High porosity biochar and its treatment of phosphate in wastewater
-
摘要: 生物炭是缺氧状态下生物质热解的产物;然而,常见的生物炭表面积小、孔隙结构不发达、表面活性基团少,吸附效果差。本文以高粱(GC)和柚子皮(YC)为原料,利用4种物质进行表面处理制备得到生物炭,其中制备的高粱/KOH(GC-KH)和柚子皮/KOH(YC-K)粉末表面孔状明显,证实了该工艺的可行性。GC-KH比表面积为2096.05 m2/g,平均孔径4.12 nm,在其表面含有丰富的含氧官能团,为吸附提供了良好的结构空间和活性位点。通过批量实验,探讨了不同因素对磷酸盐吸附的影响,评估了离子强度。等温线结果表明GC-KH对磷酸盐的吸附发生在单分子层表面,在pH值为7时GC-KH对磷酸盐最大吸附能力为74.73 mg/g,具有反应迅速等显著优势,为废水中磷酸盐的高效去除提供了创新路径。Abstract: Biochar is a product of pyrolysis of biomass under anoxic conditions; however, common biochar has a small surface area, underdeveloped pore structure, few surface active groups, and poor adsorption effect. In this work, biochar was prepared from sorghum (GC) and grapefruit peel (YC) by surface treatment using four substances to obtain biochar, where the prepared sorghum/KOH (GC-KH) and grapefruit peel/KOH (YC-K) powders had obvious surface porosity, confirming the feasibility of the process. With a specific surface area of 2096.05 m2/g and an average pore size of 4.12 nm, GC-KH is rich in oxygen-containing functional groups on its surface, providing a good structural space and active sites for adsorption.The effect of different factors on phosphate adsorption was explored by batch experiments to assess the ionic strength. Results of isotherms showed that the adsorption of phosphate by GC-KH occurred on the surface of the monomolecular layer, and the maximum adsorption capacity of phosphate by GC-KH was 74.73 mg/g at pH=7. It has significant advantages such as rapid response, which provides an innovative pathway for the efficient removal of phosphate from wastewater.
-
Key words:
- biochar /
- specific surface /
- phosphate /
- modified materials /
- adsorption
-
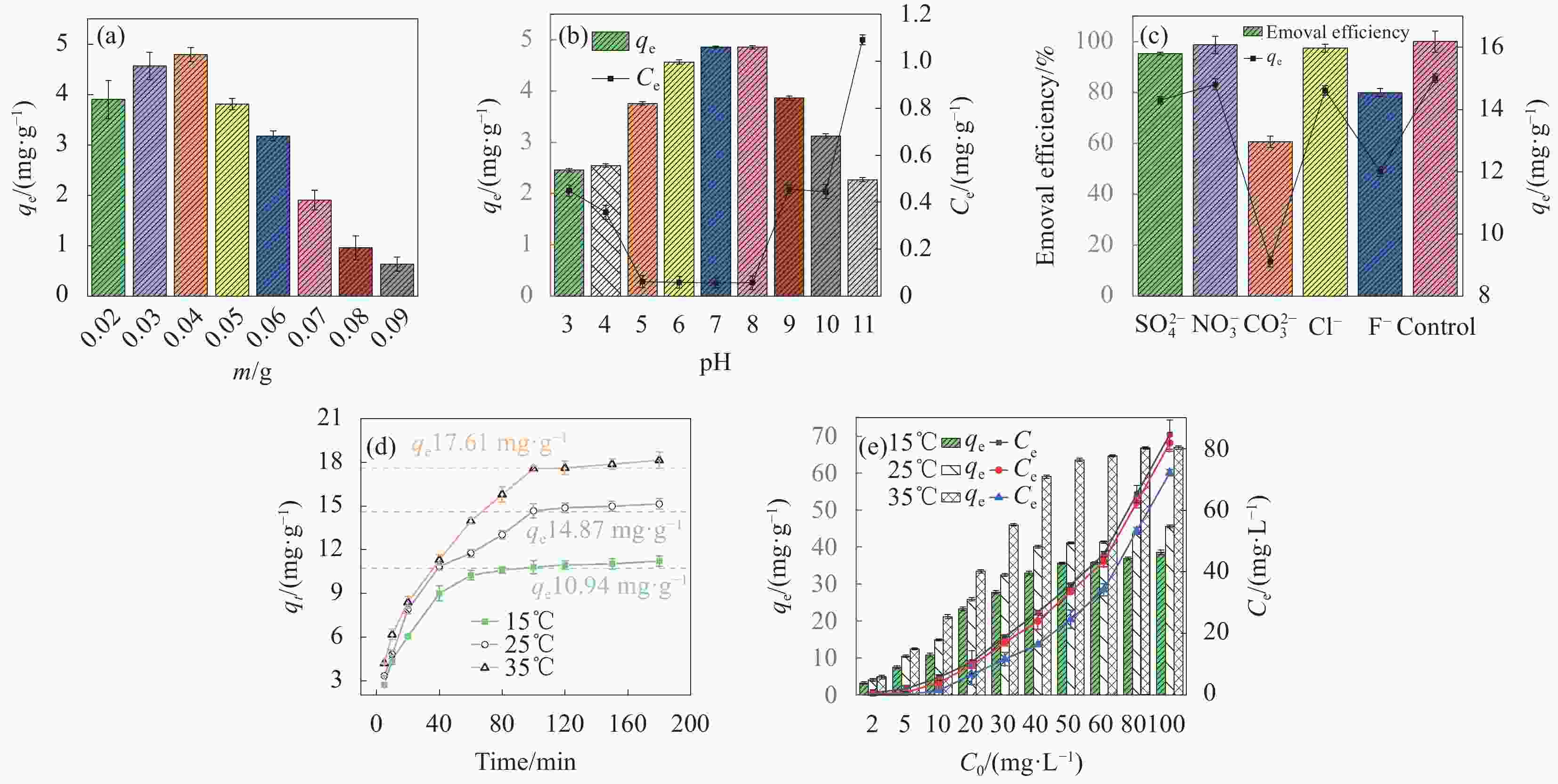
图 9 GC-KH投加量 (a)、pH (b)、共存离子 (c)、不同温度下反应时间(d)和不同温度下初始浓度 (e) 对GC-KH吸附磷酸盐效果的影响
Figure 9. Effect of GC-KH dosage (a), pH (b), coexisting ions (c), reaction time at different temperatures (d), initial concentration at different temperatures (e) on the effect of phosphate adsorption by GC-KH
m—Mass; qt—Amount adsorbed at time t; qe—Amount adsorbed when adsorption equilibrium is reached; Ce—Concentration of pollutant in the solution after removal; C0—Initial concentration
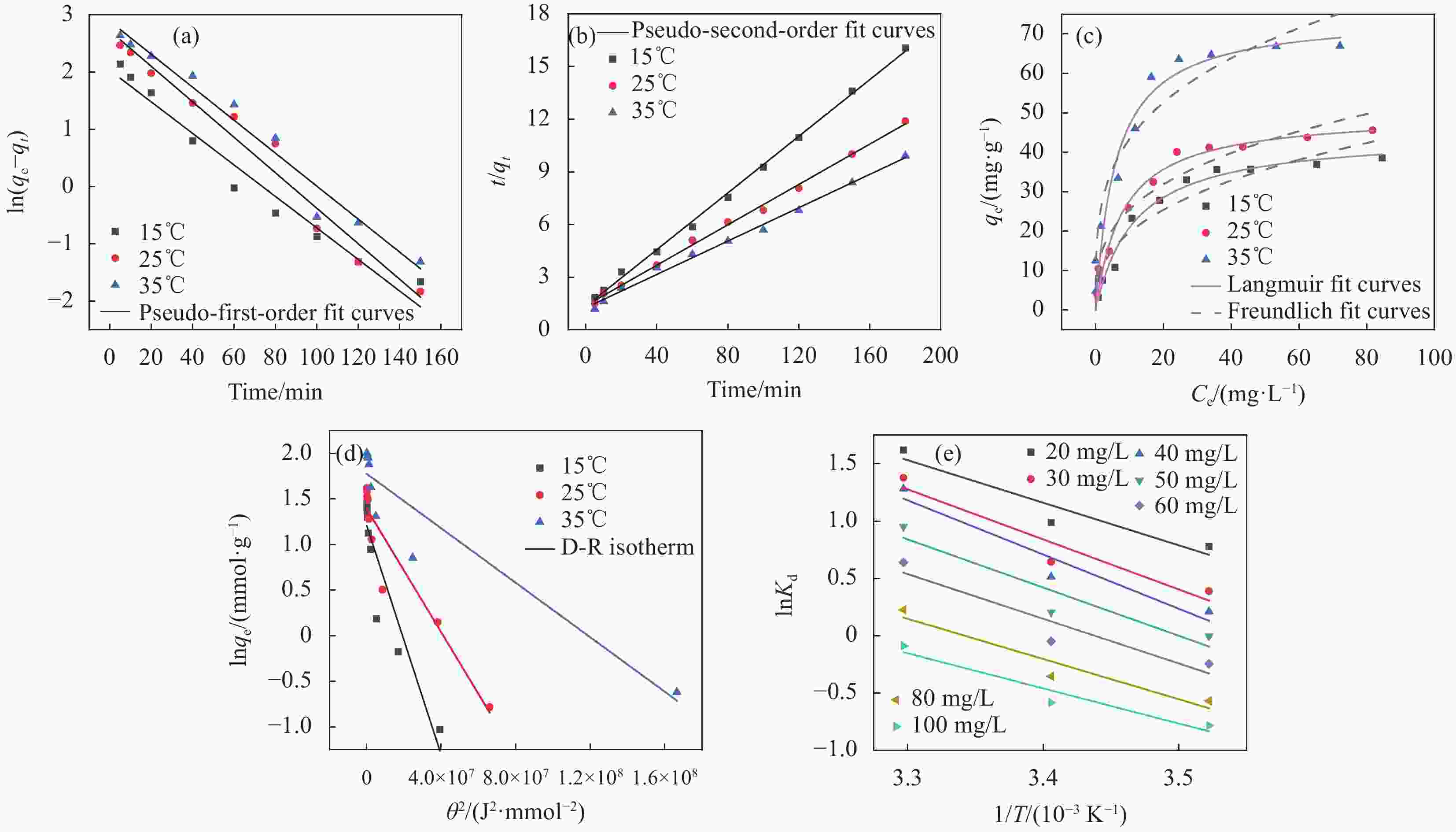
图 10 GC-KH吸附磷酸盐的伪一阶动力学模型 (a)、伪二阶动力学模型 (b);不同温度下GC-KH吸附磷酸盐的Langmuir模型和Freundlich模型 (c)、D-R模型 (d) 及热力学 (f)
Figure 10. Pseudo-first-order kinetic model (a) and Pseudo-second-order kinetic model (b) for phosphate adsorption by GC-KH; Langmuir and Freundlich models (c), D-R model (d) and thermodynamics (f) of phosphate adsorption by GC-KH at different temperatures
θ—Isotherm constant; T—Absolute temperature; Kd—Thermodynamic equilibrium constant
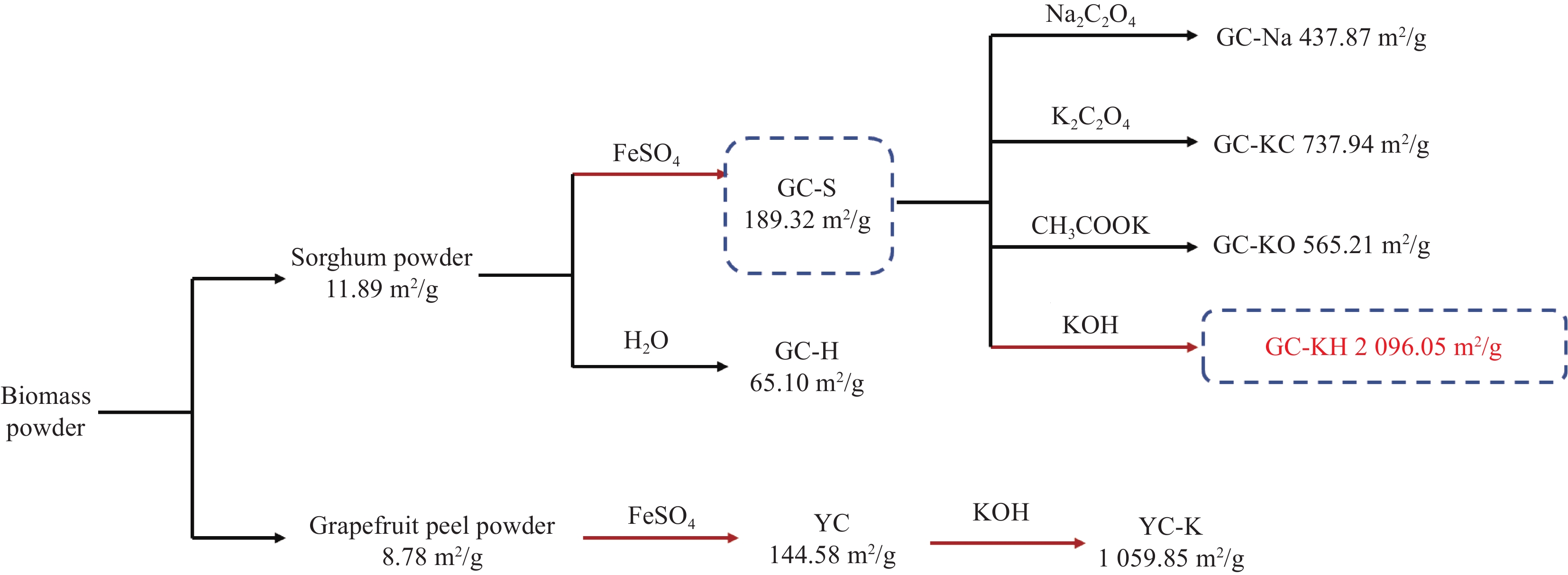
表 1 产物及其说明
Table 1. Products and their descriptions
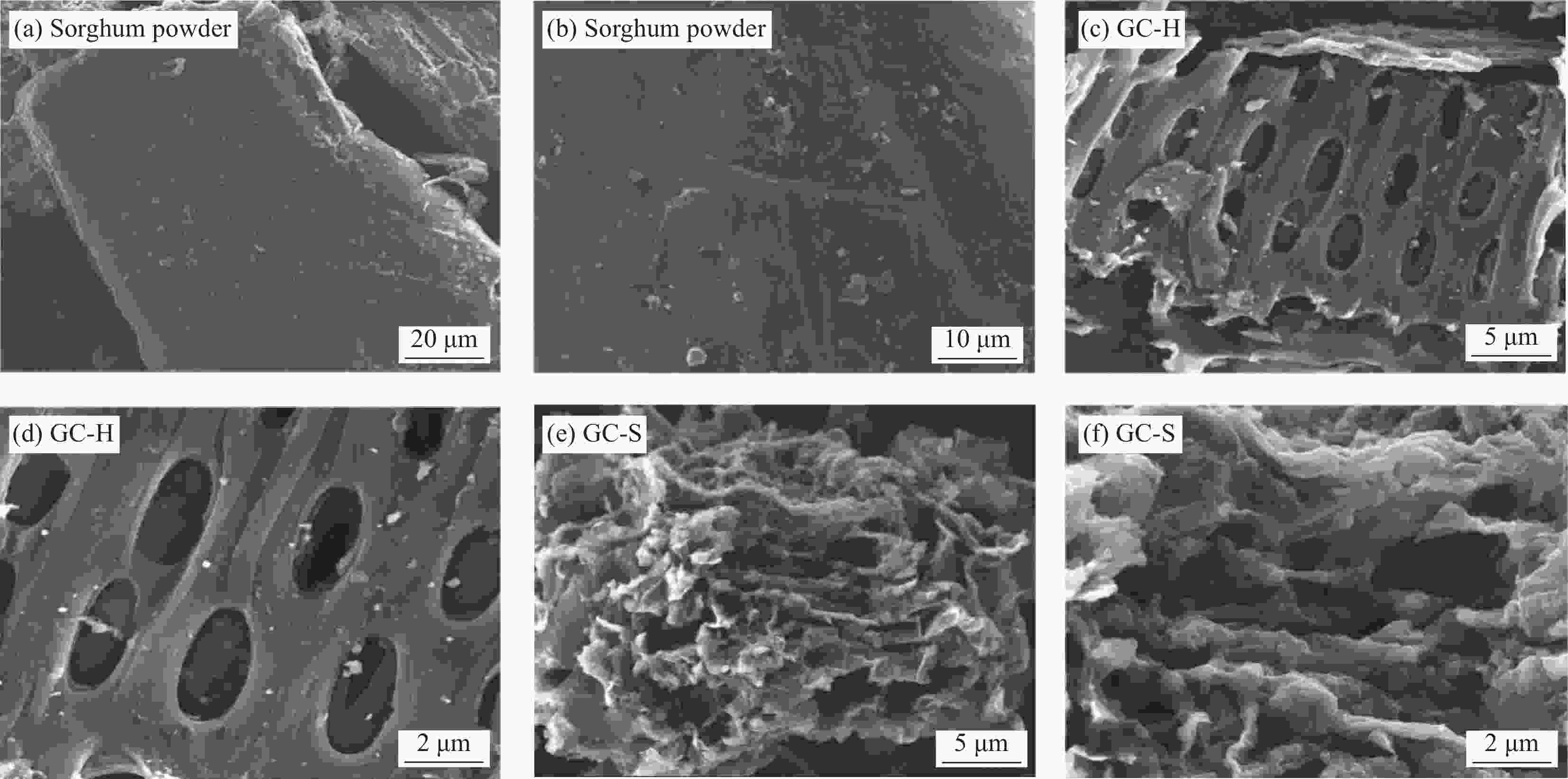
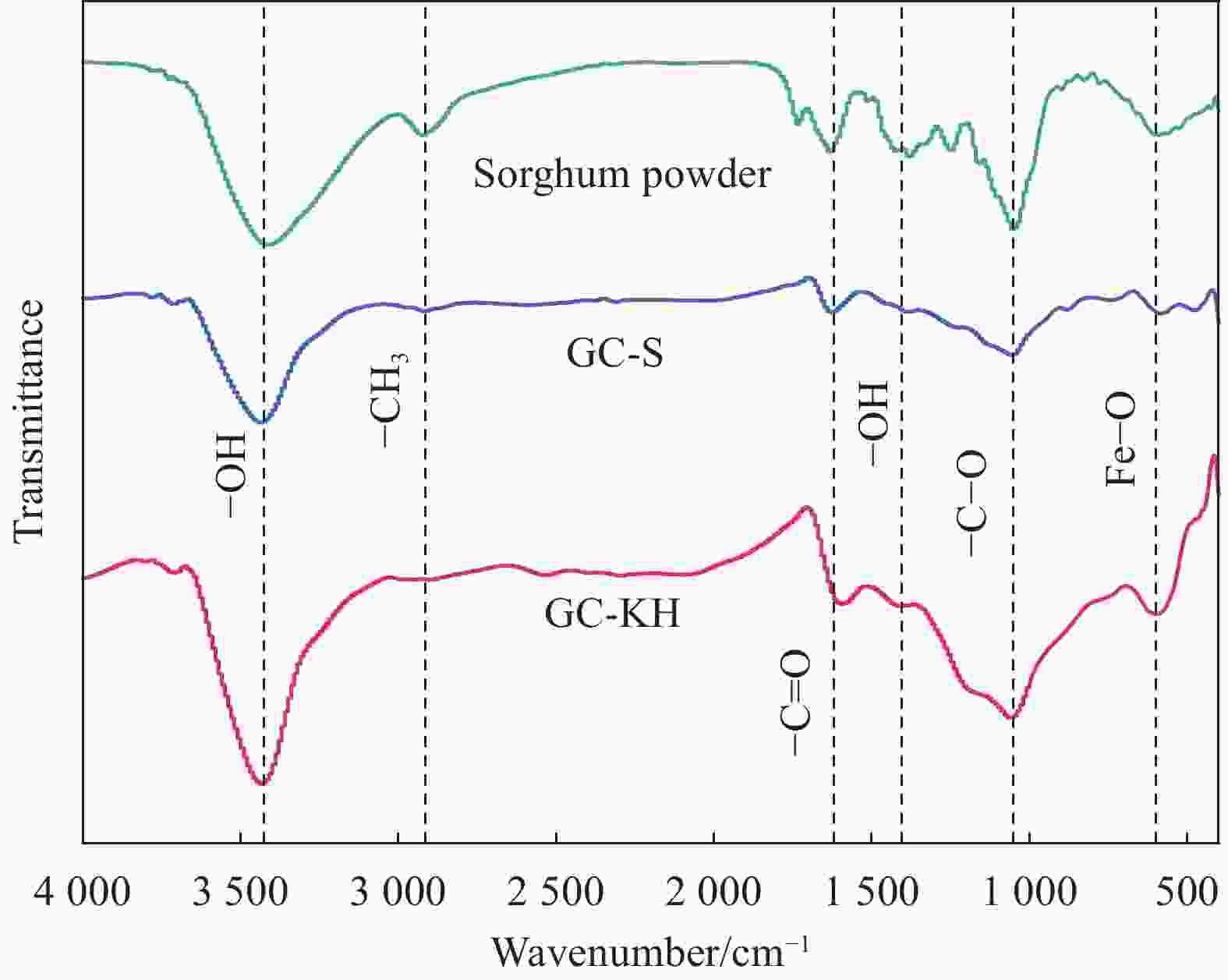
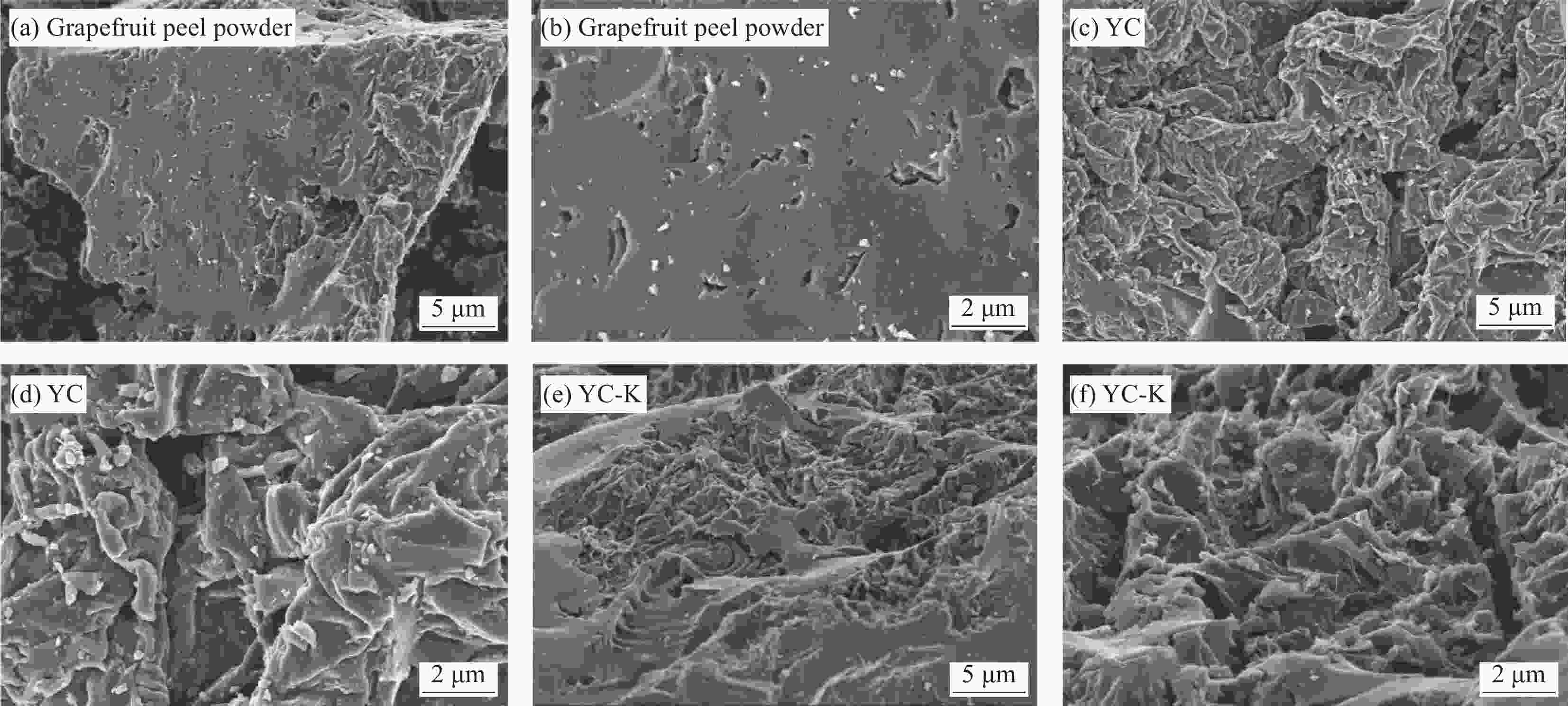
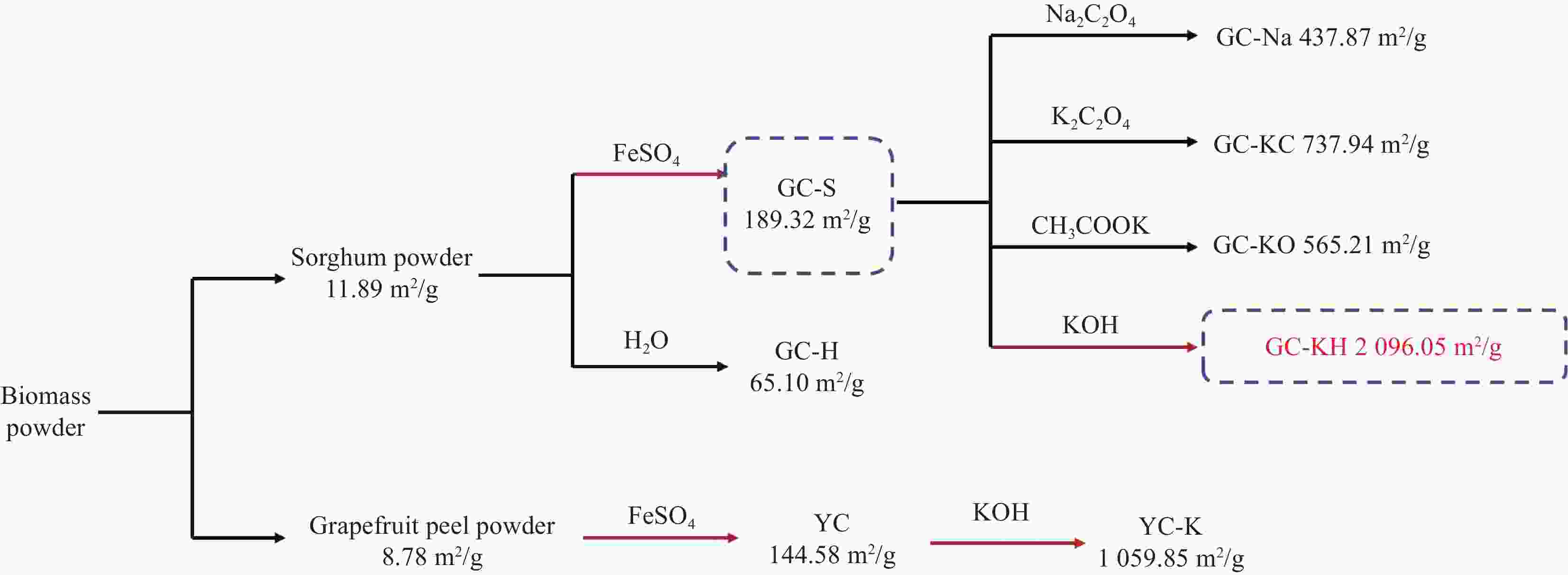
Sample Description GC-H Sorghum powder carbonised product after water treatment GC-S Sorghum powder carbonised product after FeSO4 treatment GC-Na GC-S activated product after Na2C2O4 treatment GC-KC GC-S activated by K2C2O4 treatment GC-KO GC-S activated product after CH3COOK treatment GC-KH GC-S activated by KOH treatment YC Pomelo peel powder carbonised by FeSO4 treatment YC-K YC activated product after KOH treatment 表 2 生物炭的比表面积(BET)表征结果
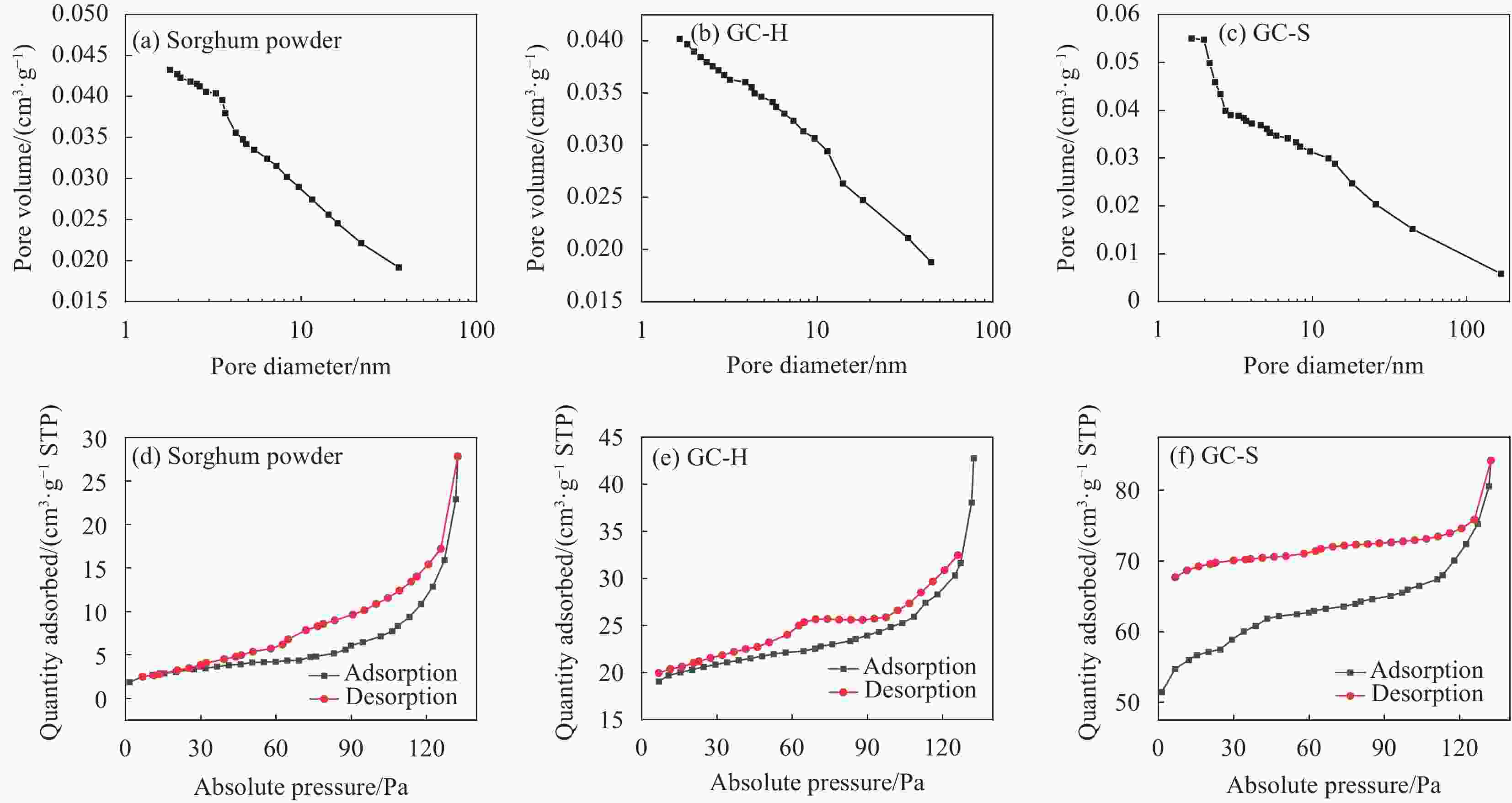
Table 2. Brunauer Emmett Teller (BET) characterization results of biochar
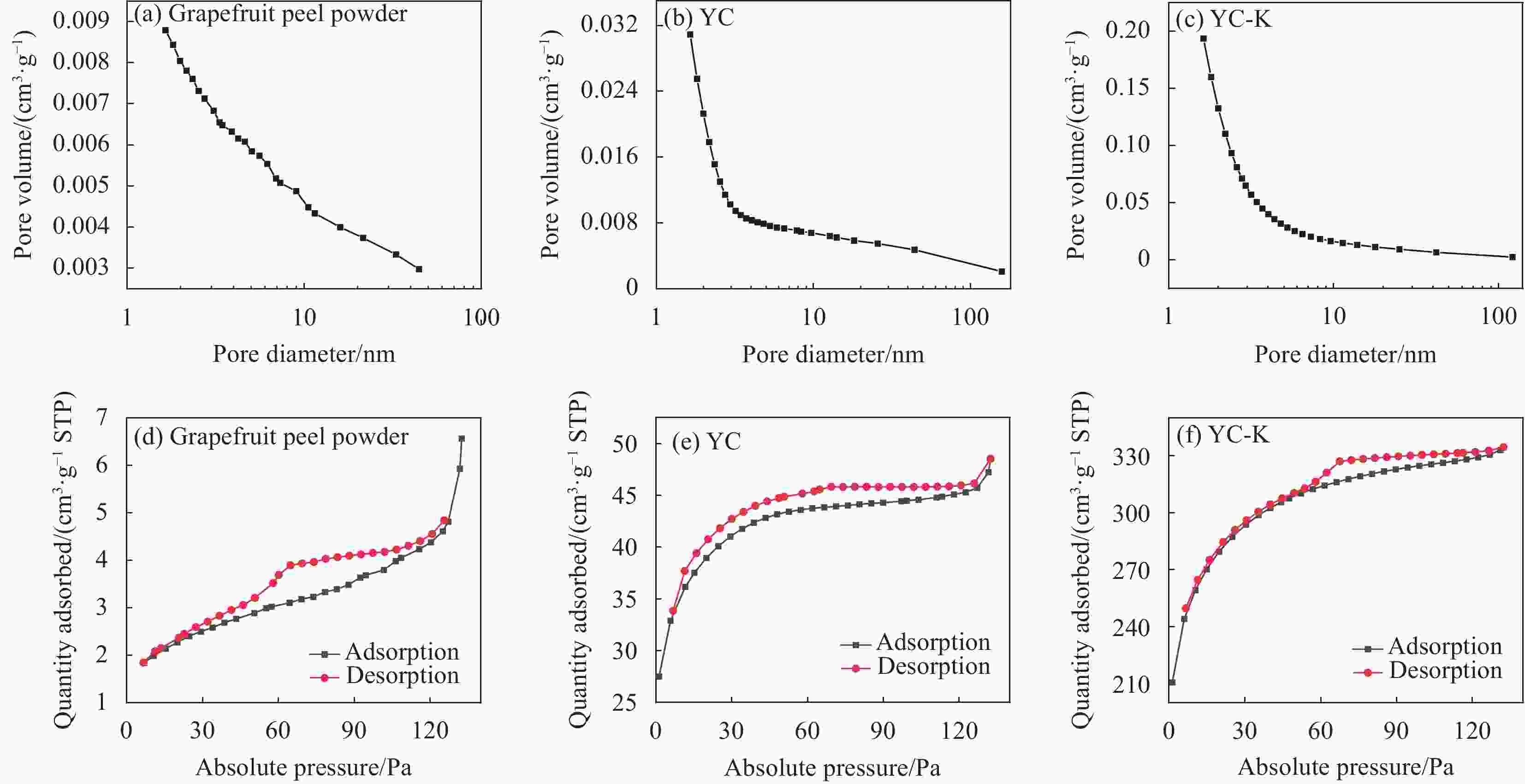
Sample Surface aeraa/
(m2·g−1)Pore diameterb/
nmPore volumec/
(cm3·g −1)Sorghum powder 11.8903000 15.61590 0.023938 GC-H 65.1037000 10.83450 0.048291 GC-S 189.3184000 5.79140 0.115718 GC-Na 437.8670000 7.69350 0.350280 GC-KC 737.9431000 3.28920 0.353356 GC-KO 565.2083000 3.85950 0.307675 GC-KH 2096.0475000 4.11660 1.000354 Grapefruit peel powder 8.7806000 5.91190 0.010157 YC 144.5847000 2.76330 0.070626 YC-K 1059.8472000 2.55870 0.511210 Notes: a—BET multi-point method specific surface; b—Barret-Joyner-Halenda (BJH) method desorption (cylindrical hole model) average hole diameter; c—BJH method desorption (cylindrical pore model, 2.0-49.6 nm) pore volume. 表 3 GC-KH吸附磷酸盐的伪一阶和伪二阶动力学参数
Table 3. Pseudo-first- and Pseudo-second-order kinetic parameters for phosphate adsorption by GC-KH
Sample Pseudo-first-order model Pseudo-second-order model qcal/(mg·g−1) k1/(min−1) R2 qcal/(mg·g−1) k2/(g·mg−1·min−1) R2 GC-KH (15℃) 7.6071 0.0275 0.9649 12.3916 0.0807 0.9948 GC-KH (25℃) 15.1653 0.0310 0.9689 17.3974 0.0575 0.9939 GC-KH (35℃) 17.9383 0.0288 0.9712 21.8818 0.0457 0.9971 Notes: qcal—Adsorption capacity at equilibrium time; k1 and k2—Reaction rate constants of pseudo-first-order and pseudo-second-order equations, respectively; R2—Correlation coefficient. 表 4 GC-KH吸附磷酸盐的Langmuir、Freundlich和D-R等温线参数
Table 4. Langmuir, Freundlich and D-R isotherm parameters for phosphate adsorption by GC-KH
Sample Langmuir Freundlich D-R qm/(mg·g−1) KL/(L·mg−1) R2 KF/(mg·g−1) n R2 qm/(mol·g−1) E/(kJ·mol−1) R2 GC-KH (15℃) 44.79 0.0879 0.9829 8.45 2.72 0.9121 3.3532 5.64 0.8702 GC-KH (25℃) 49.92 0.1250 0.9738 12.21 3.12 0.9419 4.0295 7.68 0.8997 GC-KH (35℃) 74.73 0.1694 0.9445 22.87 3.59 0.9104 5.9133 11.57 0.8876 Notes: qm—Maximum adsorption capacity of Langmuir; KL—Langmuir adsorption constant; KF—Freundlich adsorption constant; n—Constant related to the adsorption strength; E—Reactive energy. 表 5 GC-KH吸附磷酸盐的热力学参数
Table 5. Thermodynamic parameters of phosphate adsorption by GC-KH
C/(mg L−1) ΔH/(kJ·mol−1) ΔS/(J·mol−1·K−1) ΔG/(kJ·mol−1) 15℃ 25℃ 35℃ 20 86.5333 311.6134 −89.6581 −92.7743 −95.8904 30 72.9616 257.7752 −74.1663 −76.7441 −79.3218 40 30.8975 113.1529 −32.5572 −33.6887 −34.8202 50 36.3046 128.6047 −37.0018 −38.2879 −39.5739 60 39.3341 137.6547 −39.6052 −40.9818 −42.3583 80 34.9713 120.6423 −34.7100 −35.9164 −37.1229 100 32.4302 109.8654 −31.6088 −32.7075 −33.8061 Notes: C—Initial concentration; ΔG—Gibbs free energy change; ΔH—Enthalpy change; ΔS—Entropy change. -
[1] YAN L G, XU Y Y, YU H Q, et al. Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron-aluminum pillared bentonites[J]. Journal of Hazardous Materials,2010,179(1-3):244-250. doi: 10.1016/j.jhazmat.2010.02.086 [2] HUANG X, LIAO X P, SHI B. Adsorption removal of phosphate in industrial wastewater by using metal-loaded skin split waste[J]. Journal of Hazardous Materials,2009,166(2-3):1261-1265. doi: 10.1016/j.jhazmat.2008.12.045 [3] HU R. Pollution control and remediation of rural water resource based on urbanization perspective[J]. Environmental Technology & Innovation,2020,20:40-60. [4] MAYER B K, BAKER L A, BOYER T H, et al. Total value of phosphorus recovery[J]. Environmental Science & Technology,2016,50(13):6606-6620. [5] YIN Z C, CHEN Q F, ZHAO C S, et al. A new approach to removing and recovering phosphorus from livestock wastewater using dolomite[J]. Chemosphere,2020,255:80-100. [6] LAW Y, KIRKEGAARD R H, COKRO A A, et al. Integrative microbial community analysis reveals full-scale enhanced biological phosphorus removal under tropical conditions[J]. Scientific Reports,2016,6(1):1-15. doi: 10.1038/s41598-016-0001-8 [7] XIE M, LIU Z Y, XU Y H. Removal of glyphosate in neutralization liquor from the glycine-dimethylphosphit process by nanofiltration[J]. Journal of Hazardous Materials,2010,181(1-3):975-980. doi: 10.1016/j.jhazmat.2010.05.109 [8] DRENKOV-TUHTAN A, SCHNEIDER M, FRANZREB M, et al. Pilot-scale removal and recovery of dissolved phosphate from secondary wastewater effluents with reusable ZnFeZr adsorbent@Fe3O4/SiO2 particles with magnetic harvesting[J]. Water Research,2017,109:77-87. doi: 10.1016/j.watres.2016.11.039 [9] 孙健, 尚依依, 徐兆郢, 等. NaOH浓度对树脂基HFO复合吸附剂的结构及除磷影响[J]. 复合材料学报, 2022, 39(12):5678-5687. doi: 10.13801/j.cnki.fhclxb.20211207.002SUN Jian, SHANG Yiyi, XU Zhaoying, et al. Effect of NaOH concentration on structure and phosphate adsorption of polymer-based hydrated ferric oxide composite adsorbents[J]. Acta Materiae Compositae Sinica,2022,39(12):5678-5687(in Chinese). doi: 10.13801/j.cnki.fhclxb.20211207.002 [10] BLANEY L M, CINAR S, SEMGUPTA A K. Hybrid anion exchanger for trace phosphate removal from water and wastewater[J]. Water Research,2007,41(7):1603-1613. doi: 10.1016/j.watres.2007.01.008 [11] 国家环境保护总局. 国家质量监督检验检疫总局. 城镇污水处理厂污染物排放标准: GB/T 18918—2002[S]. 北京: 中国标准出版社, 2002.State Environmental Protection Administration of China. General Administration of Quality Supervision, Inspection and Quarantine of China. Pollutant emission standards for urban sewage treatment plants: GB/T 18918—2002[S]. Beijing: China Standard Press, 2002(in Chinese). [12] AMANN A, ZOBOLI O, KRAMPE J, et al. Environmental impacts of phosphorus recovery from municipal wastewater[J]. Resources Conservation and Recycling,2018,130:127-139. doi: 10.1016/j.resconrec.2017.11.002 [13] AHMAD M, RAJAPAKSHA A U, LIM J E, et al. Biochar as a sorbent for contaminant management in soil and water: A review[J]. Chemosphere,2014,99:19-33. doi: 10.1016/j.chemosphere.2013.10.071 [14] 王申宛, 钟爽, 郑丽丽, 等. 共热解法制备方解石/生物炭复合材料及其吸附Pb(II)性能和机制[J]. 复合材料学报, 2021, 38(12):4282-4293.WANG Shenwan, ZHONG Shuang, ZHENG Lili, et al. Preparation of calcite/biochar composite by co-pyrolysis and its adsorption properties and mechanism for Pb(II)[J]. Acta Materiae Compositae Sinica,2021,38(12):4282-4293(in Chinese). [15] 曾涛涛, 农海杜, 沙海超, 等. 污泥基生物炭负载纳米零价铁去除Cr(VI)的性能与机制[J]. 复合材料学报, 2023, 40(2):1037-1049.ZENG Taotao, NONG Haidu, SHA Haichao, et al. Performance and mechanism of Cr(VI) removal by sludge-derived biochar loaded with nanoscale zero-valent iron[J]. Acta Materiae Compositae Sinica,2023,40(2):1037-1049(in Chinese). [16] 刘清, 许艺文, 招国栋, 等. 生物炭负载绿色纳米铁颗粒去除水中U(Ⅵ)[J]. 复合材料学报, 2022, 39(12):5934-5945.LIU Qing, XU Yiwen, ZHAO Guodong, et al. Biochar supported green nano-iron particles to remove U(VI) from water[J]. Acta Materiae Compositae Sinica,2022,39(12):5934-5945(in Chinese). [17] DIZBAY-ONAT M, VAIDYA U K, LUNGU C T. Preparation of industrial sisal fiber waste derived activated carbon by chemical activation and effects of carbonization parameters on surface characteristics[J]. Industrial Crops and Products,2017,95:583-590. doi: 10.1016/j.indcrop.2016.11.016 [18] WANG Z H, GUO H Y, SHEN F, et al. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−)[J]. Chemosphere,2015,119:646-653. doi: 10.1016/j.chemosphere.2014.07.084 [19] WANG Z H, SHEN D K, SHEN F, et al. Phosphate adsorption on lanthanum loaded biochar[J]. Chemosphere,2016,150:1-7. doi: 10.1016/j.chemosphere.2016.02.004 [20] LI R H, WANG J J, ZHOU B Y, et al. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios[J]. Science of the Total Environment,2016,559:121-129. doi: 10.1016/j.scitotenv.2016.03.151 [21] 国家环境保护局标准处. 钼酸铵分光光度法: GB/T 11893−1989[S]. 北京: 中国标准出版社, 1989.Standards Division of State Environmental Protection Administration. Ammonium molybdate spectrophotometric method: GB/T 11893−1989[S]. Beijing: China Standards Press, 1989(in Chinese). [22] SULIMAN W, HARSH J B, ABU L N I, et al. Modification of biochar surface by air oxidation: Role of pyrolysis temperature[J]. Biomass and Bioenergy,2016,85:1-11. doi: 10.1016/j.biombioe.2015.11.030 [23] LIU Q Y, YANG F, LIU Z H, et al. Preparation of SnO2-Co3O4/C biochar catalyst as a Lewis acid for corncob hydrolysis into furfural in water medium[J]. Journal of Industrial and Engineering Chemistry,2015,26:46-54. doi: 10.1016/j.jiec.2014.11.041 [24] YAN Q G, WAN C X, LIU J, et al. Iron nanoparticles in situ encapsulated in biochar-based carbon as an effective catalyst for the conversion of biomass-derived syngas to liquid hydrocarbons[J]. Green Chemistry,2013,15(6):1631-1640. doi: 10.1039/c3gc37107g [25] THINES K R, ABDULLAH E C, MUBARAK N M, et al. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review[J]. Renewable and Sustainable Energy Reviews,2017,67:257-276. doi: 10.1016/j.rser.2016.09.057 [26] HUANG H, TANG J C, GAO K, et al. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics[J]. RSC Advances,2017,7(24):14640-14648. doi: 10.1039/C6RA27881G [27] LI B, YANG L, WANG C Q, et al. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes[J]. Chemosphere,2017,175:332-340. doi: 10.1016/j.chemosphere.2017.02.061 [28] ZHAO C Q, MA J G, LI Z Y, et al. Highly enhanced adsorption performance of tetracycline antibiotics on KOH-activated biochar derived from reed plants[J]. RSC Advances,2020,10(9):5066-5076. doi: 10.1039/C9RA09208K [29] ROMANOS J, BECKNER M, RASH T, et al. Nanospace engineering of KOH activated carbon[J]. Nanotechnology,2011,23(1):015401. [30] 黄明堦, 陈卫群, 陈燕丹, 等. 草酸钾活化法制备榴莲壳活性炭及其表征[J]. 环境工程学报, 2012, 6(10):3730-3734.HUANG Mingjie, CHEN Weiqun, CHEN Yandan, et al. Preparation and characterization of activated carbons from durian shell by potassium oxalate activation[J]. Chinese Journal of Environmental Engineering,2012,6(10):3730-3734(in Chinese). [31] YANG K B, PENG J H, SRINIVASAKANNAN C, et al. Preparation of high surface area activated carbon from coconut shells using microwave heating[J]. Bioresource Technology,2010,101(15):6163-6169. doi: 10.1016/j.biortech.2010.03.001 [32] LI Q, MU J H, ZHOU J, et al. Avoiding the use of corrosive activator to produce nitrogen-doped hierarchical porous carbon materials for high-performance supercapacitor electrode[J]. Journal of Electroanalytical Chemistry,2019,832:284-292. doi: 10.1016/j.jelechem.2018.11.013 [33] 张鹏丽, 武莉娅, 杨宗政, 等. MXene改性材料的制备及其吸附除Sr2+性能[J]. 复合材料学报, 2023, 40(10): 5678-5691.ZHANG Pengli, WU Liya, YANG Zongzheng, et al. Preparation of modified MXene material and its adsorption performance for Sr2+[J]. Acta Materiae Compositae Sinica, 2023, 40(10): 5678-5691(in Chinese). [34] WANG X M, KUBICKI J D, BOILY J F, et al. Binding geometries of silicate species on ferrihydrite surfaces[J]. ACS Earth and Space Chemistry,2018,2(2):125-134. doi: 10.1021/acsearthspacechem.7b00109 [35] PAN B J, WU J, PAN B C, et al. Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents[J]. Water Research,2009,43(17):4421-4429. doi: 10.1016/j.watres.2009.06.055 [36] GU S, FU B T, AHN J W, et al. Mechanism for phosphorus removal from wastewater with fly ash of municipal solid waste incineration, Seoul, Korea[J]. Journal of Cleaner Production,2021,280(2):20-40. [37] LIAO T W, LI T, SU X D, et al. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal[J]. Bioresource Technology,2018,263:207-213. doi: 10.1016/j.biortech.2018.04.108 [38] LIU R T, CHI L N, WANG X Z, et al. Effective and selective adsorption of phosphate from aqueous solution via trivalent-metals-based amino-MIL-101 MOFs[J]. Chemical Engineering Journal,2019,357:159-168. doi: 10.1016/j.cej.2018.09.122 [39] SU Y, CUI H, LI Q, et al. Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles[J]. Water Research,2013,47(14):5018-5026. doi: 10.1016/j.watres.2013.05.044 [40] ZHANG X, YAN L G, LI J, et al. Adsorption of heavy metals by L-cysteine intercalated layered double hydroxide: Kinetic, isothermal and mechanistic studies[J]. Journal of Colloid and Interface Science,2020,562:149-158. doi: 10.1016/j.jcis.2019.12.028 [41] MAHMOUD M E, NABIL G M, ABDEL A H, et al. Imprinting “Nano-SiO2-crosslinked chitosan-Nano-TiO2” polymeric nanocomposite for selective and instantaneous microwave-assisted sorption of Hg(II) and Cu(II)[J]. ACS Sustainable Chemistry & Engineering,2018,6(4):4564-4573. [42] ARYEE A A, MPATANI F M, KANI A N, et al. A review on functionalized adsorbents based on peanut husk for the sequestration of pollutants in wastewater: Modification methods and adsorption study[J]. Journal of Cleaner Production,2021,310:1-20. [43] YADAV A, BAGOTIA N, SHARMA A K, et al. Advances in decontamination of wastewater using biomass-based composites: A critical review[J]. Science of the Total Environment,2021,784:60-80. -





 下载:
下载: