Research progress of dispersion modification and anticorrosion mechanism of graphene and its derivatives in coatings
-
摘要: 石墨烯(Gr)具有优异的光学、电学和力学等特性,在材料、能源或生物医学领域具有重要的应用前景,特别是Gr具有超隔离性,当将其用作涂料填料使用时,可极大提高涂层的防腐性能。但是由于Gr的高比表面积及层间的范德华力,又使其非常容易团聚,限制了其实际使用。对Gr进行分散改性,促进其在基体中的均匀分布,对扩大Gr的应用范围和提高材料的性能具有重要意义。本文主要介绍了Gr及其衍生物的共价改性、非共价改性、掺杂改性和原位聚合改性等方法,通过增加Gr层间位阻效应,改变Gr表面的双亲性,增强其与涂料聚合物基之间的相容性,从而提高其在涂料中的分散性。此外,本文还分析了各种改性方法的优缺点,提出了进一步提高Gr及其衍生物分散性的改性方向;总结了Gr及其衍生物在防腐涂料中的作用机制,建议今后在实验探索的基础上,加强对防腐机制的研究。Abstract: Graphene (Gr) has excellent properties such as super-isolation, super-hydrophobicity and super-high specific surface area, making it a revolutionary material for improving the anticorrosion performance of coatings in the 21st century. However, it is easy to agglomerate due to the Van Der Waals force and the high specific surface area between Gr layers, which limits its application in the anti-corrosion coatings. The dispersion modification of Gr can promote its uniform distribution in the coating, which is of great significance to improve the anticorrosive properties of the coating. This paper introduces the structural characteristics of Gr and the anticorrosion mechanism of Gr in coatings, and summarizes the methods of Gr dispersion modification, including the covalent modification of Gr with the small organic molecules, the organic polymers and the inorganic nanoparticles, the non-covalent modification of Gr through the π-π interaction, the hydrogen bonding and the ionic bonding, doping Gr to introduce the new elements and giving it excellent performance, and through the in-situ polymerization to improve the compatibility between Gr and polymer. According to the dispersion principles such as increasing the interlayer steric hindrance of Gr, changing the amphiphilicity of its surface and increasing its compatibility with the polymer, the dispersibility of Gr can be improved remarkable. The uniformly distributed Gr forms a hydrophobic network in the coating, which can effectively improve the corrosion resistance of the coating and expand the application range of the coating. In addition, the paper also analyzed the advantages and disadvantages of various modification methods, and proposed a research direction to further improve the dispersion of Gr and its derivatives; summarized the mechanism of Gr and its derivatives in anti-corrosion coatings, and suggested to strengthen the research on the anti-corrosion mechanism based on experimental exploration in the future.
-
金属腐蚀造成大量资源和能源的浪费,其损失甚至超过了火灾、风灾和地震造成损失的总和。提高金属防腐性能的常用方法,包括涂层保护[1]、电化学保护[2]和添加缓蚀剂保护[3-4]等,其中通过防腐涂料进行金属涂层保护是目前最有效的方法。常规防腐涂料以溶剂型居多,大量挥发性有机物质的排放易导致大气污染。为践行绿色化工,防腐涂料的水性化势在必行。但是由于水性涂料具有较多亲水基团,易在涂层中形成极性通道,加快腐蚀介质的渗透,使金属腐蚀现象不能完全避免。通过在涂料中添加有机或无机纳米填料,可在改善涂层阻隔性能的同时,增强涂层的防腐性能。常用填料包括蒙脱土[5]、BN[6]、SiO2[7-8]、聚苯胺(PANI)[9-11]和石墨烯(Gr)[12-13]等。相比于其他纳米填料,Gr具有超高的比表面积,且强度高、韧性好、化学稳定性好,而其优异的超隔离性使其成为最有前途的防腐涂料用填料,但是由于Gr的超疏水性及层间存在的范德华力,又易使其在水性介质中发生团聚。如何保证Gr在水性介质中的均匀分散,是提高涂料的防腐性能的关键。本文在介绍Gr及其衍生物的结构及特征的基础上,着重对Gr分散改性方法及其在防腐涂料中的作用机制进行综述,并对其未来发展进行了展望。
1. Gr及其衍生物的结构及特征
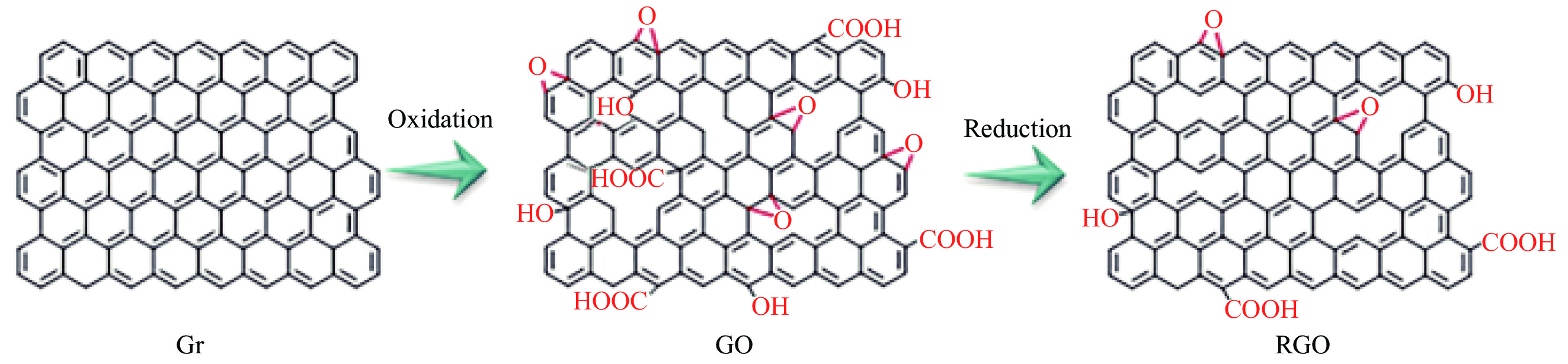
石墨烯(Gr),是一种由碳原子以sp2杂化方式形成、只有一个原子层厚度(0.34 nm)的蜂窝状准二维平面薄膜材料,因此又叫做单原子层石墨[14]。目前制备Gr的方法主要有机械剥离法[15-16]、晶体外延生长法[17]、化学气相沉积法[18-19]和氧化还原法[20-22]等。Gr通过进行氧化反应可得到氧化石墨烯(GO),GO表面带有大量的含氧基团,如羧基(—COOH)、羟基(—OH)和环氧基等[23]。由于含氧基团的存在,使GO表面存在结构缺陷,相比于Gr,GO的导电性和力学性能下降、厚度增加、亲水性增加。GO表面带有的大量活性位点,又可为GO的共价改性提供方便。还原氧化石墨烯(RGO),是通过化学还原和热还原等多种还原手段,不完全去除GO表面的含氧基团得到的。RGO表面带有少量的含氧基团,仍可为接枝反应提供活性位点。相比于GO,RGO结构缺陷减少、亲水性下降、阻隔效果更优。Gr、GO和RGO的结构如图1所示。
2. Gr及其衍生物的分散改性
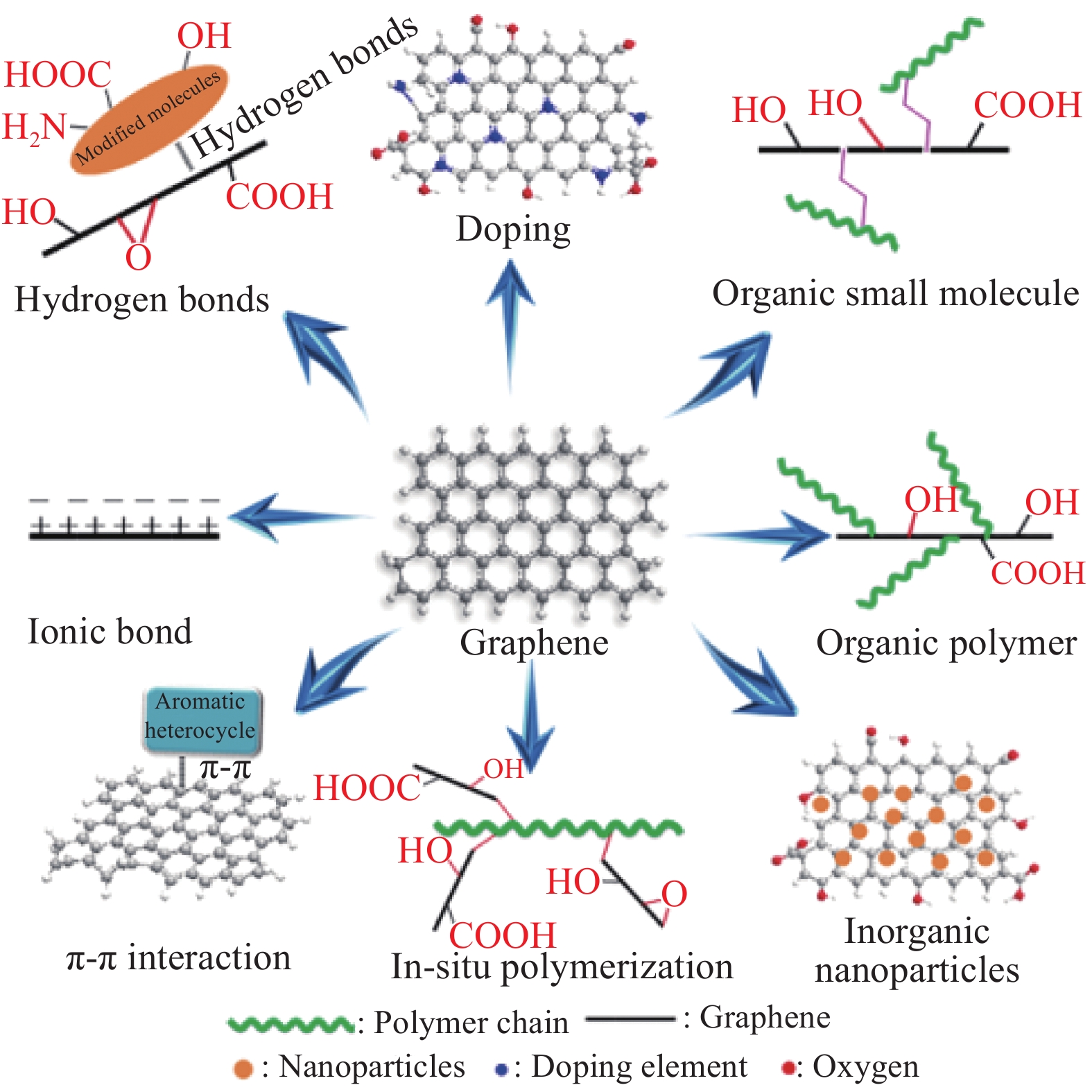
高比表面积及层间较强的范德华力,使Gr及其衍生物非常容易团聚;此外Gr及其衍生物属于无机材料,与涂料成膜物质(有机聚合物基质)的相容性较差。仅通过机械作用破坏Gr层间的范德华力,所得Gr及其衍生物的稳定性较差,在失去机械作用后仍会发生团聚,而且这种简单的机械分散虽然操作便捷,但费时。为了提高Gr及其衍生物的分散效率,可通过分散改性技术,改变Gr及其衍生物表面的亲疏水性,增加其层间的位阻效应,提高其与聚合物基质的相容性。由于GO和RGO表面带有不同比例的含氧基团,为其分散改性提供了活性位点,因此本文主要综述GO和RGO的分散改性。图2和表1分别总结了Gr衍生物(GO、RGO)的分散改性方法。
表 1 Gr及其衍生物的分散改性方法总结Table 1. Summary of dispersion modification methods of Gr derivativesModification method Modified materials Modified group Principle of dispersion References Covalent modification Organic small molecules Silane coupling agenttitanate coupling agent —OH, —COOH, epoxy group Steric hindrance, compatibility [24-31] Organic amines —COOH, epoxy group Compatibility, amphipathic [32-35] Isocyanates —OH, —COOH, epoxy group Compatibility [36-38] Organic polymer PVP, PANI, PEI, PEG, PCD, Polyisocyanate —OH,—COOH, epoxy group Compatibility, amphipathic [39-44] Inorganic nanoparticles SiO2, TiO2, Si3N4, CaCO3, Al2O3 —OH, —COOH, epoxy group Steric hindrance [45-53] Non-covalent modification π-π interaction Aniline trimer, PDA, PPy, PANI, P2BA, PAT, PGHEP, lignin, TP, BN, GO Six-membered carbon ring Steric hindrance [32, 54-64] Ionic bond IPDI, CTAB, DA —OH, —COOH, epoxy group Steric hindrance, compatibility [65-67] Hydrogen bond PPy, ATP, PANI —OH, —COOH, epoxy group Amphipathic, steric hindrance [68-70] Doping modification 3 amino-1,2,4-triazole, PA — Steric hindrance [71-72] In-situ polymerization ACAT, NABM, ABA —COOH, epoxy group, six-membered carbon ring Compatibility [73-75] Notes: PVP—Polyvinylpyrrolidone; PANI—Polyaniline; PEI—Polyethyleneimine; PEG—Polyethylene glycol; PCD—Polycarbodiimide; P2BA—Poly(2-butylaniline); PPy—Polypyrrole; PAT—Poly(2-aminothiazole); TP—Tea polyphenol; PGHEP—Hydroxy epoxy phosphate monomer; DA—Dopareine; ATP—Attapulgite; ACAT—Amino-terminated aniline trimer; NABM—N-(2-aminoethyl)-2-bromo-2-methylpropanamide; PA—Phytic acid; ABA—4-aminobenzoic acid. 2.1 共价改性
共价改性是将改性材料接枝至GO或RGO表面的活性位点,增大GO或RGO的层间距,增加其与聚合物基质的相容性,从而改善GO或RGO团聚的现象。在GO或RGO的共价改性中,共价键的断裂将破坏GO或RGO的共轭结构,而这些结构缺陷会成为H2O、O2和Cl−等腐蚀介质在涂层中的通道,最终造成被保护金属材料的腐蚀,导致GO或RGO的防腐效果下降。虽然该方法操作复杂且使GO或RGO存在结构缺陷,但所得Gr增强聚合物基复合涂料的稳定性好。共价改性所用材料多样,本文主要从有机小分子改性、有机聚合物改性和纳米粒子改性三个方面进行介绍。
2.1.1 有机小分子改性
常用于改性的有机小分子主要有偶联剂[24-31]、有机胺[32-35]和异氰酸酯类单体[36-38]。通过引入的有机小分子,将GO或RGO与聚合物连接起来,或改变GO、RGO的双亲性,进而改变其与聚合物基质的相容性,改善其在涂层中的分布,从而提高涂层的防腐性能。
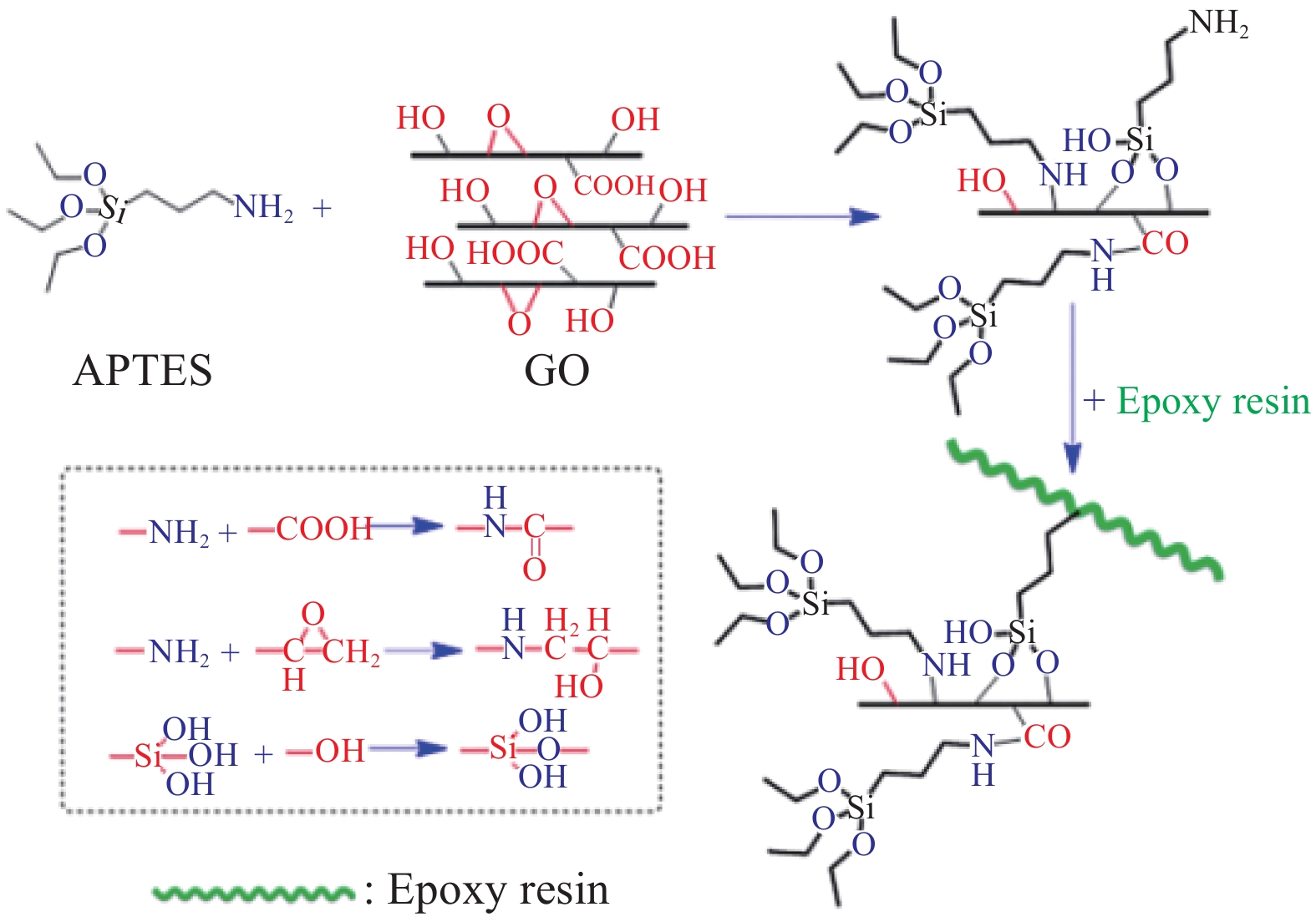
偶联剂作为“桥梁”连接GO或RGO与聚合物,增强它们之间的界面相互作用,从而改善二者相容性。偶联剂主要有硅烷偶联剂[24-30]和钛酸酯偶联剂[31]。硅烷偶联剂除了含有硅烷氧基团之外,通常还带有氨基(—NH2)或环氧基团。除了硅烷氧基团水解形成的硅羟基可与GO或RGO表面的—OH形成共价键外,偶联剂所带—NH2一方面可与GO或RGO表面的含氧基团发生反应,增加GO或RGO的层间距,另一方面还可与环氧涂料中的环氧基团形成共价键[24](图3)。常用的硅烷偶联剂主要有:γ-氨丙基三乙氧基硅烷(APTES)[24-27]、γ-氨丙基三甲氧基硅烷(APTMS)[28]、γ-(2,3-环氧丙氧)丙基三甲氧基硅烷(EPTES)[29]、(3-环氧丙氧基丙基)三甲氧基硅烷(GPTS)[30]等。硅烷偶联剂的选择与聚合物基质相关,例如APTES和EPTES对GO的改性都可提高GO在环氧树脂(EP)中的分散性,但是APTES的分散效果优于EPTES,这是由于尽管EPTES的环氧基与EP有一定相容性,但是APTES上的—NH2可与EP的环氧基形成共价键,使体系更稳定。同理,钛酸酯偶联剂也具有相类似的作用。Wang等[31]利用不同树枝状结构的钛酸酯偶联剂改性RGO,显著提高了RGO在聚合物基质中的分散稳定性。RGO与水性聚氨酯(WPUA)的界面相互作用、复合涂层的力学性能和防腐性能,均与钛酸酯偶联剂的支化程度相关,三个支化焦磷酸二辛基酯的钛酸酯偶联剂改性的RGO(T3G)效果最优,T3G/WPUA涂层的长期耐蚀性优于溶剂型聚氨酯涂层的性能。T3G/WPUA涂层的阻抗模量为1.12×105 Ω·cm2远高于纯WPUA涂层(6.93×106 Ω·cm2),其涂层电阻为8.41×104 Ω·cm2远高于纯WPUA(1.39×108 Ω·cm2)。
有机胺类物质的改性原理与偶联剂改性具有类似性。通过有机胺类小分子对GO进行氨基官能化改性,可改善GO的双亲性及与聚合物基质的相容性,从而提高GO的分散性。常用的有机胺类小分子,包括单胺和二胺,例如多巴胺[32]、十八烷基胺[33]、对苯二胺[34]、乙二胺和1, 6-己二胺[35]。Ramezanzadeh等[34]利用对苯二胺的—NH2与 GO表面的—COOH和环氧基团形成共价键,增加GO的层间距,同时氨基功能化的GO(FGO)还可与水性环氧树脂形成共价键,增强它们之间的界面相互作用。在3.5wt% NaCl溶液中浸泡40天后,FGO/EP涂层损伤指数(CDI)为56%低于纯EP涂层(92%)。单胺类小分子通常是通过改变GO的双亲性来提高其分散性;二胺类小分子通常是作为GO和聚合物连接的桥梁,通过偶联提高它们之间的相容性及稳定性。
此外,还可以通过接枝异氰酸酯类单体增强GO与聚合物的相容性,进而促进其分散。异氰酸酯类单体主要包括芳基异氰酸酯和烷基异氰酸酯[36-38]。Bai等[37]用异佛尔酮二异氰酸酯(IPDI)改性GO(MGO),MGO上的异氰酸酯基(—NCO)可与聚氨酯(PU)形成共价键,进而提高相容性。MGO的用量将影响其在水性聚氨酯基质中的分散性及涂层的耐摩擦性能,随着MGO质量分数的增加,分散性及涂层的摩擦系数先降低后升高,当MGO为1.5wt%时,MGO能均匀分散,复合涂层的摩擦系数最小为0.11。
2.1.2 有机聚合物改性
通过与GO或RGO表面的—COOH、—OH或环氧基等活性官能团反应,在其表面接枝有机聚合物,是为了在提高GO或RGO分散性的同时,发挥聚合物本身的防腐优势,两者协同作用提高涂料的防腐性能,改善涂料的力学性能、热性能和抗菌性等其他性能。
将聚乙烯吡咯烷酮(PVP)[39]、聚苯胺(PANI)[40]和聚乙烯亚胺(PEI)[41]接枝在GO或RGO表面后,可提高其在EP中的分散性。Zhang等[39]通过热合成法,将PVP接枝至RGO的表面,所得PVP-RGO可以很好地分散在水性环氧树脂基质中,两者具有良好相容性,PVP-RGO用量为0.7wt%时,涂层的力学性能和热性能得到显著提高,且随PVP-RGO用量增加,涂层可塑性下降。同理,PANI在过硫酸铵引发剂和月桂基硫酸钠表面活性剂作用下与GO共价键合,所得PANI-GO也表现出与水性环氧树脂基质良好的相容性,PANI-GO/EP涂层的阻隔性随分散性提高而改善。相比于纯EP涂层,在3.5wt% NaCl溶液浸泡450 h后,PANI-GO/EP涂层仍具有较好的耐蚀性[40]。利用PEI线型大分子链上所带的大量—NH2与GO表面—COOH或环氧基团共价反应,接枝有PEI分子链的GO表面因此引入了大量的—NH2,PEI分子链作为纽带可将GO与EP连接起来,从而提高两者相容性[41]。相比于纯EP涂层和GO/EP涂层,PEI-GO/EP涂层的初始阻抗值更高,耐腐蚀性能更优。
除上述有机聚合物外,GO也可用聚异氰酸酯[42]、聚乙二醇(PEG)[43]和聚碳化二亚胺(PCD)[44]改性,提高其在PU中的分散。聚异氰酸酯通过与GO表面的含氧基团形成酰胺键和氨基甲酸酯键接枝至GO表面(FGO),增强了GO在水性聚氨酯中的相容性和分散性。与纯PU涂层相比,GO含量为0.1wt%增加了PU涂层的低频阻抗,FGO/PU涂层的耐蚀性提高至少10倍[42]。同理,通过脲化反应可将PEG引入RGO(FRGO),将FRGO作为扩链剂、杀菌剂和增强剂,可制备超支化聚氨酯复合涂料(HPUC),提高RGO与PU的相容性,在5wt% NaCl溶液中浸泡504 h后,HPUC开始被腐蚀,而FRGO-HPUC涂层完好无损[43]。
针对不同的聚合物基质,要求用于改性GO或RGO的有机聚合物要能与聚合物基质相容,从而提高GO或RGO与聚合物基质的相容性,并改善聚合物基质的性能。例如在EP中,可选用PANI对GO进行改性,由于PANI可同时参与EP的固化反应,进一步提高GO与EP的相容性;在PU中,可选用PEG或PCD对GO进行改性,PEG可作为PU的扩链剂,进一步提高GO与PU的相容性,PCD可改善PU的交联密度,在改善相容性的同时提高涂层耐水性。
2.1.3 无机纳米粒子改性
采用无机纳米粒子改性,可增加GO或RGO片层间的位阻效应,减少其聚集现象,从而改善其在聚合物中的分散性。在复合涂料体系中,纳米颗粒不但可以堵塞涂料成膜过程中出现的微孔,提高涂层对金属的防腐性能,还可赋予其他优异性能,如紫外稳定性、抗菌性或化学惰性。常用的无机纳米粒子包括:SiO2、TiO2、Si3N4、CaCO3和Al2O3等。根据纳米粒子的来源不同,无机纳米粒子改性方法可分为两种:一种利用硅烷偶联剂将纳米粒子接枝在GO或RGO表面;另一种是在GO或RGO表面原位生长纳米粒子。
通过硅烷偶联剂可将纳米SiO2[45]、 纳米TiO2[46-47]、纳米Si3N4[48]、纳米CaCO3[49]和纳米Al2O3[50]等装饰在GO或RGO表面。Yu等[46]使用APTES先对纳米TiO2进行改性引入—NH2,再利用—NH2与GO表面的含氧基团之间的共价作用,将纳米TiO2接枝到GO表面制得TiO2-GO杂化物。也可先利用APTES的—NH2与GO表面含氧基团反应引入硅烷氧基团,纳米TiO2与硅烷氧基团形成共价键,制备TiO2-GO材料(图4)[47],GO上的纳米TiO2使其层间距增加,提高其在EP中分散性,并通过纳米TiO2对涂层微孔的堵塞,抑制腐蚀介质的渗透,纯EP的腐蚀电流密度比TiO2-GO/EP涂层高近100倍,TiO2-GO/EP涂层的防腐性能更优。此外,由于纳米TiO2本身具有很好的紫外稳定性,还能赋予复合涂层优异的抗紫外老化性能。
此外,利用含硅化合物与GO或RGO表面上的含氧基团发生原位反应生成纳米SiO2,也可实现对GO或RGO的分散改性。例如利用硅酸钾作为合成纳米SiO2的原料,经过水解和缩合在GO表面原位反应连接上纳米SiO2[51];选用硅酸四乙酯(TEOS)作为纳米SiO2的原料,在GO表面依次接枝聚丙烯酸、硅烷偶联剂APTES和TEOS,原位生长纳米SiO2。通过简单的机械搅拌和超声处理,SiO2-GO分散到水性环氧树脂涂料中,可显著提高涂层的强度和韧性[52]。不同颗粒尺寸纳米SiO2对SiO2-GO/EP涂层的增强效果不同,随着纳米SiO2直径的增加,其数目将减少,导致其与GO的相互作用力减弱,SiO2-GO/EP涂层的力学性能下降[53]。
经纳米粒子改性的GO或RGO通常适用于提高环氧涂料的防腐性能。环氧涂料在热固化成膜的过程中,溶剂的挥发形成微孔,纳米粒子堵塞微孔,从而提高防腐性能。通过硅烷偶联剂接枝在GO表面的纳米粒子,由于制备方法及纳米粒子易团聚的限制,纳米粒子对GO的改性通常会引起二次团聚;而在GO表面原位生长的纳米粒子不仅可有序排列,还可控制其形状和结构状态,具有很好的优势。
2.2 非共价改性
除了通过共价改性改善GO或RGO在涂料中的分散性能外,还可利用改性材料与GO或RGO之间的非共价相互作用提高其分散性。非共价相互作用包括:离子键相互作用、共轭π-π相互作用、氢键相互作用等。非共价改性过程中不会发生化学键断裂或开环反应,不会破坏GO或RGO原有结构和性能,且操作简单;但是非共价相互作用力弱,使改性后的GO或RGO结构不稳定,因此用其制备的复合涂料稳定性相对较差。
2.2.1 共轭π-π相互作用改性
利用GO或RGO与改性材料的π-π相互作用,破坏其层间的范德华力,增大层间距,促进GO或RGO在树脂基质中的分散,是GO或RGO非共价改性的重要手段。GO或RGO本身具有高度共轭体系,易与具有相类似结构的小分子或聚合物产生较强的π-π相互作用。可选用具有共轭结构或芳(杂)环结构的聚合物或小分子对GO或RGO进行非共价改性,如苯胺三聚体衍生物[54-55]、聚多巴胺(PDA)[32]、聚吡咯(PPy)[56]、聚苯胺[57]、聚(2-丁基苯胺)(P2BA)[58]、聚(2-氨基噻唑)(PAT)[59]、羟基环氧磷酸酯单体(PGHEP)[60]、木质素[61]、茶多酚(TP)[62]和六方氮化硼(h-BN)[63]等有机或无机化合物。GO和Gr混合使用时,GO的亲水性可促进Gr的在水性环氧树脂的分散,Gr也可弥补GO的结构缺陷,在3.5wt%NaCl溶液中浸泡100 h后,纯EP涂层在划痕处有起泡现象,浸泡200 h后,GO/EP涂层开始被腐蚀,GO-Gr/EP涂层仍具有防腐效果[64]。
利用水溶性羧化苯胺三聚体衍生物作为稳定剂,可使其通过π-π相互作用对Gr进行改性制得羧化石墨烯(G-CAT−),G-CAT−在高浓度下(>1 mg·mL−1)依然能在水中有良好分散性。将G-CAT−与EP共混所得复合防腐涂料(G-CAT−/EP),与纯EP涂层相比,G-CAT−/EP涂层腐蚀密度从5.62×10−7 A·cm−2降低到1.45×10−7 A·cm−2,防腐效率提高2.59%[54]。也可利用h-BN对气体和液体良好的不渗透性对GO进行改性,GO和h-BN之间的π-π相互作用使h-BN均匀地堆叠在GO表面上,通过协同作用提高涂料的防腐性能[63];m(h-BN)与m(GO)的比值会影响复合物在水中的分散状态,当m(h-BN)与m(GO)的比值为1或更高时,48 h后未分层;当m(h-BN)∶m(GO)=1∶1时,复合涂层可获得最高阻抗值,防腐性能最优。还可利用天然生物材料木质素的双亲性及其与RGO之间的π-π相互作用,提高RGO在水中的分散性[61]。
利用GO或RGO与改性材料之间的共轭π-π相互作用进行改性,改性过程操作简单,但所选改性材料通常应具有共轭结构或芳(杂)环结构。对于PDA、PPy等有机聚合物或PGHEP小分子,在制备过程中通常需使用有机溶剂或强酸强碱,对环境的影响较大。选用木质素和TP等可再生生物质材料对RGO进行改性,不消耗有机溶剂或强酸强碱,有利于绿色化工的发展。
2.2.2 离子键相互作用改性
通过离子键相互作用改善GO或RGO的分散是非共价修饰的另一个重要手段。离子键相互作用改性手段主要有两种:一是使GO或RGO与改性材料带有相反的电荷,两者通过静电吸附作用使改性材料附着在GO或RGO表面,增加层间距,提高分散性;二是使GO或RGO带有与离子型聚合物基质相反的电荷,通过其与聚合物基质之间形成的离子键,提高GO或RGO与聚合物基质的相容性,进而促进GO或RGO的分散。
Luo等[65]通过原位合成和成盐反应,制备了一种新型的阳离子还原氧化石墨烯 (RGO-ID+),RGO-ID+通过与水性环氧树脂的离子相互作用自对准平行排列在涂层中,进而改善涂层的防腐性能。Wang等[66]将阳离子表面活性剂CTAB改性RGO制备阳离子石墨烯(CGR),然后在相转化过程中将CGR引入羧化聚氨酯预聚物中,通过离子相互作用和氢键作用,共同改善CGR和水性聚氨酯丙烯酸酯(WPUA)之间的相互作用和相容性。与纯WPUA相比,CGR-WPUA的腐蚀防护效率提高了78.2%。多巴胺(DA)经氧化自聚合和电离反应,制备得到新型的阳离子型多巴胺还原氧化石墨烯 (DRGO+),DRGO+在电场力作用下可在水性阴离子型环氧树脂乳液中稳定分散45天,无沉淀。DRGO+的加入可以在涂层中形成屏障网络,显著提高涂层的物理屏障效果并延长腐蚀剂的渗透路径,大大提高了涂层的防腐性能。此外,DRGO+上的—NH3+可以静电吸引电子和腐蚀性负离子,并在基材表面形成致密的钝化膜。DRGO+/EP涂层的低频阻抗模量(4.79×1010 Ω·cm2)是纯EP涂层(4.07×109 Ω·cm2)的10倍[67]。
2.2.3 氢键改性
GO表面带有—OH、—COOH等含氧基团,这些基团易与其他物质的活性基团形成氢键,如—NH2、—OH和—COOH等,故可通过与GO的氢键作用对其进行非共价改性。例如PPy上的—NH—可与GO上的—COOH形成氢键,在GO表面成功生长PPy膜[68]。凹凸棒石(ATP)与GO之间存在氢键相互作用,利用ATP对GO进行改性,可改善GO与水的相容性,使GO在水性涂料中均匀分散,进一步提高涂料的防腐性能[69]。Fazli-Shokouhi等[70]采用改良的Hummers方法制备了分散良好的GO,GO表面的含氧基团可与PANI上的—NH2形成氢键制备PAIN-GO材料,并将PAIN-GO掺入水性环氧树脂基质中获得PAIN-GO/EP涂料,PAIN-GO/EP涂层在3.5wt% NaCl溶液中浸泡192 h后,其最高阻抗模量可达2.70 ×106 Ω·cm2,比纯EP涂层(1.00×104 Ω·cm2)高100倍。氢键是一种极性较强的非共价键,它的形成不涉及分子结构的变化,不会破坏其原有的结构,因此适合于GO的分散。
2.3 掺杂改性
通过掺杂改性可以赋予GO或RGO一些新的优异性能,如电性能,也可以填补其结构缺陷,形成新的化学结构,同时也可促进它的分散。主要的掺杂方法包括元素掺杂法、氧化物掺杂法和碳质材料掺杂法。
Suman等[71]以3-氨基-1,2,4-三唑为氮源,在RGO结构上掺杂了多个N原子,N原子的掺杂填补了GO的结构缺陷,同时引入了新的化学结构,例如吡啶、吡咯和石墨氮(图5)。N原子的掺杂有助于纳米Fe3O4在RGO表面上的均匀生长(Fe3O4-NRG),Fe3O4-NRG均匀分散在水性环氧基质中,并促进复合涂层牢固附着在钢表面形成抑制层。相比于纯EP涂层和Fe3O4/EP涂层,Fe3O4-NRG/EP涂层具有低的腐蚀电流密度,防腐性能更优。Wang等[72]选用植酸(PA)掺杂GO,PA上的磷酸基团与环氧基团通过开环反应,为GO引入新的含P元素的五元环结构。PA的存在使GO层间距增加,GO的分散性提高。PA-GO/EP涂层的电阻比纯EP涂层高100倍,随着在3.5wt% NaCl溶液中浸泡时间的延长,CDI增加,浸泡1 080 h时,纯EP涂层的CDI约为90%,PA-GO/EP涂层的CDI约为30%。掺杂改性可改变GO或RGO的结构,赋予其优异性能,应用范围更广,但是掺杂过程难控制,难以定量控制新结构的形成。
2.4 原位聚合改性
原位聚合通常采用改性后的GO或RGO上的功能性基团作为反应活性位点,引发单体聚合,或采用共价键或非共价键的方式与聚合物进行连接,从而改善GO或RGO与聚合物的相容性,使其均匀分散在聚合物基质中。
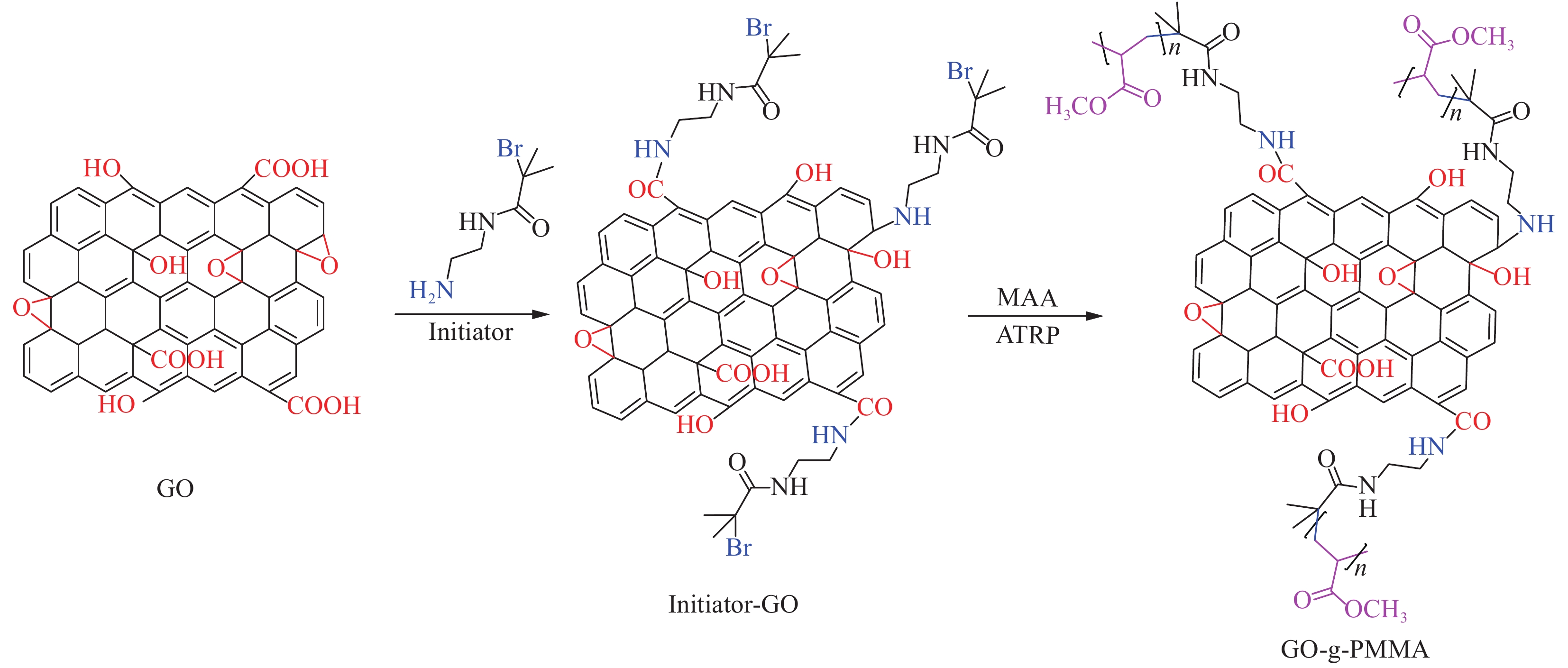
Chang等[74]先在多磷酸(PAA)和五氧化二磷(P2O5)的环境下,通过热合成制备了4-氨基苯甲酸(ABA)改性的石墨烯(ABF-G),ABF-G和苯胺进行原位聚合制得ABF-G/PANI涂层,极大地提高了对腐蚀性介质(如H2O、O2)的屏蔽性能及对金属的保护效果,但是制备ABF-G的周期长,不适合工业化生产。Chang等[73]采用羧化的GO、4’-(4,4’-异亚丙基二苯氧基)双(邻苯二甲酸酐) (BASS)和氨基封端的苯胺三聚体(ACAT)作为原料进行原位聚合,GO表面上的—COOH和ACAT上的—NH2形成共价键将其固定,使GO均匀分散在聚酰亚胺(PI)中,成功制得GO/PI涂料,但是此种方法引入的GO量较少,GO在聚合物基质中的分散性与其表面的羧基含量相关,羧基含量越高分散性越好。Qi等[75]选用N-(2-氨基乙基)-2-溴-2-甲基丙酰胺(NABM)改性GO、甲基丙烯酸甲酯单体(MMA)为原料,通过原子转移自由基聚合(ATRP),原位聚合制得GO-g-聚甲基丙烯酸甲酯(PMMA)涂料(图6)。PMMA的用量影响涂料的成膜能力及附着力,在PMMA用量为81wt%时,涂层的性能最佳,腐蚀电流密度下降了3~4个数量级,在3.5wt% NaCl溶液中浸泡100 h后,该涂层可为基材提供有效的保护。
原位聚合方法可有效提高Gr及其衍生物在聚合物中的分散稳定性,对于提高Gr/聚合物基复合材料的力学、电学性能及其他性能发挥着重要作用,但原位聚合在聚合过程中,体系的黏度变化较大,转化率下降,而且原位聚合所用溶剂也会对环境造成污染。
3. Gr及其衍生物在防腐涂料中的作用
涂层保护下的金属基材发生腐蚀,一般经过以下三个过程:首先在涂层上吸收腐蚀介质,其次通过涂层扩散腐蚀介质,然后在涂层和金属界面与腐蚀介质接触后发生腐蚀。Gr及其衍生物在涂层中的防腐作用,主要归结为物理屏蔽作用和电化学保护作用。
3.1 物理屏蔽作用
Gr及其衍生物具有超疏水性和超高比表面积,其片层结构可重叠、交织排列,在涂层中可形成复杂的疏水网络,能够有效抑制H2O、O2和Cl−等腐蚀介质的浸润、渗透和扩散;同时也可形成“迷宫”,延长腐蚀介质的扩散路径,使基底材料的腐蚀速率下降;且Gr单层厚度小,可以堵塞涂料成膜过程形成的微孔,减少涂层孔隙率,进一步提高涂层的物理屏蔽作用[24,36-37,41,51,61,66,76-79](图7)。
Li等[76]将GO、RGO和功能化石墨烯(FG)分别添加到水性聚氨酯基质中作为防腐增强剂,电化学阻抗谱(EIS)结果表明,添加0.2wt% RGO所得RGO/PU涂层的防腐性能最优,在3.5wt%的NaCl溶液中浸泡235 h未发生底漆腐蚀,0.1 Hz的阻抗模量保持在109 Ω,这是由于RGO的高长径比和出色的疏水性能,使均匀分布在聚氨酯基质中的RGO形成超疏水网络,该网络可抑制外来腐蚀介质的渗入,进而提高涂料的防腐性能。Yu等[46]借助APTES将纳米Al2O3锚固在GO上,将制得的Al2O3-GO片状杂化物引入EP中,Al2O3-GO会堵塞微孔,阻断腐蚀介质的渗透,从而提高其防腐性能。此外,有机硅树脂涂层也可通过添加GO显著增强涂层的阻隔性能,降低金属的腐蚀效率,腐蚀电位−108 mV远高于裸铜(−236 mV),腐蚀电流0.134 µA·cm−2低于裸铜(1.96 µA·cm−2)[79]。
3.2 电化学保护作用
Gr及其衍生物可以有效阻碍金属腐蚀反应的进行,其导电性可以将金属材料失去的电子传递至涂层表面,在基底材料表面形成钝化膜(Fe2O3和Fe3O4)降低腐蚀速率;同时也可吸收腐蚀介质Cl−,避免其渗透至金属表面造成腐蚀,从而为基底材料提供电化学保护[25, 34, 67-68, 80](图8)。
Zhu等[80]将水分散性石墨烯(PG)与水性丙烯酸改性醇酸树脂(AMAR)混合得到PG/AMARs防腐涂料,一方面可通过物理屏蔽作用提高涂层的防腐性能;另一方面可通过阳极保护作用提高防腐性能,Fe在腐蚀区域产生的电子能够通过导电涂层迁移出去,可有效抑制腐蚀点的氧化还原反应及腐蚀的扩散趋势。将经PG/AMARs涂层保护的钢在盐介质中360 h,基底材料钢未被腐蚀。
4. 结论与展望
石墨烯(Gr)及其衍生物在涂料中均匀分布是提高涂料防腐性能的关键。Gr及其衍生物主要是通过物理屏蔽作用和电化学保护作用提高涂层的防腐性能。共价改性、非共价改性、掺杂改性和原位聚合改性是提高Gr及其衍生物分散的常用方法,虽然可使Gr及其衍生物均匀分散,但都存在不足。通过偶联剂、有机胺、纳米粒子进行的共价改性,所得改性产物稳定性好,但操作复杂,且改性过程中形成的结构缺陷将影响防腐效果。通过共轭π-π相互作用、氢键作用和离子键作用进行的非共价改性,虽操作简单、涂层防腐效果较好,但得到的复合涂料稳定性较差。掺杂改性可以引入新的元素,修补其结构缺陷,但是难以对掺杂过程进行定量控制。原位聚合将氧化石墨烯(GO)或还原氧化石墨烯(RGO)引入到聚合物链中,GO或RGO与聚合物的相容性提高,但聚合过程中黏度变化较大,转化率下降。对于Gr及其衍生物的分散改性存在的不足之处,今后可以在以下几个方面开展工作:
(1) 改善Gr制备方法,在不破坏Gr结构的情况下,提供更多活性位点,方便对其进行共价改性,并改善其与聚合物基质的界面相容性。
(2) 寻找简单高效的分散剂,使Gr能均匀稳定地分布在聚合物基质中。
(3) 目前Gr防腐涂料的研究仍以实验探索为主,对防腐机制的研究不足,需要更深入全面地研究,进一步完善防腐机制。
-
表 1 Gr及其衍生物的分散改性方法总结
Table 1 Summary of dispersion modification methods of Gr derivatives
Modification method Modified materials Modified group Principle of dispersion References Covalent modification Organic small molecules Silane coupling agenttitanate coupling agent —OH, —COOH, epoxy group Steric hindrance, compatibility [24-31] Organic amines —COOH, epoxy group Compatibility, amphipathic [32-35] Isocyanates —OH, —COOH, epoxy group Compatibility [36-38] Organic polymer PVP, PANI, PEI, PEG, PCD, Polyisocyanate —OH,—COOH, epoxy group Compatibility, amphipathic [39-44] Inorganic nanoparticles SiO2, TiO2, Si3N4, CaCO3, Al2O3 —OH, —COOH, epoxy group Steric hindrance [45-53] Non-covalent modification π-π interaction Aniline trimer, PDA, PPy, PANI, P2BA, PAT, PGHEP, lignin, TP, BN, GO Six-membered carbon ring Steric hindrance [32, 54-64] Ionic bond IPDI, CTAB, DA —OH, —COOH, epoxy group Steric hindrance, compatibility [65-67] Hydrogen bond PPy, ATP, PANI —OH, —COOH, epoxy group Amphipathic, steric hindrance [68-70] Doping modification 3 amino-1,2,4-triazole, PA — Steric hindrance [71-72] In-situ polymerization ACAT, NABM, ABA —COOH, epoxy group, six-membered carbon ring Compatibility [73-75] Notes: PVP—Polyvinylpyrrolidone; PANI—Polyaniline; PEI—Polyethyleneimine; PEG—Polyethylene glycol; PCD—Polycarbodiimide; P2BA—Poly(2-butylaniline); PPy—Polypyrrole; PAT—Poly(2-aminothiazole); TP—Tea polyphenol; PGHEP—Hydroxy epoxy phosphate monomer; DA—Dopareine; ATP—Attapulgite; ACAT—Amino-terminated aniline trimer; NABM—N-(2-aminoethyl)-2-bromo-2-methylpropanamide; PA—Phytic acid; ABA—4-aminobenzoic acid. -
[1] SARKAR N, SAHOO G, DAS R, et al. Anticorrosion performance of three-dimensional hierarchical PANI@BN nanohybrids[J]. Industrial & Engineering Chemistry Research,2016,55(11):2921-2931.
[2] LI S N, FU J J. Improvement in corrosion protection properties of TiO2 coatings by chromium doping[J]. Corrosion Science,2013,68:101-110.
[3] PRABAKARAN M, KIM S H, HEMAPRIYA V, et al. Rhus verniciflua as a green corrosion inhibitor for mild steel in 1M H2SO4[J]. RSC Advances,2016,6(62):57144-57153.
[4] BRODINOVÁ J, STEJSKAL J, KALENDOVÁ A. Investigation of ferrites properties with polyaniline layer in anticorrosive coatings[J]. Journal of Physics and Chemistry of Solids,2007,68(5):1091-1095.
[5] CASELIS J L V, ROSAS E R, JUAREZ J D S, et al. The use of montmorillonite organoclay in preparation of UV-cured DGBA epoxy anticorrosive coatings[J]. Corrosion Engineering Science and Technology,2018,53(5):362-369.
[6] ZHANG C L, HE Y, LI F, et al. H-BN decorated with Fe3O4 Nanoparticles through mussel-inspired chemistry of dopamine for reinforcing anticorrosion performance of epoxy coatings[J]. Journal of Alloys and Compounds,2016,685:743-751.
[7] GHANBARI A, ATTAR M M. A study on the anticorrosion performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-silica on mild steel substrate[J]. Journal of Industrial and Engineering Chemistry,2015,23:145-153.
[8] BAKHSHANDEH E, JANNESARI A, RANJBAR Z, et al. Anti-corrosion hybrid coatings based on epoxy-silica nano-composites: Toward relationship between the morphology and EIS data[J]. Progress in Organic Coatings,2014,77(7):1169-1183.
[9] KALENDOVÁ A, SAPURINA I, STEJSKAL J, et al. Anticorrosion properties of polyaniline-coated pigments in organic coatings[J]. Corrosion Science,2008,50(12):3549-3560.
[10] LU J L, LIU N J, WANG X H, et al. Mechanism and life study on polyaniline anti-corrosion coating[J]. Synthetic Metals,2003,135-136:237-238.
[11] ZHAO Y Y, ZHANG Z M, YU L M, et al. Electrospinning of polyaniline microfibers for anticorrosion coatings: An avenue of enhancing anticorrosion behaviors[J]. Synthetic Metals,2016,212:84-90.
[12] JING S, ZHANG Q H, CHEN P, et al. Thermal reduced graphene oxide/polyimide nanocomposite coating: Fabrication and anticorrosive property[J]. Journal of Inorganic Materials,2017,32(12):1257-1263.
[13] LIU Q B, LI Z L, XIE C Y. Graphite nanoplatelets-epdxy composites for anti-corrosion coatings[J]. Fresenius Environ Bull,2020,29(2):1003-1011.
[14] 闻雷, 刘成名, 宋仁申, 等. 石墨烯材料的储锂行为及其潜在应用[J]. 化学学报, 2014, 72(3):333-344. WEN L, LIU C M, SONG R S, et al. Lithium storage characteristics and possible applications of graphene materials[J]. Acta Chimica Sinica,2014,72(3):333-344(in Chinese).
[15] LOTYA M, HERNANDEZ Y, KING P J, et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions[J]. Journal of the American Chemical Society,2009,131(10):3611-3620.
[16] YU P, LOWE S E, SIMON G P, et al. Electrochemical exfoliation of graphite and production of functional graphene[J]. Current Opinion in Colloid & Interface Science,2015,20(5):329-338.
[17] WANG J, WANG C B, WANG Y Y, et al. Epitaxial growth of graphene thin film by pulsed laser deposition[J]. Micro & Nano Letters,2015,10(11):649-652.
[18] REN W C, GAO L B, MA L P, et al. Preparation of graphene by chemical vapor deposition[J]. Carbon,2011,49(8):71-80.
[19] ZAITSEV E V, BOCHAROV G S, CHUPROV P N, et al. Preparation of graphene on copper substrates of various geometries by chemical vapor deposition[J]. Inorganic Materials,2018,54(12):1205-1215.
[20] BOTAS C, ÁLVAREZ P, BLANCO P, et al. Graphene materials with different structures prepared from the same graphite by the hummers and brodie methods[J]. Carbon,2013,65:156-164.
[21] POH H L, SANEK F, AMBROSI A, et al. Graphenes prepared by staudenmaier, hofmann and hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties[J]. Nanoscale,2012,4(11):3515-3522.
[22] HOU Y G, LV S H, LIU L P, et al. High-quality preparation of graphene oxide via the hummers’ method: Understanding the roles of the intercalator, oxidant, and graphite particle size[J]. Ceramics International,2020,46(2):2392-2402.
[23] 侯永刚, 吕生华, 张佳, 等. 氧化石墨烯的制备及形成机理[J]. 精细化工, 2019, 36(4):559-567. HOU Y G, LV S H, ZHANG J, et al. Preparation and formation mechanism of graphene oxide[J]. Fine Chemicals,2019,36(4):559-567(in Chinese).
[24] PARHIZKAR N, SHAHRABI T, RAMEZANZADEH B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film[J]. Corrosion Science,2017,123:55-75.
[25] LUO J, WANG J H, WEN S G, et al. Improved corrosion resistance based on APTES-grafted reduced sulfonated graphene/waterborne polyurethane coatings[J]. Journal of Coatings Technology and Research,2018,15(5):1107-1115.
[26] WANG X, XING W Y, SONG L, et al. Fabrication and characterization of graphene-reinforced waterborne polyurethane nanocomposite coatings by the sol-gel method[J]. Surface and Coatings Technology,2012,206(23):4778-4784.
[27] TIAN S, LIU Z X, SHEN L L, et al. Performance evaluation of mercapto functional hybrid silica sol-gel coating and its synergistic effect with f-GNs for corrosion protection of copper surface[J]. RSC Advances,2018,8(14):7438-7449.
[28] FENG C, ZHU L J, CAO Y Q, et al. Performance of coating based on APTMS/GO/epoxy composite for corrosion protection of steel[J]. International Journal of Electrochemical Science,2018,13(9):8827-8837.
[29] XIA W, XUE H R, WANG J W, et al. Functionlized graphene serving as free radical scavenger and corrosion protection in gamma-irradiated epoxy composites[J]. Carbon,2016,101:315-323.
[30] HAGHDADEH P, GHAFFARI M, RAMEZANZADEH B, et al. The role of functionalized graphene oxide on the mechanical and anti-corrosion properties of polyurethane coating[J]. Journal of the Taiwan Institute of Chemical Engineers,2018,86:199-212.
[31] WANG H H, HE Y, FEI G Q, et al. Functionalizing graphene with titanate coupling agents as reinforcement for one-component waterborne poly(urethane-acrylate) anticorrosion coatings[J]. Chemical Engineering Journal,2019,359:331-343.
[32] CUI M J, REN S M, ZHAO H C, et al. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating[J]. Chemical Engineering Journal,2018,335:255-266.
[33] NIYOGI S, BEKYAROVA E, ITKIS M E, et al. Solution Properties of graphite and graphene[J]. Journal of the American Chemical Society,2006,128(24):7720-7721.
[34] RAMEZANZADEH B, NIROUMANDRAD S, AHMADI A, et al. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide[J]. Corrosion Science,2016,103:283-304.
[35] YAN J L, CHEN G J, CAO J, et al. Functionalized graphene oxide with ethylenediamine and 1, 6-hexanediamine[J]. New Carbon Mater,2012,27(5):370-376.
[36] WEN J G, GENG W, GENG H Z, et al. Improvement of corrosion resistance of waterborne polyurethane coatings by covalent and noncovalent grafted graphene oxide nanosheets[J]. ACS Omega,2019,4(23):20265-20274.
[37] BAI T, LV L, DU W, et al. Improving the tribological and anticorrosion performance of waterborne polyurethane coating by the synergistic effect between modified graphene oxide and polytetrafluoroethylene[J]. Nanomaterials (Basel),2020,10(1):137-154.
[38] STANKOVICH S, PINER R D, NGUYEN S T, et al. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets[J]. Carbon,2006,44(15):3342-3347.
[39] ZHANG Z, ZHANG W, LI D, et al. Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in-situ method[J]. International Journal of Molecular Sciences,2015,16(1):2239-2251.
[40] HAYATGHEIB Y, RAMEZANZADEH B, KARDAR P, et al. A comparative study on fabrication of a highly effective corrosion protective system based on graphene oxide-polyaniline nanofibers/epoxy composite[J]. Corrosion Science,2018,133:358-373.
[41] JIANG F W, ZHAO W J, WU Y M, et al. A polyethyleneimine-grafted graphene oxide hybrid nanomaterial: synthesis and anti-corrosion applications[J]. Applied Surface Science,2019,479:963-973.
[42] RAMEZANZADEH B, GHASEMI E, MAHDAVIAN M, et al. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings[J]. Carbon,2015,93:555-573.
[43] AHMADI Y, AHMAD S. Formulation of a promising antimicrobial and anticorrosive bi-functional boronated hyperbranched oleo-polyurethane composite coating through the exploitation of functionalized reduced graphene oxide as chain extender[J]. Applied Surface Science,2019,494:196-210.
[44] CUI J C, XU J C, LI J, et al. A crosslinkable graphene oxide in waterborne polyurethane anticorrosive coatings: Experiments and simulation[J]. Composites Part B: Engineering,2020,188:107889.
[45] MA Y, DI H H, YU Z X, et al. Fabrication of silica-decorated graphene oxide nanohybrids and the properties of composite epoxy coatings research[J]. Applied Surface Science,2016,360:936-945.
[46] YU Z X, DI H H, MA Y, et al. Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings[J]. Surface and Coatings Technology,2015,276:471-478.
[47] LIU J H, YU Q, YU M, et al. Silane modification of titanium dioxide-decorated graphene oxide nanocomposite for enhancing anticorrosion performance of epoxy coatings on AA-2024[J]. Journal of Alloys and Compounds,2018,744:728-739.
[48] GE H Y, WANG T, CHENG S L. Si3N4@RGO hybrids for epoxy coatings with enhanced anticorrosion performance[J]. Polymer Composites,2018,40(5):2051-2060.
[49] DI H H, YU Z X, MA Y, et al. Anchoring calcium carbonate on graphene oxide reinforced with anticorrosive properties of composite epoxy coatings[J]. Polymers for Advanced Technologies,2016,27(7):915-921.
[50] YU Z X, DI H H, MA Y, et al. Fabrication of graphene oxide-alumina hybrids to reinforce the anti-corrosion performance of composite epoxy coatings[J]. Applied Surface Science,2015,351:986-996.
[51] SONG B J, SHI Y C, LIU Q D. An inorganic route to decorate graphene oxide with nanosilica and investigate its effect on anti-corrosion property of waterborne epoxy[J]. Polymers for Advanced Technologies,2019,31(2):309-318.
[52] LI J N, YU K J, QIAN K, et al. The situ preparation of silica nanoparticles on the surface of functionalized graphene nanoplatelets[J]. Nanoscale Research Letters,2014,9(1):172-180.
[53] YU K J, WANG M L, QIAN K, et al. The synergy effect of graphene/SiO2 hybrid materials on reinforcing and toughening epoxy resin[J]. Fibers and Polymers,2016,17(3):453-459.
[54] GU L, LIU S, ZHAO H C, et al. Facile preparation of water-dispersible graphene sheets stabilized by carboxylated oligoanilines and their anticorrosion coatings[J]. ACS Applied Materials & Interfaces,2015,7(32):17641.
[55] LU H, ZHANG S T, LI W H, et al. Synthesis of graphene oxide-based sulfonated oligoanilines coatings for synergistically enhanced corrosion protection in 3.5% NaCl solution[J]. ACS Applied Materials & Interfaces,2017,9(4):4034-4043.
[56] QIU S, LI W, ZHENG W, et al. Synergistic effect of polypyrrole-intercalated graphene for enhanced corrosion protection of aqueous coating in 3.5% NaCl solution[J]. ACS Applied Materials & Interfaces,2017,9(39):34294-34304.
[57] ZHU X M, NI Z B, DONG L L, et al. In-situ modulation of interactions between polyaniline and graphene oxide films to develop waterborne epoxy anticorrosion coatings[J]. Progress in Organic Coatings,2019,133:106-116.
[58] CHEN C, QIU S H, CUI M J, et al. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene[J]. Carbon,2017,114:356-366.
[59] QIU S H, LIU G, LI W, et al. Noncovalent exfoliation of graphene and its multifunctional composite coating with enhanced anticorrosion and tribological performance[J]. Journal of Alloys and Compounds,2018,747:60-70.
[60] DING J H, RAHMAN O U, PENG W J, et al. A novel hydroxyl epoxy phosphate monomer enhancing the anticorrosive performance of waterborne graphene/epoxy coatings[J]. Applied Surface Science,2018,427:981-991.
[61] WANG S, HU Z R, SHI J, et al. Green synthesis of graphene with the assistance of modified lignin and its application in anticorrosive waterborne epoxy coatings[J]. Applied Surface Science,2019,484:759-770.
[62] GUO Y W, XIAO G Q, ZHANG W, et al. One-step method for preparing dispersive tea polyphenol/graphene nanosheets enhanced with anticorrosion performance[J]. Coatings,2019,9(11):731-743.
[63] WU Y Q, HE Y, CHEN C L, et al. Non-covalently functionalized boron nitride by graphene oxide for anticorrosive reinforcement of water-borne epoxy coating[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2020,587:124337.
[64] ZHONG F, HE Y, WANG P Q, et al. Self-assembled graphene oxide-graphene hybrids for enhancing the corrosion resistance of waterborne epoxy coating[J]. Applied Surface Science,2019,488:801-812.
[65] LUO X H, ZHONG J W, ZHOU Q L, et al. Cationic reduced graphene oxide as self-aligned nanofiller in the epoxy nanocomposite coating with excellent anticorrosive performance and its high antibacterial activity[J]. ACS Applied Materials & Interfaces,2018,10(21):18400-18415.
[66] WANG H H, QIN S D, YANG X F, et al. A waterborne uniform graphene-poly(urethane-acrylate) complex with enhanced anticorrosive properties enabled by ionic interaction[J]. Chemical Engineering Journal,2018,351:939-951.
[67] ZHU X B, YAN Q Q, CHENG L, et al. Self-alignment of cationic graphene oxide nanosheets for anticorrosive reinforcement of epoxy coatings[J]. Chemical Engineering Journal,2020,389:124435.
[68] ZHU Q S, LI E, LIU X H, et al. Epoxy coating with in-situ synthesis of polypyrrole functionalized graphene oxide for enhanced anticorrosive performance[J]. Progress in Organic Coatings,2020,140:105488.
[69] NAN F, LIU C B, PU J B. Anticorrosive performance of waterborne epoxy coatings containing attapulgite/graphene nanocomposites[J]. Surface Topography: Metrology and Properties,2019,7(2):024002.
[70] FAZLI-SHOKOUHI S, NASIRPOURI F, KHATAMIAN M. Polyaniline-modified graphene oxide nanocomposites in epoxy coatings for enhancing the anticorrosion and antifouling properties[J]. Journal of Coatings Technology and Research,2019,16(4):983-997.
[71] CHHETRI S, GHOSH S, SAMANTA P, et al. Effect of Fe3O4-decorated N-doped reduced graphene oxide nanohybrid on the anticorrosion performance of epoxy composite coating[J]. Chemistry Select,2019,4(46):13446-13454.
[72] WANG N, GAO H, ZHANG J, et al. Phytic acid intercalated graphene oxide for anticorrosive reinforcement of waterborne epoxy resin coating[J]. Polymers (Basel),2019,11(12):31783656.
[73] CHANG K C, HSU C H, LU H I, et al. Advanced anticorrosive coatings prepared from electroactive polyimide/graphene nanocomposites with synergistic effects of redox catalytic capability and gas barrier properties[J]. Express Polymer Letters,2014,8(4):243-255.
[74] CHANG C H, HUANG T C, PENG C W, et al. Novel anticorrosion coatings prepared from polyaniline/graphene composites[J]. Carbon,2012,50(14):5044-5051.
[75] QI K, SUN Y M, DUAN H W, et al. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposites[J]. Corrosion Science,2015,98:500-506.
[76] LI J, CUI J C, YANG J Y, et al. Silanized graphene oxide reinforced organofunctional silane composite coatings for corrosion protection[J]. Progress in Organic Coatings,2016,99:443-451.
[77] ZHAN Y Q, ZHANG J M, WAN X Y, et al. Epoxy composites coating with Fe3O4 decorated graphene oxide: Modified bio-inspired surface chemistry, synergistic effect and improved anti-corrosion performance[J]. Applied Surface Science,2018,436:756-767.
[78] LIU C B, QIU S H, DU P, et al. An ionic liquid-graphene oxide hybrid nanomaterial: Synthesis and anticorrosive applications[J]. Nanoscale,2018,10(17):8115-8124.
[79] ZHU H, CHEN Y, LI H, et al. In-situ polymerization approach to graphene-oxide-reinforced silicone composites for superior anticorrosive coating[J]. Macromolecular Rapid Communications,2019,40(5):1800252.
[80] ZHU K, LI X R, WANG H H, et al. Electrochemical and anti-corrosion behaviors of water dispersible graphene/acrylic modified alkyd resin latex composites coated carbon steel[J]. Journal of Applied Polymer Science,2017,134(11):44445.
-
期刊类型引用(19)
1. 裘承,薛银,张文展,邱小林,刘定荣,陈秋香. 聚合物基石墨烯防腐材料研究进展. 现代化工. 2024(02): 58-61+66 .  百度学术
百度学术
2. 陈思宁. 展陈空间用新功能涂料研发与综合性能测试研究. 粘接. 2024(03): 89-92 .  百度学术
百度学术
3. 罗银胜,范方强. 多功能防腐涂层材料的研究进展. 电镀与涂饰. 2024(07): 115-125 .  百度学术
百度学术
4. 李红玲,原平方,谢艳新,刘振锋,褚亮亮. 新型石墨烯复合材料在金属防腐蚀领域的研究进展. 腐蚀与防护. 2024(07): 43-49 .  百度学术
百度学术
5. 李红玲,李紫祎,杨阳,张皓杰,郎五可. 苯胺还原诱导的氧化石墨烯/Fe_3O_4复合材料的防腐蚀性能及机理. 腐蚀与防护. 2024(12): 38-45 .  百度学术
百度学术
6. 国彤,王迎娣,贾志军,郭强. 不同填料对聚四氟乙烯导热性能的影响. 塑料. 2023(01): 28-33 .  百度学术
百度学术
7. 何阳,李思盈,李传强,袁小亚,郑旭煦. 热还原氧化石墨烯/环氧树脂复合涂层的防腐性能. 化工进展. 2023(04): 1983-1994 .  百度学术
百度学术
8. 陈国庆,吐尔迪·吾买尔,吴景旭,塔力哈尔·夏依木拉提,纪峰. 油相分散石墨烯/氧化石墨烯改性及其应用研究进展. 炭素技术. 2023(03): 10-15 .  百度学术
百度学术
9. 姜勇,姜洪义,熊思鹏. 还原氧化石墨烯对水性无机富锌涂料防腐性能的影响. 涂料工业. 2023(07): 68-72 .  百度学术
百度学术
10. 李通盛,詹浩成,蓝慧萍,王立杰,林益军,金日光. 氧化石墨烯改性及其在环氧防腐涂层中的应用进展. 高校化学工程学报. 2023(04): 525-536 .  百度学术
百度学术
11. 张维丽,张锐,张莲茜,闵杰,吴伟兴,檀满林,陈建军. 环氧乳液和防锈剂对水性防腐涂料性能的影响. 电镀与涂饰. 2023(22): 75-79 .  百度学术
百度学术
12. 宋志强,宋福如,游传榜,宋利强,杨立芹,赵俊,赵治巨. 石墨烯改性环氧富锌涂料的制备及性能研究. 信息记录材料. 2022(01): 14-17 .  百度学术
百度学术
13. 任小逆,张洪鑫. 高分子化工材料在化工防腐中的应用研究. 天津化工. 2022(02): 67-70 .  百度学术
百度学术
14. 尹宇航,雷浩,宋敬伏,赵盖,丁庆军. 改性石墨烯增强聚四氟乙烯摩擦学性能的分子模拟研究. 摩擦学学报. 2022(03): 598-608 .  百度学术
百度学术
15. 武书成. 艺术装饰用环保型水性贝壳粉涂料性能分析. 粘接. 2022(07): 80-84 .  百度学术
百度学术
16. 孙梦垚,燕晓宇,刘晓杰,李杰祎,贾婷,张娇,鄂彦鹏. 石墨烯应用于防腐涂料中的研究进展. 染料与染色. 2022(04): 48-51 .  百度学术
百度学术
17. 王夏. 氧化石墨烯改性防腐涂料的研制. 福建建材. 2021(05): 8-9 .  百度学术
百度学术
18. 陈双锏. 无机改性水性涂料的制备及其性能研究. 云南化工. 2021(07): 62-64 .  百度学术
百度学术
19. 宋志强,张依然,宋福如,游传榜,李东风. 石墨烯改性防腐涂料研究进展. 广东化工. 2021(19): 88-91 .  百度学术
百度学术
其他类型引用(4)
-




 下载:
下载: