Construction of nano Au-nitrogen doped carbon nanotubes integrated composite cathode and performance study for lithium-oxygen batteries
-
摘要: 高效稳定的正极对锂氧气电池至关重要。通过化学气相沉积、光还原两步合成工艺,将具有高催化活性的Au纳米粒子原位负载在具有三维贯穿结构的氮掺杂碳纳米管(N-CNT)/不锈钢(SS)网上,制备了具有互相渗透孔道的高性能一体化锂氧气电池正极Au-N-CNT/SS。通过SEM、TEM、XPS、XRD及Raman等表征手段对Au-N-CNT/SS的微观形貌、组成进行了考察。制备的Au-N-CNT/SS正极具有合适的孔道结构、高电导率、超强的力学性能、结构稳定性等,克服了传统电极机械稳定性差、碳电极易分解、副反应严重等问题。用作锂氧气电池正极,Au-N-CNT/SS一体化电极的设计避免了黏结剂的使用,极大地提高了电池的力学强度,有效降低了副反应,提升了电池的电化学/化学稳定性;正极的高导电率、充足的孔道结构提供了快速的电子输运和传质通道;Au纳米粒子高效催化剂有效提升了正极的氧还原/氧析出反应动力学,加快了放电产物的生成与分解,电池的倍率性能(1.0 mA·cm−2的高电流密度下放电电压保持在2.4 V)、放电容量(8.47 mA·h·cm−2)和循环性能(160圈)得到了较大提升。Abstract: Highly efficient, stable cathode is crucial to lithium-oxygen battery. A high performance, integrated Au-N-CNT/SS cathode with interpermeable channels was constructed by chemical vapor deposition and photoreduction, in which the high catalytic Au nanoparticles were loaded on nitrogen doped carbon nanotubes (N-CNT) with three-dimensional penetrating sturctured stainless steel (SS) mesh. The morphology and composition of the Au-N-CNT/SS were investigated by SEM, TEM, XPS, XRD and Raman spectrum. The problems of poor mechanical stability, carbonaceous cathode decomposition and serious side reactions were avoided by the suitable channel structure, high conductivity, superior mechanical properties, structural stability of Au-N-CNT/SS. Taking Au-N-CNT/SS as the integrated cathode for lithium-oxygen battery, the utilization of binder is avoided. The mechanical strength of the lithium-oxygen battery is enhanced, and the side reactions are effectively reduced, contributing to the enhanced electrochemical/chemical stability. The high conductivity, interpenetrated structure and sufficient pores provide a fast electron transport and mass transfer channel. The highly efficient Au nanoparticles are favorable to improving the oxygen reduction/oxygen evolution reaction kinetics on cathode, accelerating the generation and decomposition of discharge products. The rate performance (keeping the discharge voltage at 2.4 V with a high current density of 1.0 mA·cm−2), specific capacity (8.47 mA·h·cm−2) and cycle performance (160 cycles) of lithium-oxygen battery are greatly improved.
-
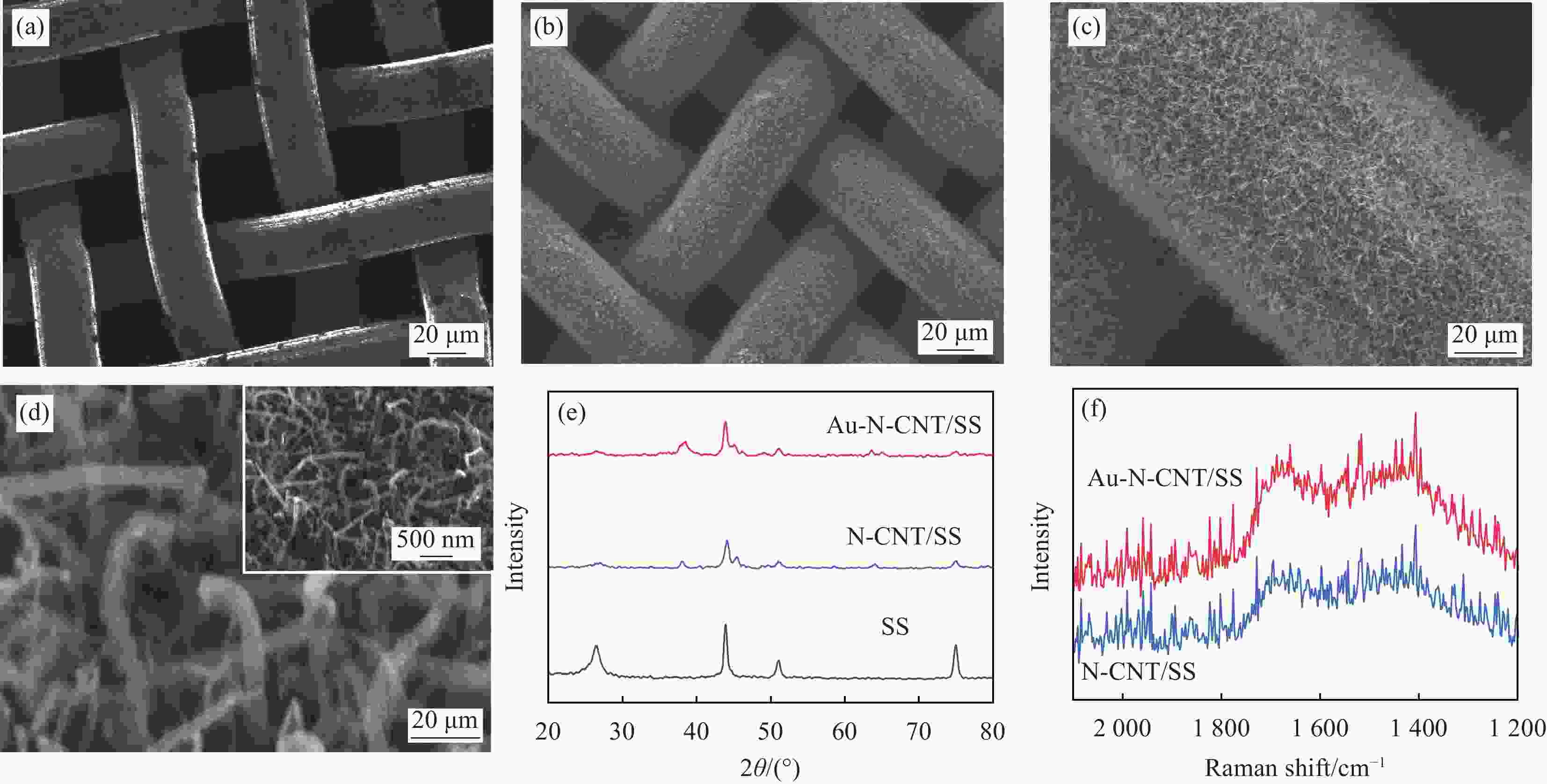
图 2 (a) SS的SEM图像;((b)~(d)) 不同放大倍数下Au-N-CNT/SS的SEM图像;(e) Au-N-CNT/SS、N-CNT/SS及SS的XRD图谱;(f) Au-N-CNT/SS及N-CNT/SS的Raman图谱
Figure 2. (a) SEM image of SS; ((b)-(d)) SEM images of Au-N-CNT/SS at different magnifications; (e) XRD patterns of Au-N-CNT/SS, N-CNT/SS and SS; (f) Raman spectra of Au-N-CNT/SS and N-CNT/SS
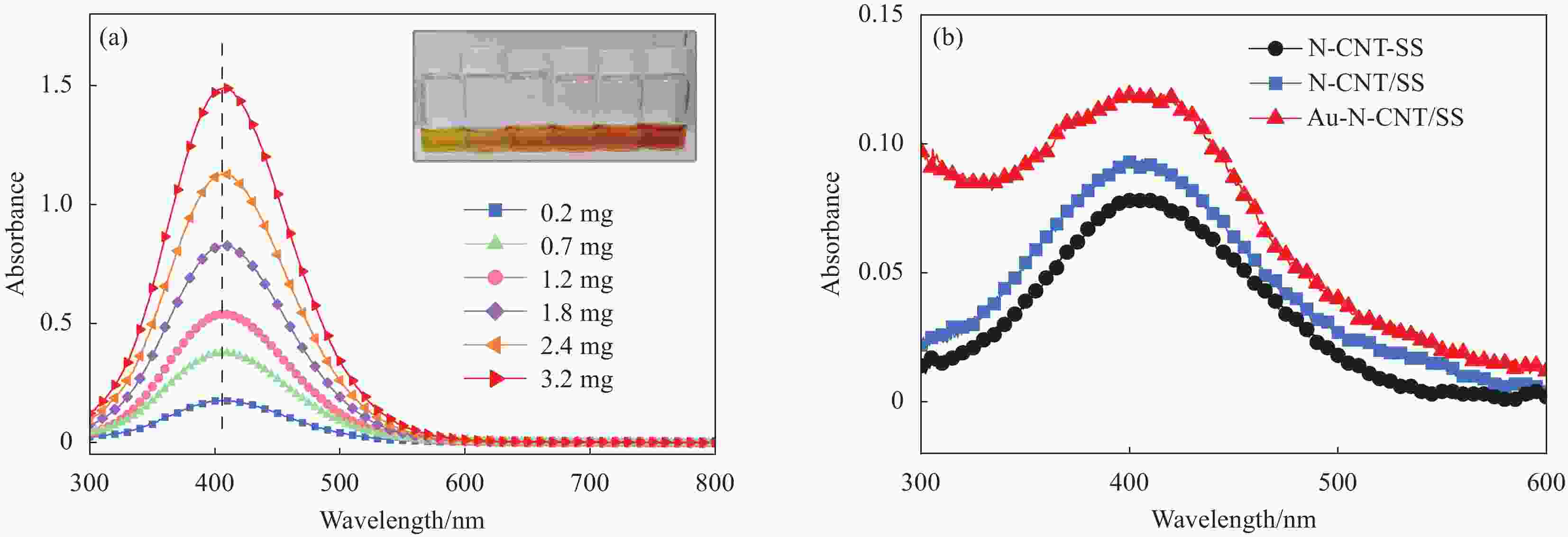
图 3 ((a), (b))不同放大倍数下氮掺杂碳纳米管/不锈钢网电极(N-CNT-SS)的SEM图像;(c) Au-N-CNT/SS的TEM图像;(d) Au-N-CNT/SS的高分辨TEM图像;(e) Au-N-CNT/SS电极中Au4f的XPS图谱;(f) Au-N-CNT/SS和N-CNT/SS的紫外可见吸收图谱
Figure 3. ((a), (b)) SEM images of nitrogen-doped carbon nanotubes/stainless steel mesh electrode (N-CNT-SS) at different magnifications; (c) TEM image of the Au-N-CNT/SS; (d) High resolution TEM image of the Au-N-CNT/SS; (e) XPS spectra of Au4f in Au-N-CNT/SS; (f) UV-Vis absorption spectra of Au-N-CNT/SS and N-CNT/SS
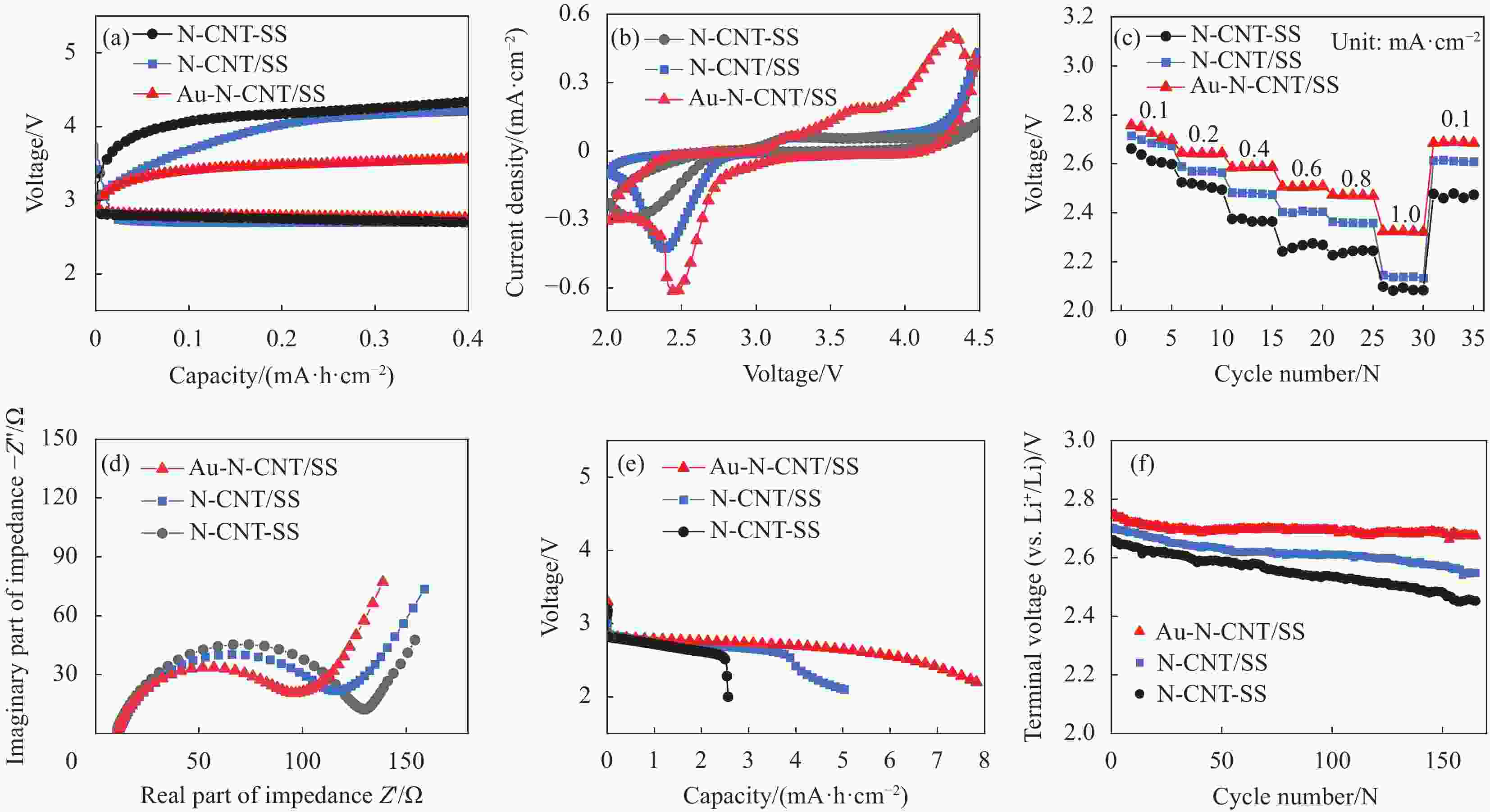
图 4 基于Au-N-CNT/SS、N-CNT/SS及N-CNT-SS电极的锂氧气电池的首圈充放电曲线 (a)、循环伏安曲线 (b)、不同电流密度下的放电电压变化 (c)、电化学交流阻抗谱 (d)、放电容量 (e)、循环性能 (f)
Figure 4. First discharge-charge curves (a), cyclic voltammetry curves (b), discharge voltage variation at different current densities (c), electrochemical impedance spectroscopy (d), discharge capacity (e), cycling performance (f) of the lithium-oxygen battery with Au-N-CNT/SS, N-CNT/SS and N-CNT-SS
图 5 首次放电和充电后Au-N-CNT/SS正极((a), (b))、N-CNT/SS正极((c), (d))和N-CNT-SS ((e), (f))正极的SEM图像;(g) 首次放电和充电后Au-N-CNT/SS、N-CNT/SS和N-CNT-SS正极的红外图谱,其中Li2O2、Li2CO3、HCO2Li和CH3CO2Li的图谱供参考
Figure 5. SEM images of the recharged Au-N-CNT/SS cathode ((a), (b)), N-CNT/SS cathode ((c), (d)) and N-CNT-SS cathode ((e), (f)) after 1st discharged and charged process; (g) FTIR spectra of the Au-N-CNT/SS, N-CNT/SS and N-CNT-SS cathodes after 1st discharged and charged process, in which the spectra for Li2O2, Li2CO3, HCO2Li and CH3CO2Li are also shown for reference
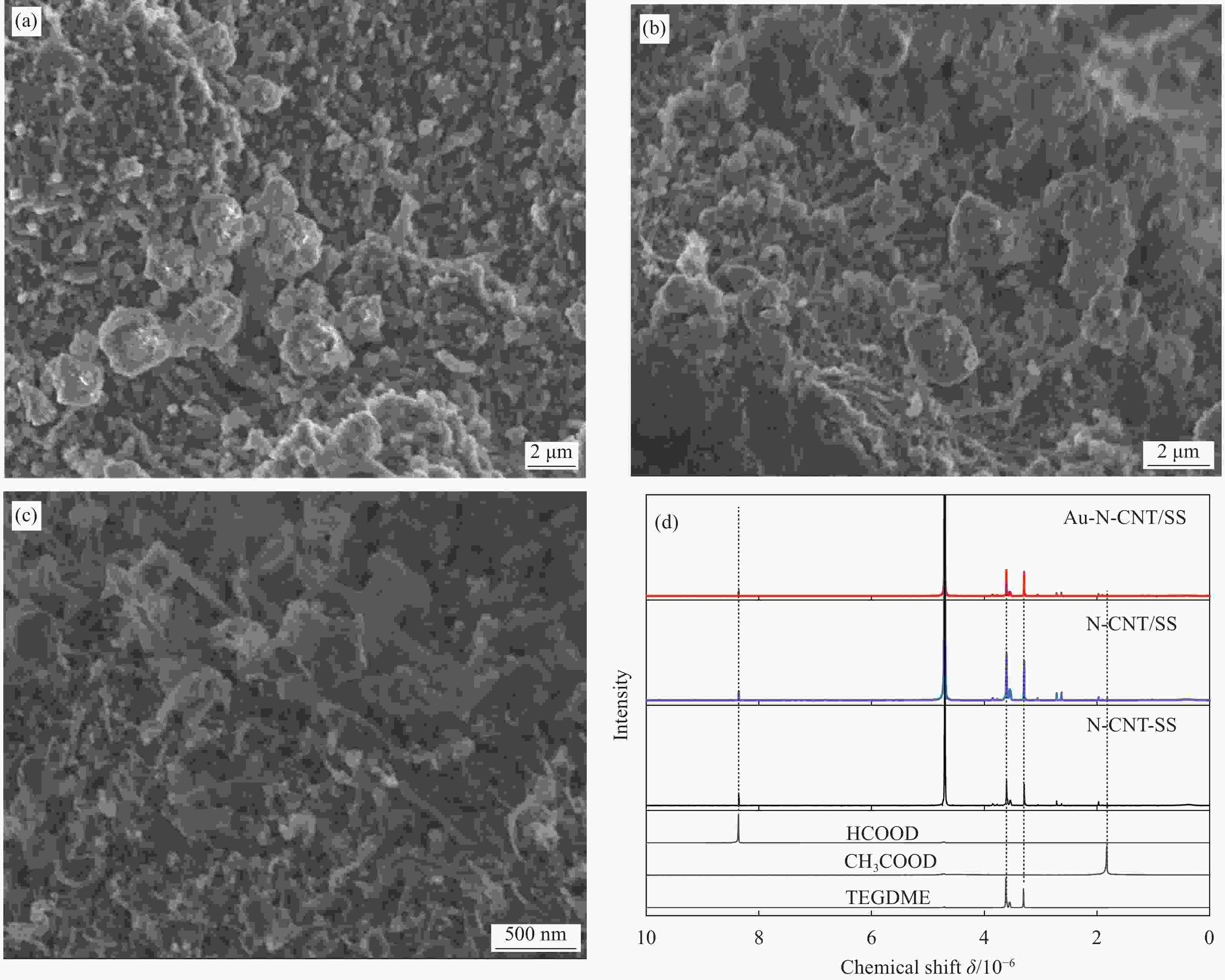
图 7 第20次充电后Au-N-CNT/SS (a)、N-CNT/SS (b) 和N-CNT-SS正极 (c) 的SEM图像(电流密度为0.2 mA·cm−2,充电容量为1.0 mA·h·cm−2);(d) 第20次充电后Au-N-CNT/SS、N-CNT/SS和N-CNT-SS正极的1H核磁共振图谱,其中四乙二醇二甲醚(TEGDME)、CH3COOD和HCOOD的图谱供参考
Figure 7. SEM images of the Au-N-CNT/SS (a), N-CNT/SS (b), and N-CNT-SS cathodes (c) at a current density of 0.2 mA·cm−2 with a charge capacity of 1.0 mA·h·cm−2 after the 20th recharge; (d) 1H NMR spectra of the Au-N-CNT/SS, N-CNT/SS and N-CNT-SS cathodes after the 20th recharge, in which the spectra for tetraethylene glycol dimethyl ether (TEGDME), CH3COOD and HCOOD are also shown for reference
-
[1] MA J M, LI Y T. Editorial for advanced energy storage and conversion materials and technologies[J]. Rare Metals, 2021, 40(2): 246-248. [2] PENG G S, HUANG J, GU Y C, et al. Self-corrosion, electrochemical and discharge behavior of commercial purity Al anode via Mn modification in Al-air battery[J]. Rare Metals,2021,40(12):3501-3511. doi: 10.1007/s12598-020-01687-9 [3] ZHU Q C, DU F H, XU S M, et al. Hydroquinone resin induced carbon nanotubes on Ni foam as binder-free cathode for Li-O2 batteries[J]. ACS Applied Materials & Interfaces,2016,8(6):3868-3873. [4] ZHAO G Y, ZHANG L, LYU J X, et al. A graphitic foam framework with hierarchical pore structure as self-supported electrodes of Li-O2 batteries and Li ion batteries[J]. Journal of Materials Chemistry A,2016,4(4):1399-1407. doi: 10.1039/C5TA09033D [5] YANG W C, QIAN Z Y, DU C Y, et al. Hierarchical ordered macroporous/ultrathin mesoporous carbon architecture: A promising cathode scaffold with excellent rate performance for rechargeable Li-O2 batteries[J]. Carbon,2017,118:139-147. doi: 10.1016/j.carbon.2017.03.037 [6] QIU Z M, BAI Y, GAO Y D, et al. MXenes nanocomposites for energy storage and conversion[J]. Rare Metals,2022,41(4):1101-1128. doi: 10.1007/s12598-021-01876-0 [7] LENG L M, LI J, ZENG X Y, et al. Enhancing the cyclability of Li-O2 batteries using PdM alloy nanoparticles anchored on nitrogen-doped reduced graphene as the cathode catalyst[J]. Journal of Power Sources,2017,337:173-179. doi: 10.1016/j.jpowsour.2016.10.089 [8] XU J J, CHANG Z W, YIN Y B, et al. Nanoengineered ultralight and robust all-metal cathode for high-capacity, stable lithium-oxygen batteries[J]. ACS Central Science,2017,3(6):598-604. doi: 10.1021/acscentsci.7b00120 [9] QIN Y C, WANG F Q, WANG X M, et al. Noble metal-based high-entropy alloys as advanced electrocatalysts for energy conversion[J]. Rare Metals,2021,40(9):2354-2368. doi: 10.1007/s12598-021-01727-y [10] LI Z, GANAPATHY S, XU Y, et al. Fe2O3 nanoparticle seed catalysts enhance cyclability on deep (dis)charge in aprotic Li-O2 batteries[J]. Advanced Energy Materials,2018,8(18):1703513. doi: 10.1002/aenm.201703513 [11] LIU X M, ZHAO L L, XU H R, et al. Tunable cationic vacancies of cobalt oxides for efficient electrocatalysis in Li-O2 batteries[J]. Advanced Energy Materials,2020,10(40):2001415. doi: 10.1002/aenm.202001415 [12] 李华, 李靖靖, 王焕锋. 多孔Co3O4纳米纤维用于锂-空气电池高性能正极催化剂[J]. 复合材料学报, 2021, 38(7): 2305–2312.LI Hua, LI Jingjing, WANG Huanfeng. Porous Co3O4 nanofibers applied as an efficient cathode catalyst for Li-air batteries[J]. Acta Materiae Compositae Sinica, 2021, 38(7): 2305-2312(in Chinese). [13] SUN Z M, HE J L, YUAN M W, et al. Li+-clipping for edge S-vacancy MoS2 quantum dots as an efficient bifunctional electrocatalyst enabling discharge growth of amorphous Li2O2 film[J]. Nano Energy,2019,65:103996. doi: 10.1016/j.nanoen.2019.103996 [14] WANG P, ZHAO D Y, HUI X B, et al. Bifunctional catalytic activity guided by rich crystal defects in Ti3C2 MXene quantum dot clusters for Li-O2 batteries[J]. Advanced Energy Materials,2021,11(32):2003069. doi: 10.1002/aenm.202003069 [15] YANG Z D, YANG X Y, LIU T, et al. In situ CVD derived Co-N-C composite as highly efficient cathode for flexible Li-O2 batteries[J]. Small,2018,14(43):1800590. doi: 10.1002/smll.201800590 [16] WANG Y, SONG L N, WANG Y F, et al. A TEMPO-grafted multi-functional cathode with strong anchoring ability towards redox mediators for high energy efficiency Li-O2 batteries[J]. Energy Storage Materials,2022,45:191-200. doi: 10.1016/j.ensm.2021.11.038 [17] LI J J, DING S Q, ZHANG S M, et al. Catalytic redox mediators for non-aqueous Li-O2 battery[J]. Energy Storage Materials,2021,43:97-119. doi: 10.1016/j.ensm.2021.08.036 [18] LIU X A, ZHANG P, LIU L L, et al. Inhibition of discharge side reactions by promoting solution-mediated oxygen reduction reaction with stable quinone in Li-O2 batteries[J]. ACS Applied Materials & Interfaces,2020,12(9):10607-10615. [19] KIM C H J, VARANASI C V, LIU J. Synergy of polypyrrole and carbon X-aerogel in lithium-oxygen batteries[J]. Nanoscale,2018,10(8):3753-3758. doi: 10.1039/C7NR08494C [20] WANG H Q, FAN X P, ZHANG X H, et al. In situ growth of NiO nanoparticles on carbon paper as a cathode for rechargeable Li-O2 batteries[J]. RSC Advances,2017,7(38):23328-23333. doi: 10.1039/C7RA02932B [21] WANG P, LI C X, DONG S H, et al. One-step route synthesized Co2P/Ru/N-doped carbon nanotube hybrids as bifunctional electrocatalysts for high-performance Li-O2 batteries[J]. Small,2019,15(30):1900001. doi: 10.1002/smll.201900001 [22] CHAI A H, JI C H, YUAN D, et al. Fluidic Ga-In liquid metal-modified cathode with improved cyclic performance and capacity of Li-O2 batteries[J]. Rare Metals,2022,41(7):2223-2229. doi: 10.1007/s12598-021-01903-0 [23] YOON K R, SHIN K, PARK J, et al. Brush-like cobalt nitride anchored carbon nanofiber membrane: Current collector-catalyst integrated cathode for long cycle Li-O2 batteries[J]. ACS Nano,2018,12(1):128-139. doi: 10.1021/acsnano.7b03794 [24] NAM J S, JUNG J W, YOUN D Y, et al. Free-standing carbon nanofibers protected by a thin metallic iridium layer for extended life-cycle Li-oxygen batteries[J]. ACS Applied Materials & Interfaces,2020,12(50):55756. [25] LIU Q C, XU J J, XU D, et al. Flexible lithium-oxygen battery based on a recoverable cathode[J]. Nature Communications,2015,6(1):7892. doi: 10.1038/ncomms8892 [26] SONG L N, ZHANG W, WANG Y, et al. Tuning lithium-peroxide formation and decomposition routes with single-atom catalysts for lithium-oxygen batteries[J]. Nature Communications,2020,11:2191. doi: 10.1038/s41467-020-15712-z [27] MENG Y, ZHANG J K, LU H Y, et al. High performance lithium oxygen batteries based on a phosphorous-doped holey graphene cathode[J]. Rare Metals,2022,41(12):4027-4033. doi: 10.1007/s12598-022-02089-9 [28] GUO X J, ZHANG Q, LI Y N, et al. Nanosized Rh grown on single-walled carbon nanohorns for efficient methanol oxidation reaction[J]. Rare Metals, 2022, 41(6): 2108-2117. [29] WANG H F, LI J F, SUN X X, et al. Stabilizing electrochemical Li-O2 batteries with a metal-based cathode of PdNi on Ni nonwoven fabric[J]. Nanoscale,2019,11(24):11513-11520. doi: 10.1039/C9NR02390A [30] WANG H F, LI J F, LI F, et al. Facile route to constructing ternary nanoalloy bifunctional oxygen cathode for metal-air batteries[J]. Chemical Research in Chinese Universities,2020,36(6):1153-1160. doi: 10.1007/s40242-020-0199-7 [31] CHEN J J, HAO R, WANG Z Y, et al. Co single atoms and nanoparticles dispersed on N-doped carbon nanotube as high-performance catalysts for Zn-air batteries[J]. Rare Metals,2022,41(6):2055-2062. doi: 10.1007/s12598-022-01974-7 [32] MANG X B, YAO L Q. Grazing-incidence small-angle X-ray scattering property of double-layered gold nanoparticle arrays[J]. Rare Metals,2022,41(10):3585-3590. doi: 10.1007/s12598-016-0736-1 [33] MANG X B, YAO L Q. Hexagonal packing lattice formed by functionalized gold nanoparticles[J]. Rare Metals,2022,41(11):3858-3864. doi: 10.1007/s12598-016-0744-1 [34] LI Y R, LI M X, LI S N, et al. A review of energy and environment electrocatalysis based on high-index faceted nanocrystals[J]. Rare Metals,2021,40(12):3406-3441. doi: 10.1007/s12598-021-01747-8 [35] WANG H F, MIN Y T, LI P C, et al. In situ integration of ultrathin PtRuCu alloy overlayer on copper foam as an advanced free-standing bifunctional cathode for rechargeable Zn-air batteries[J]. Electrochimica Acta,2018,283:54−62. doi: 10.1016/j.electacta.2018.06.097 [36] SONG L N, ZOU L C, WANG X X, et al. Realizing formation and decomposition of Li2O2 on its own surface with a highly dispersed catalyst for high round-trip efficiency Li-O2 batteries[J]. iScience,2019,14:36-46. doi: 10.1016/j.isci.2019.03.013 [37] WU H T, SUN W, WANG Y, et al. Facile synthesis of hierarchical porous three-dimensional free-standing MnCo2O4 cathodes for long-life Li-O2 batteries[J]. ACS Applied Materials & Interfaces,2017,9(14):12355-12365. -






 下载:
下载: