Investigation of the performance and reaction mechanism of tetracycline degradation by LaMnO3/GA composites activated PMS
-
摘要:
本文采用溶胶凝胶法和水热法制备了石墨烯气凝胶(GA)负载的LaMnO3复合催化剂,研究了其对过一硫酸盐(PMS)降解四环素(TC)的催化性能。采用SEM、TEM、XPS、拉曼光谱等手段对样品的形貌结构、元素组成和化学形态进行了表征,结果显示构成了LaMnO3/GA复合催化剂。实验结果表明:与LaMnO3纯样相比(降解率为58%),LaMnO3/GA25复合材料活化PMS降解TC的催化性能可提高至83%以上。这种增强效果可归因于GA的引入,加快了电荷的迁移速率并提升了活性位点的电荷浓度。自由基捕获试验验证了O2•−1、O2、 •OH作为活性物质在TC降解过程中的重要性。此外,通过探究LaMnO3/GA25/PMS体系对多种无机阴离子(如H2PO−4、SO2−4、Cl−、Urea、HCO−3)和腐殖酸(HA)的抗干扰活性及循环使用性能,证明了LaMnO3/GA25/PMS体系用于复杂水体中污染物处理的可行性,并为充分利用锰矿资源解决环境污染问题提供了新的思路。
Abstract:Graphene aerogel (GA)-loaded LaMnO3 perovskite-type oxides were prepared by sol-gel and hydrothermal methods and their catalytic performance for the degradation of tetracycline (TC) by peroxymonosulfate (PMS) was investigated. SEM, TEM, XPS, and Raman spectroscopy were employed to analyze the morphological structure, elemental composition, and chemical morphology. Characterization results showed the successful preparation of LaMnO3 and LaMnO3/GA crystalline phase heterojunction. The experimental results indicated that, when compared with the pure LaMnO3 (58% degradation rate), the catalytic performance of the LaMnO3/GA25 composites for the PMS activation for TC degradation improved to be more than 83%. This enhancement was mainly attributed to GA complexation, which promotes the charge transfer rate and increases the charge concentration at the reactive sites. The vital roles of •OH and SO4•− were certified by free radical trapping assays. In addition, the catalytic activity of the LaMnO3/GA25/PMS system in complex aqueous (HA, H2PO−4, SO2−4, Cl−, Urea, HCO−3) environments were also explored. This work demonstrated the feasibility of the LaMnO3/GA25/PMS system for wastewater treatment and provided a novel way to make full use of manganese resources to solve the problem of environmental pollution.
-
Keywords:
- LaMnO3 /
- graphene aerogel /
- peroxymonosulfate /
- tetracycline hydrochloride /
- catalysis
-
目前,全球变暖带来的负面效应极大地影响了人类的生产生活[1-2],能源的过度消耗和匮乏使得环境问题进一步增加,如何做好热管理是目前面临的主要问题[3-5]。地球表面温度为300 K,而外太空平均温度为3 K[6-7],根据热力学第二定律,地球上物体的热量由于热量差可以通过辐射的方式将热量传递到外太空。因此,辐射制冷技术是地球上的物体通过“大气窗口”波段(8~13 μm)将热量辐射到外太空[8-10],以此实现自身降温冷却的过程。
虽然辐射制冷材料在节能环保方面显示出极大的应用潜力[10],但现有材料多为白色或银白色,外观单调,利用率极低。染料会使材料的表面颜色发生改变,但染料的可见色会吸收热量,并在近红外波段内吸收额外的热量[11-12],降低了材料本身的制冷效果。到目前为止,克服这一问题的主要策略是提高在可见光区域内的反射率和在“大气窗口”波段(8~13 μm)的发射率[13-16]。为避免染料吸收热量的现象,结构色辐射制冷材料引发人们的广泛关注[17-18]。利用硅蛋白石可以制备具有结构色变化的辐射制冷材料,但这种方法不能实现亚环境冷却[19],并且生产工艺和条件复杂且苛刻,很难进行大批量生产和应用。
纤维素纳米晶体(CNC)是刚性的棒状颗粒,长度为数十至数百纳米,直径可以达到数十纳米[20],具有结晶度高、降解性好等特点。CNC可以从棉花、木材和纸浆等可再生资源中提取,具有成本低、绿色、可持续等特性[21-23]。CNC可以在水中自发地组织成手性向列结构的液晶相,这种有序结构在干燥过程中可以得到保留,直至得到具有手性向列结构的CNC薄膜[24]。双折射的CNC纳米棒在薄膜中呈现的螺旋式排列会使纳米结构的折射率产生周期性的变化,进而引起对可见光的强烈反射[25]。因此,结构色的调节与螺旋结构的周期性密切相关。
目前已有研究致力于制造色彩更为丰富的纤维素辐射制冷材料。Shanker等[26]通过将CNC自组装成结构色薄膜,与硅片基底结合,得到了一种结构色辐射制冷装置。通过控制CNC/甘油(GLU) 的质量比得到颜色由蓝紫色转变为红色的复合薄膜。结构色复合薄膜表现出在太阳光谱范围内低吸收率和“大气窗口”内高发射率,且绿色和红色样品降温效果可达9℃左右,蓝紫色样品的降温效果可达6℃左右,为制备纤维素结构色辐射制冷薄膜提供了研究基础。
本文将CNC与聚乙二醇(PEG)复合,通过自组装方法制备了高太阳光反射率和在“大气窗口”波段高发射率的结构色薄膜。通过控制CNC与PEG的质量比,调控复合薄膜的结构色,以实现不同波段的热辐射率调控。探究不同含量PEG的添加对CNC复合结构色薄膜的结构、光学性能及制冷性能的影响。并将复合结构色薄膜粘贴到醋酸纤维素滤膜上制备成双层复合膜,进一步探究双层复合膜的辐射制冷效应。
1. 实验材料及方法
1.1 原材料
醋酸纤维素:上海兴亚净化材料厂;浓硫酸(H2SO4):分析纯,阿拉丁世纪有限公司;聚乙二醇400:分析纯,天津市科密欧化学试剂有限公司。
1.2 实验过程
CNC的制备:利用酸水解法制备CNC,将200 mL 64%硫酸溶液缓慢倒入25 g硫酸盐漂白针叶浆中,在45℃的水浴加热中搅拌1 h,后加入大量蒸馏水来终止水解反应。静置2~3 d,去除上清液,加入去离子水通过高速离心机 (CT14D型,上海天美仪器有限公司) 进行多次离心洗涤。得到的CNC悬浮液,将CNC在去离子水中透析,直至pH为中性。最后将CNC悬浮液浓缩至3wt%,冷藏备用。
CNC/PEG复合薄膜的制备:称取聚乙二醇5.0 g,加入45 g超纯水中,在室温搅拌1 h,得到质量分数10wt%的聚乙二醇溶液。取一定量的质量分数为3wt%的CNC悬浮液超声5 min,取4份3.0 g CNC悬浮液分别与
0.1000 g、0.2250 g、0.3857 g和0.6000 g PEG的溶液进行混合,将上述溶液充分搅拌2 h,后将混合液体倒入圆形容器中,室温缓慢蒸发2~3 d,得到PEG浓度为10wt%、20wt%、30wt%和40wt%的CNC/PEG复合结构色薄膜。按照聚合物的种类和含量对复合薄膜进行命名,分别为CNC/PEG-10%、CNC/PEG-20%、CNC/PEG-30%、CNC/PEG-40%,具体见表1。表 1 纤维素纳米晶体/聚乙二醇(CNC/PEG)复合辐射制冷薄膜和CNC/PEG-醋酸纤维素(CA)结构色辐射制冷双层复合膜的命名Table 1. Naming of cellulose nanocrystal/polyethylene glycol (CNC/PEG) composite radiative cooling films and CNC/PEG-cellulose acetate (CA) structure-colored radiation-cooled bilayer composite filmsSample Mass fraction of
CNC/wt%Mass fraction of
PEG/wt%CA CNC/PEG-10% 3 10 — CNC/PEG-20% 3 20 — CNC/PEG-30% 3 30 — CNC/PEG-40% 3 40 — CNC/PEG-10%-CA 3 10 0.0740 g CNC/PEG-20%-CA 3 20 0.0740 g CNC/PEG-30%-CA 3 30 0.0740 g CNC/PEG-40%-CA 3 40 0.0740 g 双层复合膜的制备:将不同质量比的复合结构色薄膜与醋酸纤维素膜用双面胶粘在一起,双面胶放在醋酸纤维素膜的边缘起固定作用,不会影响双层复合结构色薄膜的结构,双层复合结构色薄膜分别命名为CNC/PEG-10%-CA、CNC/PEG-20%-CA、CNC/PEG-30%-CA,具体见表1。
1.3 测试与表征
1.3.1 CNC/PEG复合膜的性能分析
采用Malvern Zetasizer nano ZS90测试CNC的Zeta电位和粒径。利用偏光显微镜(POM,XPF-550C,上海蔡康光学仪器公司)观察复合薄膜样品的液晶特性。使用反射光谱仪(UV-vis,HR4000 CG-NIR型,海洋光学公司)对薄膜进行反射光谱测试(可见光区域)。将复合薄膜用液氮进行脆断,用双面导电胶粘到截面制样台上,喷金60 s,利用扫描电子显微镜(SEM,JSM-7500F,日本电子株式会社)观察复合薄膜的横截面微观形貌。使用带有积分球附件的紫外-可见-近红外分光光度计(UV-VIS-NIR Spectrometer,美国Perkins Elmer公司)检测样品在300~
2500 nm波长范围内的反射率变化。利用傅里叶变换红外光谱仪(FTIR,Nicolet-IS10,美国Thermo Fisher公司)检测样品在2.5~25 μm的波长范围内样品的吸收率变化。采用太阳光谱匹配良好的高功率氙灯(中教金源HXF300)来模拟太阳光照射,在聚苯乙烯(PS)泡沫箱子中裁剪一个1 cm×1 cm×1 cm的空腔,将薄膜或复合薄膜放置在空腔中固定,利用聚乙烯薄膜覆盖泡沫箱,消除环境热对流影响。将多通道温度计(JINKO,JK804)与两个k型热电偶进行连接,一个k型热电偶测试复合薄膜覆盖下空腔的内部温度,另一个k型热电偶用来测量聚乙烯膜覆盖下的装置内的环境温度。由于CNC/PEG-40%复合薄膜在表征过程中会吸收环境的水分,手性结构发生润胀,螺距在实际测量中会发生变化,不利于复合薄膜在实际环境中的应用,因此后续将不会对CNC/PEG-40%复合薄膜进行扫描电镜、光谱学和辐射制冷性能的测试与分析。1.3.2 双层复合膜的性能分析
将醋酸纤维素膜用导电胶粘贴到水平制样台上,喷金60 s,利用扫描电子显微镜观察醋酸纤维素薄膜的表面形貌。在同一氙灯光源照射下,观察不同样品在红外热成像仪(FTIR-E390,美国FLIR SYSTEMS公司)下的温度变化。利用上述自组装装置分别测量双层复合薄膜下方温度及被聚乙烯膜覆盖的整个装置内的环境温度。利用实验室自组装装置对样品进行户外降温性能测试,将室内自组装降温性能测试装置放置在用铝箔纸包裹的纸壳箱中,整体放置在泡沫箱上,用湿度计记录测试过程中的环境湿度变化。
2. 结果与讨论
2.1 纤维素纳米晶的电位粒径分析
图1为CNC的TEM图像、粒径分布和电位曲线,酸水解法制备的CNC具有棒状形态,平均粒径为144.1 nm (图1(b))。在水解过程中,CNC表面形成较多的负电荷,Zate电位高达−32.2 mV (图1(c))。CNC表面较多的负电荷会促进静电排斥作用,使CNC溶液的稳定性增强,为进一步制备结构色复合薄膜奠定基础。
2.2 纤维素纳米晶复合薄膜结构色光学特性分析
图2(a)~2(d)是PEG含量不同的复合薄膜光学照片。随着PEG含量的增加,薄膜反射颜色发生红移,逐渐由蓝绿色转变为红色。因此,复合薄膜的结构色红移现象与PEG的含量相关。通过对紫外-可见反射光谱(图2(e))分析可得,随着PEG含量的增加,4种复合薄膜分别在427 nm、487 nm、576 nm和654 nm处存在清晰的高峰,复合薄膜反射光谱中的最高峰发生红移,与薄膜结构色的红移相对应。通过图2(f)可知,复合薄膜通过反射可见光和发射热量来实现自身降温,为后续辐射制冷的研究提供理论基础。
2.3 纤维素纳米晶复合薄膜微观形貌分析
图3(a)~3(c)为PEG含量不同复合薄膜的横截断面扫描电镜图。当PEG含量为40%时,复合薄膜会吸收环境中的水分,手性结构发生润胀,螺距在实际测量中会发生变化,因此只对PEG含量为10%~30%的CNC/PEG复合薄膜进行扫描电镜的研究,不对CNC/PEG-40%复合薄膜进行扫描电镜的测试与分析。纯CNC薄膜具有周期性的层状结构,CNC通过逆时针方向旋转后形成了左旋的手性向列螺旋结构,这种左旋的手性向列结构反射特定波长的左旋圆偏振光,从而使复合薄膜表现出独特的虹彩色。聚合物的加入并不会改变CNC原有的手性向列结构,随着聚合物含量的增加,CNC手性向列结构的螺距明显增加。布拉格方程式中手性向列的螺距(P)定义为CNC棒状颗粒旋转360°产生的层间距,在电镜图(SEM)中表现为相邻两层结构的间距。
CNC/PEG复合薄膜的反射光遵循布拉格方程:
λ=nPcos(θ) (1) 其中:λ为反射波长;n为薄膜的平均折射率;θ为入射角;P为手性向列结构的层间距。因为CNC和PEG的折射率相似,分别为1.41和1.44,所以薄膜的平均折射率(n)可以认为是常量,当入射角(θ)恒定时,λ取决于手性向列结构的螺距P。图3(a)~3(c)的平均螺距分别为0.30、0.36和0.46 μm。随着平均螺距P的增加其反射波长λ也逐渐增大,主要原因是在加入PEG后,PEG高分子进入到CNC手性向列结构中,导致CNC手性向列结构的螺距P增大,复合薄膜颜色红移。
2.4 纤维素纳米晶复合薄膜折射现象
图4是PEG含量不同的复合薄膜偏光显微镜(POM)图像。通过观察图4(a)~4(d)可以得知薄膜具有明显的双折射现象,高倍POM图像(图4(e)~4(h))可以看出其具有明显的指纹结构,这说明CNC/PEG在干燥过程中CNC自组装了手性向列结构,并且在完全成膜后,仍然保留其手性向列结构。因此,适量PEG的加入并不会破坏CNC自组装所形成的手性向列结构。复合薄膜的指纹结构的纹理间隔随着PEG含量的增加逐渐变宽,分别为2.05 μm、2.33 μm、2.84 μm和3.38 μm,颜色由蓝绿色逐渐转化成蓝红色。PEG的添加占据了手性向列结构CNC之间的空间,导致螺距P增大,从而发生红移。因此,通过对POM结果分析,证明了PEG的添加不会破坏CNC的手性向列结构和双折射现象,控制PEG含量可以有效调控CNC手性向列层间距,进而调控复合薄膜结构色的变化。
2.5 纤维素纳米晶结构色复合薄膜和双层复合薄膜的光谱学分析
由基尔霍夫定律可知,样品的发射率(T)等同于吸收率(A)。通过观察图5(a)可知,在室内湿度为42%时,样品在大气窗口波段(8~13 μm)都有较高的发射率,其中当PEG的含量为30%时,复合薄膜的发射率最高可达93.0%,这样可以最大限度的向天空辐射红外热量。CNC/PEG复合薄膜具有高发射率,这是由于O—H (6.9~7.6 μm)、C—O (7.6~9.5 μm)、C—H (11.1~14.3 μm)键在大气窗口范围(8~13 μm)内产生强烈的拉伸与弯曲振动所导致的。图5(b)是PEG含量不同的复合薄膜在太阳波段(0.3~2.5 μm)范围内太阳光反射率曲线,结构色复合薄膜在近红外范围内的反射率最高可达68.5%。随着PEG的含量增加,其反射率也随之变高。
图6为不同PEG含量的双层复合制冷膜的发射率曲线和在可见光范围内的反射率曲线。通过观察图6(a)可知,在室内湿度为42%的测量环境下,双层复合膜在大气窗口的发射率高于醋酸纤维素膜的发射率,随着PEG含量的增加双层复合膜的发射率也逐步提高,当PEG含量为30%时,双层复合膜的发射率最高可达68.0%。图6(b)是PEG含量不同的双层复合膜在太阳波段(0.3~2.5 μm)范围内的太阳光反射率曲线,在近红外范围内的反射率最高可达91.8%。
2.6 纤维素纳米晶复合结构色薄膜和双层复合薄膜的辐射制冷性能分析
图7(a)为室内氙灯模拟图,利用100 mW/cm2高功率氙灯来照射,不仅可以模拟太阳光照射还可以将光均匀地分布在样品表面。图7(b)为用来测量样品温度及装置内空气温度的自组装装置,聚乙烯(PE)薄膜既可以保证氙灯的光照射到装置内部,又可以减少热对流对辐射制冷结果的影响。由图7(c)、图7(d)可知,将氙灯打开后,PE薄膜覆盖的装置内部温度迅速上升,在5 min后样品逐渐达到热稳定状态。当温度逐渐趋向平衡时,薄膜下方温度明显低于PE覆盖装置内的空气温度,不同结构色CNC/PEG复合薄膜辐射制冷性能相似,薄膜平均降温可达3.4℃。
![]() 图 7 (a)室内氙灯模拟装置图;(b)自组装温度测量装置图;CNC/PEG-10%和空气(c)、CNC/PEG-30%和空气(d) 温度对比图Figure 7. (a) Photos of indoor xenon lamp simulation device; (b) Self assembling temperature measuring device; Temperature comparison of CNC/PEG-10% and air (c), CNC/PEG-30% and air (d)PE—Polyethylene; IR—Infrared spectroscopy
图 7 (a)室内氙灯模拟装置图;(b)自组装温度测量装置图;CNC/PEG-10%和空气(c)、CNC/PEG-30%和空气(d) 温度对比图Figure 7. (a) Photos of indoor xenon lamp simulation device; (b) Self assembling temperature measuring device; Temperature comparison of CNC/PEG-10% and air (c), CNC/PEG-30% and air (d)PE—Polyethylene; IR—Infrared spectroscopy图8(a)~8(c)为不同纤维素基底在同一光源照射下的红外热成像图。在氙灯的照射下,采用红外热成像观察5 min,可以看出,醋酸纤维素薄膜的表面温度最低,滤纸的表面温度略高于醋酸纤维素薄膜,而A4纸的表面温度最高。通过观察醋酸纤维素的SEM图像(图8(d))可知,醋酸纤维素膜具有多孔结构,可以有效地反射可见光。图8(e)为醋酸纤维素膜下温度与环境温度对比曲线,膜下温度平均比环境温度低15℃左右。综上表明,醋酸纤维素薄膜具有良好的辐射降温能力,是作为双层复合薄膜的较优选择。
利用红外热成像分别观察CNC/PEG-20%、CNC/PEG-20%-CA和带有蓝色涂料的CA薄膜在相同时间和相同光照下其表面的降温能力,如图9(a)~9(c)所示。结果可知,CNC/PEG-20%-CA的表面降温能力较强,CNC/PEG-20%的降温能力次之,而带有蓝色涂料的CA薄膜的表面降温能力最差。通过分析CNC/PEG-20%和带有蓝色涂料的CA薄膜的温度曲线(图9(d)、图9(e)),进一步证实双层复合薄膜具有良好的制冷性能。由图9(f)、图9(g)可知,PE薄膜覆盖下装置内的空气温度与双层复合制冷膜下方温度的起始温度大致相同,氙灯打开后,两者温度迅速上升,5 min后双层复合制冷膜下方温度与装置内空气温度逐渐达到热稳定状态。当温度逐渐趋向平衡时,双层复合制冷膜下方温度远低于装置内空气温度,双层复合膜的辐射制冷性能几乎不受PEG含量的影响,双层复合薄膜平均降温可达14.3℃,双层复合膜的降温性能优于复合薄膜的降温性能。实验结果表明:CNC/PEG复合薄膜平均可降温3.4℃左右,醋酸纤维素膜是作为双层复合膜的理想基底,双层复合制冷膜的降温性能优于复合薄膜,平均降温可达14.3℃左右。
![]() 图 9 ((a)~(c)) CNC/PEG-20%、CNC/PEG-20%-CA与带有蓝色涂料的CA薄膜红外热成像图;带蓝色涂料的CA和空气(d)、CNC/PEG-20%-CA和空气(e)、CNC/PEG-10%-CA和空气(f)、CNC/PEG-30%-CA和空气(g)温度对比图Figure 9. ((a)-(c)) Infrared thermograms of CNC/PEG-20%, CNC/PEG-20%-CA and and CA films with blue coatings; Temperature comparison of CA with blue painting and air (d), CNC/PEG-20%-CA and air (e), CNC/PEG-10%-CA and air (f), CNC/PEG-30%-CA and air (g)
图 9 ((a)~(c)) CNC/PEG-20%、CNC/PEG-20%-CA与带有蓝色涂料的CA薄膜红外热成像图;带蓝色涂料的CA和空气(d)、CNC/PEG-20%-CA和空气(e)、CNC/PEG-10%-CA和空气(f)、CNC/PEG-30%-CA和空气(g)温度对比图Figure 9. ((a)-(c)) Infrared thermograms of CNC/PEG-20%, CNC/PEG-20%-CA and and CA films with blue coatings; Temperature comparison of CA with blue painting and air (d), CNC/PEG-20%-CA and air (e), CNC/PEG-10%-CA and air (f), CNC/PEG-30%-CA and air (g)图10(a)、图10(b)为测量CNC/PEG-30%-CA、CNC/PEG-30%及PE覆盖装置内空气温度的户外装置图,利用铝箔纸包裹整个装置以减少周围建筑物对装置热辐射的影响,在装置顶部覆盖一层PE膜来减少环境中的热对流及热传导对整个装置的影响,装置下方的泡沫箱用来隔绝地面对测量温度的热影响,利用热电偶分别记录样品覆盖空腔中的温度及PE膜覆盖下装置的空气温度。通过分析图10(c)可以看出,在平均温度为25℃,平均湿度51%的户外环境中,与PE覆盖装置中空气温度对比,复合薄膜可以实现平均2℃左右的降温效果,而双层复合薄膜可以实现平均6℃左右的降温效果。
![]() 图 10 ((a), (b))测试CNC/PEG-30%-CA、CNC/PEG-30%和空气的温差变化的户外装置图;(c) CNC/PEG-30%-CA、CNC/PEG-30%与空气的温差图Figure 10. ((a), (b)) Diagram of an outdoor installation for testing the change in temperature difference between CNC/PEG-30%-CA, CNC/PEG-30% and air; (c) Temperature comparison of CNC/PEG-30%-CA, CNC/PEG-30% and air
图 10 ((a), (b))测试CNC/PEG-30%-CA、CNC/PEG-30%和空气的温差变化的户外装置图;(c) CNC/PEG-30%-CA、CNC/PEG-30%与空气的温差图Figure 10. ((a), (b)) Diagram of an outdoor installation for testing the change in temperature difference between CNC/PEG-30%-CA, CNC/PEG-30% and air; (c) Temperature comparison of CNC/PEG-30%-CA, CNC/PEG-30% and air3. 结 论
本文将纤维素纳米晶体(CNC)与聚乙二醇(PEG)以不同比例混合,采用自组装的方法制备了具有辐射制冷性能的结构色复合薄膜,将结构色复合薄膜与具有多孔结构的醋酸纤维素膜(CA)相结合,制备具有辐射制冷和结构色特性的双层复合膜。分别对复合薄膜和双层复合膜的性能进行分析,得出的结论如下:
(1) CNC/PEG复合薄膜具有手性向列结构和鲜艳的结构色,复合薄膜出现明显的双折射特性,随着PEG含量的增加,复合薄膜手性向列结构的螺距增大,反射波长随之发生红移,最终导致薄膜结构色的变化;
(2)对CNC/PEG复合薄膜和CNC/PEG-CA双层复合膜进行FTIR和UV-vis测试可知,复合薄膜在0.25~2.5 μm的波长范围内的反射率高达93.0%,双层复合膜反射率可达68.0%,复合薄膜在“大气窗口”(8~13 μm)范围内的发射率可达68.5%,双层复合膜发射率高达91.8%;
(3)在氙灯照射下,CNC/PEG结构色复合薄膜具有辐射制冷性能,与装置内空气温度对比,平均降温可达3.4℃左右。与具有多孔结构的醋酸纤维素膜结合,双层结构色复合薄膜的辐射制冷性能得到提升,平均降温可达14.3℃左右。在户外降温性能测试中,复合薄膜可以达到平均2℃左右的降温效果,双层复合膜可以达到平均6℃左右的降温效果。
-
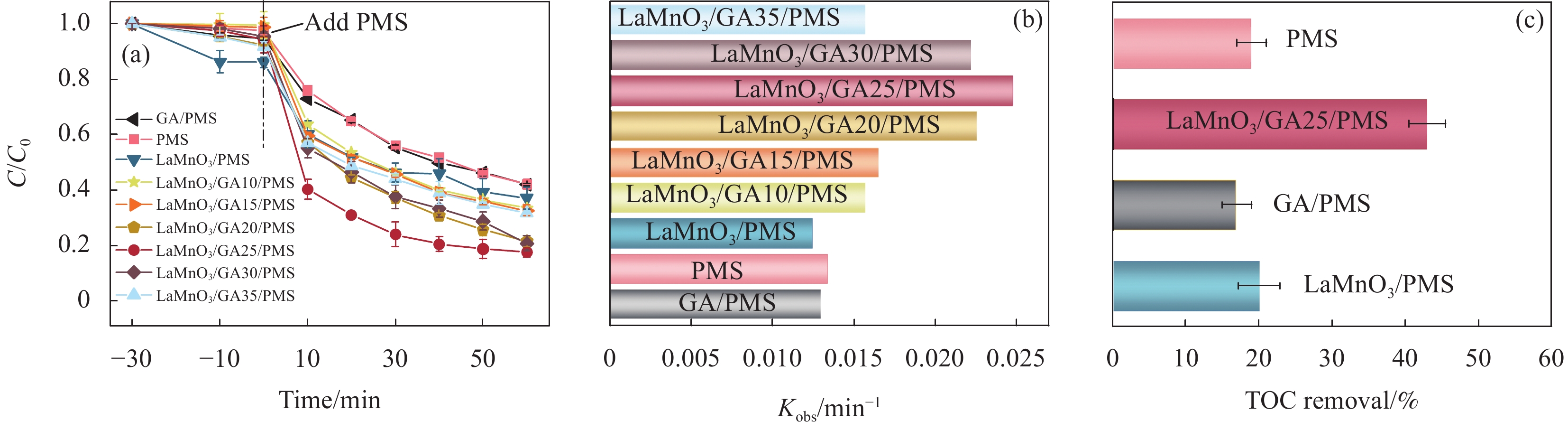
图 4 四环素(TC)在不同催化剂下的降解曲线(a)及其相关动力学速率常数(b);(c) 不同体系的TOC去除效率(实验条件:催化剂用量为0.1 g/L;过一硫酸盐(PMS)用量为0.2 g/L;初始TC浓度为0.02 g/L)
Figure 4. Degradation curves of tetracycline (TC) under different conditions (a) and associated kinetic rate constants (b); (c) TOC removal efficiency of different systems (Experimental conditions: Catalyst dosage of 0.1 g/L; Peroxymonosulfate (PMS) dosage of 0.2 g/L; Initial TC concentration of 0.02 g/L)
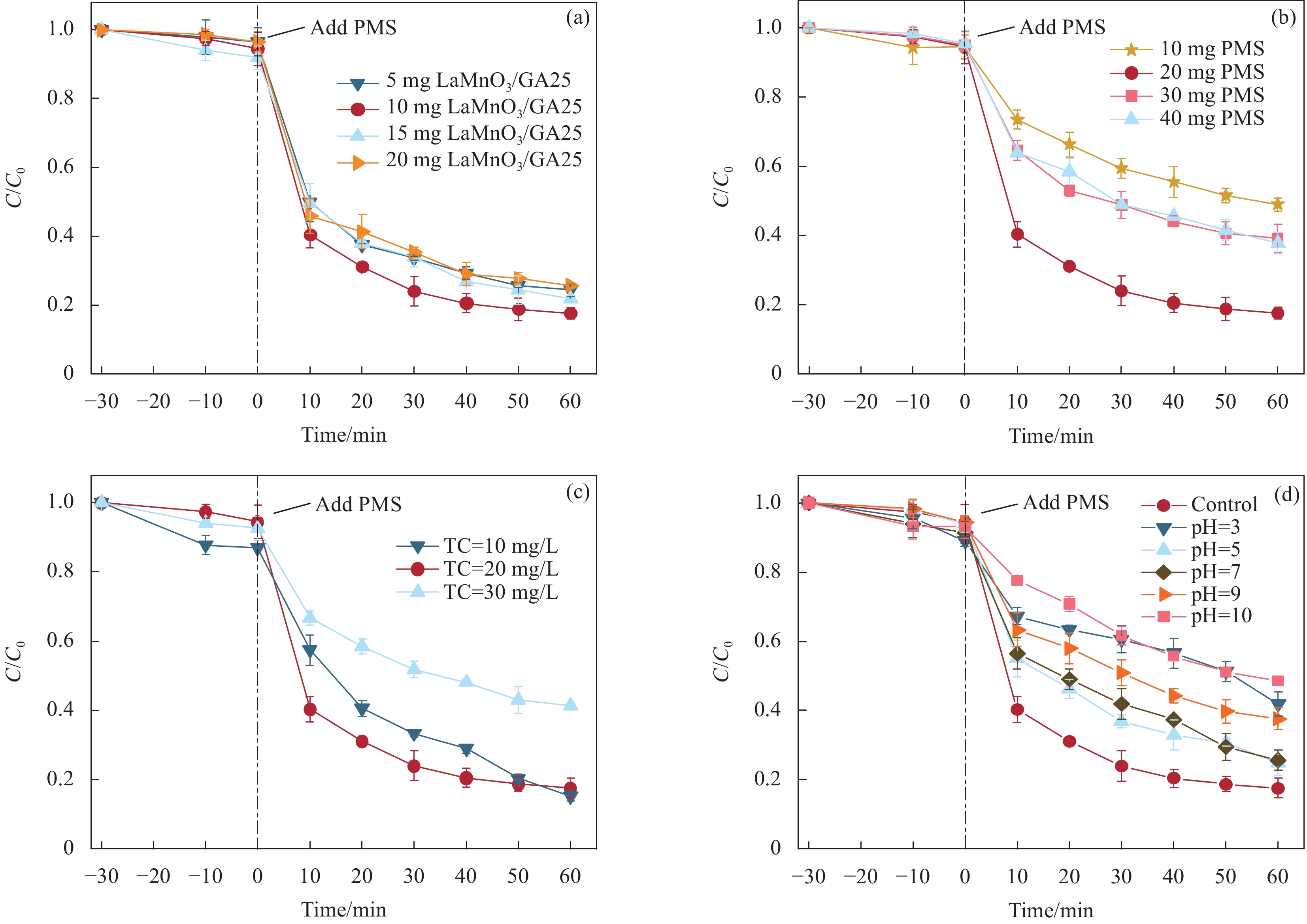
图 5 TC降解活性的影响规律:(a) LaMnO3/GA25浓度的影响;(b) PMS浓度的影响;(c) 初始TC浓度的影响;(d) 溶液pH值的影响;(实验条件:((b), (c), (d)) [LaMnO3/GA25] = 0.1 g/L; ((a), (c), (d)) [PMS] = 0.2 g/L; ((a), (b), (d)) [TC] = 100 mL,0.02 g/L )
Figure 5. Patterns of influence of TC degradation activity: (a) Effect of LaMnO3/GA25 concentration; (b) Effect of PMS concentration; (c) Effect of initial TC concentration; (d) Effect of solution pH (Experimental conditions: ((b), (c), (d)) [LaMnO3/GA25] = 0.1 g/L; ((a), (c), (d)) [PMS] = 0.2 g/L; ((a), (b), (d)) [TC] = 100 mL, 0.02 g/L
-
[1] BILGIN SIMSEK E, TUNA Ö. Boosting redox cycle and increased active oxygen species via decoration of LaMnO3 spheres with CeO2 flowers to promote Fenton-like catalytic degradation of various organic contaminants[J]. Optical Materials, 2023, 137: 113564. DOI: 10.1016/j.optmat.2023.113564
[2] LIU S, HU Q, QIU J, et al. Enhanced photocatalytic degradation of environmental pollutants under visible irradiation by a composite coating[J]. Environmental Science & Technology, 2017, 51(9): 5137-5145.
[3] WU H, XU X, SHI L, et al. Manganese oxide integrated catalytic ceramic membrane for degradation of organic pollutants using sulfate radicals[J]. Water Research, 2019, 167: 115110. DOI: 10.1016/j.watres.2019.115110
[4] SARMAH A K, MEYER M T, BOXALL A B A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment[J]. Chemosphere, 2006, 65(5): 725-759. DOI: 10.1016/j.chemosphere.2006.03.026
[5] PASTOR-NAVARRO N, MAQUIEIRA Á, PUCHADES R. Review on immuno analytical determination of tetracycline and sulfonamide residues in edible products[J]. Analytical and Bioanalytical Chemistry, 2009, 395(4): 907-920. DOI: 10.1007/s00216-009-2901-y
[6] XU R, YANG Z H, ZHENG Y, et al. Metagenomic analysis reveals the effects of long-term antibiotic pressure on sludge anaerobic digestion and antimicrobial resistance risk[J]. Bioresource Technology, 2019, 282: 179-188. DOI: 10.1016/j.biortech.2019.02.120
[7] SABIO E, ZAMORA F, GÑAN J, et al. Adsorption of p-nitrophenol on activated carbon fixed-bed[J]. Water Research, 2006, 40(16): 3053-3060. DOI: 10.1016/j.watres.2006.06.018
[8] RENE E R, KENNES C, NGHIEM L D, et al. New insights in biodegradation of organic pollutants[J]. Bioresource Technology, 2022, 347: 126737. DOI: 10.1016/j.biortech.2022.126737
[9] ZHOU J, LI X, YUAN J, et al. Efficient degradation and toxicity reduction of tetracycline by recyclable ferroferric oxide doped powdered activated charcoal via peroxymonosulfate (PMS) activation[J]. Chemical Engineering Journal, 2022, 441: 136061. DOI: 10.1016/j.cej.2022.136061
[10] GHANBARI F, MORADI M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review[J]. Chemical Engineering Journal, 2017, 310: 41-62. DOI: 10.1016/j.cej.2016.10.064
[11] GIANNAKIS S, LIN K Y A, GHANBARI F. A review of the recent advances on the treatment of industrial wastewaters by sulfate radical-based advanced oxidation processes (SR-AOPs)[J]. Chemical Engineering Journal, 2021, 406: 127083. DOI: 10.1016/j.cej.2020.127083
[12] LEE J, VON GUNTEN U, KIM J H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks[J]. Environmental Science & Technology, 2020, 54(6): 3064-3081.
[13] HU P, LONG M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications[J]. Applied Catalysis B: Environmental, 2016, 181: 103-117. DOI: 10.1016/j.apcatb.2015.07.024
[14] SAPUTRA E, PINEM J A, BUDIHARDJO M A, et al. Carbon-supported manganese for heterogeneous activation of peroxymonosulfate for the decomposition of phenol in aqueous solutions[J]. Materials Today Chemistry, 2020, 16: 100268. DOI: 10.1016/j.mtchem.2020.100268
[15] HU J, LI Y, ZOU Y, et al. Transition metal single-atom embedded on N-doped carbon as a catalyst for peroxymonosulfate activation: A DFT study[J]. Chemical Engineering Journal, 2022, 437: 135428. DOI: 10.1016/j.cej.2022.135428
[16] LU X, ZHAI T, ZHANG X, et al. WO3–x@Au@MnO2 core-shell nanowires on carbon fabric for high-performance flexible supercapacitors[J]. Advanced Materials, 2012, 24(7): 938-944. DOI: 10.1002/adma.201104113
[17] TAUJALE S, BARATTA L R, HUANG J, et al. Interactions in ternary mixtures of MnO2, Al2O3, and natural organic matter (NOM) and the impact on MnO2 oxidative reactivity[J]. Environmental Science & Technology, 2016, 50(5): 2345-2353.
[18] ZHANG H, WANG Y, ZHAI C. Construction of a novel p-n heterojunction CdS QDs/LaMnO3 composite for photodegradation of oxytetracycline[J]. Materials Science in Semiconductor Processing, 2022, 144: 106568. DOI: 10.1016/j.mssp.2022.106568
[19] LUO J, CHEN J, GUO R, et al. Rational construction of direct Z-scheme LaMnO3/g-C3N4 hybrid for improved visible-light photocatalytic tetracycline degradation[J]. Separation and Purification Technology, 2019, 211: 882-894. DOI: 10.1016/j.seppur.2018.10.062
[20] XIAO L, DENG Y, ZHOU H, et al. Activated carbon fiber mediates efficient activation of peroxymonosulfate systems: Modulation of manganese oxides and cycling of manganese species[J]. Chinese Chemical Letters, 2023, 34(12): 108407. DOI: 10.1016/j.cclet.2023.108407
[21] SHAO J J, CAI B, ZHANG C R, et al. One-pot synthesis of a cellulose-supported CoFe2O4 catalyst for the efficient degradation of sulfamethoxazole[J]. International Journal of Biological Macromolecules, 2022, 219: 166-174. DOI: 10.1016/j.ijbiomac.2022.08.003
[22] SU L, OU L, WEN Y, et al. High-efficiency degradation of quinclorac via peroxymonosulfate activated by N-doped CoFe2O4/Fe0@CEDTA hybrid catalyst[J]. Journal of Industrial and Engineering Chemistry, 2021, 102: 177-185. DOI: 10.1016/j.jiec.2021.06.040
[23] YU W, LI Y, SHU M, et al. CS/CoFe2O4 nanocomposite as a high-effective and steady chainmail catalyst for tetracycline degradation with peroxymonosulfate activation: Performance and mechanism[J]. Environmental Geochemistry and Health, 2024, 46(2): 40. DOI: 10.1007/s10653-023-01785-4
[24] JIA Y, YANG K, ZHANG Z, et al. Heterogeneous activation of peroxymonosulfate by magnetic hybrid CuFe2O4@N-rGO for excellent sulfamethoxazole degradation: Interaction of CuFe2O4 with N-rGO and synergistic catalytic mechanism[J]. Chemosphere, 2023, 313: 137392. DOI: 10.1016/j.chemosphere.2022.137392
[25] OLOWOJOBA G B, ESLAVA S, GUTIERREZ E S, et al. In situ thermally reduced graphene oxide/epoxy composites: Thermal and mechanical properties[J]. Applied Nanoscience, 2016, 6(7): 1015-1022. DOI: 10.1007/s13204-016-0518-y
[26] LIU C, LIU H, XIONG T, et al. Graphene oxide reinforced alginate/PVA double network hydrogels for efficient dye removal [J]. Polymers, 2018.
[27] LIU X, PANG K, YANG H, et al. Intrinsically microstructured graphene aerogel exhibiting excellent mechanical performance and super-high adsorption capacity[J]. Carbon, 2020, 161: 146-152. DOI: 10.1016/j.carbon.2020.01.065
[28] LIU Y, LIU X, ZHAO Y, et al. Aligned α-FeOOH nanorods anchored on a graphene oxide-carbon nanotubes aerogel can serve as an effective Fenton-like oxidation catalyst[J]. Applied Catalysis B: Environmental, 2017, 213: 74-86. DOI: 10.1016/j.apcatb.2017.05.019
[29] DONG Q, WANG J, DUAN X, et al. Self-assembly of 3D MnO2/N-doped graphene hybrid aerogel for catalytic degradation of water pollutants: Structure-dependent activity[J]. Chemical Engineering Journal, 2019, 369: 1049-1058. DOI: 10.1016/j.cej.2019.03.139
[30] ALKHOUZAAM A, QIBLAWEY H, KHRAISHEH M, et al. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method[J]. Ceramics International, 2020, 46(15): 23997-24007. DOI: 10.1016/j.ceramint.2020.06.177
[31] LUO H, GUO J, SHEN T, et al. Study on the catalytic performance of LaMnO3 for the RhB degradation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2020, 109: 15-25. DOI: 10.1016/j.jtice.2020.01.011
[32] KUMAR S R, ABINAYA C V, AMIRTHAPANDIAN S, et al. Enhanced visible light photocatalytic activity of porous LaMnO3 sub-micron particles in the degradation of rose bengal[J]. Materials Research Bulletin, 2017, 93: 270-281. DOI: 10.1016/j.materresbull.2017.05.024
[33] KARUPPIAH C, THIRUMALRAJ B, ALAGAR S, et al. Solid-state ball-milling of Co3O4 nano/microspheres and carbon black endorsed LaMnO3 perovskite catalyst for bifunctional oxygen electrocatalysis [J]. Catalysts, 2021.
[34] MARINESCU C, BEN ALI M, HAMDI A, et al. Cobalt phthalocyanine-supported reduced graphene oxide: A highly efficient catalyst for heterogeneous activation of peroxymonosulfate for rhodamine B and pentachlorophenol degradation[J]. Chemical Engineering Journal, 2018, 336: 465-475. DOI: 10.1016/j.cej.2017.12.009
[35] STANKOVICH S, DIKIN D A, PINER R D, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide[J]. Carbon, 2007, 45(7): 1558-1565. DOI: 10.1016/j.carbon.2007.02.034
[36] PRIYATHARSHNI S, RAJESH KUMAR S, VISWANATHAN C, et al. Morphologically tuned LaMnO3 as an efficient nanocatalyst for the removal of organic dye from aqueous solution under sunlight[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104146. DOI: 10.1016/j.jece.2020.104146
[37] LI J J, YU E Q, CAI S C, et al. Noble metal free, CeO2/LaMnO3 hybrid achieving efficient photo-thermal catalytic decomposition of volatile organic compounds under IR light[J]. Applied Catalysis B: Environmental, 2019, 240: 141-152. DOI: 10.1016/j.apcatb.2018.08.069
[38] KUMAR P, ŠILHAVÍK M, ZAFAR Z A, et al. Universal strategy for reversing aging and defects in graphene oxide for highly conductive graphene aerogels[J]. The Journal of Physical Chemistry C, 2023, 127(22): 10599-10608. DOI: 10.1021/acs.jpcc.3c01534
[39] ZHANG X, YANG X, CHEN S, et al. Unravelling the synergy of Eu dopant and surface oxygen vacancies confined in bimetallic oxide for peroxymonosulfate activation[J]. Chemical Engineering Journal, 2023, 452: 139192. DOI: 10.1016/j.cej.2022.139192
[40] LI Z, WANG M, JIN C, et al. Synthesis of novel Co3O4 hierarchical porous nanosheets via corn stem and MOF-Co templates for efficient oxytetracycline degradation by peroxymonosulfate activation[J]. Chemical Engineering Journal, 2020, 392: 123789. DOI: 10.1016/j.cej.2019.123789
[41] CHEN F, HUANG G X, YAO F B, et al. Catalytic degradation of ciprofloxacin by a visible-light-assisted peroxymonosulfate activation system: Performance and mechanism[J]. Water Research, 2020, 173: 115559. DOI: 10.1016/j.watres.2020.115559
[42] DU A, FU H, WANG P, et al. Enhanced catalytic peroxymonosulfate activation for sulfonamide antibiotics degradation over the supported CoSx-CuSx derived from ZIF-L(Co) immobilized on copper foam[J]. Journal of Hazardous Materials, 2022, 426: 128134. DOI: 10.1016/j.jhazmat.2021.128134
[43] MA J, CHEN L, LIU Y, et al. Oxygen defective titanate nanotubes induced by iron deposition for enhanced peroxymonosulfate activation and acetaminophen degradation: Mechanisms, water chemistry effects, and theoretical calculation[J]. Journal of Hazardous Materials, 2021, 418: 126180. DOI: 10.1016/j.jhazmat.2021.126180
[44] WANG J, WANG S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants[J]. Chemical Engineering Journal, 2021, 411: 128392. DOI: 10.1016/j.cej.2020.128392
[45] JIA J, LIU D, TIAN J, et al. Visible-light-excited humic acid for peroxymonosulfate activation to degrade bisphenol A[J]. Chemical Engineering Journal, 2020, 400: 125853. DOI: 10.1016/j.cej.2020.125853
[46] DING X, GUTIERREZ L, CROUE J P, et al. Hydroxyl and sulfate radical-based oxidation of RhB dye in UV/H2O2 and UV/persulfate systems: Kinetics, mechanisms, and comparison[J]. Chemosphere, 2020, 253: 126655. DOI: 10.1016/j.chemosphere.2020.126655
[47] ZHANG X, YANG Y, HAO N H, et al. Urea removal in reclaimed water used for ultrapure water production by spent coffee biochar/granular activated carbon activating peroxymonosulfate and peroxydisulfate[J]. Bioresource Technology, 2022, 343: 126062. DOI: 10.1016/j.biortech.2021.126062
[48] LI H, SHANG H, CAO X, et al. Oxygen vacancies mediated complete visible light NO oxidation via side-on bridging superoxide radicals[J]. Environmental Science & Technology, 2018, 52(15): 8659-8665.
[49] CAO J, YANG Z, XIONG W, et al. Three-dimensional MOF-derived hierarchically porous aerogels activate peroxymonosulfate for efficient organic pollutants removal[J]. Chemical Engineering Journal, 2022, 427: 130830. DOI: 10.1016/j.cej.2021.130830
[50] SUN H, GUO F, PAN J, et al. One-pot thermal polymerization route to prepare N-deficient modified g-C3N4 for the degradation of tetracycline by the synergistic effect of photocatalysis and persulfate-based advanced oxidation process[J]. Chemical Engineering Journal, 2021, 406: 126844. DOI: 10.1016/j.cej.2020.126844
-
其他相关附件
-
目的
四环素(TC)的广泛使用及其毒性和诱导抗生素耐药性对生物体产生严重危害,高级氧化技术可在温和条件下实现对有机污染物的有效降解和矿化。Mn基催化剂,其可调变的晶体结构、毒性低和天然丰度高等优点,被广泛用于活化过硫酸盐。LaMnO由于纳米粒子团聚、金属离子浸出等缺点活化过硫酸盐受阻。本文通过原位生长法原位生长方法制备了石墨烯气凝胶(GA)负载的LaMnO钙钛矿型氧化物。石墨烯气凝胶可作为电子受体,电子在原始碳六元环结构中较快的传输速率,有效提高过硫酸盐的利用率。
方法本实验采用通过溶胶凝胶法和水热法制备了LaMnO/GA25复合材料。采用了SEM、TEM、XPS、拉曼光谱等手段对样品的形貌结构、元素组成和化学形态进行了表征。通过一系列因素考察实验,评估实验条件对LaMnO/GA25/PMS体系降解TC能力的影响规律。自由基捕获试验验证TC降解过程中主要的活性物质。此外,通过探究LaMnO/GA25/PMS体系对多种无机阴离子(如HPO, SO, Cl, Urea, HCO)和腐殖酸(HA)的抗干扰活性以及和循环使用性能。
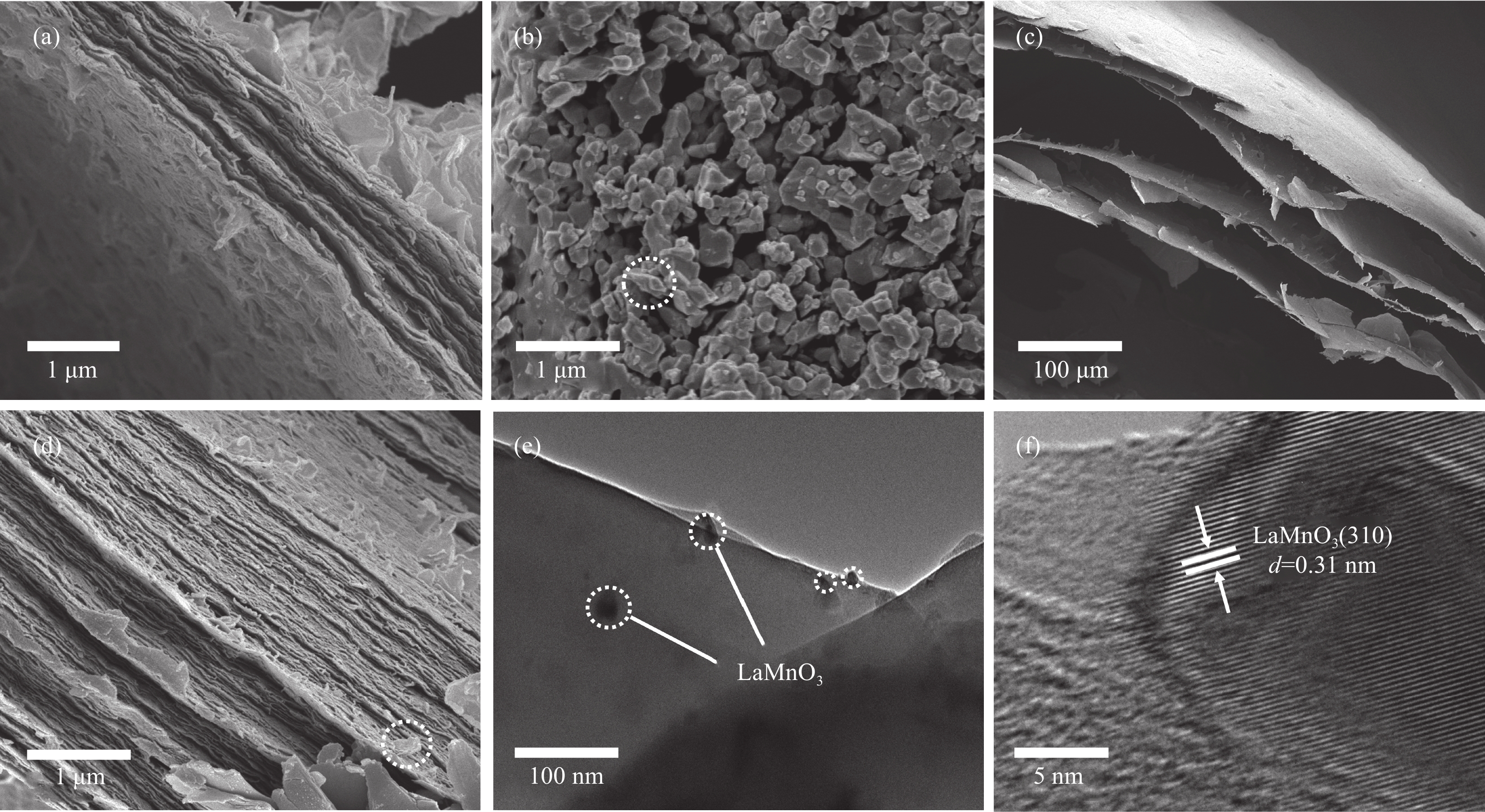
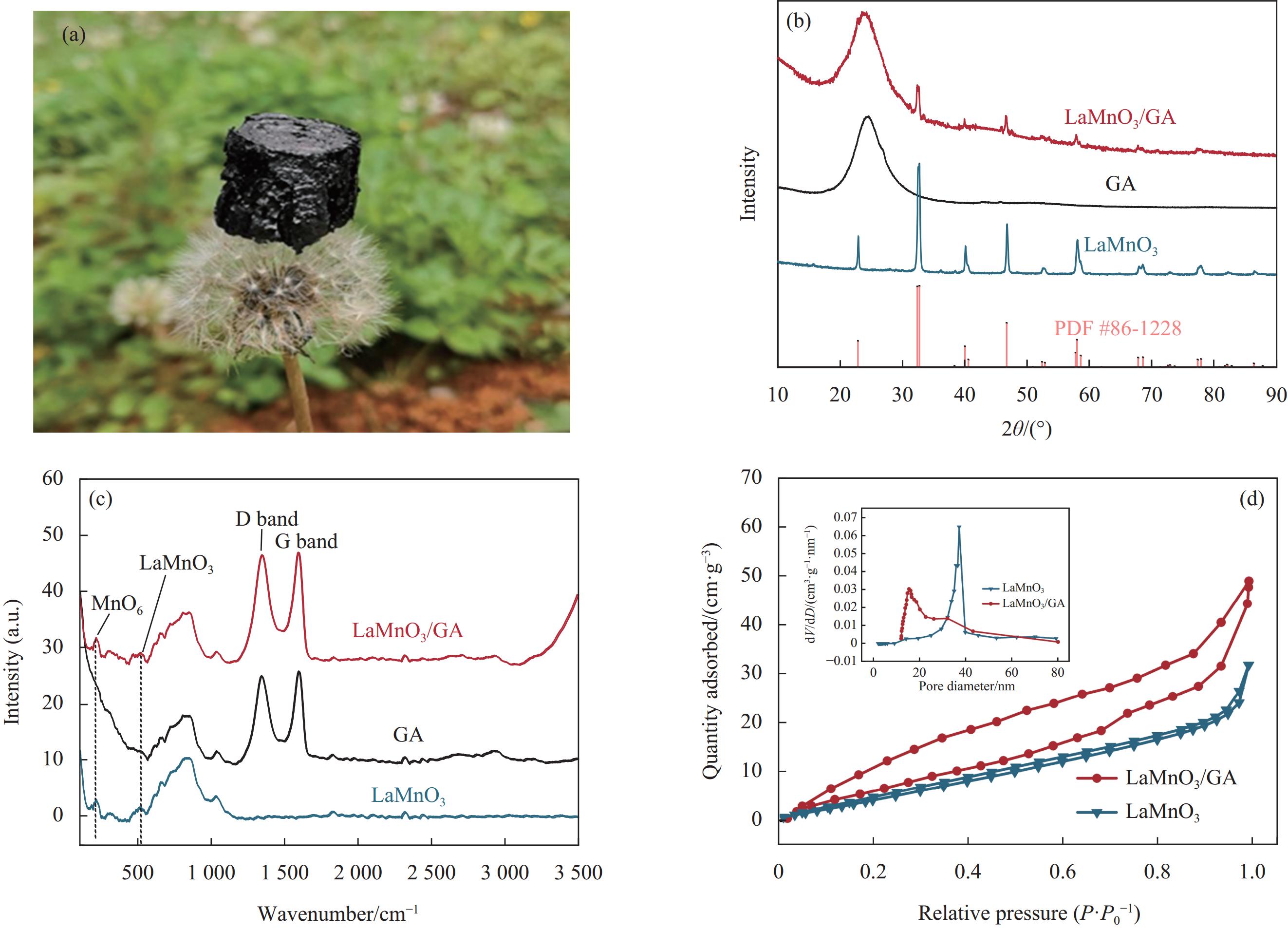
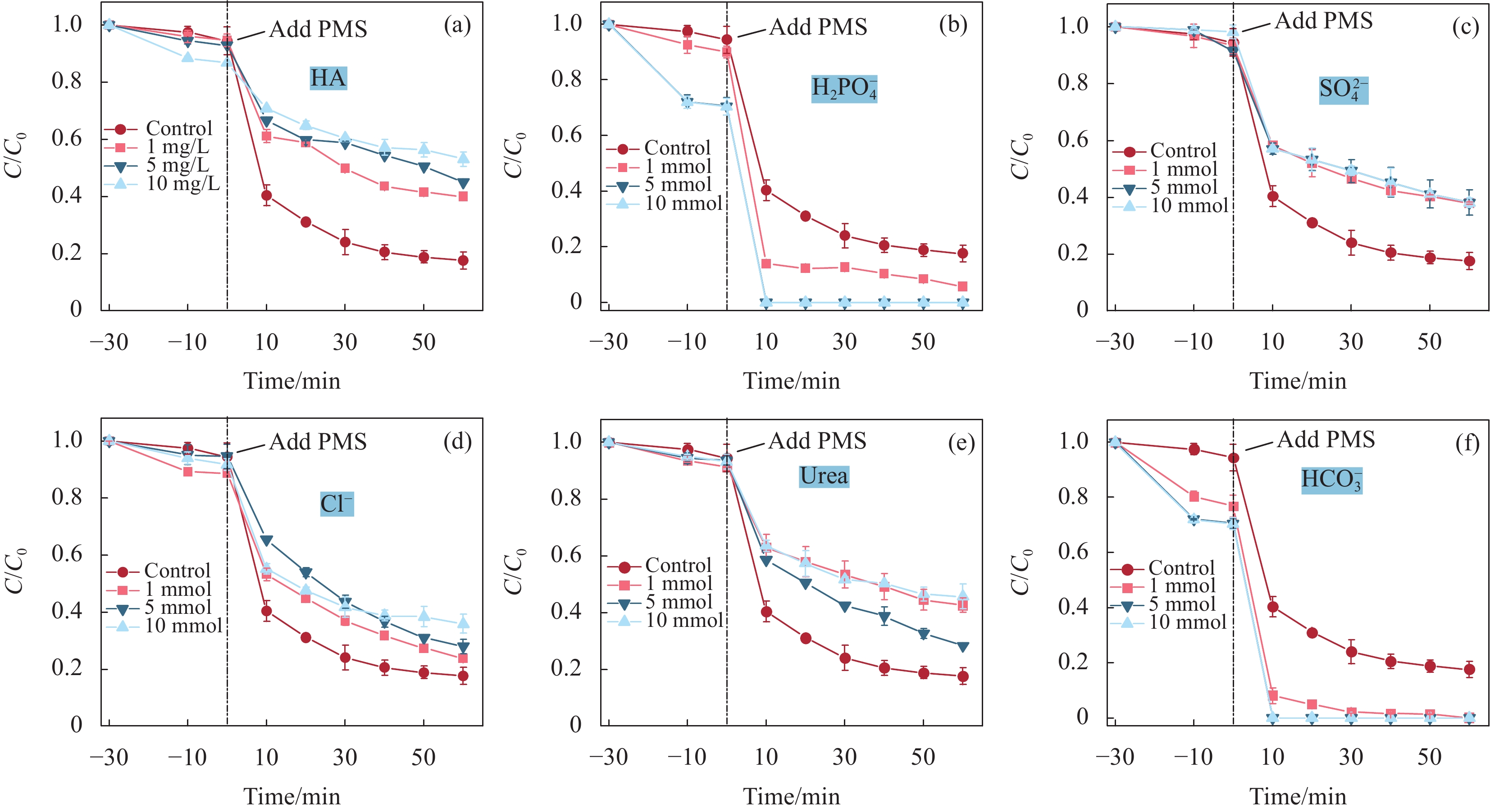
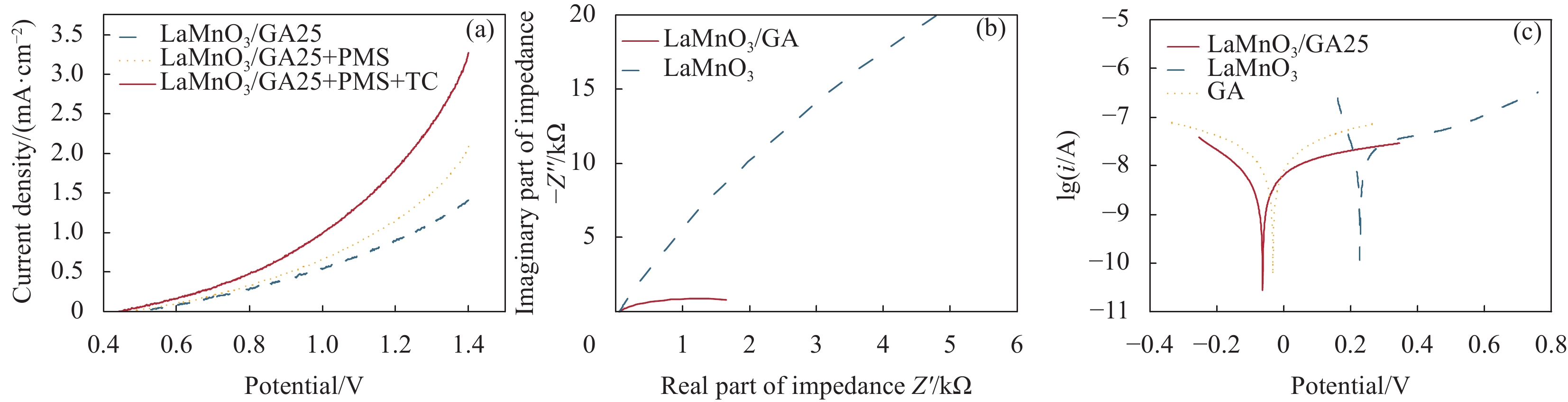
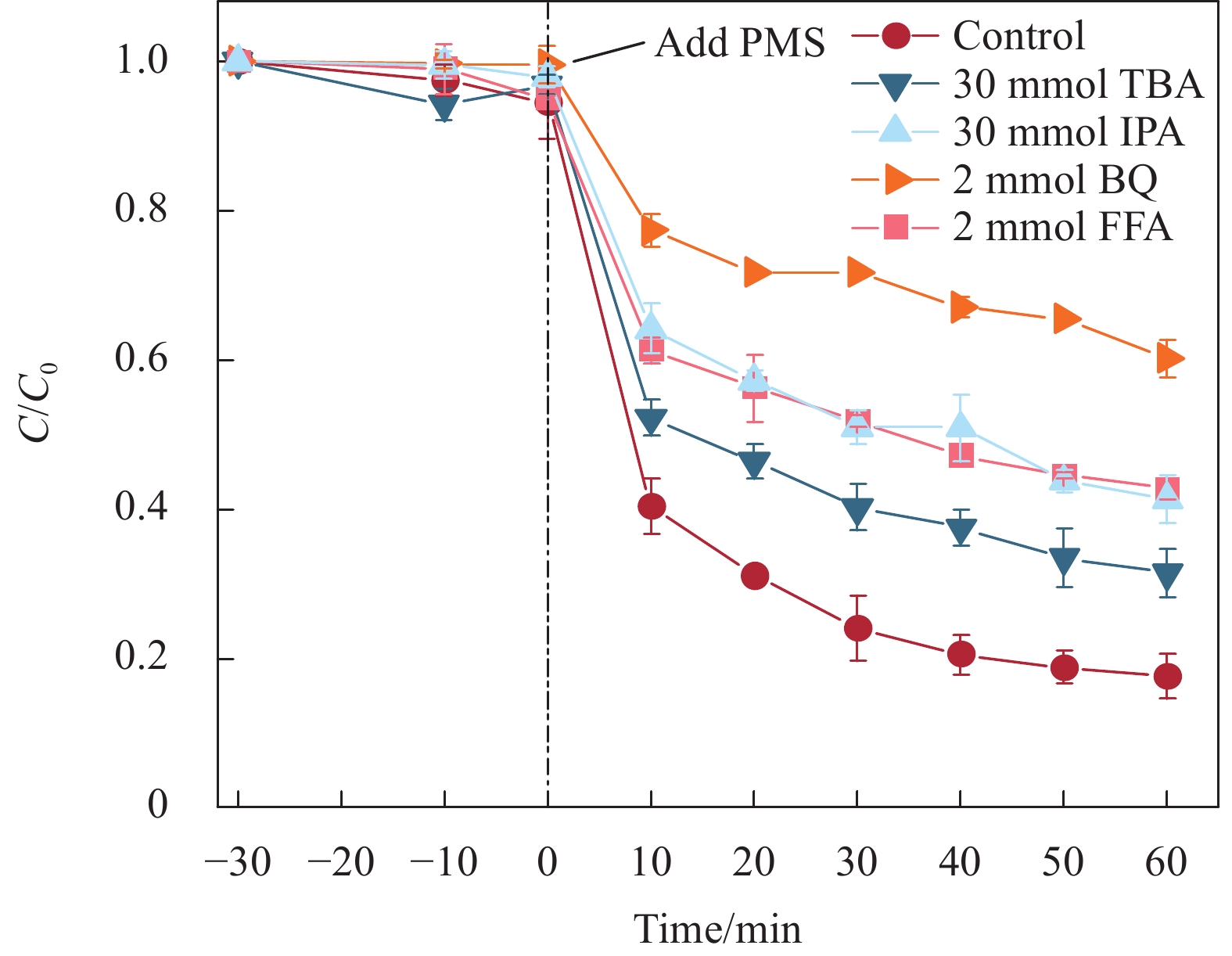
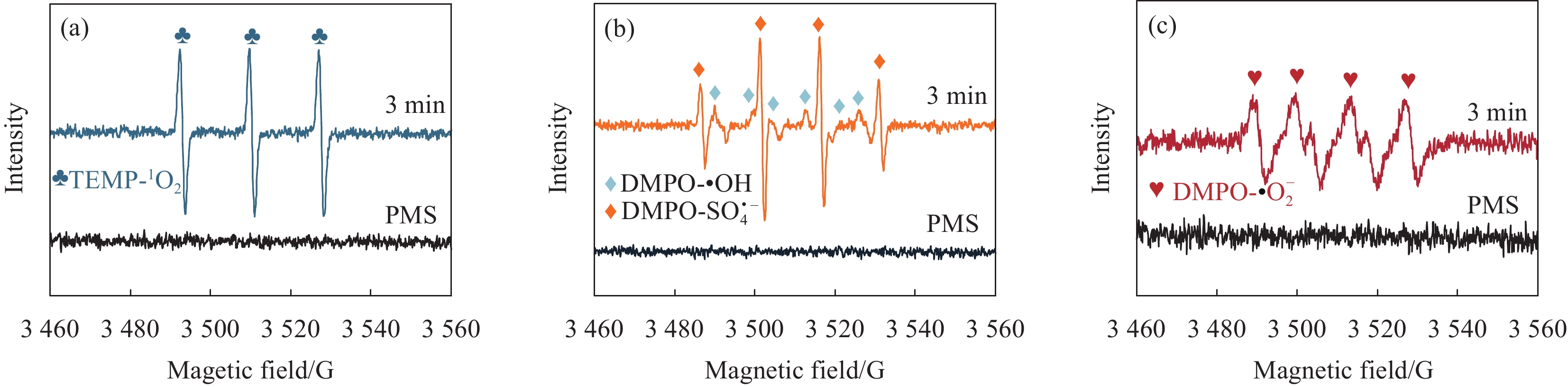
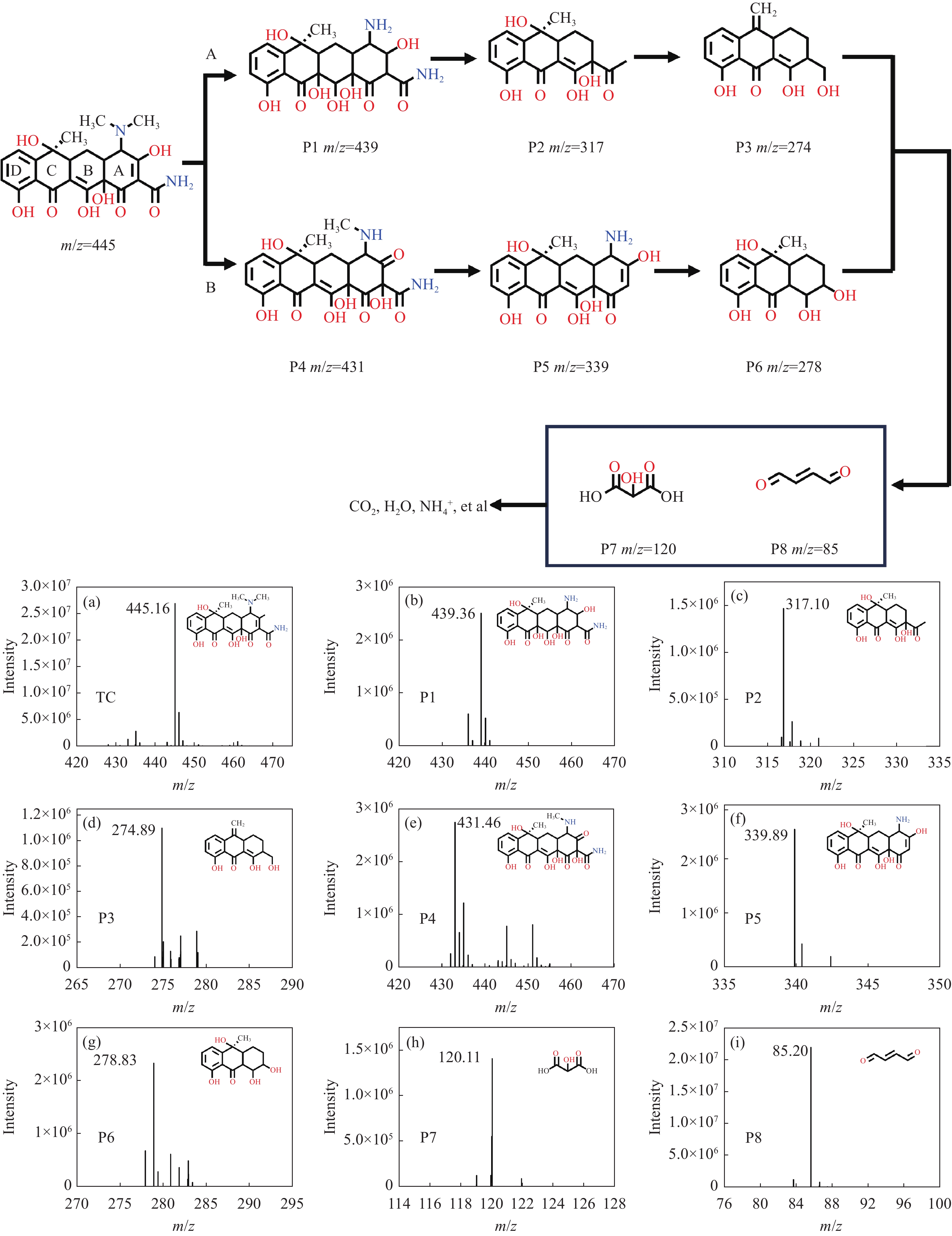
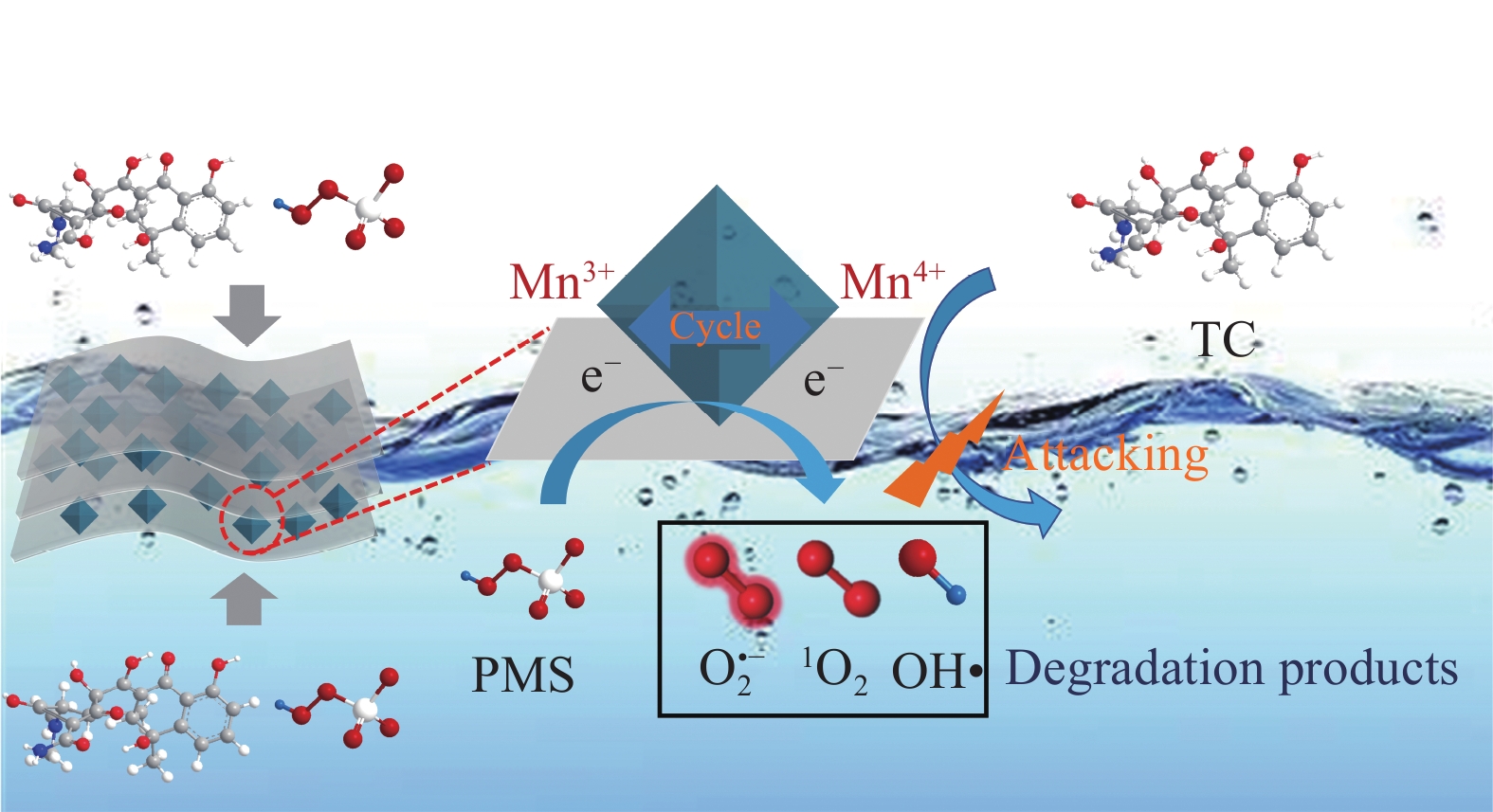
结果采用SEM与TEM对LaMnO/GA25的形貌进行了表征。所示,呈现出微观纳米片结构,LaMnO颗粒附着在GA片层表面。TEM测试结果表明,LaMnO被均匀包裹在GA的网络结构中,从HRTEM测试结果中清晰可见LaMnO的(310)晶面,其晶面间距为0.31 nm。XRD技术表征所示22.87°、32.4°、40.02°、46.71°、58.62°、68.58°和77.91°处的衍射峰可归属于LaMnO的(012)、(110)、(202)、(024)、(018)、(208)和(128)平面晶相。对于GA光谱,在22-24°处的宽峰属于石墨的(002)平面。这些结果都说明,成功构建了LaMnO/GA25复合材料。拉曼分析可以看出,LaMnO/GA25的I/I(0.99)高于GA(0.96),表明该复合材料具有更多的碳缺陷。N吸附和解吸等温线得出LaMnO和LaMnO/GA25的比表面积分别为28.32 m2/g和48.5 m2/g,这个结果可归因于GA的引入赋予了复合材料丰富的孔结构。一系列因素考察实验得出,当LaMnO/GA25添加量为10 mg、PMS为20 mg和TC浓度为20 mg/L时,LaMnO/GA25/PMS对TC的去除率为83%。结合抗干扰实验和自由基捕获实验结果,可推测在LaMnO/GA25活化PMS降解TC的实验过程中,起主导作用的自由基为•OH、O和O•,且其起作用顺序为O• ˃ O ˃ •OH。
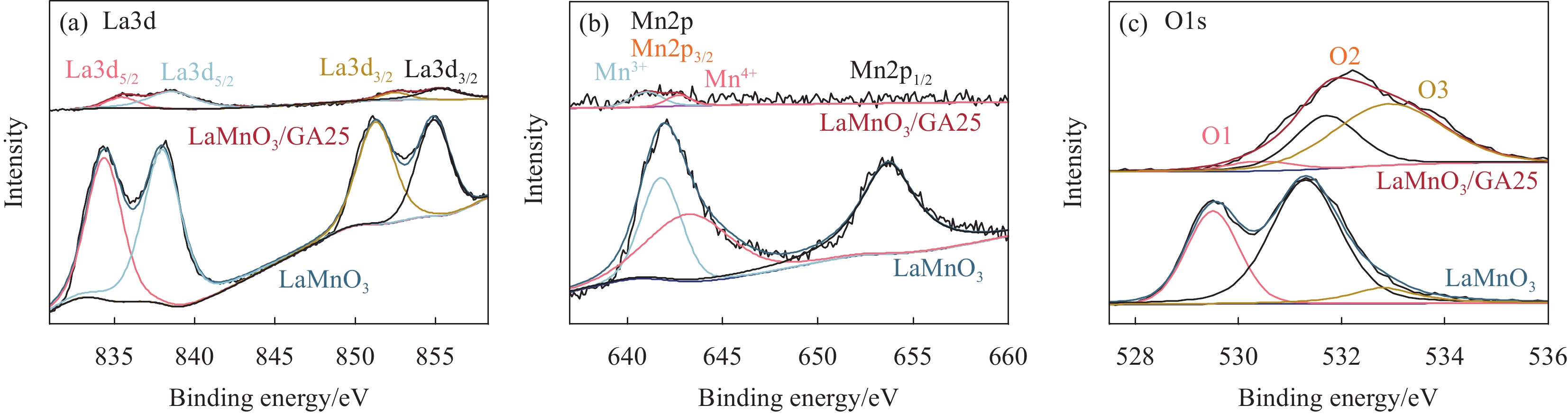
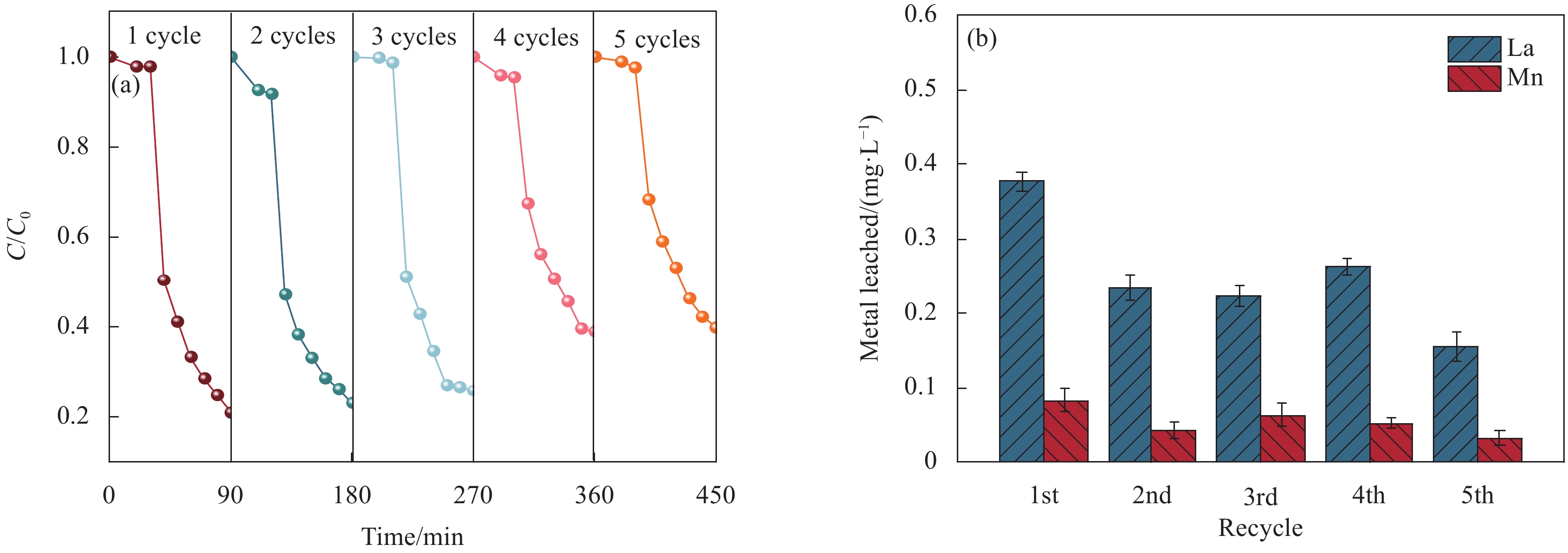
结论LaMnO/GA25复合材料对TC的降解率可达到83%,与空白LaMnO样品相比,催化活性提升了近25%,对多种无机阴离子和HA也有具有较好的抗干扰性能。另外,经过5次循环使用之后,仍能保持初始催化活性的72%。自由基捕获试验结果表明,起主导作用的自由基为•OH、O和O•,且其起作用顺序为O• ˃ O ˃ •OH。结合XPS和电化学测试结果,催化活性的提升可归因于GA的电子捕获特性,诱导电荷从LaMnO向碳基质转移,电子在原始碳六元环结构中较快的传输速率,有效提升了PMS的利用率,并为分解TC提供了丰富的活性位点。LaMnO/GA复合体系可应用于复杂水体中污染物的处理,并为消除水环境中的各种难降解污染物提供了新的思路。
-
四环素(TC)的广泛使用及其毒性和诱导抗生素耐药性对生物体产生严重危害,高级氧化技术可在温和条件下实现对有机污染物的有效降解和矿化。Mn基催化剂,其可调变的晶体结构、毒性低和天然丰度高等优点,被广泛用于活化过硫酸盐。其中,LaMnO3作为一种p型半导体,具有结构稳定、低成本和高催化活性等优点,在多相催化过程中吸引了广泛的关注。然而,LaMnO3由于纳米粒子团聚、金属离子浸出等缺点活化过硫酸盐受阻。
本文通过原位生长法制备了石墨烯气凝胶(GA)负载的LaMnO3复合催化剂。石墨烯气凝胶可作为电子受体,且电子在原始碳六元环结构中较快的传输速率,可有效提高过硫酸盐的利用率;此外,GA内部交织的网络结构极大缩短了分子的迁移路径,为污染物的扩散和分解提供了丰富的活性位点;更重要的是,其良好的机械稳定性还为开发结构稳定、重复利用率高的催化材料增加了可行性。实验结果表明,所制备的LaMnO3/GA25复合材料活化PMS降解TC的去除率可提高至83%以上,远高于纯样LaMnO3(降解率仅为58%)。





 下载:
下载: