Preparation and mechanical properties of nano-iron-graphene oxide/chitosan composites
-
摘要: 纳米铁和氧化石墨烯(GO)修饰壳聚糖(CS)复合材料对水体中重金属的优越吸附性能,在环保领域具有良好的应用前景。然而,不同含量纳米铁和/或GO修饰CS对复合材料力学性能的影响成果却十分有限。因此,以CS为聚合物基体、GO和FeCl3·6H2O为纳米填充物,采用溶液混合蒸发法制备了不同配比的纳米铁-氧化石墨烯/壳聚糖(Fe-GO/CS)复合材料。通过FTIR、XRD、SEM、TGA和力学性能测试,研究了复合材料的结构、热稳定性和力学性能。研究结果表明,纳米铁和GO在CS基体中分散性良好,具有较强的分子相互作用力,未发生团聚及形成无定型结构的铁复合物。适量的纳米铁和GO能增强CS与填料之间的氢键作用,进而提高Fe-GO/CS复合材料的热稳定性及力学性能。力学性能测试结果显示,加入1wt%纳米铁时,对比纯CS,Fe-GO/CS复合材料的拉伸强度由27.5 MPa提高到65.4 MPa、弹性模量由925.8 MPa提高到2141.4 MPa,分别提高58%和57%,但过量的纳米铁导致复合材料的拉伸强度、弹性模量、断裂伸长率及热稳定性降低。TG分析表明,1wt%纳米铁修饰利于提高Fe-GO/CS复合膜的热稳定性。Abstract: Nano-iron and graphene oxide (GO)-modified chitosan (CS) composites have the superior adsorption performance to heavy metals in water, which is environmentally friendly. The field has good application prospects. However, the effects of different contents of nano-iron and/or GO modified CS on the mechanical properties of composite materials are very limited. Therefore, CS was used as the polymer matrix, GO and FeCl3·6H2O were used as nanofillers, different ratios of nano-iron-graphene oxide/chitosan (Fe-GO/CS) composites were prepared by the solution mixing evaporation method material. By using the FTIR, XRD, SEM, TGA and mechanical property testing, the structure, thermal stability and mechanical properties of the composite material were studied. The research results show that nano-iron and GO are well dispersed in the CS matrix, and there is a strong molecular interaction force, and no agglomeration and amorphous structure of iron complex is formed. An appropriate mixture of nano-iron and GO can enhance the hydrogen bonding between CS and fillers, thereby improving the thermal stability and mechanical properties of Fe-GO/CS composites. The results of mechanical properties test show that the tensile strength and elastic modulus of Fe-GO/CS composites increase from 27.5 MPa to 65.4 MPa and 925.8 MPa to 2141.4 MPa, respectively, which are 58% and 57% higher than those of pure CS. But excessive nano-iron reduces the tensile strength, elastic modulus, breaking elongation and thermal stability. TG analysis shows that 1wt% nano-iron modification is beneficial to improve the stability of Fe-GO/CS composite film.
-
壳聚糖(Chitosan,CS)化学名称为聚葡萄糖胺(1-4)-2-氨基-B-D葡萄糖,是甲壳质的部分脱乙酰化产物,也是一种天然可降解的高分子材料[1-2]。作为一种分子链上富含游离氨基(—NH2)和羟基(—OH)的天然聚合物,CS具有良好的生物相容性、生物可降解性、抗菌性、无毒性和吸附性等,使其在食品、化工、医药和环保等领域具有良好的应用前景[3-5]。例如,张中勋等[3]研究发现,CS中引入氧化石墨烯(Graphene oxide,GO)能提高其载药性。范林林等[6]研究了CS涂膜对鲜切苹果储存品质的影响,发现当CS的质量分数为1wt%时鲜切苹果保鲜效果最佳。Minisy等[7]采用离子交换法制备了壳聚糖-蒙脱土/聚苯胺纳米复合材料,对水体中亚甲基蓝的最大吸附容量可达111 mg·g−1。尽管CS有很多优势和独特的功能,但其力学性能、耐热性较差,且在酸性介质中易流失,很大程度上限制着其使用价值。众多研究表明:加入纳米材料(如GO、金属纳米粒子、黏土等)形成复合材料,可显著改善CS的物理力学性能[8-10]。例如,施昌谷等[11]利用GO与CS复配涂膜,能有效增强复合涂膜食品包装纸条的拉伸强度。Nikpour等[9]制备了纳米羟基磷灰石/壳聚糖(HA/CS)复合材料,研究揭示了HA与CS之间有很强的界面相互作用,在生物相容性良好的基体中加入HA纳米颗粒,能提高复合产物的机械强度。此外,CS作为一种有机聚合物,能有效改善土基聚合物的韧性和脆性。偏高岭土基聚合物(MKG)中加入1wt%的CS后,其弯曲强度和弯曲韧性系数分别提高了33.3%和83.63%[12]。
石墨烯(Graphene)是一种具有二维结构的新型碳纳米材料,因其光学、电学、力学性能良好而备受关注,为开发各种新型功能材料开辟了新的途径[13]。GO作为石墨烯的衍生物,其碳骨架结构边缘含有丰富的含氧官能团,如—OH、C—O—C、—COOH、C=O等,使GO具有亲水性且容易分散在某些有机溶剂中,并通过强作用力改善GO与极性分子之间的界面相互作用,可改变极性分子的力学性能[14-15]。而良好的均匀分散性是提高纳米填充物和聚合物基体间界面相互作用的重要因素。据研究报道,GO可作为填充物分散到CS基体中,制备具有优异力学性能和热性能的GO/CS复合材料[16-18]。据研究表明,将金属离子引入内部结构形成配位键,是提高复合材料的力学性能及稳定性的有效方法[19-20]。Terzioglu等[21]将GO和ZnO作为填料加入CS基质中,与纯CS薄膜相比,GO/ZnO/CS膜的杨氏模量显著增加(1549.4 MPa到2527.7 MPa),FTIR表征证实了CS、GO和ZnO之间的相互作用。王博蔚等[22]利用冷冻干燥技术将不同质量的GO与海藻酸钠(SA)和CS复合成GO-SA-CS支架,随着GO浓度的增加,支架的机械强度随之增强。Yadav等[23]采用溶液混合蒸发法制备了Fe3O4/GO/CS复合材料,研究了Fe3O4与GO对CS基体增强的协同作用,结果表明加入0.5wt%Fe3O4和1wt%GO后,复合材料的拉伸强度和杨氏模量分别比纯CS提高28%和74%。
尽管GO/CS复合材料具有优异的力学性能和热性能,但其对水体中许多重金属离子的吸附效果差、再生性能差等问题,限制其在环境领域的应用。当前,纳米铁和/或GO修饰CS对复合材料吸附性能的影响已有大量研究报道[24-29],也证实了纳米铁和GO修饰CS复合材料对水体中重金属的优越吸附性能,在环保领域具有良好的应用前景。然而,不同含量纳米铁和/或GO修饰CS对复合材料力学性能的影响成果却十分有限。因此,本研究基于纳米铁、GO和CS,按照不同比例掺杂,采用溶液混合法制备一系列GO/CS,Fe/CS及Fe-GO/CS复合材料,通过FTIR、XRD、SEM、TGA和力学性能测试,探究复合材料的内部结构和热稳定性,重点分析纳米铁和/或GO修饰对CS复合材料力学性能的影响及其作用机制,以期为高机械强度材料研究领域提供参考。
1. 实 验
1.1 原材料
壳聚糖(CS,脱乙酰度>90%),购自上海蓝季科技发展有限公司;氧化石墨烯(GO),购自苏州碳丰石墨烯科技有限公司;醋酸、FeCl3·6H2O均为分析纯,购自西陇化工股份有限公司。实验用水均为超纯水。
1.2 材料的制备
采用简单的溶液混合蒸发法制备了Fe-GO/CS复合薄膜。称取一定质量的GO粉末于200 mL体积分数为1.5vol%的醋酸溶液中,超声搅拌30 min得GO悬浮液,加入4 g CS粉末继续超声搅拌至CS完全溶解,得到混合均匀的GO/CS混合液,超声脱泡后,倒入蒸发皿50℃下烘干,制得GO/CS复合膜。按照上述步骤,控制GO的含量,分别制得GO含量为1wt%、3wt%、5wt%和16wt%的GO/CS复合膜。向未添加GO及GO含量为16wt%的GO/CS混合液中加入一定质量的FeCl3,超声搅拌至FeCl3完全溶解,得到混合均匀的Fe/CS和Fe-GO/CS混合液,超声脱泡后,倒入蒸发皿50℃下烘干。控制FeCl3的质量,分别制得纳米铁含量为1wt%、5wt%和8wt%的Fe/CS和Fe-GO/CS复合膜(表1)。
表 1 氧化石墨烯(GO)/壳聚糖(CS)、纳米铁(Fe)/CS和Fe-GO/CS复合材料的命名Table 1. Naming of graphene oxide (GO)/ chitosan (CS), nano-iron (Fe)/CS and Fe-GO/CS compositeSample GO/wt% FeCl3/wt% CS/g 1wt%GO/CS 1 — 4 3wt%GO/CS 3 — 4 5wt%GO/CS 5 — 4 16wt%GO/CS 16 — 4 1wt%Fe/CS — 1 4 5wt%Fe/CS — 5 4 8wt%Fe-GO/CS 16 8 4 1.3 测试与表征
复合材料的官能团采用傅里叶红外光谱(FTIR,SCD-60型,美国 PE 公司)KBr压片法分析。采用X射线衍射仪(XRD,X’Pert3 Powder,荷兰帕纳科公司)对复合材料的结晶结构进行表征,测试扫描步长为0.0263°,扫描速度为0.6565° s−1,扫描角度为5°~80°。用刀片将样品切割成条状试样,尺寸为50 mm×5 mm×1 mm,采用万能试验机(Q800型,美国TA公司)在室温下以5 mm/min的拉伸速度进行测试,每组样品测5次取平均值。样品拉伸断面用场发射扫描电镜(SEM,JSM-7900F型,捷欧迪拓姆贸易有限公司)测试,所有断面测试前均喷金10 s处理。采用热重分析仪(TG 209F3型,德国耐驰公司)分析复合材料的热稳定性,温度范围为20~700℃,升温速率为10℃/min,测试过程均在N2保护下进行,气流量为30 mL/min。
2. 结果与讨论
2.1 Fe-GO/CS复合材料的微观结构
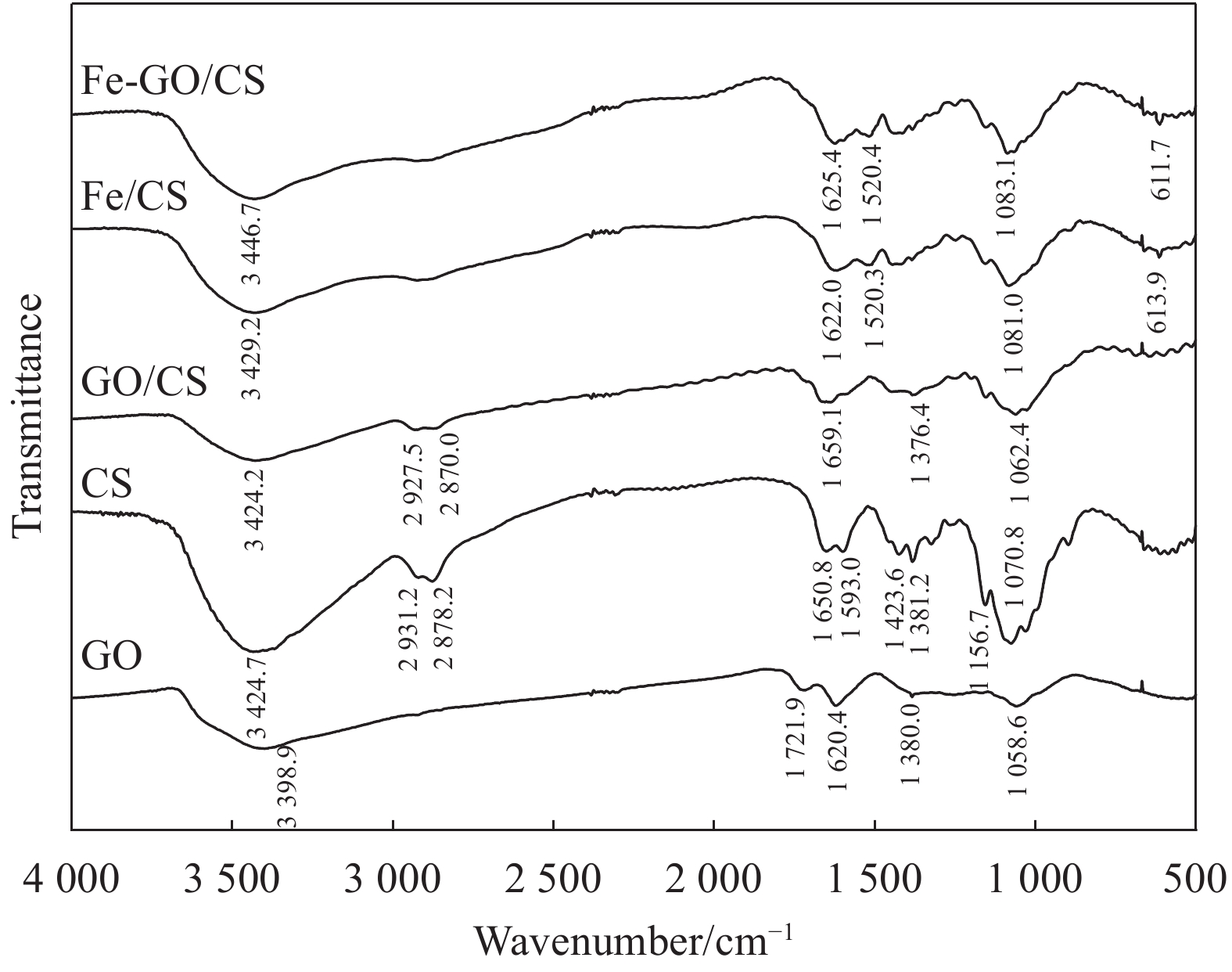
对GO、CS、GO/CS、Fe/CS和Fe-GO/CS复合材料使用傅里叶红外图谱表征,结果如图1所示。GO的FTIR图谱中,3398.9 cm−1处为O—H伸缩振动峰,1721.9 cm−1处为—COOH的C=O伸缩振动峰,1620.4 cm−1、1380.0 cm−1、1058.6 cm−1处的吸收峰分别对应C=C、C—O、C—O—C弯曲振动峰[30]。CS的FTIR图谱显示,3424.7 cm−1处的宽峰归因于O—H和N—H拉伸振动叠加[31],2931.2 cm−1和2878.2 cm−1处对应—CH3和—CH2的C—H伸缩振动峰[32],1650.8 cm−1和159 3.0 cm−1附近是酰胺I的C=O伸缩振动峰和酰胺II的N—H吸收峰[33],表明由于脱乙酰基程度不同存在各种形式的酰胺键[34],1423.6 cm−1和1381.2 cm−1处为C—H弯曲振动峰[32],1156.7 cm−1和1070.8 cm−1处属于C—O伸缩振动峰[35-36]。GO分散到CS形成GO/CS复合物后,图谱显示CS中1650.8 cm−1处酰胺I的C=O伸缩振动峰消失且1593.0 cm−1处酰胺II的N—H吸收峰变弱,GO中1620.4 cm−1处的C=C 峰和1721.9 cm−1处的C=O伸缩振动峰消失,GO/CS中出现了一个1659.1 cm−1处的新峰,由于GO的C—C和C=O键与CS的酰胺官能团的相互作用导致C=N键的形成[34];1376.4 cm−1处的吸收带可能是通过CS的C—H峰和GO的C—O峰在相似位置重叠而形成的[26],1062.4 cm−1处的一个强峰归因于CS和GO在相似位置重叠的C—O峰,证实了GO已经成功分散到CS中,形成具有丰富官能团的GO/CS。Fe/CS的FTIR图谱中,与CS的FTIR图谱相比,1650.8 cm−1处的酰胺I峰,1593.0 cm−1处的酰胺II峰和1070.8 cm−1处的C—O伸缩振动峰分别移至1622.0 cm−1、1520.3 cm−1和1081.0 cm−1处,613.9 cm−1处的峰由Fe—O伸缩振动和—OH引起[37-38],表明CS成功包裹了纳米铁。Fe-GO/CS的FTIR图谱显示,尽管1650.8 cm−1处的酰胺I的C=O和1593.0 cm−1处的酰胺II的N—H峰向低频处(1625.4 cm−1和1520.4 cm−1)偏移,但其特征峰更明显,且1070.8 cm−1处的C—O及3424.7 cm−1处的O—H、N—H峰分别向高频处(1083.1 cm−1和3446.7 cm−1)偏移,表明纳米铁参与了GO和CS间的反应,纳米铁和GO均能增强CS与填料之间的氢键作用。
2.2 Fe-GO/CS复合材料的组成
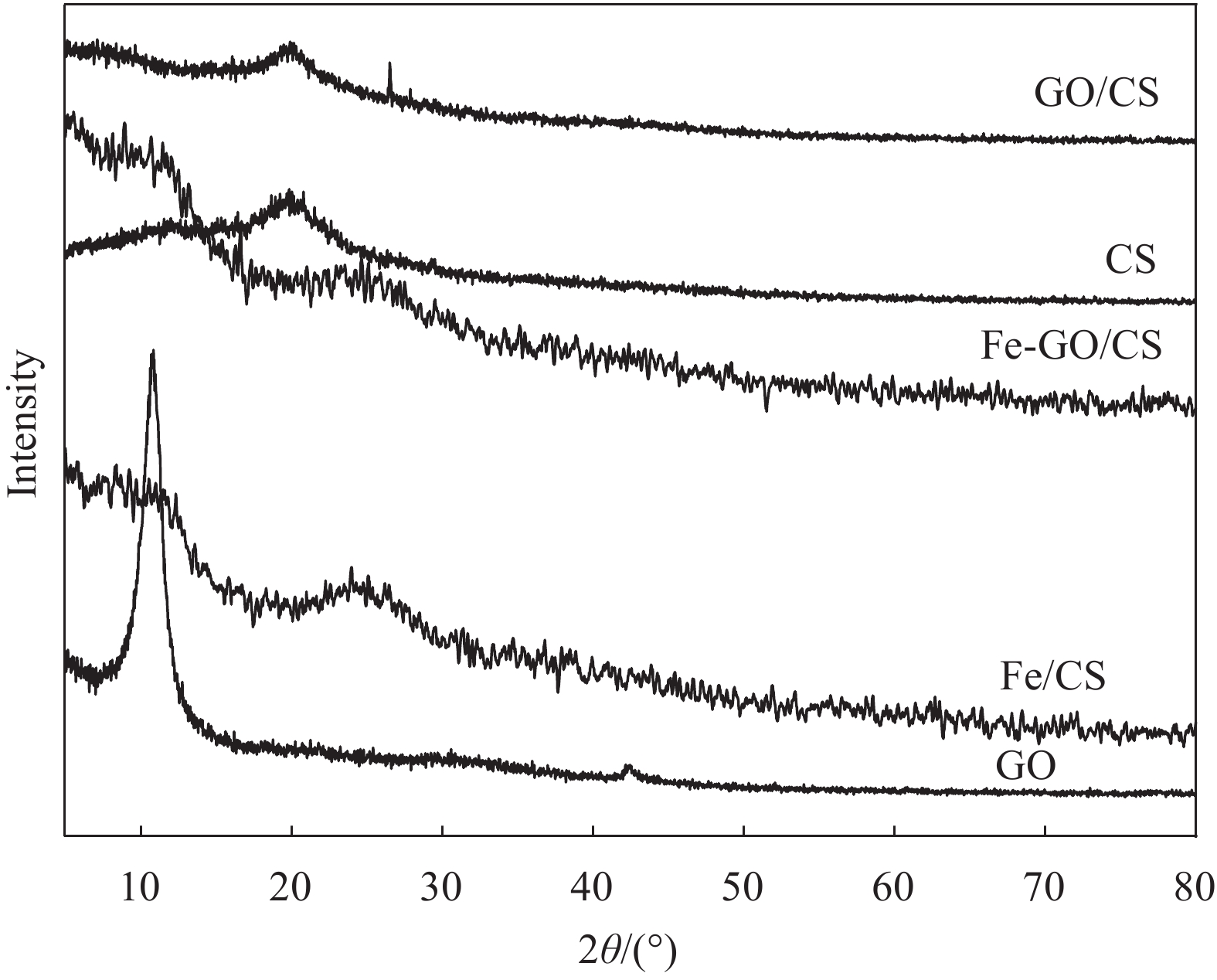
图2为GO、CS、GO/CS、Fe/CS和Fe-GO/CS复合材料的XRD图谱。GO的衍射峰出现在2θ=10.8°和42.3°处,CS的衍射峰出现在2θ=20.0°处,对应于通过分子间相互作用而排列的CS链的特征峰,为无定型结构。加入GO合成GO/CS后,仅存在2θ=20.0°的主峰,GO的特征峰消失,表明GO在CS基体分散均匀,未发生团聚而形成有序的结构[39]。加入1wt%FeCl3之后,复合材料的主峰偏移到2θ=24.5°处,表明FeCl3与GO、CS混合均匀,形成了无定型结构的铁复合物。
2.3 Fe-GO/CS复合材料的力学性能
对复合材料进行力学分析,其中图3(a)~3(c)分别为GO/CS、Fe/CS和Fe-GO/CS的拉伸应力-应变曲线,图3(d)~3(e)分别为拉伸强度和弹性模量。由图3(a)可见,加入一定含量的GO能增大CS的拉伸强度、弹性模量和断裂伸长率,且随着GO含量的增加,GO/CS的拉伸强度和弹性模量均显著增大,断裂伸长率下降。这可能归因于GO与CS分散均匀,二者间产生强烈的氢键作用及静电作用增大了力学强度,同时使链段的流动性下降,导致断裂伸长率降低[40]。当GO含量达到5wt%时,GO/CS复合材料的断裂方式由韧性断裂变成脆性断裂,这可能是由于GO和CS界面相互作用程度过强所致[39]。图3(b)~3(c)显示,CS和GO/CS在加入1wt%纳米铁时复合材料的拉伸强度和弹性模量均显著提高,但之后随着纳米铁含量的增加,其拉伸强度、弹性模量和断裂伸长率均明显下降。图3(d)~3(e)比较了CS、GO/CS和Fe-GO/CS复合材料的拉伸强度及弹性模量,Fe-GO/CS复合材料的拉伸强度分别比GO/CS和CS高55%和58%,弹性模量分别比GO/CS和CS高45%和57%。结果表明,适量的纳米铁修饰能提高GO/CS复合材料的拉伸强度和弹性模量,恰当的纳米铁和GO协同作用能提高CS的拉伸强度和弹性模量。
2.4 Fe-GO/CS复合材料的微观形貌
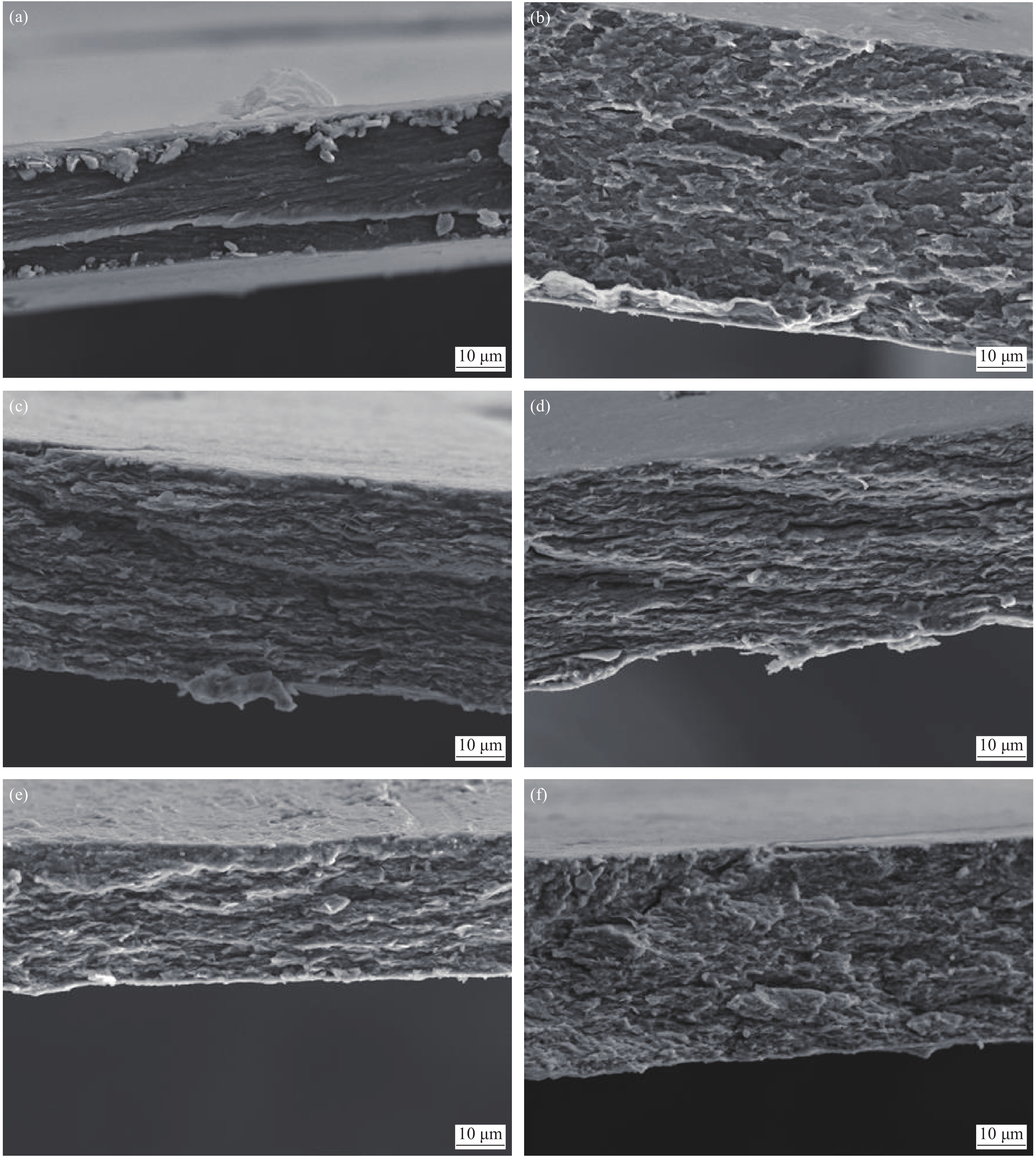
拉伸试验后CS、GO/CS和Fe-GO/CS复合材料断裂面的SEM图像如图4所示,观察倍数均为1000倍。图4(a)为纯CS膜,拉伸断裂面较紧密,表现出典型的韧性断裂特征。图4(b)和图4(c)分别为GO含量为1wt%和16wt%的GO/CS复合膜。较纯CS膜断面而言,GO的加入使GO/CS断面出现凹凸不平的波浪起伏,且随着GO含量的增加波浪起伏越明显,断面未见明显的团聚和GO片脱落现象,表明GO在CS内部分散均匀且二者间具有较强的界面附着力[40]。图4(d)~4(f)为不同纳米铁含量的Fe-GO/CS复合膜断面图像。可见纳米铁含量较低时分散较均匀,随其含量增加使复合材料内部结构变松散且分散性降低,分析认为这是导致复合材料的拉伸强度、弹性模量和断裂伸长率降低的因素之一,同时也表明良好的分散性和界面应力作用是增强复合材料力学性能的重要因素[23]。
2.5 Fe-GO/CS复合材料的热稳定性
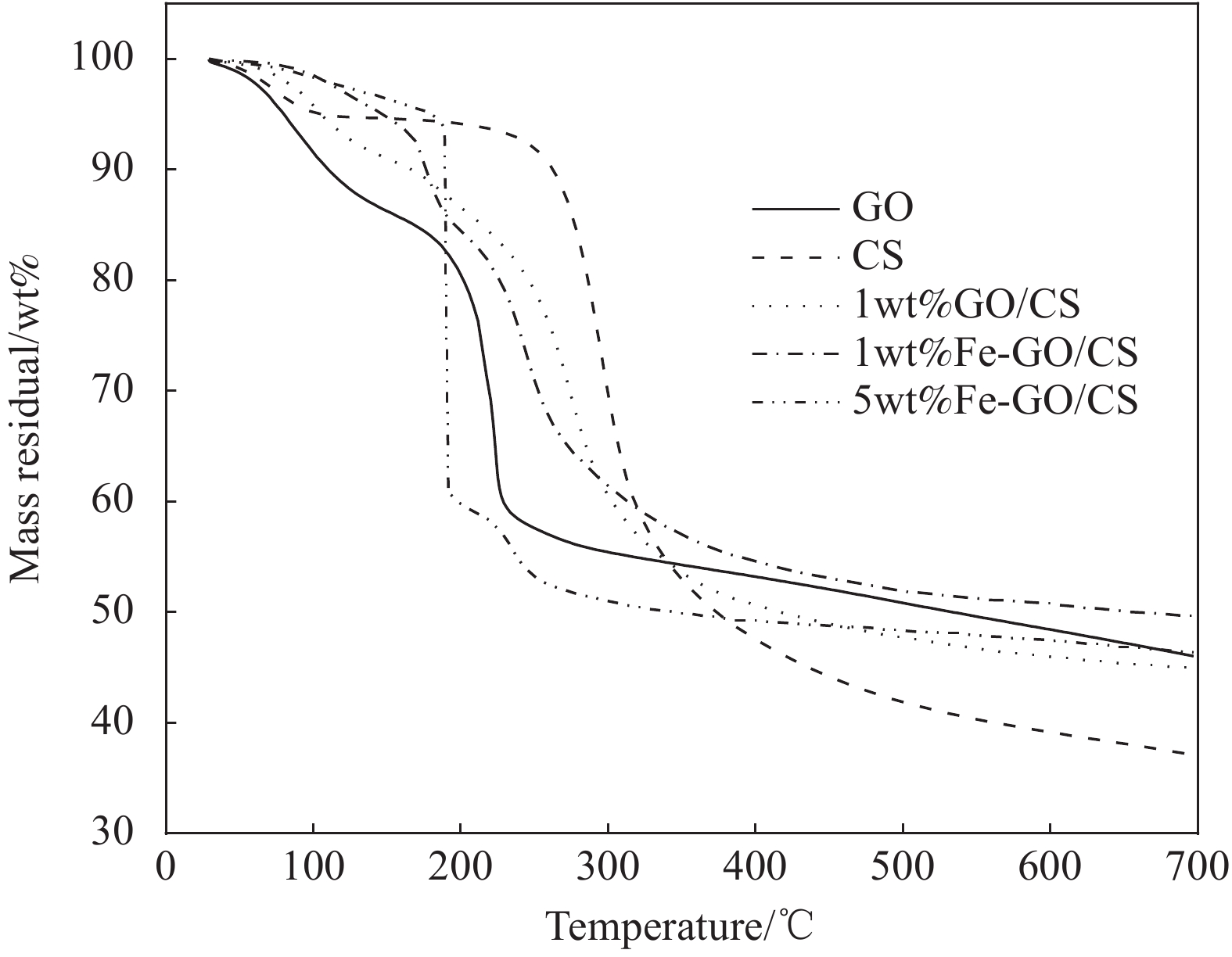
GO、CS、GO/CS和Fe-GO/CS的热重曲线如图5所示,GO在200℃前主要为GO的表面水和结合水失重;200~230℃失重急剧,不稳定官能团和碳骨架分解为主要原因,当温度达700℃,GO的残余率为46wt%[41-42]。CS膜的热分解过程可分3个阶段:第1阶段30~120℃,出现少量质量损失,主要为CS膜上残留的乙酸和水分子,CS内部结构不受影响;第2阶段为热分解主要阶段,CS膜在120~350℃大量分解,由多糖中的糖苷键热分解成乙酸和丁酸等脂肪酸所造成的[43],残余率为37wt%。GO/CS在30~220℃出现少量水分子损失,220~350℃为GO官能团和CS多糖的热分解,350~700℃为碳骨架燃烧,残余率高于纯CS膜,说明CS与GO之间存在较强相互作用,提高GO/CS膜的热稳定性[44]。通常GO的分解温度为200℃,Fe-GO/CS复合膜分解温度大于GO,700℃时残余率大于CS,表明Fe-GO/CS复合膜具有较好的热稳定性[45]。原因可能是加入纳米铁生成Fe-O键,增强了CS与GO分子间的相互作用,使Fe-GO/CS复合膜的热稳定性变强。纳米铁的增加并不能促使Fe-GO/CS复合膜热稳定性及残余率上升反而使其降低,过量的纳米铁可能破坏了复合膜的致密性[46];因此,加入适量的纳米铁利于增强Fe-GO/CS复合膜的热稳定性。
3. 结 论
采用溶液共混蒸发法按照不同配比成功制备了各种Fe-氧化石墨烯(GO)/壳聚糖(CS)复合材料,结合材料表征和材料力学测试分析,重点揭示了纳米铁、GO掺杂对CS力学性能的影响及作用机制。
(1) 纳米铁和GO均能增强CS与填料之间的氢键作用。
(2) 纳米铁和GO在CS基体分散均匀,分散性良好,具有较强的分子相互作用力,未发生团聚及形成无定型结构的铁复合物。
(3) 1wt%纳米铁可以使Fe-GO/CS复合材料的拉伸强度和弹性模量分别比纯CS提高58%和57%,但随着铁含量的增加材料的拉伸强度和弹性模量降低。
(4) 1wt%纳米铁修饰利于提高Fe-GO/CS复合膜的热稳定性,纳米铁的增加促使Fe-GO/CS复合膜热稳定性及残余率降低。
-
表 1 氧化石墨烯(GO)/壳聚糖(CS)、纳米铁(Fe)/CS和Fe-GO/CS复合材料的命名
Table 1 Naming of graphene oxide (GO)/ chitosan (CS), nano-iron (Fe)/CS and Fe-GO/CS composite
Sample GO/wt% FeCl3/wt% CS/g 1wt%GO/CS 1 — 4 3wt%GO/CS 3 — 4 5wt%GO/CS 5 — 4 16wt%GO/CS 16 — 4 1wt%Fe/CS — 1 4 5wt%Fe/CS — 5 4 8wt%Fe-GO/CS 16 8 4 -
[1] AZEEZ A A, RHEE K Y, PARK S J, et al. Application of cryomilling to enhance material properties of carbon nano-tube reinforced chitosan nanocomposites[J]. Composites Part B: Engineering,2013,50:127-134. DOI: 10.1016/j.compositesb.2013.01.010
[2] KYZAS G Z, TRAVLOU N A, DELIYANNI E A. The role of chitosan as nanofiller of graphite oxide for the removal of toxic mercury ions[J]. Colloids and Surfaces B: Biointerfaces,2014,113:467-476. DOI: 10.1016/j.colsurfb.2013.07.055
[3] 张中勋, 刘霞, 刘杨, 等. 氧化石墨烯修饰壳聚糖药物载体的构建[J]. 高分子材料科学与工程, 2019, 35(8):137-143. ZHANG Z X, LIU X, LIU Y, et al. Construction of graphene oxide modified chitosan drug carrier[J]. Polymer Materials Science and Engineering,2019,35(8):137-143(in Chinese).
[4] 陈潇, 张浩宇, 霍神焕, 等. 壳聚糖改性地聚合物的力学及吸附性能[J]. 复合材料学报, 2019, 36(12):2959-2967. CHEN X, ZHANG H Y, HUO S H, et al. Mechanical and adsorption properties of chitosan modified geopolymer[J]. Acta Materiae Compositae Sinica,2019,36(12):2959-2967(in Chinese).
[5] 何帅, 梁靖媚, 胡慧兰, 等. 氧化石墨烯/壳聚糖层层自组装复合膜的制备及其在环保领域中的应用[J]. 材料导报, 2016, 30(6):1-5. HE S, LIANG J M, HU H L, et al. Preparation of graphene oxide/chitosan layer-by-layer self-assembled composite film and its application in the field of environmental protection[J]. Materials Review,2016,30(6):1-5(in Chinese).
[6] 范林林, 李萌萌, 冯叙桥, 等. 壳聚糖涂膜对鲜切苹果贮藏品质的影响[J]. 食品科学, 2014, 35(22):350-355. DOI: 10.7506/spkx1002-6630-201422068 FAN L L, LI M M, FENG X Q, et al. The effect of chitosan coating on the storage quality of fresh-cut apples[J]. Food Science,2014,35(22):350-355(in Chinese). DOI: 10.7506/spkx1002-6630-201422068
[7] MINISY I M, SALAHUDDIN N A, AYAD M M. Adsorption of methylene blue onto chitosan-montmorillonite/polyaniline nanocomposite[J]. Applied Clay Science,2021,203:105993. DOI: 10.1016/j.clay.2021.105993
[8] LEE J H, MARRAOQUIN J, RHEE K Y, et al. Cryomilling application of graphene to improve material properties of graphene/chitosan nanocomposites[J]. Composites Part B: Engineering,2013,45(1):682-687. DOI: 10.1016/j.compositesb.2012.05.011
[9] NIKPOUR M R, RABIEE S M, JAHANSHAHI M. Synthesis and characterization of hydroxyapatite/chitosan nanocomposite materials for medical engineering applications[J]. Composites Part B: Engineering,2012,43(4):1881-1886. DOI: 10.1016/j.compositesb.2012.01.056
[10] VASILE C, DARIE R N, CHEABURU Y C N, et al. Low density polyethylene–Chitosan composites[J]. Composites Part B: Engineering,2013,55:314-323. DOI: 10.1016/j.compositesb.2013.06.008
[11] 施昌谷, 邵明聪, 李文娟, 等. 壳聚糖/氧化石墨烯复合涂膜食品包装纸的研究[J]. 中国食品学报, 2019, 19(7):192-200. SHI C G, SHAO M C, LI W J, et al. Research on chitosan/graphene oxide composite coating food packaging paper[J]. Chinese Journal of Food Science,2019,19(7):192-200(in Chinese).
[12] QIN Y, CHEN X, LI B, et al. Study on the mechanical properties and microstructure of chitosan reinforced metakaolin-based geopolymer[J]. Construction and Building Materials,2021,271:121522. DOI: 10.1016/j.conbuildmat.2020.121522
[13] LEE C, WEI X, KYSAR J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene[J]. Science,2008,321(5887):385-388. DOI: 10.1126/science.1157996
[14] CHEN Y F, QI Y Y, TAI Z X, et al. Preparation, mechanical properties and biocompatibility of graphene oxide/ultrahigh molecular weight polyethylene composites[J]. European Polymer Journal,2012,48(6):1026-1033. DOI: 10.1016/j.eurpolymj.2012.03.011
[15] LI Y, SUN J, DU Q, et al. Mechanical and dye adsorption properties of graphene oxide/chitosan composite fibers prepared by wet spinning[J]. Carbohydrate Polymers,2014,102:755-761. DOI: 10.1016/j.carbpol.2013.10.094
[16] YANG X, TU Y, LI L, et al. Well-dispersed chitosan/graphene oxide nanocomposites[J]. ACS Applied Materials & Interfaces,2010,2(6):1707-1713.
[17] AHMED J, MULLA M, ARFAT Y A, et al. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films[J]. Food Hydrocolloids,2017,71:141-148. DOI: 10.1016/j.foodhyd.2017.05.013
[18] AZARNIYA A, ESLAHI N, MAHMOUDI N, et al. Effect of graphene oxide nanosheets on the physico-mechanical properties of chitosan/bacterial cellulose nanofibrous composites[J]. Composites Part A: Applied Science and Manufacturing,2016,85:113-122. DOI: 10.1016/j.compositesa.2016.03.011
[19] HWANG S H, KANG D, S R R, et al. Poly(vinyl alcohol) reinforced and toughened with poly (dopamine)-treated graphene oxide, and its use for humidity sensing[J]. ACS Nano,2014,8(7):6739-6747. DOI: 10.1021/nn500504s
[20] PARK S, LEE K, BOZOKLU G, et al. Graphene oxide papers modified by divalent ions-enhancing mechanical propertiesvia chemical cross-linking[J]. ACS Nano,2008,2(3):572-578. DOI: 10.1021/nn700349a
[21] TERZIOGLU P, ALTIN Y, KALEMTAS A, et al. Graphene oxide and zinc oxide decorated chitosan nanocomposite biofilms for packaging applications[J]. Journal of Polymer Engineering,2020,40(2):152-157. DOI: 10.1515/polyeng-2019-0240
[22] 王博蔚, 马瑞, 吴凡, 等. 氧化石墨烯-海藻酸钠-壳聚糖复合支架的制备及表征[J]. 高等学校化学学报, 2020, 41(9):2099-2106. WANG B W, MA R, WU F, et al. Preparation and characterization of graphene oxide-sodium alginate-chitosan compo-site scaffold[J]. Chemical Journal of Chinese Universities,2020,41(9):2099-2106(in Chinese).
[23] YADAV M, RHEE K Y, PARK S J, et al. Mechanical properties of Fe3O4/GO/chitosan composites[J]. Composites Part B: Engineering,2014,66:89-96. DOI: 10.1016/j.compositesb.2014.04.034
[24] 赵超然, 单慧媚, 彭三曦, 等. 载铁氧化石墨烯壳聚糖对水中Cr(VI)的吸附研究[J]. 水处理技术, 2021, 47(4):1-7. ZHAO C R, SHAN H M, PENG S X, et al. Study on the adsorption of Cr(VI) in water by iron-loaded graphene oxide chitosan[J]. Water Treatment Technology,2021,47(4):1-7(in Chinese).
[25] 赵超然, 单慧媚, 曾春芽, 等. Fe@GOCS的制备及其对水中As(Ⅲ)的吸附[J]. 环境科学, 2020, 41(8):3665-3674. ZHAO C R, SHAN H M, ZENG C Y, et al. Preparation of Fe@GOCS and its adsorption of As(Ⅲ) in water[J]. Environmental Science,2020,41(8):3665-3674(in Chinese).
[26] SHAN H M, PENG S X, ZHAO C R, et al. Highly efficient removal of As(III) from aqueous solutions using goethite/graphene oxide/chitosan nanocomposite[J]. International Journal of Biological Macromolecules,2020,164:13-26. DOI: 10.1016/j.ijbiomac.2020.07.108
[27] SHAN H M, ZENG C Y, ZHAO C R, et al. Iron oxides decorated graphene oxide/chitosan composite beads for enhanced Cr(Ⅵ) removal from aqueous solution[J]. International Journal of Biological Macromolecules,2021,172:197-209. DOI: 10.1016/j.ijbiomac.2021.01.060
[28] LI L, LUO C, LI X, et al. Preparation of magnetic ionic liquid/chitosan/graphene oxide composite and application for water treatment[J]. International Journal of Biological Macromolecules,2014,66:172-178. DOI: 10.1016/j.ijbiomac.2014.02.031
[29] SHERLALA A I A, RAMAN A A A, BELLO M M, et al. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite[J]. Journal of Environmental Management,2019,246:547-556. DOI: 10.1016/j.jenvman.2019.05.117
[30] 张丽, 罗汉金, 方伟, 等. 改性氧化石墨烯/壳聚糖功能材料对刚果红的吸附研究[J]. 环境科学学报, 2016, 36(11):3977-3985. ZHANG L, LUO H J, FANG W, et al. Adsorption of Congo red on modified graphene oxide/chitosan functional materials[J]. Acta Scientiae Circumstantiae,2016,36(11):3977-3985(in Chinese).
[31] SHESHMANI S, NEMATZADEH M A, SHOKROLLAHZADEH S, et al. Preparation of graphene oxide/chitosan/FeOOH nanocomposite for the removal of Pb(Ⅱ) from aqueous solution[J]. International Journal of Biological Macromolecules,2015,80:475-480. DOI: 10.1016/j.ijbiomac.2015.07.009
[32] VANDE V K, KIEKENS P. Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state 13 C NMR[J]. Carbohydrate Polymers,2004,58(4):409-416. DOI: 10.1016/j.carbpol.2004.08.004
[33] NASIRI R, ARSALANI N, PANAHIAN Y. One-pot synthesis of novel magnetic three-dimensional graphene/chitosan/nickel ferrite nanocomposite for lead ions removal from aqueous solution: RSM modelling design[J]. Journal of Cleaner Production,2018,201:507-511. DOI: 10.1016/j.jclepro.2018.08.059
[34] SUBEDI N, LAHDE A, ABU D E, et al. A comparative study of magnetic chitosan (Chi@Fe3O4) and graphene oxide modified magnetic chitosan (Chi@Fe3O4GO) nanocompo-sites for efficient removal of Cr(VI) from water[J]. International Journal of Biological Macromolecules,2019,137:948-959. DOI: 10.1016/j.ijbiomac.2019.06.151
[35] SUTAR D S, SINGH G, NARAYANAM P K, et al. Spectroscopic studies of large sheets of graphene oxide and reduced graphene oxide monolayers prepared by Langmuir-Blodgett technique[J]. Thin Solid Films: An International Journal on the Science and Technology of Thin and Thick Films,2012,520(18):5991-5996.
[36] YU R M, SHI Y Z, YANG D Z, et al. Graphene oxide/chitosan aerogel microspheres with honeycomb-cobweb and radially oriented microchannel structures for broad-spectrum and rapid adsorption of water contaminants[J]. ACS Applied Materials & Interfaces,2017,9(26):21809-21819.
[37] 郭俊元, 甘鹏飞, 陈诚, 等. 磁性壳聚糖的制备及处理亚甲基蓝废水[J]. 中国环境科学, 2019, 39(6):2422-2430. DOI: 10.3969/j.issn.1000-6923.2019.06.023 GUO J Y, GAN P F, CHEN C, et al. Preparation of magnetic chitosan and treatment of methylene blue wastewater[J]. China Environmental Science,2019,39(6):2422-2430(in Chinese). DOI: 10.3969/j.issn.1000-6923.2019.06.023
[38] LEI Y, CHEN F, LUO Y, et al. Three-dimensional magnetic graphene oxide foam/Fe3O4 nanocomposite as an efficient absorbent for Cr(Ⅵ) removal[J]. Journal of Materials Science,2014,49(12):4236-4245. DOI: 10.1007/s10853-014-8118-2
[39] 赵茜, 邱东方, 王晓燕, 等. 壳聚糖/氧化石墨烯纳米复合材料的形态和力学性能研究[J]. 化学学报, 2011, 69(10):1259-1263. ZHAO Q, QIU D F, WANG X Y, et al. Morphology and mechanical properties of chitosan/graphene oxide nanocompo-sites[J]. Acta Chimica Sinica,2011,69(10):1259-1263(in Chinese).
[40] 陈元庆, 张大伟, 顾继友. 功能化氧化石墨烯/壳聚糖纳米复合材料的制备及力学性能研究[J]. 材料导报, 2015, 29(12):71-74. CHEN Y Q, ZHANG D W, GU J Y. Preparation and mechanical properties of functionalized graphene oxide/chitosan nanocomposites[J]. Materials Review,2015,29(12):71-74(in Chinese).
[41] YAN L, CHEN W, BANGAL P R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves[J]. Carbon: An International Jour-nal Sponsored by the American Carbon Society,2010,48(4):1146-1152.
[42] 黄文涛, 邓呈逊, 吉宇尘, 等. 壳聚糖功能化磁性氧化石墨烯的制备及对甲基橙的吸附[J]. 复合材料学报, 2021, 38(4):1262-1271. HUANG W T, DENG C X, JI Y C, et al. Preparation of chitosan functionalized magnetic graphene oxide and its adsorption to methyl orange[J]. Acta Materiae Compositae Sinica,2021,38(4):1262-1271(in Chinese).
[43] LOPEZ F A, MERCE A L R, ALGUACIL F J, et al. A kinetic study on the thermal behaviour of chitosan[J]. Journal of Thermal Analysis and Calorimetry,2008,91(2):633-639. DOI: 10.1007/s10973-007-8321-3
[44] HAN D, YAN L, CHEN W, et al. Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state[J]. Carbohydrate Polymers,2011,83(2):653-658. DOI: 10.1016/j.carbpol.2010.08.038
[45] LIU S, YAO F, ODERINDE O, et al. Zinc ions enhanced nacre-like chitosan/graphene oxide composite film with superior mechanical and shape memory properties[J]. Chemical Engineering Journal,2017,321:502-509. DOI: 10.1016/j.cej.2017.03.087
[46] 邢亚阁, 刘茜, 江雨若, 等. 壳聚糖/纳米TiO2复合涂膜抗菌及物理性能分析[J]. 西华大学学报(自然科学版), 2018, 37(2):34-39. DOI: 10.3969/j.issn.1673-159X.2018.02.007 XING Y G, LIU Q, JIANG Y R, et al. Antibacterial and physical properties analysis of chitosan/nano-TiO2 composite coating film[J]. Journal of Xihua University (Natural Science Edition),2018,37(2):34-39(in Chinese). DOI: 10.3969/j.issn.1673-159X.2018.02.007
-
期刊类型引用(1)
1. 蔡静宇,苗晓雨,赵玉来,肖龙强. FeOOH-RGO气凝胶光催化苯制备苯酚探索性教学实验设计. 大学化学. 2024(04): 169-177 .  百度学术
百度学术
其他类型引用(1)
-




 下载:
下载: