Effect of interfacial reaction on wear properties of Cu35Ni25Co25Cr15 multi-principal components alloy/diamond composites
-
摘要: 金刚石超硬磨具在高端芯片加工、3C陶瓷等领域发挥的作用日益重要,粘结相与金刚石的界面结合情况在很大程度上影响了金刚石超硬复合材料的力学和磨损性能。为了研究粘结相和金刚石的界面结合情况,采用放电等离子烧结方法制备了Cu35Ni25Co25Cr15多主元合金/金刚石复合材料,通过热力学计算和实验研究了粘结相和金刚石颗粒的界面反应。结果表明:烧结过程中,金属粘结相中的Cr元素与金刚石在界面处发生了化学反应,生成Cr—C化合物,且Cr—C化合物层的厚度随着烧结温度的升高而增加。当烧结温度达到950℃时,Cr—C化合物反应层均匀连续,厚度大约为1.1 μm。复合材料粘结相与金刚石颗粒的粘结系数随着Cr—C化合物层厚度的增加而增大。摩擦磨损测试表明,在900℃和950℃烧结的样品表面,粘结相在摩擦过程中首先被磨除,金刚石随后露出,而Cr—C界面反应层有助于保持对金刚石颗粒的把持能力,提高复合材料的磨削性能。因此,适当的界面反应可提升金刚石复合材料的服役性能。Abstract: Diamond superhard abrasive tools play an increasingly important role in high-end chips, 3C ceramics processing and other fields. The interface between binder phase and diamond greatly affects the mechanical and wear properties of diamond superhard composites. In order to study the interfacial bonding between binder phase and diamond, Cu35Ni25Co25Cr15 multi-principal components alloy/diamond composite was prepared by spark plasma sintering (SPS). The interfacial reaction between alloy binder phase and diamond particles was studied by thermodynamic calculation and experiments. The results show that chromium reacts with diamond at the interface to form Chromium carbides. Moreover, with the sintering temperature increasing, the thickness of Chromium carbides layer grows and the cohesion coefficient between the alloy binder phase and diamond increases. When sintering temperature reaches 950℃, the Chromium carbides layer is uniform and continuous, and the thickness is about 1.1 μm. The friction and wear tests show that on the surface of the composite sintered at 900℃ and 950℃, the alloy binder phase is removed firstly by the shear stress, and then the diamond particles expose. Due to the retention of the Chromium carbides layer, the grinding performance of the composites is improved effectively. Therefore, appropriate interfacial reaction improves the service properties of the diamond composites.
-
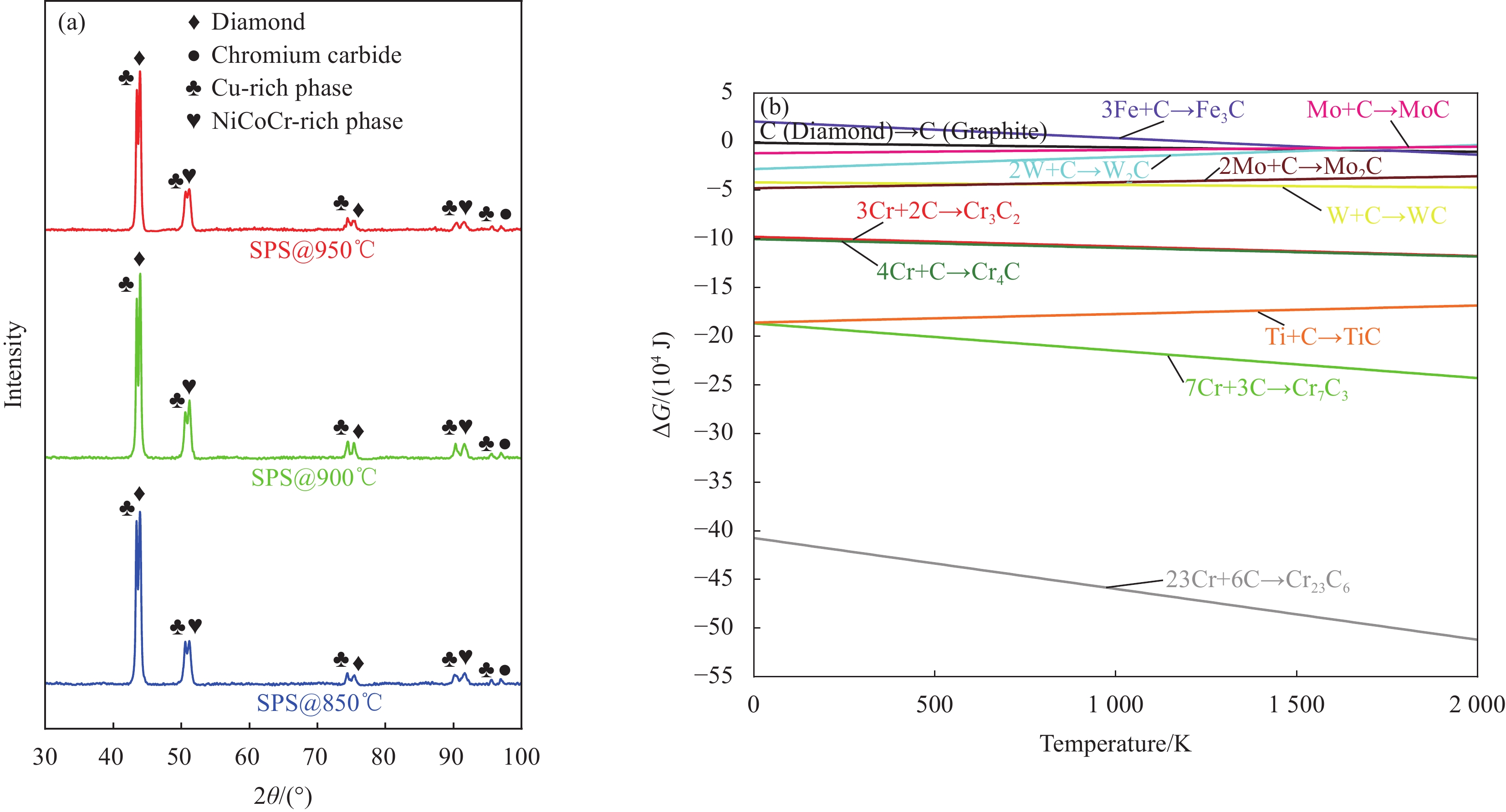
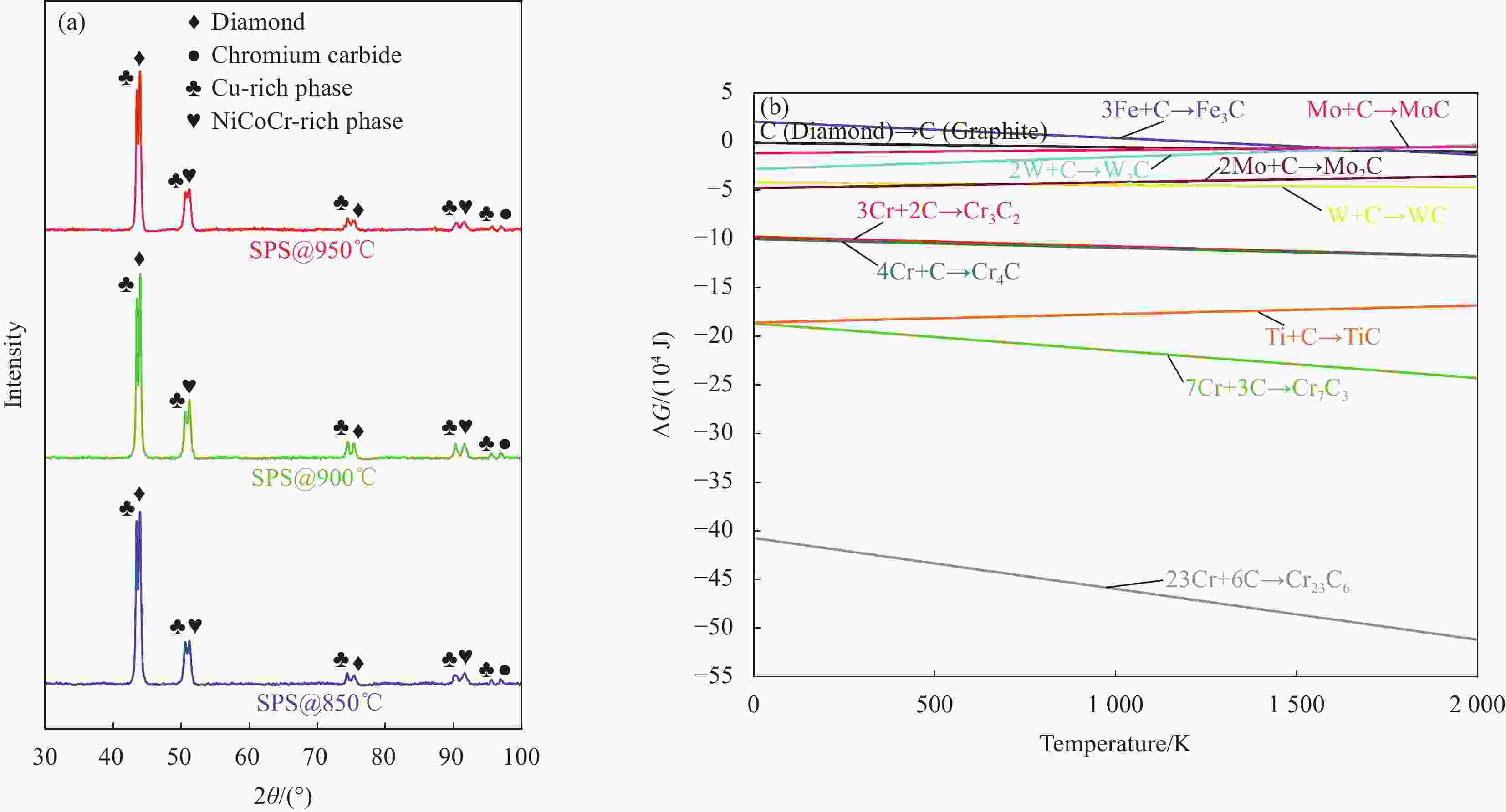
图 1 (a) 不同温度烧结的Cu35Ni25Co25Cr15多主元合金/金刚石复合材料XRD图谱;(b) 金属与金刚石反应的吉布斯自由能∆G与温度的关系
Figure 1. (a) XRD patterns of Cu35Ni25Co25Cr15 multi-principal components alloy/diamond composites sintered at different temperatures; (b) Gibbs free energy ∆G of reaction between metals and diamond as a function of temperature
图 2 不同温度烧结的Cu35Ni25Co25Cr15多主元合金/金刚石复合材料的粘结相与金刚石颗粒的界面观察及EDS线扫结果: (a) 850℃;(b) 900℃;(c) 950℃;(d) 不同复合材料界面处Cr元素含量变化
Figure 2. Interfacial microstructure between binder phase and diamond particles of Cu35Ni25Co25Cr15 multi-principal components alloy/diamond composites sintered at different temperatures and EDS line scanning results: (a) 850℃; (b) 900℃; (c) 950℃; (d) Cr content at the interface of different samples
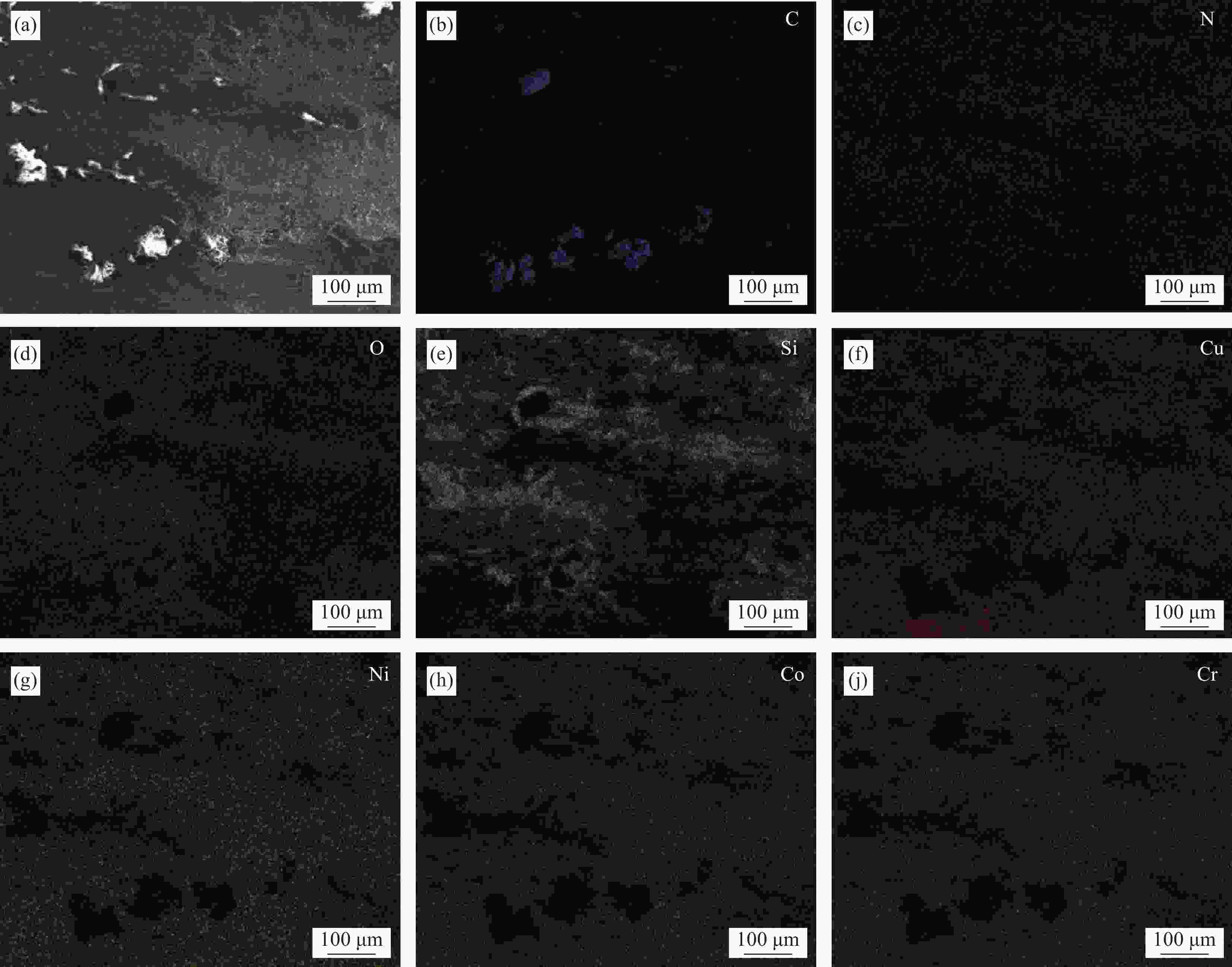
图 3 不同温度烧结的Cu35Ni25Co25Cr15多主元合金/金刚石复合材料的粘接相与金刚石颗粒界面处的场发射电子探针(EPMA)图像:((a1)~(g1)) 850℃;((a2)~(g2)) 900℃;((a3)~(g3)) 950℃
Ave—Average
Figure 3. Field emission electron probe (EPMA) images at the interface between binder phase and diamond particles of Cu35Ni25Co25Cr15 multi-principal components alloy/diamond composites sintered at different temperatures: ((a1)-(g1)) 850℃; ((a2)-(g2)) 900℃; ((a3)-(g3)) 950℃
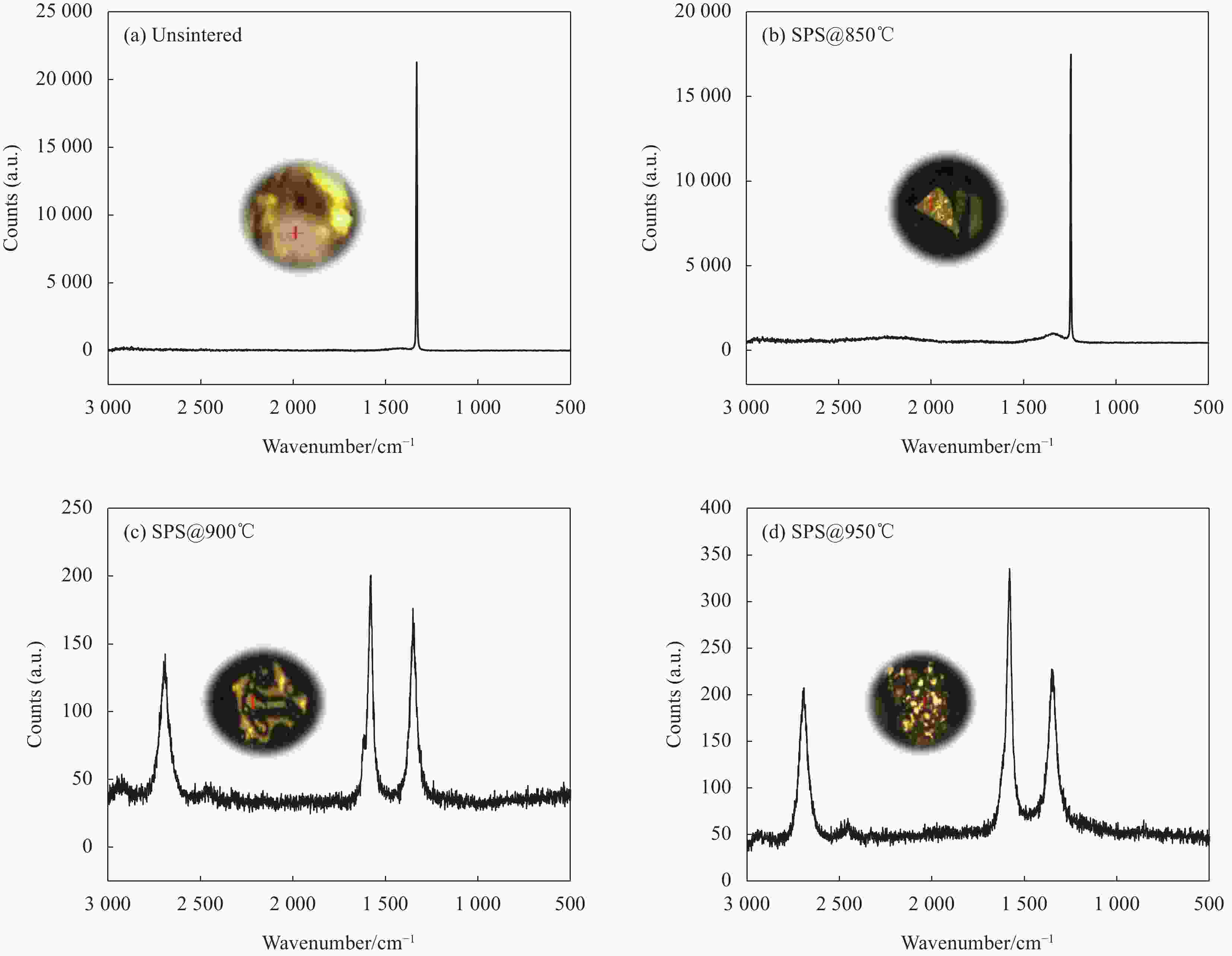
图 5 不同温度烧结的Cu35Ni25Co25Cr15多主元合金/金刚石复合材料中金刚石颗粒表面的Raman光谱:(a) 未烧结;(b) 850℃;(c) 900℃;(d) 950℃
Figure 5. Raman spectra on the surface of diamond particles in Cu35Ni25Co25Cr15 multi-principal components alloy/diamond composites sintered at different temperatures: (a) Unsintered; (b) 850℃; (c) 900℃; (d) 950℃
表 1 Cu35Ni25Co25Cr15多主元合金粘结相的名义成分
Table 1. Nominal composition of Cu35Ni25Co25Cr15 multi-principal components alloy binder phase
at% Cu Ni Co Cr 35 25 25 15 表 2 样品名称缩写
Table 2. Sample name abbreviation
Sample Sintering temperature/℃ SPS@850℃ 850 SPS@900℃ 900 SPS@950℃ 950 Note: SPS—Spark plasma sintering. 表 3 不同温度烧结的Cu35Ni25Co25Cr15多主元合金/金刚石复合材料的密度、硬度、横向断裂强度(TRS)及粘结系数
Table 3. Density, hardness, transverse rupture strength (TRS) and cohesion coefficient of Cu35Ni25Co25Cr15 multi-principal components alloy/diamond composites sintered at different temperatures
Composite Density/(g·cm−3) Hardness of composites
(HB)Hardness of binder phase
(HV)TRS of binder phase/MPa TRS of composites/MPa Cohesion coefficient SPS@850℃ 7.8378±0.0028 161.2±5.5 202.23±4.14 1068.86 868.79 0.187 SPS@900℃ 8.1232±0.0074 205.2±4.7 202.55±3.28 1486.08 1016.48 0.316 SPS@950℃ 8.0187±0.0081 208.7±6.2 205.16±2.37 1898.94 1046.84 0.449 表 4 不同温度烧结的Cu35Ni25Co25Cr15多主元合金/金刚石复合材料的磨耗比
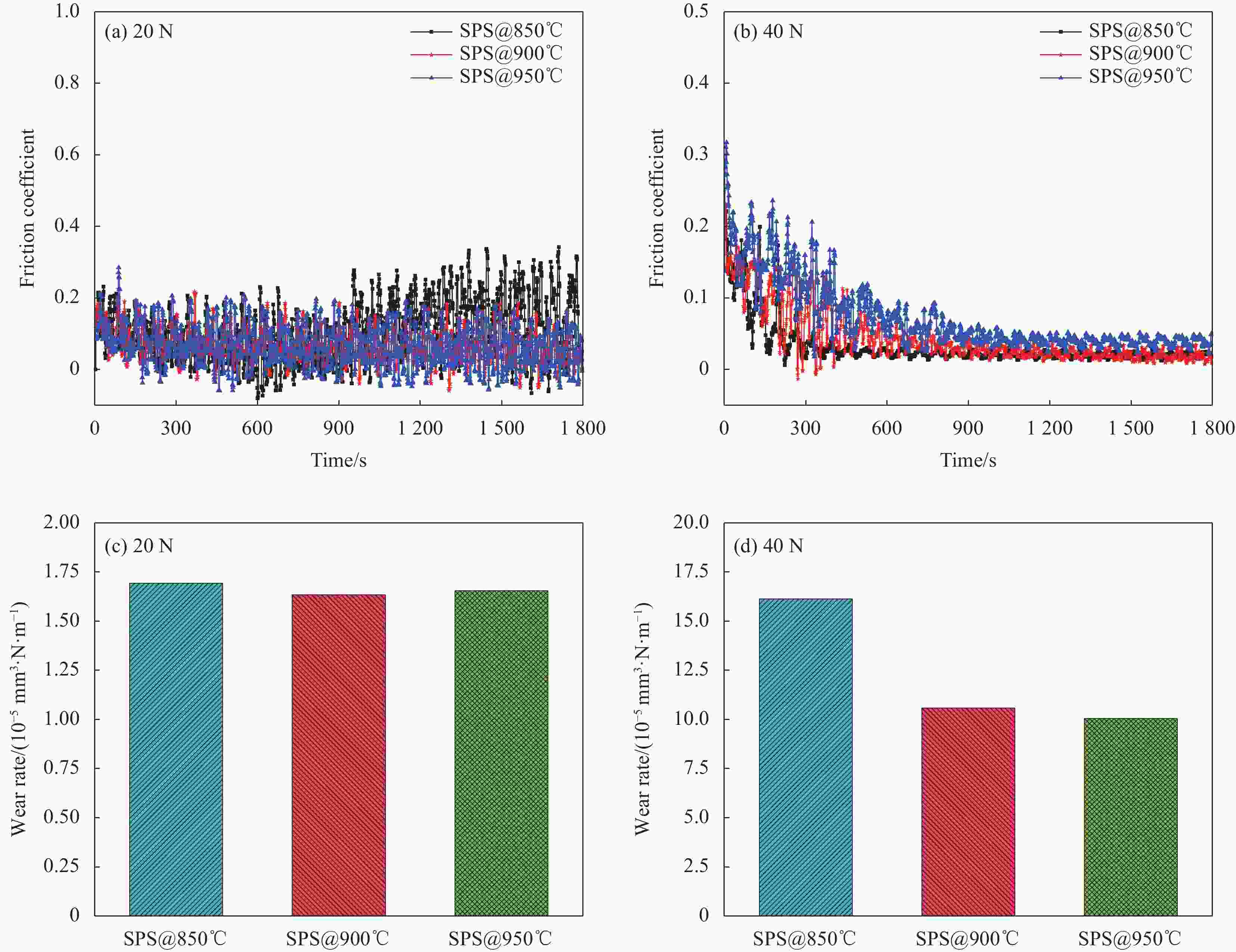
Table 4. Abrasion ratios of Cu35Ni25Co25Cr15 multi-principal components alloy/diamond composites sintered at different temperatures
Composite Abrasion ratio SPS@850℃ 766.7±57.7 SPS@900℃ 1033.3±208.1 SPS@950℃ 1766.7±251.6 -
[1] CHON K S, TAKAHASHI H, NAMBA Y. Wear inspection of a single-crystal diamond tool used in electroless nickel turning[J]. Optical Engineering,2014,53(3):034102. doi: 10.1117/1.OE.53.3.034102 [2] ZHAO X, LI J, DUAN L, et al. Effect of Fe-based pre-alloyed powder on the microstructure and holding strength of impregnated diamond bit matrix[J]. International Journal of Refractory Metals & Hard Materials,2019,79:115-122. [3] LU C, FENG X, SHEN Y, et al. Wear resistance and thermal conductivity of diamond/Cu-Cr mechanical milled coatings after high temperature annealing[J]. Diamond and Related Materials,2019,97:107438. doi: 10.1016/j.diamond.2019.05.023 [4] KOBARU Y, KONDO E, IWAMOTO R. Ultra-precision cutting of single crystal silicon using diamond tool with large top corner radius[J]. Key Engineering Materials,2012,523:81-86. [5] ZHANG Y, HAN T, XIAO M, et al. Tribological behavior of diamond reinforced FeNiCoCrTi0.5 carbonized high-entropy alloy coating[J]. Surface and Coatings Technology,2020,401:126233. doi: 10.1016/j.surfcoat.2020.126233 [6] TILLMANN W, FERREIRA M, STEFFEN A, et al. Carbon reactivity of binder metals in diamond-metal composites—Characterization by scanning electron microscopy and X-ray diffraction[J]. Diamond and Related Materials,2013,38:118-123. doi: 10.1016/j.diamond.2013.07.002 [7] KONSTANTY J. Powder metallurgy diamond tools–A review of manufacturing routes[J]. Materials Science Forum,2007,534-536:1121-1124. [8] DUAN D, SUN L, FANG X, et al. Microstructure and processing performance of brazed diamond drill bits with Ni–Cr + Cu–Ce composite solder[J]. Diamond and Related Materials,2019,93:216-223. doi: 10.1016/j.diamond.2019.01.023 [9] DGL A, LIANG Z A, LI Z A, et al. Effect of W-coated diamond on the microstructure and thermal conductivity of diamond/W matrix composites for plasma-facing materials (PFMs)[J]. Fusion Engineering and Design,2019,144:141-147. doi: 10.1016/j.fusengdes.2019.05.005 [10] PING H, XIAO F R, ZOU W J, et al. Effect of different oxides addition on the thermal expansion coefficients and residual stresses of Fe-based diamond composites[J]. Ceramics International,2014,40(3):5007-5013. doi: 10.1016/j.ceramint.2013.08.080 [11] OLIVEIRA L J D, CABRAL S C, FILGUEIRA M. Study hot pressed Fe-diamond composites graphitization[J]. International Journal of Refractory Metals and Hard Materials,2012(35):228-234. [12] JIE G A, HUI G A, SW B, et al. Simulation, forming process and mechanical property of Cu-Sn-Ti/diamond composites fabricated by selective laser melting[J]. International Journal of Refractory Metals and Hard Materials, 2020, 87: 105144. [13] MECHNIK V A, BONDARENKO N A, DUB S N, et al. A study of microstructure of Fe-Cu-Ni-Sn and Fe-Cu-Ni-Sn-VN metal matrix for diamond containing composites[J]. Materials Characterization,2018,146:209-216. doi: 10.1016/j.matchar.2018.10.002 [14] ZUO Q, WANG W, GU M S, et al. Thermal conductivity of the diamond-Cu composites with chromium addition[J]. Advanced Materials Research,2011,311-313:287-292. doi: 10.4028/www.scientific.net/AMR.311-313.287 [15] MA S, ZHAO N, SHI C, et al. Mo2C coating on diamond: Different effects on thermal conductivity of diamond/Al and diamond/Cu composites[J]. Applied Surface Science,2017,402(Complete):372-383. [16] YANG T, ZHAO Y L, TONG Y, et al. Multicomponent intermetallic nanoparticles and superb mechanical behaviors of complex alloys[J]. Science,2018,362(6417):933-937. doi: 10.1126/science.aas8815 [17] LI C W, CHANG K C, YEH A C. On the microstructure and properties of an advanced cemented carbide system processed by selective laser melting[J]. Journal of Alloys and Compounds,2018,782:440-450. [18] ZHANG W, ZHANG M Y, PENG Y B, et al. Interfacial structures and mechanical properties of a high entropy alloy-diamond composite[J]. International Journal of Refractory Metals and Hard Materials,2019,86:105-109. [19] LI Z, PRADEEP K G, DENG Y, et al. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off[J]. Nature,2016,534(7606):227-230. doi: 10.1038/nature17981 [20] LAPLANCHE G, BERGLUND S, REINHART C, et al. Phase stability and kinetics of σ-phase precipitation in CrMnFeCoNi high-entropy alloys[J]. Acta Materialia,2018,161:338-351. doi: 10.1016/j.actamat.2018.09.040 [21] SHANG C, AXINTE E, SUN J, et al. CoCrFeNi(W1xMox) high-entropy alloy coatings with excellent mechanical properties and corrosion resistance prepared by mechanical alloying and hot pressing sintering[J]. Materials & Design,2017,117(3):193-202. [22] CHEN C S, YANG C C, CHAI H Y, et al. Novel cermet material of WC/multi-element alloy[J]. International Journal of Refractory Metals and Hard Materials,2014,43(12):200-204. [23] CHEN J N P, WEI T, ET A L. Fabrication and mechanical properties of AlCoNiCrFe high-entropy alloy particle reinforced Cu matrix composites[J]. Journal of Alloys and Compounds: An Interdisciplinary Journal of Materials Science and Solid-state Chemistry and Physics,2015,649:630-634. [24] ZHANG W, ZHANG M, PENG Y, et al. Effect of Ti/Ni coating of diamond particles on microstructure and properties of high-entropy alloy/diamond composites[J]. Entropy,2019,21(2):164-174. doi: 10.3390/e21020164 [25] WANG H, ZHANG W, PENG Y, et al. Microstructures and wear resistance of FeCoCrNi-Mo high entropy alloy/diamond composite coatings by high speed laser cladding[J]. Coatings,2020,10(3):300-315. doi: 10.3390/coatings10030300 [26] 中华人民共和国工业和信息化部. 聚晶金刚石磨耗比测定方法: JB/T 3235—2013[S]. 北京: 机械工业出版社, 2013.Ministry of Industry and Information Technology of the People's Republic of China. Testing method for abrasion ratio of polycrystalline diamond: JB/T 3235—2013[S]. Beijing: China Machine Press, 2013(in Chinese). [27] 叶大伦, 胡建华. 实用无机物热力学数据手册[M]. 北京: 冶金工业出版社, 1980.YE Dalun, HU Jianhua. Practical inorganic thermodyna-mic data manual[M]. Beijing: Metallurgical Industry Press, 1980(in Chinese). [28] LÜTTGE A. Crystal dissolution kinetics and Gibbs free energy[J]. Journal of Electron Spectroscopy and Related Phenomena,2006,150(2-3):248-259. doi: 10.1016/j.elspec.2005.06.007 [29] BUNDY F P, BOVENKERK H P, STRONG H M, et al. Diamond-graphite equilibrium line from growth and graphitization of diamond[J]. Journal of Chemical Physics,2004,35(2):383-391. [30] 朱瑞华, 刘金龙, 陈良贤. 金刚石自支撑膜拉曼光谱1420 cm-1特征峰研究[J]. 人工晶体学报, 2015, 44(4):6-12.ZHU Ruihua, LIU Jinlong, CHEN Liangxian. Research on 1420 cm-1 characteristic peak of free-standing diamond films in Raman spectrum[J]. Journal of Synthetic Crystals,2015,44(4):6-12(in Chinese). [31] IRAVANITABRIZIPOUR M. Laser direct deposition of metal matrix diamond composite[M]. Ontario: Waterloo, 2016: 77-85. [32] WILHELM H, LELAURAIN M. Raman spectroscopic studies on well-defined carbonaceous materials of strong two-dimensional[J]. Journal of Applied Physics,1998,84(12):6552-6564. doi: 10.1063/1.369027 [33] KNIGHT D S, WHITE W B. Raman and fluorescence spectroscopic characterization of diamonds and CVD diamond films[J]. Proceedings of Spie the International Society for Optical Engineering,1989,1055:144-151. [34] 吴颖. 新型金刚石工具铜基结合剂及其性能的研究[D]. 重庆: 重庆大学, 2014.WU Ying. Study on a new Cu-matrix binding agent of diamond tools and its properties[D]. Chongqing: Chongqing University, 2014(in Chinese). [35] ANDERSSON J, ALMQVIST A, LARSSON R. Numerical simulation of a wear experiment[J]. Wear,2011,271(11):2947-2952. -





 下载:
下载: