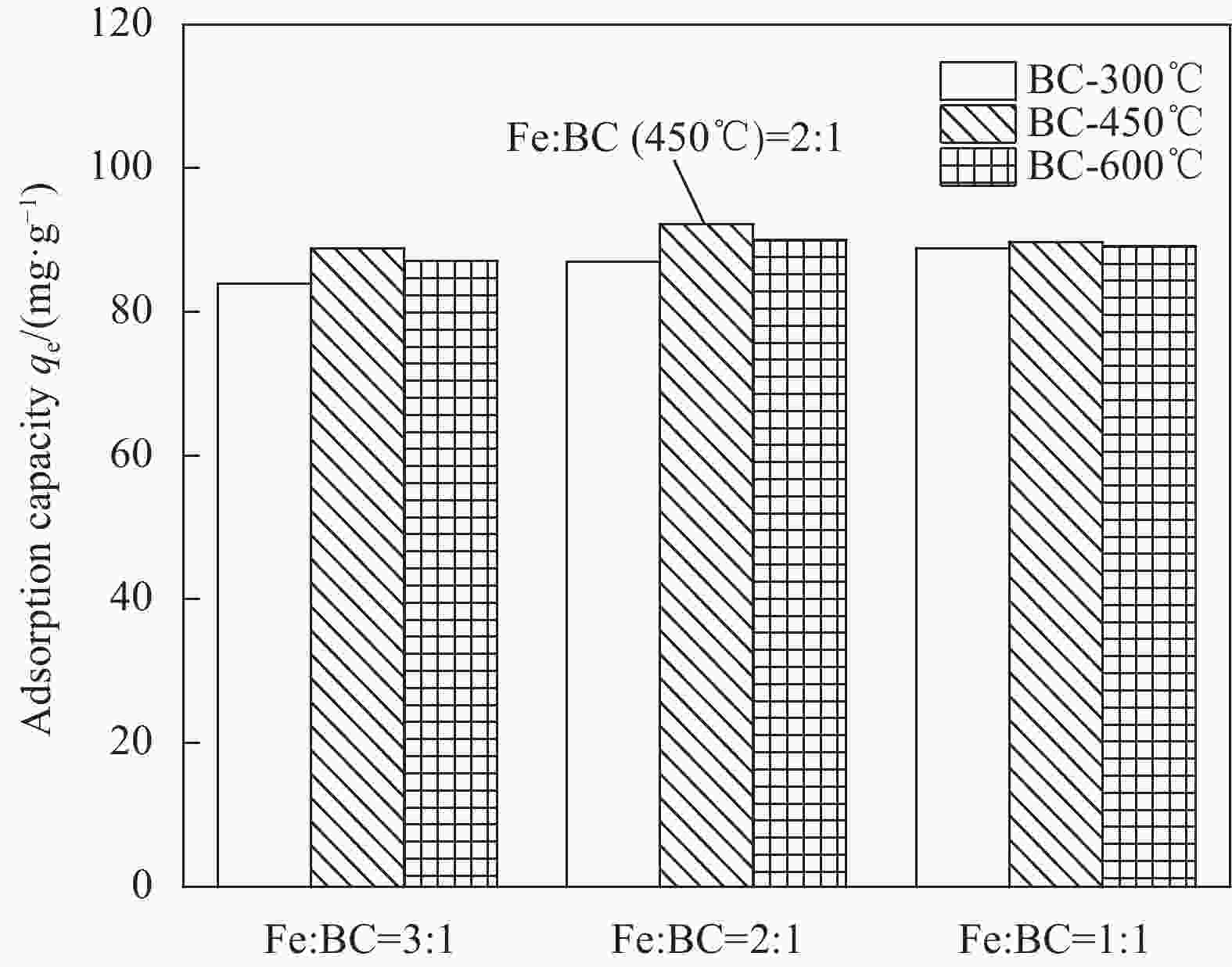
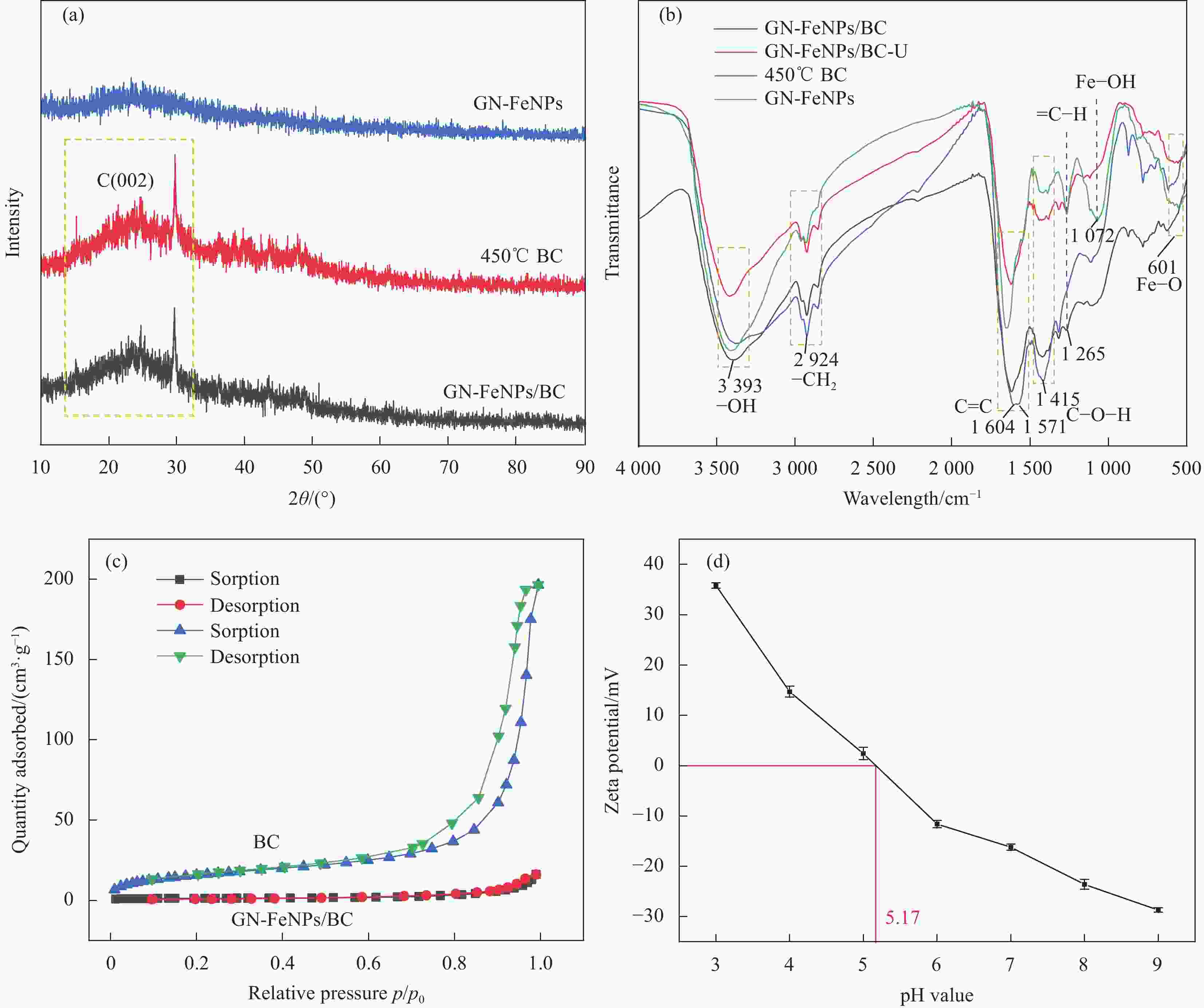
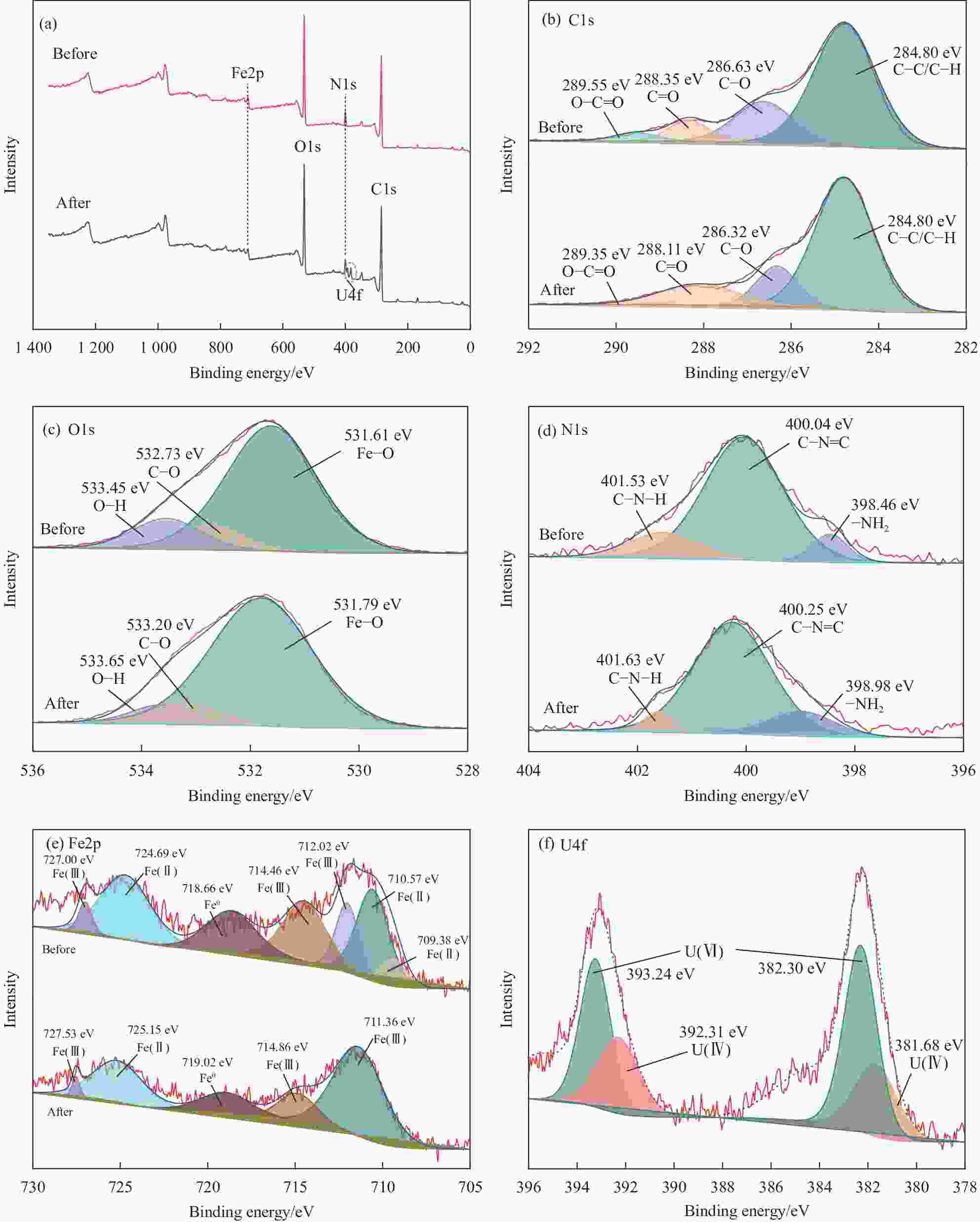
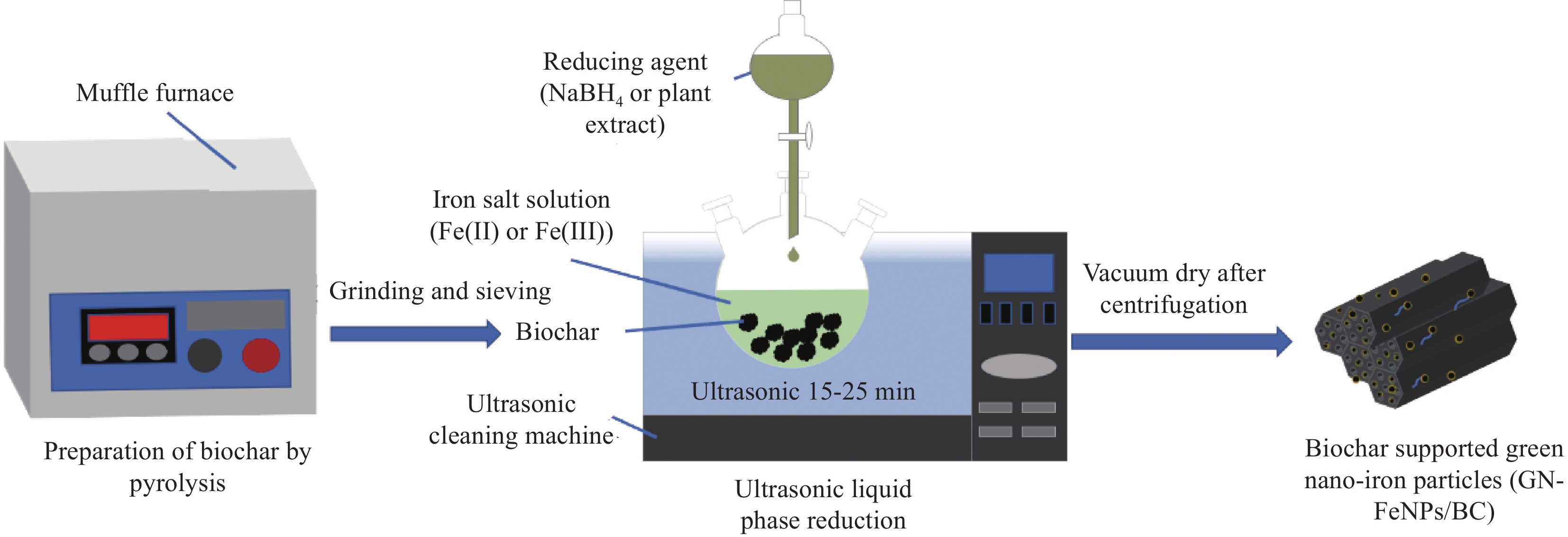
Biochar supported green nano-iron particles to remove U(VI) from water
-
摘要: 铀矿开采与水冶过程产生大量铀废水,对周边生态环境造成污染,因此高效绿色治理是保障核工业可持续发展及生态安全的重要基础。本文以向日葵叶为原料绿色合成生物炭负载纳米铁颗粒(GN-FeNPs/BC),并用于去除水中的U(VI)。利用向日葵叶制备植物提取液,然后将残渣热解制备成生物炭,最后将七水硫酸亚铁溶液、生物炭和植物提取液混合,成功制备出绿色纳米铁复合材料。探究了生物炭碳化温度、铁碳比、pH值、温度、时间和U(VI)浓度对除铀的影响。在298 K、pH=5时,最大吸附量为96.43 mg·g−1,并进行动力学和热力学研究。结果表明,准二级动力学模型和Langmuir等温吸附模型拟合良好。热力学常数表明GN-FeNPs/BC对U(VI)的吸附是一个自发吸热的过程。XPS分析表明去除机制包括吸附作用和还原作用。Abstract: Uranium mining and hydrometallurgy produce a large amount of uranium wastewater, which causes pollution to the surrounding ecological environment. Therefore, efficient and green treatment is an important basis to guarantee the sustainable development and ecological security of nuclear industry. In this study, sunflower leaves were used as raw materials for green synthesis of biochar-loaded nano-iron particles (GN-FeNPs/BC) that used to remove U(VI) in water. Sunflower leaves were used to prepare plant extract, and then the residue was pyrolyzed to prepare biochar. Finally, ferrous sulfate heptahydrate solution, biochar and plant extract were mixed to successfully prepare a green nano-iron composite material. The effects of biochar carbonization temperature, iron-to-carbon ratio, pH value, temperature, time and U(VI) concentration on uranium removal were explored. When the pH is 5 at 298 K, the maximum adsorption capacity is 96.43 mg·g−1. The kinetics and thermodynamics are studied. The results show that the pseudo-second order model and Langmuir model fit well. The thermodynamic constants indicate that the adsorption of U(VI) by GN-FeNPs/BC is a spontaneous endothermic process. XPS analysis shows that the removal mechanism includes adsorption and reduction.
-
Key words:
- sunflower leaves /
- biochar /
- nano-iron particles /
- uranium /
- adsorption /
- reduction
-
图 3 450℃ BC (a)、GN-FeNPs (b)、GN-FeNPs/BC (c) 和GN-FeNPs/BC吸附铀后 (d) 的SEM图像;GN-FeNPs/BC吸附铀反应前 (e) 和反应后 (f) 的EDS图谱
Figure 3. SEM images of 450℃ BC (a), GN-FeNPs (b), GN-FeNPs/BC (c) and GN-FeNPs/BC-U (d) after uranium adsorption; EDS spectra of GN-FeNPs/BC before uranium adsorption reaction (e) and after reaction (f)
图 5 GN-FeNPs/BC吸附U(Ⅵ)的影响因素及动力学研究:(a) pH值的影响(U(Ⅵ)的初始浓度(C0)=10 mg/L);(b) 时间的影响(pH=5.0、 GN-FeNPs/BC的质量(m)/U(Ⅵ)的体积(V)=0.1 g/L、温度(T)=298 K);(c) 拟一级动力学;(d) 拟二级动力学
Figure 5. Study on influencing factors and kinetics of U(Ⅵ) adsorption by GN-FeNPs/BC: (a) Influence of pH value (Initial concentration of U(Ⅵ) (C0)=10 mg/L); (b) Influence of time (pH=5.0, mass of GN-FeNPs/BC (m)/volume of U(Ⅵ) (V)=0.1 g/L, temperature (T)=298 K); (c) Pseudo-first model; (d) Pseudo-second model
qe—Adsorption capacity at equilibrium; qt—Adsorption capacity at time t; t—Reaction time
图 6 (a) GN-FeNPs/BC的吸附等温线(pH=5、m/V=0.1 g/L);(b) Langmuir等温线;(c) Freundlich等温线;(d) ln(qe/Ce) vs qe线性拟合;(e) lnK0 vs 1/T线性关系图(K0为分配系数);(f) GN-FeNPs/BC吸附-解吸次数对U(Ⅵ)去除率的影响(pH=5、m/V=0.1 g/L、T=298 K、C0=10 mg/L)
Figure 6. (a) Adsorption isotherm of GN-FeNPs/BC (pH=5, m/V=0.1 g/L); (b) Langmuir isotherm; (c) Freundlich isotherm; (d) ln(qe/Ce) ) vs qe linear fitting; (e) lnK0 vs 1/T linear relationship diagram (K0 is the distribution coefficient); (f) Influence of GN-FeNPs/BC adsorption-desorption times on U(Ⅵ) removal rate (pH=5, m/V=0.1 g/L, T=298 K, C0=10 mg/L)
表 1 GN-FeNPs/BC吸附U(Ⅵ)的动力学常数
Table 1. Kinetic constants of U(Ⅵ) adsorption by GN-FeNPs/BC
Adsorbent Pseudo-first order model Pseudo-second order model qe/(mg·g−1) k1/(min−1) R2 qe/(mg·g−1) k2/(g·mg−1·min−1) R2 GN-FeNPs/BC 95.495 0.073 0.869 101.644 0.0011 0.999 Notes: qe—Amount of adsorption at equilibrium; k1—Quasi-first-order kinetic model constant; k2—Quasi-second-order kinetic model constant; R—Correlation coefficient. 表 2 Langmuir和Freundlich等温吸附模型拟合参数
Table 2. Fitting parameters of Langmuir and Freundlich isotherm adsorption models
T/K Langmuir model Freundlich model qm/(mg·g−1) KL/(L·mg−1) R2 n KF/(mg1-n·Ln·g−1) R2 298 214.639 0.093 0.999 1.314 19.562 0.892 308 194.140 0.135 0.984 1.374 24.069 0.929 318 231.252 0.139 0.935 1.299 28.452 0.908 Notes: T—Absolute temperature; qm—Maximum adsorption capacity; KL—Langmuir coefficient related to binding site affinity; KF and n—Constants related to the adsorption strength and adsorption capacity in the Freundlich model. 表 3 GN-FeNPs/BC吸附U(Ⅵ)的热力学参数
Table 3. Thermodynamic parameters of U(Ⅵ) adsorption by GN-FeNPs/BC
T/K ΔG/(kJ·mol−1) ΔH/(kJ·mol−1) ΔS/(J·mol−1·K−1) 298 −2.72 308 −3.04 5.45 27.47 318 −3.27 Notes: ΔH—Enthalpy; ΔS—Entropy; ΔG—Gibbs free energy. -
[1] LIU B, PENG T J, SUN H J, et al. Release behavior of uranium in uranium mill tailings under environmental conditions[J]. Journal of Environmental Radioactivity,2017,171:160-168. doi: 10.1016/j.jenvrad.2017.02.016 [2] DING L, TAN W F, XIE S B, et al. Uranium adsorption and subsequent re-oxidation under aerobic conditions by Leifsonia sp-Coated biochar as green trapping agent[J]. Envi-ronmental Pollution,2018,242:778-787. doi: 10.1016/j.envpol.2018.07.050 [3] LIU B, PENG T J, SUN H J, et al. Mobility and risk assessment of uranium and associated heavy metals in uranium mill Tailings[J]. Journal of Nanoscience and Nanotechnology,2017,17(9):6746-6753. doi: 10.1166/jnn.2017.14449 [4] ZHANG Q, ZHAO D L, FENG S J, et al. Synthesis of nanoscale zero-valent iron loaded chitosan for synergistically enhanced removal of U(VI) based on adsorption and reduction[J]. Journal of Colloid and Interface Science,2019,552:735-743. doi: 10.1016/j.jcis.2019.05.109 [5] JIN J, LI S W, PENG X Q, et al. HNO3 modified biochars for uranium(VI) removal from aqueous solution[J]. Bioresource Technology,2018,256:247-253. doi: 10.1016/j.biortech.2018.02.022 [6] KLUEPFEL L, KEILUWEIT M, KLEBER M, et al. Redox pro-perties of plant biomass-derived black carbon (Biochar)[J]. Environmental Science & Technology,2014,48(10):5601-5611. [7] WANG S S, ZHAO M Y, ZHOU M, et al. Biochar-supported nZVI(nZVI/BC) for contaminant removal from soil and water: A critical review[J]. Journal of Hazardous Materials,2019,373:820-834. doi: 10.1016/j.jhazmat.2019.03.080 [8] 姜记威, 张诗轩, 曾文炉, 等. 生物炭基材料在抗生素废水处理中的研究进展[J]. 化工进展, 2021, 40(S2):389-401.JIANG Jiwei, ZHANG Shixuan, ZENG Wenlu, et al. Research progress on biochar-based materials for the treatment of antibiotic wastewater[J]. Chemical Industry and Engineering Progress,2021,40(S2):389-401(in Chinese). [9] DIAO Z H, DU J J, JIANG D, et al. Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: Coexistence effect and mechanism[J]. Science of the Total Environment,2018,642:505-515. doi: 10.1016/j.scitotenv.2018.06.093 [10] YANG F, ZHANG S, SUN Y, et al. Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal[J]. Bioresource Technology,2018,265:490-497. doi: 10.1016/j.biortech.2018.06.029 [11] CHEN L, YANG J Y, WANG D. Phytoremediation of uranium and cadmium contaminated soils by sunflower (Helianthus annuus L. ) enhanced with biodegradable chelating agents[J]. Journal of Cleaner Production,2020,263:121491. doi: 10.1016/j.jclepro.2020.121491 [12] CHEN L, HU W F, LONG C, et al. Exogenous plant growth regulator alleviate the adverse effects of U and Cd stress in sunflower (Helianthus annuus L. ) and improve the efficacy of U and Cd remediation[J]. Chemosphere,2021,262:127809. doi: 10.1016/j.chemosphere.2020.127809 [13] CHEN A W, SHANG C, SHAO J H, et al. The application of iron-based technologies in uranium remediation: A review[J]. Science of the Total Environment,2017,575:1291-1306. doi: 10.1016/j.scitotenv.2016.09.211 [14] TSAREV S, COLLINS R N, ILTON E S, et al. The short-term reduction of uranium by nanoscale zero-valent iron(nZVI): Role of oxide shell, reduction mechanism and the formation of U(V)-carbonate phases[J]. Environmental Science: Nano,2017,4(6):1304-1313. doi: 10.1039/C7EN00024C [15] LI J, ZHANG L B, PENG J H, et al. Removal of uranium from uranium plant wastewater using zero-valent iron in an ultrasonic field[J]. Nuclear Engineering and Technology,2016,48(3):744-750. doi: 10.1016/j.net.2016.01.021 [16] WANG Y N, O'CONNOR D, SHEN Z T, et al. Green synthesis of nanoparticles for the remediation of contaminated waters and soils: Constituents, synthesizing methods, and influencing factors[J]. Journal of Cleaner Production,2019,226:540-549. doi: 10.1016/j.jclepro.2019.04.128 [17] MACHADO S, PINTO S L, GROSSO J P, et al. Green production of zero-valent iron nanoparticles using tree leaf extracts[J]. Science of the Total Environment,2013,445:1-8. [18] CHEN R, YI G H, WU S S, et al. Controlled green synthesis of Au-Pt bimetallic nanoparticles using chlorogenic acid[J]. Research on Chemical Intermediates,2021,47(10):4051-4066. doi: 10.1007/s11164-021-04513-8 [19] LIU C M, DIAO Z H, HUO W Y, et al. Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: Synthesis, reactivity and mechanism[J]. Environmental Pollution,2018,239:698-705. doi: 10.1016/j.envpol.2018.04.084 [20] MANDAL S, PU S Y, HE L L, et al. Biochar induced modification of graphene oxide & nZVI and its impact on immobilization of toxic copper in soil[J]. Environmental Pollution,2020,259:113851. doi: 10.1016/j.envpol.2019.113851 [21] WEI Y F, FANG Z Q, ZHENG L C, et al. Biosynthesized iron nanoparticles in aqueous extracts of Eichhornia crassipes and its mechanism in the hexavalent chromium removal[J]. Applied Surface Science,2017,399:322-329. doi: 10.1016/j.apsusc.2016.12.090 [22] ZHANG Q, WANG Y Y, WANG Z, et al. Active biochar support nano zero-valent iron for efficient removal of U(VI) from sewage water[J]. Journal of Alloys and Compounds: An Interdisciplinary Journal of Materials Science and Solid-state Chemistry and Physics,2021,852:156993. doi: 10.1016/j.jallcom.2020.156993 [23] WANG G H, LIU J S, WANG X G, et al. Adsorption of uranium(VI) from aqueous solution onto cross-linked chitosan[J]. Journal of Hazardous Materials,2009,168(2-3):1053-1058. doi: 10.1016/j.jhazmat.2009.02.157 [24] 司子彦, 谢水波, 朱奥琦, 等. 蒙脱土/Fe3O4/腐殖酸复合材料对U(Ⅵ)的作用机制[J]. 材料工程, 2021, 49(3):158-166.SI Ziyan, XIE Shuibo, ZHU Aoqi, et al. Action mechanism of montmorillonit/Fe3O4/humic acid composite on U(Ⅵ)[J]. Journal of Materials Engineering,2021,49(3):158-166(in Chinese). [25] SUN Y B, YANG S T, SHENG G D, et al. Comparison of U(VI) removal from contaminated groundwater by nanoporous alumina and non-nanoporous alumina[J]. Separation and Purification Technology,2011,83:196-203. doi: 10.1016/j.seppur.2011.09.050 [26] ELTAWEIL A S, MOHAMED H A, ABD EL-MONAEM E M, et al. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms[J]. Advanced Powder Technology,2020,31(3):1253-1263. doi: 10.1016/j.apt.2020.01.005 [27] HO Y, MCKAY G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat[J]. Water Research,2000,34(3):735-742. doi: 10.1016/S0043-1354(99)00232-8 [28] 荣丽杉, 夏麟, 周书葵, 等. UiO-66/壳聚糖的制备及其对U(VI)的吸附机制[J]. 复合材料学报, 2022, 39(10): 4879-4888.RONG Lishan, XIA Lin, ZHOU Shukui, et al. Preparation of UiO-66/chitosan and its adsorption mechanism of U(VI)[J]. Acta Materiae Compositae Sinica, 2022, 39(10): 4879-4888(in Chinese). [29] FEBRIANTO J, KOSASIH A N, SUNARSO J, et al. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies[J]. Journal of Hazardous Materials,2009,162(2-3):616-645. doi: 10.1016/j.jhazmat.2008.06.042 [30] AHMED W, MEHMOOD S, NUNEZ-DELGADO A, et al. Utilization of Citrullus lanatus L. seeds to synthesize a novel MnFe2O4-biochar adsorbent for the removal of U(VI) from wastewater: Insights and comparison between modified and raw biochar[J]. Science of the Total Environment,2021,771:144955. doi: 10.1016/j.scitotenv.2021.144955 [31] 杨金辉, 胡世琴, 杨斌, 等. 氨化烟末生物碳吸附剂的制备及对Cr(VI)的吸附行为[J].复合材料学报, 2022, 39(1): 222-231.YANG Jinhui, HU Shiqin, YANG Bin, et al. Preparation of bio-carbon adsorbent for ammoniated tobacco dust and its adsorption behavior for Cr(VI)[J]. Acta Materiae Compositae Sinica, 2022, 39(1): 222-231(in Chinese). [32] FAN Q H, HAO L M, WANG C L, et al. The adsorption behavior of U(VI) on granite[J]. Environmental Science-Processes & Impacts,2014,16(3):534-541. [33] HU H, ZHANG X, WANG T, et al. Bamboo (acidosasa longiligula) shoot shell biochar: Its potential application to isolation of uranium(VI) from aqueous solution[J]. Journal of Radioanalytical and Nuclear Chemistry,2018,316(1):349-362. doi: 10.1007/s10967-018-5731-6 [34] SAWADA K, HIRABAYASHI D, ENOKIDA Y. Kinetics of chlorination of uranium-antimony composite oxide for uranium removal from waste catalyst used for acrylonitrile synthesis[J]. Journal of Nuclear Science and Technology,2019,56(4):317-321. doi: 10.1080/00223131.2019.1580622 [35] ZHANG Q, ZHAO D, DING Y, et al. Synthesis of Fe-Ni/graphene oxide composite and its highly efficient removal of uranium(VI) from aqueous solution[J]. Journal of Cleaner Production,2019,230:1305-1315. doi: 10.1016/j.jclepro.2019.05.193 [36] GUO D, SONG X, ZHANG L, et al. Recovery of uranium(VI) from aqueous solutions by the polyethyleneimine-functionalized reduced graphene oxide/molybdenum disulfide composition aerogels[J]. Journal of the Taiwan Institute of Chemical Engineers,2020,106:198-205. doi: 10.1016/j.jtice.2019.09.029 [37] LI J, ZHANG X, LIU M, et al. Enhanced reactivity and electron selectivity of sulfidated zerovalent iron toward Chromate under aerobic conditions[J]. Environmental Science & Technology,2018,52(5):2988-2997. -






 下载:
下载: