Preparation of magnetic nitrogen-doped fir sawdust biochar to activate peroxymonosulfate for Levofloxacin degradation
-
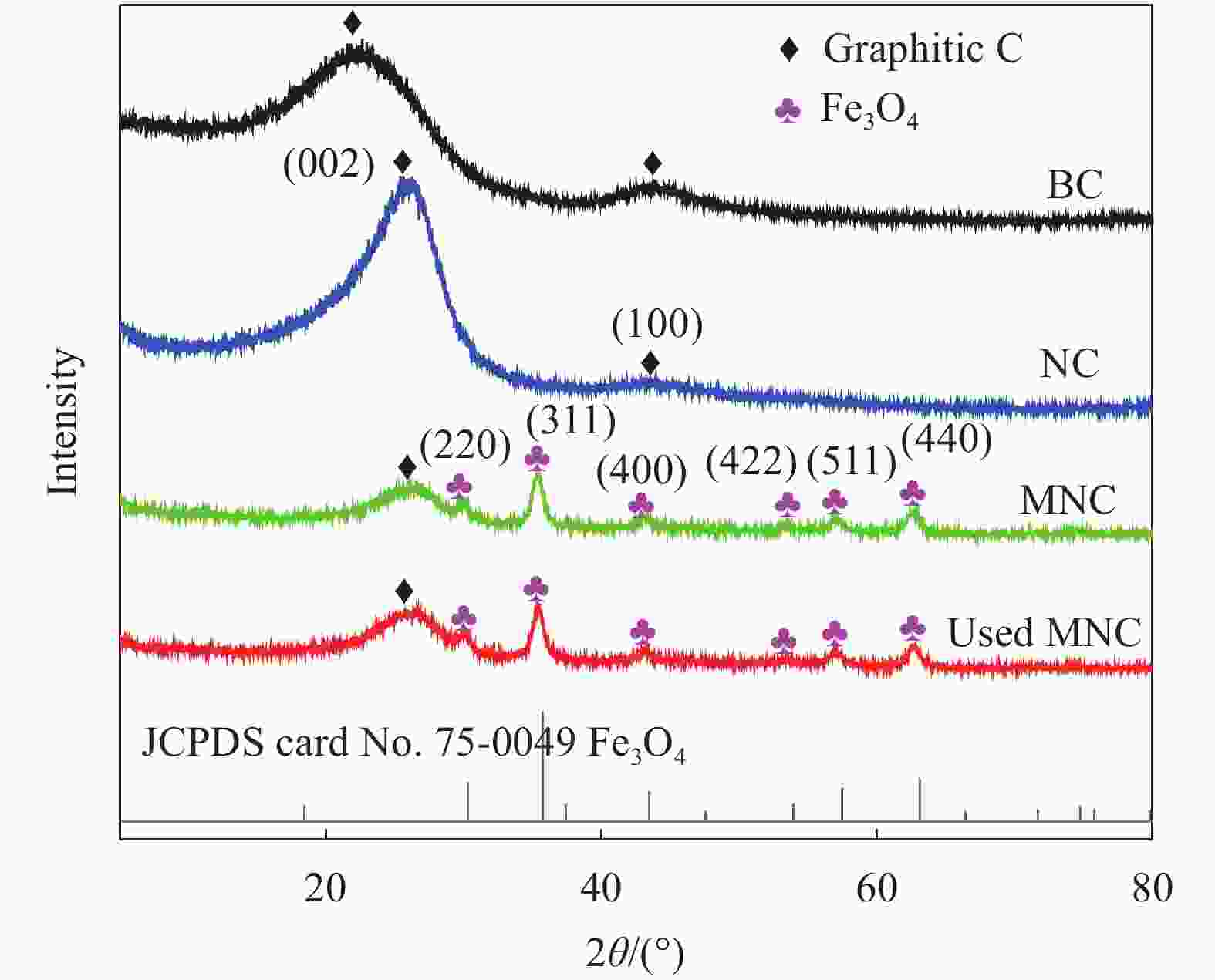
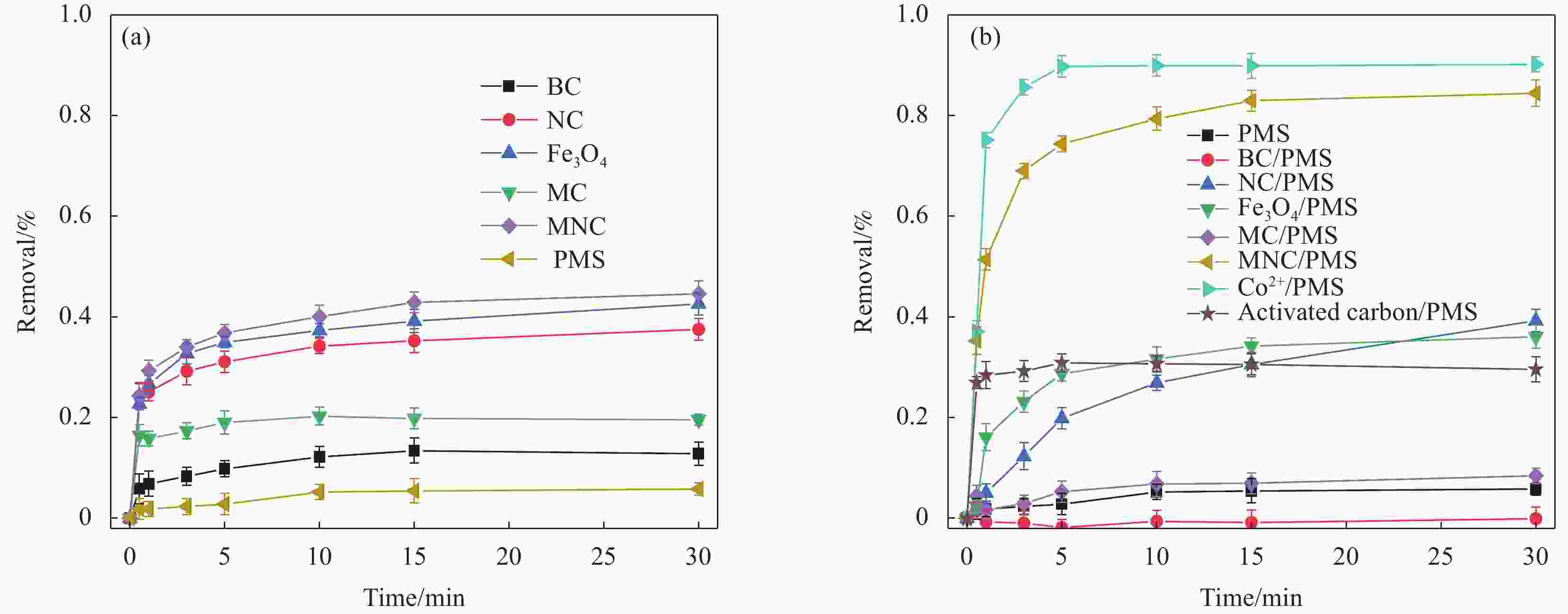
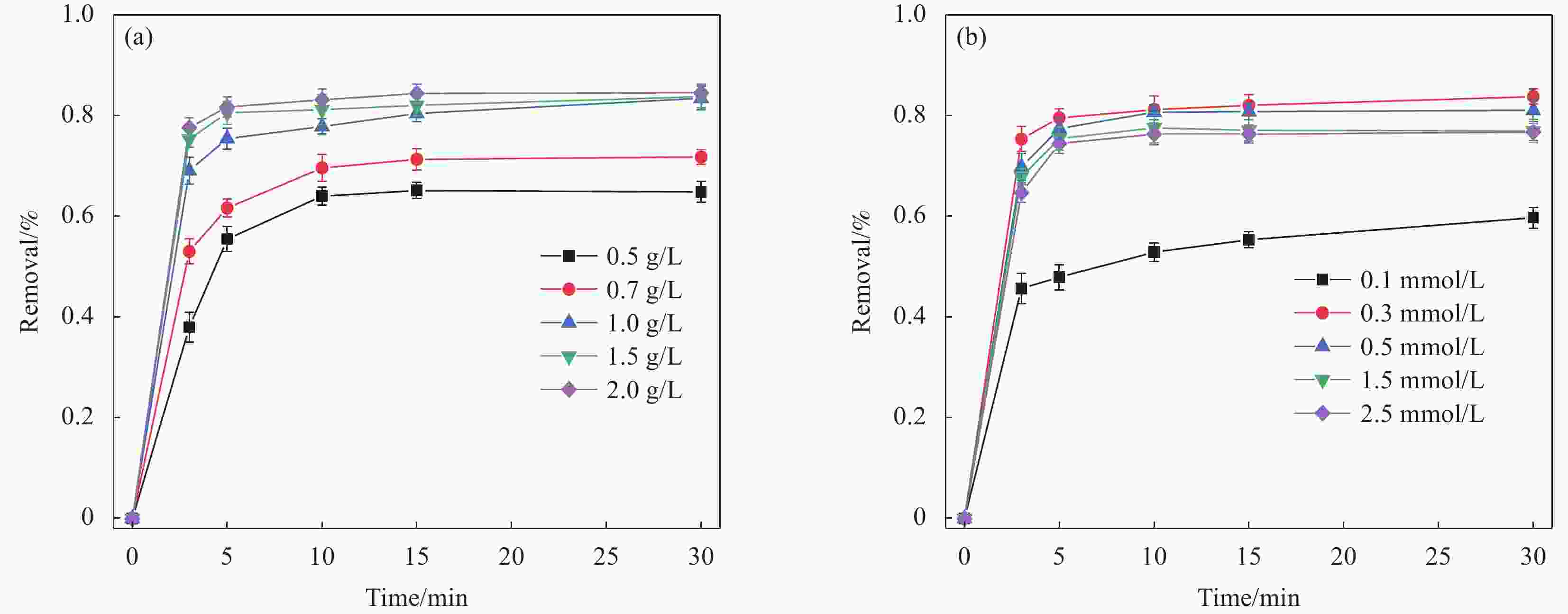
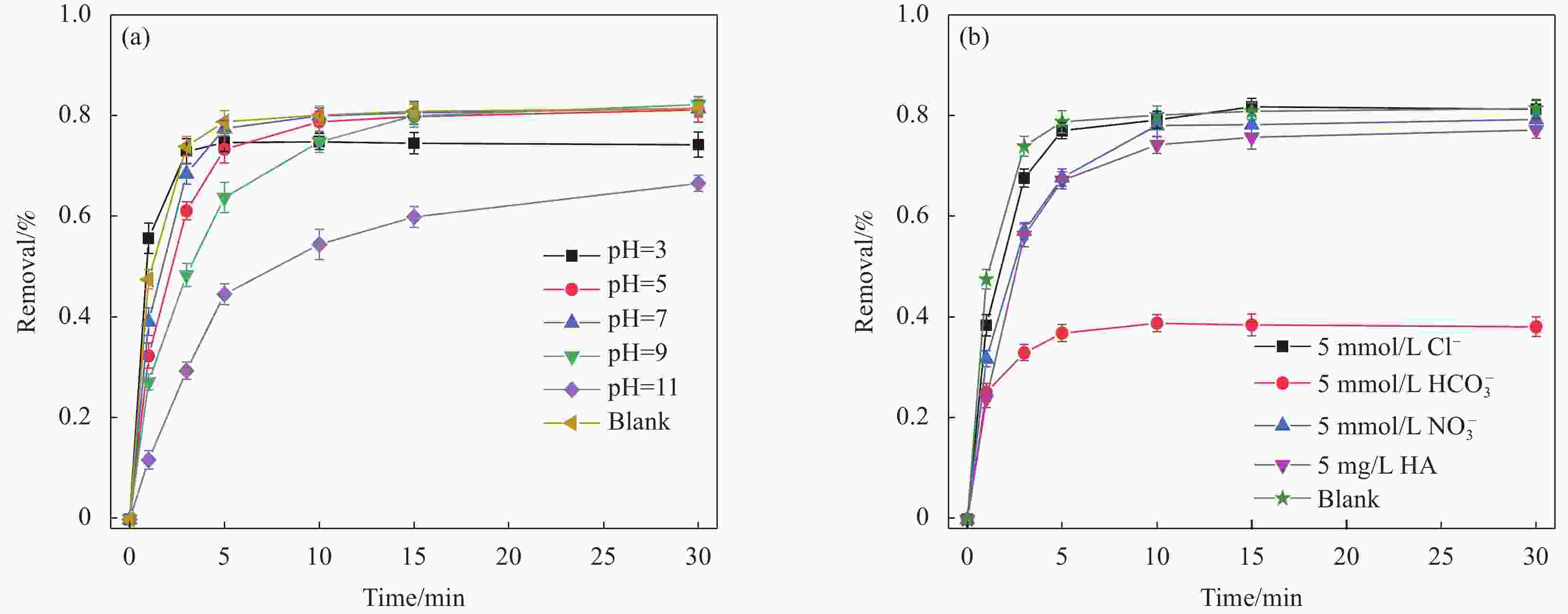
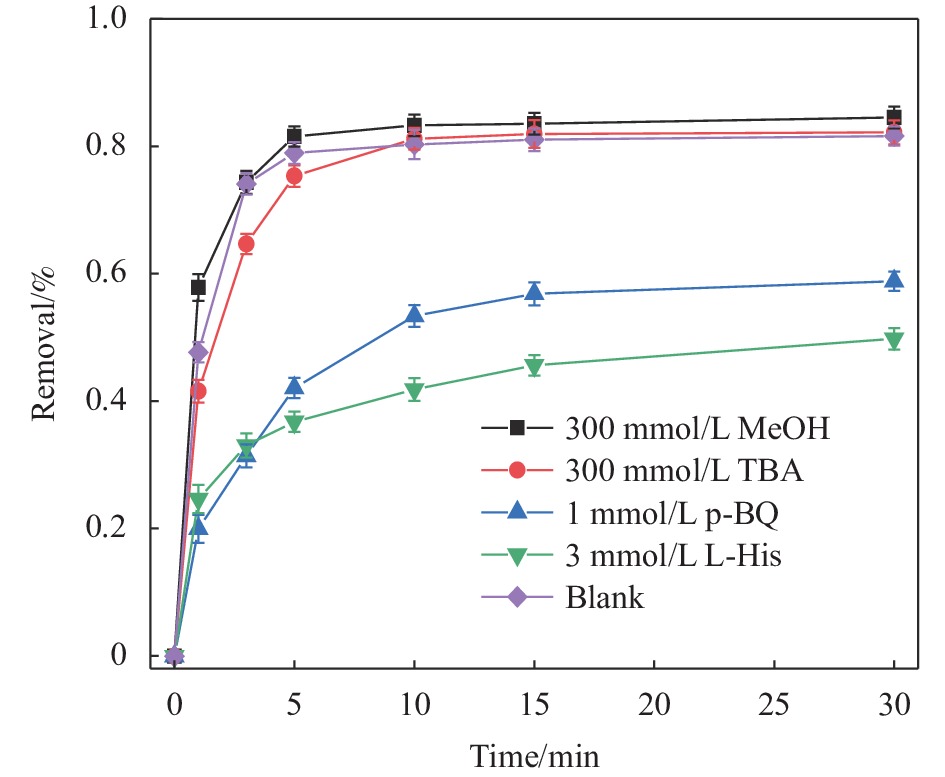
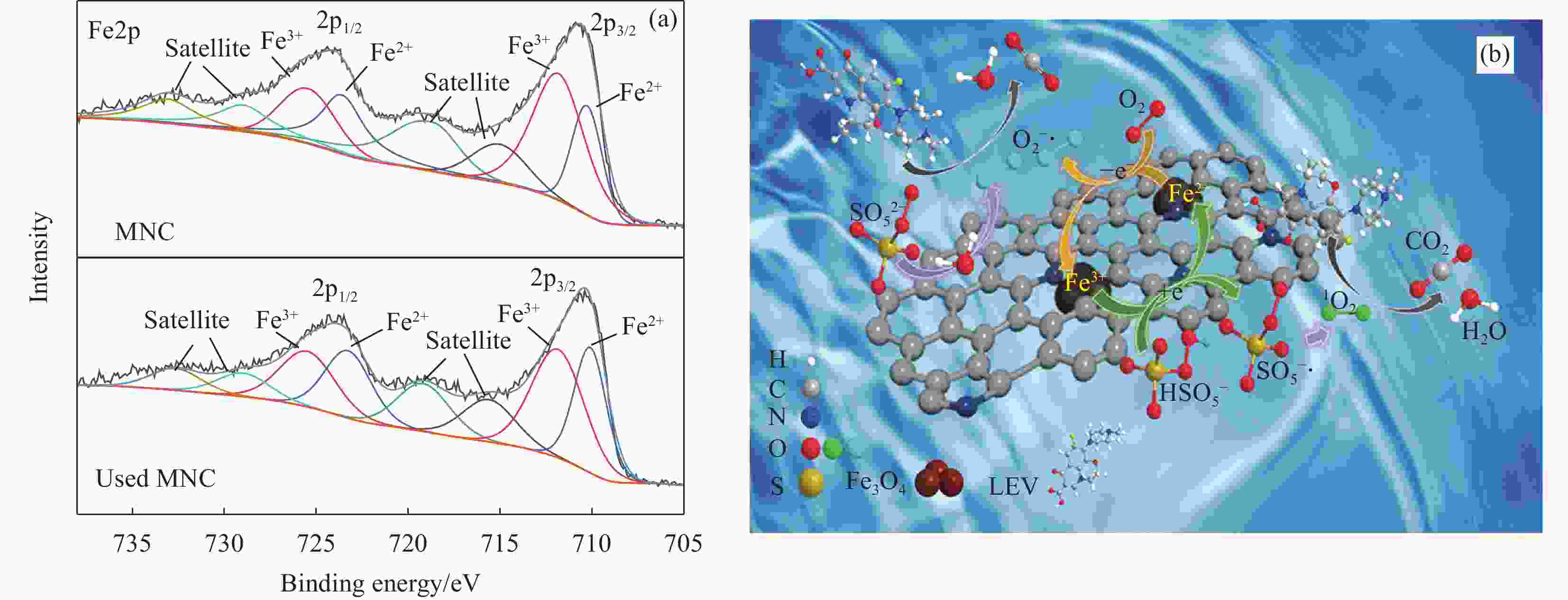
摘要: 广西大量的废弃杉木屑是放错位置的宝贵资源。为达到“以废治废”目的,本文以废弃杉木屑为原料制备合成了具有磁回收能力的生物炭复合材料,并研究其活化过一硫酸盐(Peroxymonosulfate,PMS)降解左氧氟沙星(Levofloxacin,LEV)抗生素的性能。通过对杉木屑生物炭(Fir sawdust biochar, BC)进行氮掺杂、Fe3O4负载制备出具有高效PMS活化能力和优异磁分离性能的磁性氮掺杂杉木屑生物炭(Magnetic nitrogen doped fir sawdust biochar, MNC)。相比BC,MNC的石墨化程度提高,缺陷活性位点增多,比表面积也得到显著改善,且具备超顺磁性和大的磁饱和强度,饱和磁化值达到10.45 emu·g−1;主要考察了PMS浓度、MNC投加量、溶液初始pH、无机阴离子与腐殖酸对MNC降解LEV的影响。研究表明:相较于BC、磁性生物炭(Magnetic fir sawdust biochar,MC)和氮掺杂生物炭(Nitrogen doped fir sawdust biochar,NC),MNC活化PMS降解LEV的效率显著提升。当MNC投加量为1.0 g/L,PMS浓度为0.3 mmol/L,初始pH为7,LEV浓度为10 mg/L的条件下,LEV去除率在30 min达到84%;同等条件下,对双酚A(Bisphenol A,BPA)、罗丹明B(Rhodamine B,RhB)和四环素(Tetracycline,TC)的去除率分别为94%、98%和87%。Cl−、NO3−和腐殖酸(Humic acid,HA)对MNC活化PMS降解LEV无明显影响。淬灭实验证实,自由基途径和非自由基途径生成的O2−•与1O2主导了MNC/PMS体系对LEV的降解。此外,MNC循环使用4次后,活化PMS去除LEV的效率仍能达到75%左右。本文为废弃杉木屑高效、绿色的资源化利用提供了新策略和借鉴意义。Abstract: Guangxi's large amount of waste fir sawdust is a valuable resource in the wrong place. In order to realize cyclic utilization of fir sawdust, biochar composites with magnetic recovery capability were prepared from waste fir sawdust, and the performance of activated peroxymonosulfate (PMS) to degrade levofloxacin (LEV) antibiotics was studied in this study. Magnetic nitrogen-doped fir sawdust biochar (MNC) with high PMS activation ability and excellent magnetic separation performance was synthesized by nitrogen doping and loading Fe3O4. Several characterizations confirm that compared with fir sawdust biochar (BC), MNC has higher graphitization, more defect active sites, significantly improved specific surface area, superparamagnetism and large magnetic saturation intensity, with a saturation magnetization value of 10.45 emu·g−1. In addition, the effects of various environmental factors on the degradation of LEV by MNC are simulated. The effects of PMS concentration, MNC dosage, initial pH of solution, inorganic anions and humic acid are mainly investigated. The results shows that compares with BC, magnetic biochar (MC) and nitrogen doped biochar (NC), the efficiency of degradation of LEV by MNC activated PMS is significantly improved. Under the conditions of MNC dosage of 1.0 g/L, PMS concentration of 0.3 mmol/L, initial pH of 7, and LEV concentration of 10 mg/L, the removal rate of LEV reachs 84% in 30 minutes, and the removal rates of bisphenol A (BPA), rhodamine B (RhB), and tetracycline (TC) are 94%, 98% and 87%, respectively. Cl−, NO3− and humic acid have no obvious effect on the degradation of LEV by MNC activated PMS. The quenching experiments show the generation of O2−• and 1O2 through free radical and non-free radical pathways dominate the degradation of LEV in MNC/PMS system. In addition, after 4 cycles of MNC, the efficiency of activating PMS to remove LEV can still reach about 75%. This study provides a new strategy and reference for the efficient and green resource utilization of waste fir sawdust.
-
Key words:
- biochar /
- degradation /
- magnetite /
- nitrogen doping /
- levofloxacin /
- peroxymonosulfate
-
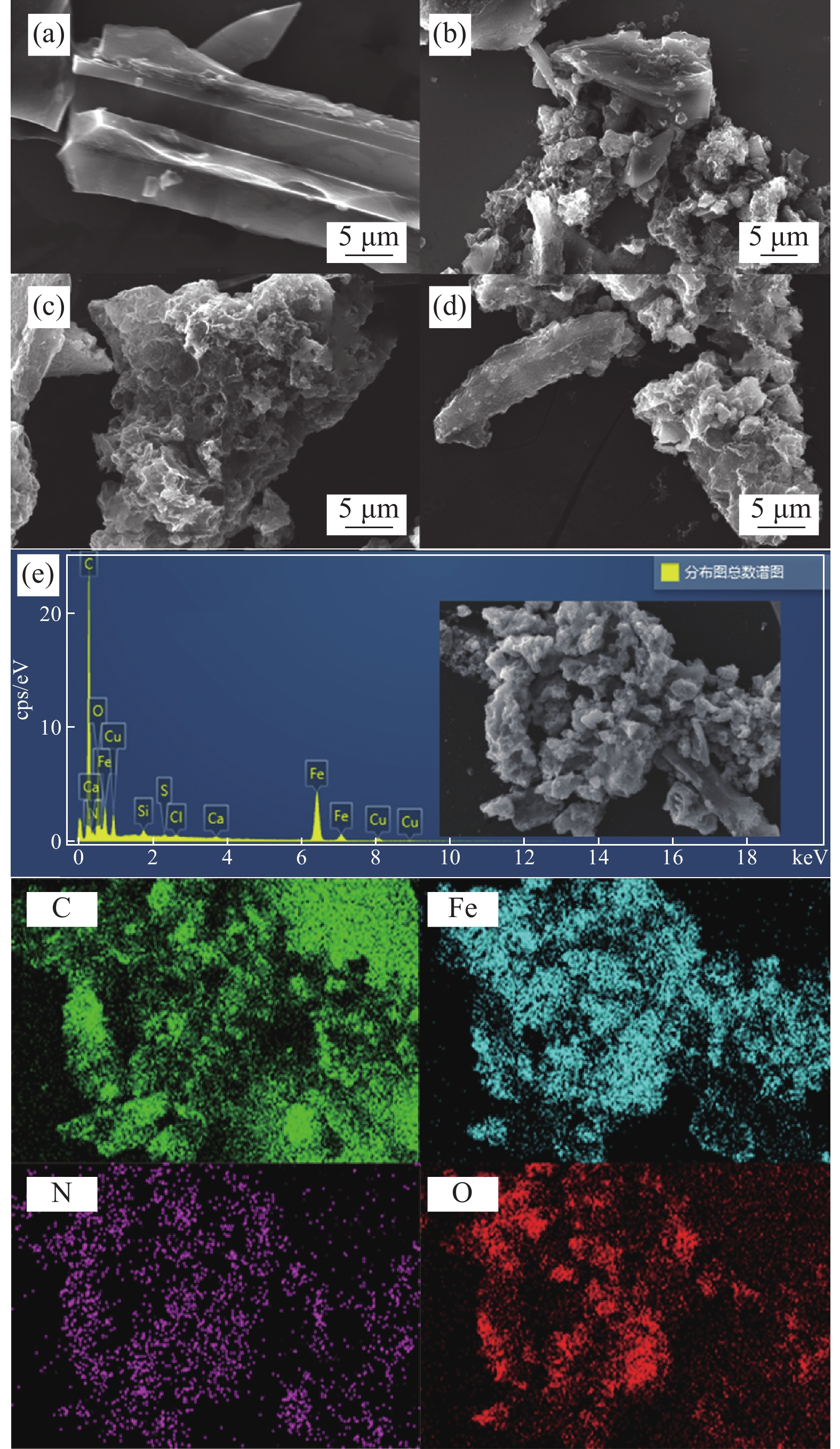
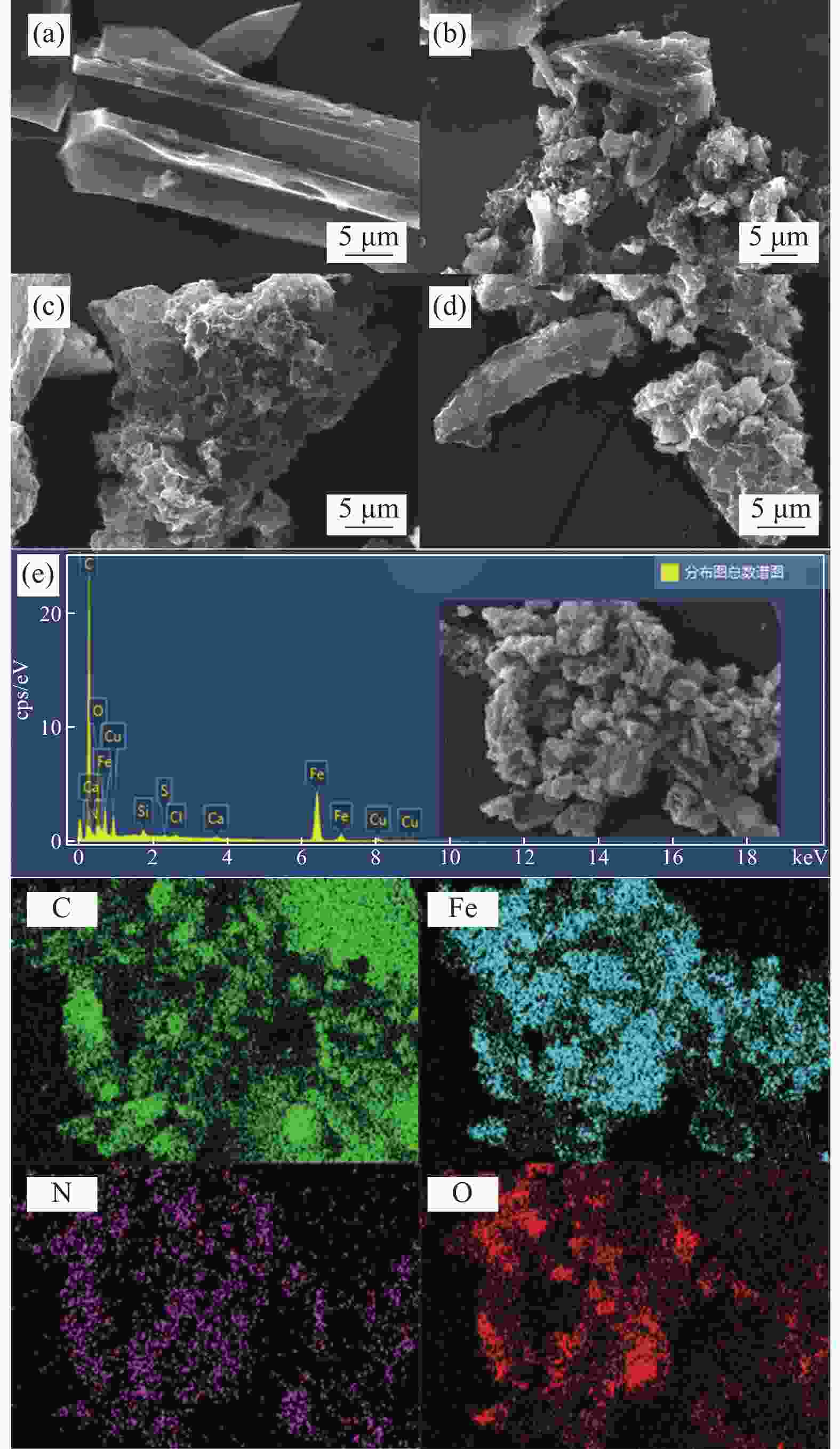
图 1 杉木屑生物炭(BC) (a)、氮掺杂生物炭(NC) (b)、磁性氮掺杂杉木屑生物炭(MNC) (c)和循环后MNC (d)的SEM图像及MNC的EDS能谱和元素分布图 (e)
Figure 1. SEM images of fir sawdust biochar (BC) (a), nitrogen doped fir sawdust biochar (NC) (b), magnetic nitrogen doped fir sawdust biochar (MNC) (c) and MNC after recycling (d), EDS and element distribution diagram of MNC (e)
表 1 BC、NC和MNC比表面积和孔隙结构
Table 1. Specific surface area and pore structure of BC, NC and MNC
Sample Surface area/
(m2·g−1)Pore volume/
(cm3·g−1)Average pore diameter/nm BC 2.180 0.002 37.560 NC 38.151 0.180 13.650 MNC 46.667 0.161 11.125 -
[1] HE X X, HUANG Y Z, ZHANG Q C, et al. Distribution of organic carbon fractions in soil aggregates in Chinese fir plantations with different stand ages[J]. Ecological Process,2021,10(1):49. doi: 10.1186/s13717-021-00321-5 [2] SAYA L, MALIK V, GAUTAM D, et al. A comprehensive review on recent advances toward sequestration of levofloxacin antibiotic from wastewater[J]. The Science of the Total Environment,2021,813:152529. [3] LIU L L, ZHAN R, ZHANG M, et al. Insights into the performance, mechanism, and ecotoxicity of levofloxacin degradation in CoFe2O4 catalytic peroxymonosulfate process[J]. Journal of Environmental Chemical Engineering,2022,10(3):107435. doi: 10.1016/j.jece.2022.107435 [4] ZHANG X Y, GAO Y J, LI Y H, et al. Synthesis of magnetic NiFe2O4/CuS activator for degradation of lomefloxacin via the activation of peroxymonosulfate under simulated sunlight illumination[J]. Separation and Purification Technology,2022,288:120664. doi: 10.1016/j.seppur.2022.120664 [5] 梁锦芝, 许伟城, 赖树锋, 等. 磁性生物炭的制备及其活化过一硫酸盐的研究进展[J]. 环境化学, 2021, 40(9):2901-2911. doi: 10.7524/j.issn.0254-6108.2021022301LIANG Jinzhi, XU Weicheng, LAI Shufeng, et al. Research progress on preparation and peroxymonosulfate activation of magnetic biochar[J]. Environmental Chemistry,2021,40(9):2901-2911(in Chinese). doi: 10.7524/j.issn.0254-6108.2021022301 [6] LI J J, LIANG Y Q, JIN P L, et al. Heterogeneous metal-activated persulfate and electrochemically activated persulfate: A review[J]. Catalysts,2022,12(9):1024. doi: 10.3390/catal12091024 [7] WU L P, WEI C B, ZHANG S R, et al. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil[J]. Journal of Cleaner Production,2019,235:901-909. doi: 10.1016/j.jclepro.2019.07.043 [8] BOMBUWALA D N, FOWLER R E, PITTMAN C U, et al. Lead (Pb2+) sorptive removal using chitosan-modified biochar: Batch and fixed-bed studies[J]. RSC Advances,2018,8(45):25368-25377. doi: 10.1039/C8RA04600J [9] LIU C, CHEN L, DING D, et al. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor[J]. Applied Catalysis B: Environmental,2019,254:312-320. doi: 10.1016/j.apcatb.2019.05.014 [10] LI S, WANG Z, ZHAO X, et al. Insight into en hanced carbamazepine photodegradation over biochar-based magnetic photocatalyst Fe3O4/BiOBr/BC under visible LED light irradiation[J]. Chemical Engineering Journal,2019,360:600-611. doi: 10.1016/j.cej.2018.12.002 [11] BAI Y Y, SUN X Q, DANG Y, et al. A self-circulating electro-fenton-like process over Fe3O4-CaO2 cathode for highly efficient degradation of levofloxacin[J]. Chemosphere,2023,313:137520. doi: 10.1016/j.chemosphere.2022.137520 [12] 黄仕元, 林森焕, 董雯, 等. 锰氮共掺杂稻壳生物炭活化过二硫酸盐降解酸性橙[J]. 复合材料学报, 2023, 40(2): 1077-1090.HUANG Shiyuan, LIN Senhuan, DONG Wen, et al. Manganese-nitrogen co-doped rice husk biochar activated peroxydisul fate to degrade acid orange[J]. Acta Materiae Compositae Sinica, 2023, 40(2): 1077-1090(in Chinese). [13] LI M L, LINS V, ZANTA C L P S, et al. MgAl-LDH/biochar composites for methylene blue removal by adsorption[J]. Applied Clay Science,2019,168:11-20. doi: 10.1016/j.clay.2018.10.012 [14] WU J Q, WANG T S, LIU Y Y, et al. Norfloxacin adsorption and subsequent degradation on ball-milling tailored N-doped biochar[J]. Chemosphere,2022,303(3):135264. [15] DING D H, YANG S J, QIAN X Y, et al. Nitrogen-doping positively whilst sulfur-doping negatively affect the catalytic activity of biochar for the degradation of organic contaminant[J]. Applied Catalysis B: Environmental,2020,263(C):118348. [16] 鞠梦灿, 严丽丽, 简铃, 等. 氮掺杂生物炭材料的制备及其在环境中的应用[J]. 化工进展, 2022, 41(10):5588-5598. doi: 10.16085/j.issn.1000-6613.2021-2498JU Mengcan, YAN Lili, JIAN Ling, et al. Preparation of nitrogen-doped biochar and its environmental applications[J]. Chemical Industry and Engineering Progress,2022,41(10):5588-5598(in Chinese). doi: 10.16085/j.issn.1000-6613.2021-2498 [17] YE S J, ZENG G M, TAN X F, et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer[J]. Applied Catalysis B: Environmental,2020,269:118850. doi: 10.1016/j.apcatb.2020.118850 [18] YU Y J, GUO H B, ZHONG Z J, et al. Fe3O4 loaded on ball milling biochar enhanced bisphenol a removal by activating persulfate: Performance and activating mechanism[J]. Journal of Environmental Management,2022,319:115661. doi: 10.1016/j.jenvman.2022.115661 [19] LI G, CAO X Q, MENG N, et al. Fe3O4 supported on water caltrop-derived biochar toward peroxymonosulfate activation for urea degradation: The key role of sulfate radical[J]. Chemical Engineering Journal,2022,433(2):133595. [20] HUANG P, ZHANG P, WANG C P, et al. Enhancement of persulfate activation by Fe-biochar composites: Synergism of Fe and N-doped biochar[J]. Applied Catalysis B: Environmental,2022,303:120926. doi: 10.1016/j.apcatb.2021.120926 [21] LI J Q, LIN Q T, LUO H Y, et al. The effect of nanoscale zero-valent iron-loaded N-doped biochar on the generation of free radicals and nonradicals by peroxydisulfate activation[J]. Journal of Water Process Engineering,2022,47:102681. doi: 10.1016/j.jwpe.2022.102681 [22] HUANG H X, GUO T, WANG K, et al. Efficient activation of persulfate by a magnetic recyclable rape straw biochar catalyst for the degradation of tetracycline hydrochloride in water[J]. Science of the Total Environment,2021,758:143957. doi: 10.1016/j.scitotenv.2020.143957 [23] LIU T, WANG Q, LI C X, et al. Synthesizing and characterizing Fe3O4 embedded in N-doped carbon nanotubes-bridged biochar as a persulfate activator for sulfamethoxazole degradation[J]. Journal of Cleaner Production,2022,353:131669. doi: 10.1016/j.jclepro.2022.131669 [24] ZHONG Q F, LIN Q T, HE W J, et al. Study on the nonradical pathways of nitrogen-doped biochar activating persulfate for tetracycline degradation[J]. Separation and Purification Technology,2021,276:119354. doi: 10.1016/j.seppur.2021.119354 [25] YANG H W, ZHOU J, YANG E X, et al. Magnetic Fe3O4-N-doped carbon sphere composite for tetracycline degradation by enhancing catalytic activity for peroxymonosulfate: A dominant non-radical mechanism[J]. Chemosphere,2021,263:128011. doi: 10.1016/j.chemosphere.2020.128011 [26] WANG L, YAN W, HE C, et al. Microwave-assisted preparation of nitrogen-doped biochars by ammonium acetate activation for adsorption of acid red 18[J]. Applied Surface Science,2018,433:222-231. doi: 10.1016/j.apsusc.2017.10.031 [27] YU J F, TANG L, PANG Y, et al. Non-radical oxidation by N, S, P co-doped biochar for persulfate activation: Different roles of exogenous P/S doping, and electron transfer path[J]. Journal of Cleaner Production,2022,374:133995. doi: 10.1016/j.jclepro.2022.133995 [28] GUO L J, ZHAO L M, TANG Y L, et al. Switching the free radical based peroxydisulfate activation to the nonradical pathway by a chrome shaving-derived biochar for the efficient degradation of tetracycline[J]. Chemical Engineering Journal,2022,435(2):135189. [29] 杨贤, 梁嘉林, 曾刘婷, 等. 负载磁性废茶生物炭活化过一硫酸盐高效降解水中腐殖酸和富里酸[J]. 环境科学学报, 2021, 41(8):3185-3199. doi: 10.13671/j.hjkxxb.2021.0043YANG Xian, LIANG Jialin, ZENG Liuting, et al. Efficient degradation of humic acid and fulvic acid in water by magnetic waste tea biochar activated persulfate[J]. Acta Scientiae Circumstantiae,2021,41(8):3185-3199(in Chinese). doi: 10.13671/j.hjkxxb.2021.0043 [30] SONG B, ZENG Z T, ALMATRAFI E, et al. Pyrite-mediated advanced oxidation processes: Applications, mechanisms, and enhancing strategies[J]. Water Research,2022,211:118048. doi: 10.1016/j.watres.2022.118048 [31] LI H M, LI D, LONG M Y, et al. Solvothermal synthesis of MIL-53 Fe@g-C3N4 for peroxymonosulfate activation towards enhanced photocatalytic performance[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2023,658:130646. doi: 10.1016/j.colsurfa.2022.130646 [32] HUANG Y H, HUANG Y F, HUANG C, et al. Efficient decolorization of azo dye reactive black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2+-catalyst[J]. Journal of Hazardous Materials,2009,170(2-3):1110-1118. doi: 10.1016/j.jhazmat.2009.05.091 [33] WEI T Q, MENG Y, AI D, et al. Ball milling Fe3O4@biochar cathode coupling persulfate for the removal of sulfadiazine from water: Effectiveness and mechanisms[J]. Journal of Environmental Chemical Engineering,2022,10(6):108879. doi: 10.1016/j.jece.2022.108879 [34] WANG T, LU J, LEI J Y, et al. Highly Efficient activation of peroxymonosulfate for rapid sulfadiazine degradation by Fe3O4@Co3S4[J]. Separation and Purification Technology,2022,307:122755. [35] PAN J W, GAO B Y, DUAN P J, et al. Recycling exhausted magnetic biochar with adsorbed Cu2+ as a cost-effective permonosulfate activator for norfloxacin degradation: Cu contribution and mechanism[J]. Journal of Hazardous Materials,2021,413:125413. doi: 10.1016/j.jhazmat.2021.125413 [36] ZHU K, BIN Q, SHEN Y Q, et al. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants[J]. Chemical Engineering Jour-nal,2020,402:126090. doi: 10.1016/j.cej.2020.126090 [37] DU W Y, ZHANG Q Z, SHANG Y A, et al. Sulfate saturated biosorbent-derived Co-S@NC nanoarchitecture as an efficient catalyst for peroxymonosulfate activation[J]. Applied Catalysis B: Environmental,2020,262:118302. doi: 10.1016/j.apcatb.2019.118302 [38] YANG Y, BANERJEE G, BRUDVIG G W, et al. Oxidation of organic compounds in water by unactivated peroxymonosulfate[J]. Environmental Science & Technology,2018,52(10):5911-5919. [39] NITESH K, PRAVEEN K, STEVEN G, et al. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review[J]. Biochar,2022,4(1):17. doi: 10.1007/s42773-022-00145-2 [40] WANG Q R, SHI Y X, LYU S Y, et al. Peroxymonosulfate activation by tea residue biochar loaded with Fe3O4 for the degradation of tetracycline hydrochloride: Performance and reaction mechanism[J]. RSC Advances,2021,11(30):18525. doi: 10.1039/D1RA01640G -





 下载:
下载: