Preparation and properties of elliptic leaves SiO2/polyvinyl alcohol pervaporation composite membranes
-
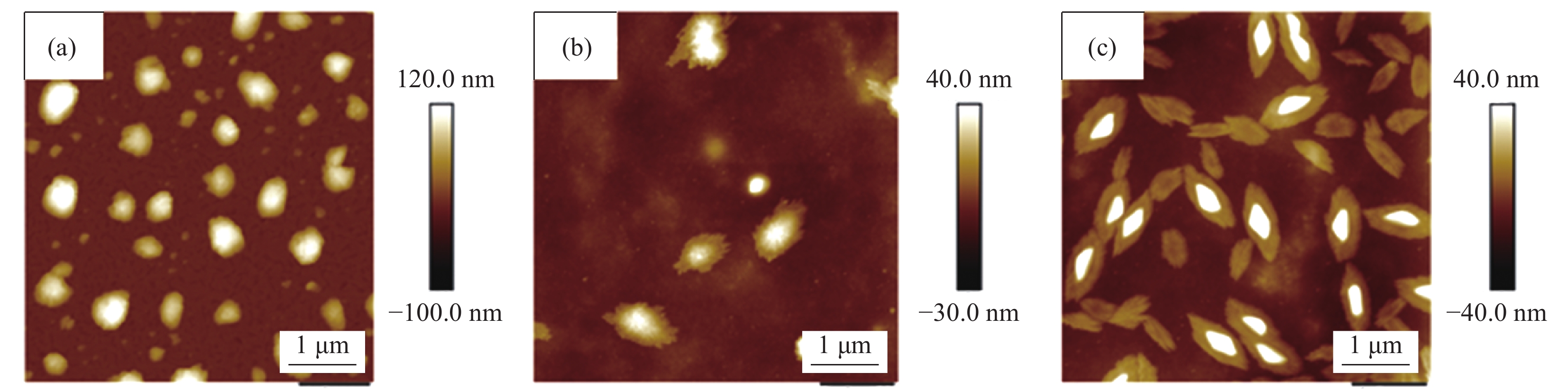
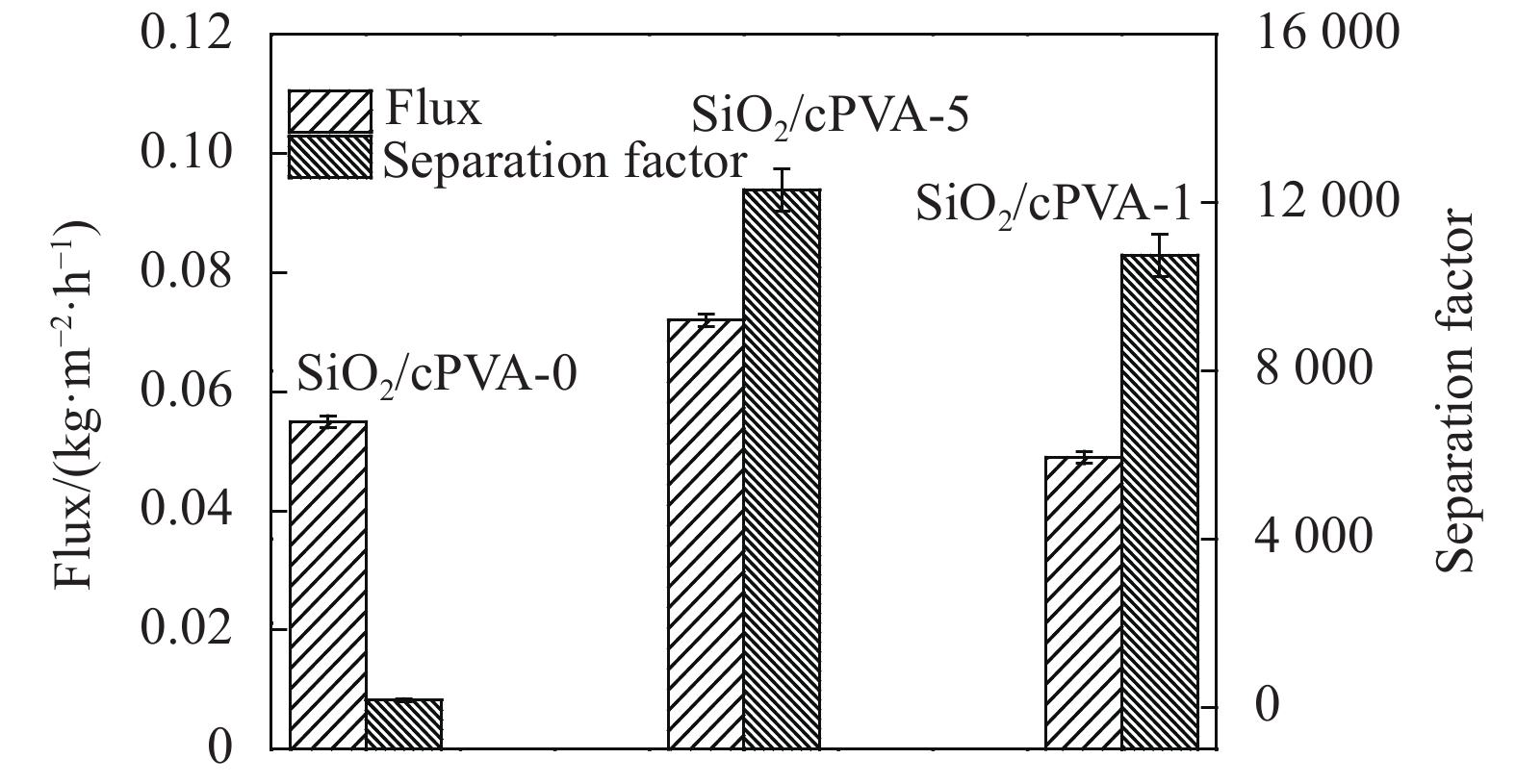
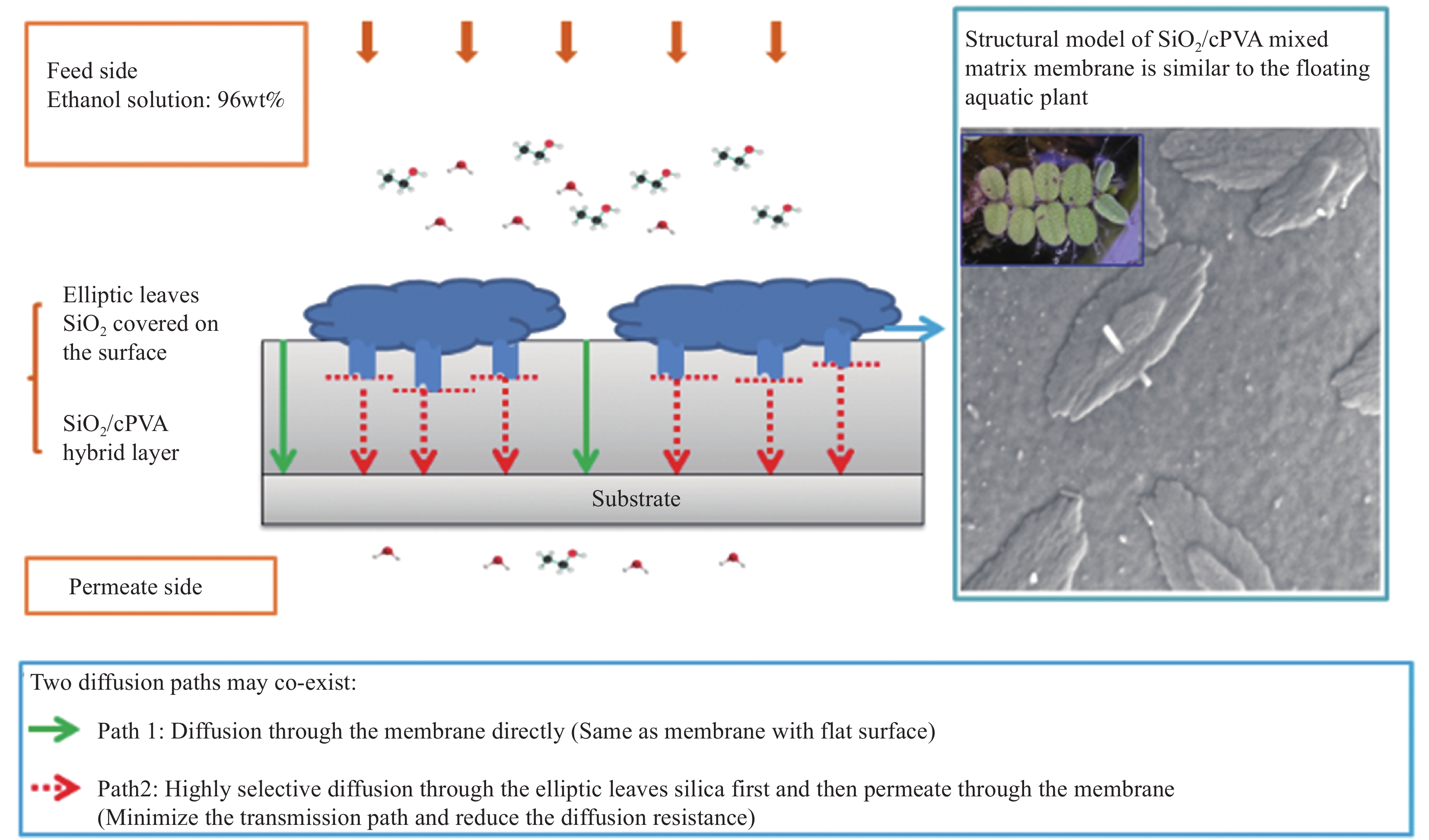
摘要: 如何制备含形貌可控且高度分散无机纳米颗粒的高性能复合分离膜,是当前膜分离领域的研究热点和难点。本文采用溶胶-凝胶和溶液刮涂法将聚乙烯醇(PVA)、马来酸(MA)和SiO2三者交联制备得到混合基质膜。通过SEM、FTIR、XRD对SiO2/交联PVA混合基质膜进行结构表征,在50℃下对97wt%乙醇水溶液进行渗透汽化性能测试。结果表明,含椭圆叶片状SiO2聚集体的SiO2/交联PVA混合基质膜,椭圆叶片状SiO2纳米颗粒聚集体可作为表面预筛选层,且在基体内高度分散,能够同时增加对醇水溶液的渗透通量和选择性。对97wt%乙醇水溶液的渗透通量和选择性分别高达 0.072 kg·m−2·h−1和12301。分离性能提高的原因可能是由于该混合基质膜具有表面预筛功能和更致密的网络结构。该结果将促进纳米SiO2/PVA复合材料的研究及该类材料在分离领域的应用。Abstract: How to prepare high-performance separation membranes with morphology controlled and well-dispersed nanoparticles, has been a hotspot and difficulty in the field of membrane separation. Mixed matrix membranes were prepared by crosslinking polyvinyl alcohol (PVA), maleic acid (MA) and SiO2 via an aqueous sol-gel route and solution casting method. The membrane was characterized by SEM, FTIR, XRD and tested for its perfor-mance in pervaporation separation of ethanol/water mixtures with feed ethanol concentration of 97wt% at 50℃. Membrane characterization results reveal that SiO2/crosslinked PVA membrane shows that elliptic leaves SiO2 nano-particles cover the surface and act as a water prescreening layer, are well disperse within the polymer matrix. It results in a significantly enhanced effect in both flux and selectivity. The membranes achieve high water flux of 0.072 kg·m−2·h−1 and separation factor of 12301 for separation of 97wt% ethanol aqueous solution. The enhanced membrane performance can be attributed to the surface prescreening ability and dense crosslinking membrane network. The research results would promote the study of SiO2/PVA composite materials, and their application in the field of membrane separation.
-
Keywords:
- pervaporation /
- mixed matrix membrane /
- SiO2 /
- polyvinyl alcohol /
- sol-gel route
-
颗粒增强Al基复合材料是一种先进的结构材料,因其优异的性能(低密度、高比强度和比刚度、良好的耐磨性、良好的导热性和导电性等)和廉价的增强相颗粒,在汽车制造、军事、体育运动、精密仪表、电子封装、航空航天等行业中有大规模应用的广阔前景[1-4]。复合材料的制备方法主要有粉末冶金法、喷射沉积、挤压铸造和搅拌铸造等[5-7]。粉末冶金法制备的复合材料具有优异的性能,但其工艺流程长、工序复杂,制品的尺寸难以控制,工业上大规模生产颗粒增强型复合材料仍然面临许多的困难。喷射沉积制备法成本高,设备复杂,不利于实现产业化。挤压铸造法工艺的缺点在于预制块在压力作用下易变形,因而会出现制得的复合材料微观组织不均匀、晶粒尺寸比较大、有害界面反应难以控制、后续机加工困难等问题。搅拌铸造法所用的设备简单,工艺流程短,生产成本低,具有近终形成型等优点,易于实现大规模的产业化生产。但其制备的复合材料组织粗大、成分偏析严重、陶瓷颗粒与基体之间结合强度低,采用搅拌铸造法制备复合材料的工艺还需持续改进。研究者通过对增强相的加热、球磨、喷吹等辅助方法与传统的搅拌铸造法相结合来完善复合材料的制备工艺[8-10],如Mazahery等[8]采用球磨-涡流的方式制备高性能A356/nano-Al2O3 Al基复合材料,其拉伸性能、屈服强度、延伸率均得到提高;MOHSEN等[10]利用加热增强相来增强复合材料的性能等。
颗粒增强Al基复合材料的磨损特性也引起了学术界的重视,如刹车片、发动机引擎等,颗粒增强Al基复合材料的出现,在相同磨损条件下,可减轻重量,提高经济效益[11-15]。王凤梅等[16]发现Al基复合材料的磨损机制主要为磨粒磨损、黏着磨损和氧化磨损。目前大多数增强颗粒的研究集中在微米级,纳米级别的研究甚少。并有部分学者认为,增强颗粒平均直径过小时,易引发颗粒团聚现象,导致复合料力学性能降低,增强效果不佳,故耐磨性提升有限。在本研究中,制备了转喷微注的设备,采用球磨-转喷微注的工艺将纳米Al2O3颗粒(Al2O3p)加入到Al(7075)基体中并对其进行摩擦磨损实验,探索不同载荷下细晶组织及纳米Al2O3p对纳米Al2O3p/Al(7075)复合材料耐磨性的影响,为开发耐磨性好的Al基复合材料提供实验与理论依据。
1. 实验材料及方法
实验设计了包含有可调速的搅拌设备、惰性气体隔绝保护装置、颗粒增强体的加入装置、温度控制系统的一整套可控的转喷微注装置(如图1所示),其优点是将搅拌与喷射注入增强颗粒的过程合为一体,它可以避免增强颗粒与空气直接接触,带入杂质气体,颗粒由Ar通过空心的搅拌杆直接送入Al熔体的内部,再由搅拌叶片分散到熔体中,增加增强相与Al熔体的接触时间,同时增强相的微量连续注入过程能够得到保证,使纳米级陶瓷颗粒与Al合金良好的复合,以达到更好的分散度。
采用球磨-转喷微注的工艺制备纳米Al2O3p/Al(7075)复合材料,具体制备过程如下:(1)球磨:将30~100 nm的Al2O3p和5~10 μm的Al粉按质量比为1∶1的比例置于充满Ar的球磨罐中,球粉比为10∶1,球磨机设置转速为250 r/min,每隔0.5 h,停止运行球磨机0.5 h,如此间断进行,以减轻粉末高温凝结,经6 h球磨备用;(2)转喷微注:将Al(7075)加热到700℃,待熔化后,将混合粉体通过Ar连续微量注入熔融态Al(7075)中,同时对金属液加以旋转搅拌,使喷射与搅拌过程同时进行,Al2O3p的质量分数为1.5wt%;(3)精炼浇注成型:待转喷微注过程完成约3 min后,升温至720℃加入ZnCl2进行精炼除气,最后浇注成尺寸为Φ50 mm×10 mm的圆柱形试样。采用相同的工艺参数,制备不含纳米Al2O3p的Al(7075)作为对比。
摩擦磨损实验采用的设备为CFT-I型多功能摩擦磨损测试仪,磨损方式为室温干摩擦,对Al(7075)和纳米Al2O3p/Al(7075)复合材料进行测试,加载载荷分别为15 N、25 N、35 N,实验时间为10 min,采用旋转摩擦,摩擦副选用Φ5 mm的钢球,旋转半径为50 mm,运行速度为600 r/min。实验原理如图2所示,a为WC-Co硬质合金球,b为盘形试样,尺寸为Φ30 mm×10 mm,通过旋转摩擦,产生磨痕,再用磨损测试仪扫描出磨痕,并计算出磨损的体积量。每一组测试均用3个试样。通过SEM观察微观组织、磨损表面形貌。
2. 结果与讨论
2.1 纳米Al2O3p/Al(7075)复合材料铸态组织
图3为Al粉和Al-Al2O3混合粉体球磨后的SEM图像。可见,Al2O3p经过与Al粉球磨混合后可将Al2O3p黏贴在Al粉表面,在复合材料制备的转喷微注过程中能起到更好的分散作用。
图4为Al(7075)和纳米Al2O3p/Al(7075)复合材料的金相显微组织。可见,Al(7075)的铸锭显微组织为蔷薇形枝晶组织(图4(a)),晶粒尺寸较大,平均晶粒度为137 μm。纳米Al2O3p/Al(7075)复合材料的晶粒尺寸最小,晶粒形态逐渐趋于等轴,粗大的树枝晶消失,逐渐变为大小均匀的等轴晶。Al(7075)的平均晶粒尺寸由137 μm细化至77 μm,这是由于金属结晶时可依附在纳米Al2O3p的表面,纳米Al2O3p可能在熔体中起到非自发生核质点的作用[17],经二维晶格错配度(δ)作为经验判据来验证:当δ<6%时,表明外来相可以作为结晶相最有效的异质形核核心;当δ=6%~12%时,外来相可以作为结晶相中等有效的异质形核核心;当δ>12%时,外来相不能够作为结晶相的异质形核核心。两个不同晶体的二维错配度常用计算公式如下[18]:
δ(hkl)s(hkl)n=3∑i=113di[uvw]n|di[uvw]scosθ−di[uvw]n|×100 式中:(hkl)s为基底的低指数晶面;(hkl)n为结晶相的低指数晶面;[uvw]s为基底晶面(hkl)s上的低指数晶向;[uvw]n为结晶相晶面(hkl)n上的低指数晶向;
d[uvw]s 为沿[uvw]s晶向上的原子间距;d[uvw]n 为沿[uvw]n晶向的原子间距;θ为[uvw]s与[uvw]n两晶向之间的夹角。通过上述二位错配度常用计算公式计算,可知α-Al2O3p(11-20)面和α-Al(111)面的δ=8.2%,因此纳米Al2O3p可以作为α-Al晶粒中等有效的异质形核核心,这可能是α-Al晶粒细化的原因之一。另外,由于复合材料凝固过程中部分纳米Al2O3p被固液界面前沿捕获,被推移到枝晶间或晶界处,纳米Al2O3p阻碍α-Al晶粒生长,这可能是α-Al晶粒细化的另一个原因[19-21]。图5是转喷微注法和传统搅拌铸造法制备Al2O3p/Al(7075)复合材料的SEM图像。可以看到,传统的搅拌铸造法所制备的复合材料中有颗粒的团聚现象,而转喷微注法制备的复合材料中Al2O3p分布比较均匀,主要归因于以下几点:(1)实验通过粉末混合将硬脆纳米Al2O3p镶嵌在Al粉表面,纳米Al2O3p具有较高的比表面能,因此原始状态下颗粒通常呈团聚状,如果直接将这些团聚的纳米Al2O3p加入到Al(7075)熔体中,很难获得分散均匀的纳米Al2O3p增强Al基复合材料,因此,首先通过球磨将纳米颗粒分散;(2)使用转喷微注装置,将混合粉随Ar直接连续地、微量地注入到Al熔体内部,当复合粉体加入到基体合金熔体中时,Al粉首先开始在基体合金熔体内溶解,同时逐渐把纳米Al2O3p释放到基体合金熔体中,同时随着叶片不断搅拌分散,以改善纳米Al2O3p在基体中的分布状态。因此,球磨-转喷微注的工艺能够改善增强体颗粒的分散性,组织中没有明显的团聚发生。但是,图中出现了一些微孔,这主要与转喷微注过程和精炼浇注过程有关,转喷微注过程中会带入气体杂质,在浇铸前未能将微孔去除而留在基体当中。
图6(a)为纳米Al2O3p/Al(7075)复合材料的TEM图像,深色颗粒为Al2O3p。可看出Al2O3p与基体的结合良好,图6(b)是纳米Al2O3p/Al(7075)复合材料的高分辨图像。可见,更清晰地看到良好的界面结合,这主要有以下两个方面原因:(1)纳米Al2O3p在加入前经过清洗、球磨等预处理,起到净化颗粒表面及强化与Al粉接触界面的结合;(2)在转喷微注过程中,纳米Al2O3p能够通过惰性气体缓慢注入到Al熔体中,同时搅拌的过程给其一个动能,促使颗粒与Al熔体具有相对运动,对其与基体的结合有益。
2.2 纳米Al2O3p/Al(7075)复合材料磨损量
图7为Al(7075)合金和纳米Al2O3p/Al(7075)复合材料在不同载荷(15 N、25 N和35 N)下磨损量的变化曲线。可见,随着载荷的增大,基体的磨损量变化较复合材料多。图8为Al(7075)合金和纳米Al2O3p/Al(7075)复合材料磨损表面形貌。可知,低载荷下基体与复合材料磨损量及磨痕相差不大,随载荷增大,基体合金磨损量及磨痕变化较复合材料明显,这说明Al2O3p的加入能够改善材料的摩擦性能。
2.3 纳米Al2O3p对Al2O3p/Al(7075)磨屑形貌的影响
图9为Al(7075)合金和纳米Al2O3p/Al(7075)复合材料在磨损过程中产生的磨屑形貌。可以看到,Al(7075)合金基体的磨屑很大,大多以不规则片状出现(图8(a)),在表面可以清晰的看见一些塑性变形产生的流线。随着纳米Al2O3p增强相的加入,对Al(7075)合金基体有了一定的强化作用,复合材料磨损过程中塑性流变受到纳米Al2O3p的阻碍,纳米Al2O3p/Al(7075)复合材料被剥落下来的磨屑变小,磨屑呈小的碎片状和小的颗粒状(图8(b)),对比材料磨损面的SEM图像可知,同载荷下Al(7075)合金的磨面呈现较大的凹坑,而纳米Al2O3p/Al(7075)复合材料的磨面较浅且相对平滑。这主要是由于复合材料中纳米Al2O3p与基体的结合良好(图4、图5),能够起到细化晶粒的作用,同时在磨损过程中能够起到良好的承载作用;另一方面,由于纳米Al2O3p的奥罗万强化作用,因此磨损量较少,磨损面积小,磨屑的尺寸也较小,复合材料的磨损性能得到改善[22-25]。这也与其磨损性能变化的趋势相一致。
3. 结 论
(1)转喷微注设备的设计对复合材料的制备具有很重要的作用,利用球磨-转喷微注的工艺制备纳米Al2O3颗粒(Al2O3p)/Al(7075)复合材料能够得到细小的晶粒组织,纳米Al2O3p在Al(7075)基体中分布均匀,且与基体结合较好,这为复合材料的制备提供了一种新的思路。
(2)经球磨-转喷微注的工艺制备纳米Al2O3p/Al(7075)复合材料,在磨损过程中,随着外加载荷的增大,纳米Al2O3p/Al(7075)复合材料和Al(7075)基体的磨损量都增大,但纳米Al2O3p/Al(7075)复合材料磨损量的增大幅度小于基体Al(7075)合金的。
(3)纳米Al2O3p/Al(7075)复合材料在高载荷下进行磨损实验时,磨损面上没有出现较大的磨粒磨损的痕迹,磨屑表面出现一些塑性变形产生的流线,且尺寸较小,复合材料的磨损性能提高主要得益于纳米Al2O3p与基体的结合及支撑作用和材料的细晶强化作用。
-
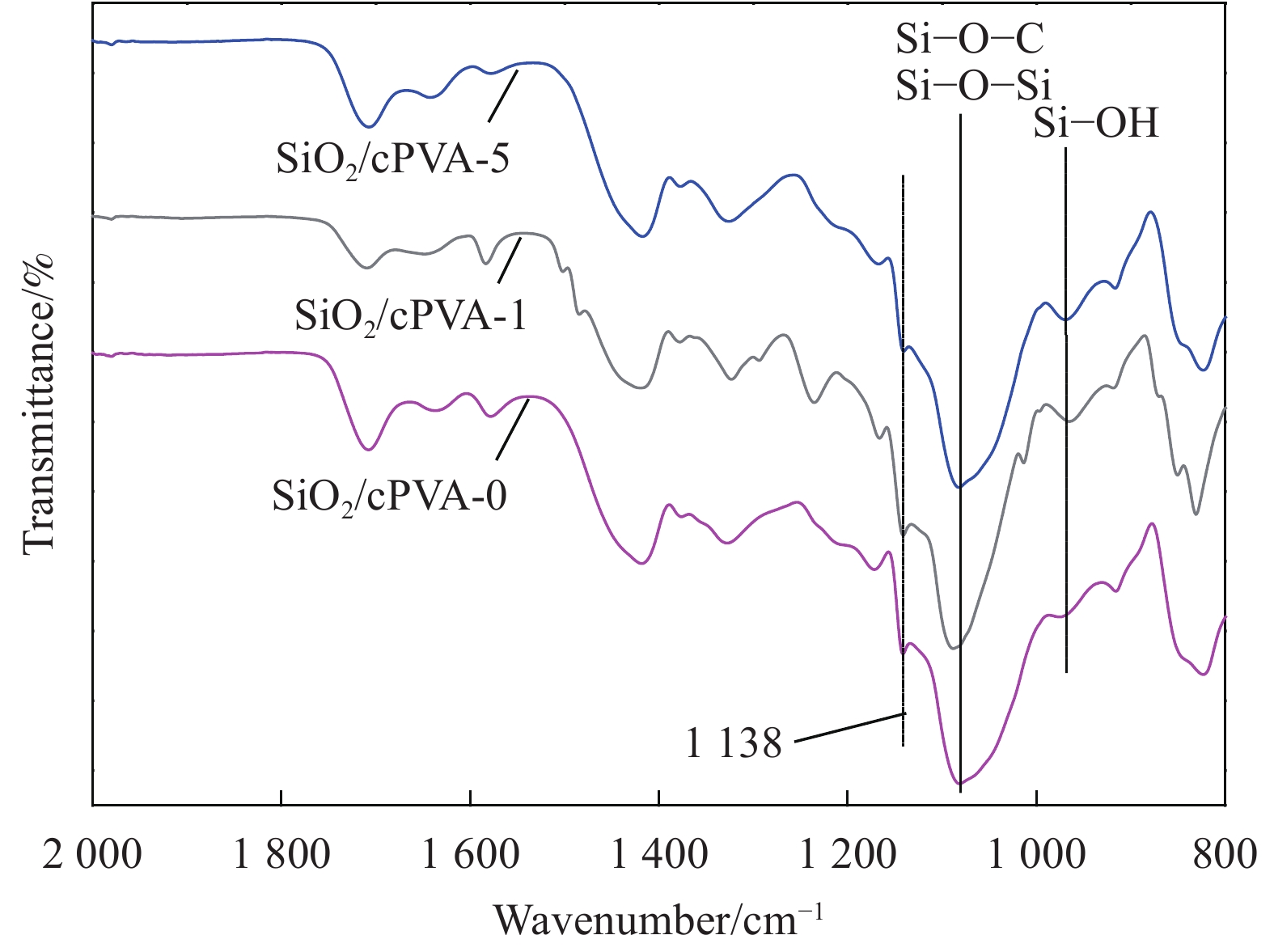
表 1 SiO2/交联聚乙烯醇(cPVA)混合基质膜的命名
Table 1 Naming of SiO2/crosslinked polyvinyl alcohol (cPVA) mixed matrix membranes
Sample Mass ratio of SiO2 to PVA/
%Maleic acid (MA)/g PVA/g 0.1 mol/L HCl/mL SiO2/cPVA-0 5 3.2 16.0 0 SiO2/cPVA-1 5 3.2 16.0 1 SiO2/cPVA-5 5 3.2 16.0 5 表 2 50℃水扩散系数DWater的计算
Table 2 Diffusion coefficients of water DWater at 50℃
Di×108 /(m2·s−1) SiO2/cPVA-5 SiO2/cPVA-1 SiO2/cPVA-0 DWater 14.4 9.8 10.6 Note: Di—Apparent diffusion coefficient. 表 3 SiO2/cPVA混合基质膜与其他渗透汽化膜的比较
Table 3 Comparison of SiO2/cPVA mixed matrix membranes with other pervaporation membranes
Nanofiller Polymer matrix Application Temperature/℃ Flux/(g·m−2·h−1) Separation Factor Ref. Titanate nanotubes PVA Water/isopropanol 50 ~30 5520 [26] GO Polyimide Water/isopropanol 60 161.5 >5000 [27] MXene CS Water/ethanol 50 1424 1421 [28] SiO2 PDMS Recover phenol 50 7.16 4.29 [29] GOF PVA Water/ethanol 70 ~300 330 [30] PMA PDMS Water/butanol 70 923 42 [31] Elliptic
silicaPVA Water/ethanol 50 72 12301 This work Notes: GO—Graphite oxide; CS—Chitosan; PDMS—Polydimethylsiloxane; GOF—Graphene oxide framework; PMA—Propylene glycol methyl ether acetate. -
[1] VANE L M. Review of pervaporation and vapor permeation process factors affecting the removal of water from industrial solvents[J]. Journal of Chemical Technology and Biotechnology,2020,95(3):495-512. DOI: 10.1002/jctb.6264
[2] CASTRO-MUNOZ R, GALIANO F, FILA V, et al. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: Current state of the art[J]. Reviews in Chemical Engineering,2019,35(5):565-590. DOI: 10.1515/revce-2017-0115
[3] ZENG G Y, WANG B, ZHANG J, et al. Construction of two-dimensional MXene membrane and its research progress of application in water treatment[J]. Acta Materiae Compositae Sinica,2021,38(7):2078-2091.
[4] LIU G P, JIN W Q, XU N P. Graphene-based membranes[J]. Chemical Society Reviews,2015,44(15):5016-5030. DOI: 10.1039/C4CS00423J
[5] KARDANI R, ASGHARI M, MOHAMMADI T, et al. Effects of nanofillers on the characteristics and performance of PEBA-based mixed matrix membranes[J]. Reviews in Chemical Engineering,2018,34(6):797-836. DOI: 10.1515/revce-2017-0001
[6] LIU G, SHEN J, LIU Q, et al. Ultrathin two-dimensional MXene membrane for pervaporation desalination[J]. Journal of Membrane Science,2018,548:548-558. DOI: 10.1016/j.memsci.2017.11.065
[7] LIU G, CHERNIKOVA V, LIU Y, et al. Mixed matrix formulations with MOF molecular sieving for key energy-intensive separations[J]. Nature Materials,2018,17(3):283-289. DOI: 10.1038/s41563-017-0013-1
[8] LIANG B, LI Q, CAO B, et al. Water permeance, permeability and desalination properties of the sulfonic acid functionalized composite pervaporation membranes[J]. Desalination,2018,433:132-140. DOI: 10.1016/j.desal.2018.01.028
[9] ZHU Z Y, WAND L, JIANG J L, etal. Preparation and properties of nano SiO2-GO/poly vinylidene fluoride hybrid membrane[J]. Acta Materiae Compositae Sinica,2018,35(4):785-792.
[10] WANG X Y, CHU W D, GE M N, et al. Fabrication of sulfhydryl grafted graphene oxide/polyamide composite membranes for reverse osmosis desalination[J]. Acta Materiae Compositae Sinica,2021,38(8):2479-2488.
[11] YANG D C, CHENG C, BAO M T, et al. The pervaporative membrane with vertically aligned carbon nanotube nanochannel for enhancing butanol recovery[J]. Journal of Membrane Science,2019,577:51-59. DOI: 10.1016/j.memsci.2019.01.032
[12] YANG J, GONG D, LI G, et al. Self-assembly of thiourea-crosslinked graphene oxide framework membranes toward separation of small molecules[J]. Advanced Mater-ials,2018,30(16):1705775. DOI: 10.1002/adma.201705775
[13] YANG H, CHENG X P, CHENG X X, et al. Highly water-selective membranes based on hollow covalent organic frameworks with fast transport pathways[J]. Journal of Membrane Science,2018,565:331-341. DOI: 10.1016/j.memsci.2018.08.043
[14] WANG J, LI M, ZHOU S, et al. Graphitic carbon nitride nanosheets embedded in poly(vinyl alcohol) nanocompo-site membranes for ethanol dehydration via pervaporation[J]. Separation and Purification Technology,2017,188:24-37. DOI: 10.1016/j.seppur.2017.07.008
[15] RODENAS T, LUZ I, PRIETO G, et al. Metal-organic framework nanosheets in polymer composite materials for gas separation[J]. Nature Materials,2014,14:48.
[16] JEON M Y, KIM D, KUMAR P, et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets[J]. Nature,2017,543:690. DOI: 10.1038/nature21421
[17] ARIF Z, SETHY N K, MISHRA P K, et al. Investigating the influence of sol gel derived PVA/SiO2 nano composite membrane on pervaporation separation of azeotropic mixture I. Effect of operating condition[J]. Journal of Porous Materials,2018,25(4):1203-1211. DOI: 10.1007/s10934-017-0530-y
[18] WU Y, XIE Z, NG D, et al. Poly(ether sulfone) supported hybrid poly(vinyl alcohol)-maleic acid-silicone dioxide membranes for the pervaporation separation of ethanol-water mixtures[J]. Journal of Applied Polymer Science,2017,134(20):44839.
[19] YANG H, WU H, XU Z, et al. Hierarchical pore architectures from 2D covalent organic nanosheets for efficient water/alcohol separation[J]. Journal of Membrane Science,2018,561:79-88. DOI: 10.1016/j.memsci.2018.05.036
[20] YANG G, XIE Z, DOHERTY C M, et al. Understanding the transport enhancement of poly (vinyl alcohol) based hybrid membranes with dispersed nanochannels for pervaporation application[J]. Journal of Membrane Science,2020,603:118005. DOI: 10.1016/j.memsci.2020.118005
[21] KIM H G, NA H R, LEE H, et al. Distillation-pervaporation membrane hybrid system for epichlorohydrin and isopropyl alcohol recovery in epoxy resin production process[J]. Separation and Purification Technology,2021,254:117678. DOI: 10.1016/j.seppur.2020.117678
[22] TENG S H, NIU N, CHEN F W, et al. Polyvinyl alcohol/silica hybrid microspheres: Synthesis, characterization and potential applications as adsorbents[J]. Materials Research Express,2019,6(5):055201. DOI: 10.1088/2053-1591/aaffa7
[23] LUO R, DING H, LYU J, et al. Fabrication of sandwiched silicalite-1 membrane at 2D confined space for enhanced alcohol/water separation[J]. Chemical Communications,2020,56:125686-125688.
[24] PRIHATININGTYAS I, HARTANTO Y, BALLESTEROS M S R, et al. Cellulose triacetate/LUDOX-SiO2 nanocomposite for synthesis of pervaporation desalination membranes[J]. Journal of Applied Polymer Science,2021,138(11):50000. DOI: 10.1002/app.50000
[25] FLYNN E J, KEANE D A, TABARI P M, et al. Pervaporation performance enhancement through the incorporation of mesoporous silica spheres into PVA membranes[J]. Separation and Purification Technology,2016,118:73-80.
[26] RAEISI Z, MOHEB A, SADEGHI M, et al. Titanate nano-tubes-incorporated poly(vinyl alcohol) mixed matrix membranes for pervaporation separation of water-isopropanol mixtures[J]. Chemical Engineering Research & Design,2019,145:99-111.
[27] SALEHIAN P, CHUNG T S. Thermally treated ammonia functionalized graphene oxide/polyimide membranes for pervaporation dehydration of isopropanol[J]. Journal of Membrane Science,2017,528:231-242. DOI: 10.1016/j.memsci.2017.01.038
[28] XU Z, LIU G, YE H, et al. Two-dimensional MXene incorporated chitosan mixed-matrix membranes for efficient solvent dehydration[J]. Journal of Membrane Science,2018,563:625-632. DOI: 10.1016/j.memsci.2018.05.044
[29] YAO J, LI D, LIU S, et al. Pervaporation process with SiO2/PDMS/PVDF composite membrane for the recycle of phenol from coal chemical wastewater[J]. Technology of Water Treatment,2017,43(2):85-89.
[30] LI G, SHI L, ZENG G, et al. Sharp molecular-sieving of alcohol-water mixtures over phenyldiboronic acid pillared graphene oxide framework (GOF) hybrid membrane[J]. Chemical Communications,2015,51:7345-7348. DOI: 10.1039/C5CC00924C
[31] HU M J, SUN L, WU Z Y, et al. Preparation and pervaporation performance of PDMS-PMA semi-interpenetrating polymer network membrane[J]. Chemistry & Bioengineering,2019,36(10):41-46.
-
期刊类型引用(2)
1. 郇昌宝,贺毅强,苏前航,任昌旭,丁云飞,冯文,穆昱学,刘勇,左立杰,尚峰,冯立超. 喷射沉积颗粒增强铝基复合材料显微组织调控与强韧化机制的研究现状. 材料导报. 2022(S2): 302-307 .  百度学术
百度学术
2. 廖先金,连晨姿,张贵锋. SiC颗粒增强铝基复合材料的纯铜中间层液相扩散焊. 复合材料学报. 2021(02): 572-582 .  本站查看
本站查看
其他类型引用(1)
-





 下载:
下载: