Research progress on preparation and energy storage properties of Sb2S3-based anode materials
-
摘要: 由于在低电位范围内的合金化/脱合金化反应机制,硫化锑(Sb2S3)材料的理论放电比容量高达946 mA·h·g−1,是一种有发展前景的锂/钠/钾离子电池负极材料。然而,在电化学反应过程中Sb2S3材料的聚集性和较差的导电性限制了离子/电子转移,导致了较差的电化学性能,严重阻碍了其实际应用。有必要对Sb2S3基负极材料的结构设计和储锂/钠/钾机制及近几年来的一些重要工作进行总结。本文综述了近年来Sb2S3基化合物材料的研究进展,主要包括合理的结构设计和/或与碳基材料结合等策略及所涉及的电化学反应机制,并提出了进一步改善Sb2S3化合物负极材料的展望。
-
关键词:
- Sb2S3基负极材料 /
- 电化学性能 /
- 锂离子电池(LIBs) /
- 钠离子电池(SIBs) /
- 钾离子电池(PIBs)
Abstract: Due to the alloying/dealloying reaction mechanism in the low potential range, the theoretical discharge specific capacity of antimony sulfide (Sb2S3) material is as high as 946 mA·h·g−1, which is a promising anode mater-ial for lithium/sodium/potassium ion batteries. However, the aggregation and poor conductivity of Sb2S3 materials limit ion/electron transfer, resulting in poor electrochemical performance and severely hindering its practical application. It is necessary to summarize the structural design and lithium/sodium/potassium storage mechanism of Sb2S3-based anode materials and some important work in recent years. This article reviews the research progress of Sb2S3 based compound materials in recent years, mainly including reasonable structure design and/or combining with carbon-based materials and the electrochemical reaction mechanism involved, and puts forward the prospect of further improving Sb2S3 compound anode materials. -
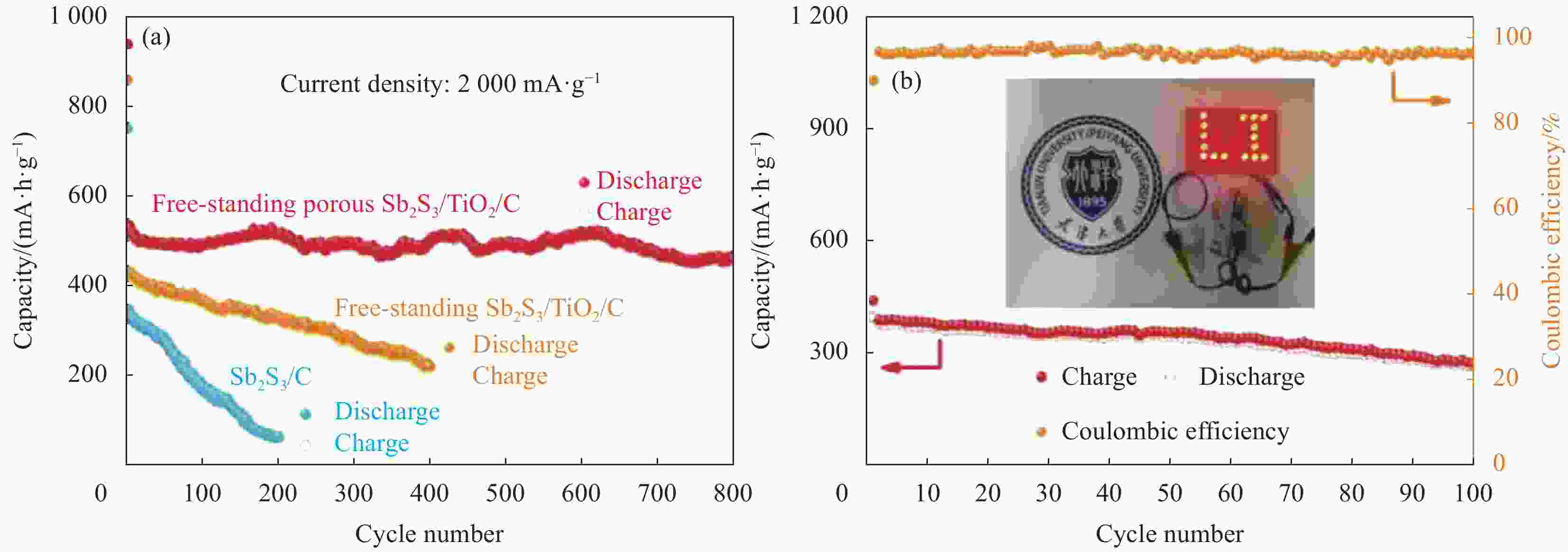
图 3 (a) 自支撑多孔Sb2S3/TiO2/C纳米纤维在LIBs中2000 mA·g−1下的循环性能;(b) 自支撑多孔Sb2S3/TiO2/C-LiFePO4全电池的循环性能图(插图:可点亮16个LED的全电池的数字照片)[33]
Figure 3. (a) Cycle performance of free-standing porous Sb2S3/TiO2/C nanofibers at 2000 mA·g−1 in LIBs; (b) Cycle performance of free-standing porous Sb2S3/TiO2/C-LiFePO4 full-cell (Inset: Digital photograph of a full cell that lights 16 LEDs)[33]
图 4 (a) MoS2-Sb@Sb2S3@C样品的制备示意图;(b) LIBs中MoS2-Sb@Sb2S3@C电极的倍率性能图;(c) 在电流密度为1 A·g−1时,LIBs中MoS2-Sb@Sb2S3@C电极的长期循环性能图[40]
Figure 4. (a) Schematic illustration of the preparation of MoS2-Sb@Sb2S3@C sample; (b) Rate performance of the MoS2-Sb@Sb2S3@C electrode in LIBs; (c) Long-term cycling performance of the MoS2-Sb@Sb2S3@C electrode at a current density of 1 A·g−1 in LIBs[40]
图 7 (a) Sb2S3-Bi2S3@C@还原氧化石墨烯(rGO)微棒形成示意图;(b) SIBs中Sb2S3-Bi2S3@rGO电极在8 A·g−1条件下的长期循环稳定性[58]
Figure 7. (a) Schematic illustration of the formation of the Sb2S3-Bi2S3@C@reduced graphene oxide (rGO) microrods; (b) Long-term cycling stability of Sb2S3-Bi2S3@rGO electrode at 8 A·g−1 in SIBs[58]
PDA—Polydopamine; GO—Graphene oxide
表 1 最近报道的Sb2S3基材料作为LIBs负极材料的合成方法和电化学性能
Table 1. Synthetic method and electrochemical property of Sb2S3-based electrodes used as anode for LIBs from recent reported
Sample Synthesis method Voltage
range/VCurrent
density/(A·g−1)Cycle number Final capacity/
(mA·h·g−1)Sb2S3[23] Two-step oxidation-sulfuration route 0.01-3.0 0.1 100 548 Sb2S3[13] Hydrothermal method 0.01-2.0 1 100 469 Sb2S3 nanosheet[26] Exfoliation assisted by Li intercalation 0.01-3.0 0.2 200 800 Sb2S3@CNT[27] Vapor transport deposition system 0.01-3.0 0.47 160 845 Sb2S3-C[28] Plasma assisted milling 0.01-3.0 1 500 496.1 Sb2S3@EG′-S[10] Sulfur-mediated route 0.01-3.0 5 100 548 S-rGO/Sb2S3[29] In-situ sulfuration process 0.01-3.0 0.5 800 451 Sb2S3/CS[32] Electrospinning coupled with hydrothermal 0.01-2.0 0.2 200 566 Sb2S3/TiO2/C[33] Electrospinning coupled with hydrothermal 0.01-2.5 2 800 454.1 NSSCs[38] Electrospinning technology 0.01-3.0 1 1000 490.3 CPC/Sb2S3[39] Hydrothermal method 0.01-3.0 0.1 200 1100 MoS2-Sb@Sb2S3@C[40] Semi-sacrificial template and thermal carbonization 0.01-3.0 1 100 760 Notes: CNT—Carbon nanotube; NSSCs—N doped Sb2S3-carbon fiber; CPC—Carbon derived from coconut pulp. 表 2 最近报道的Sb2S3基材料作为SIBs负极材料的合成方法和电化学性能
Table 2. Synthetic methods and electrochemical properties of Sb2S3-based electrodes used as anode for SIBs from recent reported
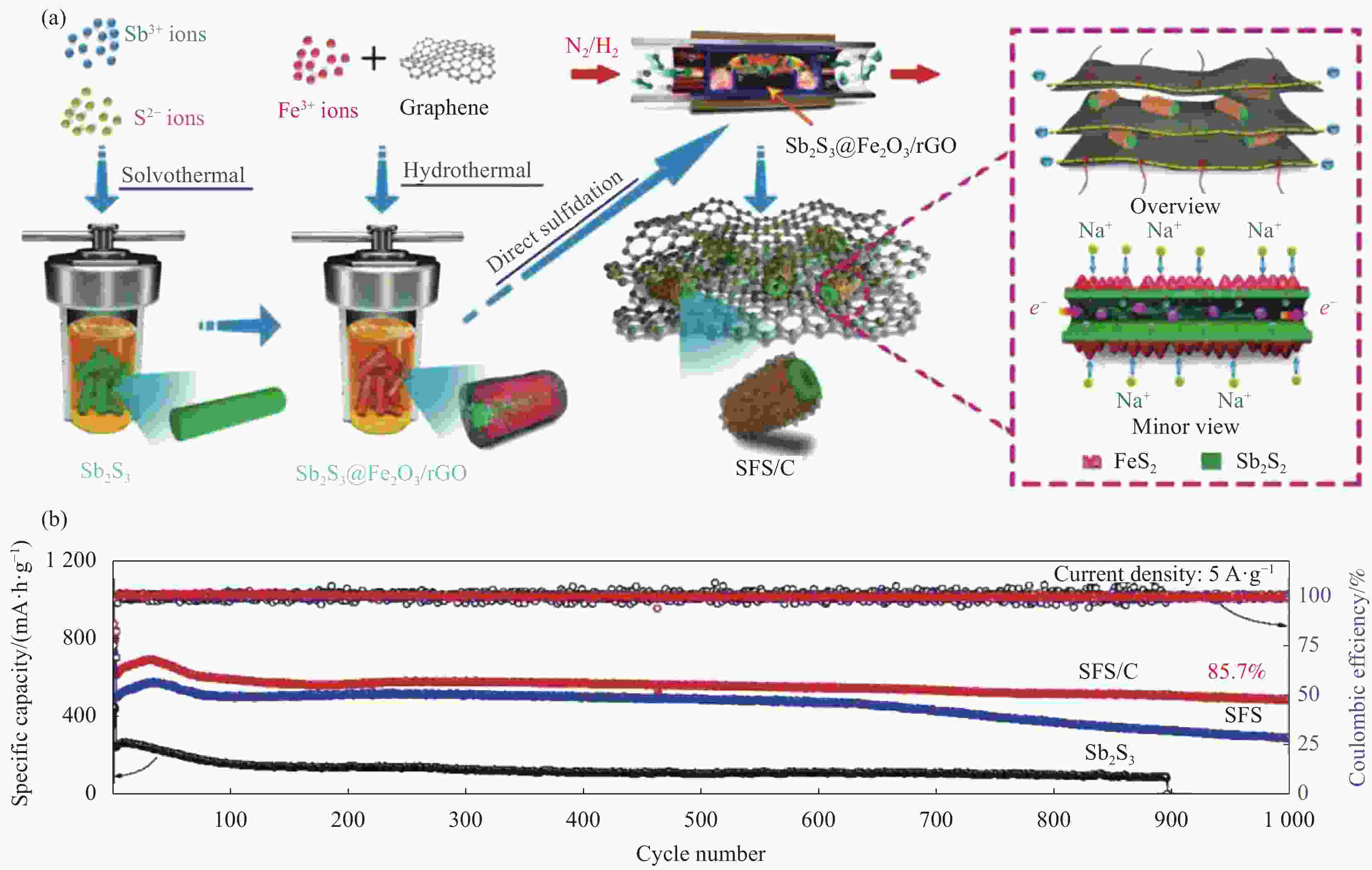
Sample Synthesis method Voltage
range/VCurrent
density/(A·g−1)Cycle
numberFinal capacity/
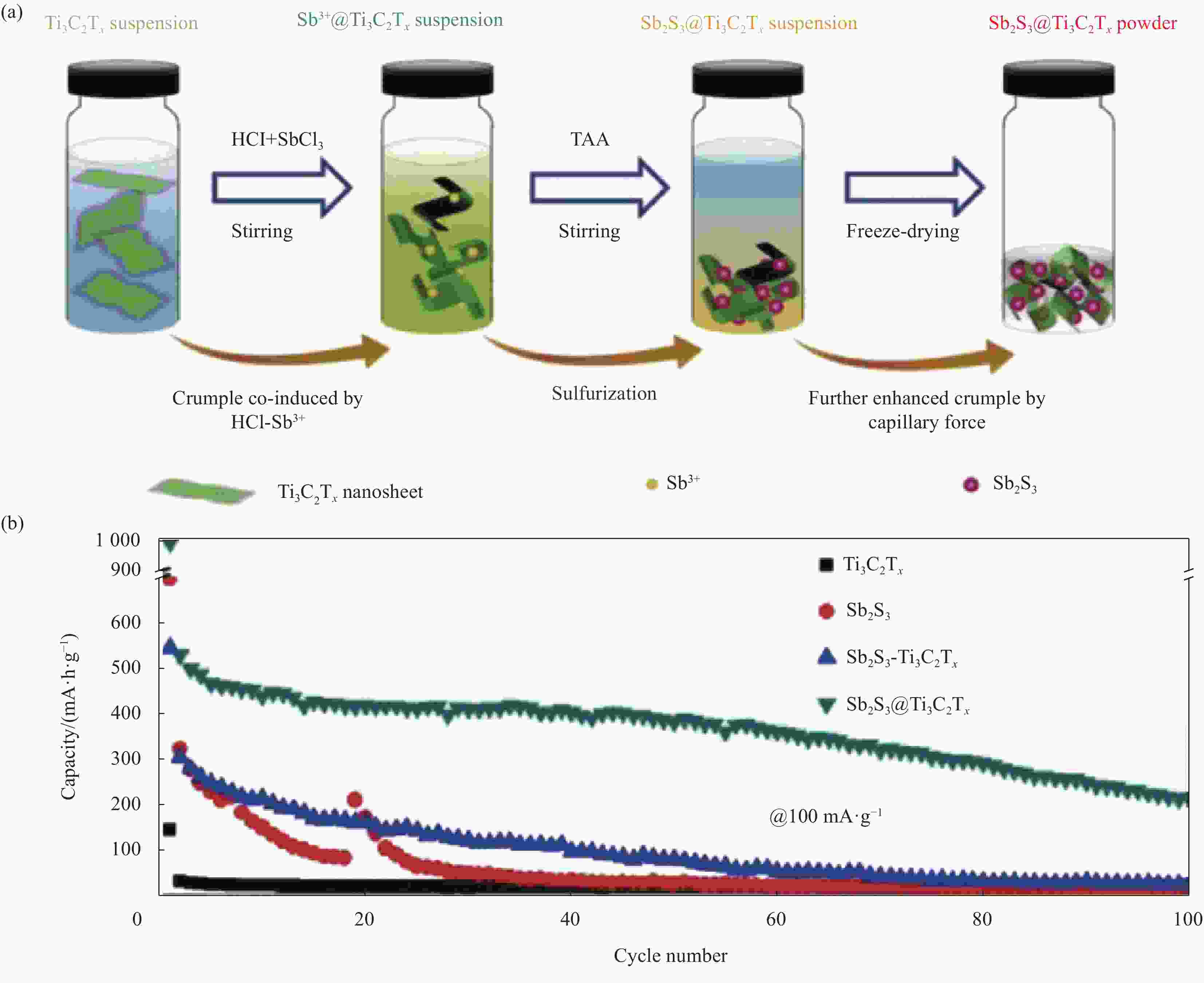
(mA·h·g−1)Sb2S3 nanosheeets[26] Exfoliation assisted by Li intercalation 0.01-3.0 0.2 200 500 More shells Sb2S3[46] Template method 0.01-2.0 1 50 >500 rGO/Sb2S3[47] Hydrothermal and solvothermal method 0.01-2.0 0.1 60 652 Sb2S3/CNT[48] Self-assembly method 0.01-1.5 0.1 50 704 Sb2S3@PPy[49] Hydrothermal method 0.01-3.0 0.5 150 632 Sb2S3@YP[50] Vaporization-condensation method 0.01-3.0 1.162 1000 476.5 Sb2S3@N-C[53] Coating method and heat treatment 0.01-3.0 1 1000 625 Sb2S3/SnO2[57] Hydrothermal-solution method 0.01-2.0 0.05 100 582.9 SFS/C[54] Solvothermal method 0.1-3.0 5 1000 534.8 Sb2S3-Bi2S3@C@rGO[58] Cation exchange treatment 0.01-3.0 8 1100 460.5 Sb2S3 after precycling Li[60] — 0.001-2.5 0.1 200 195 a-Sb2S3@CuSbS2[61] Closed-space sublimation method 0.01-2.5 0.05 50 506.7 Sb-CNTs[62] Electrochemical approach 0.01-2.0 1 100 425 Sb2S3@Ti3C2Tx[63] Wet-chemistry synthesis method 0.01-3.0 0.1 100 215 Notes: PPy—polypyrrole; YP—YP80F carbon. 表 3 最近报道的Sb2S3基材料作为钾离子电池(PIBs)负极材料的合成方法和电化学性能
Table 3. Synthetic methods and electrochemical properties of Sb2S3-based electrodes used as anode for potasssium ion batteries (PIBs) from recent reported
Sample Synthesis method Voltage
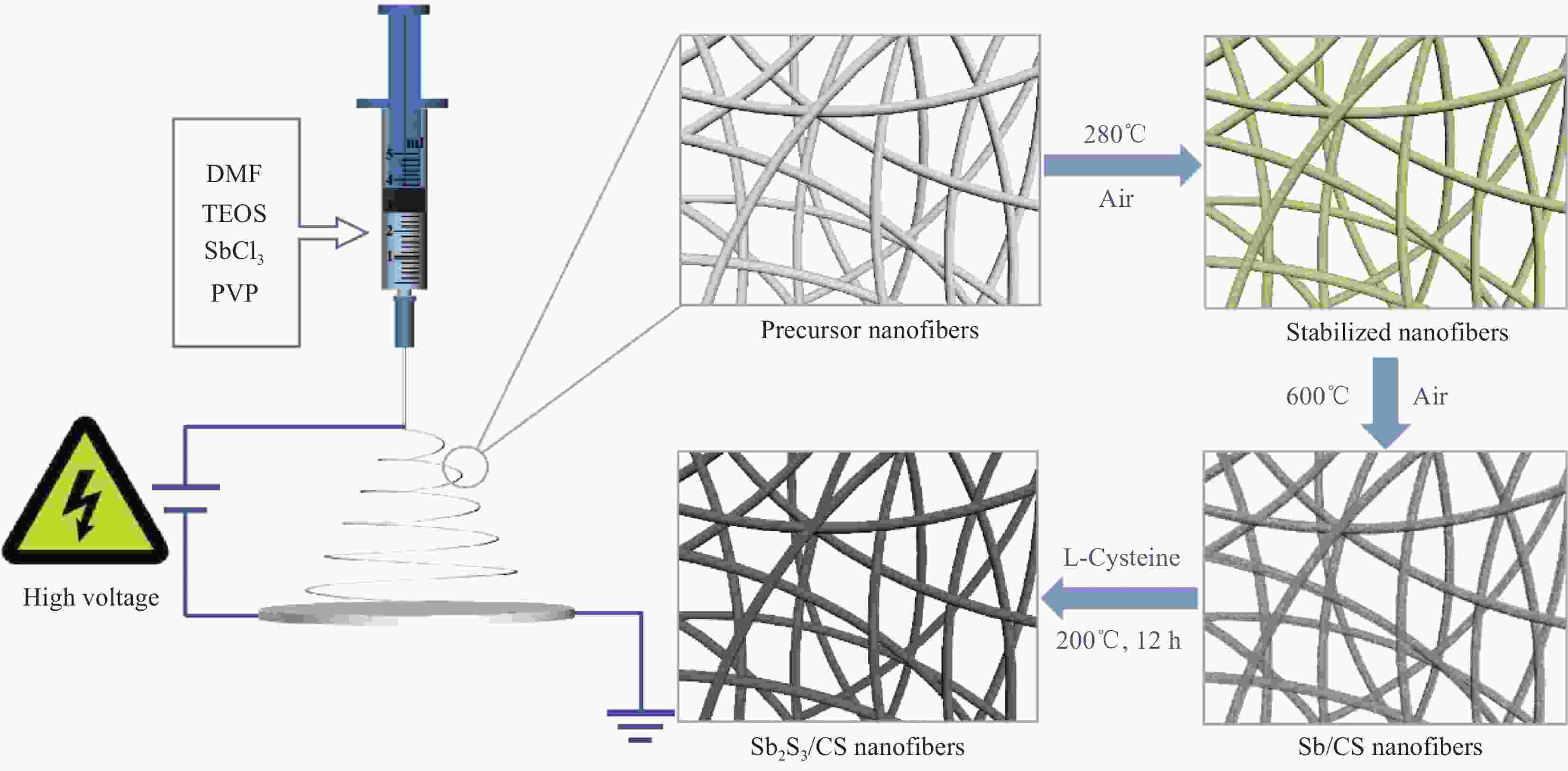
range/VCurrent
density/(A·g−1)Cycle
numberFinal capacity/
(mA·h·g−1)Sb2S3/CNT[48] Self-assembly method 0.01-2.5 0.5 50 212.4 Sb2S3@PPy[49] Hydrothermal reaction 0.01-3.0 0.1
118
50487
157Sb2S3-Bi2S3@C@rGO[58] Cation exchange treatment 0.01-3.0 0.2 80 294.6 Sb2S3-SNG[66] Hydrothermal reaction 0.01-3.0 0.05 100 480 Ti3C2-Sb2S3[64] Solvothermal and calcination method 0.01-2.0 0.1 500 286 Sb2S3-C@Nb2O5-C NFs[72] Electrospinning technology 0.01-3.0 0.1
2100
2200347.5
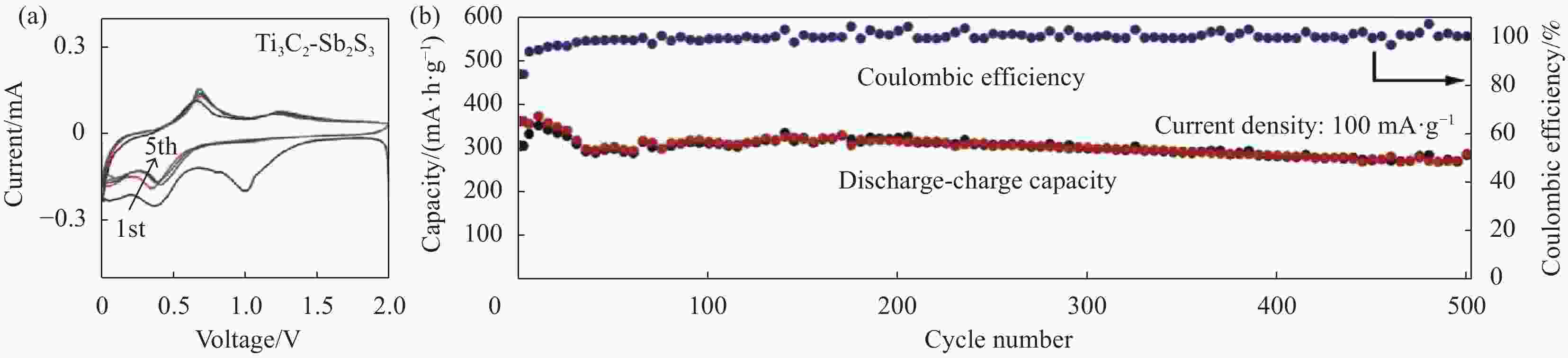
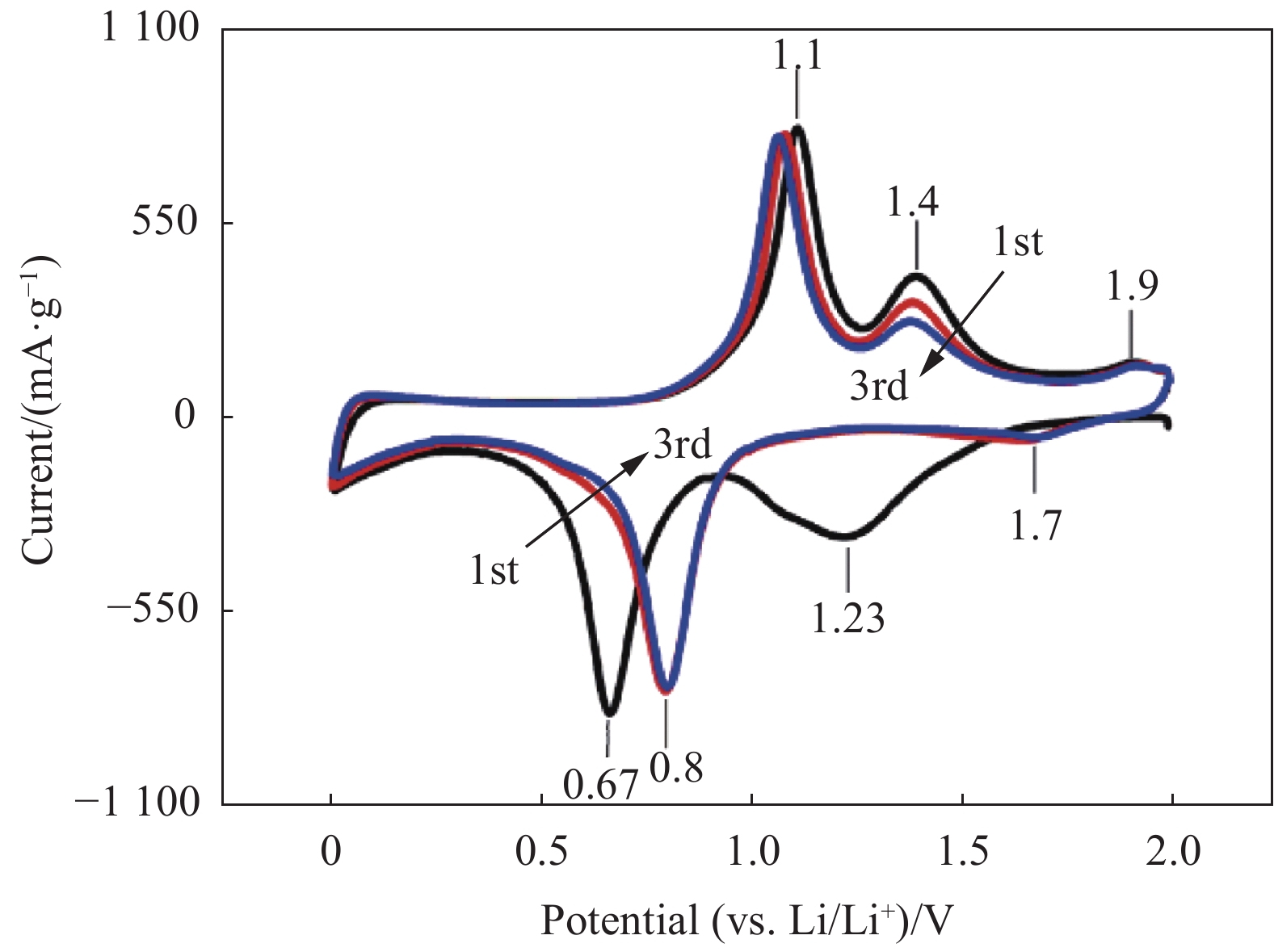
96.1Notes: SNG—S, N-codoped graphene framework; CNFs—Carbon nanofibers. -
[1] XIE D H, ZHANG M, WU Y, et al. A flexible dual-ion battery based on sodium-ion quasi-solid-state electrolyte with long cycling life[J]. Advanced Functional Materials,2020,30(5):1906770. doi: 10.1002/adfm.201906770 [2] WU J X, LIU J P, CUI J, et al. Dual-phase MoS2 as a high-performance sodium-ion battery anode[J]. Journal of Mater-ials Chemistry A,2020,8(4):2114-2122. doi: 10.1039/C9TA11913B [3] LIANG S Z, CHENG Y J, ZHU J, et al. A chronicle review of nonsilicon (Sn, Sb, Ge)-based Lithium/Sodium-ion battery alloying anodes[J]. Small Methods,2020,4(8):2000218. doi: 10.1002/smtd.202000218 [4] LIU Z M, WANG J, LU B A. Plum pudding model inspired KVPO4F@3DC as high-voltage and hyperstable cathode for potassium ion batteries[J]. Science Bulletin,2020,65(15):1242-1251. doi: 10.1016/j.scib.2020.04.010 [5] ZHANG Q, WANG Z J, ZHANG S L, et al. Cathode materials for potassium-ion batteries: Current status and perspective[J]. Electrochemical Energy Reviews,2018,1(4):625-658. doi: 10.1007/s41918-018-0023-y [6] YANG C, XIN S, MAI L Q, et al. Materials design for high-safety sodium-ion battery[J]. Advanced Energy Materials,2021,11(2):2000974. doi: 10.1002/aenm.202000974 [7] SARKAR A, MANOHAR C, MITRA S. A simple approach to minimize the first cycle irreversible loss of sodium titanate anode towards the development of sodium-ion battery[J]. Nano Energy,2020,70:104520. doi: 10.1016/j.nanoen.2020.104520 [8] MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry[J]. Nature Communications,2020,11(1):1-9. doi: 10.1038/s41467-019-13993-7 [9] ZHANG W L, MING J, ZHAO W L, et al. Graphitic nanocarbon with engineered defects for high-performance potassium-ion battery anodes[J]. Advanced Functional Materials,2019,29(35):1903641. doi: 10.1002/adfm.201903641 [10] WANG S, CHENG Y, XUE H, et al. Multifunctional sulfur-mediated strategy enabling fast-charging Sb2S3 micro-package anode for lithium-ion storage[J]. Journal of Materials Chemistry A,2021,9(12):7838-7847. doi: 10.1039/D0TA11954G [11] ZHAI H L, JIANG H F, QIAN Y, et al. Sb2S3 nanocrystals embedded in multichannel N-doped carbon nanofiber for ultralong cycle life sodium-ion batteries[J]. Materials Chemistry and Physics,2020,240:122139. doi: 10.1016/j.matchemphys.2019.122139 [12] ZAKAZNOVA-HERZOG V P, HARMER S L, NESBITT H W, et al. High resolution XPS study of the large-band-gap semiconductor stibnite (Sb2S3): Structural contributions and surface reconstruction[J]. Surface Science,2006,600(2):348-356. doi: 10.1016/j.susc.2005.10.034 [13] XIE J J, LIU L, XIA J, et al. Template-free synthesis of Sb2S3 hollow microspheres as anode materials for lithium-ion and sodium-ion batteries[J]. Nano-Micro Letters,2018,10(1):12. doi: 10.1007/s40820-017-0165-1 [14] ZHOU X Z, BAI L H, YAN J, et al. Solvothermal synthesis of Sb2S3/C composite nanorods with excellent Li-storage performance[J]. Electrochimica Acta,2013,108:17-21. doi: 10.1016/j.electacta.2013.06.049 [15] DONG Y C, YANG S L, ZHANG Z Y, et al. Enhanced electrochemical performance of lithium ion batteries using Sb2S3 nanorods wrapped in graphene nanosheets as anode materials[J]. Nanoscale,2018,10(7):3159-3165. doi: 10.1039/C7NR09441H [16] PAN J, WANG N N, ZHOU Y L, et al. Simple synthesis of a porous Sb/Sb2O3 nanocomposite for a high-capacity anode material in Na-ion batteries[J]. Nano Research,2017,10(5):1794-1803. doi: 10.1007/s12274-017-1501-y [17] WANG S, YUAN S, YIN Y B, et al. Green and facile fabrication of MWNTs@Sb2S3@PPy coaxial nanocables for high-performance Na-ion batteries[J]. Particle & Particle Systems Characterization,2016,33(8):493-499. [18] 李志华, 凌云, 姜文娟, 等. Sb2S3纳米棒的制备及其性能研究[J]. 化工新型材料, 2009, 37(5):57-60. doi: 10.3969/j.issn.1006-3536.2009.05.021LI Z H, LING Y, JIANG W J, et al. Preparation and properties of Sb2S3 nanorods[J]. New Chemical Materials,2009,37(5):57-60(in Chinese). doi: 10.3969/j.issn.1006-3536.2009.05.021 [19] JARAMILLO-QUINTERO O, BENITEZ-CRUZ M, GARCIA-OCAMPO J, et al. Enhanced performance of S-doped Sb/Sb2O3/CNT/GNR nanocomposite as anode material in lithium-ion batteries[J]. Journal of Alloys and Compounds,2019,807:151647. doi: 10.1016/j.jallcom.2019.151647 [20] YIN W H, CHAI W W, WANG K, et al. Facile synthesis of Sb nanoparticles anchored on reduced graphene oxides as excellent anode materials for lithium-ion batteries[J]. Journal of Alloys and Compounds,2019,797:1249-1257. doi: 10.1016/j.jallcom.2019.04.329 [21] DENG M X, LI S J, HONG W W, et al. Octahedral Sb2O3 as high-performance anode for lithium and sodium storage[J]. Materials Chemistry and Physics,2019,223:46-52. doi: 10.1016/j.matchemphys.2018.10.043 [22] LAKSHMI K, DEIVANAYAGAM R, SHAIJUMON M. Carbon nanotube ‘wired’octahedral Sb2O3/graphene aerogel as efficient anode material for sodium and lithium ion batteries[J]. Journal of Alloys and Compounds,2021,857:158267. doi: 10.1016/j.jallcom.2020.158267 [23] YI Z, HAN Q G, CHENG Y, et al. Facile synthesis of symmetric bundle-like Sb2S3 micron-structures and their application in lithium-ion battery anodes[J]. Chemical Communications,2016,52(49):7691. doi: 10.1039/C6CC03176E [24] WANG Q H, ZHU L X, SUN L Q, et al. Facile synthesis of hierarchical porous ZnCo2O4 microspheres for high-performance supercapacitors[J]. Journal of Materials Chemistry A,2014,3(3):982-985. [25] SHEN L F, YU L, YU X Y, et al. Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors[J]. Angewandte Chemie International Edition,2015,54(6):1868-1872. doi: 10.1002/anie.201409776 [26] YAO S S, CUI J, DENG Y, et al. Ultrathin Sb2S3 nanosheet anodes for exceptional pseudocapacitive contribution to multi-battery charge storage[J]. Energy Storage Materials,2019,20:36-45. doi: 10.1016/j.ensm.2018.11.005 [27] WANG Q, DU Y Y, LAI Y Q, et al. Three-dimensional antimony sulfide anode with carbon nanotube interphase modified for lithium-ion batteries[J]. International Jour-nal of Minerals, Metallurgy and Materials,2021,28(10):1629-1635. doi: 10.1007/s12613-021-2249-7 [28] LIU Y X, LU Z, CUI J, et al. Plasma milling modified Sb2S3-graphite nanocomposite as a highly reversible alloying-conversion anode material for lithium storage[J]. Electrochimica Acta,2019,310:26-37. doi: 10.1016/j.electacta.2019.04.104 [29] ZHOU X Z, ZHANG Z F, YAN P F, et al. Sulfur-doped reduced graphene oxide/Sb2S3 composite for superior lithium and sodium storage[J]. Materials Chemistry and Physics,2020,244:122661. doi: 10.1016/j.matchemphys.2020.122661 [30] JING X A, LI L, SJ A, et al. Free-standing SnS/C nanofiber anodes for ultralong cycle-life lithium-ion batteries and sodium-ion batteries[J]. Energy Storage Materials,2019,17:1-11. doi: 10.1016/j.ensm.2018.08.005 [31] XIA J, JIANG K Z, XIE J J, et al. Tin disulfide embedded in N-, S-doped carbon nanofibers as anode material for sodium-ion batteries[J]. Chemical Engineering Journal,2019,359:1244-1251. doi: 10.1016/j.cej.2018.11.053 [32] XIE J J, XIA J, YUAN Y T, et al. Sb2S3 embedded in carbon-silicon oxide nanofibers as high-performance anode materials for lithium-ion and sodium-ion batteries[J]. Journal of Power Sources,2019,435:226762. doi: 10.1016/j.jpowsour.2019.226762 [33] XIA J, ZHANG X, YANG Y G, et al. Electrospinning fabrication of flexible, foldable, and twistable Sb2S3/TiO2/C nano-fiber anode for lithium ion batteries[J]. Chemical Engineering Journal,2021,413:127400. doi: 10.1016/j.cej.2020.127400 [34] YUAN Y, CHEN Z W, YU H X, et al. Heteroatom-doped carbon-based materials for lithium and sodium ion batteries[J]. Energy Storage Materials, 2020, 32: 65-90. [35] SAROJA A P V K, GARAPATI M S, SHYIAMALADEVI R, et al. Facile synthesis of heteroatom doped and undoped graphene quantum dots as active materials for reversible lithium and sodium ions storage[J]. Applied Surface Science,2020,504:144430. doi: 10.1016/j.apsusc.2019.144430 [36] XIA J, YUAN Y T, YAN H X, et al. Electrospun SnSe/C nano-fibers as binder-free anode for lithium-ion and sodium-ion batteries[J]. Journal of Power Sources,2020,449:227559. doi: 10.1016/j.jpowsour.2019.227559 [37] LIU J D, LIANG J J, WANG C Y, et al. Electrospun CoSe@N-doped carbon nanofibers with highly capacitive Li storage[J]. Journal of Energy Chemistry,2019,33:160-166. doi: 10.1016/j.jechem.2018.09.006 [38] YIN H, HUI K S, ZHAO X, et al. Eco-friendly synthesis of self-supported N-doped Sb2S3-carbon fibers with high atom utilization and zero discharge for commercial full lithium-ion batteries[J]. ACS Applied Energy Materials,2020,3(7):6897-6906. doi: 10.1021/acsaem.0c00984 [39] MULLAIVANANATHAN V, KALAISELVI N. Sb2S3 added bio-carbon: Demonstration of potential anode in lithium and sodium-ion batteries[J]. Carbon,2019,144:772-780. doi: 10.1016/j.carbon.2019.01.001 [40] LI C R, SONG H, MAO C M, et al. A novel MoS2 nanosheets-decorated Sb@Sb2S3@C tubular composites as anode material for high performance lithium ion battery[J]. Journal of Alloys and Compounds,2019,786:169-176. doi: 10.1016/j.jallcom.2019.01.315 [41] PAN J, ZUO Z L, DEENG J Q, et al. Sb2S3 single crystal nanowires with comparable electrochemical properties as an anode for sodium ion batteries[J]. Surfaces and Interfaces,2018,10:170-175. doi: 10.1016/j.surfin.2017.10.010 [42] DONG S H, LI C X, GE X L, et al. ZnS-Sb2S3@C core-double shell polyhedron structure derived from metal-organic framework as anodes for high performance sodium ion batteries[J]. ACS Nano,2017,11(6):6474-6482. doi: 10.1021/acsnano.7b03321 [43] YAN C S, CHEN G, CHEN D H, et al. Double surfactant-directed controllable synthesis of Sb2S3 crystals with comparable electrochemical performances[J]. CrystEngComm,2014,16(33):7753-7760. doi: 10.1039/C4CE00871E [44] XIONG X H, WANG G H, LIN Y W, et al. Enhancing sodium ion battery performance by strongly binding nanostructured Sb2S3 on sulfur-doped graphene sheets[J]. ACS Nano,2016,10(12):10953-10959. doi: 10.1021/acsnano.6b05653 [45] LUO W, GAUMET J J, MAI L Q. Antimony-based intermetallic compounds for lithium-ion and sodium-ion batteries: Synthesis, construction and application[J]. Rare Metals,2017,36(5):321-338. doi: 10.1007/s12598-017-0899-4 [46] XIE F X, ZHANG L, GU Q F, et al. Multi-shell hollow structured Sb2S3 for sodium-ion batteries with enhanced energy density[J]. Nano Energy,2019,60:591-599. doi: 10.1016/j.nanoen.2019.04.008 [47] WEN S Y, ZHAO J C, ZHAO Y, et al. Reduced graphene oxide (RGO) decorated Sb2S3 nanorods as anode material for sodium-ion batteries[J]. Chemical Physics Letters,2019,716:171-176. doi: 10.1016/j.cplett.2018.12.031 [48] LI M, HUANG F B, PAN J, et al. Amorphous Sb2S3 nanospheres in-situ grown on carbon nanotubes: Anodes for NIBs and KIBs[J]. Nanomaterials,2019,9(9):1323. doi: 10.3390/nano9091323 [49] SHI Y, LI F, ZHANG Y, et al. Sb2S3@PPy coaxial nanorods: A versatile and robust host material for reversible storage of alkali metal ions[J]. Nanomaterials,2019,9(4):560. doi: 10.3390/nano9040560 [50] CHANG G L, YIN X P, SHI S S, et al. Sb2S3@YP nanostructured anode material synthesized by a novel vaporization-condensation method for long cycle-life sodium-ion battery[J]. Journal of the Electrochemical Society,2020,167(14):140531. doi: 10.1149/1945-7111/abc658 [51] WANG Z Y, DONG K Z, WANG D, et al. Monodisperse multicore-shell SnSb@SnOx/SbOx@C nanoparticles space-confined in 3D porous carbon networks as high-performance anode for Li-ion and Na-ion batteries[J]. Chemical Engineering Journal,2019,371:356-365. doi: 10.1016/j.cej.2019.04.045 [52] ZHAN W W, ZHU M, LAN J L, et al. 1D Sb2S3@ nitrogen-doped carbon coaxial nanotubes uniformly encapsulated within 3D porous graphene aerogel for fast and stable sodium storage[J]. Chemical Engineering Journal,2021,408:128007. doi: 10.1016/j.cej.2020.128007 [53] DONG Y C, HU M J, ZHANG Z Y, et al. Nitrogen-doped carbon-encapsulated antimony sulfide nanowires enable high rate capability and cyclic stability for sodium-ion batteries[J]. ACS Applied Nano Materials,2019,2(3):1457-1465. doi: 10.1021/acsanm.8b02335 [54] CAO L, GAO X W, ZHANG B, et al. Bimetallic sulfide Sb2S3@FeS2 hollow nanorods as high-performance anode materials for sodium-ion batteries[J]. ACS Nano,2020,14(3):3610-3620. doi: 10.1021/acsnano.0c00020 [55] YANG C H, LIANG X H, OU X, et al. Heterostructured nanocube-shaped binary sulfide (SnCo)S2 interlaced with S-doped graphene as a high-performance anode for advanced Na+ batteries[J]. Advanced Functional Materials,2019,29(9):1807971. doi: 10.1002/adfm.201807971 [56] ZHANG Z D, ZHAO J C, XU M L, et al. Facile synthesis of Sb2S3/MoS2 heterostructure as anode material for sodium-ion batteries[J]. Nanotechnology,2018,29(33):335401. doi: 10.1088/1361-6528/aac645 [57] CHANG G L, YIN X P, SHI S S, et al. Sb2S3@SnO2 hetero-nanocomposite as high-performance anode material for sodium-ion battery[J]. International Journal of Green Energy,2020,17(15):1044-1050. doi: 10.1080/15435075.2020.1821692 [58] LI K, LIU X F, QIN Y C, et al. Sb2S3-Bi2S3 microrods with the combined action of carbon encapsulation and rGO confinement for improving high cycle stability in sodium/potassium storage[J]. Chemical Engineering Journal,2021,414:128787. doi: 10.1016/j.cej.2021.128787 [59] WANG S J, XIONG P, GUO X, et al. A stable conversion and alloying anode for potassium-ion batteries: A combined strategy of encapsulation and confinement[J]. Advanced Functional Materials,2020,30(27):2001588. doi: 10.1002/adfm.202001588 [60] FU L, SHANG C Q, LI G C, et al. Lithium pre-cycling induced fast kinetics of commercial Sb2S3 anode for advanced sodium storage[J]. Energy & Environmental Materials,2019,2(3):209-215. [61] ZHOU J, DOU Q R, ZHANG L J, et al. A novel and fast method to prepare a Cu-supported α-Sb2S3@CuSbS2 binder-free electrode for sodium-ion batteries[J]. Rsc Advances,2020,10(49):29567-29574. doi: 10.1039/D0RA05623E [62] LI X Y, QU J K, HU Z J, et al. Electrochemically converting Sb2S3/CNTs to Sb/CNTs composite anodes for sodium-ion batteries[J]. International Journal of Hydrogen Energy,2021,46(33):17071-17083. doi: 10.1016/j.ijhydene.2021.02.157 [63] REN M X, CAO D, JIANG W, et al. Hierarchical composite of Sb2S3 decorated on highly crumpled Ti3C2Tx nanosheets for enhanced sodium storage properties[J]. Electrochimica Acta,2021,373:137835. doi: 10.1016/j.electacta.2021.137835 [64] WANG T H, SHEN D Y, LIU H, et al. A Sb2S3 nanoflower/MXene composite as an anode for potassium-ion batteries[J]. ACS Applied Materials & Interfaces,2020,12(52):57907-57915. [65] LIU Y J, TAI Z X, ZHANG J, et al. Boosting potassium-ion batteries by few-layered composite anodes prepared via solution-triggered one-step shear exfoliation[J]. Nature Communications,2018,9(1):1-10. doi: 10.1038/s41467-017-02088-w [66] LU Y Y, CHEN J. Robust self-supported anode by integrating Sb2S3 nanoparticles with S, N-codoped graphene to enhance K-storage performance[J]. Science China Chemistry,2017,60(12):1533-1539. doi: 10.1007/s11426-017-9166-0 [67] ZHANG W C, LIU Y J, GUO Z P. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering[J]. Science Advances,2019,5(5):eaav7412. doi: 10.1126/sciadv.aav7412 [68] LI P, ZHENG X B, YU H X, et al. Electrochemical potassium/lithium-ion intercalation into TiSe2: Kinetics and mechanism[J]. Energy Storage Materials,2019,16:512-518. doi: 10.1016/j.ensm.2018.09.014 [69] DU C F, DINH K N, LIANG Q H, et al. Self-assemble and in situ formation of Ni1−xFexPS3 nanomosaic-decorated MXene hybrids for overall water splitting[J]. Advanced Energy Materials,2018,8(26):1801127. doi: 10.1002/aenm.201801127 [70] ER D Q, LI J W, NAGUIB M, et al. Ti3C2 MXene as a high capacity electrode material for metal (Li, Na, K, Ca) ion batteries[J]. Acs Applied Materials & Interfaces,2014,6(14):11173-11179. [71] FAN L, MA R F, ZHANG Q F, et al. Graphite anode for a potassium-ion battery with unprecedented performance[J]. Angewandte Chemie International Edition,2019,58(31):10500-10505. doi: 10.1002/anie.201904258 [72] LIU H Q, HE Y N, CAO K Z, et al. Stimulating the reversibility of Sb2S3 anode for high-performance potassium-ion batteries[J]. Small,2021,17(10):2008133. doi: 10.1002/smll.202008133 [73] SHE L N, LI Q, ZHANG F, et al. Sulfur doping induced anionic oxidation of niobium-pentoxide-based anode for ultralong-life and high energy-density Na-ion capacitors[J]. Journal of Power Sources,2020,451:227744. doi: 10.1016/j.jpowsour.2020.227744 [74] LIU Z C, DONG W J, WANG J B, et al. Orthorhombic Nb2O5-x for durable high-rate anode of Li-ion batteries[J]. Iscience,2020,23(1):100767. doi: 10.1016/j.isci.2019.100767 [75] DENG Q L, CHEN F, LIU S, et al. Advantageous functional integration of adsorption-intercalation-conversion hybrid mechanisms in 3D flexible Nb2O5@hard carbon@MoS2@soft carbon fiber paper anodes for ultrafast and super-stable sodium storage[J]. Advanced Functional Materials,2020,30(10):1908665. doi: 10.1002/adfm.201908665 -






 下载:
下载: