Research progress on preparation and modification technology of conductive polymer corrosive protection coatings
-
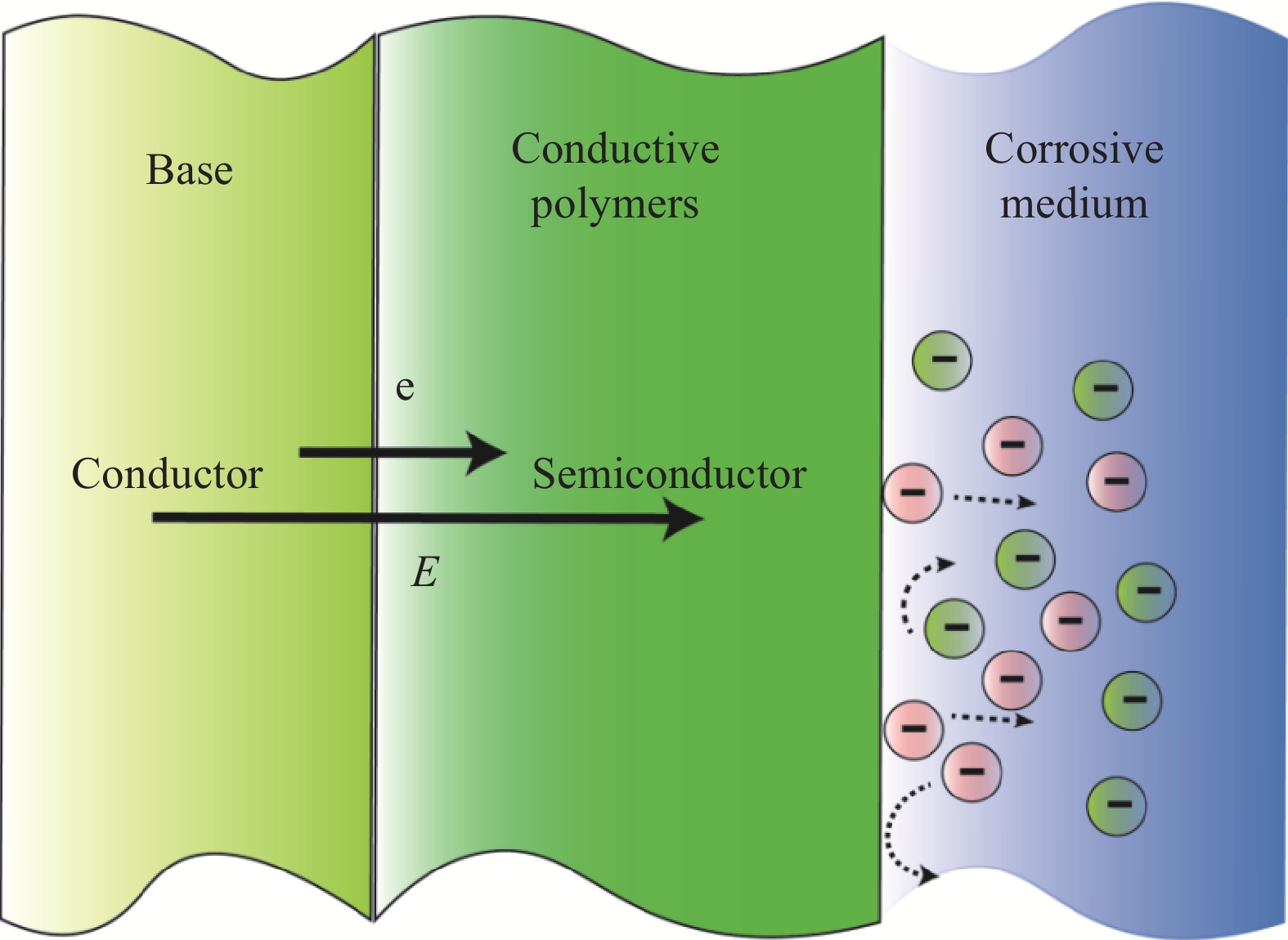
摘要: 我国海洋工程装备制造业正处在生存与发展的关键阶段,防腐涂层是降低基材腐蚀速率、提升其服役寿命最有效的方式之一。导电聚合物涂层由于其绿色环保、制备简单等优点及独特的导电与防腐机制,使其在金属腐蚀防护领域得到了广泛的应用。本文归纳总结了导电聚合物涂层的防腐机制,介绍了采用化学氧化和电化学合成两种方法制备导电聚合物涂层的现状,重点阐述了导电聚合物涂层的掺杂改性、共聚改性、分层设计3种改性技术对涂层耐蚀性能的提升效果,最后提出了导电聚合物涂层在腐蚀防护领域可能存在的研究热点和发展趋势。Abstract: The marine engineering equipment manufacturing industry in China is in the critical stage of survival and development. The anti-corrosion coating is one of the most effective ways to reduce the corrosion rate of the substrate and improve its service life. Conductive polymers (CPs) coatings have been widely used in the field of metal corrosion protection due to its advantages of environmental protection, simple preparation and unique conductive and anticorrosive mechanism. This work summarizes the anticorrosion mechanism of CPs coatings, introduces the current situation of preparing conductive polymer coating by two methods of chemical oxidation and electrochemical synthesis, and focuses on the improvement effect of doping modification, copolymerization modification and layered design of CPs coatings on the corrosion resistance of the coating. Finally, the possible research hotspots and development trends of CPs coatings in the field of corrosion protection are proposed.
-
膜分离技术具有高能效、易操作、环境友好和占地面积小等优点,近年来在气体分离领域受到广泛关注[1-4]。传统气体分离膜多以聚合物膜为主,然而由于聚合物分离膜固有的选择性和渗透性的制约关系(Trade-off效应),使其性能很难再提升[5-6]。研究者发现将多孔材料与聚合物基体共混制成混合基质膜,通过结合两种材料的优点,能够同时提升膜的气体渗透性和选择性,从而突破聚合物膜的Trade-off效应[7-8]。因此,制备混合基质膜是改善膜气体分离性能的一种有效方法。
对于混合基质膜,填料和聚合物基体材料的选择尤为重要。聚酰亚胺由于其优异的热稳定性、良好的力学性能以及可加工性,已经在气体分离膜领域发展多年,是混合基质膜聚合物基体候选材料之一[9-11]。对于填料材料的选择,共价有机框架材料(COFs)是一种由有机单元通过共价键构成的多孔材料,由于其具有优异的稳定性,易功能化、永久空隙率以及高比表面积等优点,在气体分离领域展现出不俗的潜力[12]。由于COFs全有机的性质,使其能够均匀地分散在聚合物基质中,减少了混合基质膜中由于界面缺陷产生的非选择性孔[13-14]。然而,大部分COFs的孔径很难做到2 nm以下,相对于气体分子动力学直径(N2:0.36 nm;O2:0.35 nm;CO2:0.33 nm)还是较大,难以实现对气体的高效分离,从而降低了气体选择性[15-16]。因此,需要对COFs的孔径大小调控或引入一些功能性吸附位点进行改善。研究表明,引入氟原子能够有效改善COFs的孔径大小且能够提供与气体相互作用的吸附位点。Alahakoon等[17]通过使用含氟单体制备出两种氟化COFs,将氟化COFs与未氟化的相比,发现氟化COFs具有更大的比表面积、更小且更明确的孔径。Gao等[18]报道了3种具有—H、—Me和—F取代基的同构三维共价有机骨架,对比不含氟的COFs,氟化COFs具有更高的CO2亲和力,对CO2/N2有着更高的IAST选择性。Yang等[19]制备了一种氟化CTF,通过氟原子的强静电作用以及C—F键与CO2的偶极-四极作用,使其具有优异的CO2吸附能力。
基于上述讨论,本文合成出一种具有较小孔径、高比表面积的氟化共价有机框架材料(TpPa-CF3)。随后,以TpPa-CF3为填料,聚酰亚胺(6FDA-ODA)为基体,制备出不同负载量的TpPa-CF3/6FDA-ODA混合基质膜。表征了其结构和表面、截面的微观形貌,探究了其热性能、力学性能以及疏水性能,最后讨论了混合基质膜的气体渗透性以及在烟道气分离(CO2/N2)和空气分离(O2/N2)上的应用前景。
1. 实验材料及方法
1.1 原材料
4,4-二氨基二苯醚(ODA,98%)、4,4-(六氟异丙烯)二酞酸酐(6FDA,98%+)、2,4,6-三甲酰间苯三酚(Tp,98%)、2-三氟甲基-1,4-苯二胺(Pa-CF3,97%)、1,3,5-三甲基苯(99%+)、1,4-二氧六环(99%)均购自上海阿达玛斯试剂有限公司;乙酸(AR)、N,N-二甲基甲酰胺(DMF,AR)均购自西陇科学股份有限公司;间甲酚(m-Cresol,99%)、异喹啉(97%)均购自上海阿拉丁试剂有限公司;丙酮(Acetone,AR),成都市科隆化学品有限公司;工业酒精(95%),弘昊实验设备有限公司。
1.2 TpPa-CF3的合成
将Tp (63.0 mg,0.30 mmol)、Pa-CF3 (79.0 mg,0.45 mmol)、1,3,5-三甲基苯(1.5 mL)、1,4-二氧六环(1.5 mL)依次加入到Pyrex管(25 mL)中。为使混合物均匀分散,超声处理0.5 h,再加入3 mol/L乙酸溶液(0.5 mL)。随后,用液氮将Pyrex管骤冻抽出空气,再在室温下解冻,此操作循环3次。密闭封管,将Pyrex管在120℃下油浴3天。反应完毕后冷却至室温,过滤收集产物,先用DMF溶液搅拌洗涤3次,再通过索氏提取法进行提纯(提纯溶剂采用丙酮)。随后,收集产物,在真空烘箱中120℃下干燥12 h后,得到橘红色粉末样品TpPa-CF3。
1.3 聚酰亚胺(6FDA-ODA)的合成
在N2氛围下,向装有机械搅拌、冷凝回流的150 mL三口烧瓶内依次加入ODA (2.00 g,9.99 mmol)、间甲酚(28 mL),待ODA完全溶解后再依次加入6FDA (4.44 g,9.99 mmol)、间甲酚(28 mL),随后将温度升到50℃待反应物完全溶解后,滴加5~6滴异喹啉后升温至80℃反应3 h,120℃反应3 h,180℃反应3 h,最后200℃反应12 h。反应结束冷却至室温后,将聚酰亚胺溶液缓慢倒入大量工业酒精中拉丝沉淀,过滤收集产物,在真空烘箱中150℃干燥8 h。随后,使用适量DMF重新溶解并进行二次沉淀以除去聚酰亚胺中残留杂质。
1.4 TpPa-CF3/6FDA-ODA混合基质膜的制备
取一定量的TpPa-CF3粉末分散在DMF (3 mL)中,使用细胞粉碎机,在300 W功率下超声0.5 h后再搅拌6 h,保证TpPa-CF3粉末在DMF溶液中分散均匀。同时,称取0.2 g 6FDA-ODA溶解在DMF (2 mL)中,用针式滤头(0.45 μm,尼龙)过滤除去杂质。随后,将TpPa-CF3的分散液滴加到6FDA-ODA溶液中搅拌12 h,确保TpPa-CF3和6FDA-ODA充分混合。最后,将混合溶液缓慢流延到光滑平整的玻璃板(5 cm×5 cm)上,在80℃下蒸发12 h除去溶剂,待冷却至室温后在温水中脱膜,最后在150℃的真空烘箱中干燥12 h以除去残留的溶剂分子。按以上步骤分别制备含量为0wt%、1wt%、3wt%、5wt%、7wt%的TpPa-CF3/6FDA-ODA混合基质膜。
1.5 表征测试
X射线衍射(XRD):采用荷兰帕纳科公司的X'Pert Pro型X射线衍射仪对制备的TpPa-CF3粉末与薄膜进行晶型及结构表征,扫描范围在3°~40°,扫描速度为2°/min。
傅里叶变换红外光谱(FTIR):采用美国尼高力公司的Nicolette 6700-NXR型傅里叶变换红外光谱仪分析TpPa-CF3和薄膜的化学键组成及官能团。对于粉末样品通过溴化钾压片的方式测试,对于薄膜样品通过制成厚度约为20 μm的薄膜直接测试。扫描范围为400~
4000 cm−1,扫描次数64次以上。固态核磁共振(ssNMR):通过德国布鲁克公司的Avance Neo 400 WB型固体核磁共振波谱仪测试TpPa-CF3的13C NMR,分析其化学键连接方式。所需样品压实后的体积应多于0.5 cm3。
X射线光电子能谱(XPS):采用美国热电公司的Escalab 250 Xi型X射线光电子能谱仪分析TpPa-CF3的化学元素及化学态。采用粉末压片的方式制样。
扫描电子显微镜(SEM):通过日本日立公司的SU 4800型扫描电子显微镜表征TpPa-CF3和薄膜表面、截面的微观形貌。对于粉末样品,用牙签将少量样品涂在导电胶上制样;对于薄膜样品,膜表面直接粘在导电胶上,膜断面通过液氮脆断选取平整截面制样。全部样品在测试前通过喷金处理提高样品导电性。
N2吸附-脱附测试:通过美国康塔公司的Autosorb IQ型比表面及孔隙度分析仪器表征TpPa-CF3的比表面积及孔径分布。采用BET (Brunauer-Emmett-Teller)法计算比表面积,密度泛函理论(DFT)计算孔径分布。
热重分析(TGA):通过德国耐驰公司的STA 449C型综合热分析仪测试TpPa-CF3和薄膜的热稳定性。在N2氛围下测试,升温速率为10℃/min,测试范围在50~800℃。
差示扫描量热分析(DSC):通过德国耐驰公司的DSC 214型差示扫描量热仪测试薄膜的玻璃化转变温度,在N2氛围下以10℃/min的速率升温,测试范围在30~350℃,所有结果均采用消除热历史后的二次升温曲线。
力学性能:通过美国美特斯公司的CMT2103型万能试验机来表征薄膜的力学性能。薄膜样品尺寸为50 mm×10 mm,拉伸载荷为5 kN,拉伸速率为2 mm/min,标距为20 mm。
水接触角测试:通过中国承德优特仪器有限公司JY-PHb型接触角分析仪测定薄膜的亲疏水性能。薄膜样品尺寸为20 mm×20 mm,测试次数至少3次。
气体渗透性测试:通过中国济南兰光公司的VAC-V1型气体渗透仪测试薄膜的气体渗透性能。测试方法为恒体积变压法,测试气体为高纯气体(CO2、O2、N2),测试条件为4 bar,35℃。测试过程如下,将厚度均匀的待测薄膜装入膜腔中,测试前将上下腔气压抽至20 Pa以下,随后下腔关闭,上腔通入待测纯气体形成压差。压差推动气体自上腔(高压侧)向下腔(低压侧)渗透,通过系统计算得到膜的气体渗透系数P。
2. 结果与讨论
2.1 TpPa-CF3的表征
通过粉末X射线衍射(PXRD)对合成的TpPa-CF3的晶体结构进行分析,图1(a)中2θ=4.79°处出现的强峰对应于COF的(100)晶面,其他峰也出现在2θ=7.81°、26.14°处,分别对应于(200)、(001)晶面,其中(001)晶面也是其π-π堆叠峰,通过布拉格方程计算得出其堆叠层间距为0.33 nm。将测试结果与模拟的晶体模型的衍射峰进行对比,结果表明二者衍射峰的位置与强度均匹配良好。TpPa-CF3在Pawley精修后得到的晶胞参数为a=
2.290351 nm,b=2.236760 nm,c=0.423813 nm,α=89.56415 ,β=89.73479 ,γ=120.51471 。实验结果与精修后的PXRD之间的残差值较小,Rwp=1.24%,Rp=0.91%。以上结果初步说明成功合成出了目标晶体结构,且具有良好的结晶性。为进一步说明TpPa-CF3成功合成,通过FTIR对TpPa-CF3以及其构筑单元测试,从图1(b)中可以看到,构筑单元2,4,6-三甲酰间苯三酚(Tp)在
2894 cm−1处醛基的CH=O特征峰和构筑单元2-三氟甲基-1,4-苯二胺(Pa-CF3)在3318 cm−1和3210 cm−1处的—NH2特征峰在产物TpPa-CF3中消失,表明醛胺缩合反应完全。在1282 cm−1处的C—N的特征吸收峰表明烯醇-酮异构的发生。因为框架是以酮的形式存在,结构中有强的分子内氢键及共轭作用,所以在1592 cm−1处的C=C的特征峰和1610 cm−1处的C=O特征峰合并呈肩状[20]。在1128 cm−1处出现了C—F的特征峰。13C固体NMR分析如图1(c)所示,图中显示化学位移在184.2×10−6和108.1×10−6处有两个较明显的信号峰,分别对应于烯醇-酮异构反应所形成的C=O键和C—N键上的C原子,123.6×10−6处归属于C—F上的C原子。其余在119.0×10−6、134.1×10−6和146.5×10−6处的信号峰则归属于芳香单元上的C原子。
FTIR和固体核磁分析结果证实TpPa-CF3的成功合成且以稳定的β-酮胺形式存在。
通过XPS测量TpPa-CF3的全谱和各个元素的光谱,由图2(a)的全谱可知TpPa-CF3是由C、N、O、F 4种元素组成的。图2(b)是C1s的高分辨率XPS光谱,其能够被卷积为4个峰,分别对应于TpPa-CF3中的C=C/C—C(284.8 eV)、C—N(286.2 eV)、C=O(288.9 eV)和C—F(292.9 eV)键,N1s的高分辨率XPS如图2(c)所示,其被卷积为2个峰,分别归属于N—C(400.2 eV)和N—H(403.9 eV)键,F1s的高分辨率XPS如图2(d)所示,其只有一个卷积峰归属于C—F(688.3 eV)。所有以上结果说明TpPa-CF3形成目标结构,由C1s和N1s证明该结构发生了烯醇-酮异构。
通过扫描电镜(SEM)观察TpPa-CF3的微观形貌。如图3(a)所示,TpPa-CF3具有均匀的微观形貌,呈现为“米粒”形颗粒堆积形成的团簇,每一颗“米粒”的尺寸在(100±30) nm。
为了解TpPa-CF3的多孔性,对其进行N2吸附-脱附测试。图3(b)中N2吸脱附曲线呈现出I型曲线特征,TpPa-CF3在相对压力较低的区域(p/p0<0.1),N2的吸附量快速增加,说明材料中存在丰富的微孔结构。通过计算分析得出TpPa-CF3具有较大的比表面积(791.83 m2·g−1),图3(c)显示TpPa-CF3具有较小的孔径(1.18 nm)。这归因于TpPa-CF3中氟原子的高电负性增强了框架中芳香环之间的相互作用力,这种相互作用力有助于COF形成较大的比表面积以及较小的孔径[17]。
通过热重分析TpPa-CF3的热性能。热重曲线如图3(d)所示,从图中看到热损失分为两个阶段,大约在400℃之前的损失可能为残留在孔道里的高沸点溶剂(DMF)的挥发。400℃后出现明显的质量损失,从DTG曲线上可以看到在416℃质量损失的速度最快,这主要归因于TpPa-CF3框架的分解。以上结果可以看出TpPa-CF3具有较好的热稳定性。
2.2 TpPa-CF3/6FDA-ODA混合基质膜的表征
通过XRD对膜结构表征,评价了填料对聚合物链排列的影响。从图4(a)中可以看到所有曲线在2θ=15°左右均出现典型的聚合物宽峰。通过布拉格方程计算,得到膜的分子链间距。纯6FDA-ODA膜的分子链间距为0.574 nm,随着填料TpPa-CF3负载量的增加,链间距呈现先增大后下降的趋势,7%TpPa-CF3/6FDA-ODA膜的链间距最小(0.566 nm)。分子链间距先增大主要是由于小负载量的掺入破坏了分子链的堆积,随着负载量的增大,填料与聚合物基质的相互作用逐渐增强,限制了分子链的迁移率,链间距减小有利于提高气体的选择性。同时,在TpPa-CF3/6FDA-ODA混合基质膜中没有观察到TpPa-CF3粉末的特征峰,这主要是由于在超声搅拌过程中COF填料的部分剥落[21]。
混合基质膜的FTIR图谱如图4(b)所示,所有膜都表现出6FDA-ODA的特征峰,包括C=O的对称(
1783 cm−1)和不对称拉伸(1733 cm−1)、C—N的拉伸振动(1378 cm−1)、C—O—C的拉伸振动(1157 cm−1)、C—F键的吸收峰(1110 cm−1),以及酰亚胺环的弯曲振动(721 cm−1),值得注意的是在1597 cm−1处的特征峰,随着填料的增加而增强,这主要归因于TpPa-CF3和6FDA-ODA中芳香环上的C=C的吸收峰重叠[22]。以上结果说明聚酰亚胺基体和填料之间具有良好的相容性,填料的加入并没有破坏聚酰亚胺的结构。为分析TpPa-CF3的加入对混合基质膜热稳定性的影响,对6FDA-ODA及TpPa-CF3/6FDA-ODA混合基质膜进行了热重测试。如图5(a)所示,混合基质膜的分解分为两个阶段,第一阶段是400℃左右TpPa-CF3框架的分解,第二阶段是500℃左右6FDA-ODA基体膜的分解,填料的加入对膜的热稳定性影响不大。所有混合基质膜都表现出高达500℃的良好热稳定性,远高于工业中膜的操作温度,表明这些膜具有良好的适用性。DSC曲线用于分析膜的玻璃化转变温度(Tg)。如图5(b)所示,6FDA-ODA膜的Tg出现在297.5℃。随着TpPa-CF3负载量的增加,TpPa-CF3/6FDA-ODA膜的Tg从297.5℃逐渐增加到302.1℃,说明TpPa-CF3与6FDA-ODA之间具有良好的界面相互作用,这有利于提升混合基质膜的气体选择性[23]。
膜表面、截面的扫描电镜表征能够反映出填料在膜内的分散情况。如图6(a1)~6(e1)所示,与表面光滑平整的纯膜相比,混合基质膜的表面随着填料负载量的增加逐渐变得粗糙,在负载量达到7wt%时可以看到膜表面出现不平整及大颗粒团聚的现象。图6(a2)~6(e2)为纯膜及其混合基质膜的截面扫描电镜图,纯膜的截面表现出均匀、致密的微观结构,在1wt%~5wt%混合基质膜的截面图中能够观察到随着负载量的增加其截面形貌逐渐变粗糙,同时在膜内能够观察到TpPa-CF3颗粒很好地被聚合物包裹且分散均匀。当填料负载量达到7wt%时膜内出现填料与聚合物基质相分离的现象,说明此时负载量已经达到聚合物基质所能承受的上限,5wt%为其最优负载量。
通过接触角测试仪分析纯膜及其混合基质膜的水接触角(θw)。如图7(a)和表1所示,6FDA-ODA膜的水接触角为79.9°,TpPa-CF3/6FDA-ODA混合基质膜的水接触角为81.4°~89.1°,呈现逐渐增大的趋势。这主要归因于TpPa-CF3框架中含有—CF3疏水基团,因此随着TpPa-CF3含量的增加相应负载量的混合基质膜水接触角也逐渐增加。提升膜的疏水性能有助于阻止水汽进入,提升其气体传输性能。
表 1 不同负载量下TpPa-CF3/6FDA-ODA混合基质膜的力学性能Table 1. Mechanical properties of TpPa-CF3/6FDA-ODA mixed matrix membranes at different loadingsTpPa-CF3 loadings/wt% Tensile strength/MPa Elongation at break/% Young's modulus/GPa θw/(°) 0 74.1 10.1 1.59 79.9 1 79.6 9.7 1.63 81.4 3 82.9 8.6 1.70 83.1 5 93.0 7.8 1.82 84.1 7 84.5 7.3 1.76 89.1 Note: θw—Water contact angle. 对混合基质膜进行拉伸实验以此来检验其力学性能。测试结果如图7(b)和表1所示。从表中可以看出,TpPa-CF3/6FDA-ODA混合基质膜的抗拉强度和杨氏模量随着TpPa-CF3负载量的增加呈现出先增加后下降的趋势,而断裂伸长率呈现逐渐下降的趋势。这主要归因于,在混合基质膜中TpPa-CF3与6FDA-ODA之间较好的相互作用力使得填料与聚合物之间具有良好的界面相容性,增强了膜的刚性。然而,当负载量达到7wt%时,抗拉强度和杨氏模量略微下降,这主要是过量的TpPa-CF3颗粒之间发生团聚,使得界面出现缺陷导致应力集中,降低了膜的力学性能[24]。
利用3种纯气体(CO2、O2、N2)渗透测试来评估不同负载量下TpPa-CF3/6FDA-ODA混合基质膜的渗透性及CO2/N2和O2/N2的理想选择性。结果如表2所示,每一种膜气体渗透系数的大小均与气体分子动力学直径呈反比,即膜的3种气体渗透系数大小排列为P(CO2)>P(O2)>P(N2),3种气体分子动力学直径大小排列为N2(0.36 nm)>O2(0.35 nm)>CO2(0.33 nm)。同6FDA-ODA膜相比所有混合基质膜的气体渗透性都有所提升。由图8(a)中可得,随着TpPa-CF3含量的增加,膜的气体渗透性呈现出先增大后下降的趋势,其中5%TpPa-CF3/6FDA-ODA膜气体渗透性能最佳,P(CO2)提升了149%,P(O2)提升了138%,P(N2)提升了98%。这主要归因于TpPa-CF3的高孔隙率提高了TpPa-CF3/6FDA-ODA膜的比表面积及固有孔隙率,为气体传输提供了快速通道。TpPa-CF3负载量到7wt%时,气体的渗透性明显下降,但仍然比6FDA-ODA膜高。这主要是由于负载量过大,造成TpPa-CF3在膜内团聚堵塞了气体传输的孔道。
表 2 6FDA-ODA及TpPa-CF3/6FDA-ODA混合基质膜的气体渗透系数P和理想选择性Table 2. Gas permeability coefficient P and ideal selectivity of 6FDA-ODA and TpPa-CF3/6FDA-ODA mixed matrix membranesMembrane Permeability/Barrer Ideal selectivity α CO2 O2 N2 α(CO2/N2) α(O2/N2) 6FDA-ODA 12.47 2.55 0.64 19.5 4.0 1%TpPa-CF3/6FDA-ODA 16.91 3.76 0.98 17.2 3.8 3%TpPa-CF3/6FDA-ODA 22.77 4.43 1.03 22.0 4.3 5%TpPa-CF3/6FDA-ODA 31.08 6.08 1.27 24.5 4.8 7%TpPa-CF3/6FDA-ODA 18.62 4.16 1.11 16.8 3.8 Notes: 1 Barrer=10−10 cm3(STP)·cm·cm−2·s−1·cmHg−1; Ideal selectivity α=P(A)/P(B), A and B are two different pure gases. TpPa-CF3/6FDA-ODA膜的气体选择性变化趋势和气体渗透性变化趋势不同,如图8(b)所示,CO2/N2和O2/N2均呈现出先下降后上升再下降的趋势,CO2/N2及O2/N2的理想选择性范围分别在16.8~24.4和3.8~4.8。其中,当TpPa-CF3负载量为5wt%时,混合基质膜的CO2/N2和O2/N2选择性最好,分别是6FDA-ODA膜的125%和119%。理想选择性的提高主要归因于两个方面:一个方面是CO2和O2的分子动力学直径要小于N2的分子动力学直径,从而CO2和O2分子倾向于优先通过。另一个方面,TpPa-CF3中富含大量对CO2具有亲和力的N、O和F等电负性原子,同时框架内还存在能与CO2发生偶极-四极相互作用的强极性C—F键,因此CO2/N2选择性相较于O2/N2的提升更明显[25]。然而,当负载量到7wt%时,混合基质膜的CO2/N2和O2/N2选择性大幅下降,略低于6FDA-ODA膜,这主要归因于当TpPa-CF3的负载量增加一定程度时,其在膜内发生团聚,并和聚合物基质产生部分相分离,产生一些非选择的孔,从而造成CO2/N2和O2/N2理想选择性的大幅下降。
对TpPa-CF3/6FDA-ODA混合基质膜进行72 h的连续气体渗透性测试,以验证膜的稳定性。如图8(c)所示,该膜在72 h的运行试验中P(CO2)下降了18%,CO2/N2的选择性下降了16%,总体表现出了良好的分离稳定性。
为了评估混合基质膜的气体分离性能,图9显示了不同负载量的TpPa-CF3/6FDA-ODA混合基质膜的气体分离性能与Robeson上限的对比。当负载量为5wt%时,其气体分离性能更靠近Robeson上限,气体的渗透性与选择性同步提升。说明适量的引入TpPa-CF3能够改善聚合物膜的气体分离性能。此外,表3显示了文献[26-30]中报道的混合基质膜气体分离性能与本工作的对比,TpPa-CF3/6FDA-ODA混合基质膜显示出适中的气体渗透性以及适中的气体选择性,说明TpPa-CF3/6FDA-ODA混合基质膜还有进一步提升的潜力。
表 3 文献中报道的混合基质膜气体分离性能与本工作的对比Table 3. Comparison of gas separation performance of mixed matrix membranes reported in the literature with the present workMembrane type P(CO2)/Barrer P(O2)/Barrer α(CO2/N2) α(O2/N2) Ref. TpPa-1-nc/Pebax 21 — 72 — [26] COFp-PVAm 270 — 86 — [27] TpBD@PBI-BuI 14.8 — 23 — [28] ZIF-7-I/(BPDA/6FDA-ODA) — 2.9 — 0.19 [29] PBI-PI-based carbon 293.5 93.1 8.3 2.6 [30] 5%TpPa-CF3/6FDA-ODA 31.08 6.08 24.4 4.8 This work Notes: TpPa-1-nc—; COFp—; TpBD—; BPDA—; Pebax—Poly(ether-block-amide); PVAm—Polyvinylamine; PBI-BuI—Tert-butylpolybenzimidazole; ZIF-7-I—Wide-pore ZIF-7; PBI—Polybenzimidazoles; PI—Polyimide. 3. 结 论
(1)采用溶剂热法合成了一种氟化共价有机框架材料(TpPa-CF3),其具有高比表面积(791.83 m2·g−1),较小且均一的孔径(1.18 nm)以及良好的热稳定性。
(2)采用共混法成功制备TpPa-CF3/聚酰亚胺(6FDA-ODA)混合基质膜。通过表征得出,所得膜具有良好的界面相容性以及较高的热稳定性(热分解温度在500℃左右)。水接触角的范围在81.4°~89.1°,且膜具有良好的力学性能,有利于膜在分离过程中的稳定性。
(3) TpPa-CF3的掺入提高了混合基质膜的气体渗透性,随着膜中TpPa-CF3负载量的增加,混合基质膜的气体渗透性呈现先减小后增大再减小的趋势。其中,5%TpPa-CF3/6FDA-ODA膜的气体分离性能最好,其CO2和O2的渗透性能分别提高了149%和138%,CO2/N2和O2/N2的分离性能分别是6FDA-ODA基体膜的125%和119%。
-
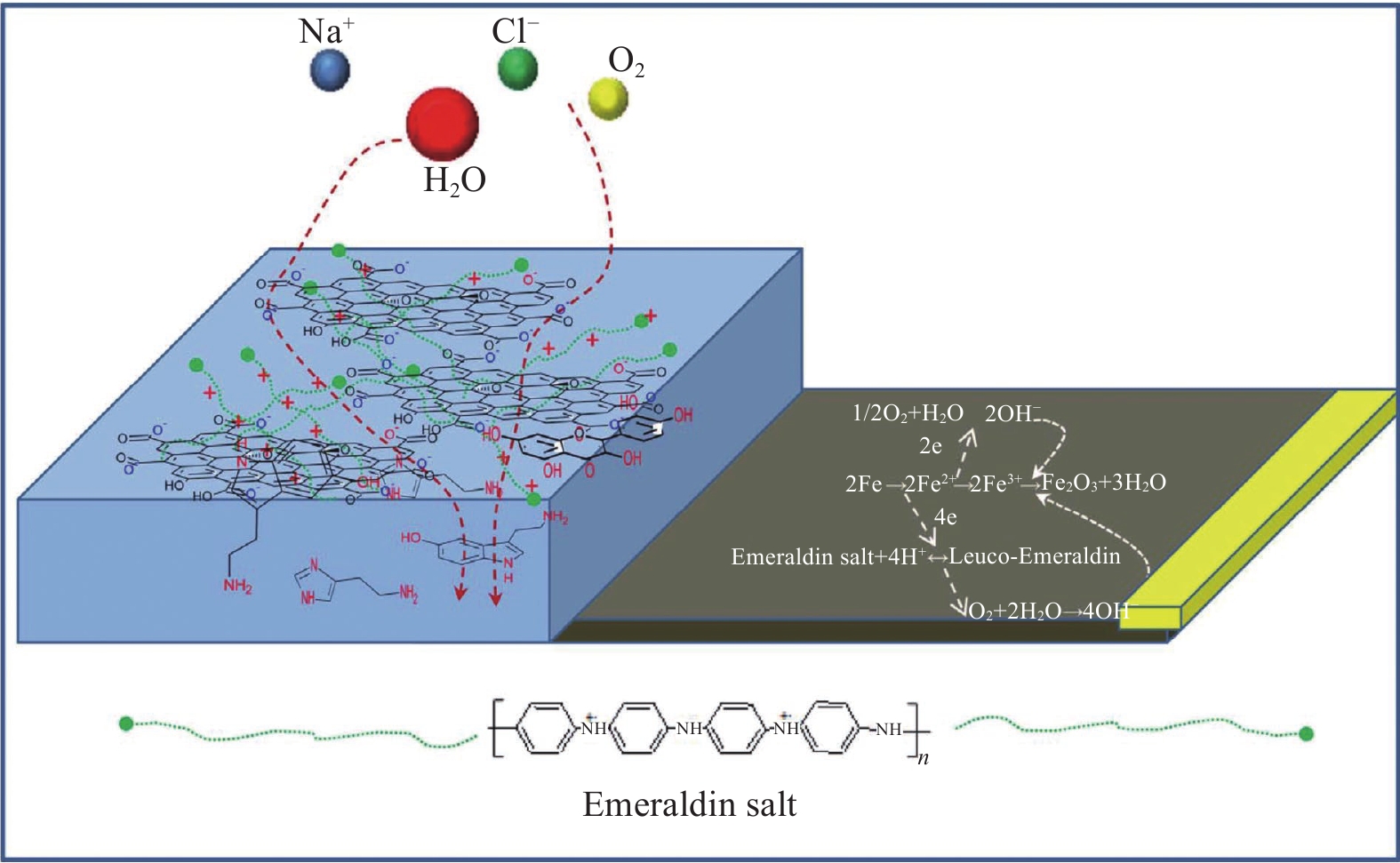
图 4 不同掺杂剂对PANI涂层防护性能和导电性的影响[45]
APS—Ammonium persulfate; PANI-HCl—Hydrochloric acid modified polyaniline; PANI-TSA—p-toluenesulfonic acid modified polyaniline; PANI-DBSA—Dodecylbenzenesulfonic acid modified polyaniline; EP—Epoxy; PEC—Polyaniline epoxy coating; CS—Carbon steel; I—Corrosion current density
Figure 4. Effect of different dopants on the protective properties and conductivity of PANI coatings[45]
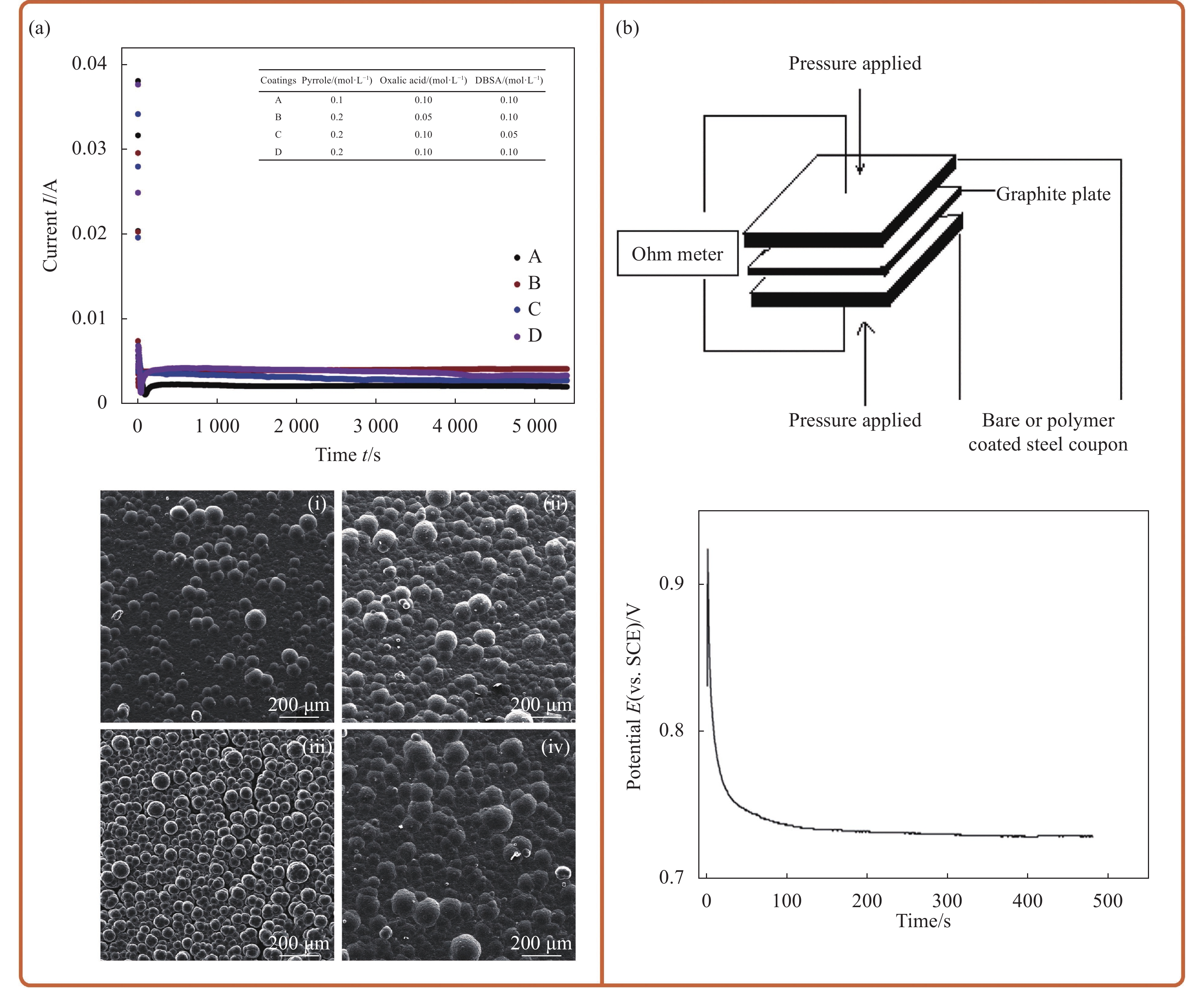
图 6 化学氧化法合成不同形态的复合材料:(a) PANI-PA-NFs/BTA[48];(b) SnO2/PPy/MoO4/PDA[31];(c) Tl2O3-SiO2/PANI[49];(d) GO-PANI-CeO2[50]
BTA—Benzotriazole; SDS—Sodium dodecylbenzene sulfonate; CPS—Counts per second
Figure 6. Composites of different forms synthesized by chemical oxidation method: (a) PANI-PA-NFs/BTA[48]; (b) SnO2/PPy/MoO4/PDA[31]; (c) Tl2O3-SiO2/PANI[49]; (d)GO-PANI-CeO2[50]
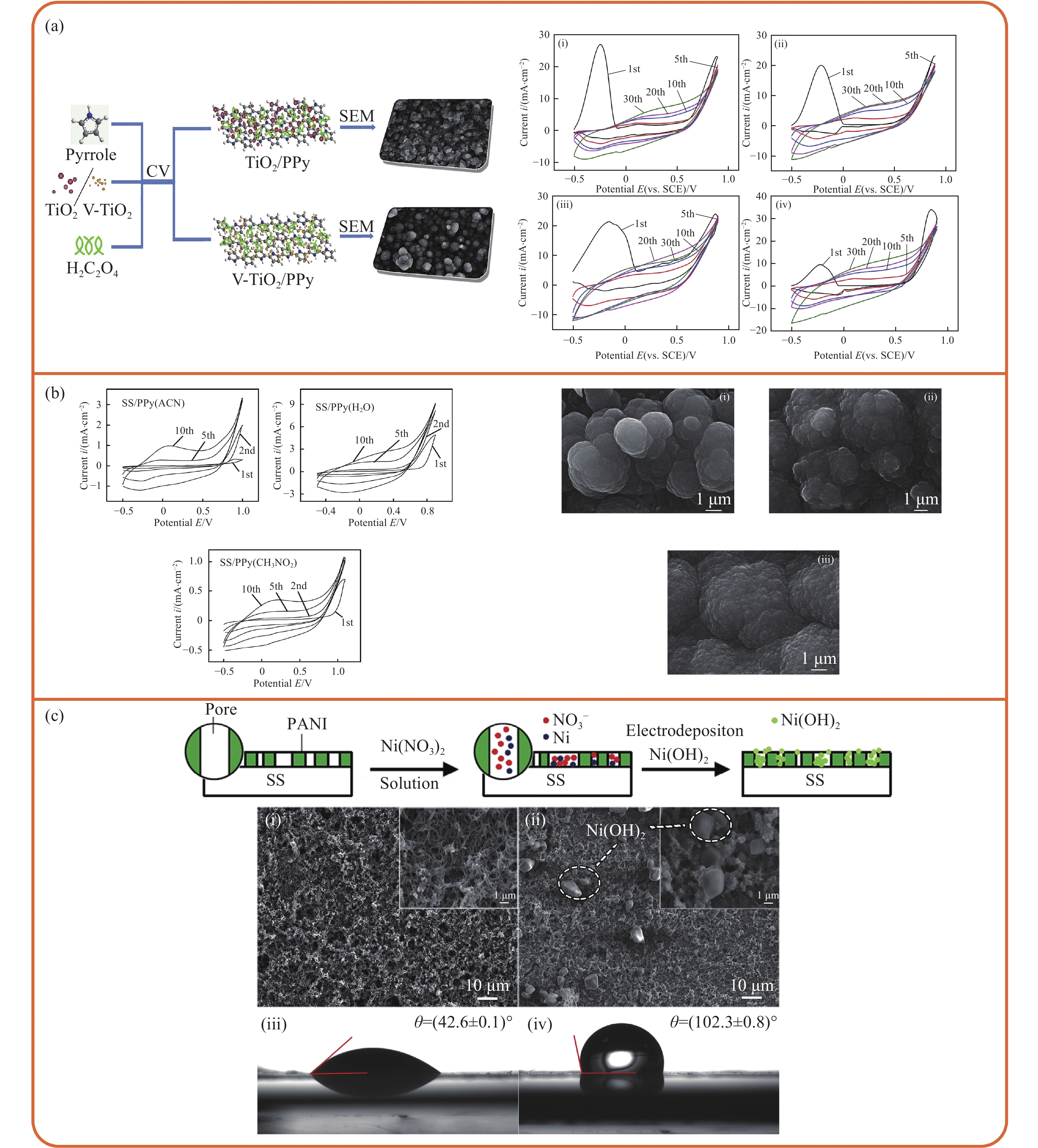
图 8 采用CV法合成的导电聚合物涂层:(a) TiO2和V-TiO2掺杂的PPy涂层[32];(b)水、乙腈和硝基甲烷三种溶液中合成PPy[55];(c) PANI-Ni(OH)2涂层[40]
CV—Cyclic voltammetric; ACN—Acetonitrile; θ—Contact angle; SS—Stainless steel
Figure 8. Conductive polymer coating synthesized by CV method: (a) TiO2 and V-TiO2 doped PPy coating[32]; (b) Synthesis of PPy in three solutions of water, acetonitrile and nitromethane[55]; (c) PANI-Ni(OH)2 coating[40]
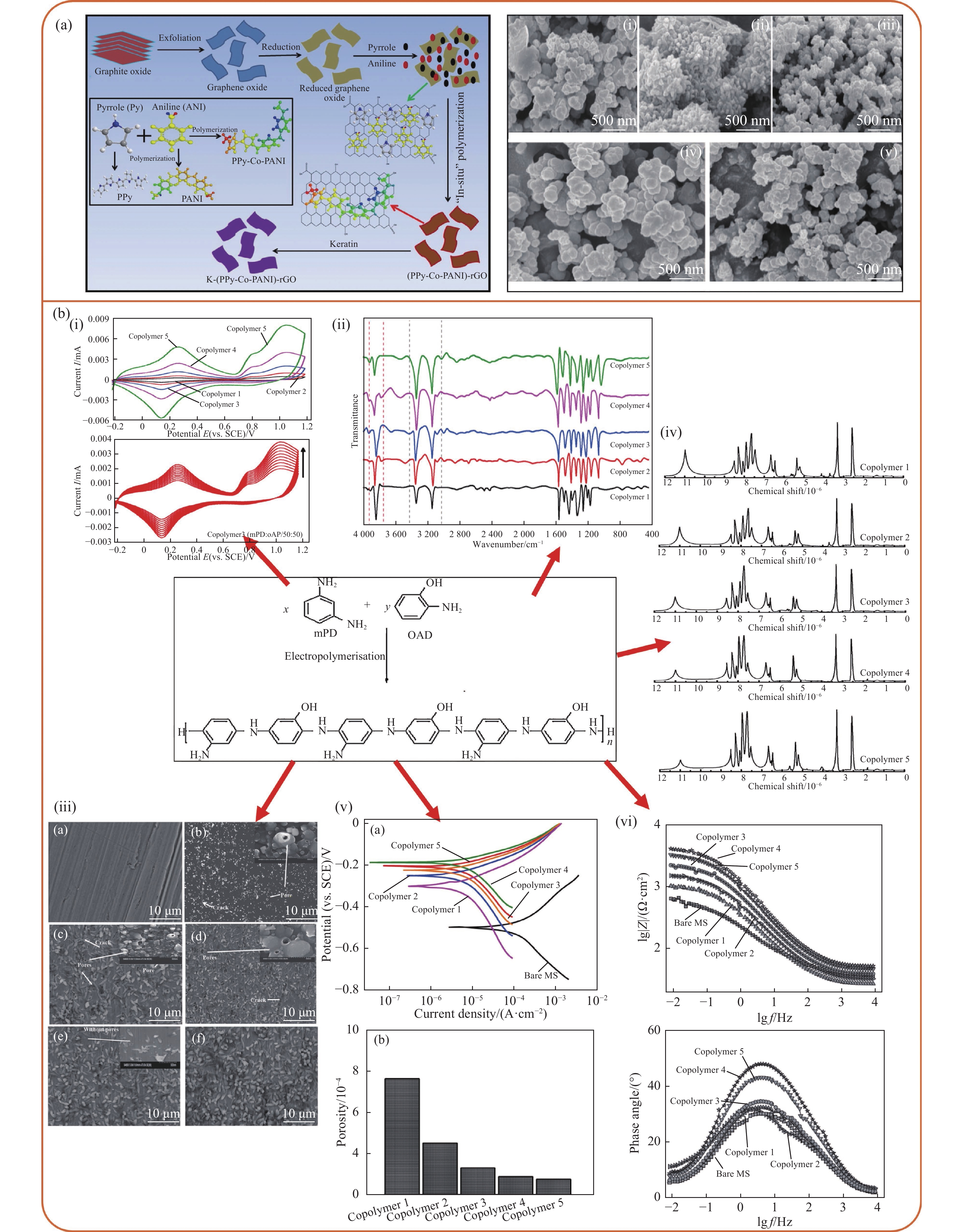
图 9 (a) 角蛋白修饰还原氧化石墨烯(rGO)负载(聚苯胺-共-聚吡咯)复合材料(K-(PPy-co-PANI)-rGO)[81];(b)聚(间苯二胺-共-邻氨基苯酚)共聚物涂层[82]
Z—Impedance; f—Frequency; MS—Mild steel
Figure 9. (a) Keratin modified reduced graphene oxide (rGO) loading (polyaniline-co-polypyrrole)copolymer composite(K-(PPy-co-PANI)-rGO)[81]; (b) Poly(m-phenylenediamine-co-o-aminophenol) copolymer coating[82]
表 1 双层或多层涂层的制备和防护效率
Table 1 Preparation and protection efficiency of double-layer or multi-layer coating
Coatings Substrate Preparation Corrosive environment Protection efficiency/% References PPy-SA-/PPy-MoO42−-NO3− 316 SS Potentiostatic 0.15 mol/L NaCl 91.2 [86] PNMA/PPy-DS− MS Cyclic voltammetry 0.5 mol/L HCl 97.4 [87] PANI/PNMA MS Cyclic voltammetry 3.5wt%NaCl 77.2 [88] PANI/PTH-DS− 304 SS Cyclic voltammetry 0.5 mol/L HCl 95.6 [89] PANI/Ni, PANI/Zn MS Cyclic voltammetry 3.5wt%NaCl 97.1 [91] PANI/PAA/PEI 316 SS Chemical oxidation 3.5wt%NaCl 94.7 [92] Notes: SA—Salicylic acid; PTH—Polythiophene; PNMA—Poly(N-methylaniline); PAA—Polyacrylic acid; PEI—Polyethyleneimine; 316 SS—316 stainless steel; DS–—Dodecylbenzene sulfonate. -
[1] ZHANG F, JU P, PAN M, et al. Self-healing mechanisms in smart protective coatings: A review[J]. Corrosion Science,2018,144:74-88. DOI: 10.1016/j.corsci.2018.08.005
[2] BOOPATHIRAJA R, PARTHIBAVARMAN M. Desert rose like heterostructure of NiCo2O4/NF@PPy composite has high stability and excellent electrochemical performance for asymmetric super capacitor application[J]. Electrochimica Acta, 2020, 346: 136270.
[3] SADEGHINIA M, SHAYEH J S, FATEMI F, et al. Electrochemical study of perlite-barium ferrite/conductive polymer nano composite for super capacitor applications[J]. International Journal of Hydrogen Energy,2019,44(52):28088-28095. DOI: 10.1016/j.ijhydene.2019.09.085
[4] HUSSAIN S K, DUDEM B, YU J S. Enhanced electrochemical performance via PPy encapsulated 3D flower-like bismuth molybdate nanoplates for high-performance supercapacitors[J]. Applied Surface Science,2019,478:846-856. DOI: 10.1016/j.apsusc.2019.01.196
[5] JAMNANI S R, MOGHADDAM H M, LEONARDI S G, et al. PANI/Sm2O3 nanocomposite sensor for fast hydrogen detection at room temperature[J]. Synthetic Metals,2020,268:116493. DOI: 10.1016/j.synthmet.2020.116493
[6] LV D, SHEN W, CHEN W, et al. PSS-PANI/PVDF composite based flexible NH3 sensors with sub-ppm detection at room temperature[J]. Sensors and Actuators B: Chemical,2021,328:129085. DOI: 10.1016/j.snb.2020.129085
[7] YU W, ZHANG G, LIU Y, et al. Selective electromagnetic interference shielding performance and superior mechanical strength of conductive polymer composites with oriented segregated conductive networks[J]. Chemical Engineering Journal,2019,373:556-564. DOI: 10.1016/j.cej.2019.05.074
[8] 高利民, 骈岩杰, 杨建光, 等. 掺杂聚苯胺制备及其在水性防腐防静电涂料中的应用[J]. 涂料工业, 2009, 39(5):14-18. DOI: 10.3969/j.issn.0253-4312.2009.05.004 GAO Liming, PIAO Yanjie, YANG Jianguang, et al. Preparation of doped-polyaniline and its application in waterborne anticorrosive and antistatic epoxy coatings[J]. Paint & Coatings Industry,2009,39(5):14-18(in Chinese). DOI: 10.3969/j.issn.0253-4312.2009.05.004
[9] 易波, 王群, 郭红霞. 聚苯胺/环氧树脂防静电涂料的研制[J]. 化工新型材料, 2010(6):112-114. DOI: 10.3969/j.issn.1006-3536.2010.06.039 YI Bo, WANG Qun, GUO Hongxia. Development research of electrostatic conductive coating of polyaniline/epoxy resin[J]. New Chemical Materials,2010(6):112-114(in Chinese). DOI: 10.3969/j.issn.1006-3536.2010.06.039
[10] KAMRAN M, ULLAH H, SHAH A A, et al. Combined experimental and theoretical study of poly(aniline-co-pyrrole) oligomer[J]. Polymer,2015,72:30-39. DOI: 10.1016/j.polymer.2015.07.003
[11] SAMBYAL P, RUHI G, DHAWAN S K, et al. Enhanced anticorrosive properties of tailored poly(aniline-anisidine)/chitosan/SiO2 composite for protection of mild steel in aggressive marine conditions[J]. Progress in Organic Coatings,2018,119:203-213. DOI: 10.1016/j.porgcoat.2018.02.014
[12] HU C, LI Y, YIN Y, et al. Fabrication of poly(alkyl-aniline)-SiC/zinc bilayer coatings and evaluation of their corrosion resistance properties[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2018,551:137-147.
[13] ZHU A, WANG H, ZHANG C, et al. A facile, solvent-free and scalable method to prepare poly(aniline-co-5-aminosalicylic acid) with enhanced electrochemical activity for corrosion protection[J]. Progress in Organic Coatings,2017,112:109-117. DOI: 10.1016/j.porgcoat.2017.07.006
[14] DENG F, MIN L, LUO X, et al. Visible-light photocatalytic degradation performances and thermal stability due to the synergetic effect of TiO2 with conductive copolymers of polyaniline and polypyrrole[J]. Nanoscale,2013,5(18):8703-8710. DOI: 10.1039/c3nr02502k
[15] SHIRAKAWA H, LOUIS E J, MACDIARMID A G, et al. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene[J]. Journal of the Chemical Society, Chemical Communications,1977(16):578-580. DOI: 10.1039/c39770000578
[16] 狄婧, 刘海霞, 姜永强, 等. 聚吡咯/壳聚糖复合膜的制备及其对Cu(Ⅱ)和Cr(Ⅵ)吸附机制[J]. 复合材料学报, 2021, 38(1):221-231. DI Jing, LIU Haixia, JIANG Yongqiang, et al. Preparation of polypyrrole/chitosan composite membrane and its adsorption mechanism for Cu(II) and Cr(VI)[J]. AMCS,2021,38(1):221-231(in Chinese).
[17] 王杰, 李莹, 邵亮, 等. 聚乙烯醇/聚吡咯复合导电水凝胶应变传感器的制备及性能[J]. 高等学校化学学报, 2021, 42(3):929-936. WANG Jie, LI Ying, SHAO Liang, et al. Preparation and properties of polyvinyl alcohol/polypyrrole composite conductive hydrogel strain sensor[J]. Chemical Journal of Chinese Universities,2021,42(3):929-936(in Chinese).
[18] 冯江涛, 王睎, 赵旭阳, 等. 改性聚吡咯材料去除水中氟离子的性能[J]. 化工进展, 2021, 40(7):4036-4046. FENG Jiangtao, WANG Hui, ZHAO Xuyang, et al. Performance of modified polypyrrole materials in removing fluoride ions in water[J]. Chemical Industry and Engineering Progress,2021,40(7):4036-4046(in Chinese).
[19] 李晓丹, 刘小平, 刘宏宇, 等. 原位聚合法制备聚苯胺改性MoS2及其环氧复合涂层的防腐性能[J]. 材料科学与工艺, 2021, 29(6):18-26. DOI: 10.11951/j.issn.1005-0299.20210186 LI Xiaodan, LIU Xiaoping, LIU Hongyu, et al. Corrosion resistance of polyaniline modified MoS2 and its epoxy composite coating prepared by in situ polymerization[J]. Materials Science and Technology,2021,29(6):18-26(in Chinese). DOI: 10.11951/j.issn.1005-0299.20210186
[20] 甘孟瑜, 李志春, 马利, 等. 聚苯胺的表面修饰及防腐性能[J]. 高等学校化学学报, 2012, 33(3):630-634. DOI: 10.3969/j.issn.0251-0790.2012.03.037 GAN Mengyu, LI Zhichun, MA Li, et al. Surface modification and antiseptic properties of polyaniline[J]. Chemical Journal of Chinese Universities,2012,33(3):630-634(in Chinese). DOI: 10.3969/j.issn.0251-0790.2012.03.037
[21] 魏治洋, 邹飞林, 赖竹林, 等. 植酸掺杂聚苯胺对水中Cr(Ⅵ)的去除研究[J]. 环境科学研究, 2021, 34(2):337-345. WEI Zhiyang, ZOU Feilin, LAI Zhulin, et al. Removal of Cr(VI) from water by phytic acid-doped polyaniline[J]. Environmental Science Research,2021,34(2):337-345(in Chinese).
[22] MARTI M, ARMELIN E, IRIBARREN J I, et al. Soluble polythiophenes as anticorrosive additives for marine epoxy paints[J]. Materials and Corrosion-Werkstoffe Und Korrosion,2015,66(1):23-30. DOI: 10.1002/maco.201307132
[23] 徐守斌, 江龙, 杨海刚, 等. 光诱导聚合制备聚噻吩/二氧化钛复合粒子的结构及光催化性能[J]. 催化学报, 2011, 32(4):536-545. XU Shoubin, JIANG Long, YANG Haigang, et al. Structure and photocatalytic performance of polythiophene/titanium dioxide composite particles prepared by photo-induced polymerization[J]. Chinese Journal of Catalysis,2011,32(4):536-545(in Chinese).
[24] 李宝铭, 吴洪才, 刘效增, 等. 离子注入改性聚对苯乙炔的光学及电学性能[J]. 高分子材料科学与工程, 2005, 21(5):114-117. DOI: 10.3321/j.issn:1000-7555.2005.05.029 LI Baoming, WU Hongcai, LIU Xiaozeng, et al. Optical and electrical properties of ion implantation modified polytextrine[J]. Polymer Materials Science and Engineering,2005,21(5):114-117(in Chinese). DOI: 10.3321/j.issn:1000-7555.2005.05.029
[25] 孙建平, 马琳璞, 黄小珠. 聚对苯乙炔二元共聚物P(DHOPV-co-MOBOPV)的制备与性能[J]. 高分子材料科学与工程, 2011, 27(6):162-165. SUN Jianping, MA Linpu, HUANG Xiaozhu. Preparation and properties of poly-p-phenylacetylene binary copolymer P(DHOPV-co-MOBOPV)[J]. Polymer Materials Science and Engineering,2011,27(6):162-165(in Chinese).
[26] GIL L K, BACA E, MORÁN O, et al. Influence of polyparaphenylene on the magneto transport of manganite/polymer composites[J]. Physica B: Condensed Matter,2008,403(10):1813-1818.
[27] GOLOVTSOV I, BEREZNEV S, TRAKSMAA R, et al. Thin composite films consisting of polypyrrole and polyparaphenylene[J]. Thin Solid Films,2007,515(19):7712-7715. DOI: 10.1016/j.tsf.2006.11.098
[28] 路亮, 侯文鹏, 关士友, 等. 聚吡咯的绿色制备及其环氧树脂复合涂层对Q235钢的防腐性能[J]. 化工进展, 2013, 32(3):617-623. LU Liang, HOU Wenpeng, GUAN Shiyou, et al. Green route to prepare polypyrrole and the anticorrosion property of polypyrrole-epoxy composite coating on Q235 steel[J]. Progress in Chemical Industry,2013,32(3):617-623(in Chinese).
[29] DINIZ F B, DE ANDRADE G F, MARTINS C R, et al. A comparative study of epoxy and polyurethane based coatings containing polyaniline-DBSA pigments for corrosion protection on mild steel[J]. Progress in Organic Coatings,2013,76(5):912-916. DOI: 10.1016/j.porgcoat.2013.02.010
[30] CHEN Z, JIN Y, YANG W, et al. Fabrication and characterization of polypyrrole coatings by embedding antimony modified SnO2 nanoparticles[J]. Journal of Industrial and Engineering Chemistry,2019,75:178-186. DOI: 10.1016/j.jiec.2019.03.021
[31] CHEN Z, YANG W, CHEN Y, et al. Smart coatings embedded with polydopamine-decorated layer-by-layer assembled SnO2 nanocontainers for the corrosion protection of 304 stainless steels[J]. Journal of Colloid and Interface Science,2020,579:741-753. DOI: 10.1016/j.jcis.2020.06.118
[32] CHEN Z, YANG W, XU B, et al. Corrosion protection of carbon steels by electrochemically synthesized V-TiO2/polypyrrole composite coatings in 0.1 M HCl solution[J]. Journal of Alloys and Compounds,2019,771:857-868. DOI: 10.1016/j.jallcom.2018.09.003
[33] CHEN Z, YANG W, XU B, et al. Corrosion behaviors and physical properties of polypyrrole-molybdate coating electropolymerized on carbon steel[J]. Progress in Organic Coatings,2018,122:159-169. DOI: 10.1016/j.porgcoat.2018.05.022
[34] CHEN Z, YANG W, YIN X, et al. Corrosion protection of 304 stainless steel from a smart conducting polypyrrole coating doped with pH-sensitive molybdate-loaded TiO2 nanocontainers[J]. Progress in Organic Coatings,2020,146:105750. DOI: 10.1016/j.porgcoat.2020.105750
[35] CHEN Z, ZHANG G, YANG W, et al. Superior conducting polypyrrole anti-corrosion coating containing functionalized carbon powders for 304 stainless steel bipolar plates in proton exchange membrane fuel cells[J]. Chemical Engineering Journal,2020,393:124675. DOI: 10.1016/j.cej.2020.124675
[36] HAYATGHEIB Y, RAMEZANZADEH B, KARDAR P, et al. A comparative study on fabrication of a highly effective corrosion protective system based on graphene oxide-polyaniline nanofibers/epoxy composite[J]. Corrosion Science,2018,133:358-373. DOI: 10.1016/j.corsci.2018.01.046
[37] HERMAS A A. XPS analysis of the passive film formed on austenitic stainless steel coated with conductive polymer[J]. Corrosion Science,2008,50(9):2498-2505. DOI: 10.1016/j.corsci.2008.06.019
[38] REN Y J, CHEN J, ZENG C L, et al. Electrochemical corrosion characteristics of conducting polypyrrole/polyaniline coatings in simulated environments of a proton exchange membrane fuel cell[J]. International Journal of Hydrogen Energy,2016,41(20):8542-8549. DOI: 10.1016/j.ijhydene.2016.03.184
[39] REN Y J, ZENG C L. Effect of conducting composite polypyrrole/polyaniline coatings on the corrosion resistance of type 304 stainless steel for bipolar plates of proton-exchange membrane fuel cells[J]. Journal of Power Sources,2008,182(2):524-530. DOI: 10.1016/j.jpowsour.2008.04.056
[40] JIANG L, SYED J A, GAO Y, et al. Electrodeposition of Ni(OH)2 reinforced polyaniline coating for corrosion protection of 304 stainless steel[J]. Applied Surface Science,2018,440:1011-1021. DOI: 10.1016/j.apsusc.2018.01.145
[41] JIANG L, SYED J A. In-situ electrodeposition of conductive polypyrrole-graphene oxide composite coating for corrosion protection of 304 SS bipolar plates[J]. Journal of Alloys and Compounds,2019,770:35-47. DOI: 10.1016/j.jallcom.2018.07.277
[42] JIANG L, SYED J A, ZHANG G, et al. Enhanced anticorrosion performance of PPY-graphene oxide/PPY-camphorsulfonic acid composite coating for 304 SS bipolar plates in proton exchange membrane fuel cell[J]. Journal of Industrial and Engineering Chemistry,2019,80:497-507. DOI: 10.1016/j.jiec.2019.08.032
[43] WANG Y, ZHANG S, WANG P, et al. Synthesis and corrosion protection of Nb doped TiO2 nanopowders modified polyaniline coating on 316 stainless steel bipolar plates for proton-exchange membrane fuel cells[J]. Progress in Organic Coatings,2019,137:105327. DOI: 10.1016/j.porgcoat.2019.105327
[44] HUNG H M, LINH D K, CHINH N T, et al. Improvement of the corrosion protection of polypyrrole coating for CT3 mild steel with 10-camphor sulfonic acid and molyb-date as inhibitor dopants[J]. Progress in Organic Coatings,2019,131:407-416. DOI: 10.1016/j.porgcoat.2019.03.006
[45] HU C, LI T, YIN H, et al. Preparation and corrosion protection of three different acids doped polyaniline/epoxy resin composite coatings on carbon steel[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2021:612.
[46] XU J, ZHANG Y, ZHANG D, et al. Electrosynthesis of PANI/PPy coatings doped by phosphotungstate on mild steel and their corrosion resistances[J]. Progress in Orga-nic Coatings,2015,88:84-91. DOI: 10.1016/j.porgcoat.2015.06.024
[47] LIANG B, QIN Z, ZHAO J, et al. Controlled synthesis, core-shell structures and electrochemical properties of polyaniline/polypyrrole composite nanofibers[J]. Journal of Materials Chemistry A,2014,2(7):2129-2135. DOI: 10.1039/C3TA14460G
[48] HAO Y, ZHAO Y, YANG X, et al. Self-healing epoxy coating loaded with phytic acid doped polyaniline nanofibers impregnated with benzotriazole for Q235 carbon steel[J]. Corrosion Science,2019,151:175-189. DOI: 10.1016/j.corsci.2019.02.023
[49] HANY M, KHALAF M. Novel dispersed Tl2O3-SiO2/polyaniline nanocomposites: In-situ polymerization, characterization and enforcement as a corrosion protective layer for carbon-steel in acidic chloride medium[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2019,573:95-111.
[50] BAHRAM R, BAHLAKEH G, RAMEZANZADEH M. Polyaniline-cerium oxide (PANI-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild steel[J]. Corrosion Science,2018,137:111-126. DOI: 10.1016/j.corsci.2018.03.038
[51] POUR-ALI S, DEHGHANIAN C, KOSARI A. In situ synthesis of polyaniline-camphorsulfonate particles in an epoxy matrix for corrosion protection of mild steel in NaCl solution[J]. Corrosion Science,2014,85:204-214. DOI: 10.1016/j.corsci.2014.04.018
[52] LEHR I L, SAIDMAN S B. Characterisation and corrosion protection properties of polypyrrole electropolymerised onto aluminium in the presence of molybdate and nitrate[J]. Electrochimica Acta,2006,51(16):3249-3255. DOI: 10.1016/j.electacta.2005.09.017
[53] MERVE M, OZKAZANC H. Electrodeposition of polypyrrole on copper surfaces in OXA-DBSA mix electrolyte and their corrosion behaviour[J]. Progress in Organic Coatings,2019,130:149-157. DOI: 10.1016/j.porgcoat.2019.01.058
[54] KAMARAJ K, KARPAKAM V, SATHIYANARAYANAN S, et al. Synthesis of tungstate doped polyaniline and its usefulness in corrosion protective coatings[J]. Electrochimica Acta,2011,56(25):9262-9268. DOI: 10.1016/j.electacta.2011.08.005
[55] YAN Q, PAN W, ZHONG S, et al. Effect of solvents on the preparation and corrosion protection of polypyrrole[J]. Progress in Organic Coatings,2019,132:298-304. DOI: 10.1016/j.porgcoat.2019.04.014
[56] OZYILMAZ A T, AVSAR B, OZYILMAZ G, et al. Different copolymer films on ZnFeCo particles: Synthesis and anticorrosion properties[J]. Applied Surface Science,2014,318:262-268. DOI: 10.1016/j.apsusc.2014.04.177
[57] 曹祥康, 孙晓光, 肖松, 等. 聚苯并噁嗪基三维超疏水涂层的制备及抗磨损腐蚀性能[J]. 复合材料学报, 2022, 39(2):617-627. CAO Xiangkang, SUN Xiaoguang, XIAO Song, et al. Preparation and wear corrosion resistance of polybenzoxazine 3D superhydrophobic coating[J]. Acta Materiae Compositae Sinica,2022,39(2):617-627(in Chinese).
[58] 汪雨微, 欧宝立, 鲁忆, 等. 功能化纳米TiO2/环氧树脂超疏水防腐复合涂层的制备与性能[J]. 复合材料学报, 2021, 38(12):3971-3985. WANG Yuwei, Opelli, LU Yi, et al. Preparation and properties of functionalized nano TiO2/epoxy superhydrophobic and anti-corrosion composite coatings[J]. Acta Materiae Compositae Sinica,2021,38(12):3971-3985(in Chinese).
[59] LEI Y, SHENG N, HYONO A, et al. Influence of pH on the synthesis and properties of polypyrrole on copper from phytic acid solution for corrosion protection[J]. Progress in Organic Coatings,2014,77(4):774-784. DOI: 10.1016/j.porgcoat.2014.01.002
[60] PLESU N, ILIA G, PASCARIU A, et al. Preparation, degradation of polyaniline doped with organic phosphorus acids and corrosion essays of poly aniline-acrylic blends[J]. Synthetic Metals,2006,156(2-4):230-238. DOI: 10.1016/j.synthmet.2005.11.006
[61] 宋航, 李栋, 王金福, 等. 共掺杂态聚苯胺/环氧树脂复合涂层的耐蚀性分析[J]. 全面腐蚀控制, 2020, 34(12):19-27. SONG Hang, LI Dong, WANG Jinfu, et al. Corrosion resistance analysis of Co-doped polyaniline/epoxy composite coatings[J]. Total Corrosion Control,2020,34(12):19-27(in Chinese).
[62] 宋航, 王华, 钱晨. 掺杂态聚苯胺的乳液聚合及其与环氧树脂所制涂层的耐蚀性[J]. 电镀与涂饰, 2018, 37(2):54-60. SONG Hang, WANG Hua, QIAN Chen. Emulsion polymerization of doped polyaniline and corrosion resistance of coatings made with epoxy resin[J]. Electroplating and Finishing,2018,37(2):54-60(in Chinese).
[63] JAFARZADEH S, ADHIKARI A, SUNDALL P E, et al. Study of PANI-MeSA conducting polymer dispersed in UV-curing polyester acrylate on galvanized steel as corrosion protection coating[J]. Progress in Organic Coatings,2011,70(2):108-115.
[64] ADHIKARI A, CLAESSON P, PAN J, et al. Electrochemical behavior and anticorrosion properties of modified polyaniline dispersed in polyvinylacetate coating on carbon steel[J]. Electrochimica Acta,2008,53(12):4239-4247. DOI: 10.1016/j.electacta.2007.12.069
[65] SAMUI A B, PHADNIS S M. Polyaniline-dioctyl phosphate salt for corrosion protection of iron[J]. Progress in Orga-nic Coatings,2005,54(3):263-267. DOI: 10.1016/j.porgcoat.2005.07.002
[66] HUANG H Y, HUANG T C, LIN J C, et al. Advanced environmentally friendly coatings prepared from amine-capped aniline trimer-based waterborne electroactive polyurethane[J]. Materials Chemistry and Physics,2013,137(3):772-780. DOI: 10.1016/j.matchemphys.2012.09.063
[67] PENG C, HSU C, LIN K H, et al. Electrochemical corrosion protection studies of aniline-capped aniline trimer-based electroactive polyurethane coatings[J]. Electrochimica Acta,2011,58:614-620. DOI: 10.1016/j.electacta.2011.10.002
[68] 刘玮, 安成强, 郝建军, 等. 钼酸钠对AZ91 D镁合金钒/锆复合转化膜性能的影响[J]. 电镀与精饰, 2019, 41(8):10-13. DOI: 10.3969/j.issn.1001-3849.2019.08.003 LIU Wei, AN Chengqiang, HAO Jianjun, et al. Effect of sodium molybdate on the properties of vanadium/zirconium composite transformation film of AZ91 D magnesium alloy[J]. Electroplating and Finishing,2019,41(8):10-13(in Chinese). DOI: 10.3969/j.issn.1001-3849.2019.08.003
[69] 伍良银, 姜雪婷, 张菊, 等. AZ31镁合金表面钼酸盐-高铁酸盐转化膜的制备及腐蚀行为研究[J]. 材料保护, 2020, 53(9):70-75, 81. WU Liangyin, JIANG Xueting, ZHANG Ju, et al. Preparation and corrosion behavior of molybdenate-perferrate conversion film on the surface of AZ31 magnesium alloy[J]. Materials Protection,2020,53(9):70-75, 81(in Chinese).
[70] 程明月, 林颖菲, 王超, 等. Al2O3溶胶对铝基磷酸盐涂层组织及性能的影响[J]. 涂料工业, 2021, 51(9):1-8. DOI: 10.12020/j.issn.0253-4312.2021.9.1 CHENG Mingyue, LIN Yingfei, WANG Chao, et al. Effect of Al2O3 sol on microstructure and properties of aluminum-based phosphate coating[J]. Coatings Industry,2021,51(9):1-8(in Chinese). DOI: 10.12020/j.issn.0253-4312.2021.9.1
[71] 曾荣昌, 胡艳, 张芬, 等. AZ31镁合金表面铈掺杂锌钙磷酸盐化学转化膜的腐蚀性能[J]. 中国有色金属学报, 2016(2):472-483. ZENG Rongchang, HU Yan, ZHANG Fen, et al. Corrosion properties of cerium-doped zinc-calcium phosphate chemical conversion film on the surface of AZ31 magnesium alloy[J]. Chinese Journal of Nonferrous Metals,2016(2):472-483(in Chinese).
[72] JENSEN M B, PETERSON M J, JADHAV N, et al. SECM investigation of corrosion inhibition by tungstate- and vanadate-doped polypyrrole/aluminum flake composite coatings on AA2024-T3[J]. Progress in Organic Coatings,2014,77(12):2116-2122. DOI: 10.1016/j.porgcoat.2014.05.019
[73] 杨浩, 王成, 肖小波, 等. 添加硼酸和钨酸铵对取向硅钢无铬绝缘涂层的影响[J]. 材料导报, 2021, 35(22):22141-22145. DOI: 10.11896/cldb.20080274 YANG Hao, WANG Cheng, XIAO Xiaobo, et al. Effect of addition of boric acid and ammonium tungstate on chromium-free insulation coating of oriented silicon steel[J]. Materials Reports,2021,35(22):22141-22145(in Chinese). DOI: 10.11896/cldb.20080274
[74] 向可友, 杨晓波, 肖革, 等. 几种元素在金属表面化学转化中的作用研究进展[J]. 电镀与涂饰, 2022, 41(2):96-102. XIANG Keyou, YANG Xiaobo, XIAO Ge, et al. Research progress on the role of several elements in the chemical transformation of metal surfaces[J]. Electroplating and Finishing,2022,41(2):96-102(in Chinese).
[75] KAMARAJ K, SATHIYANARAYANAN S, VENKATACHARI G. Electropolymerised polyaniline films on AA 7075 alloy and its corrosion protection performance[J]. Progress in Organic Coatings,2009,64(1):67-73. DOI: 10.1016/j.porgcoat.2008.07.008
[76] SATHIYANARAYANAN S, DEVI S, VENKATACHARI G. Corrosion protection of stainless steel by electropolymerised PANI coating[J]. Progress in Organic Coatings,2006,56(2-3):114-119. DOI: 10.1016/j.porgcoat.2006.01.003
[77] 刘学佳, 王伟龙, 张云霞, 等. 苯甲酸钠对双相不锈钢在草酸溶液中腐蚀行为的影响[J]. 当代化工, 2021, 50(5):1064-1068. DOI: 10.3969/j.issn.1671-0460.2021.05.016 LIU Xuejia, WANG Weilong, ZHANG Yunxia, et al. Effect of sodium benzoate on corrosion behavior of duplex stainless steel in oxalic acid solution[J]. Contemporary Chemi-cal Industry,2021,50(5):1064-1068(in Chinese). DOI: 10.3969/j.issn.1671-0460.2021.05.016
[78] BONASTRE J, GARCÉS P, GALVÁN J C, et al. Characterisation and corrosion studies of steel electrodes covered by polypyrrole/phosphotungstate using electrochemical impedance spectroscopy[J]. Progress in Organic Coatings,2009,66(3):235-241. DOI: 10.1016/j.porgcoat.2009.07.012
[79] GOPI D, KARTHIKEYAN P, KAVITHA L, et al. Development of poly(3, 4-ethylenedioxythiophene-co-indole-5-carboxylic acid) co-polymer coatings on passivated low-nickel stainless steel for enhanced corrosion resistance in the sulphuric acid medium[J]. Applied Surface Science,2015,357:122-130. DOI: 10.1016/j.apsusc.2015.09.001
[80] SIVA T, KAMARAJ K, SATHIYANARAYANAN S. Electrosynthesis of poly(aniline-co-o-phenylenediamine) film on steel and its corrosion protection performance[J]. Progress in Organic Coatings,2014,77(11):1807-1815. DOI: 10.1016/j.porgcoat.2014.06.003
[81] PATTANAYAK P, PRAMANIK N, PAPIYA F, et al. Metal-free keratin modified poly(pyrrole-co-aniline)-reduced graphene oxide based nanocomposite materials: A pro-mising cathode catalyst in microbial fuel cell application[J]. Journal of Environmental Chemical Engi-neering,2020,8(3):103813. DOI: 10.1016/j.jece.2020.103813
[82] MADHANKUMAR A, RAJENDRAN N. Poly(m-phenylen diamine-co-o-aminophenol) coatings on mild steel: Effect of comonomers feed ratio on surface and corrosion protection aspects[J]. Progress in Organic Coatings,2013,76(10):1445-1453. DOI: 10.1016/j.porgcoat.2013.05.033
[83] WANG H, ZHANG P, FEI G, et al. Design and properties of environmental anticorrosion coating based on m-aminobenzenesulfonic acid/aniline/p-phenylenediamine terpolymer[J]. Progress in Organic Coatings,2019,137:105274. DOI: 10.1016/j.porgcoat.2019.105274
[84] JIN Y, CHEN Z, YANG W, et al. Electrochemical and quantum mechanical investigation of various small molecule organic compounds as corrosion inhibitors in mild steel[J]. Journal of the Taiwan Institute of Chemical Engi-neers, 2020, 117: 171-181
[85] OWCZAREK E, ADAMCZYK L. Electrochemical and anticorrosion properties of bilayer polyrhodanine/isobutyltriethoxysilane coatings[J]. Journal of Applied Electroche-mistry,2016,46(6):635-643. DOI: 10.1007/s10800-016-0946-0
[86] GONZÁLEZ M B, SAIDMAN S B. Electrodeposition of bilayered polypyrrole on 316 L stainless steel for corrosion prevention[J]. Progress in Organic Coatings,2015,78:21-27. DOI: 10.1016/j.porgcoat.2014.10.012
[87] ZEYBEK B, ÖZÇIÇEK N, KILIÇ E. Electrochemical synthesis of bilayer coatings of poly(N-methylaniline) and polypyrrole on mild steel and their corrosion protection performances[J]. Electrochimica Acta,2011,56(25):9277-9286. DOI: 10.1016/j.electacta.2011.08.003
[88] NARAYANASAMY B, RAJENDRAN S. Electropoly-merized bilayer coatings of polyaniline and poly(N-methylaniline) on mild steel and their corrosion protection performance[J]. Progress in Organic Coatings,2010,67(3):246-254. DOI: 10.1016/j.porgcoat.2009.12.001
[89] PEKMEZ N Ö, ABACI E, CINKILLI K, et al. Polybithiophene and its bilayers with polyaniline coatings on stainless steel by electropolymerization in aqueous medium[J]. Progress in Organic Coatings,2009,65(4):462-468. DOI: 10.1016/j.porgcoat.2009.04.012
[90] HASANOV R, BILGIC S. Monolayer and bilayer conducting polymer coatings for corrosion protection of steel in 1 M H2SO4 solution[J]. Progress in Organic Coatings,2009,64(4):435-445. DOI: 10.1016/j.porgcoat.2008.08.004
[91] KUMAR S A, MEENAKSHI K S, SANKARANARAYANAN T S N, et al. Corrosion resistant behaviour of PANI-metal bilayer coatings[J]. Progress in Organic Coatings,2008,62(3):285-292. DOI: 10.1016/j.porgcoat.2008.01.005
[92] SYED J A, TANG S, MENG X. Intelligent saline enabled self-healing of multilayer coatings and its optimization to achieve redox catalytically provoked anti-corrosion ability[J]. Applied Surface Science,2016,383:177-190. DOI: 10.1016/j.apsusc.2016.04.178
-





 下载:
下载: