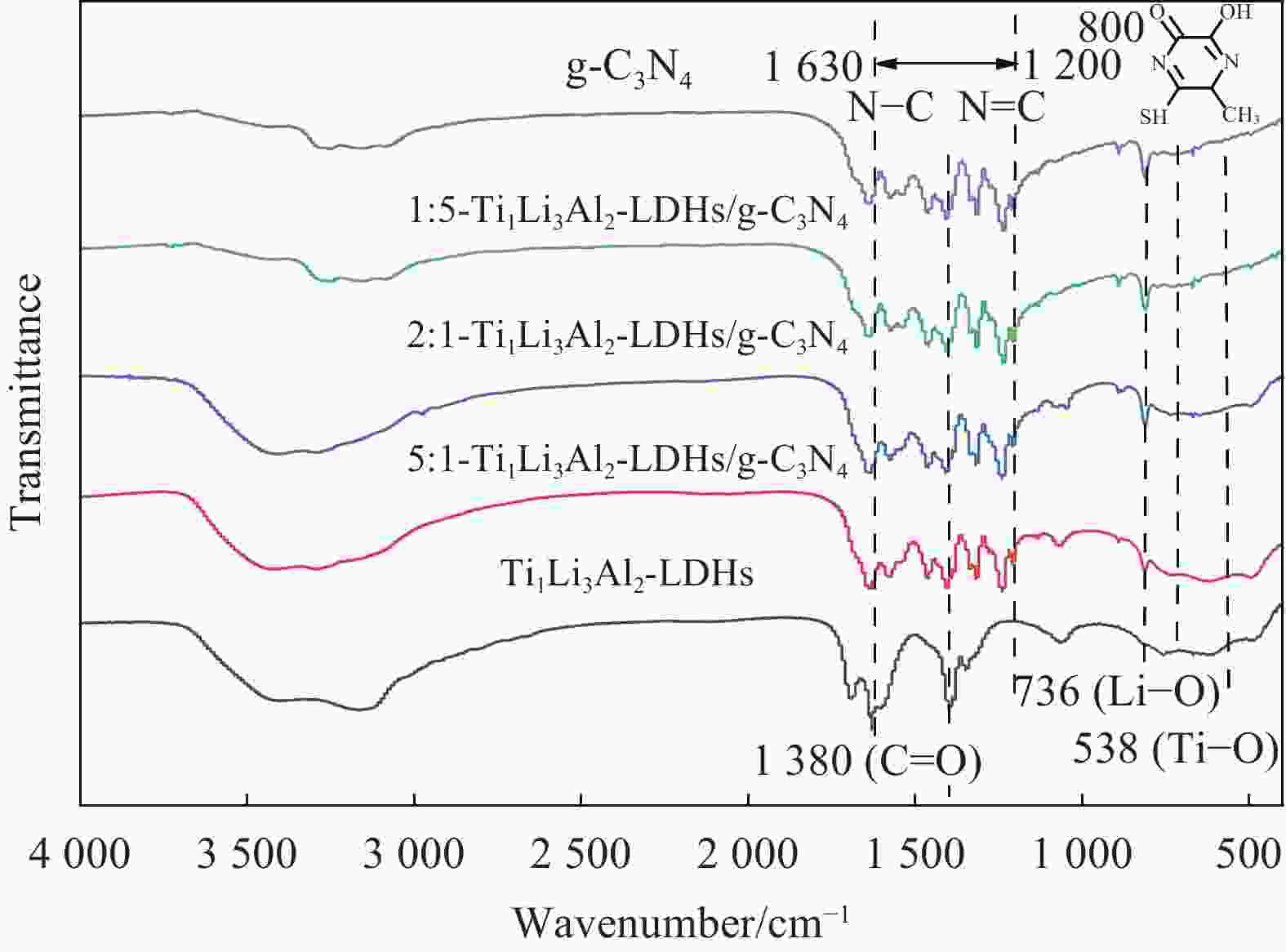
Preparation of Ti1Li3Al2-LDHs/g-C3N4 composites and its photocatalytic properties in CO2-toluene reaction system
-
摘要: 光催化CO2还原是实现CO2绿色转化利用的重要途径之一,但一直受其反应转化效率低的制约。开发新的CO2还原反应体系和提高光催化剂的可见光利用率及光生电子与空穴的分离效率是解决上述问题的有效方法。本文利用甲苯作为底物,构建了光催化CO2-甲苯耦合反应的新体系,并通过静电组装法合成了Ti1Li3Al2-层状双氢氧化物(LDHs)/石墨相氮化碳(g-C3N4)复合光催化剂。重点研究了该复合光催化剂的光电性质及在CO2-甲苯耦合反应体系中的光催化反应特性。结果表明,在光催化CO2-甲苯耦合体系中,Ti1Li3Al2-LDHs/g-C3N4作用下,CO2被还原为CO,甲苯被氧化为苯甲醇、苯甲醛及苯甲酸苄酯,其中苯甲醛和苯甲醇的含量可达到4.80和4.70 mmol/gcat。这主要归因于Ti1Li3Al2-LDHs/g-C3N4中,g-C3N4将Ti1Li3Al2-LDHs在紫外区的吸收扩展到了可见光区,并提高了Ti1Li3Al2-LDHs的分散性,从而为光催化反应提供更多的活性位点;Ti1Li3Al2-LDHs/g-C3N4的界面处形成了S型异质结,有利于界面处的光生电子的转移,提高了其光生电子与空穴的分离效率,而甲苯可作为有机底物加快空穴的消耗速度促进了CO2还原反应的进行。为CO2与小分子有机物协同转化提供了一种新思路。
-
关键词:
- Ti1Li3Al2-层状双氢氧化物(LDHs) /
- 石墨相氮化碳 /
- 光催化 /
- 异质结 /
- CO2-甲苯
Abstract: Photocataltyic reduction of CO2 is one of the promising routes in CO2 conversion and utilization, but the very low CO2 conversion rate is the biggest hurdle for the process. Developing a new CO2 reduction reaction system and improving the visible light utilization and separation efficiency of photogenerated electrons and holes are effective ways to solve the above problems. In this work, we designed a CO2-toluene coupling reaction system to promote the CO2 reduction reaction. The Ti1Li3Al2-layered dihydroxides (LDHs)/graphite phase carbon nitride (g-C3N4) composite with heterojunction structure were synthesized by electrostatic assembly method. And the photoelectric property and photocatalytic properties of Ti1Li3Al2-LDHs/g-C3N4 composite were explored in CO2-toluene reduction reaction system. The results show that CO2 is reduced to CO, and toluene is oxidized to benzyl alcohol, benzaldehyde and benzyl benzoate in the photocataltyic coupling reaction system. Benzaldehyde and benzyl alcohol content can reach 4.80 and 4.70 mmol/gcat. This is mainly because the g-C3N4 can extend the absorption of Ti1Li3Al2-LDHs from the ultraviolet region to the visible region, and improve the dispersion of Ti1Li3Al2-LDHs which provide more active sites for photocatalytic reactions. Moreover, the S-type heterojunction is formed in the interface of Ti1Li3Al2-LDHs/g-C3N4, which is conducive to the transfer of photogenerated electrons at the interface and improves the separation efficiency of photogenerated electrons and holes. And toluene can be used as an organic substrate to accelerate the rate of hole consumption and stimulate the CO2 reduction reaction. This work provides a new idea for the synergistic reaction between CO2 reduction and small molecular organics. -
图 4 5∶1-Ti1Li3Al2-LDHs/g-C3N4 (a)、2∶1-Ti1Li3Al2-LDHs/g-C3N4 (b)、1∶5-Ti1Li3Al2-LDHs/g-C3N4 (c) 的TEM图像和Ti1Li3Al2-LDHs (d)、2∶1-Ti1Li3Al2-LDHs/g-C3N4 (e) 的TEM mapping图像
Figure 4. TEM images of 5∶1-Ti1Li3Al2-LDHs/g-C3N4 (a), 2∶1-Ti1Li3Al2-LDHs/g-C3N4 (b), 1∶5-Ti1Li3Al2-LDHs/g-C3N4 (c) and TEM mapping images of Ti1Li3Al2-LDHs (d), 2∶1-Ti1Li3Al2-LDHs/g-C3N4 (e)
Ratio before the composite name is the mass ratio of Ti1Li3Al2-LDHs to g-C3N4
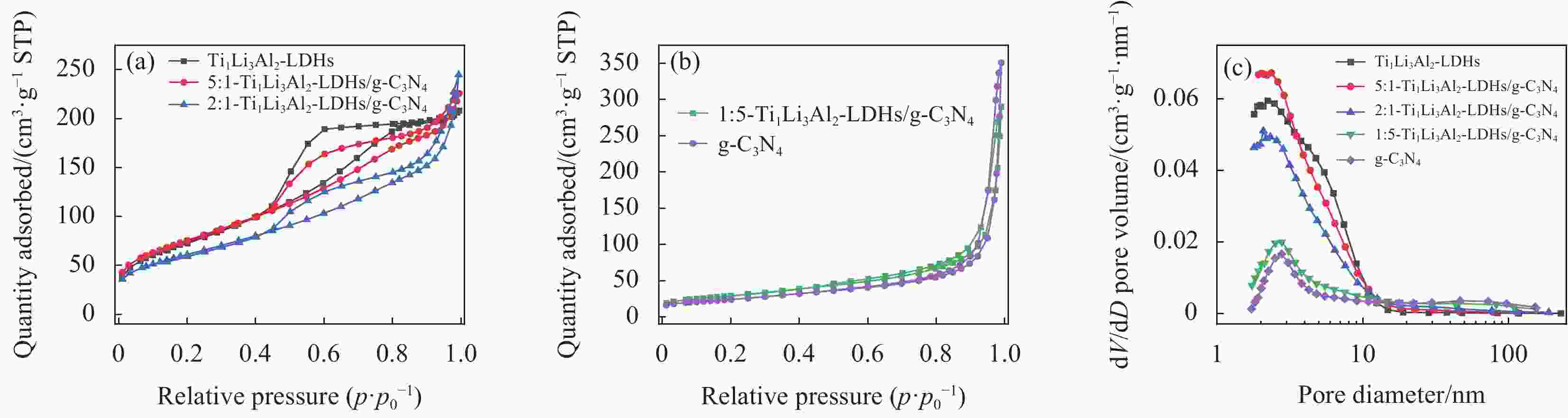
图 7 g-C3N4、Ti1Li3Al2-LDHs和不同质量比的Ti1Li3Al2-LDHs/g-C3N4的N2吸附-解析等温线 ((a), (b)) 和孔结构分布图 (c)
Figure 7. N2 adsorption-desorption isotherms ((a), (b)) and the pore size distribution diagram (c) of g-C3N4, Ti1Li3Al2-LDHs and Ti1Li3Al2-LDHs/g-C3N4 with different mass ratios
STP—Standard temperature and pressure
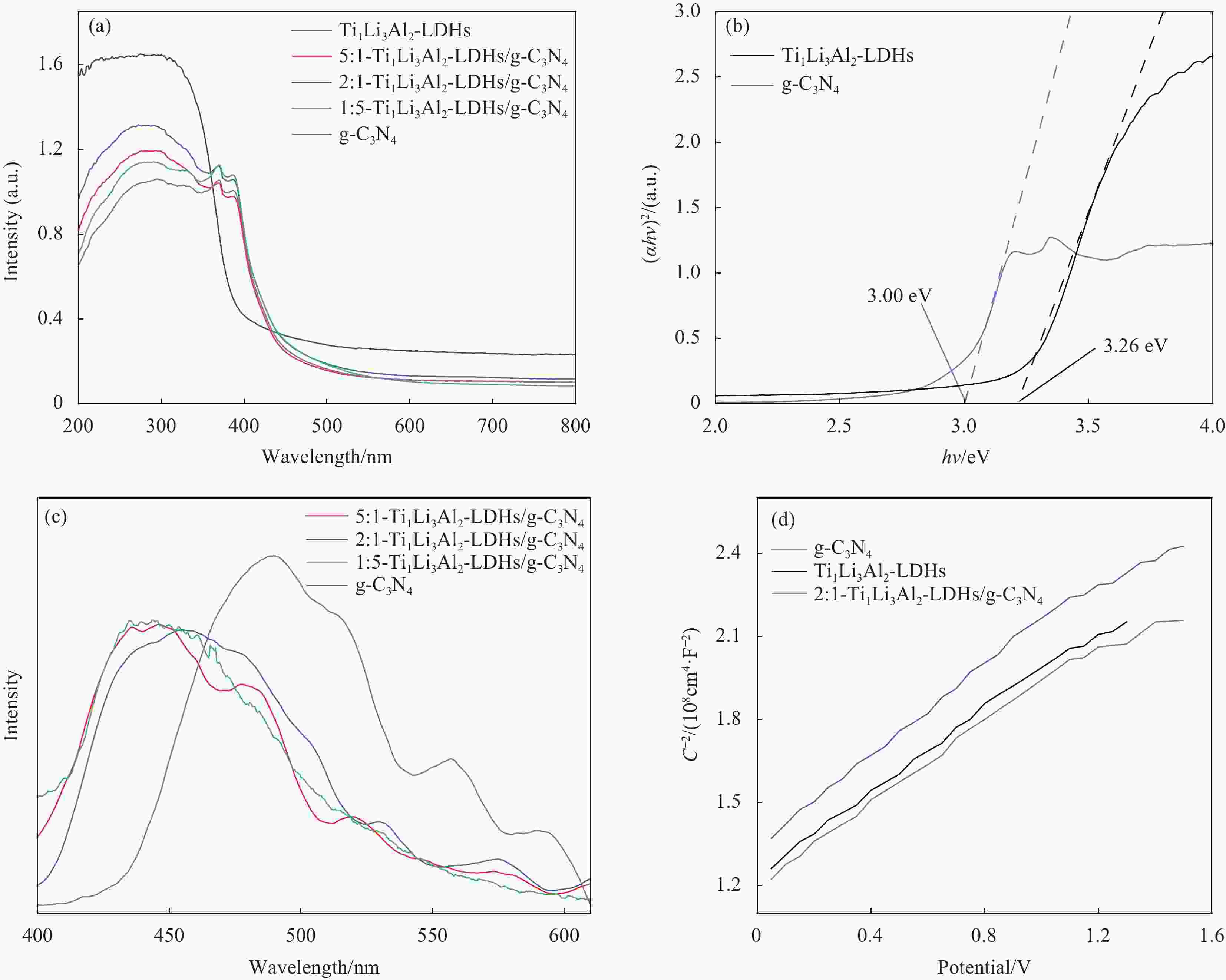
图 8 g-C3N4、Ti1Li3Al2-LDHs和不同质量比的Ti1Li3Al2-LDHs/g-C3N4的UV-Vis DRS谱图 (a)、(αhν)2和hν的关系图 (b)、PL谱图 (c) 和莫特-肖特基(M-S)测试曲线 (d)
Figure 8. UV-Vis DRS spectra (a), relationship between (ahν)2 and hν (b), PL spectra (c) and Mott-Schottky (M-S) curves (d) of g-C3N4, Ti1Li3Al2-LDHs and Ti1Li3Al2-LDHs/g-C3N4 with different mass ratios
α—Absorption coefficient; hν—Photon energy; C—Capacitance
图 10 g-C3N4、Ti1Li3Al2-LDHs和不同质量比的Ti1Li3Al2-LDHs/g-C3N4的CO2-程序升温脱附(TPD)曲线 (a)、产物分布图 (b)和2∶1-Ti1Li3Al2-LDHs/g-C3N4不同时间的产物分布图 (c)、反应前后的XRD图谱 (d)
Figure 10. CO2-temperature programmed desorption (TPD) curves (a), product distribution (b) of g-C3N4, Ti1Li3Al2-LDHs and Ti1Li3Al2-LDHs/g-C3N4 with different mass ratios and product distribution at different time (c), XRD patterns before(used) and after(fresh) reaction (d) of 2∶1-Ti1Li3Al2-LDHs/g-C3N4
表 1 不同样品的比表面积及孔结构参数
Table 1. Specific surface area and pore structure parameters of different samples
Sample SBET/(m2·g−1) Vpore/(cm3·g−1) dpore/nm Ti1Li3Al2-LDHs 347.5 0.32 4.60 5∶1-Ti1Li3Al2-LDHs/g-C3N4 266.0 0.35 4.64 2∶1-Ti1Li3Al2-LDHs/g-C3N4 214.5 0.38 5.46 1∶5-Ti1Li3Al2-LDHs/g-C3N4 104.2 0.45 9.72 g-C3N4 85.6 0.54 12.06 Notes: SBET—BET surface area; Vpore—Pore volume; dpore—Pore size. -
[1] GONG E, ALI S, HIRAGOND C B, et al. Solar fuels: Research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels[J]. Energy & Environmental Science,2022,15(3):887-930. [2] FU J, LIU K, LI H, et al. Bimetallic atomic site catalysts for CO2 reduction reactions: A review[J]. Environmental Chemistry Letters,2021,20(1):243-262. [3] LIU D, ZHANG M, HUANG H H, et al. CoII–ZnII heterometallic dinuclear complex with enhanced photocatalytic activity for CO2-to-CO conversion in a water-containing system[J]. ACS Sustainable Chemistry & Engineering,2021,9(28):9273-9281. [4] GONG X, YU S, GUAN M, et al. Pyrene-functionalized polymeric carbon nitride with promoted aqueous-organic biphasic photocatalytic CO2 reduction[J]. Journal of Materials Chemistry A,2019,7(13):7373-7379. doi: 10.1039/C8TA09801H [5] RIEMER D, SCHILLING W, GOETZ A, et al. CO2-catalyzed efficient dehydrogenation of amines with detailed mechanistic and kinetic studies[J]. ACS Catalysis,2018,8(12):11679-11687. doi: 10.1021/acscatal.8b03059 [6] YU H, HAVIV E, NEUMANN R. Visible-light photochemical reduction of CO2 to CO coupled to hydrocarbon dehydrogenation[J]. Angewandte Chemie International Edition,2020,59(15):6219-6223. doi: 10.1002/anie.201915733 [7] YUAN L, LI Y H, TANG Z R, et al. Defect-promoted visible light-driven C—C coupling reactions pairing with CO2 reduction[J]. Journal of Catalysis,2020,390:244-250. doi: 10.1016/j.jcat.2020.07.036 [8] DONG G X, ZHANG W, MU Y F, et al. A halide perovskite as a catalyst to simultaneously achieve efficient photocatalytic CO2 reduction and methanol oxidation[J]. Chem Commun,2020,56(34):4664-4667. doi: 10.1039/D0CC01176B [9] 孔婷婷, 董羿蘩, 张颖萍, 等. 类水滑石Ti/Li/Al-LDHs的制备及其CO2吸附性能[J]. 燃料化学学报, 2016, 44(8):1017-1024. doi: 10.3969/j.issn.0253-2409.2016.08.017KONG Tingting, DONG Yifan, ZHANG Yingping, et al. Preparation of hydrotalcite-like Ti/Li/Al-LDHs and its performance in CO2 adsorption[J]. Journal of Fuel Chemistry and Technology,2016,44(8):1017-1024(in Chinese). doi: 10.3969/j.issn.0253-2409.2016.08.017 [10] BIAN X, ZHANG S, ZHAO Y, et al. Layered double hydroxide-based photocatalytic materials toward renewable solar fuels production[J]. InfoMat,2021,3(7):719-738. doi: 10.1002/inf2.12192 [11] WANG K, WANG T, ISLAM Q A, et al. Layered double hydroxide photocatalysts for solar fuel production[J]. Chinese Journal of Catalysis,2021,42(11):1944-1975. doi: 10.1016/S1872-2067(21)63861-5 [12] 高超民, 于海瀚, 赵悦含, 等. ZnO@SnO2异质结复合纳米管的可控构筑及其光催化性能[J], 复合材料学报, 2022, 39(12): 5856-5867.GAO Chaomin, YU Haihan, ZHAO Yuehan, et al. Controllable construction of ZnO@SnO2 heterojunction composite nano-tubes and their photocatalytic properties[J]. Acta Materiae Compositae Sinica, 2022, 39(12): 5856-5867(in Chinese). [13] SONG B, ZENG Z, ZENG G, et al. Powerful combination of g-C3N4 and LDHs for enhanced photocatalytic performance: A review of strategy, synthesis, and applications[J]. Advances in Colloid and Interface Science,2019,272:101999. doi: 10.1016/j.cis.2019.101999 [14] KUMAR S, ISAACS M A, TROFIMOVAITE R, et al. P25@CoAl layered double hydroxide heterojunction nanocomposites for CO2 photocatalytic reduction[J]. Applied Catalysis B: Environmental,2017,209:394-404. doi: 10.1016/j.apcatb.2017.03.006 [15] GUO Q, ZHANG Q, WANG H, et al. Core-shell structured ZnO@Cu-Zn-Al layered double hydroxides with enhanced photocatalytic efficiency for CO2 reduction[J]. Catalysis Communications,2016,77:118-122. doi: 10.1016/j.catcom.2016.01.019 [16] 崔言娟, 徐红赟, 祝玉鑫, 等. SnO2/C3N4二维复合光催化剂制备及其光催化还原性能[J]. 复合材料学报, 2022, 39(8):3852-3862.CUI Yanjuan, XU Hongyun, ZHU Yuxin, et al. Preparation and photocatalytic reduction performance of 2D SnO2/C3N4 composite photocatalyst[J]. Acta Materiae Compositae Sinica,2022,39(8):3852-3862(in Chinese). [17] RAIZADA P, KUMAR A, SINGH P. Graphitic carbon nitride-based new advanced materials for photocatalytic applications[J]. Current Analytical Chemistry,2021,17(2):150-165. doi: 10.2174/1573411016666191230152919 [18] 段飞阳, 周安宁, 陈福欣, 等. 石墨相氮化碳纳米片的可控制备及光催化性能[J]. 硅酸盐学报, 2021, 49(10):2053-2060.DUAN Feiyang, ZHOU Anning, CHEN Fuxin, et al. Controllable preparation and photocatalytic performance of graphitic carbon nitride nanosheets[J]. Journal of the Chinese Ceramic Society,2021,49(10):2053-2060(in Chinese). [19] 孔婷婷, 张颖萍, 张亚刚, 等. 锂铝基类水滑石的制备及其光催化性能表征[J]. 功能材料, 2016, 47(12):12255-12260.KONG Tingting, ZHANG Yingping, ZHANG Yagang, et al. Preparation and photocatalytic properties of hydrotalcite-like Li/Al-LDHs[J]. Journal of Functional Materials,2016,47(12):12255-12260(in Chinese). [20] ZHANG C, ZHOU Y, BAO J, et al. Structure regulation of ZnS@g-C3N4/TiO2 nanospheres for efficient photocatalytic H2 production under visible-light irradiation[J]. Chemical Engineering Journal,2018,346:226-237. doi: 10.1016/j.cej.2018.04.038 [21] QIN J, ZHANG X, YANG C, et al. ZnO microspheres-reduced graphene oxide nanocomposite for photocatalytic degradation of methylene blue dye[J]. Applied Surface Science,2017,392:196-203. doi: 10.1016/j.apsusc.2016.09.043 [22] LEI Y G, YANG C, HOU J H, et al. Strongly coupled CdS/graphene quantum dots nanohybrids for highly efficient photocatalytic hydrogen evolution: Unraveling the essential roles of graphene quantum dots[J]. Applied Catalysis B: Environmental,2017,216:59-69. doi: 10.1016/j.apcatb.2017.05.063 [23] WEI R B, HUANG Z L, GU G H, et al. Dual-cocatalysts decorated rimous CdS spheres advancing highly-efficient visible-light photocatalytic hydrogen production[J]. Applied Catalysis B: Environmental,2018,231:101-107. doi: 10.1016/j.apcatb.2018.03.014 [24] GUO Y, LI J, GAO Z, et al. A simple and effective method for fabricating novel p-n heterojunction photocatalyst g-C3N4/Bi4Ti3O12 and its photocatalytic performances[J]. Applied Catalysis B: Environmental,2016,192:57-71. doi: 10.1016/j.apcatb.2016.03.054 [25] DAHL M, LIU Y, YIN Y. Composite titanium dioxide nanomaterials[J]. Chemical Reviews,2014,114(19):9853-9889. doi: 10.1021/cr400634p [26] MOUSAVI M, HABIBI-YANGJEH A, ABITORABI M. Fabrication of novel magnetically separable nanocomposites using graphitic carbon nitride, silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visible-light irradiation[J]. Journal of Colloid and Interface Science,2016,480:218-231. doi: 10.1016/j.jcis.2016.07.021 [27] NING P, CHEN H, PAN J, et al. Surface defect-rich g-C3N4/TiO2 Z-scheme heterojunction for efficient photocatalytic antibiotic removal: Rational regulation of free radicals and photocatalytic mechanism[J]. Catalysis Science & Technology,2020,10(24):8295-8304. [28] MEI Z, WANG G, YAN S, et al. Rapid microwave-assisted synthesis of 2D/1D ZnIn2S4/TiO2 S-scheme heterojunction for catalyzing photocatalytic hydrogen evolution[J]. Acta Physico-Chimica Sinica,2021,37(6):2009097. [29] LIU Y, HAO X, HU H, et al. High efficiency electron transfer realized over NiS2/MoSe2 S-scheme heterojunction in photocatalytic hydrogen evolution[J]. Acta Physico-Chimica Sinica,2020,37(6):2008030. [30] HE Z, SHI Y, GAO C, et al. BiOCl/BiVO4 p–n heterojunction with enhanced photocatalytic activity under visible-light irradiation[J]. The Journal of Physical Chemistry C,2013,118(1):389-398. [31] PENG Y, YAN M, CHEN Q G, et al. Novel one-dimensional Bi2O3-Bi2WO6 p-n hierarchical heterojunction with enhanced photocatalytic activity[J]. Journal of Materials Chemistry A,2014,2(22):8517-8524. doi: 10.1039/C4TA00274A [32] LI X, LIU J, HUANG J, et al. All organic S-scheme heterojunction PDI-Ala/S-C3N4 photocatalyst with enhanced photocatalytic performance[J]. Acta Physico-Chimica Sinica,2020,37(6):2010030. [33] REN J, MEBRAHTU C, PALKOVITS R. Ni-based catalysts supported on Mg-Al hydrotalcites with different morphologies for CO2 methanation: Exploring the effect of metal-support interaction[J]. Catalysis Science & Technology,2020,10(6):1902-1913. [34] RAZZAQ R, ZHU H, JIANG L, et al. Catalytic methanation of CO and CO2 in coke oven gas over Ni-Co/ZrO2-CeO2[J]. Industrial & Engineering Chemistry Research,2013,52(6):2247-2256. [35] LIU L J , ZHAO C Y, MILLER, et al. Mechanistic study of CO2 photoreduction with H2O on Cu/TiO2 nanocomposites by in situ X-ray absorption and infrared spectroscopies[J]. The Journal of Physical Chemistry C: Nanomaterials and Interfaces, 2017, 121(1): 490-499. [36] ZHAO C, LIU L, RAO G, et al. Synthesis of novel MgAl layered double oxide grafted TiO2 cuboids and their photocatalytic activity on CO2 reduction with water vapor[J]. Catalysis Science & Technology,2015,5(6):3288-3295. doi: 10.1039/C5CY00216H [37] YE T, HUANG W M, ZENG L M, et al. CeO2-x platelet from monometallic cerium layered double hydroxides and its photocatalytic reduction of CO2[J]. Applied Catalysis B: Environmental,2017,210:141-148. doi: 10.1016/j.apcatb.2017.03.051 [38] IDE Y, OGINO R, SADAKANE M, et al. Effects of Au loading and CO2 addition on photocatalytic selective phenol oxidation over TiO2-supported Au nanoparticles[J]. ChemCatChem,2013,5(3):766-773. doi: 10.1002/cctc.201200661 [39] HIROSHI I, MIKIO Y, YONEYAMA H. Photocatalytic conversion of Lacltic acid to Malic acid through pyruvic acid in the presence of Malic enzyme and semiconductor photocatalysts[J]. Journal of the Chemical Society, Faraday Transactions,1992,88:2215-2219. doi: 10.1039/ft9928802215 [40] UR RASHID H, SADIQ M, ABID ZIA M, et al. Photooxidation of toluene: Correlation of noble metal loading on titania and activation energy[J]. Journal of Chemistry,2016(1):4231467. [41] HUANG H, ZHAO J, DU Y, et al. Direct Z-scheme heterojunction of semicoherent FAPbBr3/Bi2WO6 interface for photoredox reaction with large driving force[J]. ACS Nano,2020,14:16689-16697. doi: 10.1021/acsnano.0c03146 [42] ZHANG R, WANG H, TANG S, et al. Photocatalytic oxidative dehydrogenation of ethane using CO2 as a soft oxidant over Pd/TiO2 catalysts to C2H4 and syngas[J]. ACS Catalysis,2018,8(10):9280-9286. doi: 10.1021/acscatal.8b02441 [43] LI X, WEI D, YE L, et al. Fabrication of Cu2O-RGO/BiVO4 nanocomposite for simultaneous photocatalytic CO2 reduction and benzyl alcohol oxidation under visible light[J]. Inorganic Chemistry Communications,2019,104:171-177. doi: 10.1016/j.inoche.2019.04.012 [44] SEKI T, NAKAJO T, ONAKA M. The Tishchenko reaction: A classic and practical tool for ester synthesis[J]. Chemistry Letters,2006,35(8):824-829. doi: 10.1246/cl.2006.824 [45] CHERYL M, MILLER S P, WHITE P S, et al. Frst catalytic asymmetric Aldol-Tishchenko reaction-insight into the catalyst structure and reaction mechanism[J]. Angewandte Chemie,2001,40(3):601-603. doi: 10.1002/1521-3773(20010202)40:3<601::AID-ANIE601>3.0.CO;2-W -





 下载:
下载: