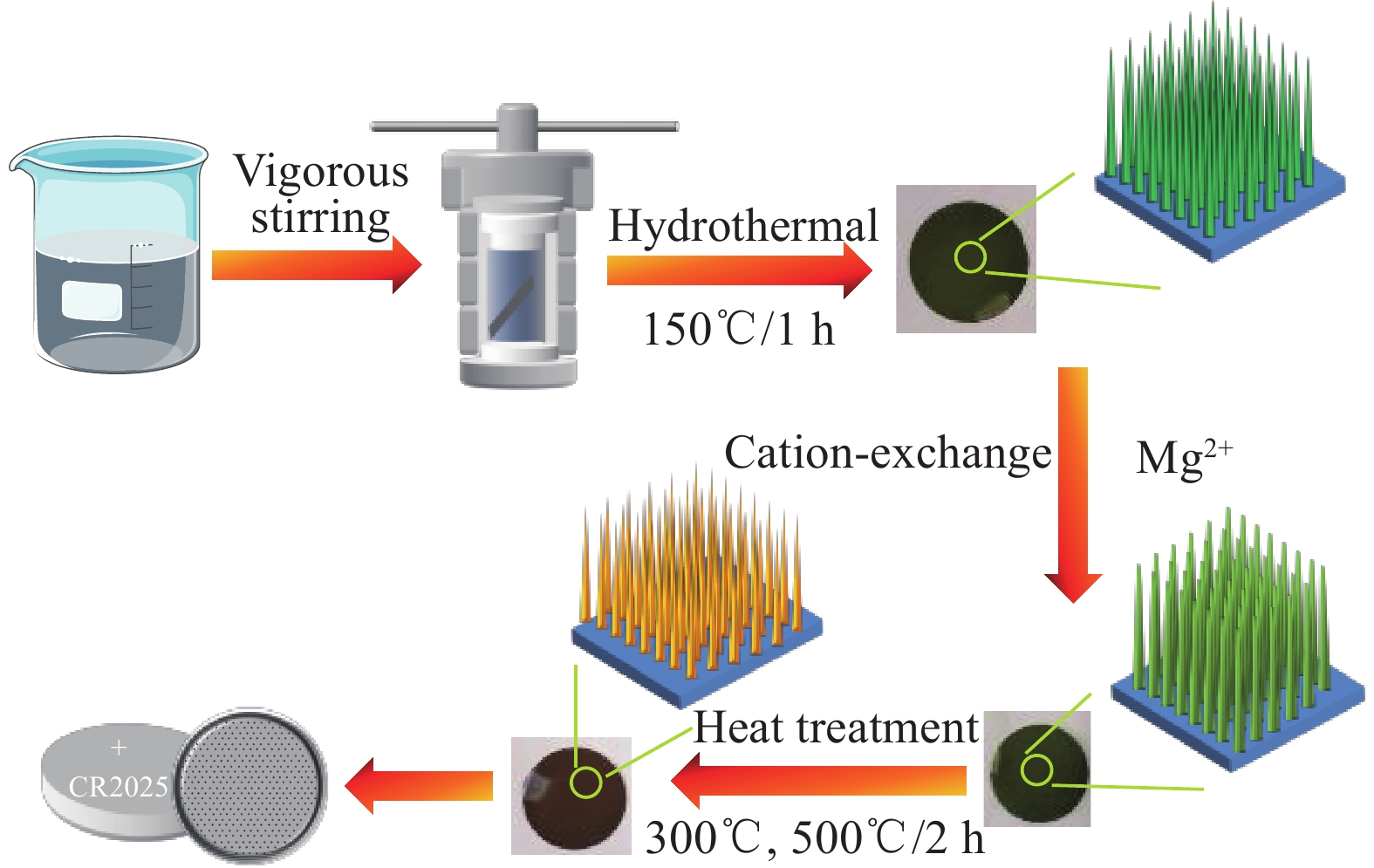
Synthesis of magnesium vanadate-sodium vanadate composite nanowires by cation exchange as electrode materials for lithium ion batteries
-
摘要: 为了满足对锂离子电池性能更好和更多样化的要求,研究了用于改善电池性能的电极材料,在众多电极材料中,钒基材料的价态变化丰富和种类繁多等优点使其适用于锂离子电池电极材料。以钒酸钠纳米线阵列为前驱体,采用离子交换法合成了钛箔上的钒酸镁(MgV2O6)-钒酸钠(NaV6O15)复合材料。后续在空气中煅烧,温度分别为300℃和500℃,随着煅烧温度的升高,纳米线的直径变大。对所制备样品的晶体结构、化学成分和微观形貌进行了详细的表征。其中,300℃煅烧制备的钒酸镁-钒酸钠复合材料的电化学储锂性能较好,在电流密度为50 mA·g−1下首次放电容量为1144 mA·h·g−1,经过100次循环后的放电比容量仍有837 mA·h·g−1,表现出良好的循环稳定性,较钒酸钠前驱体的储锂性能有大幅提升,为镁离子合成碱土金属钒酸盐应用于电化学储能领域的研究提供了新思路。Abstract: In order to meet the requirements for better and more diversified performance of lithium-ion batteries, electrode materials for improving battery performance were studied. Among many electrode materials, vanadium-based materials are suitable for lithium-ion battery electrode materials due to their rich valence changes and various types. Magnesium vanadate (MgV2O6)-sodium vanadate (NaV6O15) composites on titanium foil were synthesized by ion exchange method using sodium vanadate nanowar arrays as precursors. After calcination in air, the temperature was 300℃ and 500℃, respectively. With the increase of the calcination temperature, the diameter of the nanowires became larger. The crystal structure, chemical composition and microstructure of the prepared samples were characterized in detail. Among them, the magnesium vanadate and sodium vanadate composite prepared by calcination at 300℃ had better electrochemical lithium storage performance. The first discharge capacity was 1144 mA·h·g−1 at the current density of 50 mA·g−1, and the specific discharge capacity was still 837 mA·h·g−1 after 100 cycles, showing good cycling stability. Compared with sodium vanadate precursor, the lithium storage performance was significantly improved. It provides a new idea for the application of alkaline earth vanadate synthesized by magnesium ions in the field of electrochemical energy storage.
-
Key words:
- cation exchange /
- hydrothermal method /
- magnesium vanadate /
- sodium vanadate /
- lithium ion battery
-
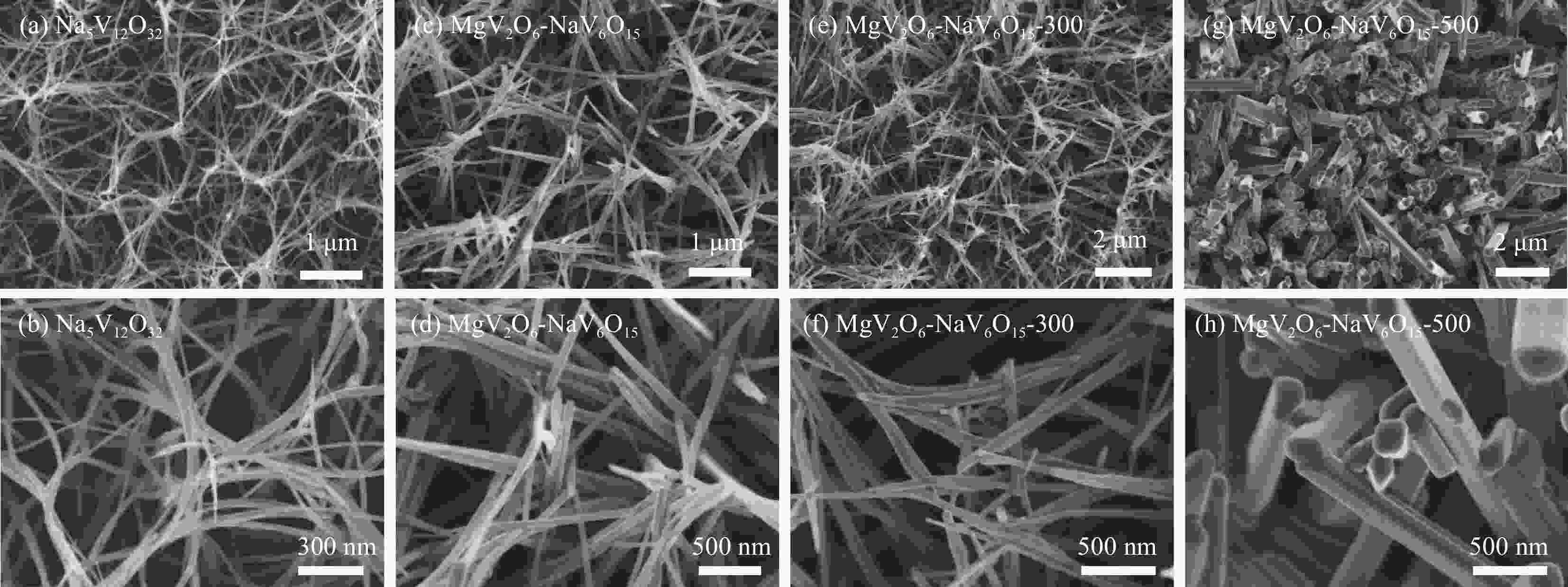
图 3 原始制备的Na5V12O32 ((a), (b)) 和MgV2O6-NaV6O15复合物纳米线 ((c), (d))、MgV2O6-NaV6O15-300 ((e), (f)) 和MgV2O6-NaV6O15-500 ((g), (h)) 复合材料的SEM图像
Figure 3. SEM images of pristine Na5V12O32 ((a), (b)) and MgV2O6-NaV6O15 nanowires ((c), (d)), MgV2O6-NaV6O15-300 ((e), (f)) and MgV2O6-NaV6O15-500 ((g), (h)) composites
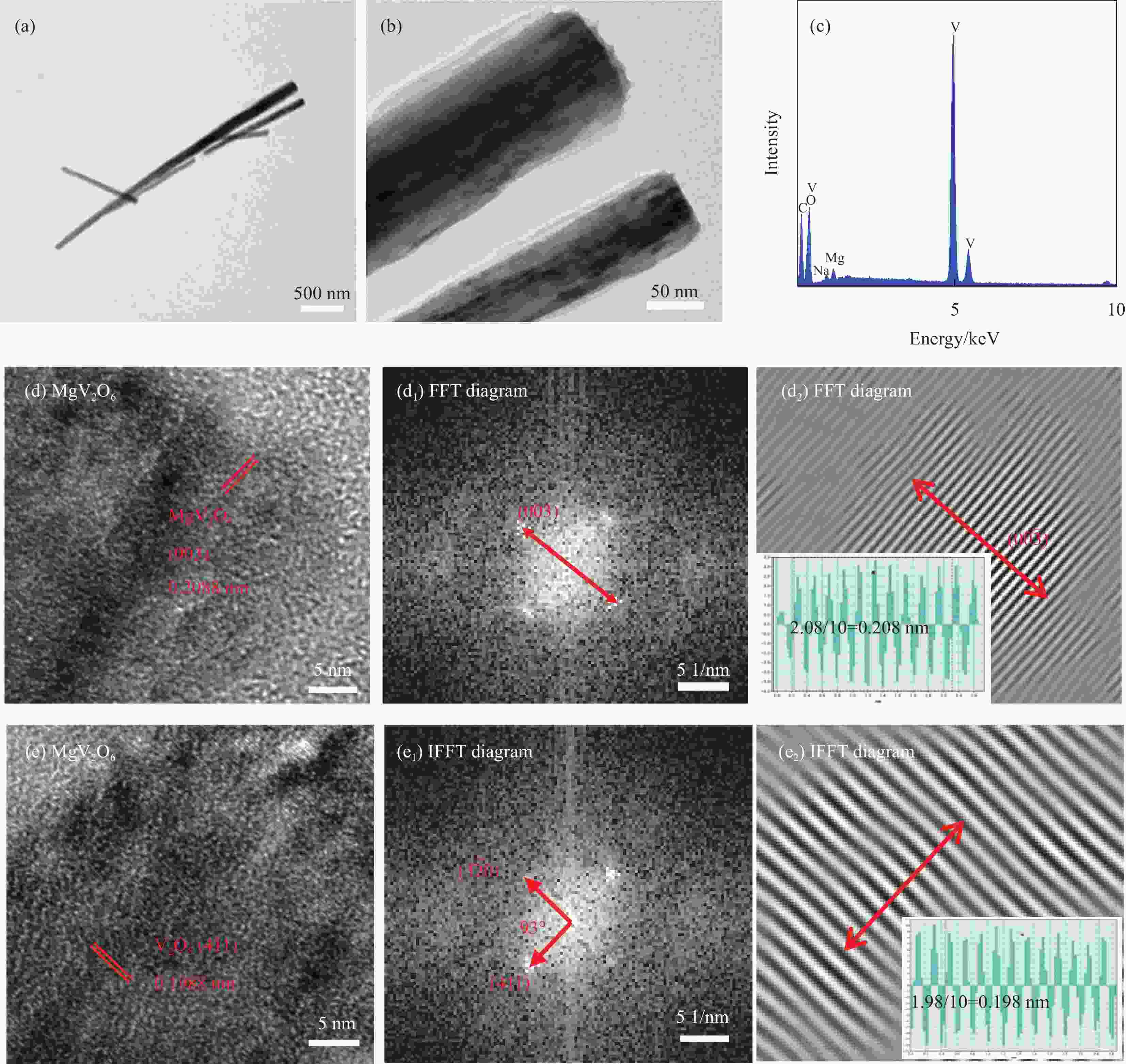
图 5 MgV2O6-NaV6O15-300复合材料的TEM图像 ((a), (b))、EDS (c) 和HRTEM图像 ((d), (e)) 及傅里叶变换图(FFT) ((d1), (d2)) 和反傅里叶变换图(IFFT) ((e1), (e2))
Figure 5. TEM images ((a), (b)), EDS images (c), HRTEM images ((d), (e)) of MgV2O6-NaV6O15-300 composite; Regions reveal the existence of MgV2O6 and V2O5 by fast fourier transform (FFT) ((d1), (d2)) and inverse fast fourier transform (IFFT) ((e1), (e2)) methods, respectively
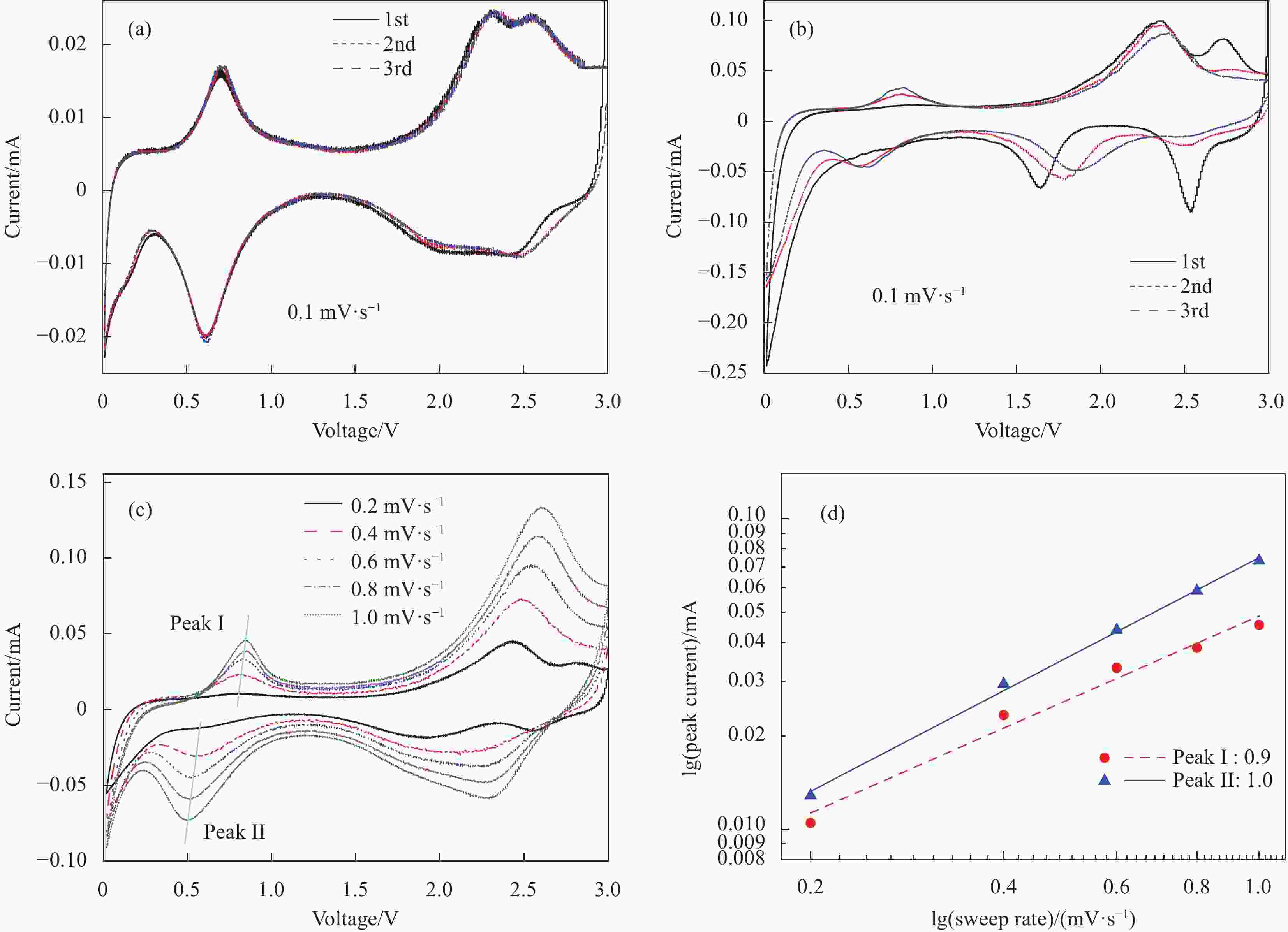
图 7 电极在0.01~3 V电位范围内的动力学分析:(a) MgV2O6-NaV6O15-300电极前3圈CV曲线(0.1 mV·s−1);(b) 钒酸钠前驱体前3圈CV曲线(扫速: 0.1 mV·s−1); (c) MgV2O6-NaV6O15-300电极不同扫描速率下的CV曲线(0.2~1.0 mV·s−1);(d) MgV2O6-NaV6O15-300电极每个氧化还原峰的lgi和lgv图
i—Peak current; v—Sweep rate
Figure 7. Kinetic analysis electrode within the potential range of 0.01-3 V: (a) CV curves of the first three turns of MgV2O6-NaV6O15-300 electrode (0.1 mV·s−1); (b) CV curves of the first three turns of Na5V12O32 electrode (0.1 mV· s−1); (c) CV curves in different scan rates of MgV2O6-NaV6O15-300 electrode (0.2-1.0 mV·s−1); (c) lgi vs lgv plots at each redox peak of MgV2O6-NaV6O15-300 electrode
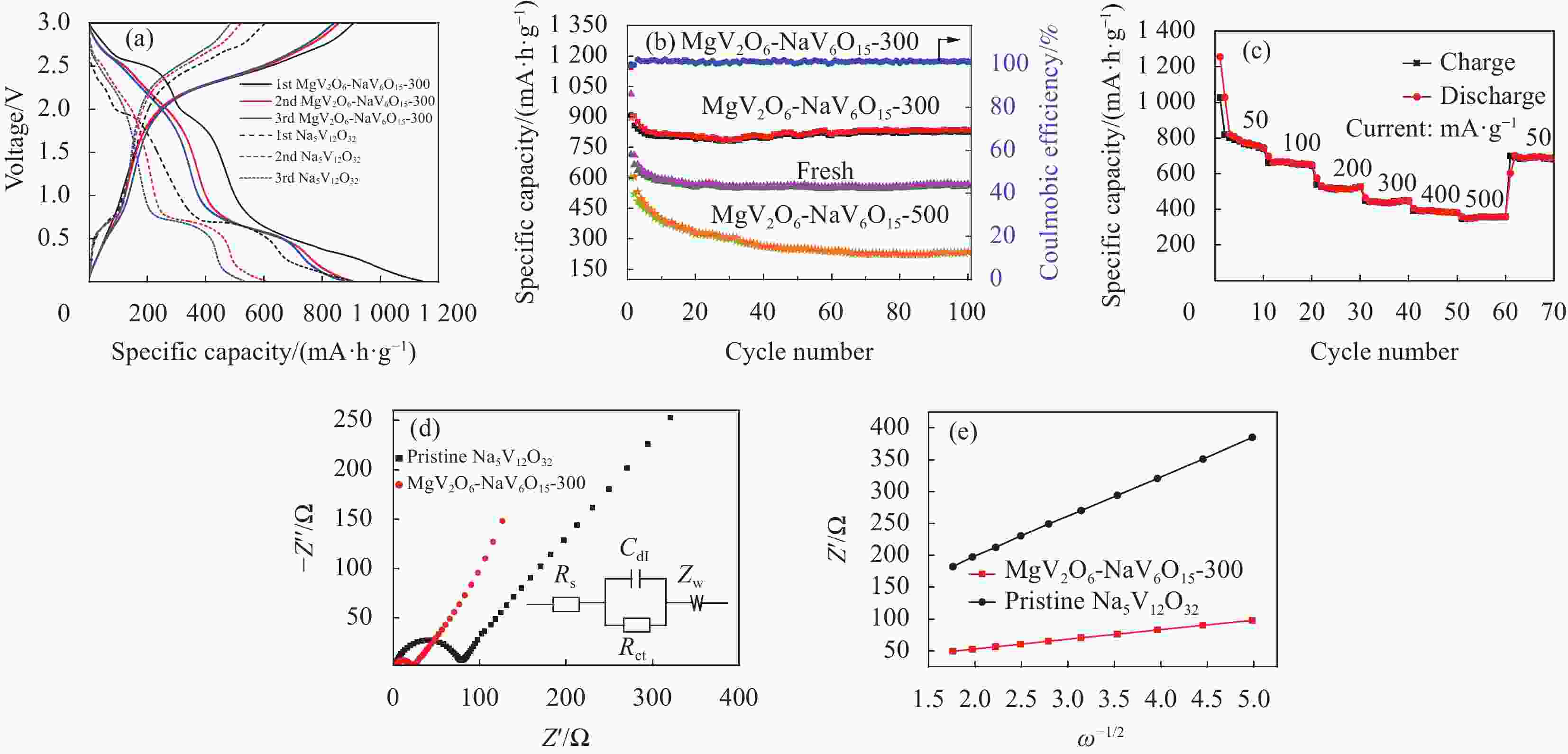
图 8 电化学性能:(a) 充放电;(b) 长周期;(c) 速率能力;(d) 电化学阻抗谱(EIS);(e) Na5V12O32和MgV2O6-NaV6O15-300电极Warburg阻抗的线性拟合
Rs—Ohmic resistance of the electrolyte and electrode; Rct—Charge-transfer resistance; Zw—Warburg impedance; Cdl—Double layer capacitor; ω—Angular frequency
Figure 8. Electrochemical performance: (a) Charge-discharge; (b) Long cycle; (c) Rate capability; (d) Electrochemical impedance spectra (EIS); (e) Linear fitting of the Warburg impedance for Na5V12O32 and MgV2O6-NaV6O15-300 electrodes
表 1 不同温度处理的MgV2O6-NaV6O15电极材料
Table 1. MgV2O6-NaV6O15 electrode materials treated at different temperatures
Sample Treated temperature/℃ MgV2O6-NaV6O15-300 300 MgV2O6-NaV6O15-500 500 -
[1] LI X, LIN Z, JIN N, et al. Perovskite-type SrVO3 as high-performance anode materials for lithium-ion batteries[J]. Advanced Materials,2022,34(46):2107262. [2] XU Y H, ZHU Y J, LIU Y H, et al. Electrochemical performance of porous carbon/tin composite anodes for sodium-ion and lithium-ion batteries[J]. Advanced Energy Materials,2013,3(1):128-133. doi: 10.1002/aenm.201200346 [3] LI M, LU J, CHEN Z W, et al. 30 years of lithium-ion batteries[J]. Advanced Materials,2018,30(33):1800561. doi: 10.1002/adma.201800561 [4] XIA Y, YANG P, SUN Y, et al. One-dimensional nanostructures: Synthesis, characterization, and applications[J]. Advanced Materials,2003,15(5):353-389. doi: 10.1002/adma.200390087 [5] WANG Y, CAO G Z. Developments in nanostructured cathode materials for high-performance lithium-ion batteries[J]. Advanced Materials,2008,20(12):2251-2269. doi: 10.1002/adma.200702242 [6] AKOLKAR R. Modeling dendrite growth during lithium electrodeposition at sub-ambient temperature[J]. Journal of Power Sources,2014,246:84-89. doi: 10.1016/j.jpowsour.2013.07.056 [7] BELOV D, YANG M H. Failure mechanism of Li-ion battery at overcharge conditions[J]. Journal of Solid State Electrochemistry,2008,12(7):885-894. [8] FAN Z Y, LIANG J, YU W, et al. Ultrathin NiO nanosheets anchored on a highly ordered nanostructured carbon as an enhanced anode material for lithium ion batteries[J]. Nano Energy,2015,16:152-162. doi: 10.1016/j.nanoen.2015.06.009 [9] LIU C L, LUO S H, HUANG H B, et al. Potassium vanadate K0.23V2O5 as anode materials for lithium-ion and potassium-ion batteries[J]. Journal of Power Sources,2018,389:77-83. doi: 10.1016/j.jpowsour.2018.04.014 [10] ZHU K, ZHANG Y, QIU H L, et al. Hierarchical Fe3O4 microsphere/reduced graphene oxide composites as a capable anode for lithium-ion batteries with remarkable cycling performance[J]. Journal of Alloys and Compounds,2016,675:399-406. doi: 10.1016/j.jallcom.2016.02.214 [11] ETACHERI V, MAROM R, ELAZARI R, et al. Challenges in the development of advanced Li-ion batteries: A review[J]. Energy & Environmental Science,2011,4(9):3243-3262. [12] MCDOWELL M T, LEE S W, NIX W D, et al. 25th anniversary article: Understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries[J]. Advanced Materials,2013,25(36):4966-4985. doi: 10.1002/adma.201301795 [13] REDDY M V, SUBBA RAO G V, CHOWDARI B V R. Metal oxides and oxysalts as anode materials for Li ion batteries[J]. Chemical Reviews,2013,113(7):5364-5457. doi: 10.1021/cr3001884 [14] NITTA N, WU F X, LEE J T, et al. Li-ion battery materials: Present and future[J]. Materials Today,2015,18(5):252-264. doi: 10.1016/j.mattod.2014.10.040 [15] CUI M M, LU X J, ZENG T F, et al. Effect of anions on the copper vanadate structure during ion-exchange and its lithium storage performance[J]. Journal of Alloys and Compounds,2021,862:158576. doi: 10.1016/j.jallcom.2020.158576 [16] YIN C, ZHU S M, CHEN Z X, et al. One step fabrication of C-doped BiVO4 with hierarchical structures for a high-performance photocatalyst under visible light irradiation[J]. Journal of Materials Chemistry A,2013,1(29):8367-8378. doi: 10.1039/c3ta11833a [17] ANDRUKAITIS E, COOPER J P, SMIT J H. Lithium intercalation in the divalent metal vanadates MeV2O6 (Me=Cu, Co, Ni, Mn or Zn)[J]. Journal of Power Sources,1995,54(2):465-469. doi: 10.1016/0378-7753(94)02126-N [18] CHAE O B, KIM J, PARK I, et al. Reversible lithium storage at highly populated vacant sites in an amorphous vanadium pentoxide electrode[J]. Chemistry of Materials,2014,26(20):5874-5881. doi: 10.1021/cm502268u [19] CHEN H D, HUANG J J, TIAN S H, et al. Interlayer modification of pseudocapacitive vanadium oxide and Zn(H2O)n2+ migration regulation for ultrahigh rate and durable aqueous zinc-ion batteries[J]. Advanced Science,2021,8(14):2004924. doi: 10.1002/advs.202004924 [20] HAO J X, WU W J, WANG Q, et al. Effect of grain size on electrochemical performance and kinetics of Co3O4 electrode materials[J]. Journal of Materials Chemistry A,2020,8(15):7192-7196. doi: 10.1039/D0TA02032J [21] QIN T F, PENG S L, HAO J X, et al. Flexible and wearable all-solid-state supercapacitors with ultrahigh energy density based on a carbon fiber fabric electrode[J]. Advanced Energy Materials,2017,7(20):1700409. doi: 10.1002/aenm.201700409 [22] ZHANG L, CHANG S Y, LU X J, et al. Vapor phosphorus-coated cobalt vanadate as a high-performance anode for a lithium-ion battery[J]. Journal of Solid State Electrochemistry,2022,26(4):917-927. doi: 10.1007/s10008-022-05127-9 [23] CAO Y H, FANG D, WANG C, et al. Novel aligned sodium vanadate nanowire arrays for high-performance lithium-ion battery electrodes[J]. RSC Advances,2015,5(53):42955-42960. doi: 10.1039/C5RA01102G [24] MORISHITA T, NOMURA K, INAMASU T, et al. Synthesis of anhydrous manganese vanadate powder by coprecipitation and its anodic performance for lithium secondary battery[J]. Solid State Ionics,2005,176(29-30):2235-2241. doi: 10.1016/j.ssi.2005.06.013 [25] ZHANG S Y, HOU M H, HOU L L, et al. Synthesis and electrochemical performance of cable-like copper vanadates/polypyrrole nanobelts as anode materials for lithium-ion batteries[J]. Chemical Physics Letters,2016,658:203-206. [26] HUA K, LI X J, FU Z W, et al. Cation-exchange synthesis of manganese vanadate nanosheets and its application in lithium-ion battery[J]. Journal of Solid State Chemistry,2019,273:287-294. doi: 10.1016/j.jssc.2019.02.026 [27] XU X, XIONG F, MENG J, et al. Multi-electron reactions of vanadium-based nanomaterials for high-capacity lithium batteries: Challenges and opportunities[J]. Materials Today Nano,2020,10:100073. doi: 10.1016/j.mtnano.2020.100073 [28] ZHANG L, LUO Y D, ZHANG R F, et al. Lithium storage performance and investigation of electrochemical mecha-nism of cobalt vanadate nanowires assembled by nanosheets[J]. ACS Applied Energy Materials,2021,4(12):13401-13409. doi: 10.1021/acsaem.1c02247 [29] GUO Z, SU Y, LI Y X, et al. Porous single-crystalline CdSe nanobelts: Cation-exchange synthesis and highly selective photoelectric sensing toward Cu2+[J]. Chemistry– A European Journal,2018,24(39):9877-9883. doi: 10.1002/chem.201801215 [30] YIN J F, PELLICCIONE C J, LEE S H, et al. Communication-sol-gel synthesized magnesium vanadium oxide, MgxV2O5·nH2O: The role of structural Mg2+ on battery performance[J]. Journal of the Electrochemical Society,2016,163(9):A1941. doi: 10.1149/2.0781609jes [31] SUN J Z. Study of MgV2O6 as cathode material for secondary magnesium batteries[J]. Asian Journal of Chemistry,2011,23:1399-1400. [32] DIAS A P S, DIMITROV L D, OLIVEIRA M C R, et al. Oxidative dehydrogenation of butane over substoichiometric magnesium vanadate catalysts prepared by citrate route[J]. Journal of Non-Crystalline Solids,2010,356(28-30):1488-1497. doi: 10.1016/j.jnoncrysol.2010.04.042 [33] VASIĆ M M, MILOVIĆ M, BAJUK-BOGDANOVIĆ D, et al. Simply prepared magnesium vanadium oxides as cathode materials for rechargeable aqueous magnesium ion batteries[J]. Nanomaterials,2022,12(16):2767. doi: 10.3390/nano12162767 [34] SILVERSMIT G, DEPLA D, POELMAN H, et al. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+)[J]. Journal of Electron Spectroscopy & Related Phenomena,2004,135(2-3):167-175. [35] MENDIALDUA J, CASANOVA R, BARBAUX Y. XPS studies of V2O5, V6O13, VO2 and V2O3[J]. Journal of Electron Spectroscopy and Related Phenomena,1995,71(3):249-261. doi: 10.1016/0368-2048(94)02291-7 [36] HUA K, CUI M M, LUO Z P, et al. Fabrication of zinc pyrovanadate (Zn3(OH)2V2O7·2H2O) nanosheet spheres as an ethanol gas sensor[J]. Journal of Alloys and Compounds,2019,801:581-588. doi: 10.1016/j.jallcom.2019.06.015 [37] XU X L, FANG D, YI J H, et al. EuVO4-V2O5 composite as high-performance electrode material of lithium-ion battery[J]. Rare Metal Materials and Engineering,2022,51(4):1488-1496. [38] HUA K, FANG D, CUI M M, et al. In-situ deposition of Co nanoparticles in discharged TiO2 nanotube array with enhanced magnetic property[J]. Journal of Magnetism and Magnetic Materials,2019,485:217-223. doi: 10.1016/j.jmmm.2019.04.029 [39] ZHOU Q F, WANG J Z, ZHENG R R, et al. One-step mild synthesis of Mn-based spinel MnIICrIII2O4/MnIIMnIII2O4/C and Co-based spinel CoCr2O4/C nanoparticles as battery-type electrodes for high-performance supercapacitor application[J]. Electrochimica Acta,2018,283:197-211. doi: 10.1016/j.electacta.2018.06.164 [40] YU L, ZHANG X G. Electrochemical insertion of magnesium ions into V2O5 from aprotic electrolytes with varied water content[J]. Journal of Colloid and Interface Science,2004,278(1):160-165. doi: 10.1016/j.jcis.2004.05.028 [41] WANG X A, ZHANG Z, XIONG S L, et al. A high-rate and ultrastable aqueous zinc-ion battery with a novel MgV2O6·17H2O nanobelt cathode[J]. Small,2021,17(20):2100318. [42] LIANG L Y, LIU H M, YANG W S. Synthesis and characterization of self-bridged silver vanadium oxide/CNTs compo-site and its enhanced lithium storage performance[J]. Nanoscale,2013,5(3):1026-1033. doi: 10.1039/C2NR33091A [43] YANG K W, FANG G Z, ZHOU J, et al. Hydrothermal synthesis of sodium vanadate nanobelts as high-performance cathode materials for lithium batteries[J]. Journal of Power Sources,2016,325:383-390. doi: 10.1016/j.jpowsour.2016.06.023 [44] IMAMURA D, MIYAYAMA M, HIBINO M, et al. Mg intercalation properties into V2O5 gel/carbon composites under high-rate condition[J]. Journal of the Electrochemical Society,2003,150(6):A753. doi: 10.1149/1.1571531 [45] HUANG H J, NIEDERBERGER M. Towards fast-charging technologies in Li+/Na+ storage: From the perspectives of pseudocapacitive materials and non-aqueous hybrid capacitors[J]. Nanoscale,2019,11(41):19225-19240. doi: 10.1039/C9NR05732C [46] YANG G J, HAN T, LU X J, et al. "Powder electrodeposition" synthesis of NiO-Ni/CNTs composites with high performances of lithium storage battery[J]. Journal of Alloys and Compounds,2022,898:163005. [47] LIU T, YAO T H, LI L, et al. Embedding amorphous lithium vanadate into carbon nanofibers by electrospinning as a high-performance anode material for lithium-ion batteries[J]. Journal of Colloid and Interface Science,2020,580:21-29. doi: 10.1016/j.jcis.2020.06.111 [48] ZHANG Y, WANG Y H, WANG L, et al. A fiber-shaped aqueous lithium ion battery with high power density[J]. Journal of Materials Chemistry A,2016,4(23):9002-9008. doi: 10.1039/C6TA03477B [49] JIANG Y Q, LIU J P. Definitions of pseudocapacitive materials: A brief review[J]. Energy & Environmental Materials,2019,2(1):30-37. doi: 10.1002/eem2.12028 [50] CHEN X H, XU G H, REN X H, et al. A black/red phosphorus hybrid as an electrode material for high-performance Li-ion batteries and supercapacitors[J]. Journal of Materials Chemistry A,2017,5(14):6581-6588. doi: 10.1039/C7TA00455A [51] JI Y S, FANG D, WANG C, et al. Cobalt-doped V2O5 nanowire arrays on Ti foil for enhanced lithium-ion storage[J]. Journal of Alloys and Compounds,2018,742:567-576. doi: 10.1016/j.jallcom.2018.01.293 [52] LI Z, DENG S F, LI H J, et al. Explore the influence of coverage percentage of sulfur electrode on the cycle performance of lithium-sulfur batteries[J]. Journal of Power Sources,2017,347(15):238-246. [53] CAO H L, ZHENG Z Y, MENG J, et al. Examining the effects of nitrogen-doped carbon coating on zinc vanadate nanoflowers towards high performance lithium anode[J]. Electrochimica Acta,2020,356:136791. doi: 10.1016/j.electacta.2020.136791 -






 下载:
下载: