One-step preparation of self-imaging Fe3O4@chitosan drug-loaded embolic microspheres by electrostatic spraying
-
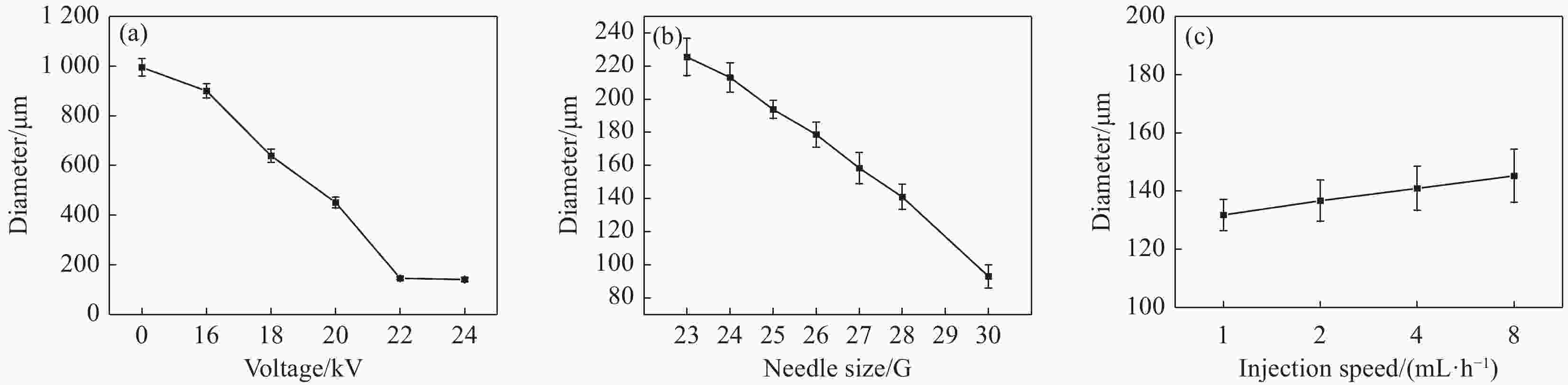
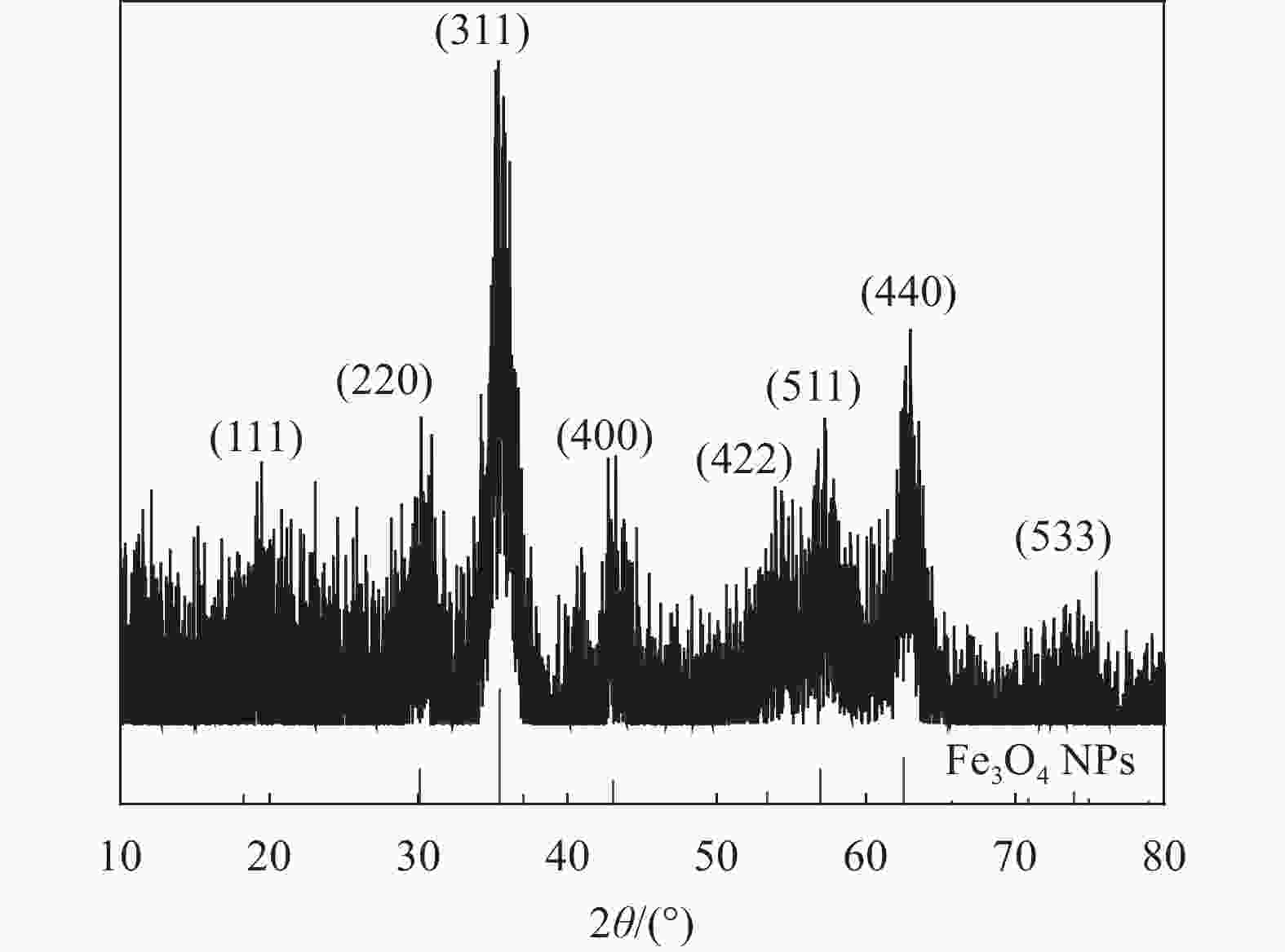
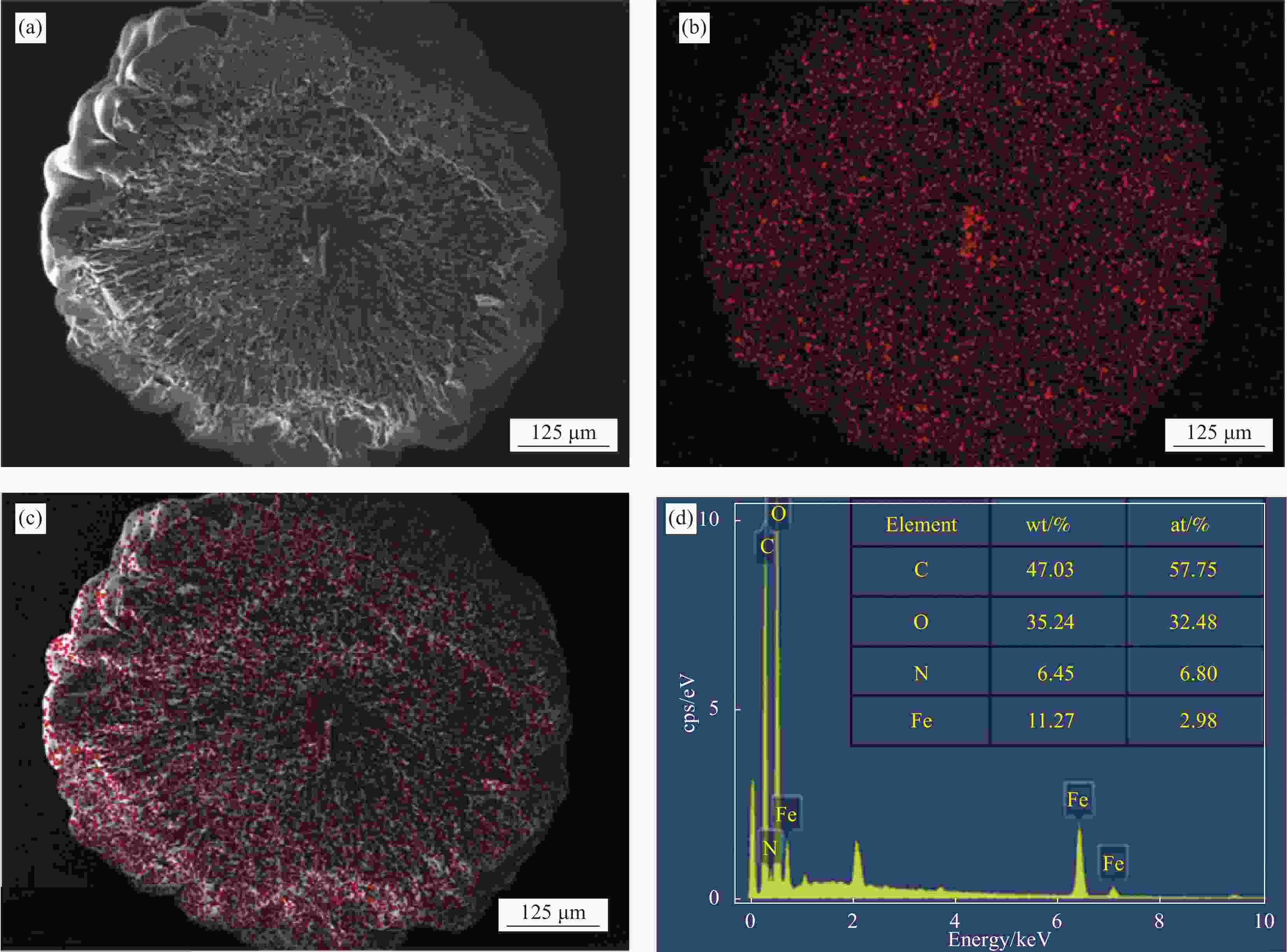
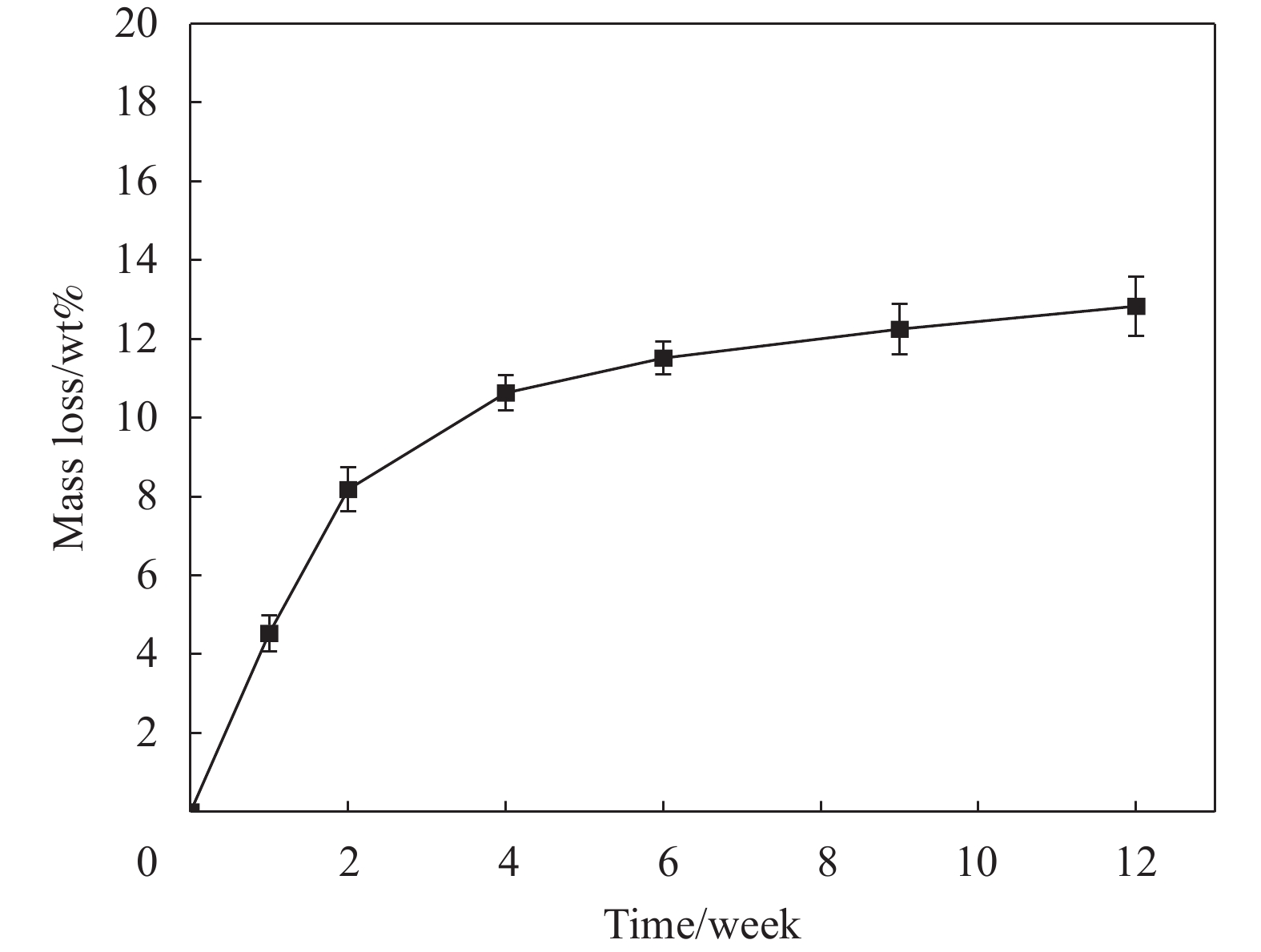
摘要: 采用静电喷雾法一步制备包裹着Fe3O4纳米粒子的壳聚糖复合微球(Fe3O4@CS微球),实现Fe3O4纳米粒子与微球同时合成。还可以按需制备粒径范围为90~1000 μm的Fe3O4@CS微球,以满足不同部位血管的临床栓塞要求。SEM显示微球形貌均匀且粒径分布均一((94±3) μm),体外降解实验证明了微球具有生物可降解性,磁共振成像测试表明所制备的Fe3O4@CS微球具有良好的临床成像能力,血液、细胞相容性评估证实Fe3O4@CS微球具有良好的生物相容性。负载盐酸阿霉素(DOX)的载药微球显示出典型的药物缓释曲线,72 h内DOX的累计释放率为28.82%。结果表明,这一步可控制备的自显影栓塞剂在经导管动脉栓塞术(TACE)未来应用中展示了巨大的潜力。Abstract: Chitosan composite microspheres encapsulating Fe3O4 nanoparticles (Fe3O4@CS microspheres) were prepared in one step by the electrostatic spraying, enabling simultaneous synthesis of Fe3O4 nanoparticles and microspheres. Additionally, Fe3O4@CS microspheres with particle sizes ranging from 90 to 1000 μm could be prepared on demand to meet the clinical embolization requirements of different blood vessels. SEM images showed that the microspheres were homogeneous in shape and uniform in size distribution ((94±3) μm). In vitro degradation experiments proved that the microspheres were biodegradable. Magnetic resonance imaging showed that the prepared Fe3O4@CS microspheres had excellent clinical imaging capabilities. Moreover, blood and cytocompatibility assessments confirmed the good biocompatibility of Fe3O4@CS microspheres. The drug-loaded microspheres loaded with doxorubicin hydrochloride (DOX) showed a typical drug sustained release curve, and the cumulative release rate of DOX was 28.82% within 72 h. These results demonstrate that one-step controllable preparation of self-imaging embolic agents show great potential for future applications in transcatheter arterial embolization (TACE).
-
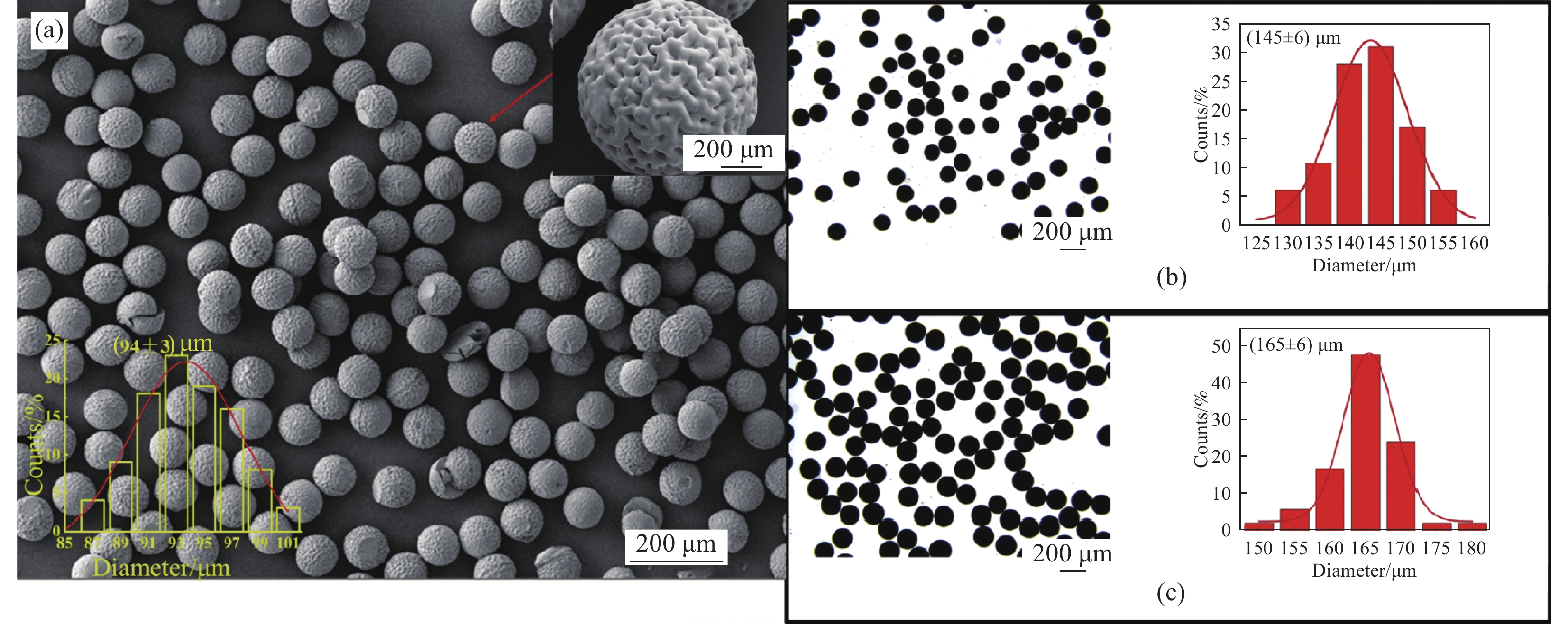
图 1 (a) Fe3O4@壳聚糖(CS)微球的SEM图像及粒径分布直方图;((b), (c)) 微球溶胀前后的光学显微镜图及粒径分布直方图
Figure 1. (a) SEM image and histogram of particle size distribution of Fe3O4@chitosan (CS) microspheres; ((b), (c)) Optical microscope images of Fe3O4@CS microspheres before and after swelling and particle size distribution histograms
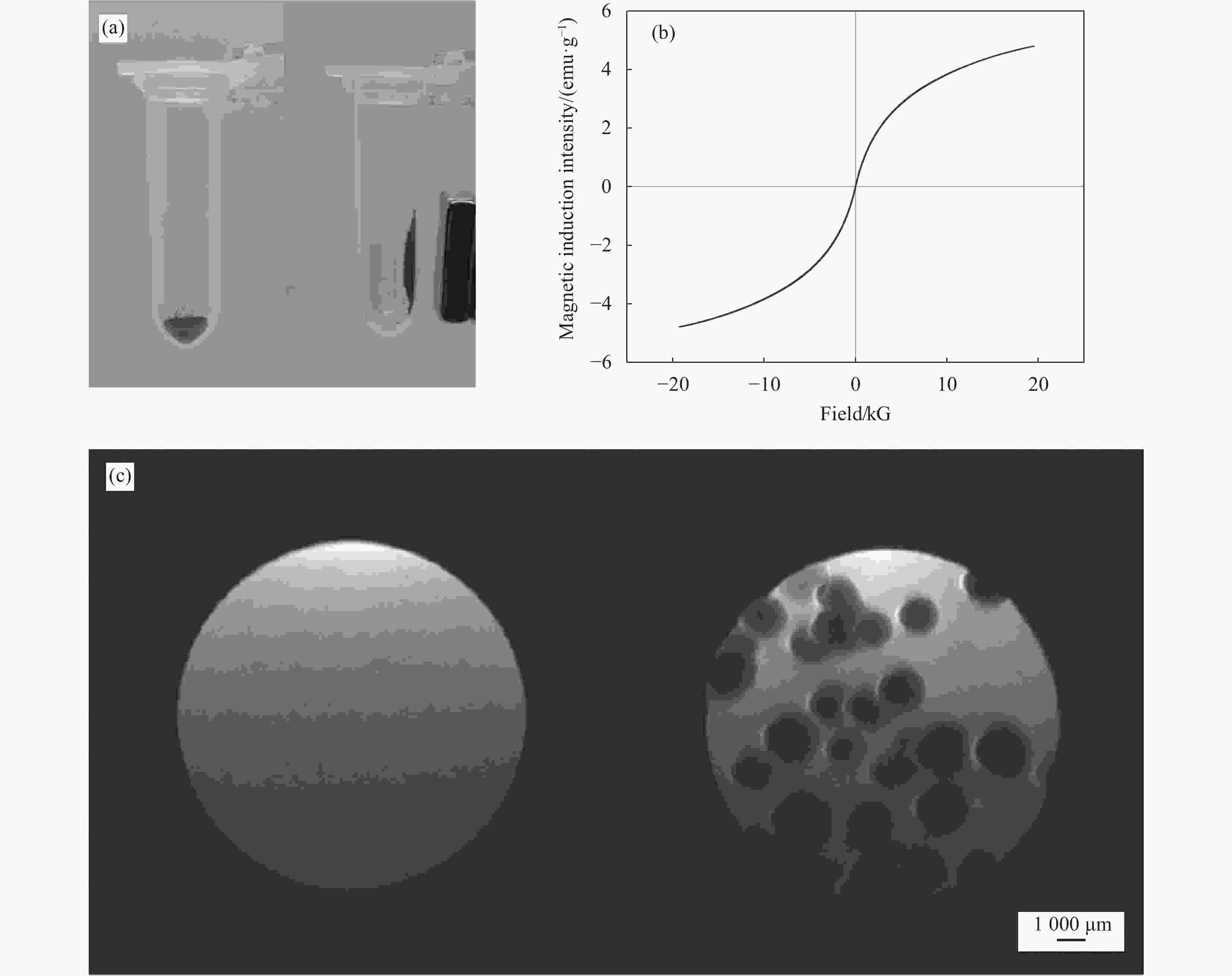
图 6 (a) Fe3O4@CS微球在磁场存在下的迁移图;(b) Fe3O4@CS微球的磁滞曲线图;(c) Fe3O4@CS微球体外模型的T2加权核磁共振成像(MRI)图像
Figure 6. (a) Migration diagram of Fe3O4@CS microspheres in the presence of a magnetic field; (b) Hysteresis curve diagram of Fe3O4@CS microspheres; (c) T2-weighted magnetic resonance imaging (MRI) images of Fe3O4@CS microspheres in a vitro model
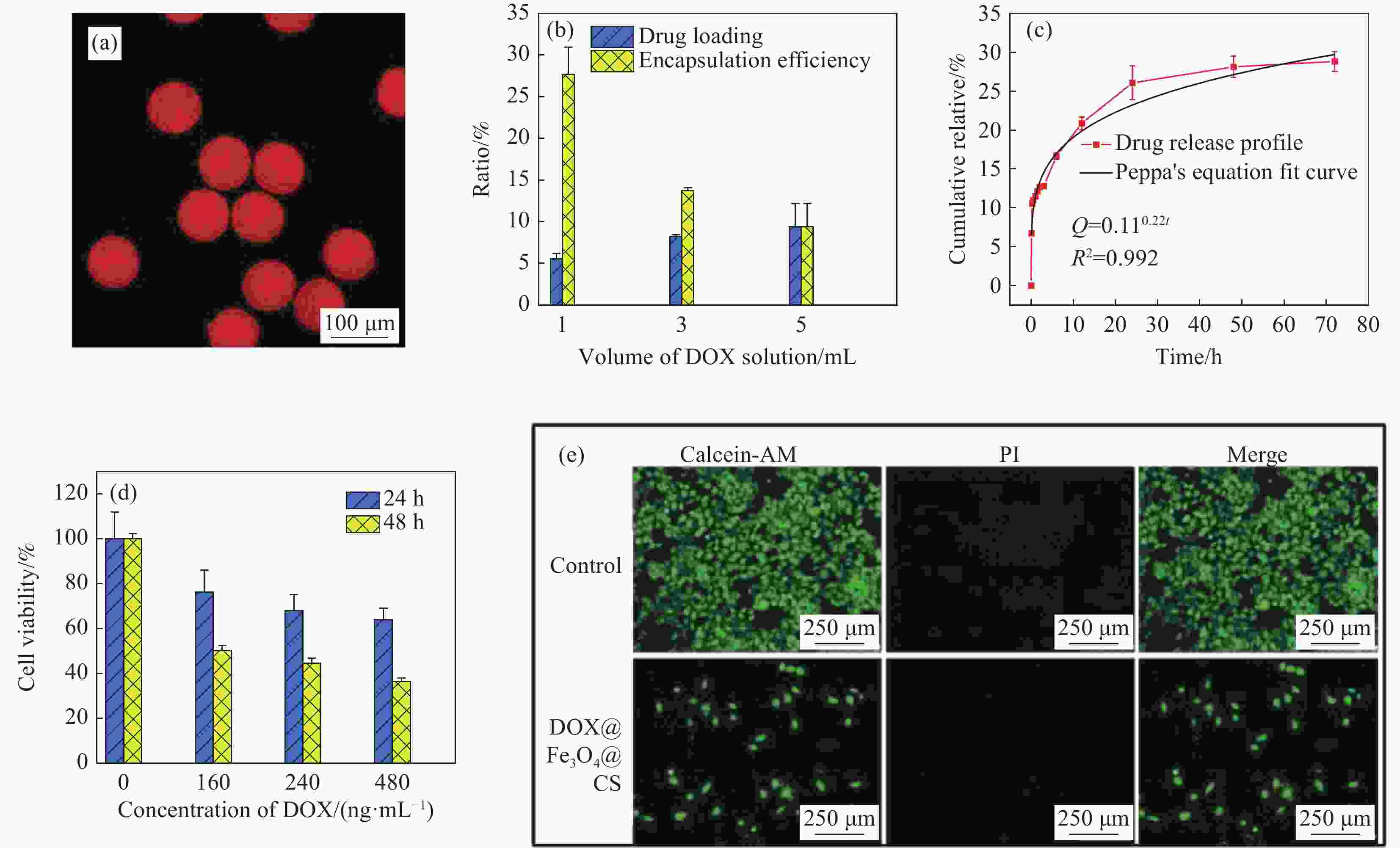
图 9 (a) 载药Fe3O4@CS微球的荧光成像图;(b) 载药Fe3O4@CS微球的载药率;(c) 载药Fe3O4@CS微球的药物体外释放;(d) 载药Fe3O4@CS微球孵育HepG2细胞24 h和48 h的细胞活力;(e) HepG2癌细胞的活/死染色荧光成像测定
Figure 9. (a) Fluorescence imaging of drug-loaded Fe3O4@CS microspheres; (b) Drug-loading capacities; (c) Drug release in vitro of drug-loaded Fe3O4@CS microspheres; (d) Cell viability of HepG2 cells incubating with drug-loaded Fe3O4@CS microspheres at 24 h and 48 h; (e) Live/dead staining fluorescence imaging assay of HepG2 cancer cells
DOX—Doxorubicin hydrochloride; AM—Calcein-AM; PI—Propidium iodide; Q—Diffusion flux; R2—Correlation coefficient
-
[1] ZHAO X T, HUANG W Q, LI X F, et al. One-step preparation of photoclick method for embolic microsphere synthesis and assessment for transcatheter arterial embolization[J]. European Journal of Pharmaceutics and Biopharmaceutics,2021,166:94-102. doi: 10.1016/j.ejpb.2021.06.002 [2] SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA: A Cancer Journal for Clinicians,2021,71(3):209-249. doi: 10.3322/caac.21660 [3] CHOI H, CHOI B, YU B, et al. On-demand degradable embolic microspheres for immediate restoration of blood flow during image-guided embolization procedures[J]. Biomaterials,2021,265:120408. doi: 10.1016/j.biomaterials.2020.120408 [4] LIU L, LIANG X X, XU X X, et al. Magnetic mesoporous embolic microspheres in transcatheter arterial chemoembolization for liver cancer[J]. Acta Biomaterialia,2021,130:374-384. doi: 10.1016/j.actbio.2021.05.031 [5] HSU M Y, HUANG Y T, WENG C J, et al. Preparation and in vitro/in vivo evaluation of doxorubicin-loaded poly [lactic-co-glycol acid] microspheres using electrospray method for sustained drug delivery and potential intratumoral injection[J]. Colloids and Surfaces B: Biointerfaces,2020,190:110937. doi: 10.1016/j.colsurfb.2020.110937 [6] YI Z H, SUN Z C, SHEN Y, et al. The sodium hyaluronate microspheres fabricated by solution drying for transcatheter arterial embolization[J]. Journal of Materials Chemistry B,2022,10(21):4105-4114. doi: 10.1039/D2TB00413E [7] LENG F, LEI S, LUO B, et al. Size-tunable and biodegradable thrombin-functionalized carboxymethyl chitin microspheres for endovascular embolization[J]. Carbohydrate Polymers,2022,286:119274. doi: 10.1016/j.carbpol.2022.119274 [8] CHEN M J, SHU G F, LYU X L, et al. HIF-2α-targeted interventional chemoembolization multifunctional microspheres for effective elimination of hepatocellular carcinoma[J]. Biomaterials,2022,284:121512. doi: 10.1016/j.biomaterials.2022.121512 [9] LIU K L, JIN Z C, HU X L, et al. A biodegradable multifunctional porous microsphere composed of carrageenan for promoting imageable trans-arterial chemoembolization[J]. International Journal of Biological Macromolecules,2020,142:866-878. doi: 10.1016/j.ijbiomac.2019.10.026 [10] LI J J, WANG J H, LI J Y, et al. Fabrication of Fe3O4@PVA microspheres by one-step electrospray for magnetic resonance imaging during transcatheter arterial embolization[J]. Acta Biomaterialia,2021,131:532-543. doi: 10.1016/j.actbio.2021.07.006 [11] DOUCET J, KIRI L, O’CONNELL K, et al. Advances in degradable embolic microspheres: A state of the art review[J]. Journal of Functional Biomaterials,2018,9(1):14. doi: 10.3390/jfb9010014 [12] FUCHS K, DURAN R, DENYS A, et al. Drug-eluting embolic microspheres for local drug delivery—State of the art[J]. Journal of Controlled Release,2017,262:127-138. doi: 10.1016/j.jconrel.2017.07.016 [13] WEI C X, WU C W, JIN X, et al. CT/MR detectable magnetic microspheres for self-regulating temperature hyperthermia and transcatheter arterial chemoembolization[J]. Acta Biomaterialia,2022,153:453-464. doi: 10.1016/j.actbio.2022.09.054 [14] LI X H, JI X F, CHEN K, et al. Immobilized thrombin on X-ray radiopaque polyvinyl alcohol/chitosan embolic microspheres for precise localization and topical blood coagulation[J]. Bioactive Materials,2021,6(7):2105-2119. doi: 10.1016/j.bioactmat.2020.12.013 [15] JIA G, VAN VALKENBURGH J, CHEN A Z, et al. Recent advances and applications of microspheres and nanoparticles in transarterial chemoembolization for hepatocellular carcinoma[J]. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology,2022,14(2):e1749. [16] D’ABADIE P, HESSE M, LOUPPE A, et al. Microspheres used in liver radioembolization: From conception to clinical effects[J]. Molecules,2021,26(13):3966. doi: 10.3390/molecules26133966 [17] TALAIE R, TORKIAN P, AMILI O, et al. Particle distribution in embolotherapy, how do they get there? A critical review of the factors affecting arterial distribution of embolic particles[J]. Annals of Biomedical Engineering,2022,50(8):885-897. doi: 10.1007/s10439-022-02965-6 [18] KALANTARI K, AFIFI A M, JAHANGIRIAN H, et al. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review[J]. Carbohydrate Polymers,2019,207:588-600. doi: 10.1016/j.carbpol.2018.12.011 [19] 许浩, 魏延, 徐双梦, 等. 基于壳聚糖/β-甘油磷酸钠(CS/β-GP)温敏型水凝胶的细胞表面壳化及对癌细胞行为的影响研究[J]. 功能材料, 2021, 52(5):5121-5126.XU Hao, WEI Yan, XU Shuangmeng, et al. Cell surface shellization with chitosan/β-glycerol phosphate sodium (CS/β-GP) temperature-sensitive hydrogel and its effect on cancer cell behavior[J]. Journal of Functional Materials,2021,52(5):5121-5126(in Chinese). [20] REN S, SONG L, TIAN Y, et al. Emodin-conjugated PEGylation of Fe3O4 nanoparticles for FI/MRI dual-modal imaging and therapy in pancreatic cancer[J]. International Journal of Nanomedicine,2022,17:711-712. doi: 10.2147/IJN.S361728 [21] 王旭东, 吴鹏, 金淑萍, 等. 原位共沉淀法制备载药Fe3O4/壳聚糖磁性复合微球及其体外药物释放行为[J]. 复合材料学报, 2014, 31(1):158-165. doi: 10.3969/j.issn.1000-3851.2014.01.023WANG Xudong, WU Peng, JIN Shuping, et al. Preparation of Fe3O4/chitosan magnetic composite particle loading drug by co-precipitation in situ and its release behavior in vitro[J]. Acta Materiae Compositae Sinica,2014,31(1):158-165(in Chinese). doi: 10.3969/j.issn.1000-3851.2014.01.023 [22] XIE W S, GUO Z H, GAO F, et al. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics[J]. Theranostics,2018,8(12):3284. doi: 10.7150/thno.25220 [23] MA X H, WANG S A, HU L B, et al. Imaging characteristics of USPIO nanoparticles (<5 nm) as MR contrast agent in vitro and in the liver of rats[J]. Contrast Media & Molecular Imaging, 2019, 2019: 3687537. [24] PÉREZ-LÓPEZ A, MARTÍN-SABROSO C, GÓMEZ-LÁZARO L, et al. Embolization therapy with microspheres for the treatment of liver cancer: State-of-the-art of clinical translation[J]. Acta Biomaterialia,2022,149:1-15. doi: 10.1016/j.actbio.2022.07.019 [25] CAINE M, CARUGO D, ZHANG X, et al. Review of the development of methods for characterization of microspheres for use in embolotherapy: Translating bench to cathlab[J]. Advanced Healthcare Materials,2017,6(9):1601291. doi: 10.1002/adhm.201601291 -





 下载:
下载: