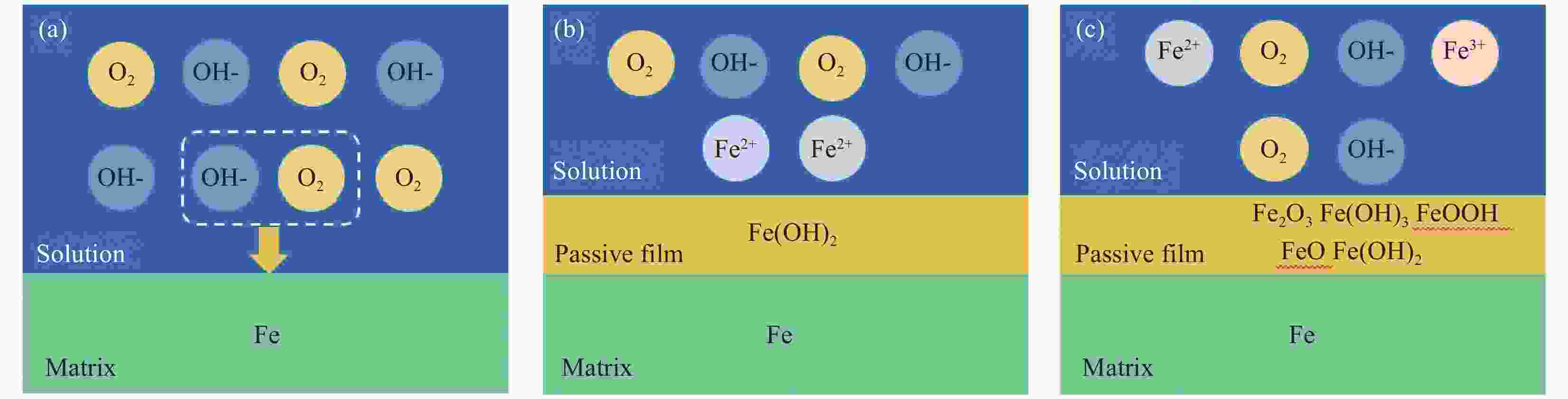
Electrochemical response and mechanism of steel rebar passivation in simulated concrete pore solutions with different pH values
-
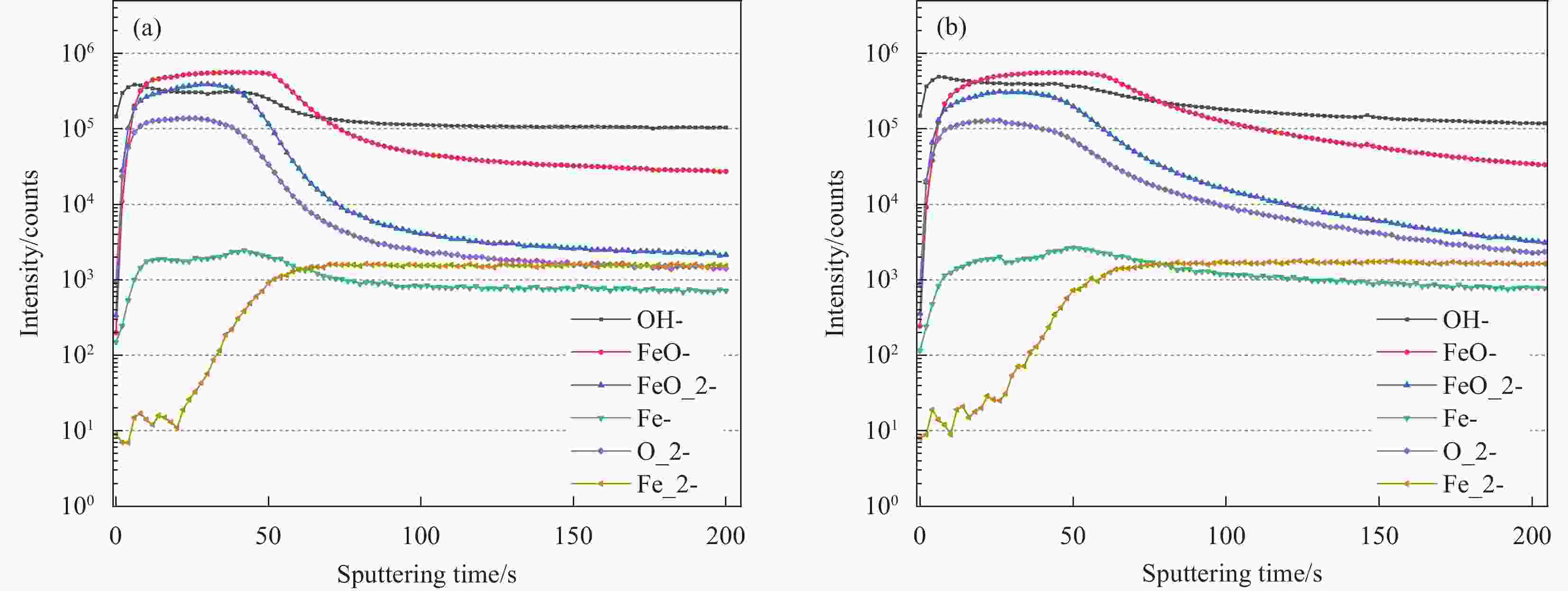
摘要: 本研究旨在探讨普通低碳钢在不同pH值的混凝土孔隙溶液中的钝化行为及其机制。通过开路电位、线性极化电阻、电化学阻抗谱以及ToF-SIMS分析,深入探究了pH值对低碳钢钝化行为的影响。结果表明,在pH值较低的CH溶液中,极化电阻(Rp)缓慢增加最终稳定在120 kΩ·cm²,腐蚀电流密度(Icorr)保持在较高水平0.15 μA/cm²。表明CH溶液对低碳钢钝化效果不明显;而在pH值较高的ST溶液中,电容电抗弧一天内发生明显变化,表明其在一天内即发生钝化。且无论混凝土模拟液的pH值如何,低碳钢均能自发形成钝化膜,其表面钝化膜都经历了从快速初始生长到后期逐渐稳定的过程。pH值是促进低碳钢钝化的关键因素且与钝化膜性能存在正相关性,CH溶液中,钝化膜厚度从最初的1.930 nm上升至3.733 nm。而在ST溶液中一天内从4.786 nm增至9.187 nm,说明随着模拟液pH值的升高,钝化膜厚度为增加的趋势,形成更加稳定的钝化膜,从而使其钝化性能得到增强。Abstract: This study aims to investigate the passivation behavior and mechanisms of low carbon steel in concrete pore solutions with different pH values. Through open circuit potential, linear polarization resistance, electrochemical impedance spectroscopy and ToF-SIMS analysis, the effect of pH on the passivation behavior of low carbon steel was examined in detail. The results demonstrate that mild steel can spontaneously form a passivation film in concrete pore solutions of any pH value, with the film on its surface undergoing a transition from rapid initial growth to a more gradual stabilization over time. pH is a crucial factor in promoting the passivation of mild steel and shows a positive correlation with the performance of the passivation film. As the pH of the simulated solution increases, there is a tendency for the thickness of the passivation film to increase, resulting in the formation of a more stable passivation layer, thereby enhancing its passivation properties.
-
Key words:
- low carbon steel /
- passivation /
- pH values /
- electrochemical /
- concrete
-
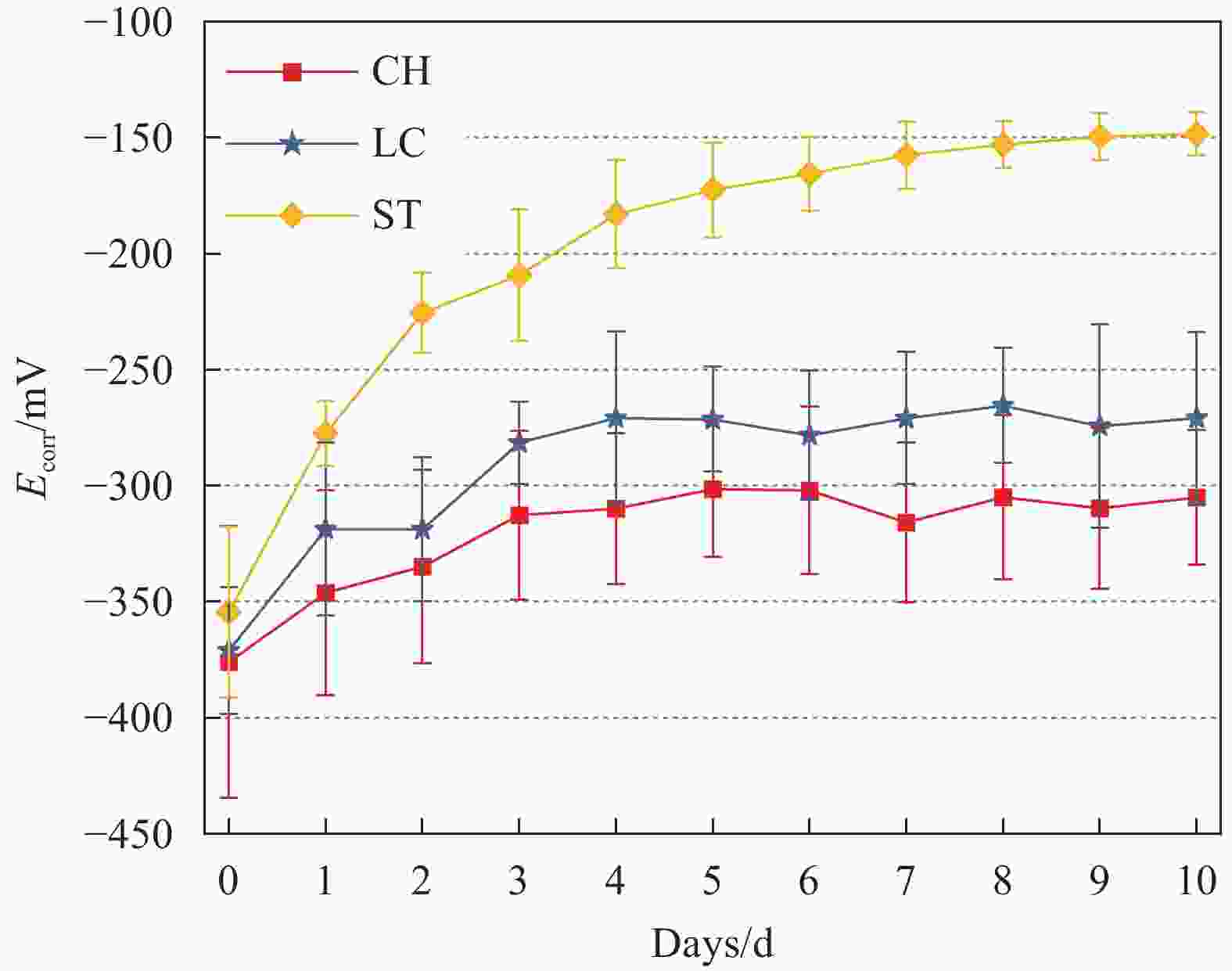
图 1 钢试样在 CH、LC 和 ST 溶液钝化阶段的腐蚀电位曲线
Figure 1. Corrosion potential curves of steel specimens in the passivation stage of CH, LC and ST solutions.
The y-axis shows the corrosion potential (Ecorr) in volts (V), indicating the material’s tendency to corrode. More negative values of Ecorr suggest a higher corrosion tendency
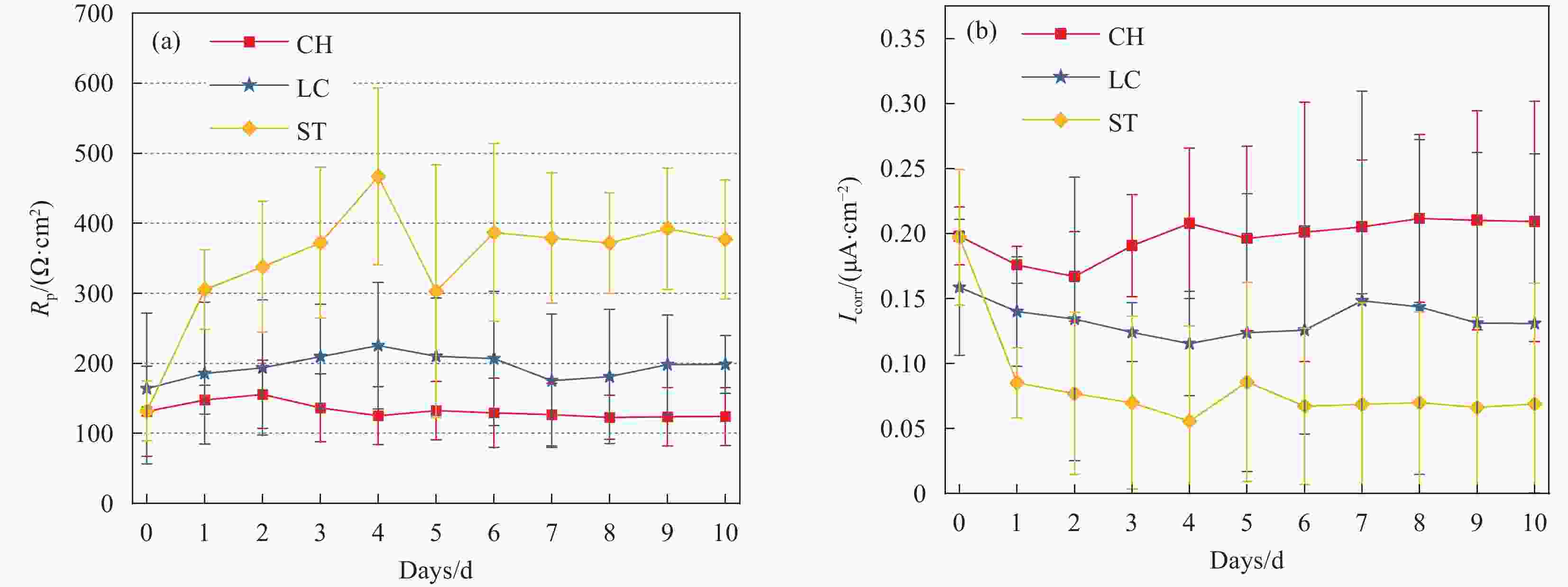
图 2 钢试样在CH、LC和ST溶液钝化阶段的Rp和Icorr曲线:(a)Rp;(b)Icorr
Figure 2. Rp and Icorr curves of steel specimens in the passivation stage of CH, LC and ST solutions: (a) Rp; (b) Icorr
(a) Polarization resistance (Rp) in ohms (Ω), indicating the material’s resistance to corrosion. Higher Rp suggests better corrosion resistance. (b) Corrosion current density (Icorr) in µA/cm², reflecting the rate of corrosion. Higher Icorr indicates a faster corrosion rate.
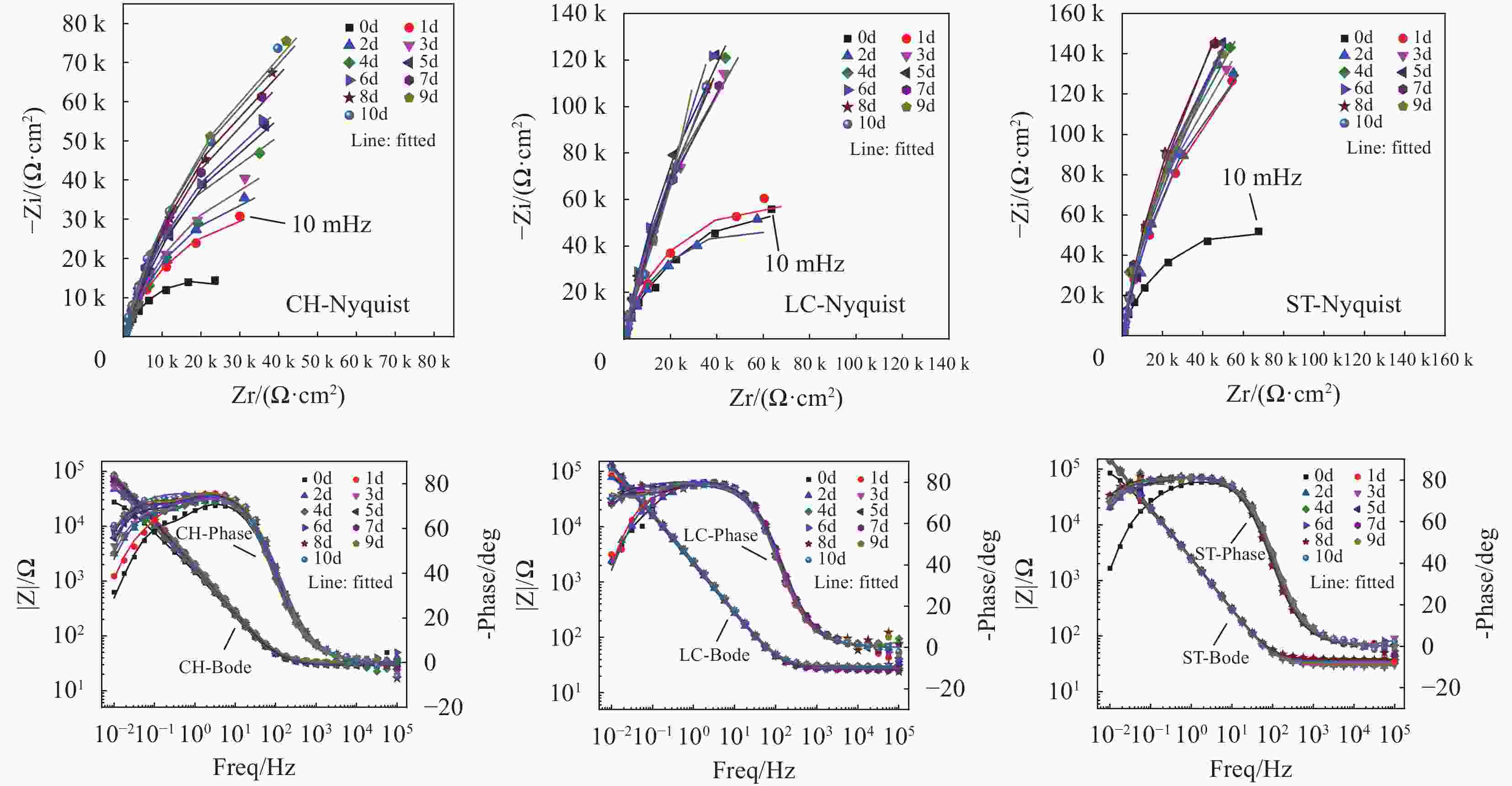
图 3 钢试样钝化阶段的Nyquist图、Bode图和Phase图及其在CH、LC和ST溶液中的等效电路拟合结果
Figure 3. Nyquist, Bode and Phase plots of steel specimens in passivation phase and their equivalent circuit fitting results in CH, LC and ST solutions
Zi: The negative imaginary part of the impedance, representing the capacitive behavior of the system. A larger value indicates higher capacitive reactance. Zr: The real part of the impedance, representing the resistive component of the system. Higher values suggest greater resistance to current flow. |Z|: The absolute value of the impedance, which combines both the real and imaginary components. It reflects the overall opposition to the flow of current in the system
表 1 钢筋成分表
Table 1. Chemical composition of steel bars
Element C Si Mn S P Fe Wt.% 0.2 0.55 1.42 0.028 0.026 97.776 表 2 EIS数据拟合结果
Table 2. Fitting results of EIS data of steel specimens
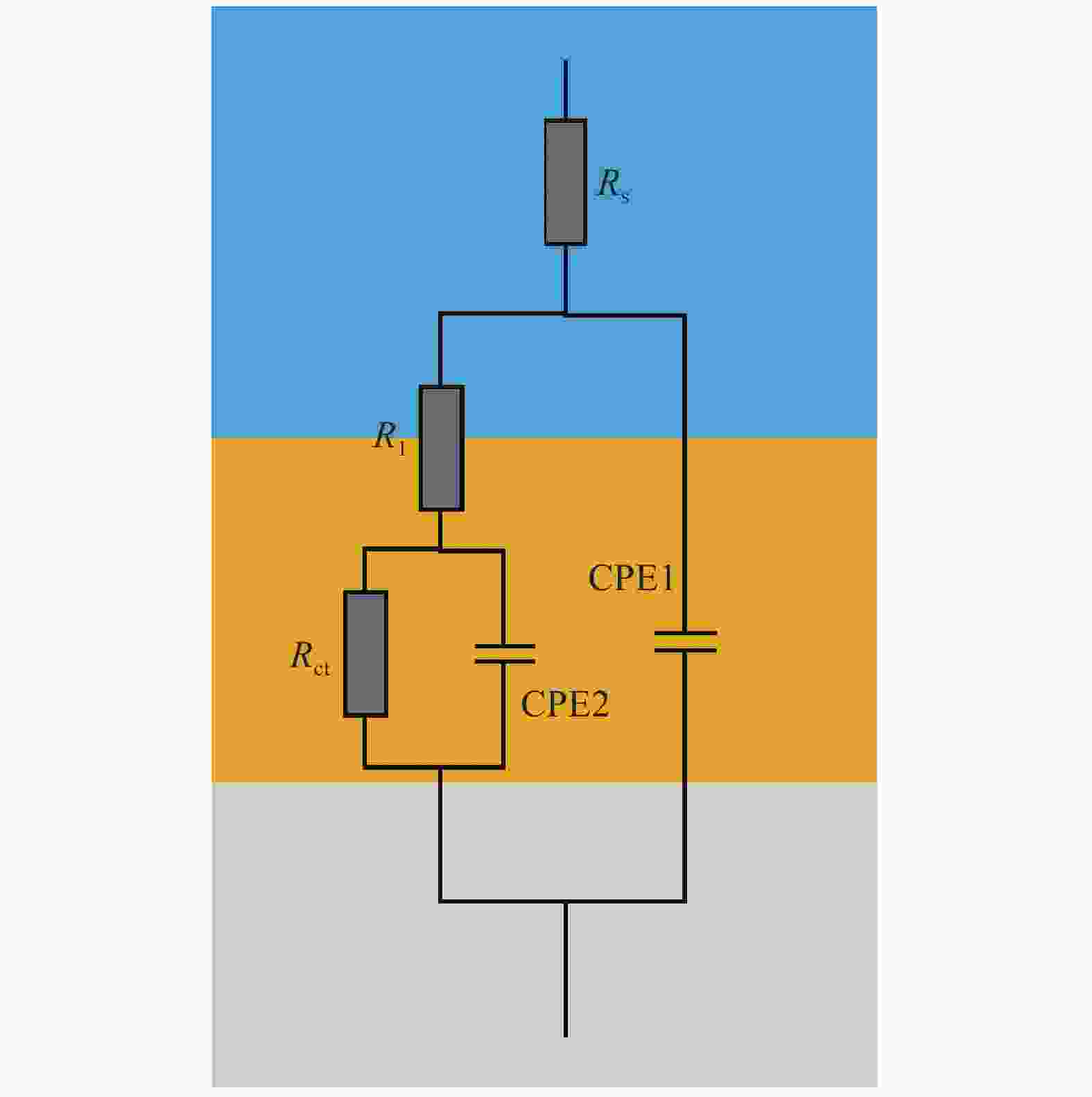
$ {R}_{S} $(Ω·cm2) CPE1,$ Q $
(S·secn·cm−2)CPE1,$ a $
[0<a<1]$ {R}_{1} $
(Ω·cm2)CPE2,$ Q $
(S·secn·cm−2)CPE2,$ a $
[0<a<1]$ {R}_{ct} $
(Ω·cm2)Chi-squared CH 0 d 30.99 0.000181 0.8801 3524 0.00007947 0.6681 37950 1. 62E-03 1 d 34.79 0.0006243 1 4439 0.0001044 0.6391 79980 4.18E-03 2 d 30.94 0.0001127 0.8815 5494 0.00004949 0.6548 117700 4.44E-04 3 d 33.37 0.0001092 0.8853 4838 0.00004493 0.6353 155900 6.45E-04 4 d 32.86 0.0002026 0.8861 5367 0.00004023 0.6493 186520 5.36E-04 5 d 29.14 0.0009702 0.8869 6947 0.00003695 0.6698 225400 4.11E-04 6 d 31.38 0.0001019 0.8840 7993 0.00003565 0.6095 218800 7.27E-04 7 d 29.54 0.0009796 0.8898 7881 0.00003274 0.6943 284900 5.77E-04 8 d 30.53 0.0009453 0.8914 7976 0.00002633 0.6764 316300 1.07E-04 9 d 33.21 0.0008241 0.9017 7023 0.00002888 0.6980 367900 1.69E-03 10 d 31 0.0008867 0.8954 8369 0.0000271 0.6853 338700 1.67E-03 LC 0 d 26.88 0.00007963 0.9061 68810 0.0001285 0.8469 55570 1.76E-03 1 d 29.81 0.00008169 0.9162 49580 0.00003099 0.8537 80470 3.31E-03 2 d 27.02 0.00008597 0.9147 34970 0.00003930 0.8694 96460 1.57E-03 3 d 27.35 0.00007808 0.9202 35500 0.00001436 0.9261 71310 8.15E-04 4 d 28.73 0.00007633 0.9234 25330 0.00001419 0.8907 78030 1.06E-04 5 d 29.51 0.00003119 1 13280 0.00005319 0.8801 1417000 1.18E-03 6 d 26.56 0.00007546 0.9189 29240 0.00001400 0.8347 1114000 1.39E-03 7 d 26.03 0.00007776 0.9115 37020 0.00002071 0.8937 704000 1.58E-03 8 d 29.48 0.00007773 0.9123 31290 0.00001734 0.8714 2148000 4.01E-03 9 d 30.64 0.00007588 0.9165 21900 0.00001732 0.9250 4468000 1.22E-03 10 d 30.25 0.00007609 0.9173 23960 0.000018 0.8852 7130000 6.82E-04 ST 0 d 38.06 0.00007504 0.9183 54850 0.0000255 0.2326 686100 9.78E-04 1 d 33.09 0.00003052 1 15580 0.00005107 0.8384 619000 1.38E-03 2 d 30.92 0.00003169 1 25560 0.00004324 0.8214 656700 1.84E-03 3 d 29.45 0.00002717 1 12940 0.00005259 0.8538 741300 5.99E-04 4 d 33.62 0.00002766 1 13730 0.00004696 0.8482 846000 9.22E-04 5 d 34.40 0.00007253 0.9229 14380 0.00001506 0.8328 562500 1.20E-04 6 d 35.79 0.00002826 1 14090 0.00004813 0.8487 988800 4.91E-04 7 d 38.06 0.00007504 0.9183 14850 0.00003524 0.8326 6861000 9.78E-04 8 d 38.24 0.00002682 1 12490 0.00005017 0.8504 0.851237000 7.70E-04 9 d 31.27 0.00007871 0.9116 13360 0.00005955 0.7915 1256000 7.13E-04 10 d 34.21 0.00004021 1 18620 0.00001925 0.7912 1145000 6.35E-03 Notes: Rs: Solution resistance. CPE1, Q1: Constant phase element 1 (CPE), describing the non-ideal capacitance behavior. CPE1, n1: Exponent of CPE1, indicating deviation from ideal capacitive behavior. R1: Charge transfer resistance. CPE2, Q2: Constant phase element 2 (CPE), associated with surface processes. CPE2, n2: Exponent of CPE2, indicating deviation from ideal capacitive behavior. Rct: Corrosion resistance. Chi-squared: Indicator of the goodness-of-fit for the model.) 表 3 钝化阶段钝化膜厚度计算(nm)
Table 3. Calculation of passivation film thickness (nm) at passivation stage
0 d 1 d 2 d 3 d 4 d 5 d 6 d 7 d 8 d 9 d 10 d CH 2.16 2.252 2.681 2.536 2.693 2.795 2.884 3.287 3.564 3.629 3.757 LC 2.412 2.699 3.285 3.997 3.869 3.789 3.854 3.905 3.881 3.798 3.773 ST 3.572 4.379 4.317 4.296 4.271 4.165 4.137 4.364 4.152 4.312 4.288 -
[1] PANG L, LI Q. Service life prediction of RC structures in marine environment using long term chloride ingress data: comparison between exposure trials and real structure surveys[J]. Construction and Building Materials, 2016, 113: 979-987. doi: 10.1016/j.conbuildmat.2016.03.156 [2] 金伟良, 赵羽习. 混凝土结构耐久性 [M]. 2 版. 北京: 科学出版社, 2014: 15.JIN Weiliang, ZHAO Yuxi. Durability of concrete structures (2nd ed. ). Beijing: Science Press. 2014: 15(in Chinese). [3] 李嘉伦, 刘国建, 佘伟, 等. 模拟混凝土孔隙溶液中氯离子和硫酸根离子对钢筋锈蚀的影响[J/OL]. 复合材料学报, 复合材料学报, https://doi.org/10.13801/j.cnki.fhclxb.20240719.003.LI Jialun, LIU Guojian, SHE Wei, et al. Influence of chloride and sulfate on steel corrosion in simulated concrete pore solutions[J]. Acta Materiae Compositae Sinica. (in Chinese). [4] 张伦馨, 刘国建, 刘志勇, 等. 模拟混凝土孔隙液碱性环境对 Cr10Mo 合金钢钝化行为的影响[J/OL]. 硅酸盐学报, https://doi.org/10.14062/j.issn.0454-5648.20240216.ZHANG Lunxin, LIU Guojian, LIU Zhiyong, et al. Effect of An Alkaline Environment in Simulated Concrete Pore Solution onthe Passivation Behavior of Cr10Mo Alloy Steel[J/OL]. Journal of the Chinese Ceramic Society. (in Chinese). [5] BERTOLINI L, ELSENER B, PEDEFERRI P. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair[M]. 2nd Ed, Weinheim: John Wiley & Sons, 2013: 172. [6] FENG Z, CHENG X, DONG C, XU L, LI X. Passivity of 316L stainless steel in borate buffer solution studied by Mott–Schottky analysis, atomic absorption spectrometry and X-ray photoelectron spectroscopy[J]. Corrosion Science, 2010, 52(11): 3646-3653. doi: 10.1016/j.corsci.2010.07.013 [7] KULAKOWSKI M P, PEREIRA F M, DAL MOLIN D C C. Carbonation-induced reinforcement corrosion in silica fume concrete[J]. Construction and Building Materials, 2009, 23(3): 1189-1195. doi: 10.1016/j.conbuildmat.2008.08.005 [8] SUN W, ZHANG Y, LIU S, ZHANG Y. The influence of mineral admixtures on resistance to corrosion of steel bars in green high-performance concrete[J]. Cement and Concrete Research, 2004, 34(10): 1781-1785. doi: 10.1016/j.cemconres.2004.01.008 [9] HE Z, LI L, DU S. Mechanical properties, drying shrinkage, and creep of concrete containing lithium slag[J]. Construction and Building Materials, 2017, 147: 296-304. doi: 10.1016/j.conbuildmat.2017.04.166 [10] ZHANG J, YANG J, YING Z. Study on Mechanical Properties of Metakaolin-Based Concretes and Corrosion of Carbon Steel Reinforcement in 3.5% NaCl[J]. International Journal of Electrochemical Science, 2020, 15(4): 2883-2893. doi: 10.20964/2020.04.25 [11] NGUYEN Q D, AFROZ S, CASTEL A. Influence of Calcined Clay Reactivity on the Mechanical Properties and Chloride Diffusion Resistance of Limestone Calcined Clay Cement (LC3) Concrete[J]. Journal of Marine Science and Engineering, 2020, 8(5): 301. doi: 10.3390/jmse8050301 [12] ZOU G, WANG Q, WANG G, LIU W, ZHANG S, AI Z, CHEN H, MA H, SONG D. Revealing excellent passivation performance of a novel Cr-alloyed steel rebar in carbonized concrete environment[J]. Journal of Materials Research and Technology, 2023, 23: 1848-1861. doi: 10.1016/j.jmrt.2023.01.118 [13] ZHANG D, LIU T, SHAO Y. Weathering carbonation behavior of concrete subject to early-age carbonation curing[J]. Journal of Materials in Civil Engineering, 2020, 32(4): 04020038. doi: 10.1061/(ASCE)MT.1943-5533.0003087 [14] RAMMELT U, KOEHLER S, REINHARD G. Use of vapour phase corrosion inhibitors in packages for protecting mild steel against corrosion[J]. Corrosion Science, 2009, 51(4): 921-925. doi: 10.1016/j.corsci.2009.01.015 [15] FREIRE L, CARMEZIM M J, FERREIRA M G S, MONTEMOR M F. The electrochemical behaviour of stainless steel AISI 304 in alkaline solutions with different pH in the presence of chlorides[J]. Electrochimica Acta, 2011, 56(14): 5280-5289. doi: 10.1016/j.electacta.2011.02.094 [16] FREIRE L, CATARINO M A. , GODINHO M I. , FERREIRA M J, FERREIRA M G S, SIMOES A M P, MONTEMOR M F. Electrochemical and analytical investigation of passive films formed on stainless steels in alkaline media[J]. Cement and Concrete Composites, 2012, 34(9): 1075-1081. [17] ZHOU Y, ZUO Y. The Passivation Behavior of Mild Steel in CO2 Saturated Solution Containing Nitrite Anions[J]. Journal of the Electrochemical Society, 2014, 162(1): C47-C54. [18] SHI J, WU M, MING J. Degradation effect of carbonation on electrochemical behavior of 2304 duplex stainless steel in simulated concrete pore solutions[J]. Corrosion Science, 2020, 177: 109006. doi: 10.1016/j.corsci.2020.109006 [19] SHI J, MING J, SUN W. Electrochemical behaviour of a novel alloy steel in alkali-activated slag mortars[J]. Cement and Concrete Composites, 2018, 92: 110-124. doi: 10.1016/j.cemconcomp.2018.06.004 [20] SHI J, MING J, WU M. Passivation and corrosion behavior of 2304 duplex stainless steel in alkali-activated slag materials[J]. Cement and Concrete Composites, 2020, 108: 103532. doi: 10.1016/j.cemconcomp.2020.103532 [21] CHEN Z, NONG Y, CHEN Y, CHEN J, YU B. Study on the adsorption of OH − and CaOH + on Fe (100) surface and their effect on passivation of steel bar: Experiments and DFT modelling[J]. Corrosion Science, 2020, 174: 108804 doi: 10.1016/j.corsci.2020.108804 [22] SIVAI BHARASI N, PUJAR M G, MALLIKA C, KAMACHI MUDALI U. Corrosion and Passive Film Formation Studies on Modified 9Cr-1Mo Steel in Different Sodium Hydroxide Concentrations at Room Temperature and in Boiling Condition[J]. Transactions of the Indian Institute of Metals, 2017, 70(8): 1953-1963. doi: 10.1007/s12666-016-0958-9 [23] 刘国建, 朱航, 张云升, 等. 混凝土孔溶液中不同侵蚀离子对钢筋的腐蚀行为[J]. 硅酸盐学报, 2022, 50(2): 413-419 (in Chinese).LIU G, ZHU H, ZHANG Y. et al. Corrosion Behavior of Steel Subjected to Different Corrosive lons in Simulated Concrete Pore Solution[J]. Journal of the Chinese Ceramic Society, 2022, 50(2): 413-419. [24] 刘国建, 张云升, 刘诚, 等. 模拟混凝土孔溶液中钢筋腐蚀与等效电路选取[J]. 材料导报, 2021, 35(14): 14072-14078 (in Chinese). doi: 10.11896/cldb.20040138LIU G, ZHANG Y, LIU C, et al. Corrosion of Steel in Simulated Concrete Pore Solution and Equivalent Circuits Selection[J]. Materials Reports, 2021, 35(14): 14072-14078. doi: 10.11896/cldb.20040138 [25] VOLPI E, OLIETTI A, STEFANONI M, TRASATTI S P. Electrochemical characterization of mild steel in alkaline solutions simulating concrete environment[J]. Journal of Electroanalytical Chemistry, 2015, 736: 38-46. doi: 10.1016/j.jelechem.2014.10.023 [26] GHODS P, ISGOR O B, BROWN J R, BENSEBAA F, KINGSTON D. XPS depth profiling study on the passive oxide film of carbon steel in saturated calcium hydroxide solution and the effect of chloride on the film properties[J]. Applied Surface Science, 2011, 257(10): 4669-4677. doi: 10.1016/j.apsusc.2010.12.120 [27] LIU G, ZHANG Y, WU M, HUANG R. Study of depassivation of carbon steel in simulated concrete pore solution using different equivalent circuits[J]. Construction Building Materials 2017, 157: 357-362. [28] LIU G, ZHANG Y, NI Z, HUANG R. Corrosion behavior of steel submitted to chloride and sulphate ions in simulated concrete pore solution[J]. Construction Building Materials 2016, 115: 1-5. [29] LIU R, JIANG L, XU J, XIONG C, SONG Z. Influence of carbonation on chloride-induced reinforcement corrosion in simulated concrete pore solutions[J]. Construction and Building Materials, 2014, 56: 16-20. doi: 10.1016/j.conbuildmat.2014.01.030 [30] YANG L, CHIANG K T, YU H, PABALAN R T, DASGUPTA B, IBARRA L. Threshold Chloride Levels for Localized Carbon Steel Corrosion in Simulated Concrete Pore Solutions Using Coupled Multielectrode Array Sensors[J]. Corrosion, 2014, 70(8): 850-857. doi: 10.5006/0830 [31] ZHOU X, LI M, GUAN X, et al. Insights into the enhanced corrosion resistance of carbon steel in a novel low-carbon concrete with red mud and phytic acid[J]. Corrosion Science, 2023, 211: 110903. doi: 10.1016/j.corsci.2022.110903 [32] SHI J, ZOU Y, MING J, et al. Effect of DC stray current on electrochemical behavior of low-carbon steel and 10%Cr steel in saturated Ca(OH)2 solution[J]. Corrosion Science, 2020, 169: 108610. doi: 10.1016/j.corsci.2020.108610 [33] GHODS P, ISGOR O, BROWN J, et al. XPS depth profiling study on the passive oxide film of carbon steel in saturated calcium hydroxide solution and the effect of chloride on the film properties[J]. Applied Surface Science, 2011, 257(10): 4669-4677. doi: 10.1016/j.apsusc.2010.12.120 [34] H. GUNAY, P GHODS, O ISGOR, et al. Characterization of atomic structure of oxide films on carbon steel in simulated concrete pore solutions using EELS[J]. Applied Surface Science, 2013, 274: 195-202. -

 点击查看大图
点击查看大图
计量
- 文章访问数: 18
- HTML全文浏览量: 11
- 被引次数: 0




 下载:
下载: