Study on the progress of chitosan composite pervaporation membrane for the separation of organic solvents
-
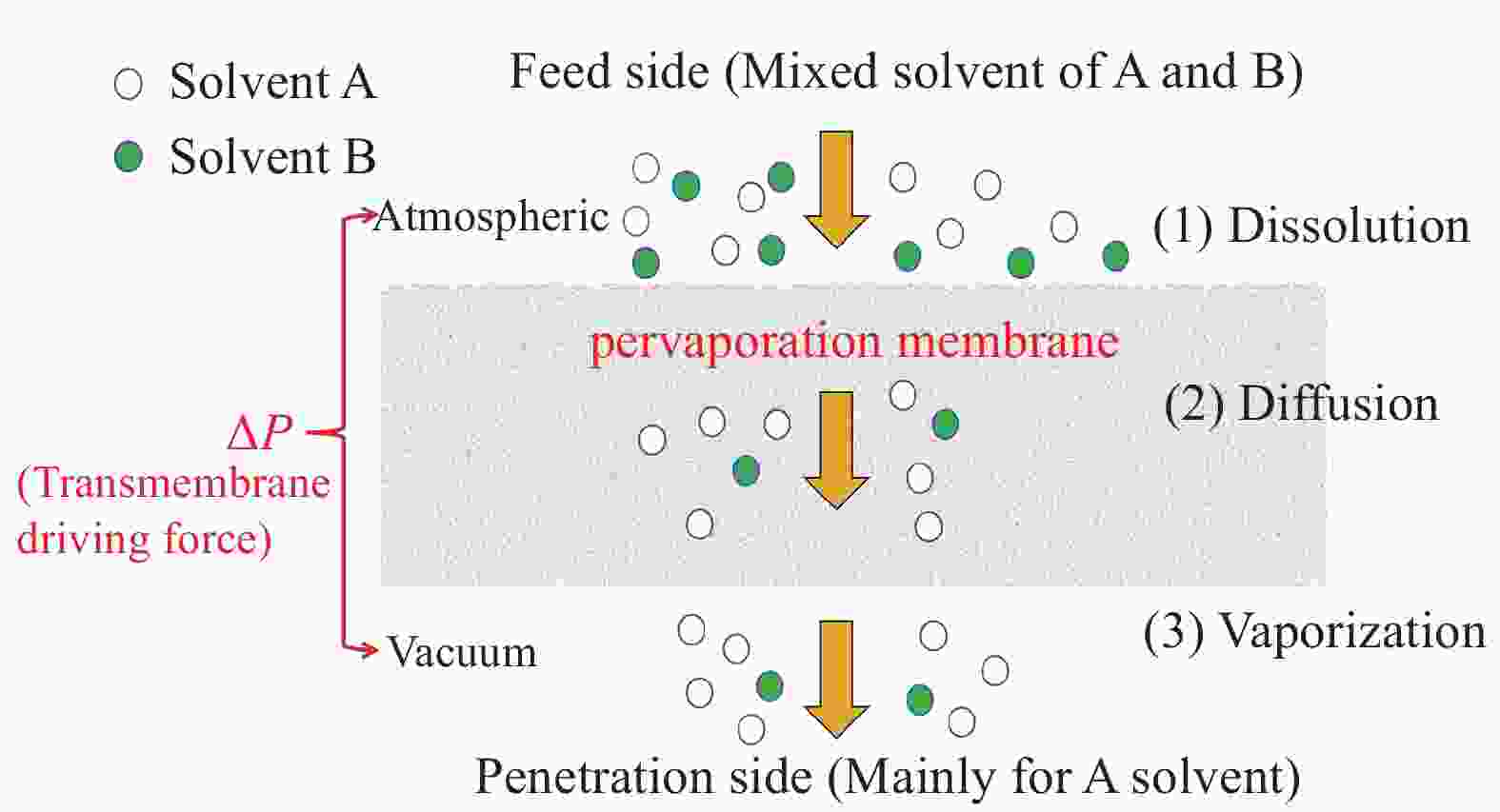
摘要: 有机溶剂的高效分离与纯化是化学合成、生物技术、药物研发等领域中不可或缺的重要环节,含有机溶剂的废水若直接排放会对环境和人类健康造成极大危害,并造成资源浪费。渗透汽化(PV)是一种新型膜分离技术,利用膜对不同组分的选择性和渗透速率差异实现对有机溶剂的分离。本文介绍了渗透汽化分离有机溶剂的原理以及常用渗透汽化膜材料,详细阐述了壳聚糖渗透汽化膜应用于有机溶剂分离的研究进展,从乙醇、异丙醇、丙酮等有机溶剂与水的分离和有机溶剂混合物分离两个方面进行了总结。归纳了壳聚糖复合渗透汽化膜用于提高有机溶剂分离与提纯效率的方法与分离机制。最后,对利用天然生物质壳聚糖基复合渗透汽化膜分离有机溶剂的发展方向进行了展望。Abstract: The efficient separation and purification of organic solvents is an important and indispensable link in the fields of chemical synthesis, biotechnology, and drug discovery, etc. Direct discharge of wastewater containing organic solvents can cause great harm to the environment and human health, and result in resource waste. Pervaporation (PV) is a novel membrane separation technology that utilizes membrane selectivity for different components and permeation rate differences to achieve separation of organic solvents. This paper introduces the principles of pervaporation for organic solvent separation and details commonly used pervaporation membrane materials. It reviews the research progress on chitosan pervaporation membranes for organic solvent separation, focusing on separating solvents like ethanol, isopropanol, and acetone from water, as well as the separation of organic solvents mixtures. Furthermore, summarized the methods and separation mechanisms of chitosan composite pervaporation membranes for improving the efficiency of organic solvent separation and purification. Finally, the development direction of using natural biomass chitosan-based composite pervaporation membranes for the separation of organic solvents is discussed.
-
Key words:
- chitosan /
- pervaporation membrane /
- organic solvent /
- separation /
- Wastewater treatment /
- Membrane material
-
表 1 常见有机渗透汽化膜材料
Table 1. Common organic permeable vapour membrane materials
Membrane materials Characteristic Examples of applications for separating organic solvents Reference Polyvinyl alcohol (PVA) The affinity for water is higher than that of organic solvents, but it is easy to contaminate and more costly. Silicotungstic acid (STA) and phosphomolybdic acid (PMA) are loaded into polyvinyl alcohol (PVA) matrix to produce hybrid membranes. The flux and selectivity values of the hybrid PVA membranes loaded with 5 wt% STA and 5 wt% PMA were 0.499 kg/m2·h,12848; 0.471 kg/m2·h, 74991,respectively. [19] Polyimide (PI) High temperature stability, excellent mechanical properties but expensive, difficult to process and not easy to degrade. PI/ZIF-8 hybrid membrane consisting of 2% (ZIF-8) mass fraction. At 40℃, the fluxes and separation factors are 242.2 g/(m2·h), 17.8 and 126.2 g/(m2·h), 55.2 for the DMF/H2O and DMAc/H2O systems, respectively. [20] Sodium alginate (SA) Excellent stability, wear resistance. However, short service life, easy to contamination and scaling. SA/phosphotungstic acid (PTA) hybrid membranes for methanol/water separation. 6 wt% PTA, 30℃, 95 wt% methanol solution, the membrane flux and separation factor were 318.2 g/(m2·h) and 656.9, respectively, which were 3.7 and 26.3 times higher than those of pure sodium alginate membrane. [21]
Polysulfone (PES)Chemical resistant and stable, but more expensive and susceptible to contaminateon. Fluoropolymer-containing composite membranes (PFHI /PES) are prepared, and the separation factor as well as the total permeate flux are 194.19 and g/(m2·h) at 20℃, 98 wt% acetic acid/water solution, respectively. [22] Chitosan (CS) Excellent antimicrobial, de-gradability and regeneration properties. However, pure chitosan membranes may have low mechanical strength and permeability may be easily affected by temperature, etc., and need to be modified to show good performance. PVA-CS-O-MWNTs/PAN hollow fibre membranes are prepared for methanol (MeOH)/dimethyl carbonate (DMC) separation by impregnation coating. 0.1 wt% O-MWNTs, thermal treatment temperature of 80℃, feed temperature of 40℃, 70 wt% MeOH, permeate flux of 210 g/(m2·h) , and methanol selectivity of 7.82. [23] 表 2 CS复合渗透汽化膜分离乙醇/水
Table 2. Chitosan composite pervaporation membrane for ethanol/water separation
Pervaporation membrane Ethnol/wt% Particle/mixture
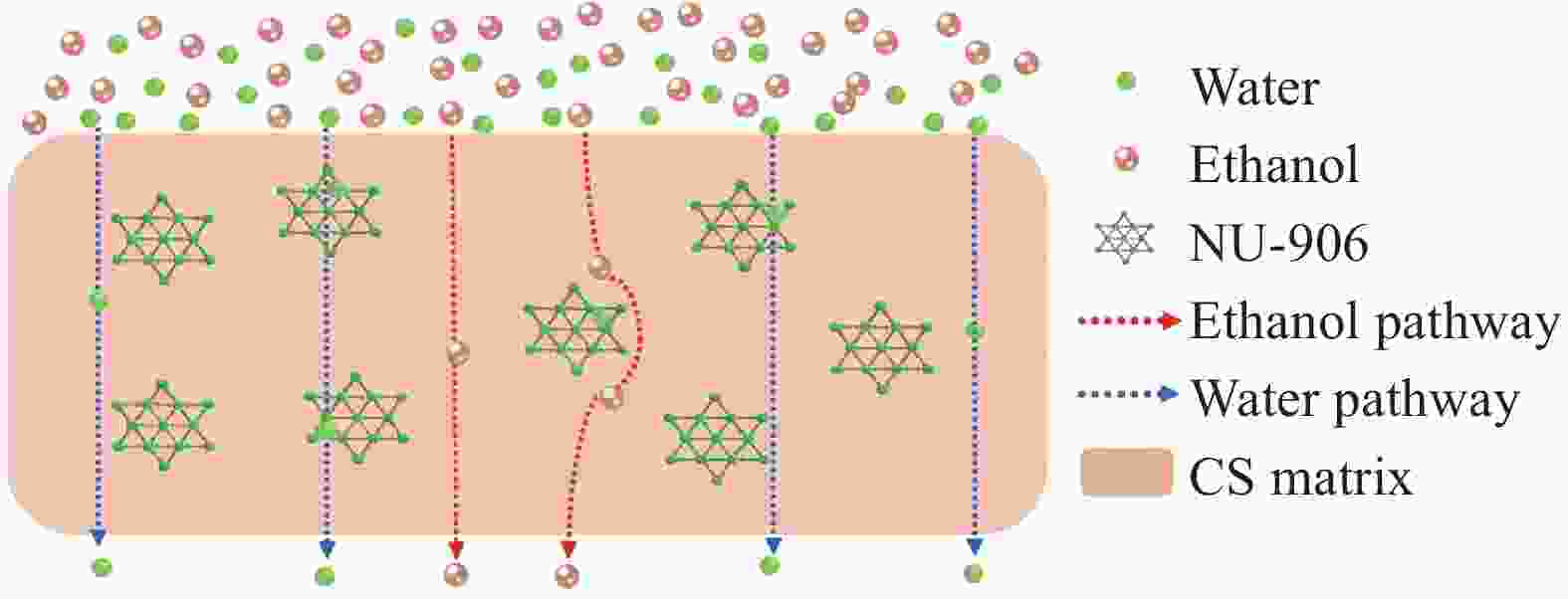
loading/wt%Temperature/℃ Fluxes/(g·m-2·h-1) Separation factor α Reference CS/ Ti3C2Tx 99.7 3%MXene(Ti3C2Tx) 50 1400 1421 [39] CS/NU-906 90 5% NU-906 76 1086 2651 [40] SiO /CS 99.5 0.05% siloxane 25 468 2182 [41] CS 90 0 60 400 500 [42] CS/PSS 90 0.75% PSS 70 495 904 [43] (CS-P)/ALG 96 10% CS-P 25 1900 136.2 [44] Al-MOF/CS 90 0.15%Al-MOF 25 378 3429 [45] Notes:Ti3C2Tx (MXene) multilayer Nanoflake (Ti3C2Tx); siloxane(SiO); poly(4-styrenesulfonic acid) (PSS); Phosphorylated chitosan (CS-P) ; alginate(ALG); Aluminum-Metal organic Framework (Al-MOF) 表 3 CS复合渗透汽化膜分离异丙醇/水
Table 3. Chitosan composite pervaporation membrane for isopropanol/water separation
Pervaporation membrane Isopropanol/wt% Particle/mixture
loading/wt%Temperature/℃ Fluxes/(g·m-2·h-1) Separation
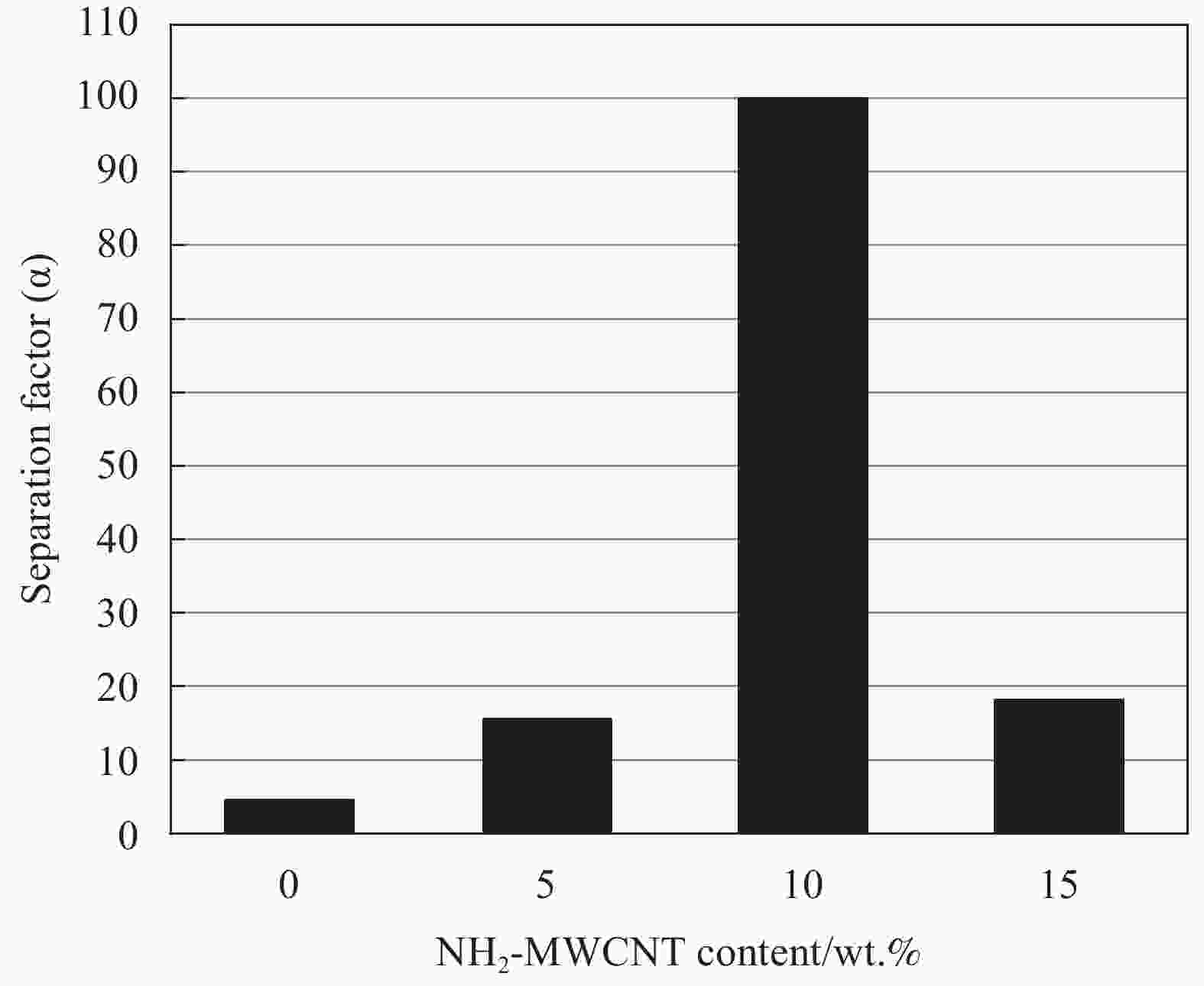
Factor αReference CS/PVA 90 75% CS 60 644 Water
permeability>99.9%[47] CS/PVA/NH2-MWCNT 70 10%NH2-MWCNT 60 795 99.5 [48] CS-PAN 90 ----- 70 330 1410 [49] GTMAC/CS 90 40% GTMAC 30 691 2133 [50] CS/NAY 95 40% NAY 30 115 2620 [51] CS/Gel 90 15%Gel 30 42.01 6330 [52] TiO2/CS 95 40% TiO2 30 121.7 94984 [53] Notes:polyvinyl alcohol (PVA); polyacrylonitrile (PAN); Glycidyltrimethylammonium chloride (GTMAC); Multi walled carbon nanotubes (MWCNT); NaY zeolite (NAY); Gelatin (Gel); Titanium Dioxide (TiO2) 表 4 CS复合渗透汽化膜分离丙酮/水
Table 4. Chitosan composite pervaporation membrane for acetone/water separation
Pervaporation
membraneActone/wt% Particle/mixture
loading/wt%Temperature/℃ Fluxes/(g·m-2·h-1) Separation
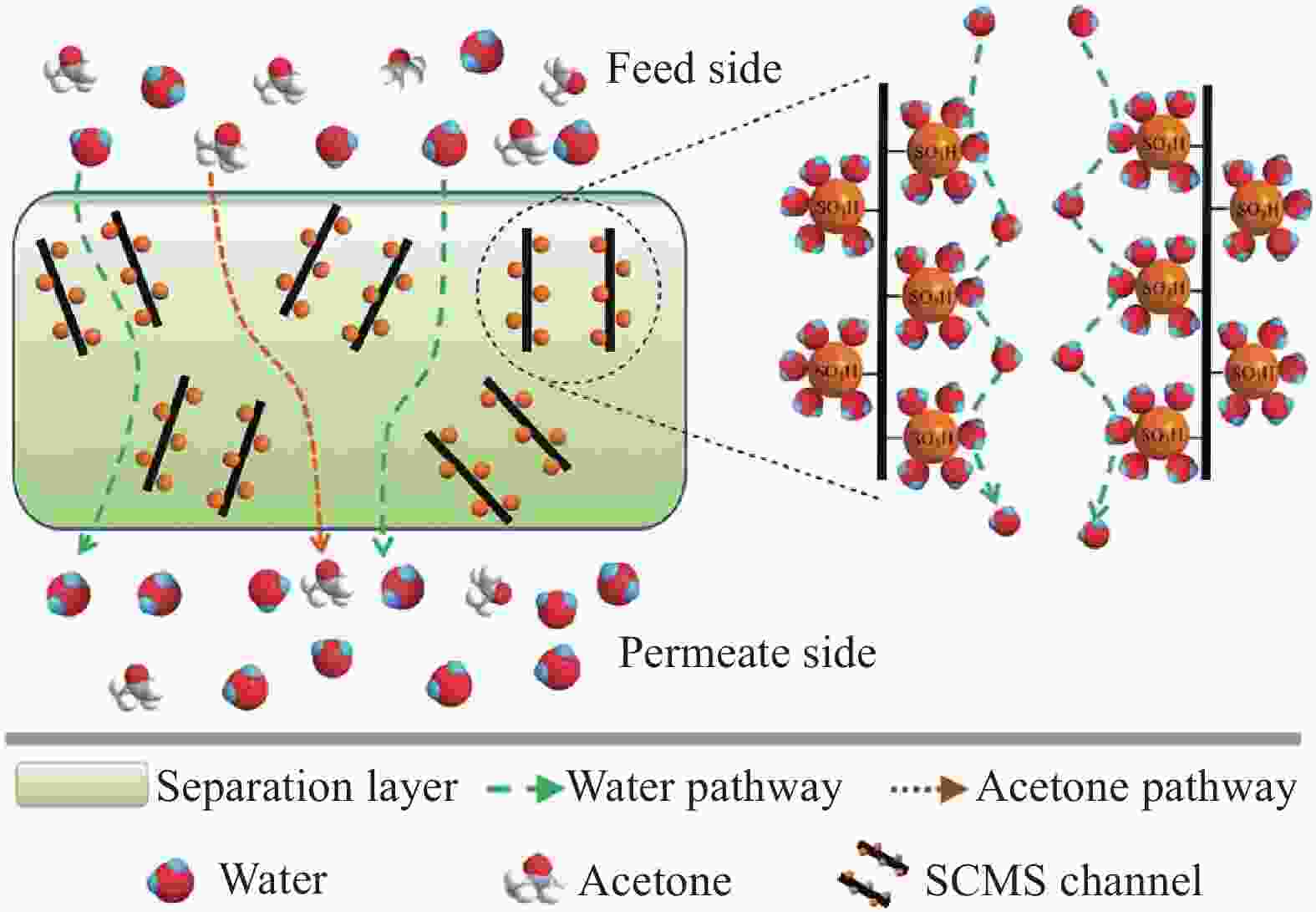
Factor αReference CS/PVA-MWCNT/PVDF 95 0.06%PVA-MWCNT 30-50 89 1450.5 [55] K+MMT-CS/PAN 95 10% K+MMT 50 1560 2200 [56] SCMS-CS/PAN 90 2% SCMS 30 1810 832 [57] NaMMT/MA/PVA-CS 99 39.3%CS;3%M;3% NaMMT 30-45 176-733 277-168 [58] Notes:Polyvinylidene fluoride (PVDF); kalium Montmorillonite (K+MMT); Sulfonated Carbon Molecular Sieves (SCMS); Natrium Montmorillonite (NaMMT); Maleic acid (MA) 表 5 CS复合渗透汽化膜分离其他有机溶剂/水
Table 5. Chitosan composite pervaporation membrane for other organic solvents/water separation
Pervaporation membrane organic solvent Particle/mixture
loading/wt%Temperature/℃ Fluxes/(g·m-2·h-1) Separation
Factor αReference PVA/CS/Zeolite-A Tert-butyl alcohol 0.9 %Zeolite-A 30 136700 1324 [60] PSSAMA/CS Tert-butyl alcohol 9% PSSAMA 30 41.45 5352 [61] SiO2 dry gel-CS Butanol 0.25% SiO2 50 736 1498 [63] PVA-CS/PVDF Butyl acetate 25% CS 40 402 27000 [65] Notes:Polystyrene sulfonic acid maleic acid (PSSAMA); Silicon dioxide (SiO2) 表 6 CS复合渗透汽化膜分离有机溶剂/有机溶剂
Table 6. Chitosan composite pervaporation membrane for organic solvents/organic solvents separation
Pervaporation
membraneOrganic
solventParticle/mixture loading/wt% Temperature/℃ Fluxes/(g·m-2·h-1) Separation
Factor αReference PVA/CS Bz/Chx 50% CS 50 51.41 49.9 [68] CG-PVA/CS Bz/Chx 6%CG;60%PVA;
40% CS50 124.2 59.8 [69] MWNT-Ag+/CS Bz/Chx MWNT-Ag+/CS=1.5% 20 357.96 7.89 [70] GA-STA-CS/PEK-C DMC/MeOH 0.3% GA 30 910.2 120.3 [72] UiO-66(ZrCl4)/CS DMC/MeOH 10%UiO-66 (ZrCl4) 50 355 337 [73] CS/PTFE/ TEOS MeOH/TOL TEOS/CS=0.08 --- 130 58.4 [76] Notes:carbon-graphite (CG); glutaraldehyde (GA); Silicotungstic acid hydrate (STA); poly(ether-ketone) (PEK-C); University of Oslo (UiO-66); Tetraethyl orthosilicate (TEOS); Polytetrafluoroethylene (PTFE) -
[1] CHINMOY B, ACHYUT K, PRARTHANA B, et al. Cellulose nanofiber-poly (ethylene terephthalate) nanocomposite membrane from waste materials for treatment of petroleum industry wastewater[J]. Journal of Hazardous Materials, 2023, 442: 129955. doi: 10.1016/j.jhazmat.2022.129955 [2] NEHA V, SOUMYA P, KUMAR P G, et al. Occupational health hazards and wide spectrum of genetic damage by the organic solvent fumes at the workplace: A critical appraisal[J]. Environmental Science and Pollution Research, 2022, 29(21): 30954-30966. doi: 10.1007/s11356-022-18889-6 [3] ABOAGYE E A, CHEA J D, YENKIE K M. Systems level roadmap for solvent recovery and reuse in industries[J]. I science, 2021, 24(10): 103114. [4] RUTHUSREE S, SUNDARRAJAN S, RAMAKRISHNA S. Progress and Perspectives on Ceramic Membranes for Solvent Recovery[J]. Membranes, 2019, 9(10): 128. doi: 10.3390/membranes9100128 [5] CASTRO-MUÑOZ R, GONZÁLEZ-VALDEZ J, FIGOLI A. New Trends in Biopolymer-Based Membranes for Pervaporation[J]. Molecules, 2019, 24(19): 3584. doi: 10.3390/molecules24193584 [6] VANE L M. Review of pervaporation and vapor permeation process factors affecting the removal of water from industrial solvents[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(3): 495-512. [7] ZHANG C , PENG L , JIANG J , et al. Mass transfer model, preparation and applications of zeolite membranes for pervaporation dehydration: A review[J]. Chinese Journal of Chemical Engineering, 2017, 25(11): 1627-1638. [8] CHEN T H , HUANG Y H . Dehydration of waste cutting oil using a pervaporation process[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 82: 75-79. [9] ONG K Y, SHI M G, LE L N, et al. Recent membrane development for pervaporation processes[J]. Progress in Polymer Science, 2016, 57: 1-31. doi: 10.1016/j.progpolymsci.2016.02.003 [10] LAKSHMY KADAVIL SUBHASH, LAL DEVIKA, NAIR ANANDU, et al. Pervaporation as a Successful Tool in the Treatment of Industrial Liquid Mixtures[J]. Polymers, 2022, 14(8): 1604. doi: 10.3390/polym14081604 [11] BAKER R W, WIJMANS J G , HUANG Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data[J]. Journal of membrane science, 2010, 348(1-2): 346-352. [12] ROBERTO C M, DE LA IGLESIA ÓSCAR, VLASTIMIL F, et al. Pervaporation-Assisted Esterification Reactions by Means of Mixed Matrix Membranes[J]. Industrial & Engineering Chemistry Research, 2018, 57(47): 15998-16011. [13] CHENG X, PAN F, WANG M , et al. Hybrid membranes for pervaporation separations[J]. Journal of Membrane Science, 2017, 541: 329-346. [14] ROBERTO CASTRO-MUÑ, OZ. Break throughs on tailoring pervaporation membranes for water desalination: A review[J]. Water Research, 2020, 18: 1164 28. [15] AND F X, HUANG M Y R. Liquid Separation by Membrane Pervaporation: A Review[J]. Ind. Eng. Chem. Res., 1997, 36(4): 1048-1066. doi: 10.1021/ie960189g [16] ONG K Y, SHI M G, LE L N, et al. Recent membrane development for pervaporation processes[J]. Progress in Polymer Science, 2016, 57: 1-31. doi: 10.1016/j.progpolymsci.2016.02.003 [17] BOLTO B, HOANG M, XIE Z. A review of membrane selection for the dehydration of aqueous ethanol by pervaporation[J]. Chemical Engineering & Processing: Process Intensification, 2011, 50(3): 227-235. [18] GOH P S, ISMAIL AF. A review oninorganic membranes for desalination and wastewater treatment[J]. Desalination, 2018, 434: 60-80. doi: 10.1016/j.desal.2017.07.023 [19] UNLU D. Fabrication and Application of Silicotungstic Acid/Polyvinyl Alcohol and Phosphomolybdic Acid/Polyvinyl Alcohol Hybrid Membrane for Pervaporative Dehydration of Isopropanol Solution[J]. Macromolecular Research, 2019, 27(10): 998-1008. doi: 10.1007/s13233-019-7134-0 [20] 李太雨, 马文中, 徐荣等. PI/ZIF-8杂化膜的制备及渗透汽化分离性能研究[J]. 高校化学工程学报, 2020, 34(3): 648-655. doi: 10.3969/j.issn.1003-9015.2020.03.012LI Taiyu, MA Wenzhong, XU Rong et al. Preparation and permeation-vapour separation performance of PI/ZIF-8 hybrid membrane[J]. Journal of Chemical Engineering in Higher Education, 2020, 34(3): 648-655(in Chinese). doi: 10.3969/j.issn.1003-9015.2020.03.012 [21] 孙晓斌, 董锐, 张建勋等. 高亲水性海藻酸钠膜渗透汽化分离甲醇水溶液[J]. 化工进展, 2013, 32(1): 72-76.SUN Xiaobin, DONG Rui, ZHANG Jianxun, et al. Separation of methanol aqueous solution by osmotic vaporisation of highly hydrophilic sodium alginate membrane[J]. Advances in Chemical Engineering, 2013, 32(1): 72-76(in Chinese). [22] 王艳, 孙俊涛, 李毅等. 含氟聚合物/聚醚砜复合膜的制备及乙酸渗透汽化脱水性能[J]. 膜科学与技术, 2023, 43(4): 118-128.WANG Yan, SUN Juntao, LI Yi et al. Preparation of fluoropolymer/ polyether sulfone composite membranes and performance of acetic acid permeation vaporisation and dehydration[J]. Mem brane Science and Technology, 2023, 43(4): 118-128(in Chinese). [23] 闫玺, 刘妍, 郎万中. 氧化多壁碳纳米管对PVA-CS-O-MWNTs/PAN渗透汽化膜性能的影响[J]. 上海师范大学学报(自然科学版), 2017, 46(4): 469-474.YAN Xi, LIU Yan, LANG Wanzhong. Effect of oxidized multi-walled carbon nanotubes on the performance of PVA-CS-O-MWNTs/PAN permeable vapour film[J]. Journal of Shanghai Normal University (Natural Science Edition), 2017, 46(4): 469-474(in Chinese). [24] KOU SHIJIE (GABRIEL), PETERS LINDA M. , MUCALO MICHAEL R. Chitosan: A review of sources and preparation methods[J]. International Journal of Biological Macromolecules, 2021, 169: 85-94. doi: 10.1016/j.ijbiomac.2020.12.005 [25] VERLEE A , MINCKE S , STEVENS V C . Recent developments in antibacterial and antifungal chitosan and its derivatives[J]. Carbohydrate Polymers, 2017, 16 4: 268-283. [26] WU F C , TSENG R L , JUANG R S . Enhanced abilities of highly swollen chitosan beads for color removal and tyrosinase immobilization[J]. Journal of Hazardous Materials, 2001, 81(1-2): 167-177. [27] DUARTE E D V, OLIVEIRA M G , SPAOLONZI M P , et al. Adsorption of pharmaceutical products from aqueous solutions on functionalized carbon nanotubes by conventional and green methods: A critical review[J]. Journal of cleaner production, 2022, 372: 133743. [28] ALUPEI L, PEPTU A C, LUNGAN A, et al. New hybrid magnetic nanoparticles based on chitosan-maltose derivative for antitumor drug delivery[J]. International Journal of Biological Macromolecules, 2016, 92: 561-572. doi: 10.1016/j.ijbiomac.2016.07.058 [29] YUAN Y , HAN X , CAI H , et al. Co-Assembled WS2 Nanosheets/Chitosan Aerogels with Oriented Micro-Channels for Efficient and Sustainable Solar Steam Generation[J]. ACS Sustainable Chem istry & Engineering, 2023, 11 (12): 4643-4651. [30] 汤薇, 董静, 赵金荣等. 壳聚糖改性及改性壳聚糖应用研究进展[J]. 济南大学学报(自然科学版), 2023, 37(1): 84-93.TANG Wei, DONG Jing, ZHAO Jinrong et al. Progress of chitosan modification and application of modified chitosan[J]. Journal of Jinan University(Natural Science Edition), 2023, 37(1): 84-93(in Chinese). [31] MORAD M H. Current status and future perspectives of efficient catalytic conversion of bioethanol to value-added chemicals and fuels[J]. Arabian Journal of Chemistry, 2024, 17(2): 105560. doi: 10.1016/j.arabjc.2023.105560 [32] URAGAMI T, SAITO T, MIYATA T. Pervaporative dehydration characteristics of an ethanol/water azeotrope through various chitosan membranes[J]. Carbohydrate Polymers, 2015, 120: 1-6. doi: 10.1016/j.carbpol.2014.11.032 [33] CASTRO-MUNOZ R, GONZALEZ-VALDEZ J, AHMAD M Z. High-performance pervaporation chitosan-based membranes: new insights and perspectives[J]. Reviews in Chemical Engineering, 2020, 37(8): 959-974. [34] SANJARI A J, ASGHARI M. Review on Chitosan Utilization in Membrane Synthesis[J]. ChemBioEng Reviews, 2016, 3(3): 134-158. doi: 10.1002/cben.201500020 [35] XIAO X , YUSAK H , DARIA N , et al. High-performance ZIF-8/biopolymerchi tos an mixed-matrix pervaporation mem brane for methanol/dimethyl carbonate separation[J]. Separation and Purific ation Technology, 2022, 293: 1–12. [36] PAWEŁ G , ŁUKASZ J , PRZEMYSŁAW B , et al. Robust and high selective chitosan asymmetric Membranes: Relation between microporous structure and pervaporative efficiency in ethanol dehydration[J]. Separation and Purification Technology, 2022, 281: 1–11. [37] CHENG X, PAN F, WANG M, et al. Hybrid membranes for pervaporation separations[J]. Journal of Membrane Science, 2017, 541: 329-346. doi: 10.1016/j.memsci.2017.07.009 [38] POLYENES. Findings on Polyenes Reported by Investigators at Chang Gung University (Highly Zeolite-Loaded Polyvinyl Alcohol Composite Membranes for Alkaline Fuel-Cell Electrolytes)[J]. Journal of Technology & Science, 2018, 10(1): 278. [39] XU Z, LIU G, YE H, et al. Two-dimensional MXene incorporated chitosan mixed-matrix membranes for efficient solvent dehydration[J]. Journal of Membrane Science, 2018, 563: 625-632. doi: 10.1016/j.memsci.2018.05.044 [40] HUAYAN F, RUIWEN L, PANPAN L, et al. Mixed matrix membrane for enhanced Ethanol/Water pervaporation separation by incorporation of hydrophilic Zr-MOF NU-906 in chitosan[J]. Separation and Purification Technology, 2023, 318: 1239 85. [41] LIN Y F , HO J C , ANDREW LIN K Y , et al. A drying-free and one-step process for the preparation of siloxane/CS mixed-matrix membranes with outstanding ethanol dehydration performances[J]. Separation &Purification Technology, 2019, 221: 325-330. [42] ZHANG W , YU Z , QIAN Q , et al. Improving the pervaporation performance of the glutaraldehyde crosslinked chitosan membrane by simultaneously changing its surface and bulk structure[J]. Journal of Membrane Science, 2010, 348(1-2): 213-223. [43] CASTRO-MUÑ R, OZ, GALIANO F, et al. Recent advances in pervaporation hollow fiber membranes for dehydration of organics[J]. Chemical Engineering Research and Design, 2020, 164: 68-85. doi: 10.1016/j.cherd.2020.09.028 [44] DUDEK G , TURCZYN R . New type of alginate/chitosan microparticle membran es for highly efficient pervaporative dehydration of ethanol[J]. Rsc Advances, 2018, 8(69): 39567-39578. [45] VINU M , PAL S , CHEN J D , et al. Microporous 3D aluminum MOF doped into chitosan-based mixed matrix membranes for ethanol/water separation[J]. Journal of the Chinese Chemical Society, 2019, 66 (9): 1165-1171. [46] SANZ M T, GMEHLING J. Study of the Dehydration of Isopropanol by a Pervaporation-Based Hybrid Process[J]. Chemical Engineering & Technology: Industrial Chemistry-Plant Equipment -Process Engineering- Biotechnology, 2006, 29 (4): 473 -480. [47] SVANG-ARIYASKUL A , HUANG R , DOUGLAS P , et al. Blended chitosan and polyvinyl alcohol membranes for the pervaporation dehydration of isopropanol[J]. Journal of Membrane Science, 2006, 280(1-2): 815-823. [48] LANGARIS, SALJOUGHIE, MOUSAVI S M. Chitosan /polyvinylalcohol/ amino functionalized multiwalled carbon nanotube pervaporation membranes: Synthesis, characterization, and performance[J]. Polymers for Advanced Technologies, 2018, 29(1): 84-94. doi: 10.1002/pat.4091 [49] XIN-PING, WANG, YUN-FANG, et al. Pervaporation properties of a three-layer structure composite membrane[J]. Journal of Applied Polymer Science, 2000, 75(6): 740-745. doi: 10.1002/(SICI)1097-4628(20000207)75:6<740::AID-APP2>3.0.CO;2-3 [50] SAJJAN A M , PREMAKSHI H G , KARIDURAGANAVAR M Y. Synthesis and characterization of GTMAC grafted chitosan membranes for the dehydration of low water content isopropanol by pervaporation[J]. Journal of Industrial and Engineering Chemistry, 2015, 25: 151-161. [51] KITTUR A , KULKARNI S , ARALAGUPPI M , et al. Preparation and characterization of novel pervaporation membranes for the separation of water–isopropanol mixtures using chitosan and NaY zeolite[J]. Journal of Membrane Science, 2005, 247(1-2): 75-86. [52] KULKARNI, A S, SAJJAN, A M, KHAN T Y, et al. Development and Characterization of Biocompatible Membranes from Natural Chitosan and Gelatin for Pervaporative[J]. Polymers, 2021, 13(17): 2868. doi: 10.3390/polym13172868 [53] KARIDURAGANAVAR M Y, VARGHESE J G, CHOUDHARI S K , et al. Organic Inorganic Hybrid Membranes: Solving the Trade-off Phenomenon Between Permeation Flux and Selectivity in Pervaporation[J]. Industrial & engineering chemistry research, 2009, 48(8): 4002-4013. [54] MANGINDAAN D W, SHI G M, CHUNG T S. Pervaporation dehydration of acetone using P84co-polyimide flat sheet membranes modified by vapor phase crosslinking[J]. Membrane Science, 2014, 458: 76-85. doi: 10.1016/j.memsci.2014.01.030 [55] YEANG W Q , ZEIN S H S , SULONG B A , et al. Comparison of the pervaporation performance of various types of carbon nanotube-based nanocomposites in the dehydration of acetone[J]. Separation and Purification Technology, 2013, 107252-107263. [56] GAO C, ZHANG M, JIANG Z, et al. Preparation of a highly water-selective membrane for dehydration of acetone by incorporating potassium montmorillonite to construct ionized water channel[J]. Chemical Engineering Science, 2015, 135: 461-471. doi: 10.1016/j.ces.2014.12.044 [57] GAO C , ZHANG M , PAN F , et al. Pervaporation dehydration of an acetone/water mixture by hybrid membranes incorporated with sulfonated carbon molecular sieves[J]. RSC Advances, 2016, 6(60): 552 72-55281. [58] DAS P , RAY K S . Separation of acetonewater mixtures by pervaporation using NaMMT-loaded PVA-chitosan nanocomposite IPN membranes[J]. Polymer Engineering and Science, 2024, 64(1): 31-51. [59] 秦玉武, 房德仁, 刘丽花, 等. 叔丁醇下游产品开发及应用进展[J]. 工业催化, 2013, 21(5): 17-22. doi: 10.3969/j.issn.1008-1143.2013.05.004QIN Yuwu, FANG Deren, LIU Lihua, et al. Progress of downstream product development and application of tert-butanol[J]. Industrial Catalysis, 2013, 21(5): 17-22(in Chinese). doi: 10.3969/j.issn.1008-1143.2013.05.004 [60] ACHARI D D , HEGDE S N , PATTANASHETTI N A , et al. Development of zeolite-A incorporated PVA/CS nanofibrous composite mem branes using the electrospinning technique for pervaporation dehydration of water/tert-butanol[J]. New Journal of Chemistry, 2021, 45(8): 3981-3996. [61] ACHARI D , RACHIPUDI P , NAIKS , et al. Polyelectrolyte complex membranes made of chitosan–PSSAMA for pervaporation separation of industrially important azeotropic mixtures[J]. Journal of Industrial and Engineering Chemistry, 2019, 78: 383-395. [62] EMMANUEL O, EZEJI T C. Exploring the synergy of nanomaterials and microbial cell factories during biohydrogen and biobutanol production from different carbon sources[J]. Sustainable Chemistry for the Environment, 2024, 6: 100098. doi: 10.1016/j.scenv.2024.100098 [63] LIN Y F, WU C Y, LIU T Y , et al. Synthesis of mesoporous SiO2 xerogel/ chitosan mixed-matrix membranes for butanol dehydration[J]. Journal of industrial and engineering chemistry, 2018, 57: 297-303. [64] HUI T, HUANG Z, TING Q, et al. Reactive distillation for producing n-butyl acetate: Experiment and simulation[J]. Chinese Journal of Chemical Engineering, 2012, 20(5): 980-987. doi: 10.1016/S1004-9541(12)60426-1 [65] ZHANG S , ZOU Y , WEI T , et al. Pervaporation dehydration of binary and ternary mixtures of n-butyl acetate, n-butanol and water using PVA-CS blended membranes[J]. Separation and Purification Technology, 2017, 173: 314-322. [66] ZHANG M, XU Q, LIU C, et al. Application of a biodegradable poly (butylene adipate-co-terephthalate) mem brane for phenol pervaporation recovery[J]. Physical Chemistry Chemical Physics, 2023, 25(26): 17657-17666. doi: 10.1039/D3CP01783D [67] 刘明. Pt/Al2O3气相苯加氢催化剂的研制[J]. 化学工业与工程技术, 2008, (2): 7-10.LIU Ming. Development of Pt/Al2O3 gas-phase benzene hydrogenation catalyst[J]. Chemical Industry and Engineering Technology, 2008, (2): 7-10(in Chinese). [68] LIANYU L, FUBING P, ZHONGYI J, et al. Poly(vinyl alcohol)/chitosan blend membranes for pervaporation of benzene/cyclohexane mixtures[J]. Journal of Applied Polymer Science, 2006, 101(1): 167-173. doi: 10.1002/app.23158 [69] LU L , SUN H , PENG F , et al. Novel graphite-filled PVA/CS hybrid membrane for pervaporation of benzene/cyclohexane mixtures[J]. Journal of Membrane Science, 2006, 281(1-2): 245-252. [70] SHEN J N , CHU Y X , RUAN H M , et al. Pervaporation of benzene/ cyclohexane mixtures through mixed matrix membranes of chitosan and Ag+/carbon nanotubes[J]. Journal of Membrane Science, 2014, 462: 160-169. [71] ZHANG F, LIU P, ZHANG K, et al. Chemical Adsorption Strategy for DMC-MeOH Mixture Separation[J]. Molecules, 2021, 26(6): 1735. doi: 10.3390/molecules26061735 [72] CHEN, HUA J, DONG , et al. Investigation into glutaraldehyde crosslinked chitosan/ cardo-poly-etherketone compo site membrane for pervaporation separation of methanol and dimethyl carbonate mixtures[J]. RSC Advances, 2016, 6(65): 60765-60772. [73] ZHU H , LI R , LIU G , et al. Efficient separation of methanol/dimethyl carbonate mixtures by UiO-66 MOF incur porated chitosan mixed-matrix membrane[J]. Journal of Membrane Science, 2022, 652: 120473. [74] BURKE D E, WILLIAMS G C, PLANK C A. Vapor-Liquid Equilibria for the Methanol-Toluene System[J]. Journal of Chemical & Engineering Data, 1964, 9(2): 212-214. [75] ALLEL A, BENGUERGOURA H, NACEUR M W, et al. Poly (styrene-co-butadiene)/Maghnia-Organo-Montmorill onite Clay Nanocomposite. Preparation, Properties and Application as Membrane in the Separation of Methanol/Toluene Azeotropic Mixture by Pervaporation[J]. Membranes, 2021, 11(12): 921. doi: 10.3390/membranes11120921 [76] MOULIK S , BUKKE V , SAJJA C S , et al. Chitosan-polytetrafluoroethylene com posite membranes for separation of methanol and toluene by pervaporation[J]. Carbohydrate Polymers, 2018, 193: 28-38. -

 点击查看大图
点击查看大图
计量
- 文章访问数: 69
- HTML全文浏览量: 66
- 被引次数: 0




 下载:
下载: