Synthesis and electrocatalytic oxygen evolution performances of Co2Ni1O4/stainless steel composites
-
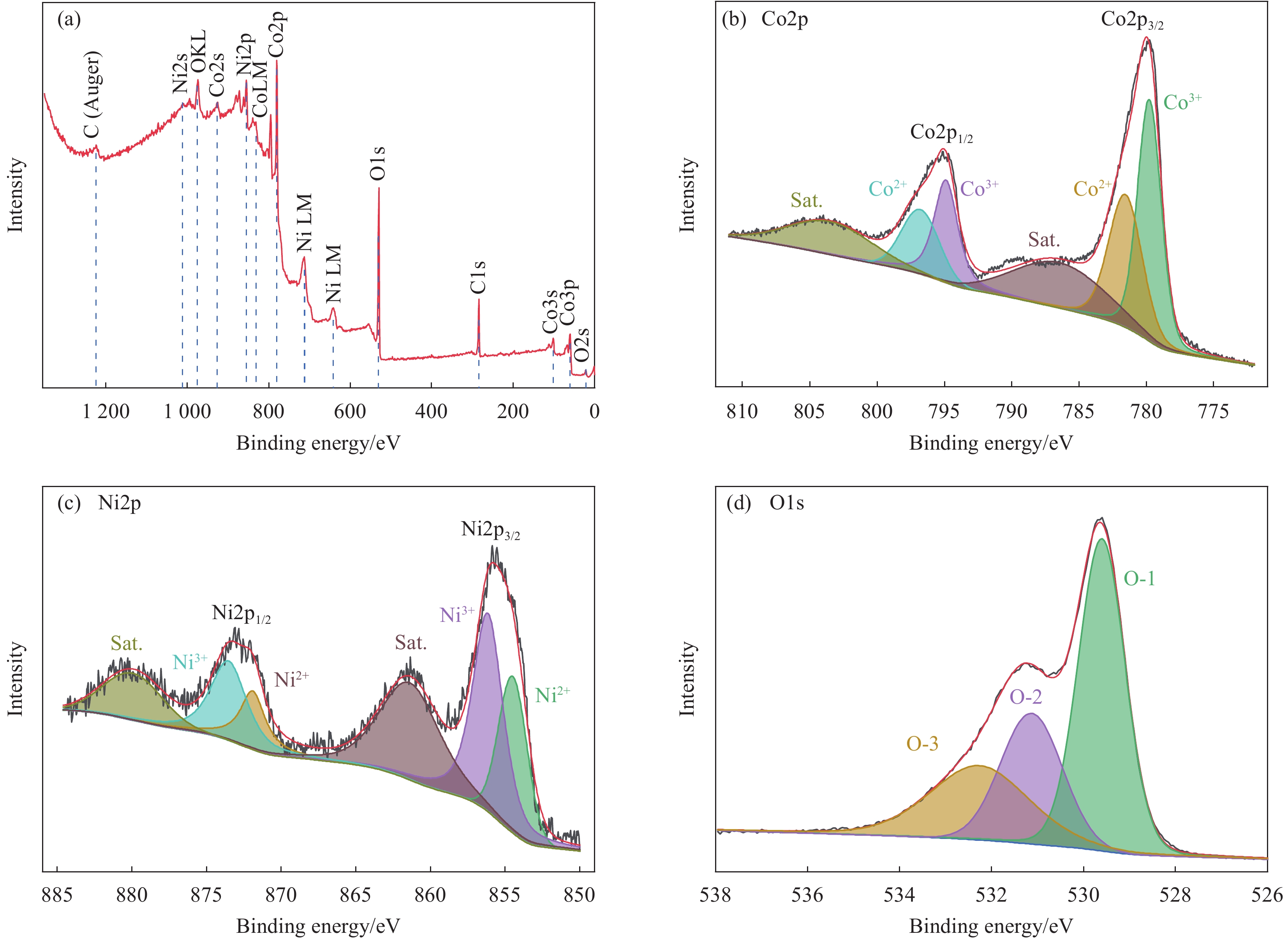
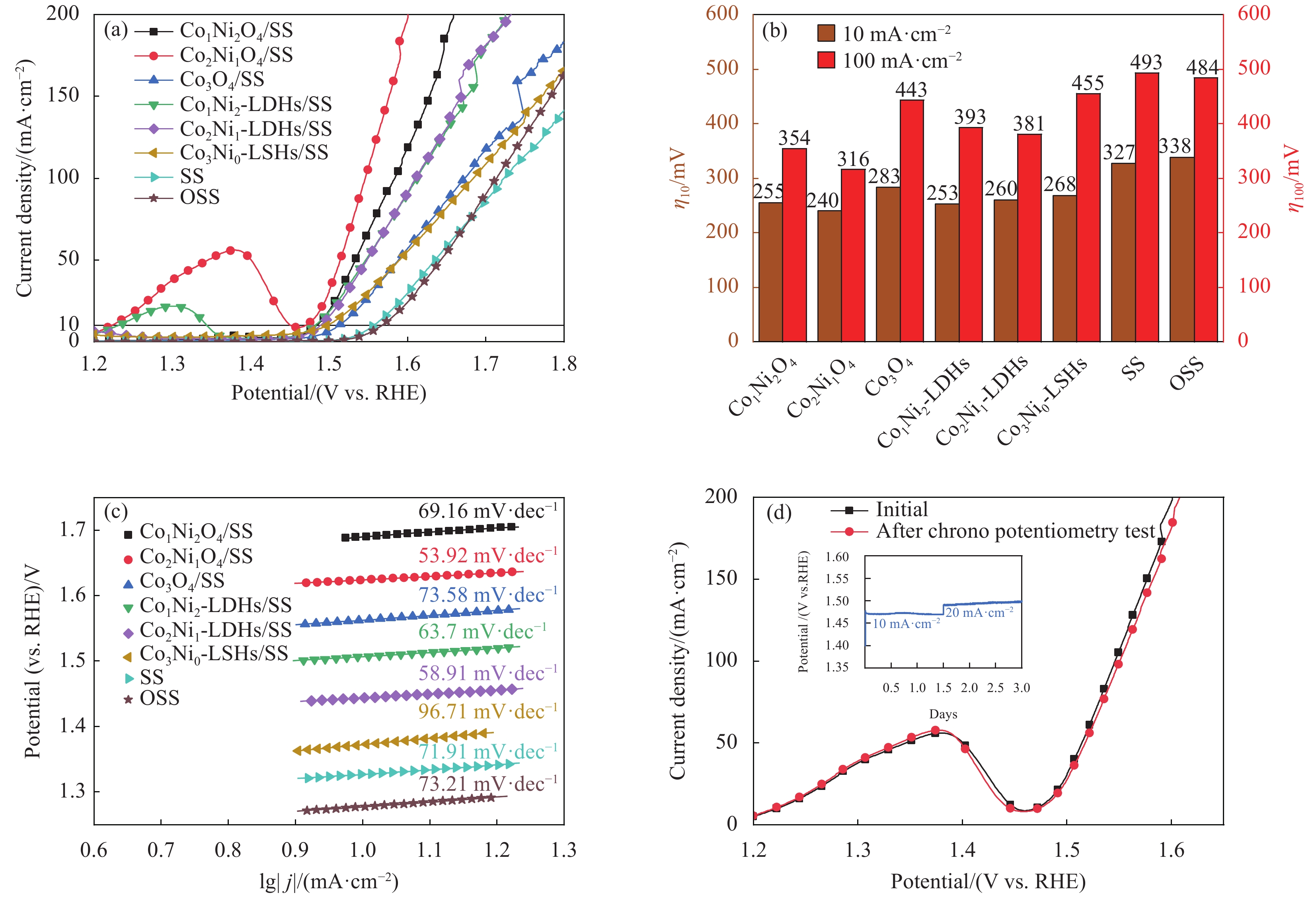
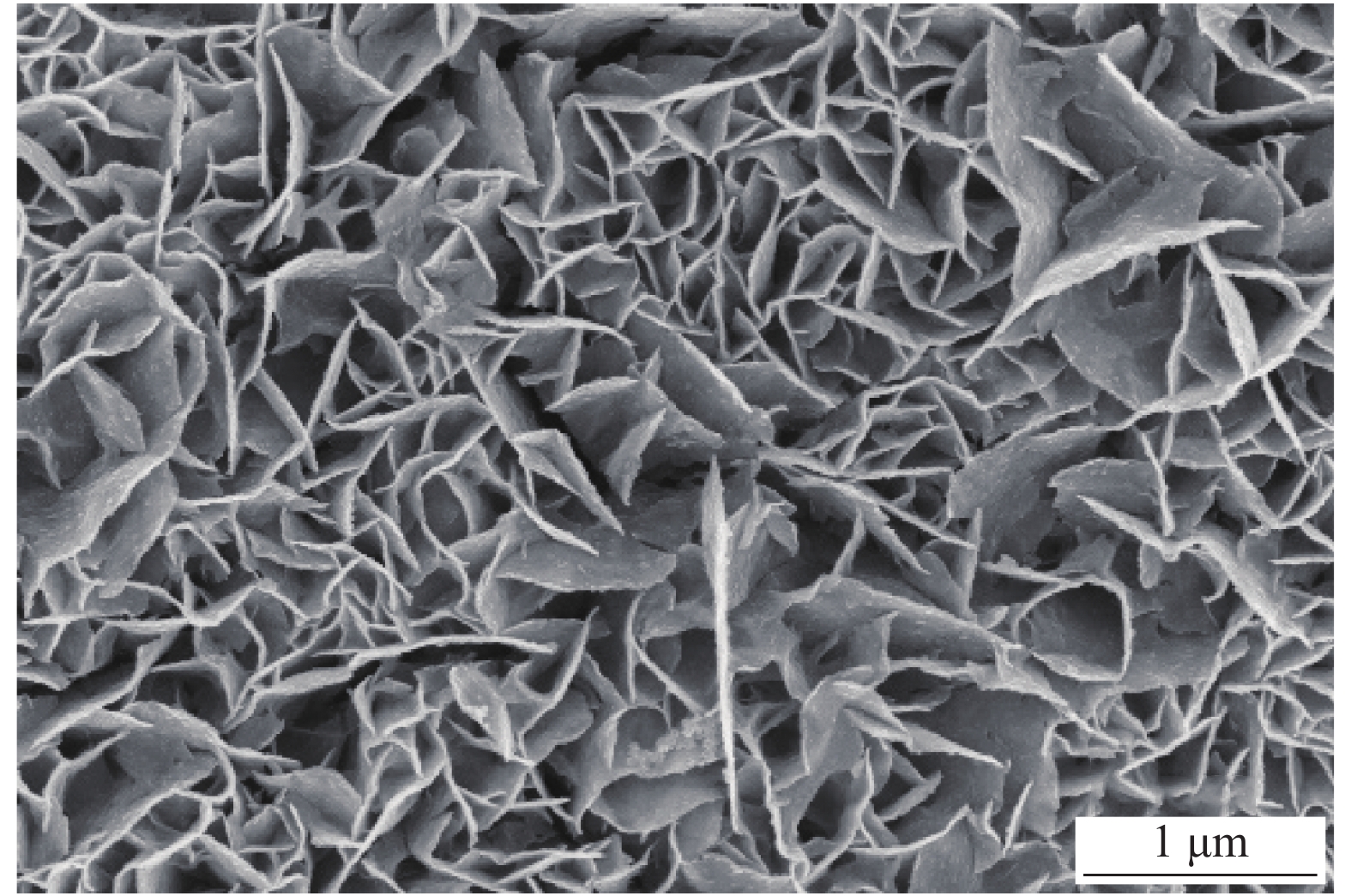
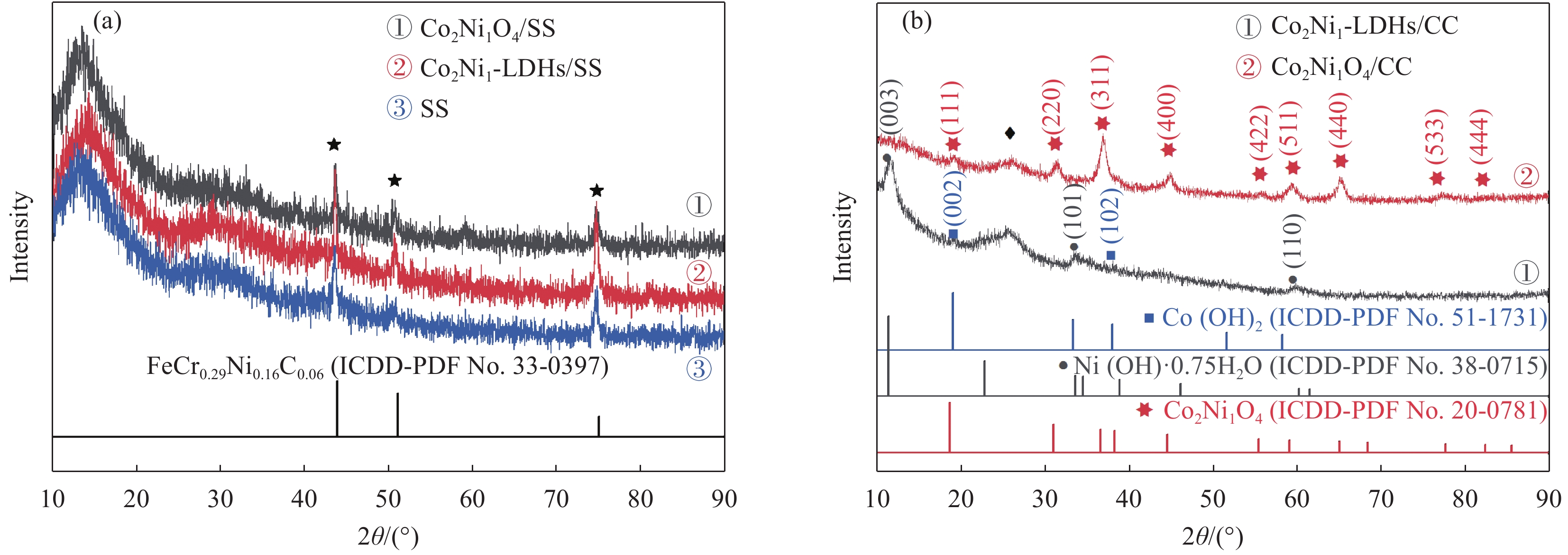
摘要: 电解水包括析氢反应(HER)与析氧反应(OER),由于OER是复杂的4电子转移过程,制作出具有优异耐久性的高活性的非贵金属OER电催化剂对于电解水至关重要。为了降低成本,选择304型不锈钢网(SS)作为基体,使用电沉积的方法制备钴-镍双氢氧化物,利用真空煅烧的方法制备钴-镍氧化物。使用XRD、SEM、TEM、XPS和电化学工作站对Co2Ni1O4/SS复合材料的晶体结构、形貌和电催化OER性能进行了研究。结果表明:电沉积制备的钴-镍双氢氧化物煅烧之后转变成尖晶石结构的钴-镍氧化物;在不锈钢表面成功合成了大量密集的层状结构;在1.0 mol/L KOH电解液中,Co2Ni1O4/SS电极表现出优异的OER电催化性能,达到10 mA·cm−2电流密度时所需要的过电位仅为240 mV,Tafel斜率为53.92 mV·dec−1,并且表现出优异的稳定性。
-
关键词:
- 电沉积 /
- 不锈钢 /
- 尖晶石化合物Co2Ni1O4 /
- 水分解 /
- 析氧反应
Abstract: Electrolytic water includes hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), because OER is a complex 4-electron transfer process, developing highly active non-precious OER electrocatalysts with superior durability is crucial to electrolytic water. In order to reduce the cost, 304 stainless steel mesh (SS) was selected as the matrix, Co-Ni double hydroxides was prepared by electrodeposition, Co-Ni oxides were produced by vacuum calcination. The crystal structure, morphology and electrocatalytic OER performance of Co2Ni1O4/ SS composite were studied by XRD, SEM, TEM, XPS and electrochemical workstation. As a result, the Co-Ni double hydroxides prepared by electrodeposition are transformed into Co-Ni oxides with spinel structure after vacuum calcination; successfully synthesized a large number of dense layered structures on the surface of stainless steel;the Co2Ni1O4/SS electrode exhibits an outstanding OER catalytic activity with an overpotentials of 240 mV at 10 mA·cm−2 and a Tafel slop as low as 53.92 mV·dec−1 in 1.0 mol/L KOH. In addition, the Co2Ni1O4/SS composites shows excellent stability in the alkaline electrolyte. -
-
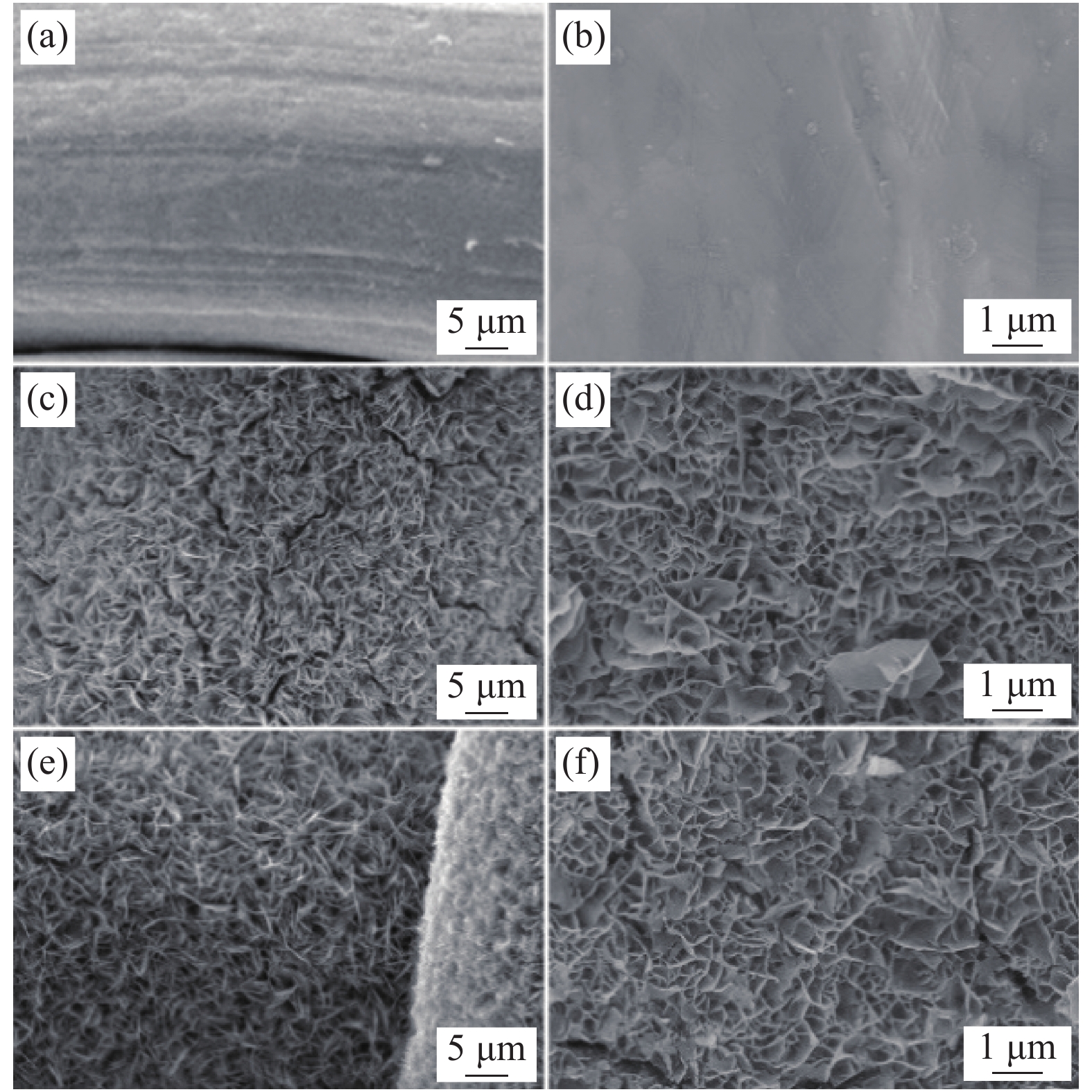
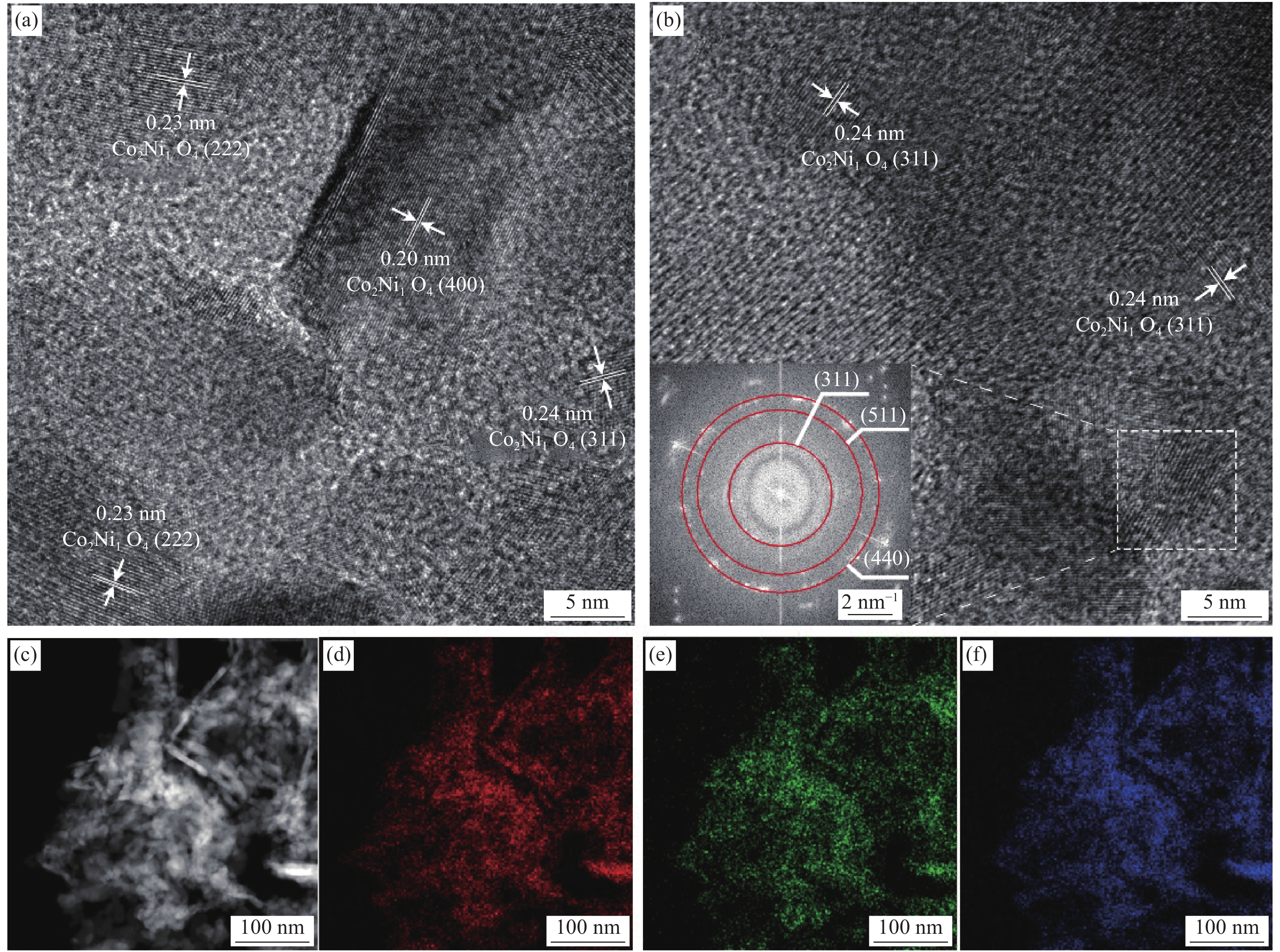
图 3 Co2Ni1O4/SS的HRTEM图像 ((a), (b)),(b)中的插图为衍射图;Co2Ni1O4/SS的HAADF-STEM图像 (c)、对应的EDS元素映射图像Co (d)、Ni (e)和O (f)
Figure 3. HRTEM image of Co2Ni1O4/SS ((a), (b)), inset (b) shows the corresponding of diffraction image; HAADF-STEM image of Co2Ni1O4/SS (c) and corresponding EDS elemental mapping images of Co (d), Ni (e) and O (f) for Co2Ni1O4/SS
图 5 Co2Ni1O4/SS等在1.0 mol/L KOH溶液中线性伏安法极化曲线 (a)、在10 mA·cm−2和100 mA·cm−2的过电位 (b)、由(a)导出的Tafel斜率图 (c)、计时电势法稳定性测试前后的极化曲线对比图 (d) 和多步计时电势相应曲线(插图)
Figure 5. Co2Ni1O4/SS in 1.0 mol/L KOH LSV polarization curves (a), overpotential at 10 mA·cm−2 and 100 mA·cm−2 (b); Tafel plots derived from (a) (c) , comparison of polarization curves before and after the chronopotentiometry stability test (d) and multistep chronopotentiometry responses curve (Inset)
η10—Overpotential at current density of 10 mA·cm-2; η100—Overpotential at current density of 100 mA·cm-2; OSS—Annealed stainless steel
图 6 Co1Ni2-LDHs/SS、Co2Ni1-LDHs/SS、Co3Ni0-层状单氢氧化物(LSHs)/SS、Co1Ni2O4/SS、Co2Ni1O4/SS、Co3Ni0O4/SS、SS和OSS的双层电容曲线 (a),电化学阻抗谱 (b) 和极化曲线归一化到电化学比表面积(ECSA) (c)
Figure 6. Capacitive current densities plotted against scan rate (a) and Nyquist plots (b) of the Co1Ni2-LDHs/SS, Co2Ni1-LDHs/SS, Co3Ni0-layered single hydroxides (LSHs)/SS, Co1Ni2O4/SS, Co2Ni1O4/SS, Co3Ni0O4/SS, SS and OSS and polarization curves normalized to the electrochemical specific surface area (ECSA) (c)
j—Current density; Z—Impedance values; Rs—Resistance of the solution; Rct—Charge transfer resistance; CPE—Capacitance
表 1 本工作与引用文献之间的OER性能对比
Table 1 Comparisons of OER performance between this work and the data in references
Sample Electrolyte Over potential (10 mA·cm−2)/mV Tafel slope/(mV·dec−1) Reference Co2Ni1O4/SS 1.0 mol/L KOH 240 53.92 This work CoFe-LDH/RGO 1.0 mol/L KOH 325 43 [5] NiFe-LDHs/RGO 1.0 mol/L KOH 245 — [7] α-(Ni-Co)(OH)X 1.0 mol/L KOH 255 — [15] MoS2/rFe-NiCo2O4 1.0 mol/L KOH 270 39 [32] N-NiCo2O4@C@NF 1.0 mol/L KOH 242 86 [33] P-NiCo2O4 NWs/NF 1.0 mol/L KOH 300 120 [34] NiCo2O4 1.0 mol/L KOH 290 102 [35] NiCo2O4@N/S-C 1.0 mol/L KOH 285 53 [36] NiCo2O4/NiO 1.0 mol/L NaOH 360 61 [37] NiCo2O4@NiWS 1.0 mol/L KOH 290 95.2 [43] Ti3C2@mNiCoP NS 1.0 mol/L KOH 237 104 [44] FeCoNiOx@NG 1.0 mol/L KOH 320 55 [45] Note: NF, RGO, NWs, NS—Nickel foram, grephene oxide, nanowire, nanosheet. -
[1] JIA Y, JIANG K, WANG H, et al. The role of defect sites in nanomaterials for electrocatalytic energy conversion[J]. Chem,2019,5(6):1371-1397. DOI: 10.1016/j.chempr.2019.02.008
[2] CAI Z, BU X, WANG P, et al. Simple and cost effective fabrication of 3D porous core-shell Ni nanochains@NiFe layered double hydroxide nanosheet bifunctional electrocatalysts for overall water splitting[J]. Journal of Materials Chemistry A,2019,7(38):2172221729.
[3] CHEN J, ZENG Q, QI X, et al. High-performance bifunctional Fe-doped molybdenum oxide-based electrocatalysts with in situ grown epitaxial heterojunctions for overall water splitting[J]. International Journal of Hydrogen Energy,2020,45(46):24828-24839. DOI: 10.1016/j.ijhydene.2020.06.283
[4] CAI Z, BU X, WANG P, et al. Recent advances in layered double hydroxide electrocatalysts for the oxygen evolution reaction[J]. Journal of Materials Chemistry A,2019,7(10):5069-5089. DOI: 10.1039/C8TA11273H
[5] HAN X, YU C, YANG J, et al. Mass and charge transfer coenhanced oxygen evolution behaviors in CoFe-layered double hydroxide assembled on graphene[J]. Advanced Materials Interfaces,2016,3(7):1500782. DOI: 10.1002/admi.201500782
[6] ZHANG W, WU Y, QI J, et al. A thin nife hydroxide film formed by stepwise electrodeposition strategy with significantly improved catalytic water oxidation efficiency[J]. Advanced Energy Materials,2017,7(9):1602547. DOI: 10.1002/aenm.201602547
[7] YOUN D H, PARK Y B, KIM J Y, et al. One-pot synthesis of NiFe layered double hydroxide/reduced graphene oxide composite as an efficient electrocatalyst for electrochemical and photoelectrochemical water oxidation[J]. Journal of Power Sources,2015,294:437-443. DOI: 10.1016/j.jpowsour.2015.06.098
[8] WU J, REN Z, DU S, et al. A highly active oxygen evolution electrocatalyst: Ultrathin CoNi double hydroxide/CoO nanosheets synthesized via interface-directed assembly[J]. Nano Research,2016,9(3):713-725. DOI: 10.1007/s12274-015-0950-4
[9] ZHU S, LI J, DENG X, et al. Ultrathin-nanosheet-induced synthesis of 3D transition metal oxides networks for lithium ion battery anodes[J]. Advanced Functional Materials,2017,27(9):1605017. DOI: 10.1002/adfm.201605017
[10] LI H, MUSHARAVATI F, ZALENEZHAD E, et al. Electrodeposited Ni Co layered double hydroxides on titanium carbide as a binder-free electrode for supercapacitors[J]. Electrochimica Acta,2018,261:178-187. DOI: 10.1016/j.electacta.2017.12.139
[11] SUN H, YAN Z, LIU F, et al. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution[J]. Advanced Materials,2019,32(3):e1806326.
[12] LIU T, LI P, YAO N, et al. Self-sacrificial template-directed vapor-phase growth of MOF assemblies and surface vulcanization for efficient water splitting[J]. Advanced Materials,2019,31(21):1806672. DOI: 10.1002/adma.201806672
[13] LYU Y, WANG R, TAO L, et al. In-situ evolution of active layers on commercial stainless steel for stable water splitting[J]. Applied Catalysis B: Environmental,2019,248:277-285. DOI: 10.1016/j.apcatb.2019.02.032
[14] LEE M, JEE M S, LEE S Y, et al. Sloughing a precursor layer to expose active stainless steel catalyst for water oxidation[J]. ACS Applied Materials & Interfaces,2018,10(29):24499-24507. DOI: 10.1021/acsami.8b04871
[15] BALOGUN M S, QIU W, HUANG Y, et al. Cost-effective alkaline water electrolysis based on nitrogen- and phosphorus-doped self-supportive electrocatalysts[J]. Advanced Materials,2017,29(34):1702095. DOI: 10.1002/adma.201702095
[16] BALRAM A, ZHANG H, SANTHANAGOPALAN S. Enhanced oxygen evolution reaction electrocatalysis via electrodeposited amorphous alpha-phase nickel-cobalt hydroxide nanodendrite forests[J]. ACS Applied Materials & Interfaces,2017,9(34):28355-28365. DOI: 10.1021/acsami.7b05735
[17] WANG F, QI X, QIN Z, et al. Construction of hierarchical Prussian Blue Analogue phosphide anchored on Ni2P@MoOx nanosheet spheres for efficient overall water splitting[J]. International Journal of Hydrogen Energy,2020,45(24):13353-13364. DOI: 10.1016/j.ijhydene.2020.03.064
[18] WANG F, CHEN J, QI X, et al. Increased nucleation sites in nickel foam for the synthesis of MoP@Ni3P/NF nanosheets for bifunctional water splitting[J]. Applied Surface Science,2019,481:1403-1411. DOI: 10.1016/j.apsusc.2019.03.200
[19] SUN H, TIAN C, LI Y, et al. Coupling NiCo alloy and CeO2 to enhance electrocatalytic hydrogen evolution in alkaline solution[J]. Advanced Sustainable Systems,2020,4(11):2000122. DOI: 10.1002/adsu.202000122
[20] CHEN J, WANG F, QI X, et al. A simple strategy to construct cobalt oxide-based high-efficiency electrocatalysts with oxygen vacancies and heterojunctions[J]. Electrochimica Acta,2019,326:134979. DOI: 10.1016/j.electacta.2019.134979
[21] ZHAN C, LIU Z, ZHOU Y, et al. Triple hierarchy and double synergies of NiFe/Co9S8/carbon cloth: A new and efficient electrocatalyst for the oxygen evolution reaction[J]. Nanoscale,2019,11(7):3378-3385. DOI: 10.1039/C8NR09740B
[22] ZHENG Z, DU X, WANG Y, et al. Efficient and stable NiCo2O4/VN nanoparticle catalyst for electrochemical water oxidation[J]. ACS Sustainable Chemistry & Engineering,2018,6(9):11473-11479.
[23] CHEN S, YANG G, JIA Y, et al. Three-dimensional NiCo2O4@ NiWO4 core–shell nanowire arrays for high performance supercapacitors[J]. Journal of Materials Chemistry A,2017,5(3):1028-1034. DOI: 10.1039/C6TA08578D
[24] LIANG C, ZOU P, NAIRAN A, et al. Exceptional performance of hierarchical Ni–Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting[J]. Energy & Environmental Science,2020,13(1):86-95.
[25] ZHANG X, KLAVER P, VAN SANTEN R, et al. Oxygen evolution at hematite surfaces: The impact of structure and oxygen vacancies on lowering the overpotential[J]. The Journal of Physical Chemistry C,2016,120(32):18201-18208. DOI: 10.1021/acs.jpcc.6b07228
[26] XIAO Y, HU T, ZHAO X, et al. Thermo-selenizing to rationally tune surface composition and evolve structure of stainless steel to electrocatalytically boost oxygen evolution reaction[J]. Nano Energy,2020,75:104949. DOI: 10.1016/j.nanoen.2020.104949
[27] ZHANG X, LI X, LI R, et al. Highly active core-shell carbon/nico2o4 double microtubes for efficient oxygen evolution reaction: Ultralow overpotential and superior cycling stability[J]. Small,2019,15(42):1903297. DOI: 10.1002/smll.201903297
[28] ZHOU X, LIU Z, WANG Y, et al. Facet effect of Co3O4 nanocrystals on visible-light driven water oxidation[J]. Applied Catalysis B: Environmental,2018,237:74-84. DOI: 10.1016/j.apcatb.2018.05.067
[29] ZHANG Y, DING F, DENG C, et al. Crystal plane-dependent electrocatalytic activity of Co3O4 toward oxygen evolution reaction[J]. Catalysis Communications,2015,67:78-82. DOI: 10.1016/j.catcom.2015.04.012
[30] ZHANG L, GAO Z, LIU C, et al. Synthesis of TiO2 decorated Co3O4 acicular nanowire arrays and their application as an ethanol sensor[J]. Journal of Materials Chemistry A,2015,3(6):2794-2801. DOI: 10.1039/C4TA06440B
[31] HU L, WU L, LIAO M, et al. Electrical transport properties of large, individual NiCo2O4 nanoplates[J]. Advanced Functional Materials,2012,22(5):998-1004. DOI: 10.1002/adfm.201102155
[32] LI J, CHU D, DONG H, et al. Boosted oxygen evolution reactivity by igniting double exchange interaction in spinel oxides[J]. Journal of the American Chemical Society,2020,142(1):50-54. DOI: 10.1021/jacs.9b10882
[33] HA Y, SHI L, YAN X, et al. Multifunctional electrocatalysis on a porous N-Doped NiCo2O4@C nanonetwork[J]. ACS Applied Materials & Interfaces,2019,11(49):45546-45553.
[34] CHU W, SHI Z, HOU Y, et al. Trifunctional of phosphorus-doped NiCo2O4 nanowire materials for asymmetric supercapacitor, oxygen evolution reaction, and hydrogen evolution reaction[J]. ACS Applied Materials & Interfaces,2020,12(2):2763-2772.
[35] CHEN Z, ZHAO B, HE Y C, et al. NiCo2O4 nanoframes with a nanosheet surface as efficient electrocatalysts for the oxygen evolution reaction[J]. Materials Chemistry Frontiers,2018,2(6):1155-1164. DOI: 10.1039/C8QM00027A
[36] YUAN Y, SUN L, LI Y, et al. Synergistic modulation of active sites and charge transport: N/S Co-doped C encapsulated NiCo2O4/NiO hollow microrods for boosting oxygen evolution catalysis[J]. Inorganic Chemistry,2020,59(6):4080-4089. DOI: 10.1021/acs.inorgchem.0c00089
[37] MAHALA C, BASU M. Nanosheets of NiCo2O4/NiO as efficient and stable electrocatalyst for oxygen evolution reaction[J]. ACS Omega,2017,2(11):7559-7567. DOI: 10.1021/acsomega.7b00957
[38] KANG Z, GUO H, WU J, et al. Engineering an earth-abundant element-based bifunctional electrocatalyst for highly efficient and durable overall water splitting[J]. Advanced Functional Materials,2019,29(9):1807031.1-1807031.10.
[39] SHINAGAWA T, GARCIA-ESPARZA A T, TAKANABE K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion[J]. Scientific Reports,2015,5:13801. DOI: 10.1038/srep13801
[40] ZHANG G, WANG H, YANG J, et al. Temperature effect on Co-based catalysts in oxygen evolution reaction[J]. Inorganic Chemistry,2018,57(5):2766-2772. DOI: 10.1021/acs.inorgchem.7b03168
[41] YAN D, LI Y, HUO J, et al. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions[J]. Advanced Materials,2017,29(48):1606459. DOI: 10.1002/adma.201606459
[42] WANG Y, ZHOU T, JIANG K, et al. Reduced mesoporous Co3O4 nanowires as efficient water oxidation electrocatalysts and supercapacitor electrodes[J]. Advanced Energy Materials,2014,4(16):1400696. DOI: 10.1002/aenm.201400696
[43] ZHAO D, DAI M, LIU H, et al. Constructing high performance hybrid battery and electrocatalyst by heterostructured NiCo2O4@NiWS nanosheets[J]. Crystal Growth & Design,2019,19(3):1921-1929.
[44] YUE Q, SUN J, CHEN S, et al. Hierarchical mesoporous MXene–NiCoP electrocatalyst for water-splitting[J]. ACS Applied Materials & Interfaces,2020,12(16):18570-18577.
[45] JIANG R, BAKER D R, TRAN D T, et al. Multimetallic FeCoNiOx nanoparticles covered with nitrogen-doped graphene layers as trifunctional catalysts for hydrogen evolution and oxygen reduction and evolution[J]. ACS Applied Nano Materials,2020,3(7):7119-7129. DOI: 10.1021/acsanm.0c01434
-
期刊类型引用(1)
1. 李佥,王添誉,孙西同,陈晓艺,李苗,韩雨擎,曾祥冰,孙芳鸿,李宪臻. 贻贝仿生修饰多孔磁性材料的制备及其在固定化脂肪酶中的应用. 复合材料学报. 2024(11): 6156-6169 .  本站查看
本站查看
其他类型引用(0)
-




 下载:
下载: