Preparation of bentonite-based Fenton composite material and its adsorption and removal of pollutants in wastewater
-
摘要: 为实现废弃物资源化及去除废水中污染物,将粉煤灰、干化污泥、牡蛎壳等3种原料按照一定比例混合为基础原料(FDO),掺入2种膨润土基无机矿物材料,制得具有去除氨氮(NH4+-N)和高锰酸盐指数(IMn)双重功能的2种新型类芬顿复合材料(SFM),分别记作活性白土型(ATC/FDO)、膨润土型(BT/FDO)。使用SEM和BET对SFM的表面形貌、孔径结构进行了表征,对比研究了2种SFM在类芬顿体系下对废水中的IMn和NH4+-N的吸附去除效果,并采用动力学和吸附等温模型分析其吸附特性。结果表明,ATC/FDO对IMn和NH4+-N的去除效果优于BT/FDO,处理5天后,相应的去除率分别高达95.76%和99.65%;ATC/FDO最优制备条件是:FDO∶ATC的质量比为5∶5,煅烧温度400℃,煅烧时间120 min;最佳使用条件是:20℃、pH=6.5,ATC/FDO∶H2O2用量比为5 g/L∶1 mL/L。2种SFM对NH4+-N的吸附过程均符合准二级动力学,且符合Freundlich吸附等温方程。研究结果能为废弃物的资源化利用和水处理领域提供新技术和新材料。Abstract: In order to realize waste recycling and remove pollutants from wastewater, two new Fenton-like compo-sites (SFM) with dual functions of removing ammonia nitrogen (NH4+-N) and permanganate index (IMn) were prepared. The two SFMs are made by mixing fly ash, dried sludge and oyster shell as basic raw materials (FDO) in a certain proportion and adding two bentonite based inorganic mineral materials, which are respectively recorded as activated clay type (ATC/FDO) and bentonite type (BT/FDO). The surface morphology and pore structure of SFM were characterized by SEM and BET. The adsorption and removal effects of IMn and NH4+-N in wastewater under the Fenton-like system of two kinds of SFM were comparatively studied, and the adsorption characteristics were analyzed by kinetics and adsorption isotherm models. The results show that the removal effect of ATC/FDO on IMn and NH4+-N is better than that of BT/FDO. After 5 days of treatment, the corresponding removal rate of ATC/FDO on IMn and NH4+-N is as high as 95.76% and 99.65% respectively. The optimum preparation conditions of ATC/FDO are as follows: the mass ratio of activated clay as basic raw material is 5∶5, calcination temperature is 400℃, calcination time is 120 min. The optimum conditions are 20℃, pH=6.5, and the dosage ratio of ATC/FDO to H2O2 is 5 g/L∶1 ml/L. The adsorption process of NH4+-N on the two SFMs conforms to the quasi-second-order kinetics and the Freundlich adsorption isotherm equation. The research results can provide new technologies and new materials for waste resource utilization and water treatment.
-
传统芬顿技术存在诸多缺点[1],如:需在强酸性范围内使用,pH范围窄,后续还需中和处理,有时需要加热,处理成本高,功能单一,处理后的污(废)水返色,限制了其使用场所和处理效果[2]。而NH4+-N和高锰酸盐指数(IMn)是评价地表水水质污染的代表性指标,其NH4+-N过高会导致水生生物中毒,破坏生态平衡[3];而IMn过高则会引起水中溶解氧降低,引发河道黑臭。因此研究出一种以废治废,成本较低,处理条件温和,无需调为酸性pH,无需加热等预处理,既能同时去除IMn和NH4+-N,又易于固液分离、方便回收再利用的类芬顿复合催化材料具有现实意义。
膨润土(BT)的主要矿物成分为蒙脱石,化学成分为(Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O,是双层硅氧四面体夹一层铝氧八面体组成的2∶1型三层晶体结构[4],其层间域内空隙大,常存在Ca2+、K+、Na+等阳离子用于补充电荷不足,表现出良好的离子吸附性能[5]。活性白土(ATC)是BT经无机酸处理,再经清水漂洗、干燥制得的高活性的吸附剂,亦称氢基BT或酸性白土,其吸附性能较强,能吸附有色物质、有机物质[6]。
我国每年有大量的粉煤灰(FA)废渣被排放并长期堆积[7]。每年从污水处理厂和河道清淤中排弃的污泥(SS)也找不到利用的途径。巨大的牡蛎市场背后隐藏着牡蛎壳废弃物带来的各种环境污染和安全隐患。这些废弃物因缺少处理技术,每年都有大量废弃,既占用土地资源,又会导致生态环境污染[8]。由于粉煤灰孔隙率高、吸附性强,能过滤截留或吸附去除废水中的污染物[9];污泥富含有机质,生物亲和性好,可作为微生物群体的载体[10];牡蛎壳富含大量的2~10 μm微孔,吸附能力强,水处理性能好[11]。以上材料价格低廉,若能加以改性利用可实现变废为宝。
以粉煤灰、污泥、牡蛎壳为基础原料(FDO),分别掺入ATC、BT等2种BT基无机矿物材料,并以Fe2+溶液拌匀,低温煅烧后制得2种结构稳定且兼具去除NH4+-N和IMn双重功能的新型类芬顿复合材料(SFM),探讨其吸附去污能力和吸附特性,以期为废弃物的资源化利用和水处理领域提供新技术和新材料。
1. 材料与方法
1.1 原材料
废水是由本地黑臭河道水中添加CH3COONa·H2O和NH4Cl后制得模拟废水,其水质如下:IMn为80 mg/L,NH4+-N为20 mg/L,总磷(TP)为7.0 mg/L、pH为6.73;用FeSO4配制20wt%的Fe2+溶液;粉煤灰取自本地燃煤发电厂,活性污泥取自本地某污水处理厂的压滤污泥;牡蛎壳购于河北某生物科技有限公司;ATC、BT均购于明光市某粘土矿物有限公司。以上材料均使用WMK-02型电热鼓风干燥箱(上海一恒)烘干后再使用DJ-04型粉碎机(上海隆拓)破碎,过0.25 mm标准筛后备用。化学分析测得上述材料的主要矿物成分见表1。
表 1 试验材料及主要成分Table 1. Main components of test materialsMaterial/wt% SiO2 Al2O3 Fe2O3 MgO CaO Na2O K2O Ignition loss Dried sludge 47.65 13.84 14.14 4.22 3.12 1.92 0.94 3.44 Fly ash 45.32 24.29 7.39 2.46 2.69 1.38 0.75 2.42 Bentonite 69.80 14.26 1.86 2.78 1.78 1.48 1.61 3.16 Activated clay 63.44 15.69 2.37 1.36 0.58 0.69 0.84 3.94 Oyster shell 7.89 0.68 0.45 1.98 86.34 0.64 0.86 4.89 1.2 试验方法
类芬顿复合材料(SFM)的最优制备条件的探索与表征:将粉煤灰、干化污泥、牡蛎壳等3种原料按5∶4∶2的比例混合为基础原料(命名为FDO),再分别与活性白土(ATC)、膨润土(BT)按照一定比例混合均匀,然后置于ZL10型圆盘造粒机(郑州尚辉)中,滴加一定量的Fe2+溶液造粒成型,经HDX型箱式马弗炉(洛阳宏达)煅烧后制得2种SFM,分别记作活性白土型(ATC/FDO)和膨润土型(BT/FDO),探究物料配比、煅烧时间、煅烧温度对IMn和NH4+-N去除效果的影响,找出最优制备条件,使用S-3400N II/EX-250型扫描电镜(日本日立)表征最优条件下的SFM结构和孔径特征。
最优水处理条件的探索:在一定温度下,取200 mL废水,分别与一定量的上述2种SFM在SHA-B型恒温震荡器(常州国华)中混合振荡反应一定时间后,使用DT5-6型低速离心机(北京医用)离心取上清液,然后用i3型分光光度计(济南海能)测定其吸光度值,平行测定3次取平均值。再进行静态吸附试验,考察废水pH(用H2SO4稀溶液调节)、投加浓度、处理温度等因素对SFM处理效果的影响,选择最优水处理条件。
IMn和NH4+-N的分析:分别采用GB/T 11892—1989[12]、GB/T7481—1987 [13]方法。
2. 结果与讨论
2.1 SFM的制备条件与微观形貌
2.1.1 SFM的最优制备条件探索
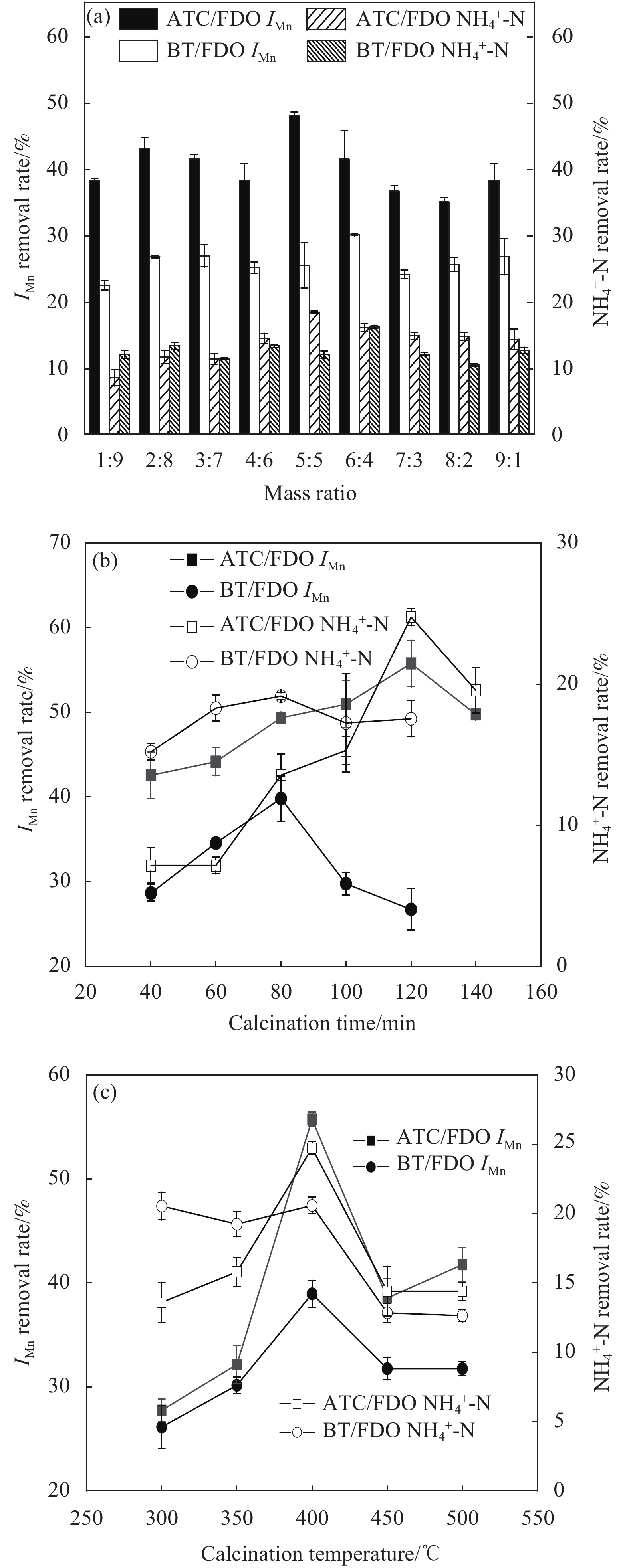
质量比对IMn和NH4+-N去除效果的影响如图1(a)所示,可知,随着物料中FDO用量的增大、矿物材料用量的减少,IMn和NH4+-N的去除效果先上升后下降。其中,在质量比FDO∶ATC=5∶5时,ATC/FDO对IMn和NH4+-N去除率达到最高,分别为47.95%和18.35%;BT/FDO的最佳制备条件为质量比6∶4时效果最好;ATC/FDO对IMn的去除率在所有比例下均高于BT/FDO,这是由于ATC是BT经过酸洗和水洗后的产物,其孔隙内的杂质少,与BT相比拥有更多的吸附点位,吸附性更强。
![]() 图 1 基础原料(FDO)与活性白土(ATC)膨润土(BT)的质量比 (a)、新型类芬顿复合材料(SFM)的煅烧时间 (b) 和煅烧温度 (c)对污染物去除率的影响Figure 1. Influences of mass ratio of basic raw material (FDO) to activated clay (ATC) bentonite (BT) (a), calcination time (b) and calcination temperature (c) of new Fenton like composite (SFM) on pollutant removal rateIMn—Permanganate index
图 1 基础原料(FDO)与活性白土(ATC)膨润土(BT)的质量比 (a)、新型类芬顿复合材料(SFM)的煅烧时间 (b) 和煅烧温度 (c)对污染物去除率的影响Figure 1. Influences of mass ratio of basic raw material (FDO) to activated clay (ATC) bentonite (BT) (a), calcination time (b) and calcination temperature (c) of new Fenton like composite (SFM) on pollutant removal rateIMn—Permanganate index煅烧时间和煅烧温度对IMn和NH4+-N去除效果的影响分别见图1(b)和图1(c)。由图1(b)可看出,2种SFM对污染物的去除率呈现出先升后降的趋势,这是由于适当的延长煅烧时间可以提高孔隙率,但煅烧时间过长会使矿物结构坍塌,破坏吸附点位,造成吸附性能的下降。另外,SFM煅烧时间过长,Fe2+会较多地转化为Fe3+,导致芬顿反应受挫。其中ATC/FDO在煅烧时间为120 min时对IMn和NH4+-N的去除率最佳,分别为55.76%和24.75%;BT/FDO在煅烧80 min时对IMn和NH4+-N去除率达到最佳,分别为39.82%和19.15%;且相同条件下ATC/FDO的去除率超过BT/FDO,说明ATC/FDO的吸附点位更多。
由图1(c)看出,随着煅烧温度的升高,去除污染物的效果增强,2种SFM材料的最佳煅烧温度均为400℃,这是由于二者成分均为BT,使煅烧温度引起的结构变化相似。相应的ATC/FDO对IMn和NH4+-N的去除率分别为55.76%和24.75%,BT/FDO对IMn和NH4+-N的去除率分别为38.96%和20.6%。试验时发现,低温时未能烧胀,机械强度较低[14],如果煅烧时间过长、或者煅烧温度过高,均会使矿物结构坍塌,内部气孔增大并连通,吸附点位被破坏并减少,且Fe2+会较多地转化为Fe3+导致催化效率降低,吸附性能下降。
2.1.2 SFM的微观形貌
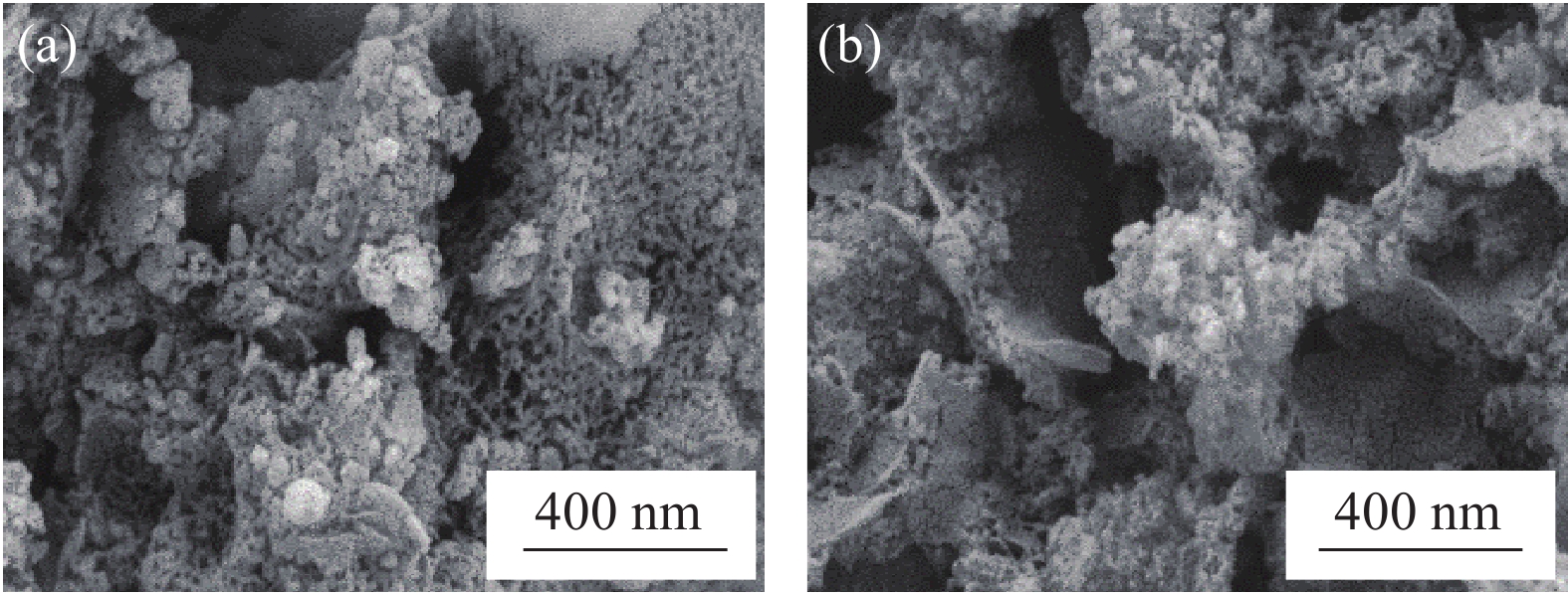
使用放大50.0 K的SEM对2种SFM材料的样貌进行扫描(图2)。可见,ATC/FDO结构紧凑,表面粗糙,有大量微孔;BT/FDO的微孔闭合,孔隙大且结构松散。BET分析表明,ATC/FDO、BT/FDO的比表面积分别为10.13、7.15 m2/g;ATC/FDO、BT/FDO的孔体积分别为0.0569、0.0743 m3/g;ATC/FDO、BT/FDO的孔径分别为3.66、3.73 nm。
不同组别的SFM孔隙率和孔径大小差异明显,主要原因在于SFM中的单质C在400℃以上可与O2反应生成CO2,活性污泥中的有机质被高温分解成CO2,各材料中CO2产生量不同,CO2溢出后产生的孔隙率也不同。表面越粗糙,孔隙越大,对污染物的吸附性越好,同时孔隙较大也适合微生物的负载和繁殖,协同去除水中的污染物。由于ATC/FDO的比表面积大于BT/FDO,而孔径小于BT/FDO,因此对于IMn和NH4+-N的去除率ATC/FDO优于BT/FDO。
2.2 SFM的水处理条件优化
pH的影响见图3(a),可知,针对IMn的去除,ATC/FDO在近中性pH为6.5时处理效果最佳,这与魏光涛等[15]用ATC催化降解甲基橙废水,在pH=5~8时的效果最好的结论基本一致,因此ATC/FDO在使用时无需调节pH至酸性范围,这一发现克服了传统类芬顿材料只在酸性和加热条件下才有效果的不足。而BT/FDO的最佳处理pH为3.5,且随着pH的升高,对污染物的去除率逐步降低。这与传统的类芬顿试剂一样,实际应用时仍然需要调节pH至强酸性。
这是由于ATC/FDO与BT/FDO的主要成分均为BT,但ATC/FDO是BT经过酸洗后得到的ATC,其层间域中的杂质较少,孔隙率较高,如果置于酸性条件下反而易使孔隙结构被腐蚀[16]。因此,ATC/FDO更适合在中性条件下使用。而BT/FDO的主要成分是未经酸洗的BT,孔隙中杂质较多,在酸性条件下使用能清除孔隙中的杂质,增强去除效果。因此BT/FDO适合在酸性条件下使用,随着pH上升,效果呈现出缓慢下降的态势。2种材料在超出各自的最佳pH范围时,都会使废水中目标污染物的去除率降低。
SFM投加浓度的影响见图3(b)所示,可知,IMn和NH4+-N的去除率走势相似,这是由于IMn去除率最佳的条件下,·OH的氧化能力也是最强。随着SFM投加量的增大,总吸附量呈增加趋势,但投加过量过大时,会使单位比表面积的吸附驱动力减弱[17],同时因为Fe2+是催化产生·OH的必要条件,SFM中过量的Fe2+又会以3.2×108 L/(mol·s)的速率被·OH氧化为Fe3+[18],导致·OH不仅数量小,而且产生速度也变慢,使IMn降解过程受到抑制[19];当SFM投加浓度值过小时,产生的·OH也少,导致IMn去除率下降。在试验条件下,SFM投加浓度值为5 g/L时污染物的去除率达到最高,这说明此时Fe2+催化产出的·OH自由基与污染物反应比较彻底。
H2O2投加浓度主要影响·OH自由基的浓度[20],其对IMn和NH4+-N去除率的影响见图3(c)。当H2O2投加不足时,无法产生足够的·OH去降解有机污染物,使Fe2+消耗氧化剂·OH生成Fe3+的量减少,直接影响催化剂Fe2+的催化过程和催化效率,也会使水样色度提高,在表观上引起IMn上升,IMn去除率变差[21]。当H2O2投加过量时,有足够量的·OH将Fe2+完全转化为Fe3+,产生较多的铁泥不利于反应的进行,同样会使芬顿催化效率降低,表现为在投加H2O2浓度值高于2.5 mL/L时IMn的去除率变化不大。至于NH4+-N的去除,当·OH产生不足时NH4+-N去除率略有下降,其原因可能是部分NH4+被氧化为NO2-N或NO3-N。ATC/FDO和BT/FDO对于IMn和NH4+-N的去除率分别在H2O2投加浓度值为1 mL/L和2.5 mL/L时,此时Fe2+的浓度比较适中,处理效率最高,之后去除率趋于平稳。
由图3(d)可知,随着反应体系温度的升高,分子的布朗运动速度加快,芬顿反应速度也会加快,在20℃时,ATC/FDO与BT/FDO对IMn和NH4+-N的去除率要高出其它温度下去除率的20%左右,因为该温度下离子较活跃,有利于氧化反应的进行和NH4+-N的吸附。随着反应体系温度的继续升高,会促使H2O2分解为O2和H2O[22],减少·OH自由基的生成,从而降低H2O2的利用率,导致处理效果变差。
反应时间对污染物去除率的影响如图3(e)所示,SFM对IMn和NH4+-N的去除率随着反应时间的延长而不断增大,其中自第2天开始ATC/FDO的去除率均高于BT/FDO。这是由于ATC/FDO相比BT/FDO经过水洗,层间域中的杂质少,拥有的吸附点位更多,能够负载更多的Fe2+构成芬顿反应体系。处理5天后,对IMn和NH4+-N的去除率分别高达95.76%和99.65%。
2.3 SFM吸附动力学
NH4+-N的去除主要依靠材料本身的吸附和·OH的氧化作用,其中吸附是主要作用。陈仕稳等[23]利用改性BT颗粒去除有机污染物和吸附NH4+-N,结果表明在投加浓度值为3 g/L、反应时间20 min、pH=7的条件下,材料对污染水体中腐殖酸(HA,20 mg/L)和NH4+-N(HA,5 mg/L)的去除率分别可达98%和20%以上。
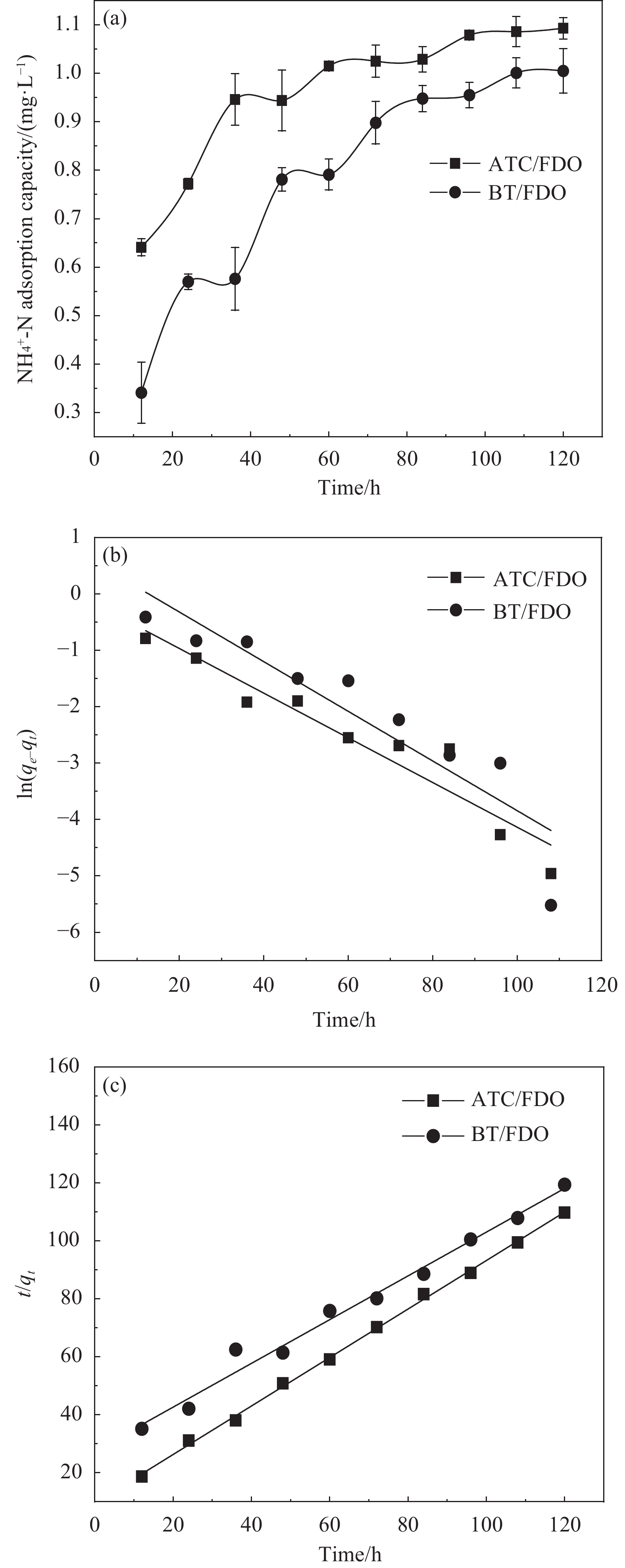
图4(a)和图4(b)分别是NH4+-N的吸附曲线和准一级动力学拟合曲线,其中,qe为平衡吸附量;qt为t时刻的吸附量。可知,ATC/FDO的R2为0.9203,而BT/FDO的R2小于0.9,相关性较差。在准二级动力学拟合曲线中的R2均大于0.98,相关性较好。且试验测得的最大吸附量qmax值与准二级动力学拟合出的平衡吸附量qe值相差较小,因此SFM对NH4+-N的吸附更加符合准二级动力学。具体动力学参数见表2。
表 2 SFM的NH4+-N吸附动力学参数Table 2. Kinetic parameters of NH4+-N adsorption of SFMModel Parameters and equations ATC/FDO BT/FDO First order kinetics Equation y=−0.0396x−0.1756 y=−0.0440x+0.5594 R2 0.9203 0.8457 qe/(mg·g−1) 1.1 1.014 qmax/(mg·g−1) 1.093 1.005 Second order kinetics Equation y=0.8365x+ 9.5827 y=0.7547x+ 27.5633 R2 0.9986 0.9853 qe/(mg·g−1) 1.091 1.016 Notes: R2—Correlation coefficient; qmax—Maximum adsorption capacity. 2.4 SFM吸附等温线
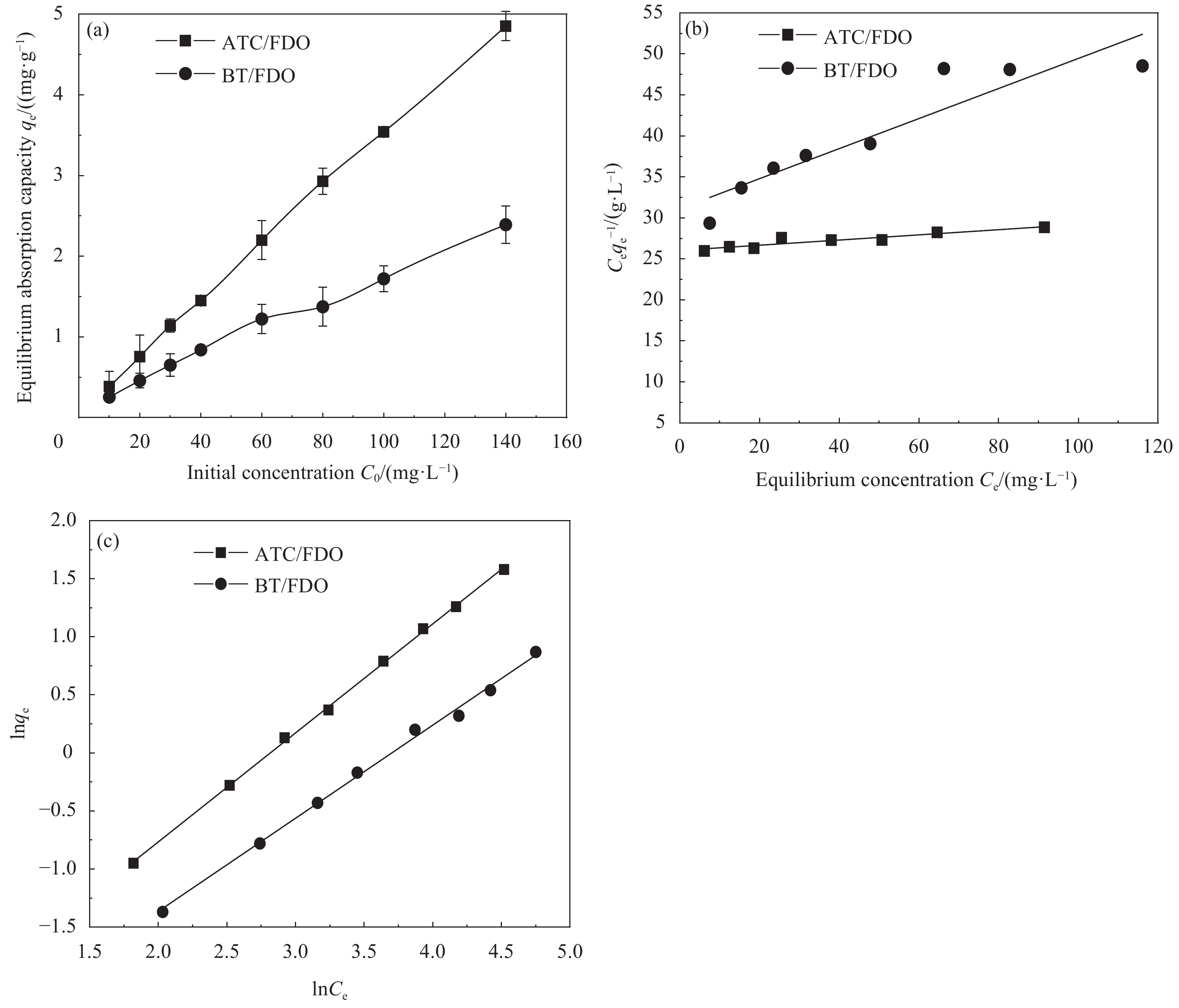
NH4+-N的吸附量曲线如图5(a)所示,在25℃时,ATC/FDO、BT/FDO的最大NH4+-N吸附量qmax分别为4.852、2.391 mg/g。图5(b)、图5(c)分别为Langmuir和Freundlich的等温线拟合结果,图中Ce为平衡浓度;C0为初始浓度。在Langmuir模型中SFM的相关系数均小于0.9。假设Langmuir吸附为单层吸附且单位位点能量均一,根据RL=1/(1+KLC0)[24],式中,RL为分离常量;KL为Langmuir的参数。当0<RL<1时,对吸附有利,而RL=0时吸附不可逆,RL=1时为线性吸附,RL>1时不利于吸附,因为方程中的Langmuir的参数KL大于0,所以0<RL<1,说明吸附过程是单层吸附且比较容易完成,属于良性吸附。SFM的相关系数均大于0.95,方程中的参数1/n小于1,也证明了吸附过程中存在少量单层吸附[25],与动力学模型中的准二级动力学相互验证。综上,SFM对NH4+-N的吸附更贴近于化学吸附。具体等温线参数见表3。
表 3 SFM的NH4+-N等温线参数Table 3. Isotherm parameters NH4+-N adsorption of SFMModel Parameters and equations ATC/FDO BT/FDO Langmuir Equation y=0.0316x+26.0459 y=0.1833x+31.1318 R2 0.8738 0.8583 qe/(mg·g−1) 3.1614 2.215 KL 0.0012 0.0059 Freundlich Equation y=0.9389x−2.6443 y=0.8022x−2.9694 R2 0.9993 0.9966 Kf 0.0711 0.0513 1/n 0.9389 0.8022 Notes: KL—Parameters of Langmuir; Kf—Parameters of Freundlich; 1/n—Parameters of Freundlich. 3. 结 论
(1) 新型类芬顿复合材料(SFM)的最佳制备条件是:将粉煤灰、污泥、牡蛎壳等3种初始原料按照5∶4∶2的固定质量比混合后作为基础原料(FDO),活性白土型复合材料(ATC/FDO)的最佳质量配比是FDO∶ATC=5∶5;膨润土型复合材料(BT/FDO)的最佳质量配比是FDO∶BT=6∶4。ATC/FDO、BT/FDO分别在400℃下煅烧120 min和80 min达到各自最佳制备条件。SFM中的类芬顿体系的催化氧化体系和较大孔隙率有利于高锰酸盐指数(IMn)的去除,而氨氮(NH4+-N)的去除主要依靠其吸附作用。
(2) SFM的最佳水处理条件是:在200 mL废水中,ATC/FDO投加浓度值为5 g/L,H2O2投加浓度值为1 mL/L,pH为6.5,20℃下ATC/FDO达到最佳水处理效果;BT/FDO投加浓度值为5 g/L,H2O2投加浓度值为2.5 mL/L,pH为3.5,20℃下BT/FDO达到其最佳水处理效果。2种SFM相比,ATC/FDO对IMn和NH4+-N的去除率均高于BT/FDO,且无需将废水调为强酸性,pH使用范围宽,其中废水经处理5天后,ATC/FDO对IMn和NH4+-N的去除率分别高达95.76%和99.65%。
(3) 2种SFM对NH4+-N的吸附过程均符合准二级动力学,也符合Freundlich吸附等温方程。在二者的互相验证下,SFM对NH4+-N的吸附以化学吸附为主,且易于完成。
-
图 1 基础原料(FDO)与活性白土(ATC)膨润土(BT)的质量比 (a)、新型类芬顿复合材料(SFM)的煅烧时间 (b) 和煅烧温度 (c)对污染物去除率的影响
Figure 1. Influences of mass ratio of basic raw material (FDO) to activated clay (ATC) bentonite (BT) (a), calcination time (b) and calcination temperature (c) of new Fenton like composite (SFM) on pollutant removal rate
IMn—Permanganate index
表 1 试验材料及主要成分
Table 1 Main components of test materials
Material/wt% SiO2 Al2O3 Fe2O3 MgO CaO Na2O K2O Ignition loss Dried sludge 47.65 13.84 14.14 4.22 3.12 1.92 0.94 3.44 Fly ash 45.32 24.29 7.39 2.46 2.69 1.38 0.75 2.42 Bentonite 69.80 14.26 1.86 2.78 1.78 1.48 1.61 3.16 Activated clay 63.44 15.69 2.37 1.36 0.58 0.69 0.84 3.94 Oyster shell 7.89 0.68 0.45 1.98 86.34 0.64 0.86 4.89 表 2 SFM的NH4+-N吸附动力学参数
Table 2 Kinetic parameters of NH4+-N adsorption of SFM
Model Parameters and equations ATC/FDO BT/FDO First order kinetics Equation y=−0.0396x−0.1756 y=−0.0440x+0.5594 R2 0.9203 0.8457 qe/(mg·g−1) 1.1 1.014 qmax/(mg·g−1) 1.093 1.005 Second order kinetics Equation y=0.8365x+ 9.5827 y=0.7547x+ 27.5633 R2 0.9986 0.9853 qe/(mg·g−1) 1.091 1.016 Notes: R2—Correlation coefficient; qmax—Maximum adsorption capacity. 表 3 SFM的NH4+-N等温线参数
Table 3 Isotherm parameters NH4+-N adsorption of SFM
Model Parameters and equations ATC/FDO BT/FDO Langmuir Equation y=0.0316x+26.0459 y=0.1833x+31.1318 R2 0.8738 0.8583 qe/(mg·g−1) 3.1614 2.215 KL 0.0012 0.0059 Freundlich Equation y=0.9389x−2.6443 y=0.8022x−2.9694 R2 0.9993 0.9966 Kf 0.0711 0.0513 1/n 0.9389 0.8022 Notes: KL—Parameters of Langmuir; Kf—Parameters of Freundlich; 1/n—Parameters of Freundlich. -
[1] ZHOU W, ZHAO H, GAO J, et al. Influence of a reagents addition strategy on the Fenton oxidation of rhodamine B: Control of the competitive reaction of OH[J]. Rsc Advances,2016,6(110):108791-108800. DOI: 10.1039/C6RA20242J
[2] 闫云涛, 张柯, 毛岩鹏, 等. Fe2O3@SCe多相芬顿催化剂的制备及其降解放热性能[J]. 中国环境科学, 2020, 40(8):3375-3384. DOI: 10.3969/j.issn.1000-6923.2020.08.015 YAN Y T, ZHANG K, MAO Y P, et al. Fe2O3@SCe Preparation of heterogeneous Fenton catalyst and its degradation exothermic performance[J]. China Environmental Science,2020,40(8):3375-3384(in Chinese). DOI: 10.3969/j.issn.1000-6923.2020.08.015
[3] SHU J, CHEN M, WU H, et al. An innovative method for synergistic stabilization/solidification of Mn2+, NH4+-N, PO43- and F- in electrolytic manganese residue and phosphogypsum[J]. Journal of Hazardous Materials,2019,376:212-222. DOI: 10.1016/j.jhazmat.2019.05.017
[4] 杨昕达, 郝林林, 常达, 等. 膨润土对垃圾渗滤液絮凝预处理的强化效果[J]. 环境工程学报, 2021(5):1549-1557. DOI: 10.12030/j.cjee.202010110 YANG X D, HAO L L, CHANG D, et al. Strengthening effect of bentonite on flocculation pretreatment of landfill leachate[J]. Journal of Environmental Engineering,2021(5):1549-1557(in Chinese). DOI: 10.12030/j.cjee.202010110
[5] LIU T, TIAN X F, ZHAO Y, et al. Swelling of K+, Na+ and Ca2+-montmorillonites and hydration of interlayer cations: A molecular dynamics simulation[J]. Chinese Physics B,2010,19(10):656-662.
[6] YIP C K, LAM L Y, HU X. A novel heterogeneous acid-activated clay supported copper catalyst for the photobleaching and degradation of textile organic pollutant using photo-Fenton-like reaction[J]. Chemical Communications,2005,41(25):3218-3220.
[7] 陈宁, 王海滨, 刘树信. 空心粉煤灰对铁氧体-炭黑/水泥基复合材料吸波性能的影响[J]. 复合材料学报, 2017, 34(6):1381-1387. CHENG N, WANG H B, LIU S X. Effect of hollow fly ash on the wave absorption properties of Ferrite-carbon black/cement-based composites[J]. Acta Materiae Compositae Sinica,2017,34(6):1381-1387(in Chinese).
[8] BOULOS R A, HAMAGEA C, DUAN X, et al. Unzipping oyster shell[J]. Rsc Advances,2013,3(10):3284-3290. DOI: 10.1039/c2ra21763e
[9] 张天永, 王智超, 姜爽, 等. 煤渣在水处理方面的应用研究进展[J]. 现代化工, 2018, 38(3):32-36. ZHANG T Y, WANG Z C, JIANG S. et. al. Research progress in the application of coal slag in water treatment[J]. Modern Chemical Industry,2018,38(3):32-36(in Chinese).
[10] 王晶, 高娜, 刘双元, 等. 活性污泥微生物解壳聚糖松江菌中菌胶团形成相关大型基因簇的鉴定和分析[J]. 微生物学通报, 2019, 46(8):1946-1953. WANG J, GAO N, LIU S Y, et al. Identification and analysis of large-scale gene clusters related to the formation of micelles in the activated sludge microbial chitosan solution of Songjiang bacteria[J]. Microbiology Bulletin,2019,46(8):1946-1953(in Chinese).
[11] 刘文, 李天华, 张滕军, 等. 牡蛎壳中钙的改性及吸附特性的研究[J]. 材料导报, 2012, 26(18):88-92. DOI: 10.3969/j.issn.1005-023X.2012.18.024 LIU W, LI T H, ZHANG T J, et al. Study on the modification of calcium in oyster shell and its adsorption characteristics[J]. Material Guide,2012,26(18):88-92(in Chinese). DOI: 10.3969/j.issn.1005-023X.2012.18.024
[12] 生态环境部. 水质 高锰酸盐指数的测定: GB/T 11892—1989[S]. 北京: 北京环保监测中心, 1989. Ministry of Ecology and Environment. The water quality Determination of permanganate index: GB/T 11892—1989[S]. Beijing: Beijing Environmental Monitoring Center, 1989(in Chinese).
[13] 生态环境部. 水质 铵的测定 水杨酸分光光度法: GB/T7481—1987[S]. 赣州: 赣州环境监测站, 1987. Ministry of Ecology and Environment. The water quality The determination of ammonium Salicylic acid spectrophotometry: GB/T7481—1987[S]. Ganzhou: Ganzhou Environmental Monitoring Station, 1987(in Chinese).
[14] 陈凤霞, 马家海. Fe3O4@PCDP纳米复合材料的制备及其在类芬顿体系中的应用(英文)[J]. 中国科学院大学学报, 2020, 37(5):593-598. DOI: 10.7523/j.issn.2095-6134.2020.05.003 CHEN F X, MA J H. Preparation of Fe3O4@PCDP nanocomposite and its application in Fenton-like system (English)[J]. Journal of University of Chinese Academy of Sciences,2020,37(5):593-598(in Chinese). DOI: 10.7523/j.issn.2095-6134.2020.05.003
[15] 魏光涛, 周萍, 韦藤幼, 等. H3PMo12O40/活性白土UV-H2O2催化氧化降解甲基橙[J]. 化工环保, 2011, 31(3):210-213. DOI: 10.3969/j.issn.1006-1878.2011.03.005 WEI G T, ZHOU P, WEI T Y, et al. Catalytic oxidative degradation of methyl orange by H3PMo12O40/activated clay UV- H2O2[J]. Chemical Environmental Protection,2011,31(3):210-213(in Chinese). DOI: 10.3969/j.issn.1006-1878.2011.03.005
[16] MAHATA B K, CHUNG K L, CHANG S M. Removal of ammonium nitrogen (NH4+-N) by Cu-loaded amino-functionalized adsorbents[J]. Chemical Engineering Journal,2021,411(7):128-139.
[17] 郭欢. 硅藻土-钨渣基多孔陶粒的制备与性能研究[D]. 赣州: 江西理工大学, 2017. GUO H. Study on the preparation and properties of diatomite-tungsten slag-based porous ceramsite[D]. Ganzhou: Jiangxi University of Science and Technology, 2017(in Chinese).
[18] 魏建平, 戴俊, 王政锦, 等. Fenton试剂氧化降解甲烷的动力学规律[J]. 煤炭学报, 2013, 38(9):1597-1603. WEI J P, DAI J, WANG Z J, et al. Kinetic law of Fenton reagent oxidative degradation of methane[J]. Journal of China Coal Society,2013,38(9):1597-1603(in Chinese).
[19] GERMÁN S M , GABRIEL R M, EDUARDO M D C, et al. Electro-Fenton and Electro-Fenton-like with in situ electrogeneration of H2O2 and catalyst applied to 4-chlorophenol mineralization[J]. Electrochimica Acta,2016,195:246-256. DOI: 10.1016/j.electacta.2016.02.093
[20] 赵雅娴, 武帅, 康宸, 等. H2O2改性对聚丙烯腈原丝化学结构的影响[J]. 复合材料学报, 2019, 36(1):85-95. ZHAO Y X, WU S, KANG C. et al. Effect of H2O2 modification on the chemical structure of polyacrylonitrile precursor[J]. Acta Materiae Compositae Sinica,2019,36(1):85-95(in Chinese).
[21] CHEN C Y, TANG C, WANG H F, et al. Oxygen reduction reaction on graphene in an Electro-Fenton system: in situ generation of H2O2 for the oxidation of organic compounds[J]. Chemsuschem,2016,9(10):1194-1199. DOI: 10.1002/cssc.201600030
[22] 赵海谦, 高杏存, 王忠华, 等. Fe2+/H2O2体系O2生成路径[J]. 化工学报, 2016, 67(6):237-245. ZHAO H Q, GAO X C, WANG Z H, et al. O2 generation path in Fe2+/H2O2 system[J]. Journal of Chemical Industry,2016,67(6):237-245(in Chinese).
[23] 陈仕稳, 聂锦旭, 谢伟楠, 等. 改性膨润土颗粒对微污染水中有机物和氨氮的吸附[J]. 环境工程学报, 2015, 9(6):2739-2744. DOI: 10.12030/j.cjee.20150632 CHEN S W, NIE J X, XIE W N, et al. Adsorption of organic matter and ammonia nitrogen in micro-polluted water by modified bentonite particles[J]. Journal of Environmental Engineering,2015,9(6):2739-2744(in Chinese). DOI: 10.12030/j.cjee.20150632
[24] 王趁义, 黄添浩, 滕丽华, 等. 绿沸石对废水中氨氮的吸附效果研究[J]. 非金属矿, 2019, 42(1):21-24. DOI: 10.3969/j.issn.1000-8098.2019.01.007 WANG C Y, HUANG T H, TENG L H, et al. Study on the adsorption effect of Green Zeolite on ammonia nitrogen in wastewater[J]. Non-Metallic Minerals,2019,42(1):21-24(in Chinese). DOI: 10.3969/j.issn.1000-8098.2019.01.007
[25] 魏世勇, 杨小洪. 针铁矿-高岭石复合体的表面性质和吸附氟的特性[J]. 环境科学, 2010, 31(9):2134-2142. WEI S Y, YANG X H. Surface properties and fluorine adsorption characteristics of goethite-kaolinite complex[J]. Environmental Science,2010,31(9):2134-2142(in Chinese).
-
期刊类型引用(4)
1. 梁鑫华,周欣然,袁辉,沈乐怡,田啸,周子楠,王趁义. 电芬顿去除垃圾渗滤液中COD运行条件的响应面法优化. 环境监测管理与技术. 2024(05): 60-63 .  百度学术
百度学术
2. 刘催萍,陶星名,姚华珍,王趁义,郑宇,游自强. 新型铁碳微电解复合材料的制备及其效果研究. 水处理技术. 2024(12): 45-51+58 .  百度学术
百度学术
3. 蔡玉福,周艳军,路君凤,张启俭,王欢,赵永华. 碱活化蒙脱土负载铁类芬顿体系去除亚甲基蓝. 复合材料学报. 2023(08): 4601-4612 .  本站查看
本站查看
4. 徐园园,李文进,林方聪,魏一恺,吕春光,王趁义. 绿沸石-牡蛎壳复合材料的制备及其性能研究. 非金属矿. 2022(06): 97-100+104 .  百度学术
百度学术
其他类型引用(1)
-




 下载:
下载: