Preparation of graphene oxide load Ag3PO4@polyaniline composite and its photocatalytic performance
-
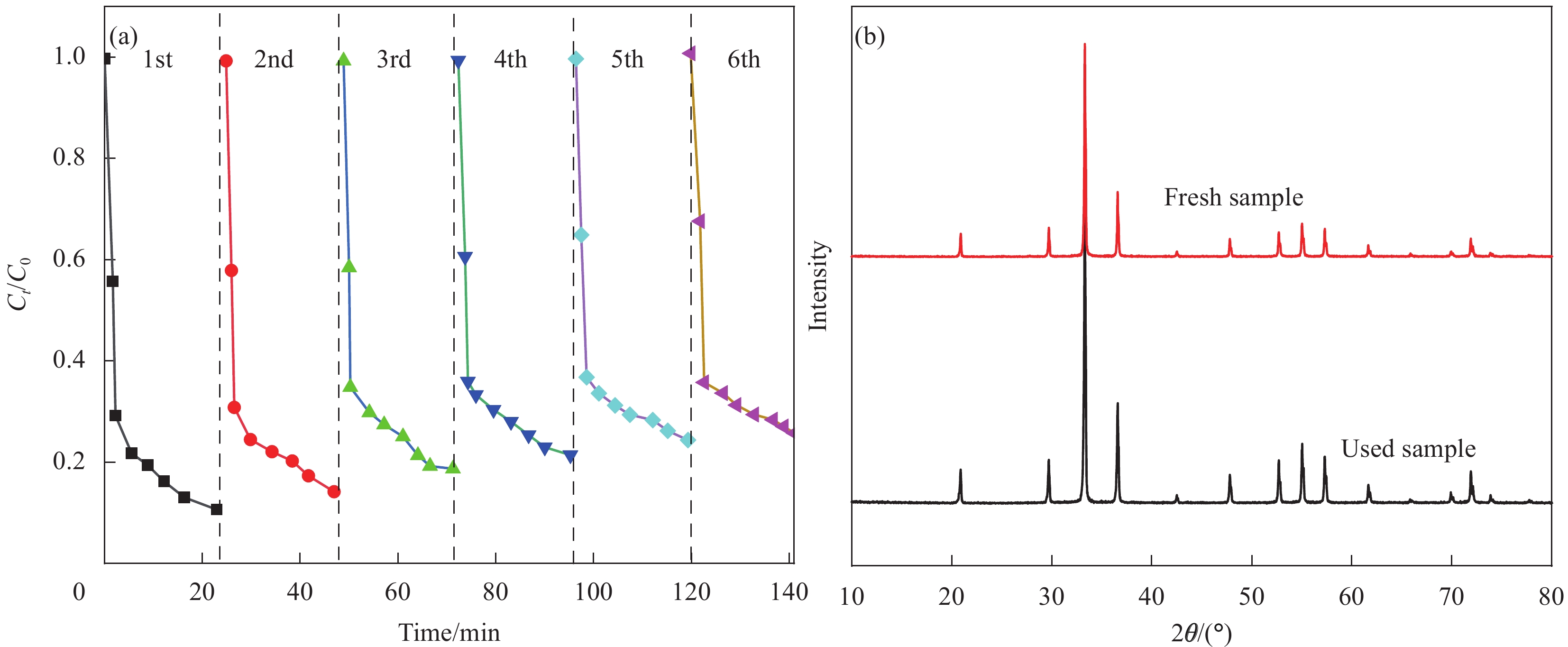
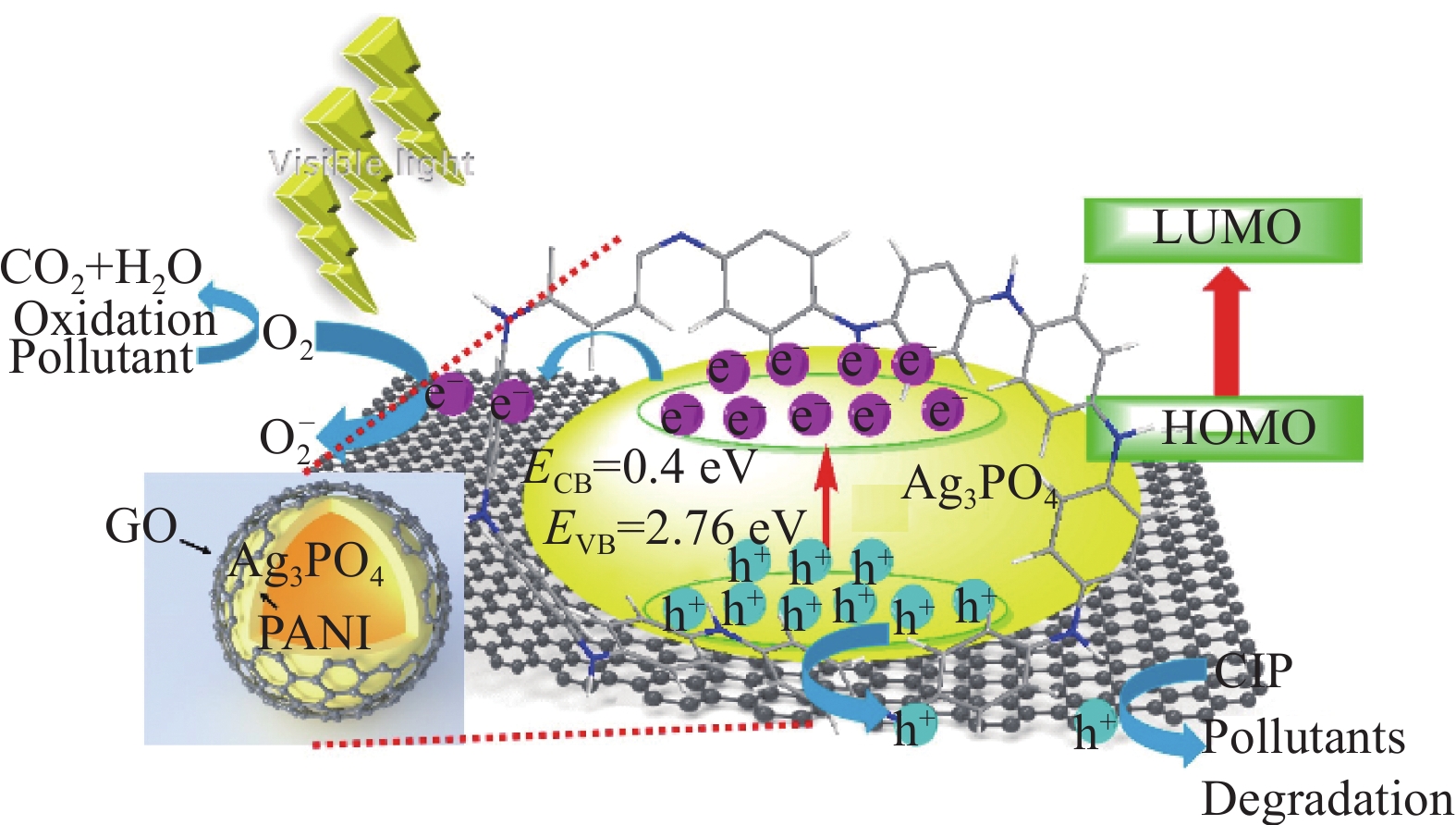
摘要: 为了解决Ag3PO4严重的光腐蚀问题,采用化学吸附法制备了核壳结构的聚苯胺(PANI)包覆磷酸银(Ag3PO4@PANI),并用氧化石墨烯(GO)作为Ag3PO4@PANI复合光催化剂的载体,通过PANI和GO的协同作用提升了载流子的分离效率。当GO与Ag3PO4@PANI质量比为4%时,催化剂在24 min内降解苯酚的去除率可达98.1%,18 min内对环丙沙星(CIP)的去除率可达90.3%,15 min内对四环素(TC)的去除率可达98.6%,在5 min内对各类染料的去除率为100%。经过6次重复反应,Ag3PO4@PANI/GO仍保持较好的稳定性。自由基捕获实验证实•h+和•O2−是光催化降解的主要活性物种。实验结果表明,PANI与Ag3PO4之间形成了核壳结构,GO的引入提升了电子的传输速率,PANI和GO对Ag3PO4的协同作用促进了光生电子-空穴的分离,进而提升了Ag3PO4的稳定性和光催化活性。Abstract: To solve severe photocorrosion of Ag3PO4, which was used to prepare a core-shell Ag3PO4@polyaniline (PANI) composite photocatalyst by chemisorption, and graphene oxide (GO) was used as the carrier of Ag3PO4@PANI composite photocatalyst, reaching the superior carrier separation efficiency via synergetic effect of GO and PANI. The photocatalyst with mass ratio of GO to Ag3PO4@PANI of 4% shows visible light activity for the degradation of phenol, ciprofloxacin (CIP), tetracycline (TC) and dyes of 98.1%, 90.3%, 98.6% and 100% in 24 min, 18 min, 15 min and 5 min, respectively. Even after six repeat reactions, Ag3PO4@PANI/GO still maintain a high degradation rate. The trapping experiments confirm that •h+ and •O2 are the main active species in the photocatalytic degradation. The experimental results show that a core-shell structure is formed between PANI and Ag3PO4, the introduction of GO increases the electron transport rate, and the synergistic effect of PANI and GO on Ag3PO4 promotes the separation of photogenerated electrons and holes, thereby improving the stability of Ag3PO4 and photocatalytic activity.
-
Keywords:
- Ag3PO4 /
- graphene oxide /
- core-shell /
- photocatalyst /
- degradation /
- phenol /
- antibiotic /
- dye
-
随着电子元器件小型化、集成化和多功能化的发展,要求基础树脂能够及时传输元器件在使用过程中产生的热量,以避免热沉积引发火灾危险的问题[1-2]。环氧树脂(EP)因其优异的粘接、耐化学腐蚀和绝缘性能而被广泛用于层压电路板、电子元件封装和热界面材料的基础树脂[3-5]。但EP本身易燃并且导热系数也非常低,约0.2 W·m−1·K−1,使其应用受限[6-8]。因此,对EP进行有效阻燃和导热改性至关重要。
石墨烯具有声子传热散射小、传热效率高等优点,导热系数高达5000 W·m−1·K−1,成为复合型导热高分子材料制备的候选填料[9],其二维层状结构具有较强的气体阻隔作用、较高的热稳定性和较大的比表面吸附能力,有利于协同阻燃[10]。石墨烯及其衍生物中,石墨烯纳米片(GNPs) 在复合材料中应用占比最大,机械剥离法比氧化还原法成本低、易制备、污染少、缺陷程度低[11-12]。报道指出[13-15],以三聚氰胺(MN)为助剥离剂,利用
π -π 相互作用,通过球磨微粉石墨可获得非共价功能化GNPs的优点是既不破坏其表面疏水性,也不产生缺陷结构[15-16]。由此,能够改善GNPs与树脂基体的界面相互作用,更好发挥其导热性能。同时,多层石墨烯显示了比单层石墨烯更好的导热效果,且与未完全剥离的微粉石墨等导热填料可以协同形成更有效的导热网络[17-18]。三嗪化合物MN具有良好的热稳定性,不仅用作助剥离剂,还可以作为制备绿色膨胀阻燃剂体系,如三聚氰胺磷酸盐(MP) [19]、二苯氧基磷酸三聚氰胺盐[20]、三聚氰胺氰尿酸盐[21]、羟基乙叉二磷酸四三聚氰胺盐[22]等的气源使用。其中MP价格低廉,其热稳定性和吸热作用优于聚磷酸铵基的膨胀阻燃剂。MP在热分解过程中能够产生MN和多磷酸,前者热解释放NH3吸热,后者在热分解过程中产生多磷酸,使基体脱水生成均匀致密的炭层,发挥隔热、隔氧、阻燃和抑烟作用[23]。MP与类石墨氮化碳杂化时,能够提高热稳定性和阻燃效率[24],与SiO2杂化时,可以提高疏水性和阻燃效率[19]。
因此,本文采用MN为助剥离剂,基于其与石墨烯之间的
π -π 相互作用及非共价修饰原理,通过机械球磨微粉石墨及磷酸液相反应,制备了兼具阻燃和导热性能的石墨烯纳米片杂化三聚氰胺磷酸盐(GMP);在对其结构和热性能进行表征的基础上,探讨了GMP对EP树脂燃烧、热分解行为及导热性能的影响。1. 实验材料及方法
1.1 原材料
环氧单体(E-51),工业纯,岳阳巴陵华兴石化有限公司;4,4-二氨基二苯甲烷(DDM),分析纯,阿拉丁试剂上海有限公司;微粉石墨 (GRA),ADT-005,D90:8~11 µm,石家庄科鹏阻燃材料厂;三聚氰胺(MN),分析纯,上海安耐吉试剂有限公司;三聚氰胺磷酸盐(MP),实验室合成,粒径小于10 μm,磷与氮含量分别为13.8%和37.5%;N,N-二甲基甲酰胺(DMF),分析纯,阿拉丁试剂上海有限公司;磷酸,分析纯,上海迈瑞尔化学技术有限公司;无水乙醇,分析纯,北京化工厂。
1.2 GMP的制备
如图1所示,将4.5 g MN和1.5 g微粉石墨加入氧化锆研磨罐,300 r/min球磨处理16 h,得到MN剥离修饰的石墨烯纳米片(MN-GNPs)。取32.0 g MN-GNPs在95℃搅拌下,溶于320 mL的去离子水中,并缓慢滴加12.7 mL磷酸进行液相反应2 h,冷却至室温后,过滤洗涤并在75℃真空环境下干燥,得到石墨烯纳米片杂化三聚氰胺磷酸盐(GMP)。将MN-GNPs溶于DMF,7000 r/min离心15 min,取上清液在0.45 μm的聚四氟乙烯滤纸上过滤,用热水洗去三聚氰胺干燥后称重计算得GNPs含量,GNPs在GMP中的占比≥1.62wt%,MP在GMP中占比84.21wt%,其他为未完全剥离微粉石墨(含有片层较薄的石墨微片)的含量。
1.3 环氧树脂(EP)复合材料的制备
按表1配方将一定量GMP、MP、GRA分别分散于无水乙醇中,超声处理2 h,将分散混合物倒入E-51中,90℃加热搅拌2 h除去乙醇,根据环氧值加入定量DDM,搅拌后抽真空,浇注于预热后的聚四氟乙烯模具中,于100℃固化2 h,150℃继续固化2 h,获得复合材料GMP/EP、MP/EP和MP-GRA/EP。纯环氧树脂在相同固化条件固化,标记为EP。
表 1 复合材料的配方及阻燃性能Table 1. Formulation and flame retardancy of compositesSample E-51/wt% DDM/wt% MP/wt% GMP/wt% GRA/wt% P/wt% LOI/% EFF UL 94 (3 mm) EP 80.0 20.0 0 0 0 0 24.5 — NR GMP20/EP 64.0 16.0 0 20.0 0 2.3 27.1 1.13 V-1 GMP25/EP 60.0 15.0 0 25.0 0 2.9 28.4 1.34 V-1 GMP30/EP 56.0 14.0 0 30.0 0 3.5 30.4 1.68 V-0 MP20/EP 64.0 16.0 20.0 0 0 2.8 26.8 0.82 V-1 MP25/EP 60.0 15.0 25.0 0 0 3.5 28.5 1.14 V-0 MP30/EP 56.0 14.0 30.0 0 0 4.2 31.0 1.55 V-0 MP-GRA20/EP 64.0 16.0 16.8 0 3.2 2.3 26.9 1.04 V-1 MP-GRA25/EP 60.0 15.0 21.1 0 3.9 2.9 28.1 1.24 V-1 MP-GRA30/EP 56.0 14.0 25.3 0 4.7 3.5 30.1 1.19 V-0 Notes: EP—Epoxy resin; E-51—Epoxy monomer; DDM—4, 4-Diaminodiphenylmethane; MP—Melamine phosphate; GMP—Graphene nanoplatelets hybrid melamine phosphate; P—Phosphorus content in composite materials; LOI—Limit oxygen index; EFF—Flame retardancy efficiency and represents the LOI increment produced by each 1wt% of phosphorus in the composites; NR—No rating. 1.4 分析与表征
采用德国布鲁克公司原子力显微镜(Dimension FastScan Bio)测定GNPs的厚度和横向尺寸;采用FEI香港有限公司扫描电子显微镜(QUNATA250)观察GMP及复合材料断面的微观形貌;采用日本电子株式会社场发射透射电镜(JEM 2100)表征GNPs形貌;采用英国雷尼绍公司拉曼光谱仪(Renishaw in Via)表征GNPs层状结构;采用美国尼高力公司傅里叶变换红外光谱仪(iS10 FT-IR Spectrometer)表征GMP的化学结构;采用日本株式会社理学X射线衍射仪(MiniFlex 600)对GMP的晶格结构进行表征;采用德国耐驰公司热重分析仪(TG 209 F1)进行GMP及复合材料的热重分析;采用美国PerkinElmer公司X射线光电子能谱仪(PHI Quantera II SXM)检测GMP表面元素的变化;采用泰思泰克(苏州)检测仪器科技有限公司氧指数仪(TTech-GBT2406-2),依据GB/T 2406.2—2009[25]测试复合材料极限氧指数(LOI)值;采用南京江宁区分析仪器厂水平垂直燃烧测定仪(CZF-3),依据GB/T 2408—2008[26]测试复合材料垂直燃烧等级;采用英国FTT公司FTT 0007型锥形量热仪(CONE)测试复合材料的燃烧行为,依据标准ISO 5660—1[27],热辐照通量为50 kW/m2;使用德国耐驰公司差示扫描量热仪(DSC 204 F1)测试复合材料比热容Cp;采用德国耐驰公司激光导热仪(LFA 467)测量直径12.7 mm,厚度1 mm样品的热扩散系数α;采用排水法测得样品密度ρ;由公式λ=αρCp计算得到导热系数。
2. 结果与讨论
2.1 GMP的形貌、结构、组成及热稳定性
首先,采用TEM和AFM表征了GNPs的结构,如图2所示。图2(a)和图2(b)为GNPs的TEM图像。呈现出半透明的GNPs图像,高倍观察堆叠边缘最大厚度约为4 nm(12层)。图2(c)和图2(d)为GNPs的AFM图像及分析。显示GNPs形状不规则,其厚度约2 nm(6层),横向尺寸在微米级。由此表明,助剥离剂MN的剥离效果良好,得到的GNPs为少层石墨烯。
其次,采用SEM表征了GMP的形貌,如图3所示。可见,与MP对比,GMP的表面形貌相对粗糙,呈不规则颗粒状。这可能由于GNPs表面吸附及MN的
π -π 相互作用,改变了晶面表面能,各向异性导致GMP晶面的生长速率不同所致[28-29]。采用Raman、XRD、FTIR、XPS及TG手段研究了GMP结构、组成及热稳定性,结果如图4所示。图4(a)的Raman曲线峰形也证实了MN助剥离得到的是少层石墨烯[14]。与微粉石墨相比,MN-GNPs的2D带下移至2687 cm−1,D峰和G峰的强度比ID/IG增至0.27,表明由
π -π 相互作用形成了缺陷较小的非共价修饰的GNPs[15,30]。![]() 图 4 (a) 微粉石墨(GRA)和三聚氰胺(MN)-GNPs的Raman光谱;(b) GRA、MN、三聚氰胺磷酸盐(MP)、MN-GNPs和GMP的XRD图谱;(c) GRA、MP、MN-GNPs和GMP的FTIR图谱;MN-GNPs (d) 和GMP (e) 的XPS N1s图谱Figure 4. (a) Raman spectra of powder graphite (GRA) and melamine (MN)-GNPs; (b) XRD patterns of GRA, MN, melamine phosphate (MP), MN-GNPs and GMP; (c) FTIR spectra of GRA, MP, MN-GNPS and GMP; XPS N1s spectra of MN-GNPs (d) and GMP (e)ID/IG—Intensity ratio of peak D to peak G
图 4 (a) 微粉石墨(GRA)和三聚氰胺(MN)-GNPs的Raman光谱;(b) GRA、MN、三聚氰胺磷酸盐(MP)、MN-GNPs和GMP的XRD图谱;(c) GRA、MP、MN-GNPs和GMP的FTIR图谱;MN-GNPs (d) 和GMP (e) 的XPS N1s图谱Figure 4. (a) Raman spectra of powder graphite (GRA) and melamine (MN)-GNPs; (b) XRD patterns of GRA, MN, melamine phosphate (MP), MN-GNPs and GMP; (c) FTIR spectra of GRA, MP, MN-GNPS and GMP; XPS N1s spectra of MN-GNPs (d) and GMP (e)ID/IG—Intensity ratio of peak D to peak G图4(b)为GRA、MN、MP、MN-GNPs和GMP的XRD图谱。可以看出,MN-GNPs和GMP在2θ=26.6°(002)形成了较石墨矮而宽的衍射峰,表明GRA被明显剥离。更重要的是GMP在2θ=17°、25.5°出现了与MP相对应的两个峰,意味着GMP的形成。FTIR和XPS结果支持了GMP的形成。由图4(c)的FTIR图谱可见,相较于MN-GNPs在3000~3500 cm−1区域的—NH2和—OH吸收峰,GMP在3392 cm−1和3131 cm−1处的吸收峰明显加宽,代表着—NH2、—NH3+及P—OH的伸缩振动;1110 cm−1和984 cm−1对应于P—OH和P—O伸缩振动[19]。同样,图4(e)为MN-GNPs和GMP的XPS N1s图谱。GMP图谱中出现了400.2 eV的—NH3+拟合峰[31],其面积近似是—NH2的二分之一,且GMP与MP的N/P质量比基本一致(表2),进一步证实了GMP的形成。
表 2 MN-GNPs、MP和GMP的表面元素组成Table 2. Surface elemental compositions of MN-GNPs, MP and GMPSample C/wt% N/wt% O/wt% P/wt% N/P MN-GNPs 66.1 28.7 5.2 — — MP 27.6 31.9 25.5 15.1 2.1 GMP 59.2 18.4 14.2 8.3 2.2 图5为氮气气氛下GRA、MP和GMP的TGA和DTG曲线。可以看出,GRA表现出较高的热稳定性,GNPs的存在导致GMP的初始热分解温度(292.6℃,5wt%失重)较MP(263.3℃)提高了29.3℃,与EP的初始热分解温度(368.1℃)更接近,EP的初始热分解温度的测试数据见表3。且最大热失重速率降低、700℃下残渣量显著提高。GMP初始热分解温度与基材匹配性更好及残渣量的提高是获得良好凝聚相阻燃效果的重要因素。
表 4 EP、GMP/EP、MP/EP复合材料锥形量热仪测试数据Table 4. Combustion parameters of EP, GMP/EP, MP/EP composites from cone testSample TTI/s PHRR/(kW·m−2) THR/(MJ·m−2) PSPR/(m2·s−1) TSP/(m2·kg−1) CR/% EP 40 954.8 90.0 0.454 41.9 5.0 GMP20/EP 37 339.5 70.2 0.144 26.1 23.6 GMP30/EP 42 297.4 62.7 0.118 19.5 31.3 MP20/EP 37 285.3 77.1 0.127 22.9 24.8 MP30/EP 37 247.7 68.6 0.116 18.7 29.6 Notes: TTI—Time to ignition; PHRR—Peak heat release rate; THR—Total heat release; PSPR—Peak smoke produce rate; TSP—Total smoke production; CR—Char residues. 2.2 复合材料的阻燃性能
表1给出了复合材料的氧指数(LOI)和UL 94垂直燃烧测试结果。当GMP的用量增加至30wt%, GMP30/EP复合材料的LOI上升至难燃级(大于30%),UL 94达到V-0级。为了进一步分析材料阻燃性能,对GMP/EP、MP/EP和MP-GRA/EP复合材料的阻燃效率(EFF)[32]进行了比较,GMP/EP的EFF最高。尽管MP添加量达25 wt%时,复合材料就可以通过V-0级,但MP/EP的EFF低于GMP/EP和MP-GRA/EP。由于GMP中MP的含量为84.21wt%,GMP/EP和MP-GRA/EP复合材料中的磷含量低于MP/EP,因此GMP/EP和MP-GRA/EP中1wt%磷产生的LOI增值更高。上述结果不仅与GMP热解吸热及热解产物多磷酸对基材的脱水交联成炭阻燃作用有关,而且与石墨微片和GNPs的阻隔机制有关。
锥形量热仪测试(CONE)是模拟真实火灾条件下材料燃烧行为的重要研究手段。图6和表4为EP和复合材料CONE燃烧测试结果,包括点燃时间(TTI)、热释放速率(HRR)及峰值HRR(PHRR)、烟释放速率(SPR)及峰值SPR(PSPR)、总热释放速率(THR)、总烟释放量(TSP)、平均有效燃烧热(Av-EHC)及残炭率(CR)。从表4可以看出,与MP对比,GMP 使TTI略有延长,与GNPs的阻隔作用有关。虽然在30wt%添加量下,GMP30/EP的PHRR、PSPR及TSP略高于MP30/EP,但与EP比较,降低幅度高达69%、74%和53%,且GMP30/EP的THR(62.7 MJ·m−2)最低。
表 3 复合材料在N2气氛下的TG和DTG数据Table 3. TG and DTG data of composites materials under N2 atmosphereSample T5%/℃ ΔT5%/℃ Tmax/℃ CR700℃/% ΔCR700℃/% Exp. Cal. Exp. Cal. EP 368.1 — — 382.9 20.1 — — GMP 292.6 — — 396.4 42.0 — — MP 263.3 — — 391.2 28.1 — — GMP20/EP 328.5 353.0 −24.5 364.0 29.7 24.2 5.5 GMP30/EP 337.8 345.4 −8.0 363.3 38.2 26.5 11.7 MP20/EP 332.8 347.1 −14.3 363.8 30.7 21.5 9.2 MP30/EP 329.5 336.7 −7.2 363.3 33.5 22.3 11.2 Notes: Exp.—Test results; Cal.—Calculated results; T5%—Temperature with mass loss of 5wt%; Tmax—Maximum decomposition temperature; CR700℃—Char residues at 700℃; ΔT5%=T5%Exp.— T5%Cal.; ΔCR700℃=CR700℃Exp.— CR700℃Cal. 另外,CR随阻燃剂含量的增大而增加,体现了稳定炭层的形成与MP促进成炭和石墨微片及GNPs阻隔作用的结合。CR的增加能够将更多的热分解产物保留在凝聚相,延缓材料燃烧过程热和烟的释放。正如图7中所示的CONE测试后残炭数码照片及相应的SEM图像,相对EP残炭,GMP20/EP的残炭表面结构完整,裂纹很少,GMP表现出了膨胀成炭效果。内嵌SEM图像显示表面致密均匀,内部以石墨微片或GNPs为骨架形成了多重网络,具有阻碍热量和物质交换的凝聚相阻燃作用,显著抑制了热和烟的释放。
2.3 复合材料的热分解行为
热分解行为的研究有助于理解GMP对复合材料燃烧性能的影响规律。从图8和表3给出的TG、DTG曲线及相关数据可见,复合材料的初始分解温度(T5%,失重5wt%对应的温度)、最大热分解温度(Tmax)低于EP基材,700℃下的残炭率显著增加。GMP30/EP与MP30/EP复合材料CR增加的幅度相对较大,与上述阻燃性能提高的规律一致,反映了阻燃剂的凝聚相作用机制。值得注意的是复合材料的T5%,通过计算值T5%Cal.的分析可见,对于实验值与计算值的差值ΔT5%,GMP/EP较MP/EP降低得更多,说明除了受阻燃剂T5%偏低的影响之外,GMP促进基材热降解的作用更强。源于GNPs的催化热降解[10]与MP促进基材脱水交联作用的结合。
2.4 复合材料的导热性能及机制
图9为EP复合材料导热性能与阻燃剂添加量的关系。可见,随阻燃剂添加量的增加GMP/EP的导热系数上升最显著。30 wt%添加量下, GMP30/EP的导热系数高达2.10 W·m−1·K−1,相对于基材EP提高了708%,相对于MP30/EP和MP-GRA30/EP分别提高了239%和275%,且优于BN[33-34]、AlN[35]、Al2O3[36-37]、石墨[38]等传统导热填料(图10),反映了石墨烯纳米片杂化阻燃剂GMP的多功能性和先进性。另外,值得注意的是GMP/EP曲线约在20wt%添加量附近呈现出导热系数变化的拐点,反映出纳米填料的逾渗现象。
GMP赋予复合材料导热性的原因主要有两方面,一是GMP含有MN非共价修饰剥离的高导热GNPs;其二是磷酸盐类化合物对环氧树脂具有良好的相容性,使GMP在基材有良好的分散性。由表5可见,复合材料的导热系数是热扩散系数、比热容、密度三者的乘积, GMP30/EP复合材料的热扩散系数最大,源于GNPs高导热的贡献。从图11复合材料的断面形貌可见,相对于图11(g)EP光滑的断面而言,复合材料的断面都显得粗糙。而图11(b)GMP30/EP中的阻燃剂与树脂界面相对模糊,说明GMP与树脂具有良好的相容性,导致GMP在基材中有良好的分散性,也使得材料具有更高的比热容Cp。相反,图11(f)的MP-GRA30/EP界面最清晰,说明MP与GRA共混的阻燃剂与树脂的相容性最差,分散性差的阻燃剂不能有效搭接形成导热网络,因此MP-GRA/EP表现出相对低的导热性能。为此,提出图11(h)~11(k)所示的导热机制,良好分散的填料使高导热石墨烯纳米片与石墨微片搭接形成热传导通道,显著降低了界面热阻,于是GMP/EP复合材料表现出相对最好的导热性能。
表 5 复合材料的热扩散系数α、比热容Cp、密度ρ及导热系数λTable 5. Thermal diffusivity α, specific heat capacity Cp, density ρ and thermal conductivity λ of compositesSample GNPs/wt% GRA/wt% α/(mm2·s−1) Cp/( J·g−1·K−1) ρ/(g·cm−3) λ/(W·m−1·K−1) EP 0 0 0.163 1.428 1.103 0.26 GMP30/EP ≥0.5 ≤4.2 0.588 2.537 1.409 2.10 MP30/EP 0 0 0.197 2.483 1.274 0.62 MP-GRA30/EP 0 4.7 0.253 1.695 1.305 0.56 ![]() 图 11 GMP30/EP ((a), (b), (h))、MP30/EP ((c), (d), (i))、MP-GRA30/EP ((e), (f), (j)) 和EP ((g), (k)) 复合材料断裂表面的SEM图像及导热机制Figure 11. SEM images of fractured surfaces and heat conductive mechanism of GMP30/EP ((a), (b), (h)), MP30/EP ((c), (d), (i)), MP-GRA30/EP ((e), (f), (j)) and EP ((g), (k)) composites
图 11 GMP30/EP ((a), (b), (h))、MP30/EP ((c), (d), (i))、MP-GRA30/EP ((e), (f), (j)) 和EP ((g), (k)) 复合材料断裂表面的SEM图像及导热机制Figure 11. SEM images of fractured surfaces and heat conductive mechanism of GMP30/EP ((a), (b), (h)), MP30/EP ((c), (d), (i)), MP-GRA30/EP ((e), (f), (j)) and EP ((g), (k)) composites3. 结 论
(1) 基于三聚氰胺和石墨烯之间的
π -π 相互作用,采用三聚氰胺为助剥离剂机械球磨的微粉石墨与磷酸液相反应,成功制备了石墨烯纳米片杂化三聚氰胺磷酸盐(GMP)。GMP中石墨烯纳米片的厚度约2 nm(6层),横向尺寸在微米级;GMP较三聚氰胺磷酸盐(MP) 初始分解温度提升了29.3℃,有更好的热稳定性。(2) 加入30wt%的GMP,环氧树脂(EP)复合材料的氧指数达到了30.4%,UL 94垂直燃烧为V-0级,峰值热释放和烟释放速率较EP分别降低了69%、74.0%。EP复合材料阻燃性能的提高与石墨微片和石墨烯纳米片良好分散、阻隔作用及三聚氰胺磷酸盐成炭作用结合有关。
(3) GMP/EP复合材料的导热系数随着GMP添加量增加而提高。当GMP含量为30 wt%时,GMP/EP复合材料的导热系数达到2.10 W·m−1·K−1,相对于EP提升了708%。
-
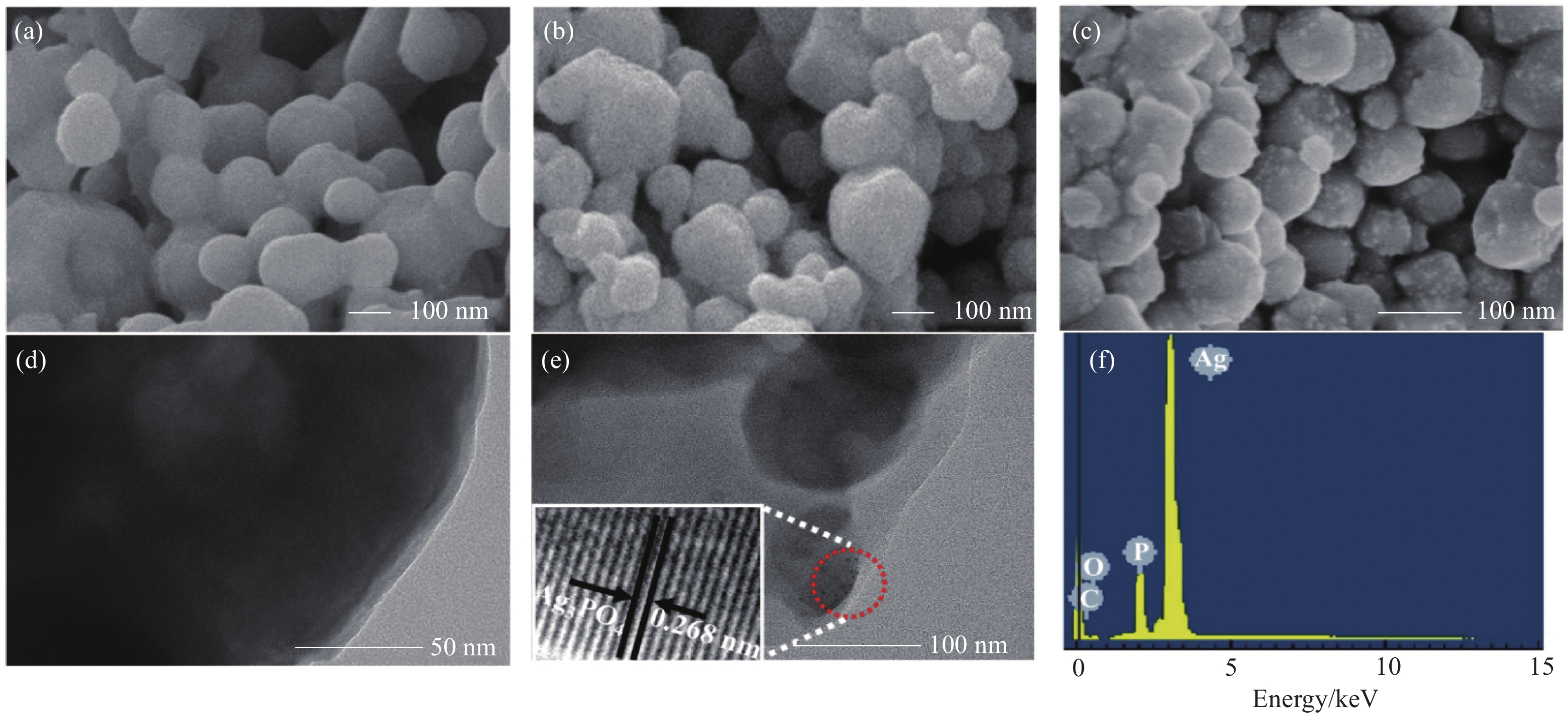
图 4 纯Ag3PO4 (a)、Ag3PO4@PANI (b) 和Ag3PO4@PANI/GO (c) 的SEM图像;Ag3PO4@PANI (d) 和Ag3PO4@PANI/4%GO (e) 的TEM图像;Ag3PO4@PANI/4%GO的EDS能谱图 (f)
Figure 4. SEM images of Ag3PO4 (a), Ag3PO4@PANI (b) and Ag3PO4@PANI/GO (c); TEM images of Ag3PO4@PANI (d) and Ag3PO4@PANI/4%GO (e); EDS spectrum of Ag3PO4@PANI/4%GO (f)
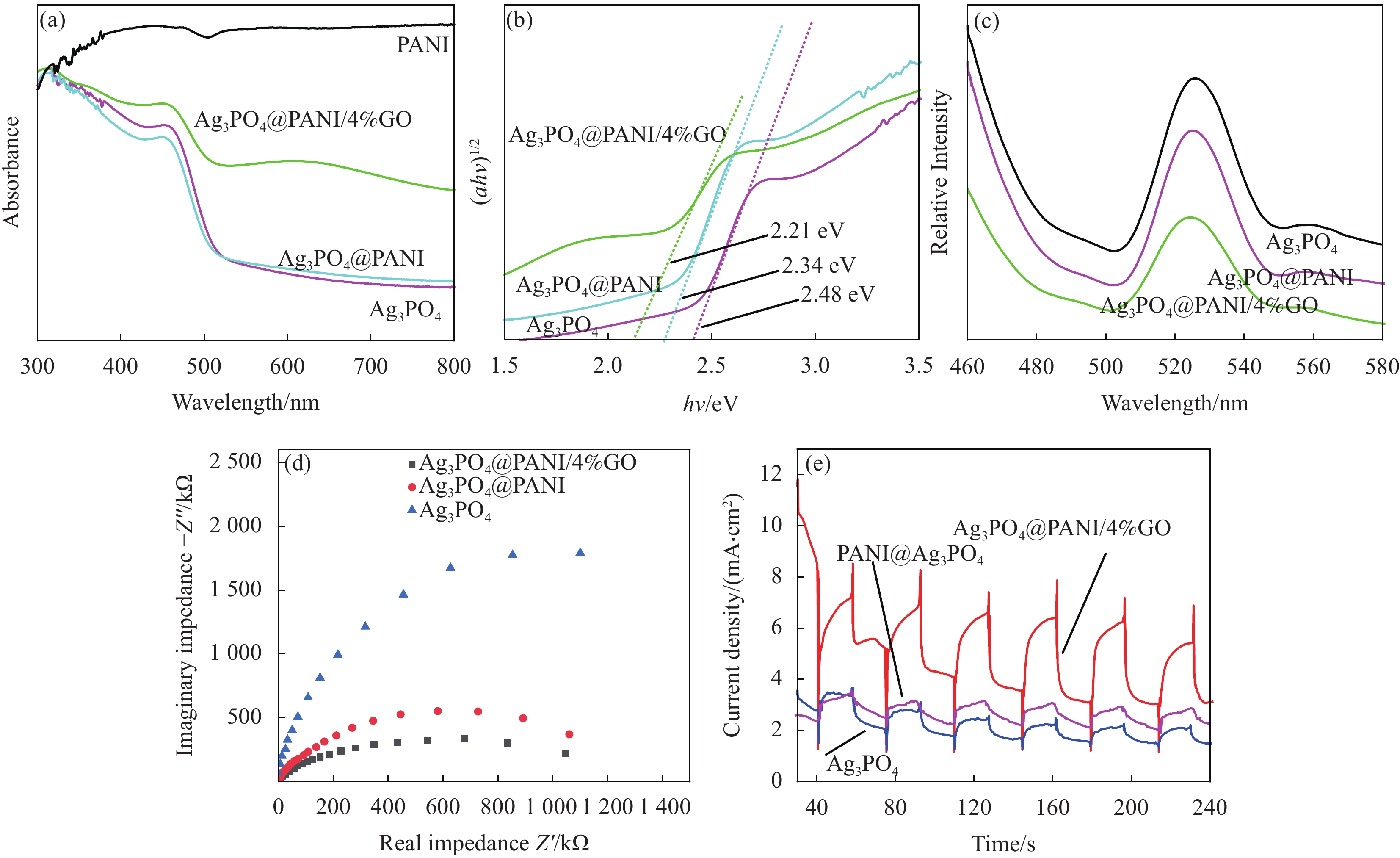
图 7 PANI、Ag3PO4、Ag3PO4@PANI和Ag3PO4@PANI/4%GO的UV-Vis (a)、Kubelka-Munk图 (b)、PL图 (c)、EIS图 (d) 和光电流响应图 (e)
Figure 7. UV-Vis diffuse reflectance spectra (a), plot of (αhν)1/2 vs. hν (b), Photoluminescence spectrum (c), EIS of Nyquist plots (d) and photocurrent responses (e) of PANI, Ag3PO4, Ag3PO4@PANI and Ag3PO4@PANI/4%GO
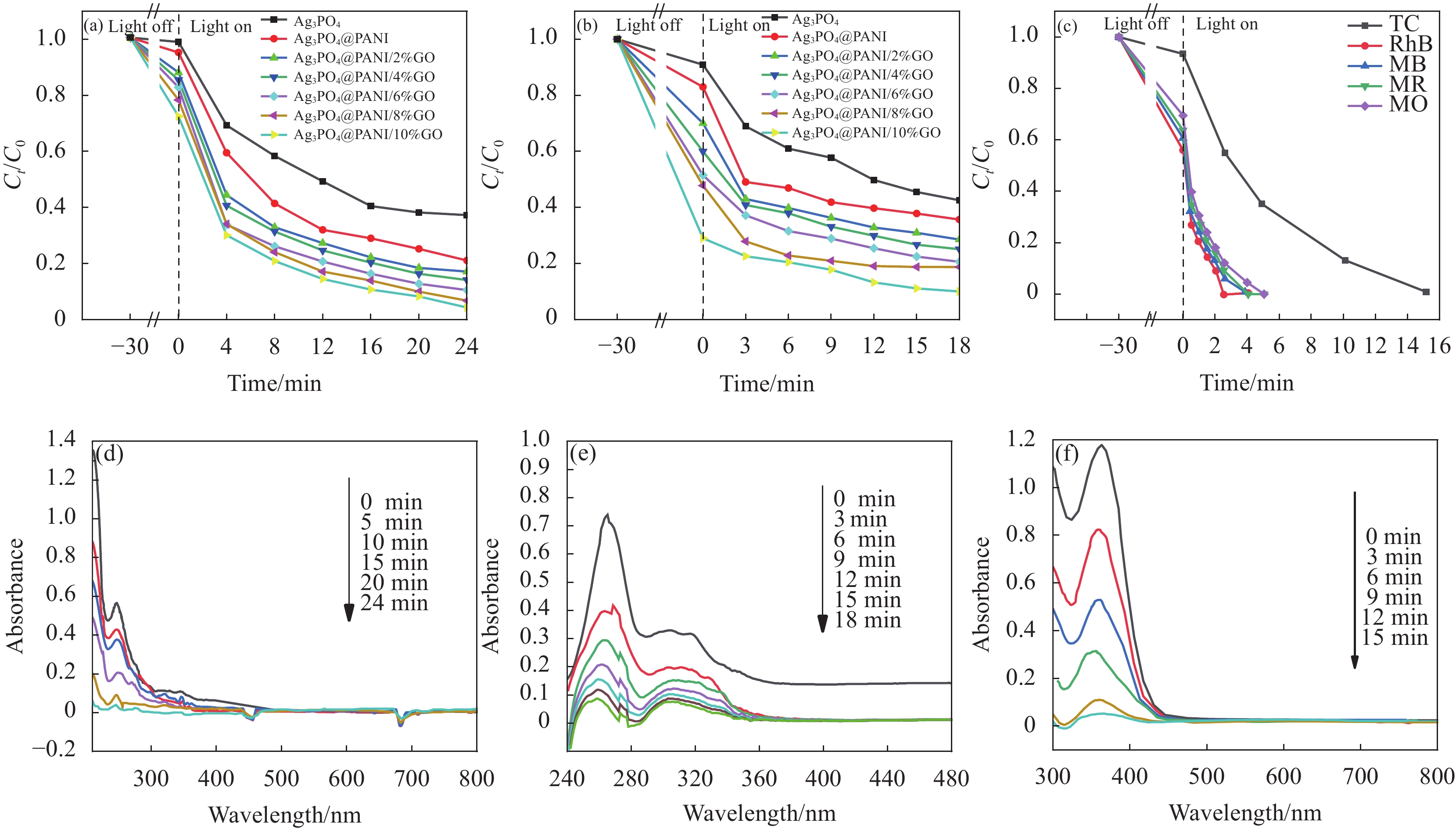
图 8 PANI、Ag3PO4、Ag3PO4@PANI和Ag3PO4@PANI/GO可见光下降解苯酚曲线 (a)、降解环丙沙星(CIP)曲线 (b);Ag3PO4@PANI/GO降解四环素(TC)、罗丹明B(RhB)、亚甲基蓝(MB)、亚甲基红(MR)和亚甲基橙 (MO)的曲线 (c);Ag3PO4@PANI/4%GO降解苯酚 (d)、CIP (e) 和TC (f) 的紫外-可见吸收光谱曲线
Figure 8. Under visible light curves of degradation of phenol (a), curves of degradation of ciprofloxacin (CIP) (b) of PANI, Ag3PO4, Ag3PO4@PANI and Ag3PO4@PANI/GO; Curves of degradation of tetracycline (TC), rhodamine B (RhB), methylene blue (MB), methylene red (MR) and methylene orange (MO) by Ag3PO4@PANI/GO (c); Degradation of phenol (d), CIP (e) and TC (f) by Ag3PO4@PANI/4%GO ultraviolet-visible absorption spectrum curves
Ct—Concentration after time t of degradation; C0—Initial concentration
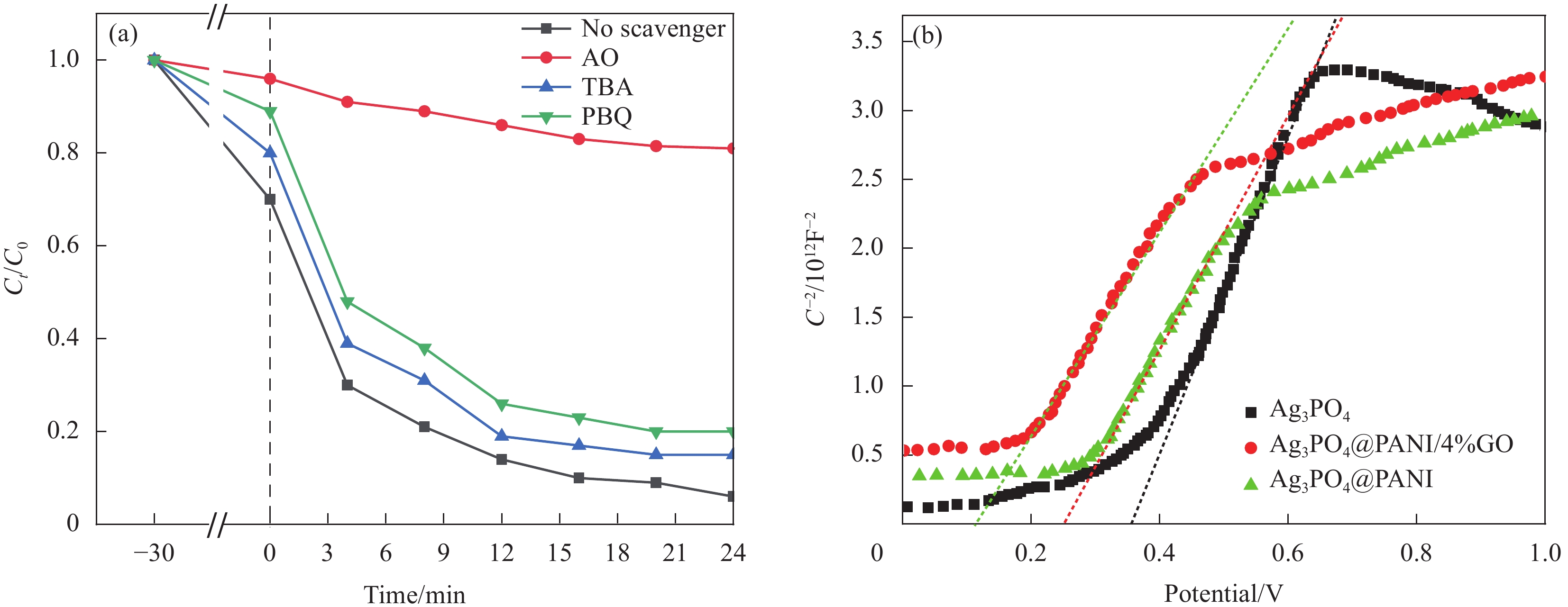
图 10 (a) Ag3PO4@PANI/4%GO的活性物种捕获实验;(b) Ag3PO4@PANI/4%GO、Ag3PO4和Ag3PO4@PANI的Mott–Schottky曲线
Figure 10. (a) Trapping experiments for active species of Ag3PO4@PANI/4%GO; (b) Mott–Schottky curves obtained for Ag3PO4@PANI/4%GO, Ag3PO4 and Ag3PO4@PANI
AO—Ammonium oxalate; TBA—Tertiary butyl alcohol; PBQ—Benzoquinone; C—Interface capacitance
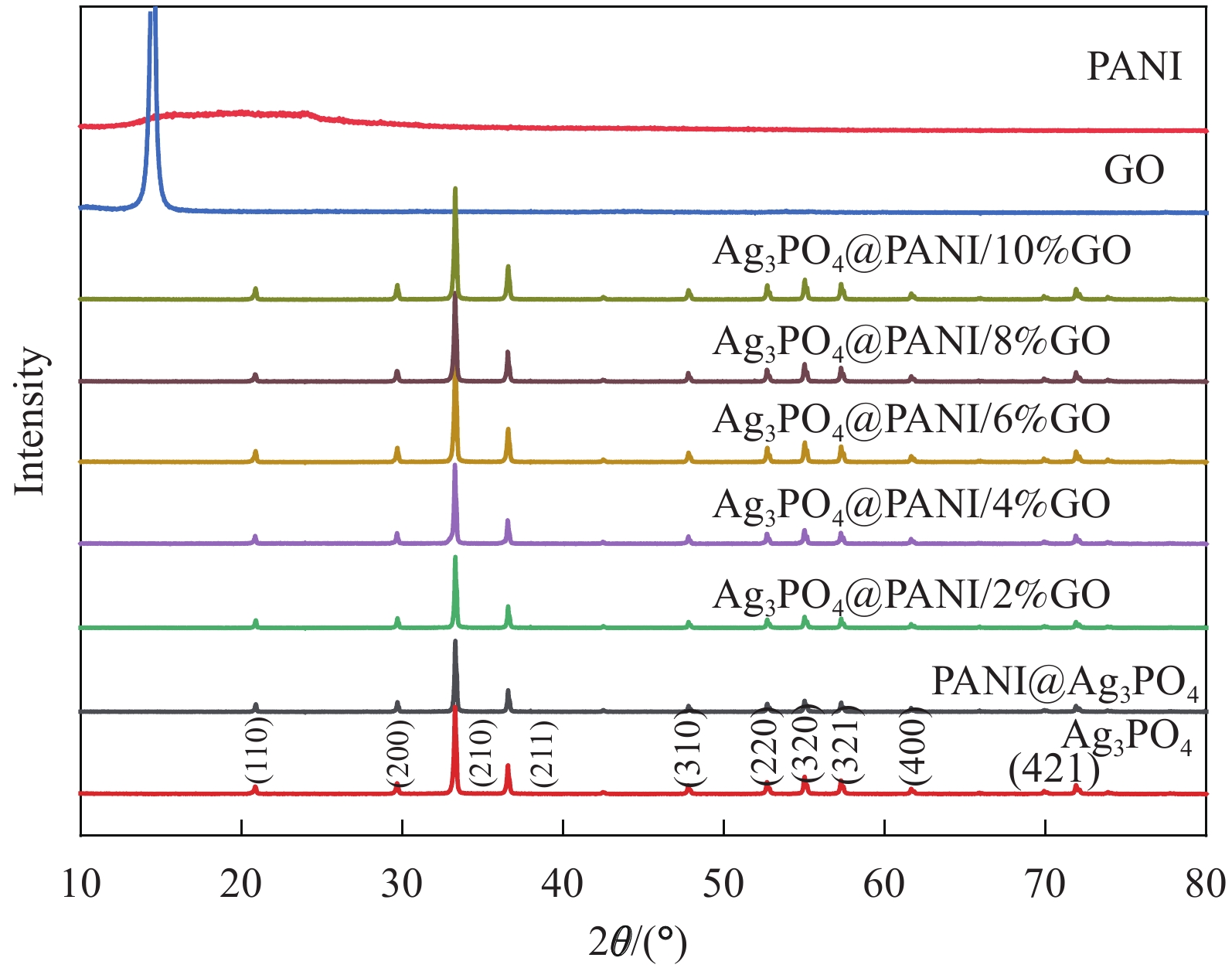
表 1 Ag3PO4@聚苯胺(PANI)/氧化石墨烯(GO)复合材料的命名
Table 1 Naming of Ag3PO4@polyaniline (PANI)/graphene oxide (GO) composites
Sample Mass ratio
of GO/%Mass ratio of
Ag3PO4@PANI/%Ag3PO4@PANI/2%GO 2 100 Ag3PO4@PANI/4%GO 4 100 Ag3PO4@PANI/6%GO 6 100 Ag3PO4@PANI/8%GO 8 100 Ag3PO4@PANI/10%GO 10 100 -
[1] KERGARAVAT S V, HERNÁNDEZ S R, GAGNETEN A M. Second-, third-and fourth-generation quinolones: Ecotoxicity effects on daphnia and ceriodaphnia species[J]. Chemosphere,2021,262:127823. DOI: 10.1016/j.chemosphere.2020.127823
[2] DORIVAL-GARCÍA N, ZAFRA-GÓMEZ A, NAVALÓN A, et al. Removal and degradation characteristics of quinolone antibiotics in laboratory-scale activated sludge reactors under aerobic, nitrifying and anoxic conditions[J]. Jour-nal of Environmental Management,2013,120:75-83.
[3] WANG H, LI J, HUO P, et al. Preparation of Ag2O/Ag2CO3/MWNTs composite photocatalysts for enhancement of ciprofloxacin degradation[J]. Applied Surface Science,2016,366:1-8. DOI: 10.1016/j.apsusc.2015.12.229
[4] LI N, ZHANG J, TIAN Y, et al. Precisely controlled fabrication of magnetic 3D γ-Fe2O3@ ZnO core-shell photocatalyst with enhanced activity: Ciprofloxacin degradation and mechanism insight[J]. Chemical Engineering Journal,2017,308:377-385. DOI: 10.1016/j.cej.2016.09.093
[5] BI Y, OUYANG S, UMEZAWA N, et al. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties[J]. Journal of the American Chemical Society,2011,133(17):6490-6492. DOI: 10.1021/ja2002132
[6] HE Y, ZHANG L, TENG B, et al. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel[J]. Environmental Science & Technology,2015,49(1):649-656.
[7] YANG X, CUI H, LI Y, et al. Fabrication of Ag3PO4-graphene composites with highly efficient and stable visible light photocatalytic performance[J]. ACS Catalysis,2013,3(3):363-369. DOI: 10.1021/cs3008126
[8] WU A, TIAN C, CHANG W, et al. Morphology-controlled synthesis of Ag3PO4 nano/microcrystals and their antibacterial properties[J]. Materials Research Bulletin,2013,48(9):3043-3048. DOI: 10.1016/j.materresbull.2013.04.054
[9] WU S, LIN Y, YANG C, et al. Enhanced activation of peroxymonosulfte by LaFeO3 perovskite supported on Al2O3 for degradation of organic pollutants[J]. Chemosphere,2019,237:124478. DOI: 10.1016/j.chemosphere.2019.124478
[10] XU Y S, ZHANG W D. Morphology-controlled synthesis of Ag3PO4 microcrystals for high performance photocatalysis[J]. CrystEngComm,2013,15(27):5407-5411. DOI: 10.1039/c3ce40172c
[11] YU H, DONG Q, JIAO Z, et al. Ion exchange synthesis of PAN/Ag3PO4 core-shell nanofibers with enhanced photocatalytic properties[J]. Journal of Materials Chemistry A,2014,2(6):1668-1671. DOI: 10.1039/C3TA14447J
[12] KHASEVANI S G, MOHAGHEGH N, GHOLAMI M R. Kinetic study of navy blue photocatalytic degradation over Ag3PO4/BiPO4@MIL-88B (Fe)@gC3N4 core@shell nanocomposite under visible light irradiation[J]. New Journal of Chemistry,2017,41(18):10390-10396. DOI: 10.1039/C7NJ01968H
[13] DENG J, LIU L, NIU T, et al. Synthesis and characterization of highly efficient and stable Pr6O11/Ag3PO4/Pt ternary hybrid structure[J]. Applied Surface Science,2017,403:531-539. DOI: 10.1016/j.apsusc.2017.01.257
[14] KIANI M, BAGHERZADEH M, KAVEH R, et al. Novel Pt- Ag3PO4/CdS/chitosan nanocomposite with enhanced photocatalytic and biological activities[J]. Nanomaterials,2020,10(11):2320. DOI: 10.3390/nano10112320
[15] PEI S, CHENG H M. The reduction of graphene oxide[J]. Carbon,2012,50(9):3210-3228. DOI: 10.1016/j.carbon.2011.11.010
[16] NACIRI Y, HSINI A, AJMAL Z, et al. Recent progress on the enhancement of photocatalytic properties of BiPO4 using π–conjugated materials[J]. Advances in Colloid and Interface Science,2020:102160.
[17] CHEN S, HUANG D, ZENG G, et al. In-situ synthesis of facet-dependent BiVO4/Ag3PO4/PANI photocatalyst with enhanced visible-light-induced photocatalytic degradation performance: Synergism of interfacial coupling and hole-transfer[J]. Chemical Engineering Journal,2020,382:122840. DOI: 10.1016/j.cej.2019.122840
[18] TAO R, YANG S, SHAO C, et al. Reusable and flexible g-C3N4/Ag3PO4/polyacrylonitrile heterojunction nanofibers for photocatalytic dye degradation and oxygen evolution[J]. ACS Applied Nano Materials,2019,2(5):3081-3090. DOI: 10.1021/acsanm.9b00428
[19] LIU L, HU P, LI Y, et al. P3HT-coated Ag3PO4 core-shell structure for enhanced photocatalysis under visible light irradiation[J]. Applied Surface Science,2019,466:928-936. DOI: 10.1016/j.apsusc.2018.10.112
[20] SUN X, LIU Z, GUO J, et al. Novel stable enhanced visible light photocatalytic system based on a Ag3PO4@polypyrrole core-shell Z-scheme with in-situ generated metallic Ag ohmic contacts[J]. Journal of Physics and Chemistry of Solids,2020,146:109572. DOI: 10.1016/j.jpcs.2020.109572
[21] LIU L, DING L, LIU Y, et al. A stable Ag3PO4@PANI core@shell hybrid: Enrichment photocatalytic degradation with π-π conjugation[J]. Applied Catalysis B: Environmental,2017,201:92-104. DOI: 10.1016/j.apcatb.2016.08.005
[22] WANG Y, WU M, LEI W, et al. Preparation of 3D grid structure rGH/α-Ag3VO4/GOQDs and its catalytic performance under visible light[J]. Journal of Alloys and Compounds, 2022, 895: 162410-162415.
[23] ISHIKAWA A, TAKATA T, KONDO J N, et al. Oxysulfide Sm2Ti2S2O5 as a stable photocatalyst for water oxidation and reduction under visible light irradiation (λ≤650 nm)[J]. Journal of the American Chemical Society,2002,124(45):13547-13553. DOI: 10.1021/ja0269643
-
期刊类型引用(7)
1. 房恩惠,杨树桐,庞瑞阳,孙忠科,兰天. 树脂预浸技术提升CFRP-混凝土界面力学性能研究. 复合材料科学与工程. 2025(02): 117-128 .  百度学术
百度学术
2. 丛龙宇,张方,钱永久. 外部粘贴CFRP-ECC粘结性能的影响因素试验. 复合材料学报. 2025(03): 1538-1554 .  本站查看
本站查看
3. 温小栋,王俊豪,殷光吉,邵璟璟,冯蕾. 海水与腐蚀电流耦合作用下铝合金与混凝土界面黏结性能. 建筑结构学报. 2024(04): 237-246 .  百度学术
百度学术
4. 朱红兵,付正昊,王烨,陈经毅. 界面剂对全轻陶粒混凝土与普通混凝土粘结界面力学性能的影响. 复合材料学报. 2024(06): 3154-3167 .  本站查看
本站查看
5. 叶华勇. 界面处理方式对CFRP-ECC复合加固混凝土梁受弯性能影响研究. 福建建筑. 2024(11): 49-54 .  百度学术
百度学术
6. 姜天华,万聪聪,颜斌. BFRP筋与钢-PVA混杂ECC粘结性能. 复合材料学报. 2023(06): 3499-3512 .  本站查看
本站查看
7. 昝鹏,陈燕萍. 水利工程混凝土复合材料的制备与力学性能分析. 塑料助剂. 2023(03): 30-33 .  百度学术
百度学术
其他类型引用(9)
-









 下载:
下载: