Manganese-nitrogen co-doped rice husk biochar activated peroxydisulfate to degrade acid orange
-
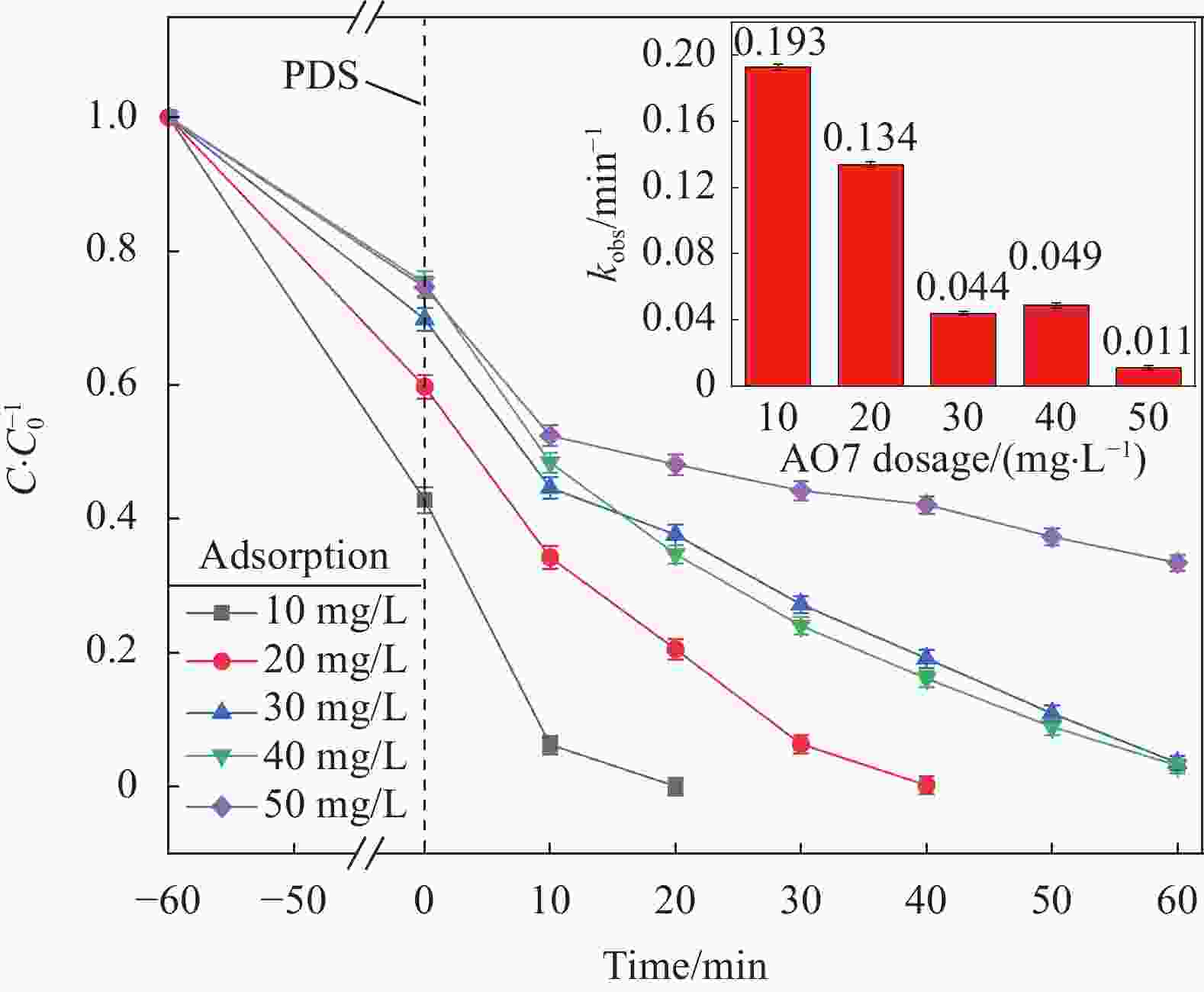
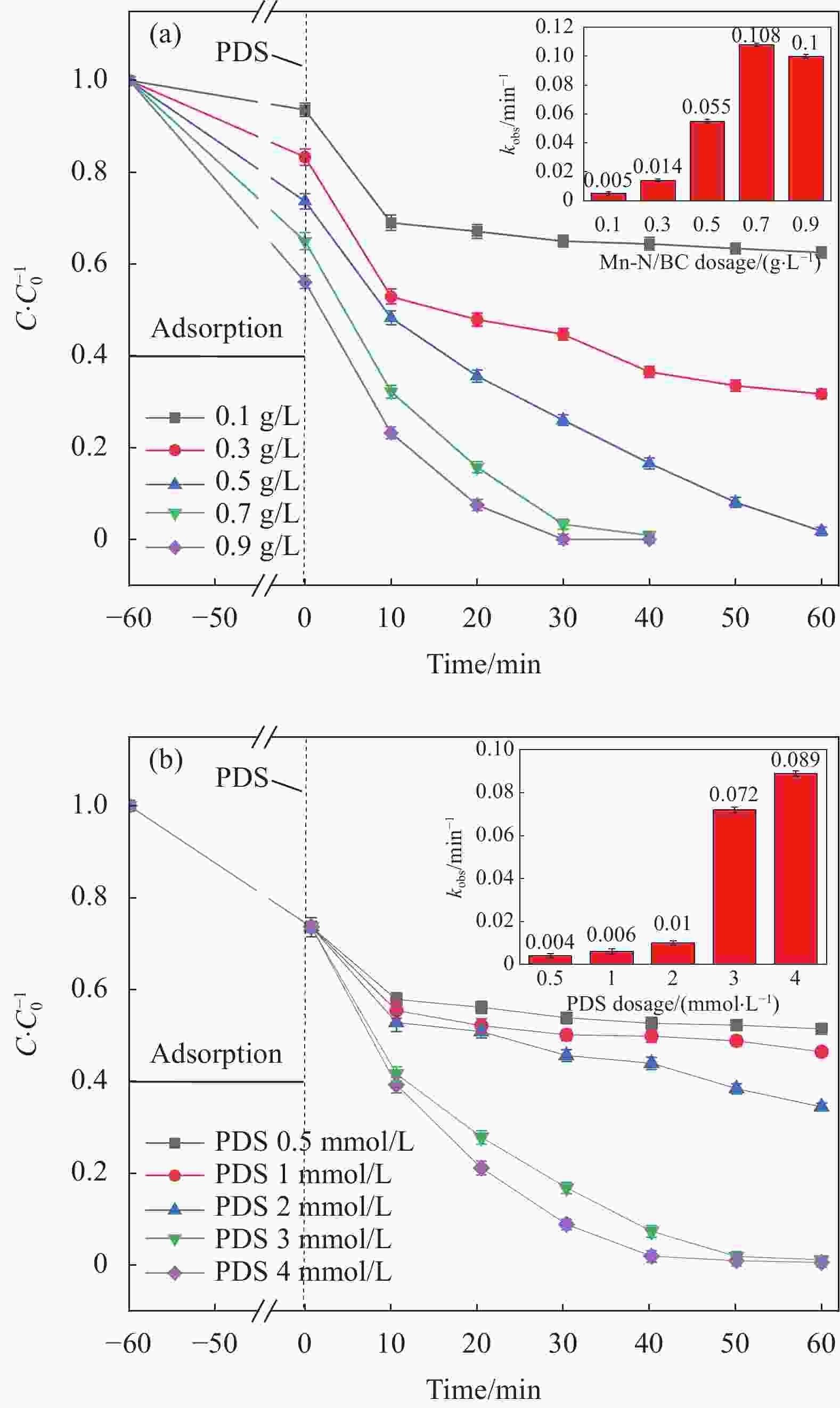
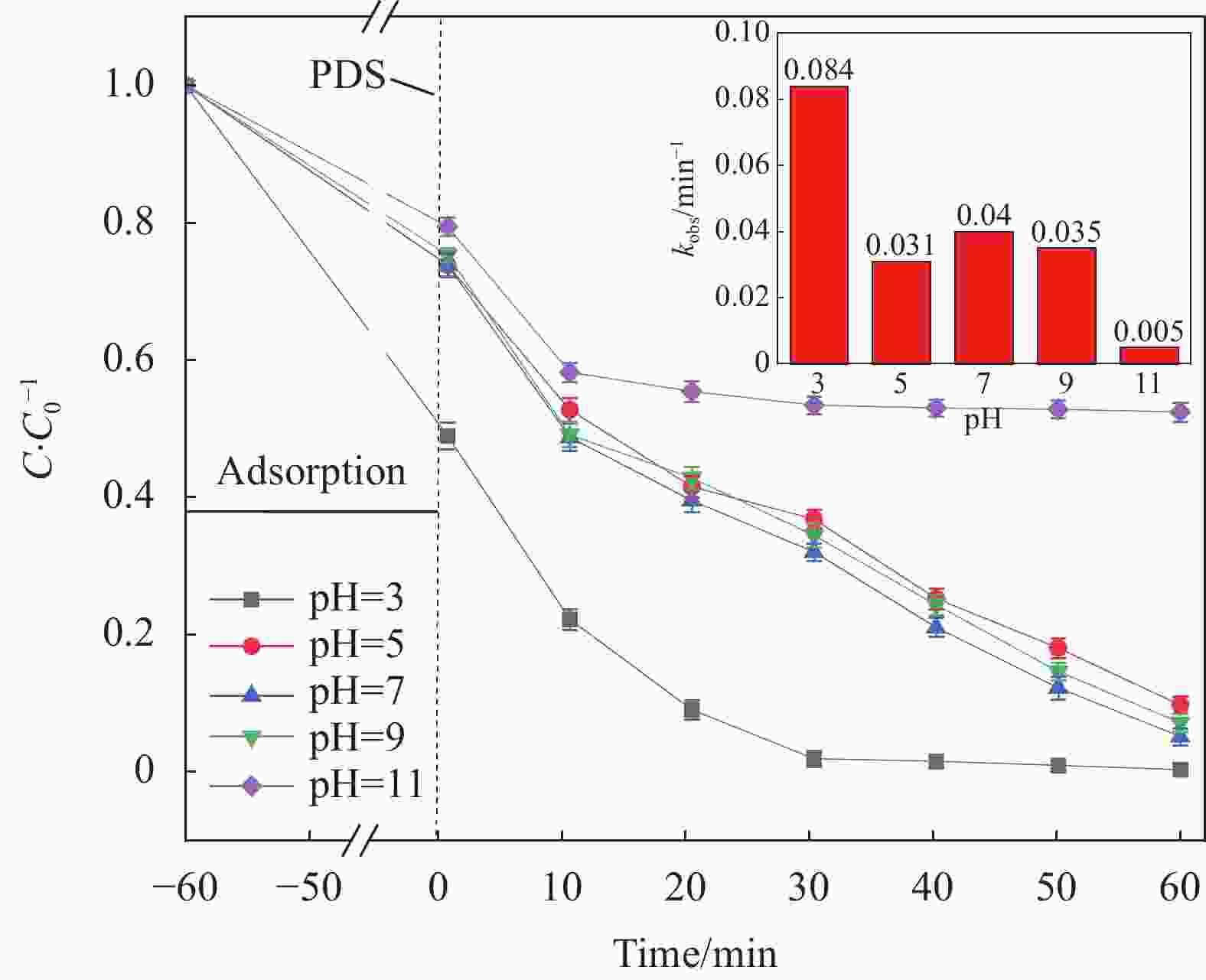
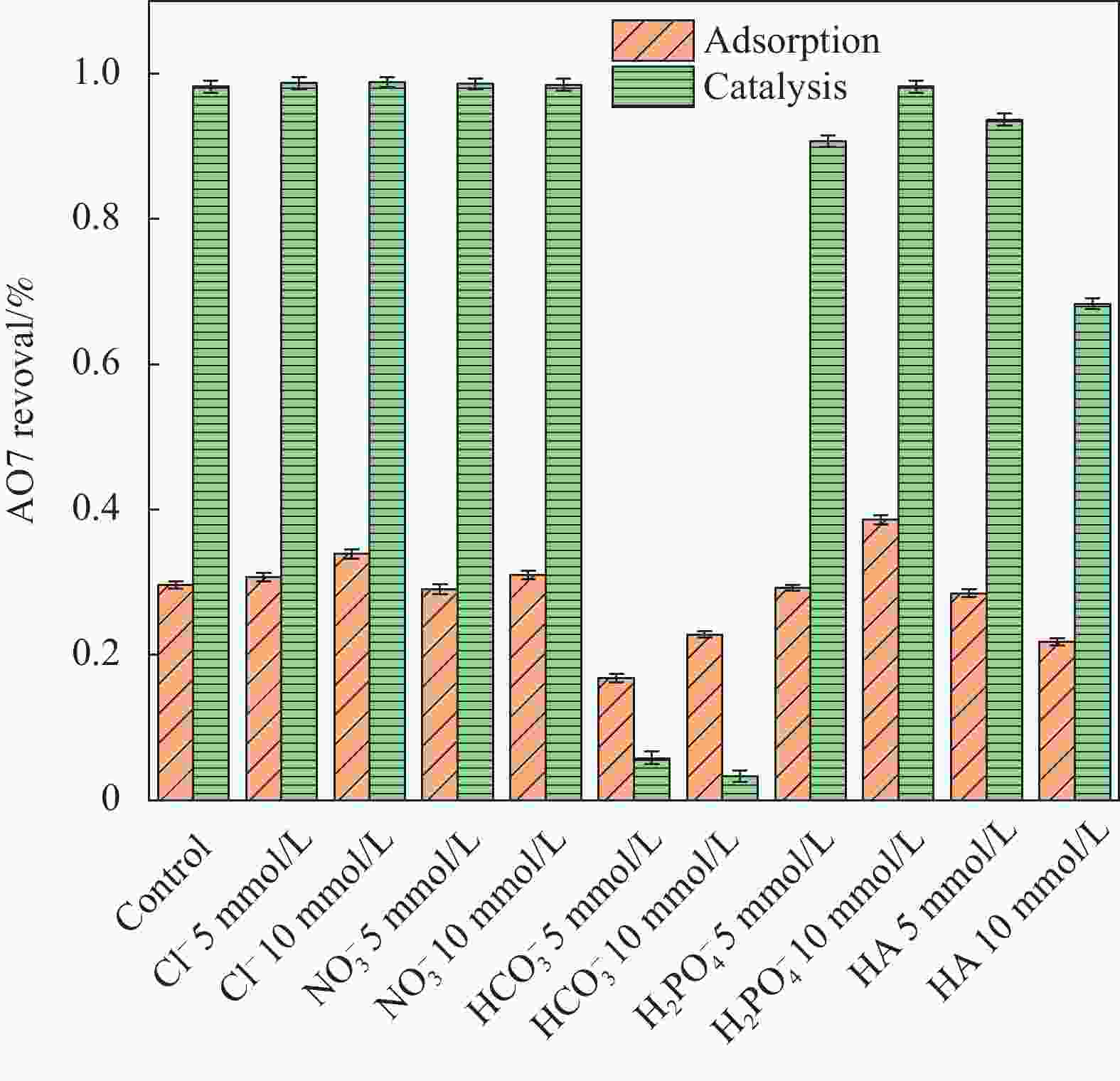
摘要: 为了更好地处理水环境中的偶氮染料(酸性橙,AO7)污染问题,以稻壳、尿素和锰盐为原料,通过热解法制备Mn、N共掺杂生物炭复合材料(Mn-N-BC),活化过二硫酸盐(PDS)降解酸性橙(AO7)染料废水。考察了AO7初始浓度、PDS浓度、催化剂投加量、初始pH值等因素对AO7去除率的影响。结果显示:Mn-N-BC/PDS体系对AO7染料具有较高的去除率,在30 min内可达为98.6%,其表观速率常数kobs为0.125 min−1;并且对水环境中的无机阴离子表现出较高的抗性。在3次循环利用后,AO7的去除率仍在75%左右,表明Mn-N-BC对有机污染物的去除具有较高的可重复利用性和稳定性。自由基淬灭研究、XPS分析表明:Mn-N-BC/PDS体系中AO7的降解机制包括自由基途径(•OH、SO4−•)和非自由基途径(O2−•、1O2和电子转移),其中非自由基途径为主要作用。Abstract: In order to better deal with the problem of azo dye (acid orange, AO7) dye pollution in water environment, Mn and N co-doped biochar composites (Mn-N-BC) were prepared by pyrolysis method using rice husk, urea and manganese salt as raw materials, and acid orange (AO7) dye wastewater was degraded by activated peroxydisulfate (PDS). The effects of initial AO7 concentration, PDS concentration, catalyst dosage and initial pH value on the removal rate of AO7 were investigated. The results show that the Mn-N-BC/PDS system has a high removal rate of AO7 dyes, which can reach 98.6% in 30 min, its apparent rate constant kobs is 0.125 min−1; and shows high resistance to inorganic anions in the water environment. After three times of recycling, the removal rate of AO7 is still about 75%, indicating that Mn-N-BC has high reusability and stability in the removal of organic pollutants. Free radical quenching and XPS analysis showed that the degradation mechanism of AO7 in Mn-N-BC/PDS system included free radical pathway (•OH, SO4−•) and non-free radical pathway (O2−•, 1O2 and electron transfer), in which non-free radical pathway was the main role.
-
Key words:
- biochar /
- impurity atoms /
- a total of doping /
- persulfate /
- degradation of dye
-
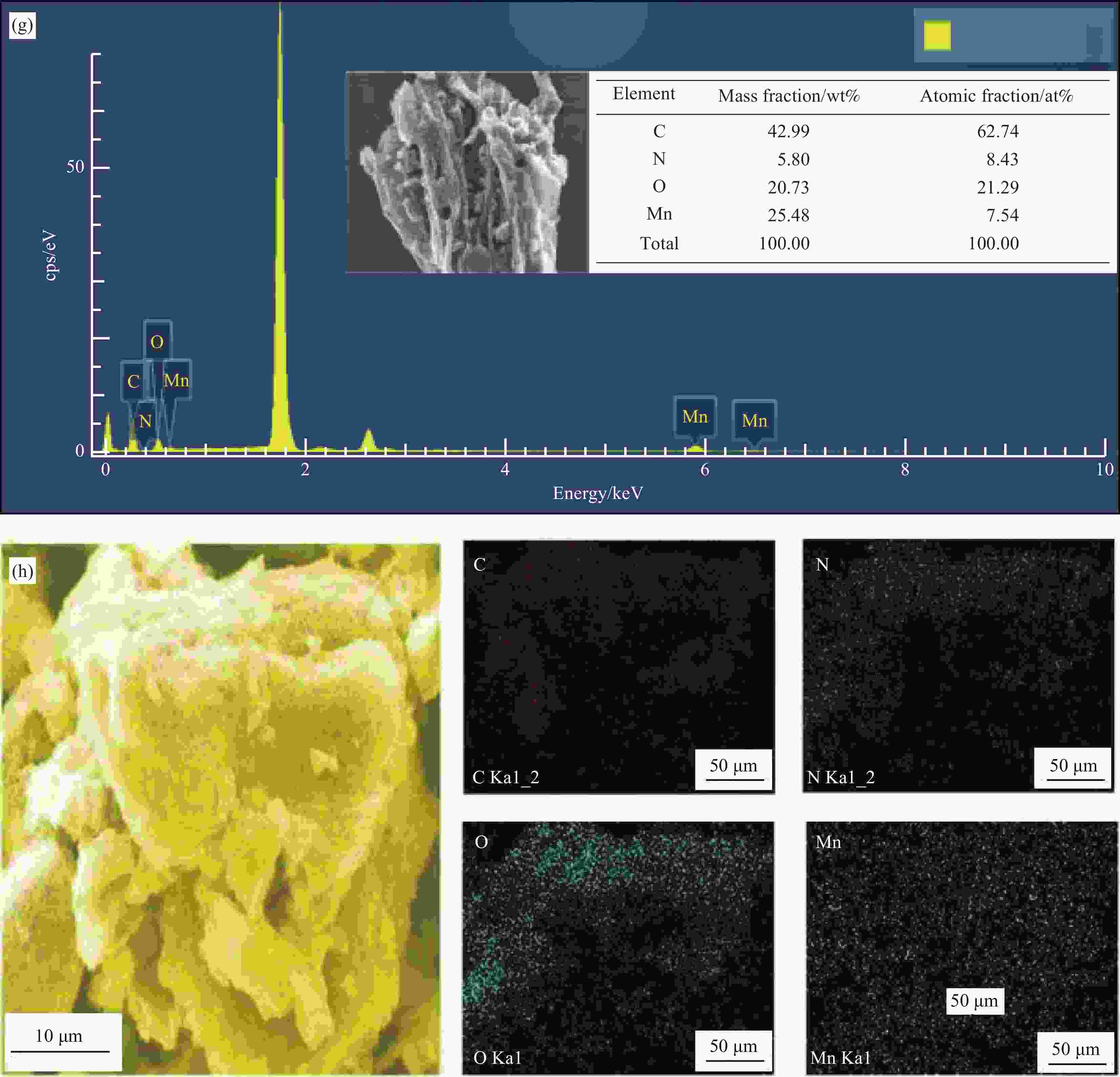
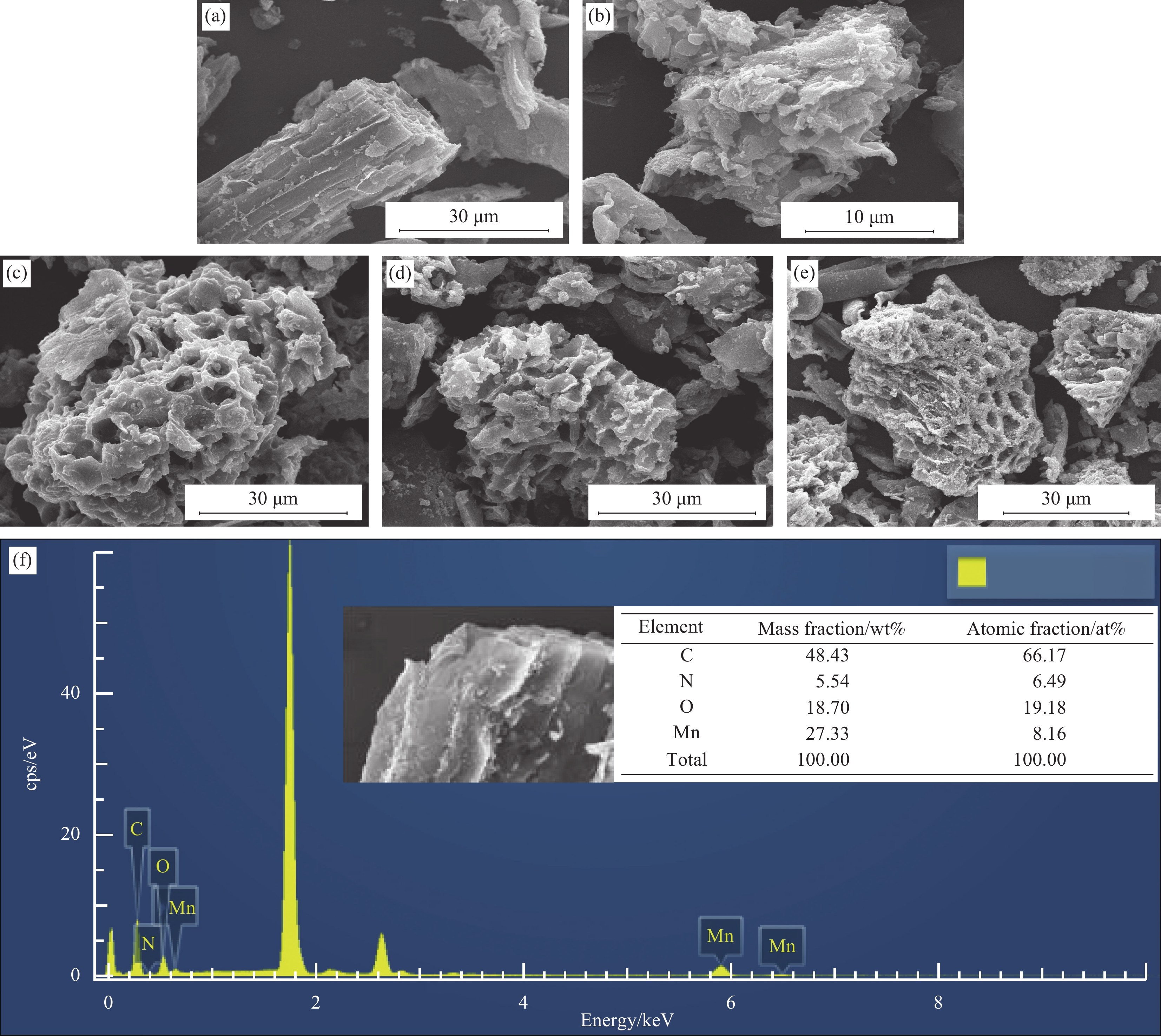
图 1 原始生物炭(BC) (a),N掺杂生物炭复合材料(N-BC) (b),Mn掺杂生物炭复合材料(Mn-BC) (c),Mn、N共掺杂生物炭复合材料(Mn-N-BC)反应前(d)和反应后(e)催化剂的SEM图像;Mn-N-BC反应前(f)和反应后(g)的EDS图谱;Mn-N-BC元素分布图(10 μm)(h)
Figure 1. SEM images of original biochar (BC) (a), N doped biochar composites (N-BC) (b), Mn doped biochar composites (Mn-BC) (c), Mn and N co-doped biochar composites (Mn-N-BC) before (d) and after (e) reaction; EDS of Mn-N-BC before (f) and after (g) reaction; Mn-N-BC element distribution diagram (h)
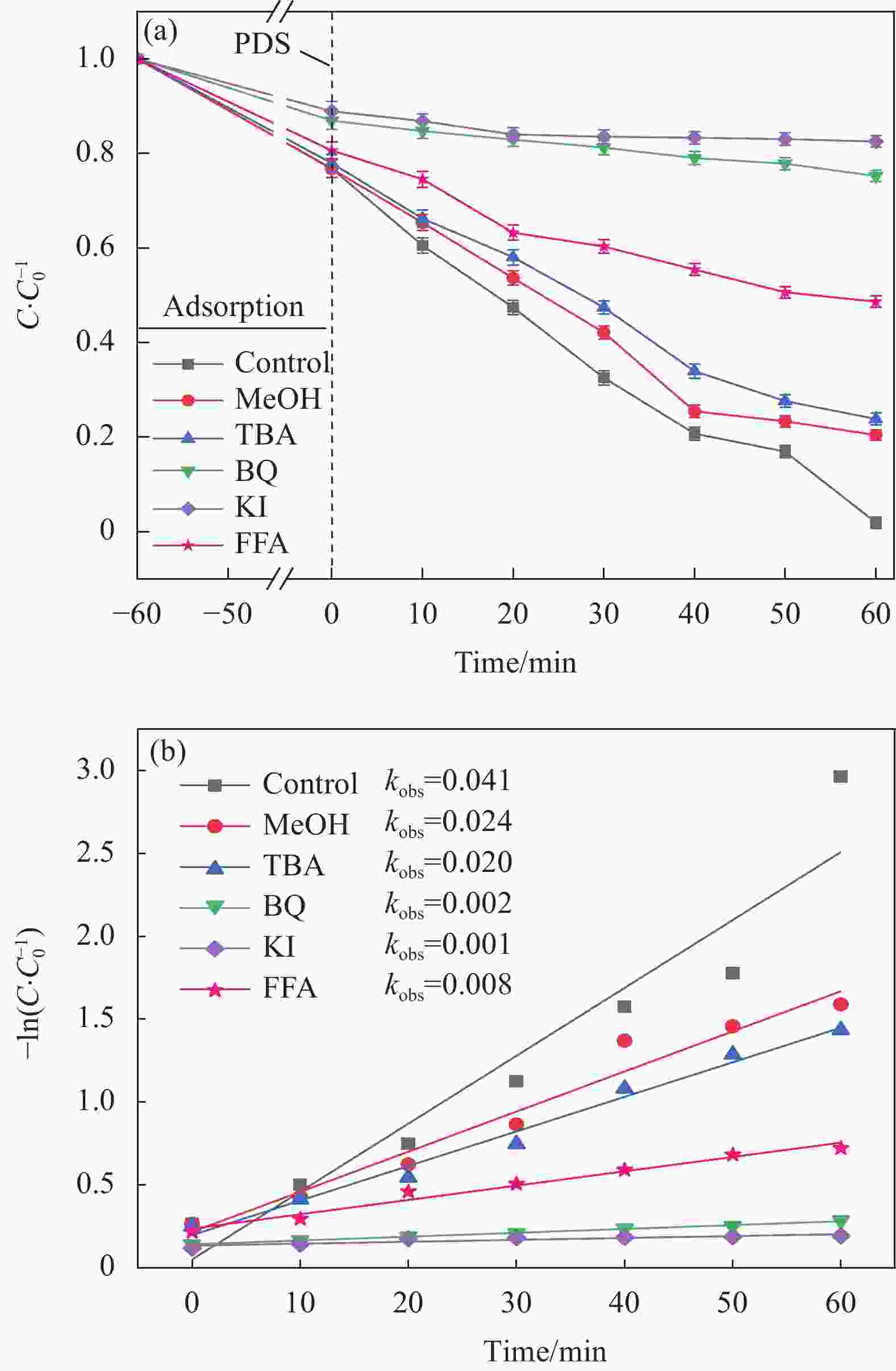
图 4 (a)不同体系催化剂对酸性橙(AO7)去除的影响;(b)准一级动力学拟合;(c)反应速率常数
PDS—Peroxydi-sulfate
Figure 4. (a) Effects of different catalysts on acid orange (AO7) removal; (b) Quasi first order dynamic fitting; (c) Reaction rate constant
kobs—Pseudo first order kinetic constant (min-1); C—Concentration of AO7 after reaction (mg/L); C0—Concentration of AO7 before reaction (mg/L)
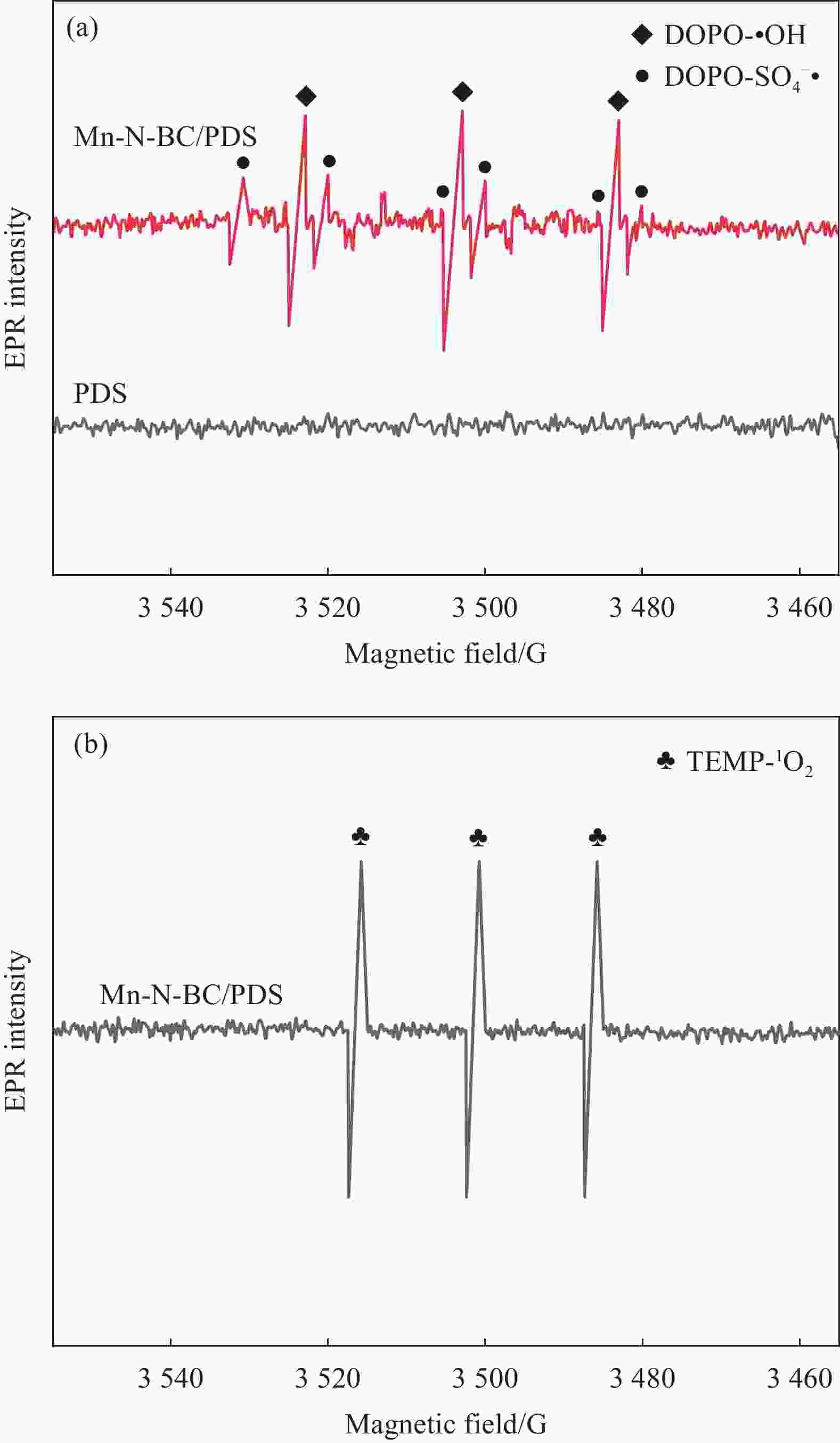
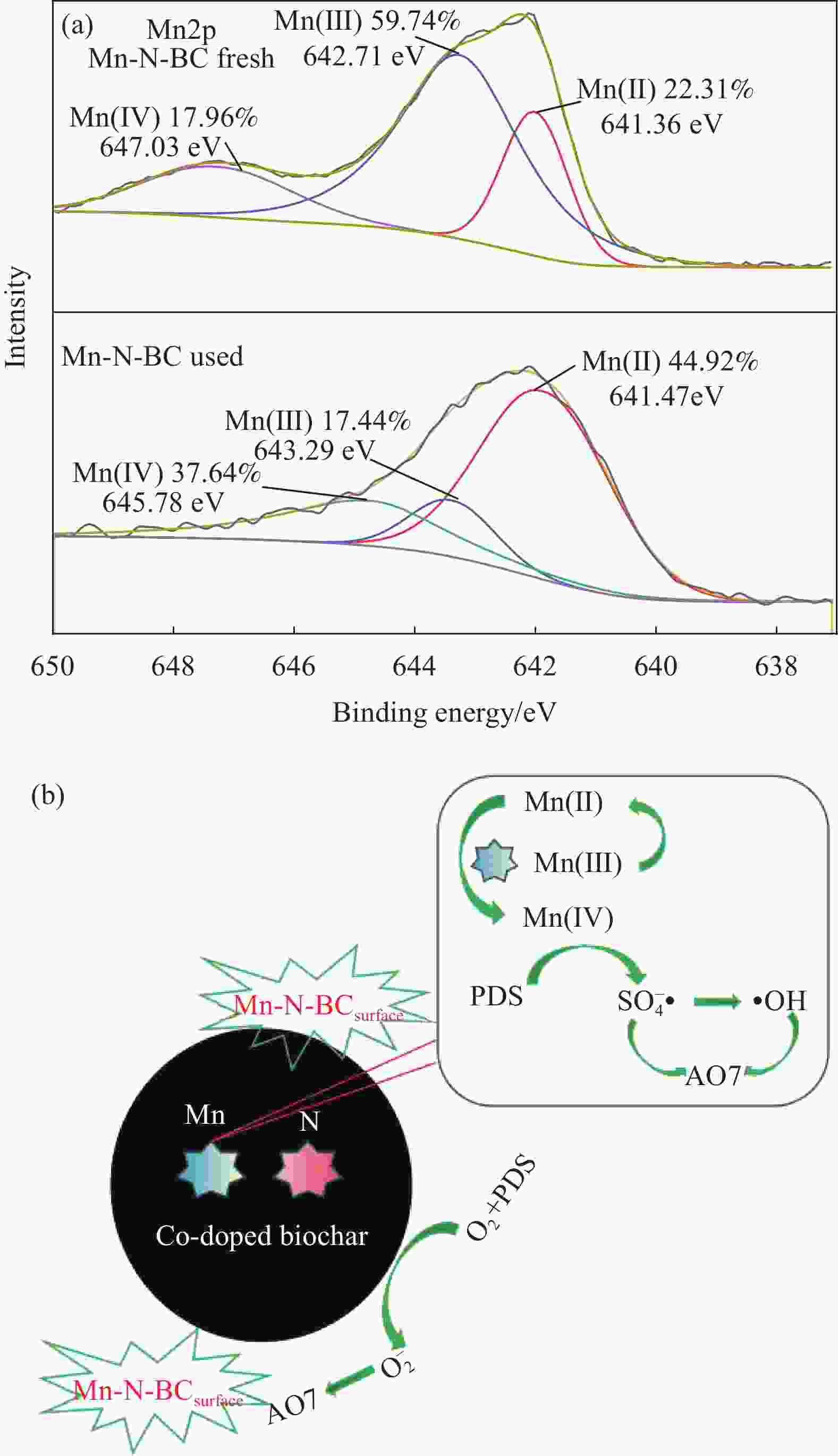
图 10 Mn-N-BC/PDS体系在 5-二甲基-1-氧化吡咯琳(DMPO)和4-氨基-2, 2, 6, 6-四甲基喉啶(TEMP)作用下的EPR光谱:(a) DOPO-SO4–•和DOPO-•OH;(b) TEPM-1O2
Figure 10. EPR spectra of the Mn-N-BC/PDS system with 5-dimethyl-1-pyrrolidine oxide (DMPO) and 4-amino-2, 2, 6, 6-tetramethylthroat (TEMP) reaction conditions: (a) DOPO-SO4–• and DOPO-•OH; (b) TEPM-1O2
表 1 不同材料/PDS体系对AO7的降解动力学参数
Table 1. Kinetic parameters of AO7 degradation by different materials/PDS systems
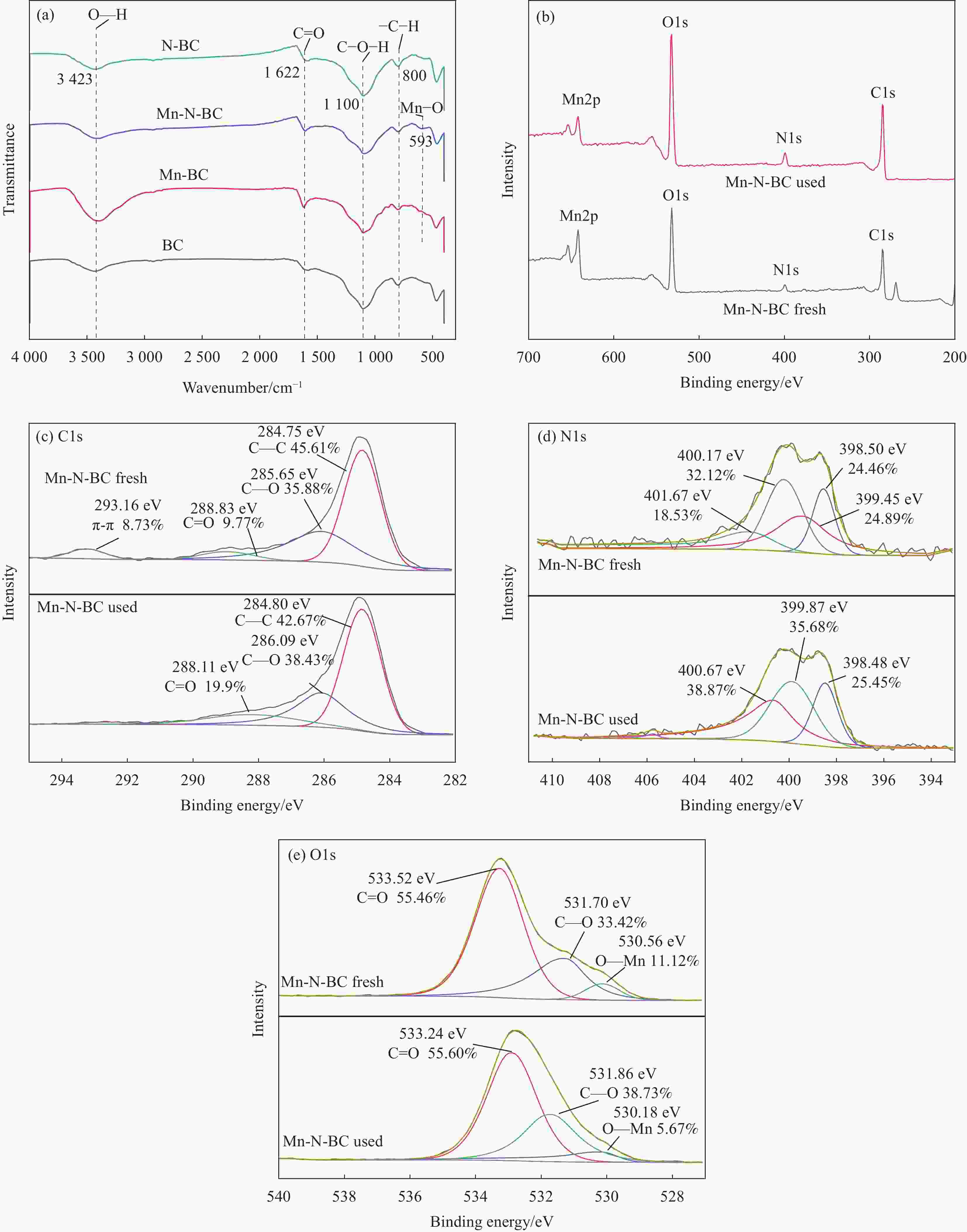
Catalytic material kobs/min–1 R2 N-BC 0.002 0.8710 Mn-BC 0.010 0.8902 Mn-N-BC 0.125 0.9789 Notes: R—Gas constant; kobs—Apparent rate constant. -
[1] PANG Y, LUO K, TANG L, et al. Carbon-based magnetic nanocomposite as catalyst for persulfate activation: A critical review[J]. Environmental Science and Pollution Research,2019,26(32):32764-32776. doi: 10.1007/s11356-019-06403-4 [2] 罗才武, 陈晴晴, 张德, 等. 多相催化剂以非自由基路线活化过二硫酸盐的研究进展[J]. 工业催化, 2021, 29(3): 11-15.LUO Caiwu, CHEN Qingqing, ZHANG De, et al. Research progress in the activation of persulfate by non-radical route over heterogeneous catalysts[J]. Industrial Catalysis, 2021, 29(3): 11-15(in Chinese). [3] 王振宇, 黄仕元, 李胜, 等. 可见光活化过二硫酸盐对染料废水的降解研究[J]. 化工新型材料, 2021, 49(10):175-178.WANG Zhenyu, HUANG Shiyuan, LI Sheng, et al. Degradation of dye wastewater by activated persulfate with visible light[J]. New Chemical Materials,2021,49(10):175-178(in Chinese). [4] 尹汉雄, 唐玉朝, 黄显怀, 等. 紫外光强化Fe(II)-EDTA活化过硫酸盐降解直接耐酸大红4BS[J]. 环境科学研究, 2017, 30(7):1105-1111.YIN Hanxiong, TANG Yuchao, HUANG Xianhuai, et al. Degradation of direct acid-fast Red 4BS by Persulfate activated by Fe(II)-EDTA enhanced by UV lightt[J]. Study Methodology of Environmental Science,2017,30(7):1105-1111(in Chinese). [5] AHN Y Y, YUN E T. Heterogeneous metals and metal-free carbon materials for oxidative degradation through persulfate activation: A review of heterogeneous catalytic activation of persulfate related to oxidation mechanism[J]. Korean Journal of Chemical Engineering,2019,36(11):1767-1779. doi: 10.1007/s11814-019-0398-4 [6] THI T, CHENG Y, INAN T, et al. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis[J]. Geoderma, 2017, 288: 79-96. [7] HUANG W, XIAO S, ZHONG H, et al. Activation of persulfate by carbonaceous materials: A review[J]. Chemical Engineering Journal,2021,418:129297. [8] 李小娟, 叶兰妹, 廖凤珍, 等. 杂原子掺杂碳材料活化过硫酸盐技术的研究进展[J]. 化工进展, 2021, 40(1):273-281.LI Xiaojuan, YE Lanmei, LIAO Fengzhen, et al. Research progress in activation of persulfate by hetero-atom doped carbon materials[J]. Chemical Industry and Engineering Progress,2021,40(1):273-281(in Chinese). [9] OH W D, VEKSHA A, CHEN X, et al. Catalytically active nitrogen-doped porous carbon derived from biowastes for organics removal via peroxymonosulfate activation[J]. Chemical Engineering Journal,2019,374:947-957. doi: 10.1016/j.cej.2019.06.001 [10] LI H, TIAN J, ZHU Z, et al. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application[J]. Chemical Engineering Journal,2018,354:507-516. doi: 10.1016/j.cej.2018.08.043 [11] LI X, JIA Y, ZHOU M, et al. High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation[J]. Journal of Hazardous Materials,2020,397:122764. doi: 10.1016/j.jhazmat.2020.122764 [12] YUAN R, HU L, YU P, et al. Co3O4 nanocrystals/3D nitrogen-doped graphene aerogel: A synergistic hybrid for peroxymonosulfate activation toward the degradation of organic pollutants[J]. Chemosphere,2018,210:877-888. doi: 10.1016/j.chemosphere.2018.07.065 [13] WANG G, NIE X, JI X, et al. Enhanced heterogeneous activation of peroxymonosulfate by Co and N codoped porous carbon for degradation of organic pollutants: The synergism between Co and N[J]. Environmental Science: Nano,2019,6(2):399-410. doi: 10.1039/C8EN01231H [14] 赵志伟, 陈晨, 梁志杰, 等. 锰氧化物改性生物炭对水中四环素的强化吸附[J]. 农业环境科学学报, 2021, 40(1):194-201.ZHAO Zhiwei, CHEN Chen, LIANG Zhijie, et al. Enhanced adsorption of tetracycline from water by manganese oxide modified biochar[J]. Journal of Agro-Environment Science,2021,40(1):194-201(in Chinese). [15] WANG H, GUO W, LIU B, et al. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism[J]. Water Research,2019,160:405-414. doi: 10.1016/j.watres.2019.05.059 [16] ZHOU Y, LIU X, XIANG Y, et al. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling[J]. Bioresource Technology,2017,245:266-273. doi: 10.1016/j.biortech.2017.08.178 [17] LAN L H, LI J, FENG Q, et al. Enhanced current production of the anode modified by microalgae derived nitrogen-rich biocarbon for microbial fuel cells[J]. International Jour-nal of Hydrogen Energy,2020,45(6):3833-3839. [18] 谭笑. 锰改性生物炭材料的制备及其对镉砷污染土壤的修复效果研究[D]. 北京: 北京化工大学, 2020.TAN Xiao. Preparation of manganese modified biochar and its remediation effect on cadmium and Arsenic contami-nated soil[D]. Beijing: Beijing University of Chemical Technology, 2020(in Chinese). [19] ZHANG K, SUN P, FAYE M C A S, et al. Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation[J]. Carbon,2018,130:730-740. doi: 10.1016/j.carbon.2018.01.036 [20] XIAO C, WEN D, ZHONG T, et al. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes[J]. Applied Catalysis B: Environmental, 2018, 225: 243-257. [21] CAZETTA A L, ZHANG T, SILVA T L, et al. Bone char-derived metal-free N-and S-co-doped nanoporous carbon and its efficient electrocatalytic activity for hydrazine oxidation[J]. Applied Catalysis B: Environmental,2018,225:30-39. doi: 10.1016/j.apcatb.2017.11.050 [22] LI L, LAI C, HUANG F, et al. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and Fe-Mn binary oxides[J]. Water Research,2019,160:238-248. doi: 10.1016/j.watres.2019.05.081 [23] LI C, WU M, LIU R. High-performance bifunctional oxygen electrocatalysts for zinc-air batteries over mesoporous Fe/Co-NC nanofibers with embedding FeCo alloy nanoparticles[J]. Applied Catalysis B: Environmental,2019,244:150-158. doi: 10.1016/j.apcatb.2018.11.039 [24] PIERRI L, GEMENETZI A, MAVROGIORGOU A, et al. Biochar as supporting material for heterogeneous Mn(II) catalysts: Efficient olefins epoxidation with H2O2[J]. Molecular Catalysis,2020,489:110946. doi: 10.1016/j.mcat.2020.110946 [25] LU Z, LIU B, DAI W, et al. Carbon network framework derived iron-nitrogen co-doped carbon nanotubes for enhanced oxygen reduction reaction through metal salt-assisted polymer blowing strategy[J]. Applied Surface Science,2019,463:767-774. doi: 10.1016/j.apsusc.2018.08.231 [26] HE C, ZHANG T, SUN F, et al. Fe/N co-doped mesoporous carbon nanomaterial as an efficient electrocatalyst for oxygen reduction reaction[J]. Electrochimica Acta,2017,231:549-556. doi: 10.1016/j.electacta.2017.01.104 [27] HUANG D, ZHANG Q, ZHANG C, et al. Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline[J]. Chemical Engineering Journal,2020,391:123532. doi: 10.1016/j.cej.2019.123532 [28] MA W, DU Y, WANG N, et al. ZIF-8 derived nitrogen-doped porous carbon as metal-free catalyst of peroxymonosulfate activation[J]. Environmental Science and Pollution Research,2017,24(19):16276-16288. doi: 10.1007/s11356-017-9191-2 [29] HAO H, ZHANG Q, QIU Y, et al. Insight into the degradation of Orange G by persulfate activated with biochar modified by iron and manganese oxides: Synergism between Fe and Mn[J]. Journal of Water Process Engineering,2020,37:101470. doi: 10.1016/j.jwpe.2020.101470 [30] ZHU K, WANG X, GENG M, et al. Catalytic oxidation of clofibric acid by peroxydisulfate activated with wood-based biochar: Effect of biochar pyrolysis temperature, performance and mechanism[J]. Chemical Engineering Journal,2019,374:1253-1263. doi: 10.1016/j.cej.2019.06.006 [31] 沈启斌. 二氧化锰改性药渣生物炭的制备及其去除水中四环素的研究[D]. 广州: 华南理工大学, 2020.SHEN Qibin. Preparation of manganese dioxide modified residue biochar and its removal of tetracycline from water[D]. Guangzhou: South China University of Technology, 2020(in Chinese). [32] HU X, ZHANG H, SUN Z. Adsorption of low concentration ceftazidime from aqueous solutions using impregnated activated carbon promoted by iron, copper and aluminum[J]. Applied Surface Science,2017,392:332-341. doi: 10.1016/j.apsusc.2016.09.047 [33] QIU Y, ZHANG Q, WANG Z, et al. Degradation of anthraquinone dye reactive blue 19 using persulfate activated with Fe/Mn modified biochar: Radical/non-radical mechanisms and fixed-bed reactor study[J]. Science of the Total Environment,2021,758:143584. doi: 10.1016/j.scitotenv.2020.143584 [34] WANG J, WANG S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal,2018,334:1502-1517. doi: 10.1016/j.cej.2017.11.059 [35] CHEN L, JIANG X, XIE R, et al. A novel porous biochar-supported Fe-Mn composite as a persulfate activator for the removal of acid red 88[J]. Separation and Purification Technology,2020,250:117232. doi: 10.1016/j.seppur.2020.117232 [36] LI H, SHAN C, PAN B. Development of Fe-doped g-C3N4/graphite mediated peroxymonosulfate activation for degradation of aromatic pollutants via nonradical pathway[J]. Science of the Total Environment,2019,675:62-72. doi: 10.1016/j.scitotenv.2019.04.171 [37] WANG W L, WU Q Y, HUANG N, et al. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species[J]. Water Research,2016,98:190-198. doi: 10.1016/j.watres.2016.04.015 [38] LONG Y, HUANG Y, WU H, et al. Peroxymonosulfate activation for pollutants degradation by Fe-N-codoped carbonaceous catalyst: Structure-dependent performance and mechanism insight[J]. Chemical Engineering Journal,2019,369:542-552. doi: 10.1016/j.cej.2019.03.097 [39] QI C, LIU X, MA J, et al. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants[J]. Chemosphere,2016,151:280-288. doi: 10.1016/j.chemosphere.2016.02.089 [40] LI X, HUANG X, XI S, et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis[J]. Journal of the American Chemical Society,2018,140(39):12469-12475. doi: 10.1021/jacs.8b05992 [41] ZHU S, LI X, KANG J, et al. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants[J]. Environmental Science & Technology,2018,53(1):307-315. [42] YU J, FENG H, TANG L, et al. Metal-free carbon materials for persulfate-based advanced oxidation process: Microstructure, property and tailoring[J]. Progress in Materials Science,2020,111:100654. doi: 10.1016/j.pmatsci.2020.100654 [43] ZHU K, BIN Q, SHEN Y, et al. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants[J]. Chemical Engineering Jour-nal,2020,402:126090. doi: 10.1016/j.cej.2020.126090 [44] DUAN P, MA T, YUE Y, et al. Fe/Mn nanoparticles encapsulated in nitrogen-doped carbon nanotubes as a peroxymonosulfate activator for acetamiprid degradation[J]. Environmental Science: Nano,2019,6(6):1799-1811. doi: 10.1039/C9EN00220K [45] WANG Y, XIE Y, SUN H, et al. 2D/2D nano-hybrids of γ-MnO2 on reduced graphene oxide for catalytic ozonation and coupling peroxymonosulfate activation[J]. Journal of Hazardous Materials,2016,301:56-64. doi: 10.1016/j.jhazmat.2015.08.031 -






 下载:
下载: