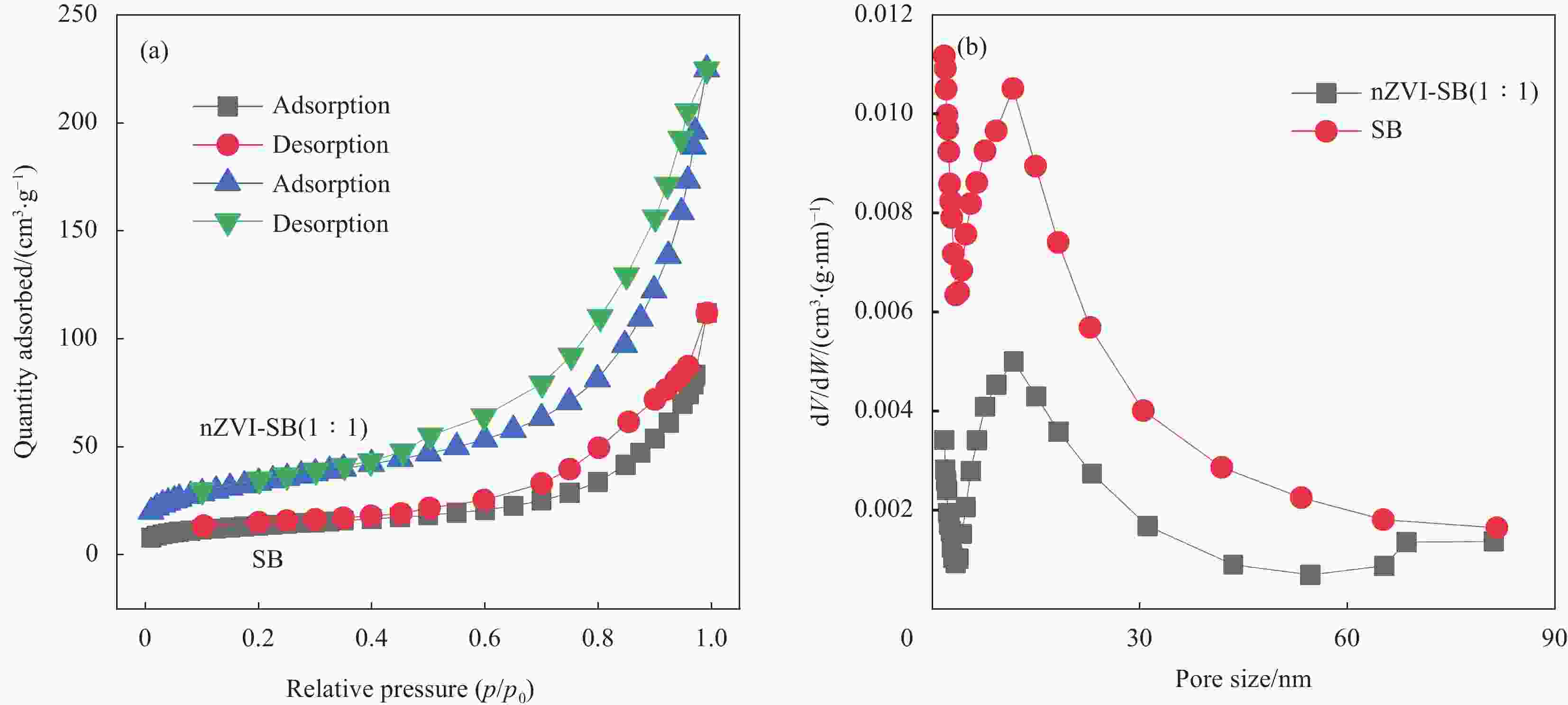
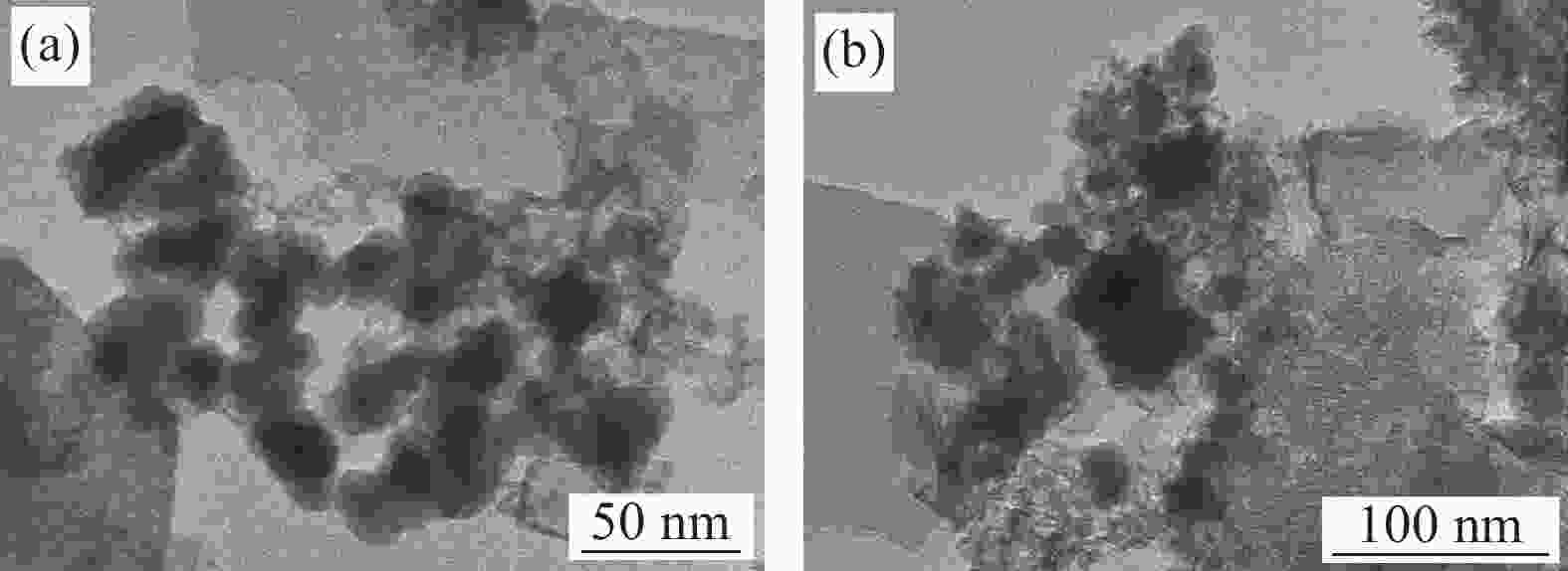
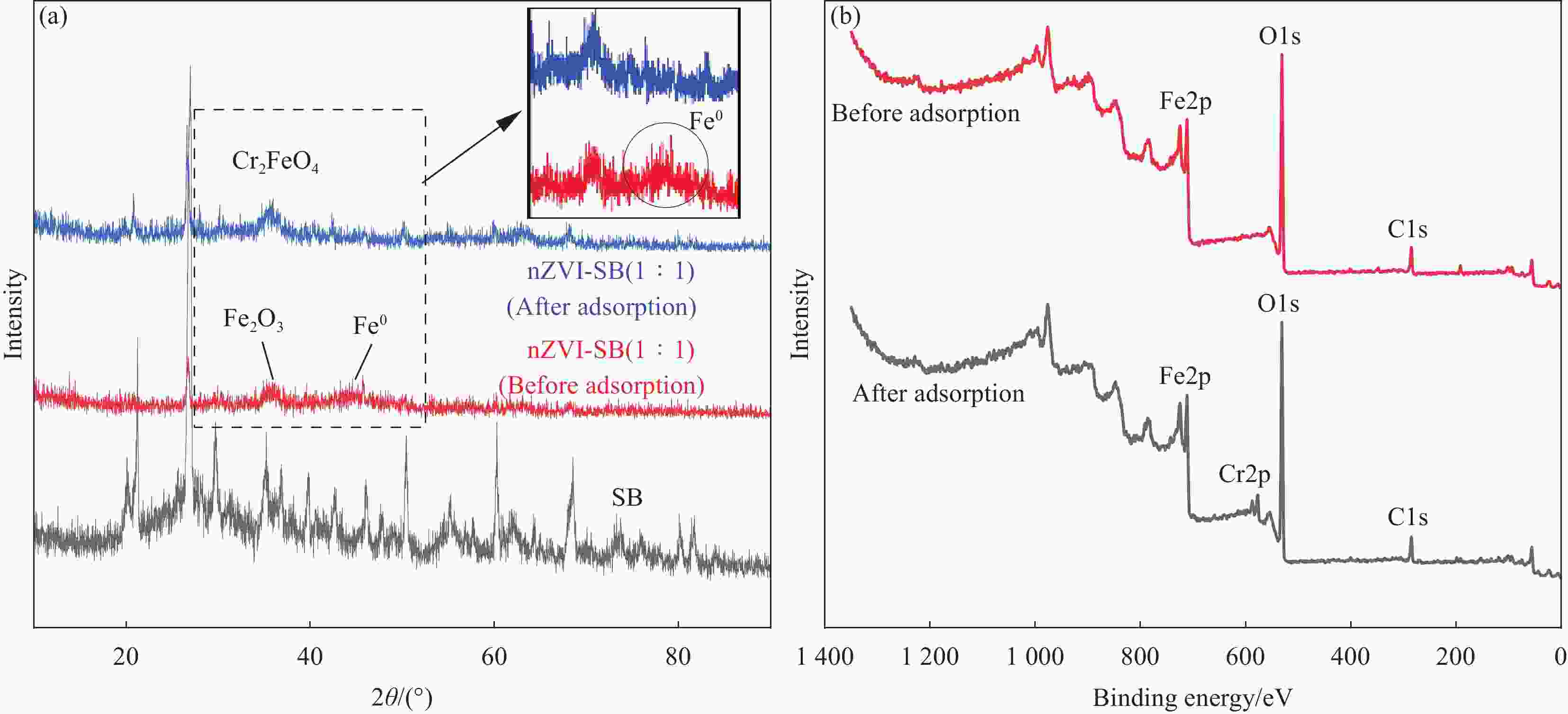
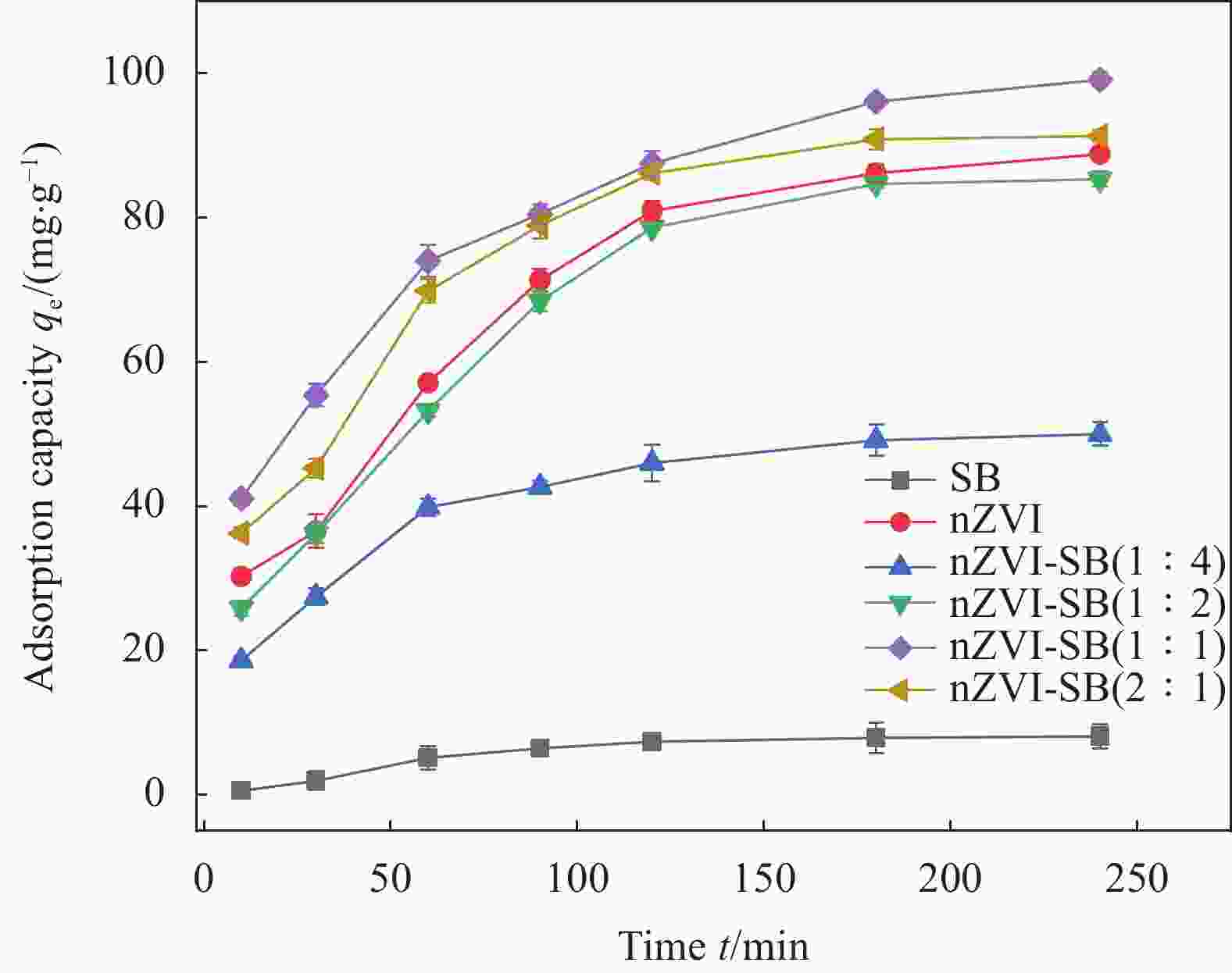
Performance and mechanism of Cr(VI) removal by sludge-derived biochar loaded with nanoscale zero-valent iron
-
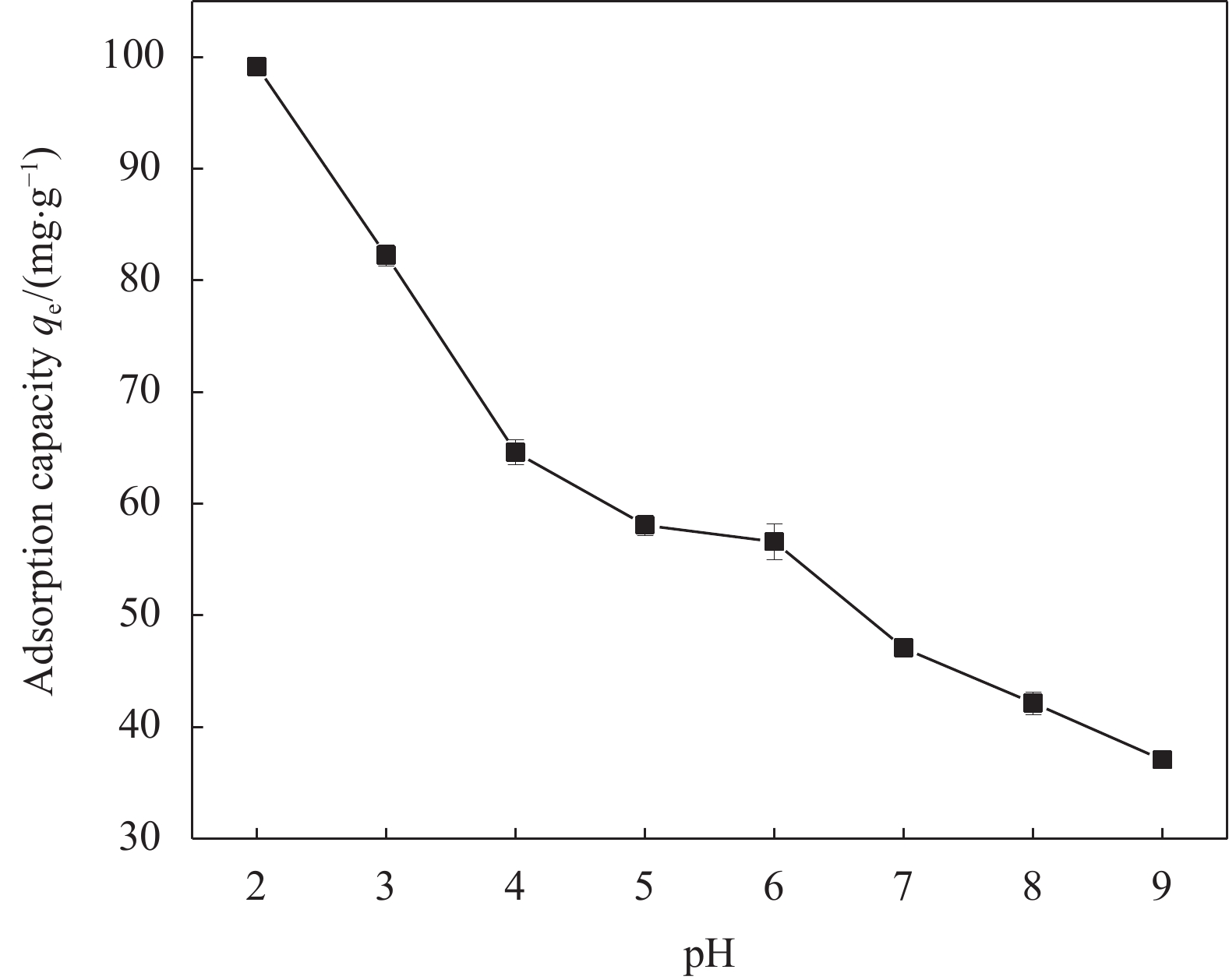
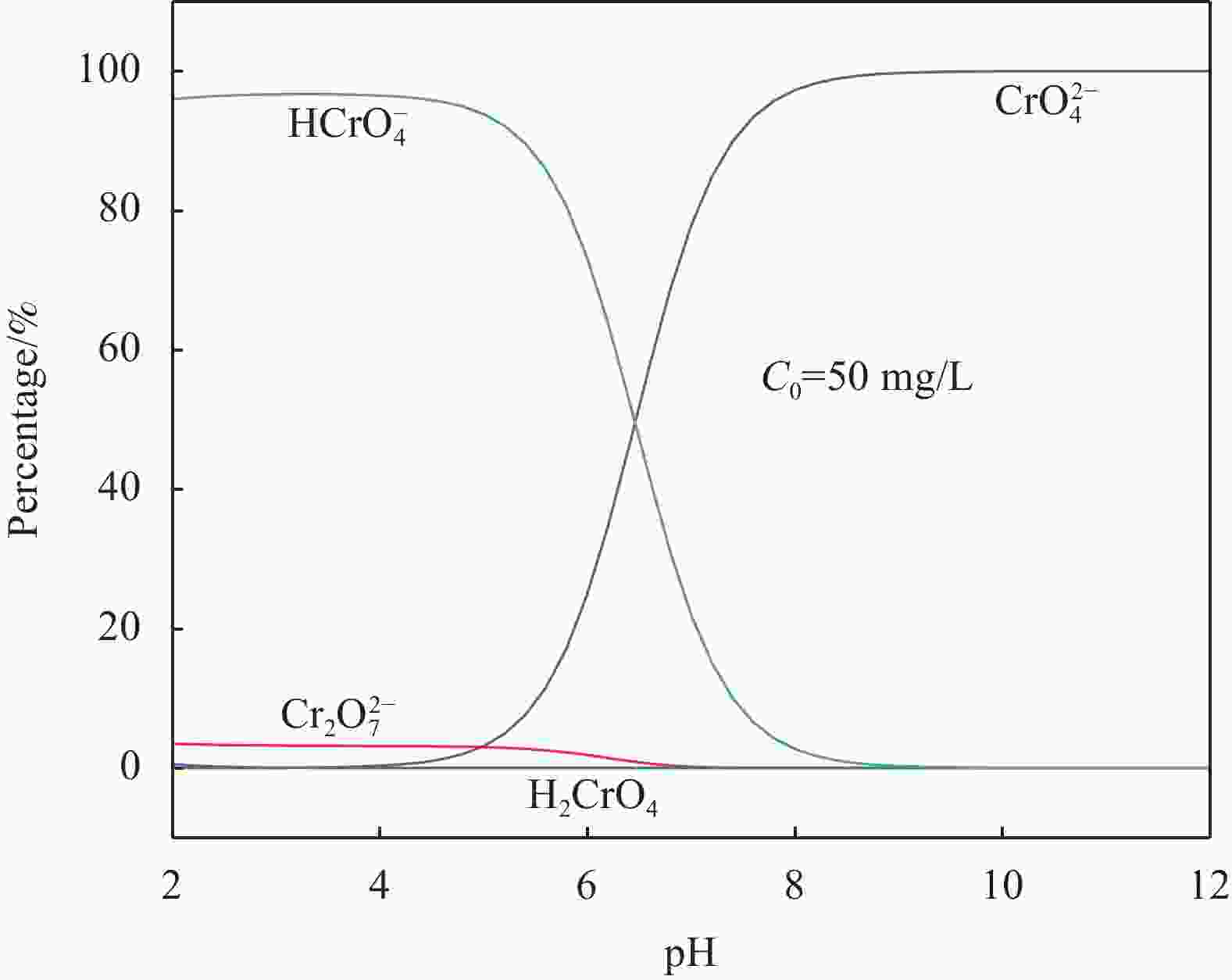
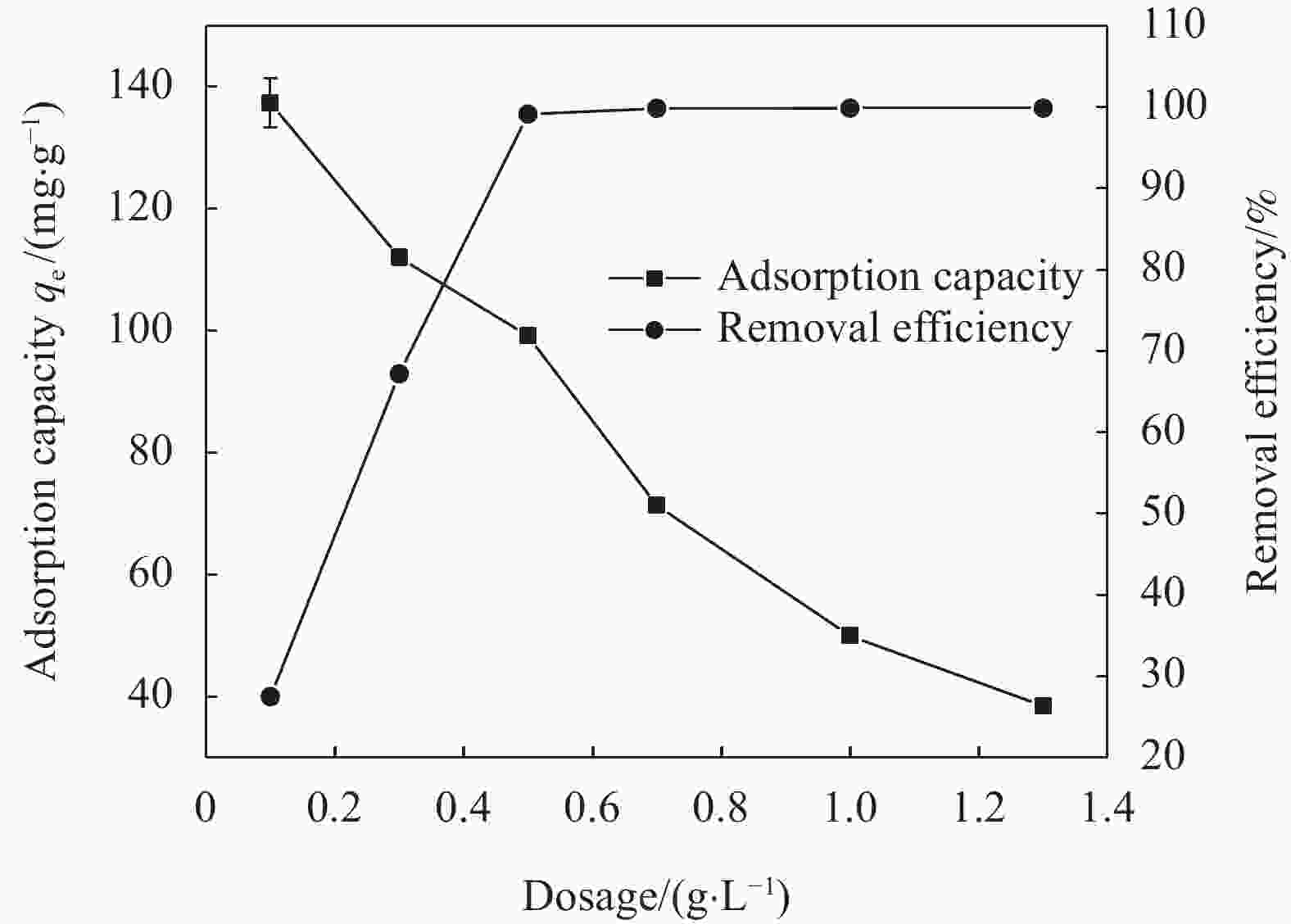
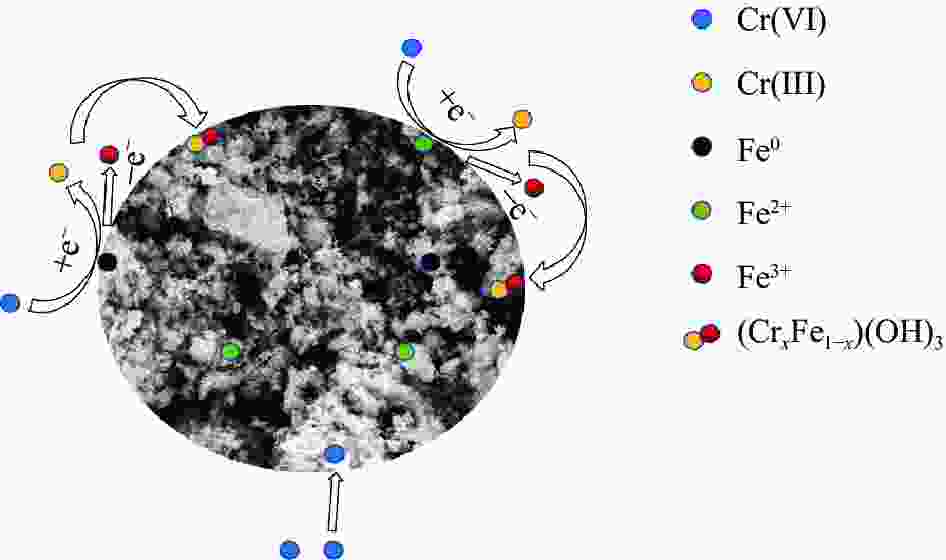
摘要: 针对电镀、冶金、印染等行业产生的含铬废水所导致的环境污染难题,以城市污泥热解获得的污泥基生物炭(SB)为载体,制备了污泥基生物炭负载纳米零价铁(nZVI-SB)材料用于去除水中的Cr(VI),探究了铁炭质量比、初始pH值、投加量、温度等因素对去除Cr(VI)的影响。通过SEM-EDS、XRD和XPS等手段对nZVI-SB去除Cr(VI)的机制进行分析。结果表明:nZVI-SB对Cr(VI)废水具有较好的去除能力。在投加量0.5 g/L、初始pH=2、温度40℃条件下, Fe与SB质量比为1∶1的nZVI-SB(1∶1)对Cr(VI)吸附量最大为150.60 mg/g。Cr(VI)去除过程可通过Langmuir吸附等温式与准二级动力学方程进行拟合。nZVI-SB对Cr(VI)去除机制主要包括吸附、还原和共沉淀。本文表明污泥基生物炭与纳米零价铁可以协同发挥除Cr(VI)作用。Abstract: Chromium-containing wastewater was generated in electroplating, metallurgy, printing and dyeing industries, which caused environmental pollution. The sludge-derived biochar (SB) was obtained from the pyrolysis of municipal sludge, and then loaded with nanoscale zero-valent iron (nZVI) to prepare sludge-derived biochar loaded with nanoscale zero-valent iron (nZVI-SB) for the removal of Cr(VI) from water. The effect of the iron to carbon mass ratio, initial pH value, dosage and temperature on the removal of Cr(VI) were explored. SEM-EDS, XRD and XPS were used to characterize the mechanisms of Cr(VI) removal. The results show that nZVI-SB has a desirable removal capacity for Cr(VI). Under the conditions of dosage 0.5 g/L, pH=2 and 40℃, the maximum adsorption capacity of Cr(VI) is 150.60 mg/g by nZVI-SB(1∶1) with a mass ratio of 1∶1 between Fe and SB. The Cr(VI) removal process can be fitted by Langmuir adsorption isotherm and pseudo-second-order kinetic equations. The removal mechanisms of Cr(VI) mainly include adsorption, reduction and co-precipitation. The present study confirms SB and nZVI can synergically remove Cr(VI).
-
Key words:
- sludge-derived biochar /
- nanoscale zero-valent iron /
- Cr(VI) /
- adsorption capacity /
- mechanism /
- reduction
-
表 1 样品名称缩写
Table 1. Abbreviation name of samples
Sample Fe/wt% SB/wt% nZVI-SB(1∶4) 20.0 80.0 nZVI-SB(1∶2) 33.3 66.7 nZVI-SB(1∶1) 50.0 50.0 nZVI-SB(2∶1) 66.7 33.3 Notes: nZVI—Nanoscale zero-valent iron; SB—Sludge-based biochar. 表 2 污泥及污泥基生物炭(SB)消解液中重金属浓度
Table 2. Heavy metal concentrations in digestion solution of sludge and sludge-based biochar (SB)
(mg·L−1) Sample Zn Pb Cu Ba Cd Cr Sludge 5.28 0.34 2.37 7.32 0.06 0.14 SB 7.81 0.51 3.01 8.67 0.09 0.13 Specified value
in GB/T
5085.3—2007[18]100.00 5.00 100.00 100.00 1.00 15.00 表 3 SB和nZVI-SB的元素组成
Table 3. Elemental composition of SB and nZVI-SB
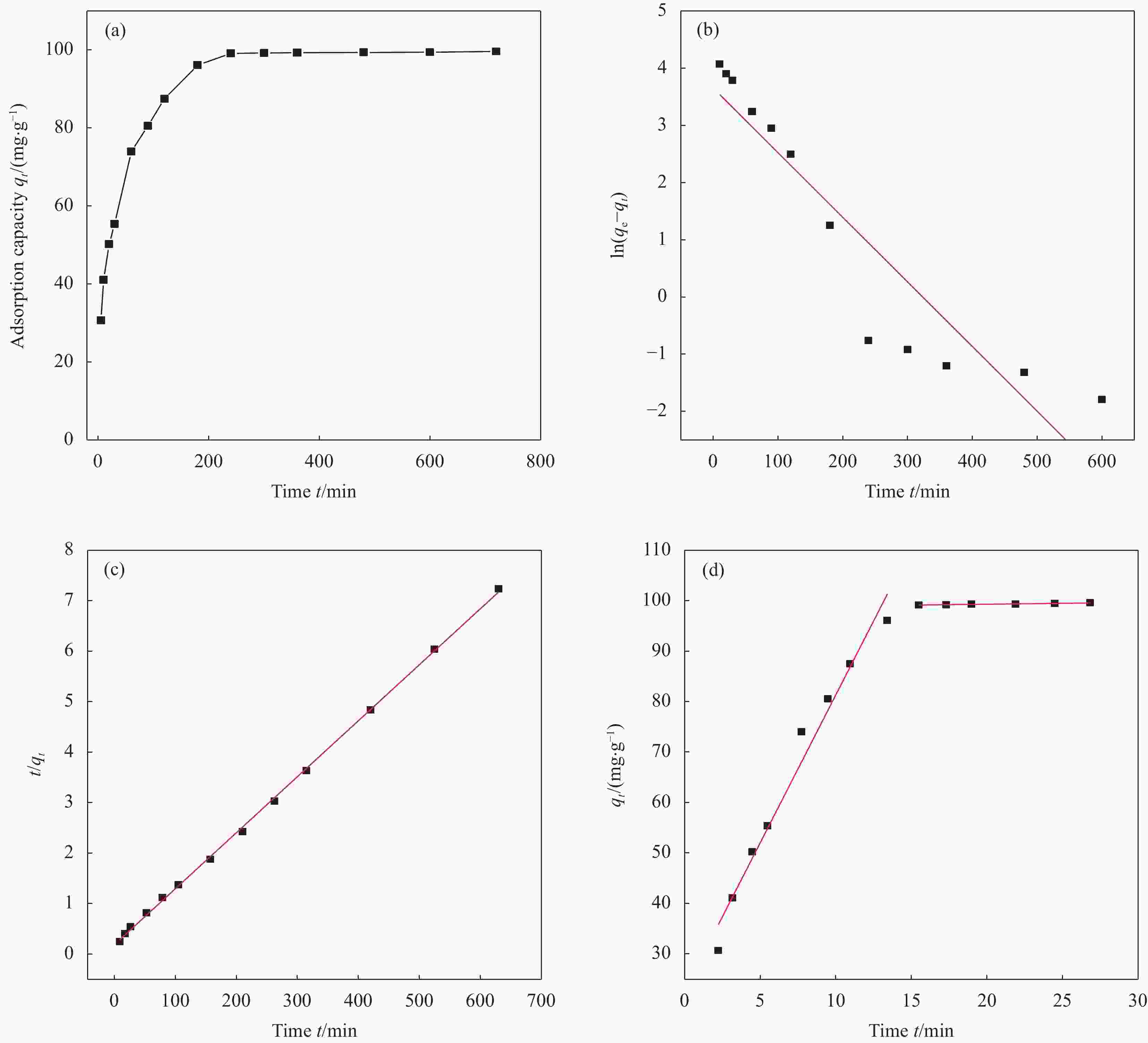
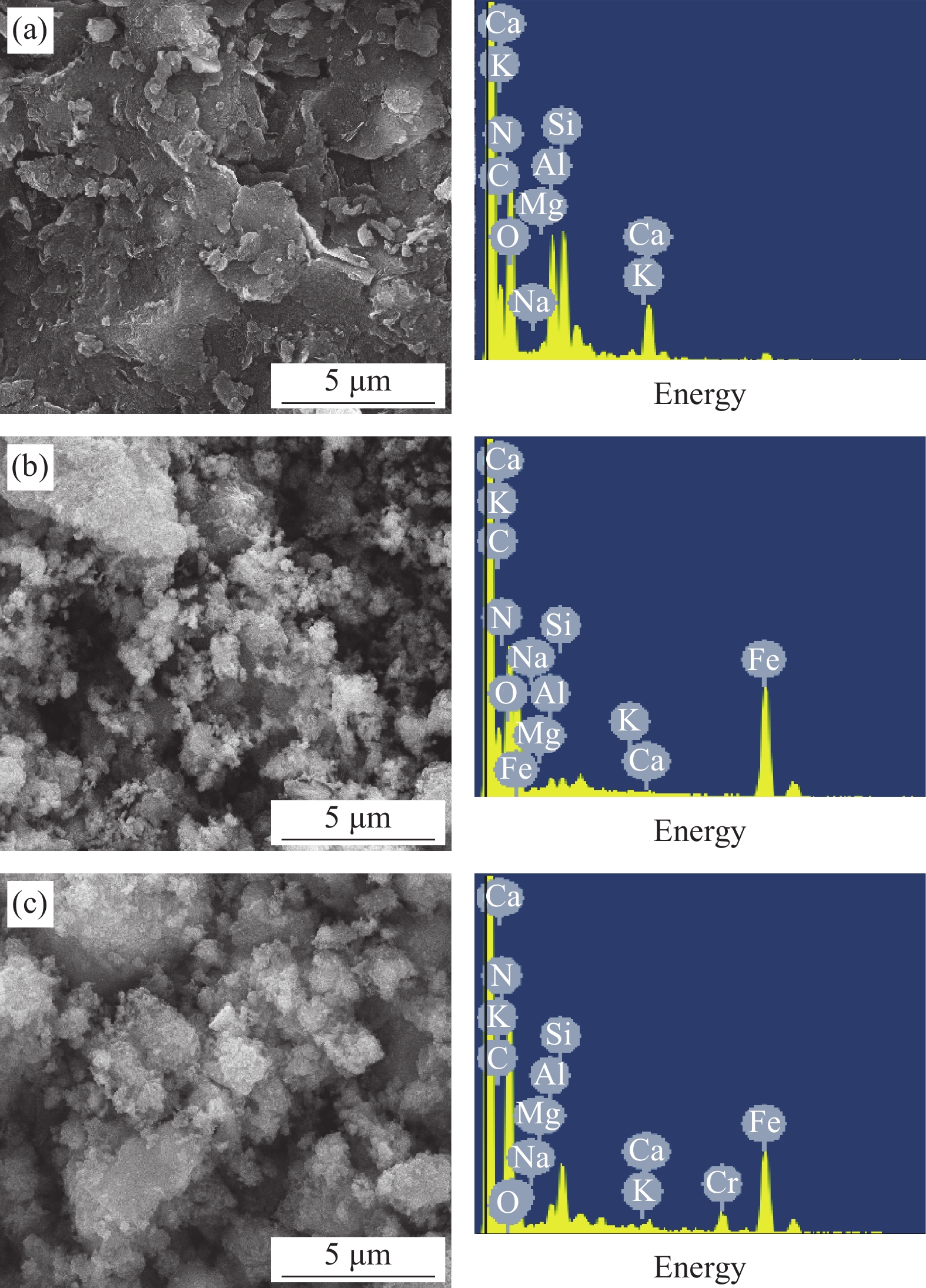
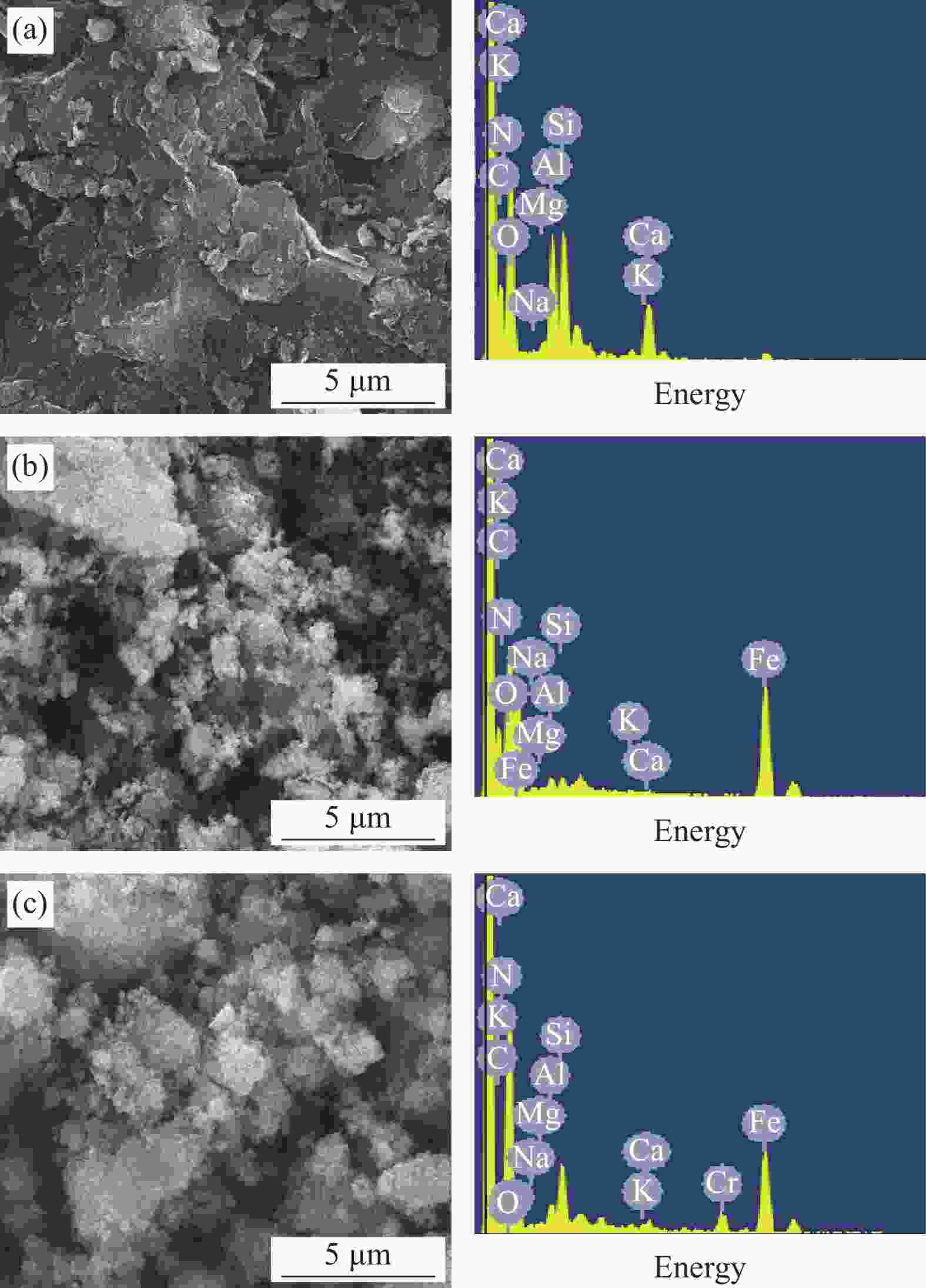
wt% Sample C N O Na Mg Al Si K Ca Cr Fe SB 40.48 8.46 34.94 0.19 0.39 3.26 6.43 0.88 3.05 0.33 1.59 nZVI-SB(1∶4) 36.63 7.56 33.05 0.22 0.48 0.63 0.76 0.28 0.30 0.22 19.87 nZVI-SB(1∶2) 30.41 6.41 27.55 0.18 0.36 0.51 0.62 0.23 0.24 0.18 33.31 nZVI-SB(1∶1) 23.92 3.11 21.45 0.14 0.27 0.28 0.35 0.19 0.21 0.16 49.92 nZVI-SB(2∶1) 16.48 1.76 14.45 0.06 0.13 0.11 0.16 0.08 0.08 0.06 66.63 表 4 nZVI-SB(1∶1)对Cr(VI)的吸附动力学参数
Table 4. Adsorption kinetic parameters of Cr(VI) adsorption by nZVI-SB(1∶1)
Intraparticle diffusion model qe/(mg·g−1) K/(min−1) R2 Kd/(mg·(m·min0.5)−1) C R2 Quasi-first order dynamics model 38.57 0.0113 0.855 5.858 22.731 0.971 Quasi-second-stage dynamics model 103.07 0.0005 0.999 0.038 98.551 0.946 Notes: qe—Equilibrium adsorption capacity; K—Adsorption rate constant; R2—Linear correlation coefficient; Kd—Particle diffusion constants; C—Constant. 表 5 nZVI-SB(1∶1)对Cr(VI)的吸附等温线拟合参数
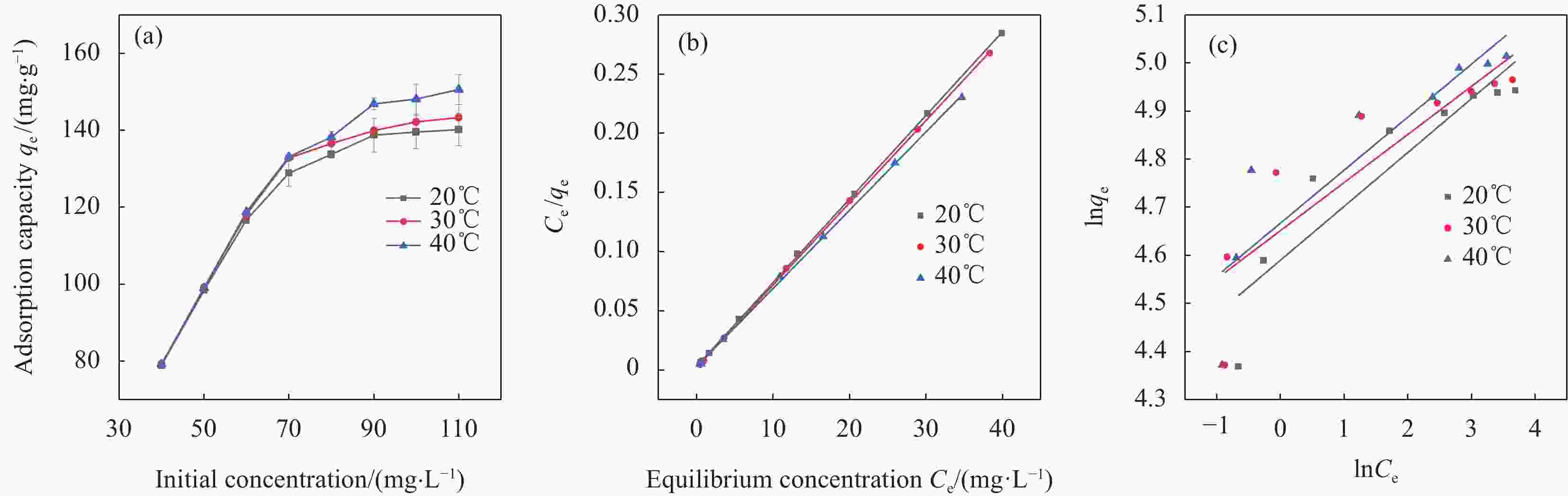
Table 5. Adsorption isotherm fitting parameters of Cr(VI) by nZVI-SB(1∶1)
Temperature/℃ Langmuir Freundlich qm/(mg·g−1) KL R2 KF n R2 20 141.55 0.457 0.999 98.48 8.93 0.821 30 143.84 0.338 0.999 104.73 9.99 0.744 40 151.23 0.433 0.999 106.38 9.07 0.766 Notes: qm—Maximum adsorption capacity; KL—Adsorption equilibrium constant of the Langmuir model; KF—Adsorption equilibrium constant of Freundlich model; n—Constants related to the adsorption intensity. 表 6 nZVI-SB(1∶1)和其他吸附剂对Cr(VI)的吸附能力比较
Table 6. Comparison of the adsorption capacity of Cr(VI) by nZVI-SB(1∶1) and other adsorbents
Adsorbent pH Temperature/℃ Adsorption
capacity/(mg·g−1)Ref. Sludge biochar (500℃) 7 25 7.93 [13] Bentonite-supported nanoscale zero-valent iron (B-nZVI) 5 25 39.48 [23] Ficus carica biosorbent 3 30 19.68 [36] Magnetic nanoparticle-Phosphorene-Titanium nano tubes (MNP-PN-TNT) 9 25 35.00 [2] Nanoscale zero-valent iron grafted on acid-activated attapulgite (A-nZVI) 7 27 4.94 [31] HNO3 modified quinoa biochar 4 — 55.85 [37] ZnO modified hyacinth biochar — 25 43.48 [38] Halloysite nanotubes/ploy composites 2 25 855.66 [39] nZVI-SB(1∶1) 2 40 150.60 This study 表 7 nZVI-SB(1∶1)去除Cr(VI)前、去除Cr(VI)后的C1s、O1s、Fe2p和Cr2p XPS光谱的成分和相应的相对百分比
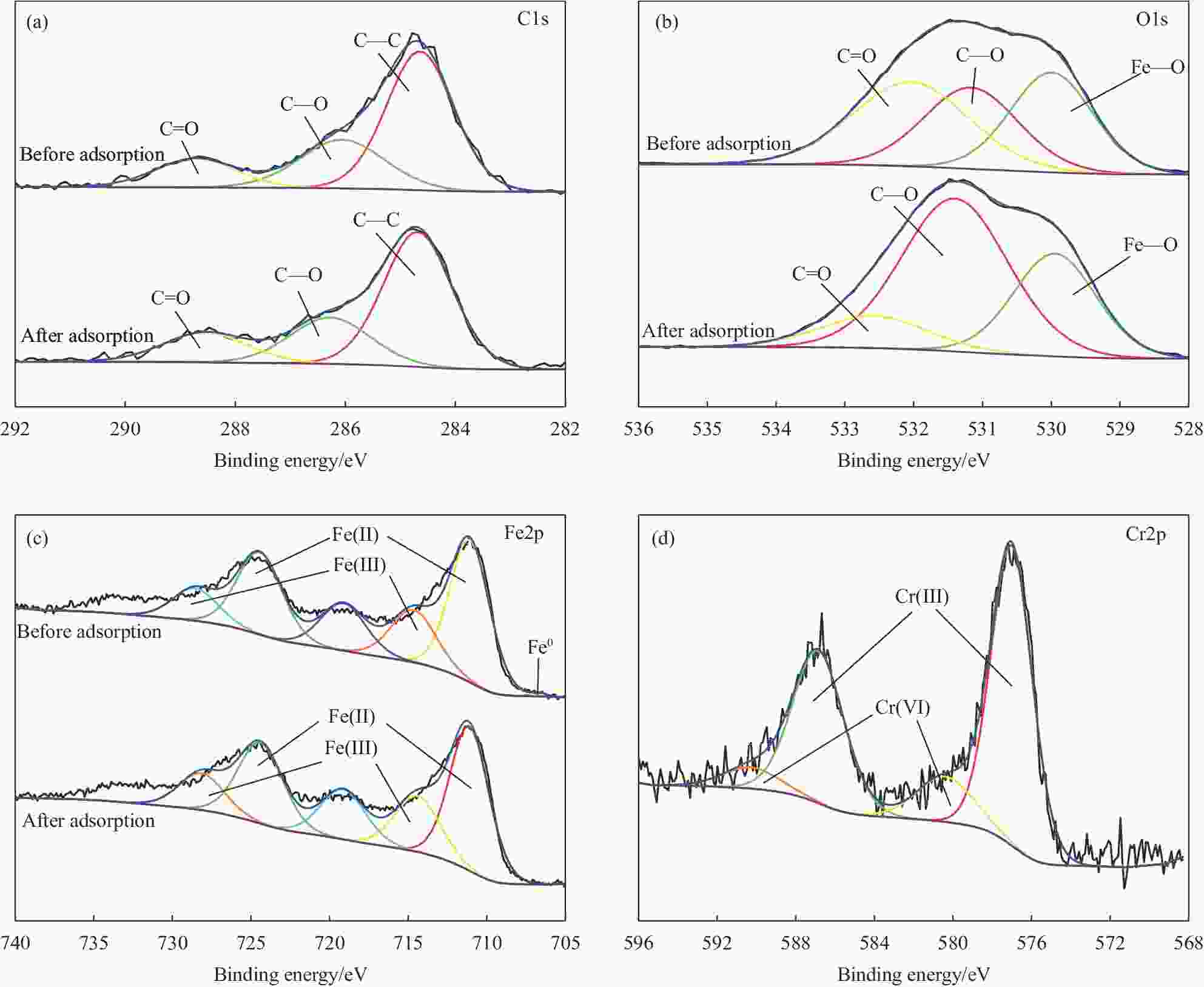
Table 7. Composition and relative percents of C1s, O1s, Fe2p and Cr2p XPS spectra before and after Cr(VI) removal by nZVI-SB(1∶1)
Components Relative percentage/% Binding energy/eV Before After Before After C1s C—C 58.99 59.78 284.64 284.69 C—O 26.24 24.00 286.06 286.27 C=O 14.77 16.22 288.54 288.72 O1s Fe—O 32.07 30.05 529.95 529.99 C—O 29.94 57.76 531.17 531.40 C=O 37.99 12.19 532.04 532.58 Fe2p Fe0 0.36 0.00 706.70 — Fe(II) 70.31 67.96 711.10/724.39 711.11/724.45 Fe(III) 29.33 32.04 714.41/728.15 714.44/728.55 Cr2p Cr(III) — 84.39 — 577.01/586.85 Cr(VI) — 15.61 — 580.40/590.28 -
[1] GAO Q Y, LIN D G, FAN Y J, et al. Visible light induced photocatalytic reduction of Cr(VI) by self-assembled and amorphous Fe-2MI[J]. Chemical Engineering Journal,2019,374:10-19. doi: 10.1016/j.cej.2019.05.151 [2] LIN Y J, CHEN J J, CAO W Z, et al. Novel materials for Cr(VI) adsorption by magnetic titanium nanotubes coated phosphorene[J]. Journal of Molecular Liquids,2019,287:110826. doi: 10.1016/j.molliq.2019.04.103 [3] GHADIKOLAEI N F, KOWSARI E, BALOU S, et al. Preparation of porous biomass-derived hydrothermal carbon modified with terminal amino hyperbranched polymer for prominent Cr(VI) removal from water[J]. Bioresource Technology,2019,288:121545. doi: 10.1016/j.biortech.2019.121545 [4] ZHANG W Y, QIAN L B, OUYANG D, et al. Effective remo-val of Cr(VI) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: Enhanced adsorption and crystallization[J]. Chemosphere,2019,221:683-692. doi: 10.1016/j.chemosphere.2019.01.070 [5] 孙建德. 含铬废水的处理现状[J]. 湖南有色金属, 2013, 29(5):59-62. doi: 10.3969/j.issn.1003-5540.2013.05.017SUN Jiande. Current situation on the treatment for chromium-containing wastewater[J]. Hunan Nonferrous Metals,2013,29(5):59-62(in Chinese). doi: 10.3969/j.issn.1003-5540.2013.05.017 [6] 秦泽敏, 董黎明, 刘平, 等. 零价纳米铁吸附去除水中六价铬的研究[J]. 中国环境科学, 2014, 34(12):3106-3111.QIN Zemin, DONG Liming, LIU Ping, et al. Removal Cr6+ from water using nanoscale zero-valent iron[J]. China Environmental Science,2014,34(12):3106-3111(in Chinese). [7] GUAN X H, SUN Y K, QIN H J, et al. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994-2014)[J]. Water Research,2015,75:224-248. doi: 10.1016/j.watres.2015.02.034 [8] SHI L N, ZHANG X, CHEN Z L. Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron[J]. Water Research,2011,45(2):886-892. doi: 10.1016/j.watres.2010.09.025 [9] PETALA E, DIMOS K, DOUVALIS A, et al. Nanoscale zero-valent iron supported on mesoporous silica: Characterization and reactivity for Cr(VI) removal from aqueous solution[J]. Journal of Hazardous Materials,2013,261:295-306. doi: 10.1016/j.jhazmat.2013.07.046 [10] ZHU H J, JIA Y F, WU X, et al. Removal of arsenic from water by supported nano zero-valent iron on activated carbon[J]. Journal of Hazardous Materials,2009,172(2-3):1591-1596. doi: 10.1016/j.jhazmat.2009.08.031 [11] SU H J, FANG Z Q, TSANG P E, et al. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles[J]. Journal of Hazardous Materials,2016,318:533-540. doi: 10.1016/j.jhazmat.2016.07.039 [12] 刘剑, 黄莉, 彭钢, 等. 颗粒活性炭载纳米零价铁去除水中的Cr(Ⅵ)[J]. 过程工程学报, 2019, 19(4):714-720.LIU Jian, HUANG Li, PENG Gang, et al. Removal of Cr(VI) from water by granular activated carbon supported nanoscale zero-valent iron[J]. The Chinese Journal of Process Engineering,2019,19(4):714-720(in Chinese). [13] 陈林, 平巍, 闫彬, 等. 不同制备温度下污泥生物炭对Cr(Ⅵ)的吸附特性[J]. 环境工程, 2020, 38(8):119-124.CHEN Lin, PING Wei, YAN Bin, et al. Adsorption characteristics of Cr(Ⅵ) by sludge biochar under different pyrolysis temperatures[J]. Environmental Engineering,2020,38(8):119-124(in Chinese). [14] 莫官海, 谢水波, 曾涛涛, 等. 污泥基生物炭处理酸性含U(VI)废水的效能与机理[J]. 化工学报, 2020, 71(5):2352-2362.MO Guanhai, XIE Shuibo, ZENG Taotao, et al. The efficiency and mechanism of U(VI) removal from acidic wastewater by sewage sludge-derived biochar[J]. CIESC Journal,2020,71(5):2352-2362(in Chinese). [15] CHEN X, FAN G J, LI H B, et al. Nanoscale zero-valent iron particles supported on sludge-based biochar for the removal of chromium (VI) from aqueous system[J]. Environmental Science and Pollution Research,2021,29(3):3853-3863. [16] ZHOU M, ZHANG C G, YUAN Y F, et al. Pinewood outperformed bamboo as feedstock to prepare biochar-supported zero-valent iron for Cr6+ reduction[J]. Environmental Research,2020,187:109695. doi: 10.1016/j.envres.2020.109695 [17] DIAO Z H, DU J J, JIANG D, et al. Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: Coexistence effect and mechanism[J]. Science of the Total Environment,2018,642:505-515. doi: 10.1016/j.scitotenv.2018.06.093 [18] 国家环境保护总局, 国家质量监督检验检疫总局. 危险废物鉴别标准浸出毒性鉴别: GB/T 5085.3—2007[S]. 北京: 中国环境科学出版社, 2007.Environmental Protection Administration of China, General Administration of Quality Supervision, Inspection and Quarantine. Identification standards for hazardous wastes identification for extraction toxicity: GB/T 5085.3—2007[S]. Beijing: China Environmental Science Press, 2007(in Chinese). [19] ZHANG Y T, JIAO X Q, LIU N, et al. Enhanced removal of aqueous Cr(VI) by a green synthesized nanoscale zero-valent iron supported on oak wood biochar[J]. Chemosphere,2020,245:125542. doi: 10.1016/j.chemosphere.2019.125542 [20] MA F F, PHILIPPE B, ZHAO B W, et al. Simultaneous adsorption and reduction of hexavalent chromium on biochar-supported nanoscale zero-valent iron (nZVI) in aqueous solution[J]. Water Science and Technology,2020,82(7):1339-1349. doi: 10.2166/wst.2020.392 [21] CHOI H, AL-ABED S R, AGARWAL S, et al. Synthesis of reactive nano-Fe/Pd bimetallic system-impregnated activated carbon for the simultaneous adsorption and dechlorination of PCBs[J]. Chemistry of Materials,2008,20(11):3649-3655. doi: 10.1021/cm8003613 [22] PHOUNGTHONG K, ZHANG H, SHAO L M, et al. Leaching characteristics and phytotoxic effects of sewage sludge biochar[J]. Journal of Material Cycles and Waste Management,2018,20(4):2089-2099. doi: 10.1007/s10163-018-0763-0 [23] DIAO Z H, XU X R, JIANG D, et al. Bentonite-supported nanoscale zero-valent iron/persulfate system for the simultaneous removal of Cr(VI) and phenol from aqueous solutions[J]. Chemical Engineering Journal,2016,302:213-222. doi: 10.1016/j.cej.2016.05.062 [24] SHI L N, DU J H, CHEN Z L, et al. Functional kaolinite supported Fe/Ni nanoparticles for simultaneous catalytic remediation of mixed contaminants (lead and nitrate) from wastewater[J]. Journal of Colloid and Interface Science,2014,428:302-307. doi: 10.1016/j.jcis.2014.04.059 [25] YI Y, WANG X Y, MA J, et al. An efficient Egeria najas-derived biochar supported nZVI composite for Cr(VI) removal: Characterization and mechanism investigation based on visual MINTEQ model[J]. Environmental Research,2020,189:109912. doi: 10.1016/j.envres.2020.109912 [26] DONG H R, DENG J M, XIE Y K, et al. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution[J]. Journal of Hazardous Materials,2017,332:79-86. doi: 10.1016/j.jhazmat.2017.03.002 [27] FANG Y, WU X G, DAI M, et al. The sequestration of aqueous Cr(VI) by zero valent iron-based materials: From synthesis to practical application[J]. Journal of Cleaner Production,2021,312:127678. doi: 10.1016/j.jclepro.2021.127678 [28] DONG H R, ZHANG C, HOU K J, et al. Removal of trichloroethylene by biochar supported nanoscale zero-valent iron in aqueous solution[J]. Separation and Purification Technology,2017,188:188-196. doi: 10.1016/j.seppur.2017.07.033 [29] HUANG L H, ZHOU S J, JIN F, et al. Characterization and mechanism analysis of activated carbon fiber felt-stabilized nanoscale zero-valent iron for the removal of Cr(VI) from aqueous solution[J]. Colloids and Surfaces A-Physicochemical and Engineering Aspects,2014,447:59-66. [30] CHOI K, LEE W. Enhanced degradation of trichloroethylene in nano-scale zero-valent iron Fenton system with Cu(II)[J]. Journal of Hazardous Materials,2012,211:146-153. [31] QUAN G X, ZHANG J, GUO J, et al. Removal of Cr(VI) from aqueous solution by nanoscale zero-valent iron grafted on acid-activated attapulgite[J]. Water Air and Soil Pollution,2014,225(6):1979. [32] QIU Y, ZHANG Q, GAO B, et al. Removal mechanisms of Cr(VI) and Cr(III) by biochar supported nanosized zero-valent iron: Synergy of adsorption, reduction and transformation[J]. Environmental Pollution,2020,265:115018. doi: 10.1016/j.envpol.2020.115018 [33] LIU L H, LIU X, WANG D Q, et al. Removal and reduction of Cr(VI) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge[J]. Journal of Cleaner Production,2020,257:120562. doi: 10.1016/j.jclepro.2020.120562 [34] WANG H B, CAI J Y, LIAO Z W, et al. Black liquor as biomass feedstock to prepare zero-valent iron embedded biochar with red mud for Cr(VI) removal: Mechanisms insights and engineering practicality[J]. Bioresource Technology,2020,311:123553. doi: 10.1016/j.biortech.2020.123553 [35] AWANG N A, SALLEH W N W, ISMAIL A F, et al. Adsorption behavior of chromium(VI) onto regenerated cellulose membrane[J]. Industrial & Engineering Chemistry Research,2019,58(2):720-728. [36] GUPTA V K, PATHANIA D, AGARWAL S, et al. Removal of Cr(VI) onto Ficus carica biosorbent from water[J]. Environmental Science and Pollution Research,2013,20(4):2632-2644. doi: 10.1007/s11356-012-1176-6 [37] IMRAN M, KHAN Z U, IQBAL M M, et al. Effect of biochar modified with magnetite nanoparticles and HNO3 for efficient removal of Cr(VI) from contaminated water: A batch and column scale study[J]. Environmental Pollution,2020,261:114231. doi: 10.1016/j.envpol.2020.114231 [38] YU J D, JIANG C Y, GUAN Q Q, et al. Enhanced removal of Cr(VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth[J]. Chemosphere,2018,195:632-640. doi: 10.1016/j.chemosphere.2017.12.128 [39] 陈泽文, 周子晗, 吴美仪, 等. 埃洛石纳米管/聚间苯二胺复合材料去除Cr(Ⅵ)的性能[J]. 复合材料学报, 2020, 37(3):493-503.CHEN Zewen, ZHOU Zihan, WU Meiyi, et al. Adsorption properties of halloysite nanotubes/poly(m-phenylenediamine) composites for Cr(VI)[J]. Acta Materiae Compo-sitae Sinica,2020,37(3):493-503(in Chinese). [40] 席冬冬, 李晓敏, 熊子璇, 等. 生物炭负载纳米零价铁对污染土壤中铜钴镍铬的协同去除[J]. 环境工程, 2020, 38(6):58-66.XI Dongdong, LI Xiaomin, XIONG Zixuan, et al. Synergis-tic removal of Cu, Co, Ni and Cr from contaminated soil by biochar-supported nanoscale zero-valent iron[J]. Environmental Engineering,2020,38(6):58-66(in Chinese). [41] YOON I H, BANG S, CHANG J S, et al. Effects of pH and dissolved oxygen on Cr(VI) removal in Fe(0)/H2O systems[J]. Journal of Hazardous Materials,2011,186(1):855-862. doi: 10.1016/j.jhazmat.2010.11.074 [42] WANG Z, CHEN G H, WANG X R, et al. Removal of hexavalent chromium by bentonite supported organosolv lignin-stabilized zero-valent iron nanoparticles from wastewater[J]. Journal of Cleaner Production,2020,267:122009. doi: 10.1016/j.jclepro.2020.122009 -





 下载:
下载: