Adsorption behavior of U(VI) on functionalized three-dimensional graphene composite aerogel
-
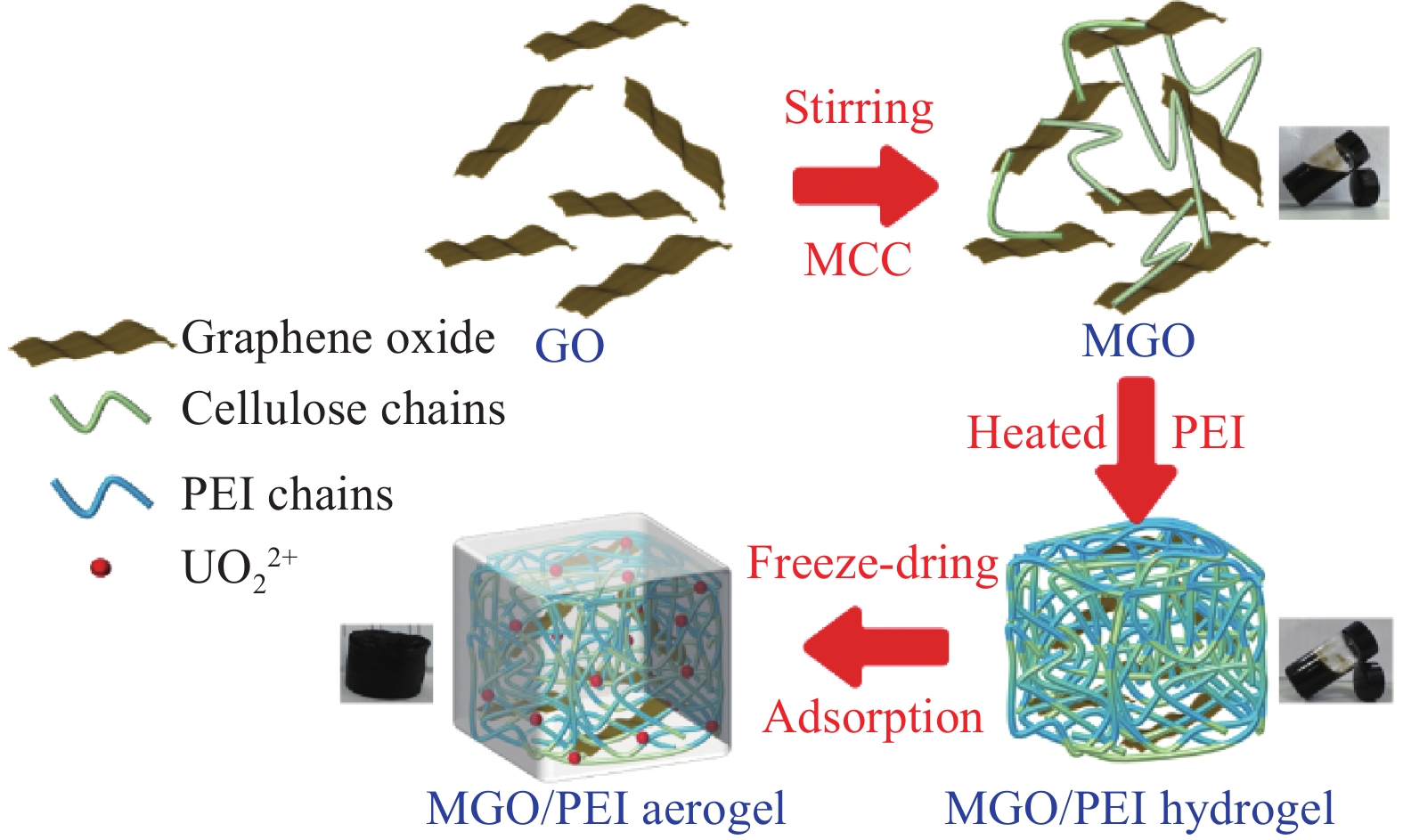
摘要: 核工业循环链中产生的大量含铀废水会对人类健康及生态环境造成损害,因此高效处理含铀废水是保障核工业可持续发展及人类生态安全的重要一环。以氧化石墨烯为前驱体自组装合成了聚乙烯亚胺(PEI)功能化的复合气凝胶(MGO/PEI),并用于去除水溶液中的U(Ⅵ)。通过探究不同PEI投放量、稳定性、pH值、时间、U(Ⅵ)浓度及温度对U(VI)的去除影响。结果表明:在298 K、pH=6时,最大吸附量为1027.01 mg·g−1,符合准二级动力学模型和Langmuir等温吸附模型。热力学常数表明MGO/PEI对U(Ⅵ)的吸附是一个自发吸热的过程。XPS分析表明去除机制主要是由于氨基及含氧官能团与U(VI)的表面络合。Abstract: A large amount of uranium-containing wastewater produced in the nuclear industry circulation chain will cause damage to human health and ecological environment. Therefore, efficient treatment of uranium-containing wastewater is an important part of ensuring the sustainable development of the nuclear industry and human ecological security. Polyethyleneimine (PEI) functionalized composite aerogel (MGO/PEI) was synthesized by self-assembly of graphene oxide as a precursor and used to remove U(VI) from aqueous solution. The effects of different PEI dosage, stability, pH value, time, U(VI) concentration and temperature on the removal of U(VI) were investigated. The results showed that the adsorption behavior was accorded with the pseudo-second-order kinetic model and the Langmuir isothermal adsorption model at 298 K and pH=6, and the maximum adsorption capacity was 1027.01 mg·g−1. The thermodynamic constants indicated that the adsorption of U(VI) by MGO/PEI was a spontaneous endothermic process. XPS analysis showed that the removal mechanism was mainly due to the surface complexation of amino and oxygen-containing functional groups with U(VI).
-
Key words:
- graphene /
- aerogel /
- polyethyleneimine /
- adsorption /
- uranium
-
图 6 (a) MGO/PEI的TG曲线;(b) 不同负荷对MGO/PEI的压缩过程;(c) MGO/PEI3的亲水接触角;(d) MGO/PEI3在U(VI)溶液中的时间稳定性
Figure 6. (a) TG curves of MGO/PEI; (b) Digital images of the compression process of MGO/PEI when loaded by different mass; (c) Water contact angel images of MGO/PEI3; (d) Time stability digital images of MGO/PEI3 in U(VI) solution
图 7 (a) pH值对MGO/PEI吸附U(VI)的影响;(b) U(VI)在不同pH下的物种形态(C0=50 mg·L−1,PCO2=38.5035 Pa);(c)接触时间对MGO/PEI吸附U(VI)的影响;(d)接触时间对MGO/PEI去除U(VI)的影响
Figure 7. (a) Effect of pH on the adsorption of U(VI) by MGO/PEI; (b) Species morphology of U(VI) at different pH (C0=50 mg·L−1, PCO2=38.5035 Pa); (c) Effect of contact time on the adsorption of U(VI) by MGO/PEI; (d) Effect of contact time on the removal rate of U(VI) by MGO/PEI
qe—Equilibrium adsorption capacity; t—Adsorption time; C0—Initial concentration; Pco2—Atmospheric pressure of dioxide in the air
表 1 不同吸附剂的PEI掺杂量
Table 1. PEI doping amount of different adsorbents
Adsorbent Doping mass ratio MCC GO PEI GO/PEI – 1 1 MGO/PEI1 1 1 1 MGO/PEI2 1 1 3 MGO/PEI3 1 1 5 MGO/PEI4 1 1 7 表 2 MGO/PEI的Mapping元素含量
Table 2. Mapping element content of MGO/PEI
Adsorbent Elements content/at% C N O MGO/PEI1 87.81 6.75 5.44 MGO/PEI2 85.47 8.71 5.72 MGO/PEI3 84.64 9.56 5.80 表 3 MGO/PEI对U(VI)的准一级、准二级和Elovich动力学参数
Table 3. Pseudo-first order kinetic, Pseudo-second order kinetic and Elovich kinetic parameters of U(VI) on MGO/PEI
Adsorbent qe, exp./
(mg·g−1)Pseudo-first order model Pseudo-second order model Elovich model qe,cal./(mg·g−1) k1/min−1 R2 qe,cal./(mg·g−1) k2/(g·mg−1·min−1) R2 α/(mg·g−1·min−1) β/(mg·g−1) R2 MGO/PEI1 104.38 93.64 1.51×10−1 0.94 102.58 1.76×10−3 0.97 5.36×101 5.89×10−2 0.95 MGO/PEI2 246.57 240.54 4.55×10−1 0.95 250.60 3.15×10−3 0.99 2.02×105 4.55×10−2 0.70 MGO/PEI3 249.47 246.70 5.71×10−1 0.96 251.58 4.18×10−3 0.98 2.24×105 5.48×10−2 0.69 Notes: qe, exp.—Experimental equilibrium adsorption capacity; qe, cal.—Calculated equilibrium adsorption capacity; k1—Rate constants of Pseudo-first-order model; k2—Rate constants of Pseudo-second-order model; α—Initial adsorption rate of Elovich model; β—Desorption constant of Elovich model; R2—Fitting constant. 表 4 MGO/PEI对U(VI)离子内扩散动力学参数
Table 4. Internal diffusion kinetic parameters of U(VI) on MGO/PEI
AdsorbentIntraparticle diffusion model ki1
/(mg·g−1·min1/2)R2 ki2
/(mg·g−1·min1/2)R2 MGO/PEI1 18.92 0.84 2.36 0.88 MGO/PEI2 35.49 0.86 0.43 0.85 MGO/PEI3 31.72 0.74 0.04 0.36 Note: ki—Rate constants of Webber-Morris model. 表 5 MGO/PEI对U(VI)的吸附等温模型参数
Table 5. Adsorption isothermal model parameters of U(VI) on MGO/PEI
Adsorbent Langmuir model Freundlich model qm/(mg·g−1) kL/(L·mg−1) R2 kF/((mg·g−1)·(mg·L−1)−1/n) n R2 MGO/PEI1 179.53 0.02 0.95 18.26 2.43 0.84 MGO/PEI2 537.29 0.84 0.91 269.05 6.25 0.89 MGO/PEI3 1027.01 0.40 0.97 392.98 4.54 0.78 Notes: qm—Constants for adsorption capacity of Langmuir model; kL—Constants for affinity of Langmuir model; kF—Constant of Freundlich model; n—Favorability factor of the adsorption. 表 6 MGO/PEI对U(VI)的热力学参数
Table 6. Thermodynamics parameters of U(VI) on MGO/PEI
Adsorbent ΔHο/(kJ·mol−1) ΔSο/(kJ·mol−1) ΔGο/(kJ·mol−1) 298 K 308 K 318 K 328 K 338 K MGO/PEI1 5.07 30.48 −33.40 −35.91 −38.47 −41.00 −43.53 MGO/PEI2 0.87 20.53 −43.65 −45.36 −47.07 −48.77 −50.48 MGO/PEI3 0.94 21.90 −46.47 −48.29 −50.11 −51.93 −53.75 Notes: ΔHο—Heat of the adsorption; ΔSο—Standard entropy change; ΔGο—Gibbs free energy of adsorption. 表 7 不同气凝胶复合吸附剂对U(VI)的吸附性能比较
Table 7. Comparison of U(VI) adsorption performance of different aerogel composite adsorbents
Adsorbent pH Adsorption time/min Adsorption capacity/(mg·g−1) Adsorption
mechanismRefs. CNFs aerogels 5.0 100 440.60 Carboxyl coordination complex [38] GONRs aerogels 4.5 60 430.60 Oxygen functional groups chemical adsorption [39] CS-PTADMA3 aerogels 6.0 480 160.00 Coordination and electrostatic adsorption [40] MgO-N aerogels 4.0 100 1061.80 Electrical interaction and surface complexation [41] GO@PDA/CS aerogels 6.0 50 415.90 Coordination of nitrogen and oxygen functional groups [42] IAA-CTSA aerogels 6.5 360 847.50 Cation-π interaction, surface complexation and electrostatic interaction [43] Pr2O3 aerogels 7.0 240 770.60 Inner-sphere surface complexation [44] MGO/PEI aerogels 6.0 40 1027.01 Complexation of amide carbonyl and hydroxyl function groups This work Notes: CNFs aerogels—Cellulose nanofibers aerogels; GONRs aerogels—Graphene oxide nanoribbons aerogels; CS-PTADMA3 aerogels—Chitosan-based aerogels; GO@PDA/CS aerogels—Chitosan crosslinked dopamine modified graphene oxide aerogels; IAA-CTSA aerogels—Indole-modified cross-linked chitosan aerogel. -
[1] BAEK J. Do nuclear and renewable energy improve the environment? Empirical evidence from the United States[J]. Ecological Indicators,2016,66:352-356. doi: 10.1016/j.ecolind.2016.01.059 [2] KONG L J, ZHU Y T, WANG M, et al. Simultaneous reduction and adsorption for immobilization of uranium from aqueous solution by nano-flake Fe-SC[J]. Journal of Hazardous Materials,2016,320:435-441. doi: 10.1016/j.jhazmat.2016.08.060 [3] LI Z J, WANG L, YUAN L Y, et al. Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite[J]. Journal of Hazardous Materials,2015,290:26-33. doi: 10.1016/j.jhazmat.2015.02.028 [4] SEMNANI F, ASADI Z, SAMADFAM M, et al. Uranium(VI) sorption behavior onto amberlite CG-400 anion exchange resin: Effects of pH, contact time, temperature and presence of phosphate[J]. Annals of Nuclear Energy,2012,48:21-24. doi: 10.1016/j.anucene.2012.05.010 [5] EBAID Y Y, NASR M M, SANTOS J K B, et al. Behavior of uranium series in groundwater of the Wajid Formation, Wadi AdDawasir, Saudi Arabia[J]. Environmental Monitoring and Assessment,2020,192(9):1-13. [6] MISHRA S, MAITY S, BHALKE S, et al. Thermodynamic and kinetic investigations of uranium adsorption on soil[J]. Journal of Radioanalytical and Nuclear Chemistry,2012,294(1):97-102. doi: 10.1007/s10967-011-1506-z [7] CACCIN M, GIACOBBO F, DA ROS M, et al. Adsorption of uranium, cesium and strontium onto coconut shell activated carbon[J]. Journal of Radioanalytical and Nuclear Chemistry,2013,297(1):9-18. doi: 10.1007/s10967-012-2305-x [8] WANG M M, QIU J, TAO X Q, et al. Effect of pH and ionic strength on U(IV) sorption to oxidized multiwalled carbon nanotubes[J]. Journal of Radioanalytical and Nuclear Chemistry,2011,288(3):895-901. doi: 10.1007/s10967-011-1018-x [9] RETHINASABAPATHY M, KANG S M, JANG S C, et al. Three-dimensional porous graphene materials for environmental applications[J]. Carbon Letters,2017,22(1):1-13. [10] WANG H, SUN K, TAO F, et al. 3D honeycomb-like structured graphene and its high efficiency as a counter-electrode catalyst for dye-sensitized solar cells[J]. Angewandte Chemie International Edition,2013,52(35):9210-9214. doi: 10.1002/anie.201303497 [11] ZHAO Q, ZHU X Y, CHEN B L. Stable graphene oxide/poly(ethyleneimine) 3D aerogel with tunable surface charge for high performance selective removal of ionic dyes from water[J]. Chemical Engineering Journal,2018,334:1119-1127. doi: 10.1016/j.cej.2017.11.053 [12] NARDECCHIA S, CARRIAZO D, FERRER M L, et al. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications[J]. Chemical Society Reviews,2013,42(2):794-830. doi: 10.1039/C2CS35353A [13] ZHAO Q A, CHEN B L. Concurrent enhancement of structure stability and adsorption capacity of freeze-dried graphene oxide aerogels via the removal of oxidation debris nanoparticles on nanosheets[J]. Environmental Science: Nano,2021,8(4):1000-1009. doi: 10.1039/D0EN01135E [14] TANGTUBTIM S, SAIKRASUN S. Adsorption behavior of polyethyleneimine-carbamate linked pineapple leaf fiber for Cr(VI) removal[J]. Applied Surface Science,2019,467-468:596-607. doi: 10.1016/j.apsusc.2018.10.204 [15] LIANG X T, LIANG B, WEI J Y, et al. A cellulose-based adsorbent with pendant groups of quaternary ammonium and amino for enhanced capture of aqueous Cr(VI)[J]. International Journal of Biological Macromolecules,2020,148:802-810. doi: 10.1016/j.ijbiomac.2020.01.184 [16] WANG X, FENG J H, CAI Y W, et al. Porous biochar modified with polyethyleneimine (PEI) for effective enrichment of U(VI) in aqueous solution[J]. Science of the Total Environment,2020,708:134575. doi: 10.1016/j.scitotenv.2019.134575 [17] KANI A N, DOVI E, ARYEE A A, et al. Polyethylenimine modified tiger nut residue for removal of Congo red from solution[J]. Desalination and Water Treatment,2021,215:209-221. doi: 10.5004/dwt.2021.26765 [18] O'CONNELL D W, BIRKINSHAW C, O'DWYER T F. Heavy metal adsorbents prepared from the modification of cellulose: A review[J]. Bioresource Technology,2008,99(15):6709-6724. doi: 10.1016/j.biortech.2008.01.036 [19] LI M Z, WANG X Y, ZHAO R, et al. A novel graphene-based micro/nano architecture with high strength and conductivity inspired by multiple creatures[J]. Scientific Reports,2021,11(1):1387. doi: 10.1038/s41598-021-80972-8 [20] HUANG B, LIN F C, TANG L R, et al. Research advances of functional cellulose-based hydrogels and its applications[J]. Journal of Forestry Engineering,2022,7(2):1-13. [21] WANG X Y, WAN K, XIE P B, et al. Ultralight, high capacitance, mechanically strong graphene-cellulose aerogels[J]. Molecules, 2021, 26(16): 4891. [22] JIANG Q S, KACICA C, SOUNDAPPAN T, et al. An in situ grown bacterial nanocellulose/graphene oxide composite for flexible supercapacitors[J]. Journal of Materials Chemistry A,2017,5(27):13976-13982. doi: 10.1039/C7TA03824K [23] STOBINSKI L, LESIAK B, MALOLEPSZY A, et al. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods[J]. Journal of Electron Spectroscopy and Related Phenomena,2014,195:145-154. doi: 10.1016/j.elspec.2014.07.003 [24] ANDONOVIC B, ADEMI A, GROZDANOV A, et al. Enhanced model for determining the number of graphene layers and their distribution from X-ray diffraction data[J]. Beilstein Journal of Nanotechnology,2015,6:2113-2122. doi: 10.3762/bjnano.6.216 [25] WANG X Y, XIE P B, HE L, et al. Ultralight, mechanically enhanced, and thermally improved graphene-cellulose-polyethyleneimine aerogels for the adsorption of anionic and cationic dyes[J]. Nanomaterials, 2022, 12(10): 1727. [26] KANG Y J, CHUN S J, LEE S S, et al. All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers, carbon nanotubes, and triblock-copolymer ion gels[J]. ACS Nano,2012,6(7):6400-6406. doi: 10.1021/nn301971r [27] KIM H, NAMGUNG R, SINGHA K, et al. Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool[J]. Bioconjugate Chemistry,2011,22(12):2558-2567. doi: 10.1021/bc200397j [28] XIE X Q, WANG Y F, XIONG Z, et al. Highly efficient removal of uranium(VI) from aqueous solution using poly(cyclotriphosphazene-co-polyethyleneimine) microspheres[J]. Journal of Radioanalytical and Nuclear Che-mistry,2020,326(3):1867-1877. doi: 10.1007/s10967-020-07455-4 [29] ZHOU X H, CHEN Z X, YAN D H, et al. Deposition of Fe-Ni nanoparticles on polyethyleneimine-decorated graphene oxide and application in catalytic dehydrogenation of ammonia borane[J]. Journal of Materials Chemistry,2012,22(27):13506-13516. doi: 10.1039/c2jm31000g [30] ZHANG Y, CHEN B, ZHANG L M, et al. Controlled assembly of Fe3O4 magnetic nanoparticles on graphene oxide[J]. Nanoscale,2011,3(4):1446-1450. doi: 10.1039/c0nr00776e [31] GENG J J, YIN Y W, LIANG Q W, et al. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: Adsorption performance and mechanism[J]. Chemical Engineering Journal,2019,361:1497-1510. doi: 10.1016/j.cej.2018.10.141 [32] LIU H Y, KUILA T, KIM N H, et al. In situ synthesis of the reduced graphene oxide-polyethyleneimine composite and its gas barrier properties[J]. Journal of Materials Chemistry A,2013,1(11):3739-3746. doi: 10.1039/c3ta01228j [33] JIANG F W, ZHAO W J, WU Y M, et al. A polyethyleneimine-grafted graphene oxide hybrid nanomaterial: Synthesis and anti-corrosion applications[J]. Applied Surface Science,2019,479:963-973. doi: 10.1016/j.apsusc.2019.02.193 [34] XU H H, ZHU S D, XIA M Z, et al. Three-dimension hierarchical composite via in-situ growth of Zn/Al layered double hydroxide plates onto polyaniline-wrapped carbon sphere for efficient naproxen removal[J]. Journal of Hazardous Materials,2022,423:127192. doi: 10.1016/j.jhazmat.2021.127192 [35] WANG X, LIU Q, LIU J Y, et al. 3D self-assembly polyethyleneimine modified graphene oxide hydrogel for the extraction of uranium from aqueous solution[J]. Applied Surface Science,2017,426:1063-1074. doi: 10.1016/j.apsusc.2017.07.203 [36] LI Y, ZHANG M C, GUO X H, et al. Growth of high-quality covalent organic framework nanosheets at the interface of two miscible organic solvents[J]. Nanoscale Horizons,2018,3(2):205-212. doi: 10.1039/C7NH00172J [37] HOU Y, CHAI D F, LI B N, et al. Polyoxometalate-incorporated metallacalixarene@graphene composite electrodes for high-performance supercapacitors[J]. ACS Applied Materials & Interfaces,2019,11(23):20845-20853. [38] WANG Y, LI Y X, ZHANG Y P, et al. Nanocellulose aerogel for highly efficient adsorption of uranium(VI) from aqueous solution[J]. Carbohydrate Polymers,2021,267:118233. doi: 10.1016/j.carbpol.2021.118233 [39] LI Y, HE H J, LIU Z C, et al. A facile method for preparing three-dimensional graphene nanoribbons aerogel for uranium(VI) and thorium(IV) adsorption[J]. Journal of Radioanalytical and Nuclear Chemistry,2021,328(1):289-298. doi: 10.1007/s10967-021-07619-w [40] YANG L R, HUANG C Q, LUO X, et al. Chitosan-based aerogel with anti-swelling for U(VI) adsorption from aqueous solution[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2021,630:127527. doi: 10.1016/j.colsurfa.2021.127527 [41] XIONG T, LI Q C, LI K D, et al. Construction of novel magnesium oxide aerogel for highly efficient separation of uranium(VI) from wastewater[J]. Separation and Purification Technology,2022,295:121296. doi: 10.1016/j.seppur.2022.121296 [42] LIAO Y, WANG M, CHEN D J. Preparation of polydopamine-modified graphene oxide/chitosan aerogel for uranium(VI) adsorption[J]. Industrial & Engineering Chemistry Research,2018,57(25):8472-8483. [43] WANG Y, AI Y Y, LIU X L, et al. Indole-functionalized cross-linked chitosan for effective uptake of uranium(VI) from aqueous solution[J]. Polymer Chemistry,2022,13(12):1751-1762. doi: 10.1039/D1PY01725J [44] LIAO J, LIU P, XIE Y, et al. Metal oxide aerogels: Preparation and application for the uranium removal from aqueous solution[J]. Science of the Total Environment,2021,768:144212. doi: 10.1016/j.scitotenv.2020.144212 -






 下载:
下载: