高分子材料科学与工程, 2005, 30(3):15-20. ZHANG J G, WANG Y, ZHANG Q, et al. Preparation of polyethyleneimine-carboxymethyl celluose and its adsorption properties for metal ions[J]. Polymer Materials Science & Engineering,2005,30(3):15-20(in Chinese).
[36] SOLOMONS T G. Organic chemistry[M]. New York: Wiley Press Inc., 1980.
[37] 杨庆, 梁伯润, 窦丰栋, 等. 以乙二醛为交联剂的壳聚糖纤维交联机理探索[J]. 纤维素科学与技术, 2005, 30(3):13-20. YANG Q, LIANG B R, DOU F D, et al. Exploration on cross-linking mechanism of chitosan fiber with glyoxal as cross-linking reagent[J]. Journal of Cellulose Science and Technology,2005,30(3):13-20(in Chinese).
[38] MA Y, WANG A, LI J, et al. Preparation of hydroxyapatite with high surface area and dispersity templated on calcium carbonate in dipeptide hydrogels[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2020,596:124740. DOI: 10.1016/j.colsurfa.2020.124740
[39] TIAN X, WANG W, WANG Y, et al. Polyethylenimine functionalized halloysite nanotubes for efficient removal and fixation of Cr(Ⅵ)[J]. Microporous and Mesoporous Materials,2015,207:46-52. DOI: 10.1016/j.micromeso.2014.12.031
[40] OCIŃSKI D, JACUKOWICZ S I, KOCIOŁEK B E. Alginate beads containing water treatment residuals for arsenic removal from water: Formation and adsorption studies[J]. Environmental Science and Pollution Research,2016,23(24):24527-24539. DOI: 10.1007/s11356-016-6768-0
[41] AI Z, CHENG Y, ZHANG L, et al. Efficient removal of Cr(Ⅵ) from aqueous solution with Fe@Fe2O3 core-shell nanowires[J]. Environmental Science & Technology,2008,42(18):6955-6960.
[42] MANNING B A, KISER J R, KWON H, et al. Spectroscopic investigation of Cr(Ⅲ)- and Cr(Ⅵ)-treated nanoscale zerovalent iron[J]. Environmental Science & Technology,2007,41(2):586-592.
[43] SUN X F, MA Y, LIU X W, et al. Sorption and detoxification of chromium(Ⅵ) by aerobic granules functionalized with polyethylenimine[J]. Water Research,2010,44(8):2517-2524. DOI: 10.1016/j.watres.2010.01.027
[44] VIEIRA R S, MENEGHETTI E, BARONI P, et al. Chromium removal on chitosan-based sorbents: An EXAFS/XANES investigation of mechanism[J]. Materials Chemistry and Physics,2014,146(3):412-417. DOI: 10.1016/j.matchemphys.2014.03.046
-
期刊类型引用(16)
1. 王新玲,李世伟,金蕾蕾,范家俊. 高强不锈钢绞线网/ECC约束高强混凝土受压本构模型. 工程力学. 2023(09): 153-160 .  百度学术
百度学术
2. 王新玲,罗鹏程,钱文文,李可. 高强不锈钢绞线网增强工程水泥基复合材料薄板受弯承载力研究. 建筑结构学报. 2022(01): 164-172 .  百度学术
百度学术
3. 姚淇耀,陆宸宇,彭林欣,滕晓丹,罗月静. 氯盐侵蚀作用下BFRP筋增强海砂ECC的拉伸及弯曲性能试验. 复合材料学报. 2022(03): 1215-1227 .  本站查看
本站查看
4. 朱俊涛,张凯,邹旭岩,李可,张普. 钢绞线与水泥基复合材料局部黏结滑移模型. 哈尔滨工程大学学报. 2022(04): 541-548 .  百度学术
百度学术
5. 王新玲,李赟璞,李苗浩夫,范家俊. 高强不锈钢绞线网增强ECC加固RC短柱轴心受压试验. 复合材料学报. 2022(05): 2308-2317 .  本站查看
本站查看
6. 朱俊涛,张凯,王新玲,李可. 高强钢绞线网增强ECC与混凝土界面黏结-滑移关系研究. 工程力学. 2022(09): 204-214 .  百度学术
百度学术
7. 李可,王宇,李志强,朱俊涛. 高强钢绞线网增强ECC加固无损RC梁受弯承载力研究. 建筑结构学报. 2022(12): 82-90 .  百度学术
百度学术
8. 李可,李思辰,周擎威,王新玲. 高强钢绞线与工程水泥基复合材料黏结性能试验. 沈阳工业大学学报. 2022(06): 706-713 .  百度学术
百度学术
9. 惠迎新,王文炜,朱忠锋. FRP-ECC复合约束混凝土圆柱反复受压力学性能. 复合材料学报. 2022(11): 5586-5598 .  本站查看
本站查看
10. 王新玲,陈永杰,钱文文,李可,朱俊涛. 高强不锈钢绞线网增强工程水泥基复合材料弯曲性能试验. 复合材料学报. 2021(04): 1292-1301 .  本站查看
本站查看
11. 王春红,左祺,支中祥,徐磊,SARANI Zakaria,SHERAZ Hussain Siddique Yousfani. 聚乙烯醇乳液改性对汉麻秸秆纤维/水泥基复合材料性能的影响. 复合材料学报. 2021(05): 1567-1575 .  本站查看
本站查看
12. 朱俊涛,赵奎,邹旭岩,王新玲,李可,张哲. 高强不锈钢绞线网在ECC中的搭接性能. 土木与环境工程学报(中英文). 2021(03): 174-182 .  百度学术
百度学术
13. 朱俊涛,侯晶强,王新玲,李可. 基于损伤演化的钢绞线网/ECC黏结滑移关系. 华中科技大学学报(自然科学版). 2021(06): 103-108 .  百度学术
百度学术
14. 范建伟,李可,王新玲. 高性能复合材料“高强钢绞线/ECC”板受弯试验分析. 建筑科学. 2021(09): 130-136 .  百度学术
百度学术
15. 王新玲,卫垚鑫,范建伟,朱俊涛. 新型复合材料“高强钢绞线网/ECC约束素混凝土”受压性能试验研究. 复合材料学报. 2021(11): 3904-3911 .  本站查看
本站查看
16. 王新玲,赵要康,周擎威,朱俊涛. 高强不锈钢绞线与ECC黏结滑移性能研究. 华中科技大学学报(自然科学版). 2020(12): 108-113 .  百度学术
百度学术
其他类型引用(2)
-




 下载:
下载: