Development of IL/MOF ternary mixed matrix membrane for CO2 separation
-
摘要: 气体膜分离技术,作为实现碳中和的一种关键气体分离方法,在二氧化碳捕集领域取得了显著进展,混合基质膜(Mixed matrix membrane, MMM)结合了聚合物膜和多孔填料的优点,使其成为传统聚合物膜的有效替代品。然而,界面不相容性问题限制了MMM的气体分离性能。在膜分离技术中,通过引入第三种组分(通常是功能化添加剂)来制备的三元MMM,能够有效克服二元MMM在结构上存在的问题,并对膜的分离性能产生积极的影响。本综述聚焦于探讨离子液体(Ionic Liquid, IL)与金属有机框架(Metal-Organic Framework, MOF)作为高效填料共同用于三元相MMM的制备过程,深入分析了IL与MOF整合入MMM后所产生的协同效应,尤其是它们对提升膜材料机械稳定性和气体吸附性能方面的显著贡献。文中详细介绍了MMM中气体分离的传输机制,总结了IL/MOF复合材料的结构和制备工艺,并对三元相MMM的CO2分离性能进行详尽的综述与分析。基于这些分离性能数据,进一步讨论了三元相MMM所面临的挑战,为未来膜分离技术的发展提供了新的思路和方向。Abstract: Gas separation membrane technology, as a key gas separation method to achieve carbon neutrality, has made significant progress in the field of carbon dioxide capture, Mixed matrix membrane (MMM) combines the advantages of polymer membranes and porous fillers, making it an effective alternative to conventional polymer membranes. However, interface incompatibility issues limit MMM's gas separation performance. In Membrane separation technology, MMM prepared by introducing a third component (usually a functional additive) can effectively overcome the structural problems of binary phase MMM and have a positive impact on the separation performance of the membrane. This review focuses on the preparation process of ternary phase MMM using Ionic Liquid (IL) and Metal-Organic Framework (MOF) as high efficiency fillers, and further analyzes the synergistic effect of integrating IL and MOF into MMM. In particular, they contribute significantly to improving the mechanical stability and gas adsorption properties of membrane materials. In this paper, the transport mechanism of gas separation in MMM is introduced in detail, the structure and preparation process of IL/MOF composites are summarized, and the CO2 separation performance of ternary phase MMM is reviewed and analyzed in detail. Based on these separation performance data, the challenges faced by ternary phase MMM are further discussed, which provides new ideas and directions for the development of membrane separation technology in the future.
-
Keywords:
- CO2 capture /
- Membrane separation /
- Mixed matrix membrane /
- Ionic liquid /
- Metal-Organic Framework
-
强韧、轻质复合多级结构材料在能源储存转化、环境治理、生物医学及航天等战略领域的应用越来越受关注[1-4],其中二维多级层状结构材料独特的电子限域效应赋予这类材料独特的物理、化学性质及丰富的科学内涵,在众多的领域都有重要的应用前景,特别是电子器件、光电器件等方面[5-6]。但人工合成材料不能像天然材料(贝壳、骨骼等)一样通过大量的相互作用对结构基元进行精准的调控,以致各结构基元在受力时应力不能有效传递导致材料断裂,影响力学性质、进而限制材料的应用[7-10]。
受自然界启发,研究工作者发展了大量具有强韧力学性能的二维层状结构材料,通常采取的方案是将高强度的无机纳米片与高柔性的有机聚合物通过层-层(LBL)组装、真空抽滤组装、空气诱导组装、冰模板等方法复合,得到类贝壳珍珠层的“砖-泥”结构[11-13]。如Shin等[14]将还原石墨烯氧化物(RGOF)与碳纳米管(SWNT)复合,用RGOF-SWNT复合体填充聚乙烯醇(PVA),获得了具有强韧性的RGOF-SWNT-PVA复合膜材料。Cao等[15]鉴于电磁屏蔽材料对厚度与力学性能的需要,选择Ti3C2Tx (MXene)与纤维素纳米纤维(CNF)为功能基元通过真空抽滤自组装的方法得到了高电磁屏蔽效率的复合膜材料,同时保证了材料的强度和韧性。Yoo等[16]通过静电组装氮化硼(BNNS)与明胶获得机械强度媲美人体骨密质的复合膜,且表现出优异的生物相容性,有望应用于组织工程与骨内植入领域。Woo等[17]利用交联剂(CA) 将片层氧化石墨烯(GO)通过化学键相连,形成仿贝壳结构的GO/CA层状复合薄膜,由于GO之间强大的共价键网络,复合薄膜表现出优异的力学性能,在航空航天、电子保护器、渗透膜等领域有潜在应用。过去研究者们采用纳米填充质增强聚合物薄膜的工作在拉伸强度上都有明显的提升,但断裂伸长率却变化不大甚至有所降低,导致复合薄膜的韧性提升被限制。究其原因在于结构基元之间可作为牺牲性成键的相互作用力较少,也将其归结为应力传递效率较低。综上所述,发展一种普适性的复合膜增强增韧方法仍然具有一定的挑战性[18-20]。
利用聚乙烯亚胺(PEI)修饰过后的纤维素纳米晶(PCNC)与蒙脱土纳米片(MMT)静电自组装得到MMT-PCNC组装体,将其分散到PVA水溶液中并搅拌均匀,随后倒入培养皿中利用溶剂蒸发法成膜。得到的MMT-PCNC/PVA复合膜拉伸强度与断裂伸长率同步增加,性能平衡。此法提高了材料的应力传递效率,有效增加了复合膜的拉伸强度;同时利用多重弱相互作用实现了断裂强度、断裂伸长率以及韧性的同步提升。
1. 实验材料及方法
1.1 原材料
蒙脱土粉末购买于浙江丰虹新材料有限公司,棉浆浆板购买于河北中国纸业公司,浓硫酸(98%)购买于北京化学试剂厂,聚乙烯醇(PVA,醇解度为87%~89%,AR)在阿拉丁上海有限公司购买,聚乙烯亚胺(PEI,Mw=10000,AR)购买于阿达玛斯试剂有限公司。实验过程中所用到的试剂都未经任何处理。
1.2 PCNC的制备
晶态纳米纤维素(Cellulose nanocrystals, CNC)悬浮液制备方法参考文献[21]制得,PEI修饰的CNC具体制备过程如下:取10 mL PEI水溶液(3.0wt%)加入到5 mL CNC悬浮液(3.0wt%)中,室温下连续搅拌1 h,随后用浓盐酸将混合溶液pH调到1.3以增强CNC与PEI之间的离子相互作用。继续搅拌十分钟后,将混合溶液高速离心20 min(13000 r/min),弃去上层清液,将沉淀用超纯水(18.2 MΩ·cm,Mill-Q Corp)洗涤三次,去掉未发生相互作用的游离PEI聚合物。最后再将沉淀加入水中,超声30 min重新分散,8000 r/min低速离心8 min,去掉CNC聚集体,收集上层清液得到单分散的CNC悬浮液,浓度为1.0wt%,命名为PCNC。
1.3 MMT悬浮液的制备
将2 g MMT粉末加入到500 mL超纯水中,连续溶解搅拌一周后静置72 h,收集上层清液得到剥离充分的MMT纳米片备用,浓度为0.2wt%。
1.4 MMT-PCNC组装体的制备
将MMT (0.2wt%)悬浮溶液逐滴加入到PCNC (1.0wt%)悬浮溶液中,持续搅拌24 h,得到MMT-PCNC组装体悬浮液,控制MMT与PCNC的质量比分别为1∶1、1∶2、1∶4。得到不同MMT、PCNC质量比的组装体,分别命名为1MMT-1PCNC、1MMT-2PCNC、1MMT-4PCNC,具体组成见表1。
1.5 MMT-PCNC/PVA复合膜的制备
将上述组装体悬浮液加入到PVA水溶液中,搅拌过夜,使组装体分散均匀,组装体含量控制为PVA质量的20%。将混合液置于直径为60 mm的聚乙烯塑料培养皿中室温干燥2天,得到柔性MMT-PCNC/PVA复合膜。
表 1 蒙脱土-纤维素纳米晶(MMT-PCNC)组装体的组成Table 1. Compositions of the montmorillonite-cellulose nanocrystal (MMT-PCNC) assembliesSample 1MMT-1PCNC 1MMT-2PCNC 1MMT-4PCNC Composition Mass fraction Mass ratio Mass ratio Mass ratio MMT 0.2% 1 1 1 PCNC 1.0% 1 2 4 1.6 样品性能及表征
SEM结果在JSM-6510A上获得,样品用导电胶粘贴于样品台表面,喷金60 s,电压设置为20 kV,电流设置为86 μA[22]。TEM结果在FEI Tecnai G2S-Twin上获得,样品滴于200 mm铜网上,室温晾干,加速电压为200 kV。溶液的Zeta电势常温下在Malvern Zetasizer Nano-ZS90上获得,样品浓度为0.1wt%[23]。傅立叶红外光谱(FTIR)测试在Bruker IFS 66v/S红外光谱仪上进行,扫描范围为 400~4000 cm−1,扫描速度为4 cm−1,制样过程参考文献[24],样品采用KBr压片,测试前置于60℃烘箱干燥1 h。力学性能测试在万能材料测试机(Instron 5944, UK)上进行,所用传感器为2000 N,拉伸速率为5 mm·min−1,夹距为10 mm。所有的样品测试均在常温下进行,样品长度约为50 mm、宽度约为5 mm、厚度约为0.3 mm,每组样品重复测量3次。
2. 结果与讨论
2.1 MMT-PCNC组装体的形成过程
图1(a)~1(c)分别为CNC、PCNC、MMT悬浮溶液置于室温下一个月之后的光学照片,从图中可以看到溶液基本都呈乳白色,无沉淀产生,说明所有的溶液均非常稳定。CNC由硫酸水解制得,表面含有磺酸基,带负电,Zeta电势分析得到CNC的电势值为−21.3 mV。加入PEI对其进行修饰后,在其表面引入了氨基,带正电,Zeta电势分析显示,PEI修饰过后的CNC (PCNC)电势值由−21.3 mV增加到35.4 mV。对MMT进行Zeta电势表征得到,其电势值为−31.3 mV,与PCNC所带电荷相反,数值匹配,为静电相互作用驱动形成MMT-PCNC组装体提供了必要的条件(图1(d))。
对修饰前后的CNC进行TEM表征,可以看到PCNC仍为棒状结构,长度与直径相比较CNC有明显的增加(图2)。经过统计,平均长度由164 nm增加到206 nm,平均直径由22 nm增加到26 nm (图3),说明PEI聚电解质已成功包覆于CNC的表面,形成PCNC。
将不同质量比的PCNC与MMT悬浮液混合,得到不同质量比的MMT-PCNC组装体悬浮液(1MMT-2PCNC、1MMT-1PCNC、1MMT-4PCNC),组装后发现三组不同配比的溶液均不同程度的发生聚沉,出现明显颗粒感,说明两种结构基元发生相互作用,且组装以后尺寸变大。以1MMT-2PCNC组装体为例,分别对组装前后的结构基元(MMT纳米片、PCNC纳米棒和1MMT-2PCNC组装体)进行TEM表征(图4)。如图4(a)所示,MMT呈分散、不规则片层结构,横向尺寸大约有100~1000 nm。在1MMT-2PCNC组装体的TEM图像中可以看到PCNC纳米棒附着于MMT纳米片的表面及边缘上,取向各异,在MMT表面上形成一层稠密的PCNC层(图4(c)及插图)。组装体的尺寸取决于原始MMT与PCNC的尺寸。
2.2 MMT-PCNC/PVA复合膜的形貌
图5为不同MMT-PCNC/PVA复合膜的SEM图像。如图所示,纯PVA薄膜截面致密,无有序结构。而由不同MMT、PCNC质量比的组装体所构成的复合膜均具有明显层状结构,为干燥成膜过程中MMT纳米片组装而成,由图5(c)、5(d)可见,层间具有明显PCNC附着(图中箭头所指),表明由于较强的静电相互作用,使MMT-PCNC组装体在成膜过程中得以有效保存,但由于层间还存在PVA分子,使得PCNC被部分覆盖,电镜下只观察到棒状尖端。图5(b)中,由于PCNC含量相对较少,未在截面处观察到明显PCNC结构。
2.3 MMT-PCNC/PVA复合膜的力学性能
对复合膜及纯PVA膜进行力学性能的测试(图6),测试结果总结于表2中。如图6所示,相比较纯PVA膜,MMT-PCNC作为增强质对复合膜的力学性能产生正影响,拉伸强度、断裂伸长率、韧性都不同程度的增加,杨氏模量均略有下降;其中,1MMT-1PCNC/PVA复合膜拉伸强度增长最为明显,为196%;1MMT-2PCNC/PVA复合膜的断裂伸长率、韧性增加最为明显(图6插图),分别为175%和900%。这些均得益于复合膜内部的弱相互作用[25-26]:MMT与PCNC之间通过静电组装,使组装体内部存在大量静电相互作用;MMT-PCNC组装体表面携带羟基和氨基,易与PVA分子侧基羟基形成氢键,增加了组装体与基体的界面相互作用[27]。这些弱相互作用在复合膜受到拉伸时首先断裂,充当牺牲性成键,使复合膜内部的MMT、PCNC产生滑移,断裂伸长率提高,进而提升韧性[28-29];其次,附着于MMT表面的PCNC通过静电与氢键相互作用将MMT与PVA连接,形成具有砖-泥结构的片层复合膜,当应力作用于膜上时,可在这些刚性结构基元之间有效传递,从而增加复合膜的强度。二者协同作用使材料单位应变时所需的应力减小,即刚性减小。
表 2 MMT-PCNC/PVA复合膜的力学性能Table 2. Mechanical properties of the MMT-PCNC/PVA hybrid filmsHybrid films of filler/PVA Elongation-at-break
/%Tensile strength
/MPaYoung’s modulus
/MPaToughness
/(MJ·m−3)PVA 28±3 26±3 101±13 3±1 1MMT-1PCNC/PVA 50±4 77±7 97±6 27±6 1MMT-2PCNC/PVA 77±4 63±8 100±3 30±9 1MMT-4PCNC/PVA 42±2 54±9 92±7 13±2 为了证实氢键的存在,对1MMT-2PCNC/PVA复合膜、纯PVA膜以及冷冻干燥的纯CNC进行了红外分析。如图7所示,在3000~3500 cm−1区域内出现的透过峰归属于PVA羟基伸缩振动,对比几个样品—OH的透过峰峰位可以发现,1MMT-2PCNC/PVA复合膜的峰位较纯CNC和PVA向低波数方向移动。过去研究表明[30-33],氢键的形成是导致羟基峰位发生偏移的主要原因。PEI修饰的CNC表面存在大量的氨基,MMT表面也有一定量的羟基,因此组装体1MMT-2PCNC与PVA之间势必会形成氢键,导致羟基峰发生移动。在1025 cm−1、1733 cm−1以及2945 cm−1波数处出现的峰分别归属于PVA的C—OH、C=O以及脂肪质C—H的伸缩振动[34],在1256 cm−1、1370 cm−1以及1446 cm−1处出现的峰分别归属于PVA的C—H、O—H的弯曲振动以及C—H非平面摇摆振动[35-37]。CNC在3382 cm−1、2915 cm−1、1649 cm−1及1055 cm−1处分别出现明显的透过峰,其中3382 cm−1、2915 cm−1归属于CNC的O—H与C—H的伸缩振动,1649 cm−1归属于CNC吡喃糖环的伸缩振动,而1055 cm−1归属于CNC中吸附了水分子的羟基伸缩振动[38]。
为了进一步研究自组装体改善聚合物薄膜力学性能的强韧化机制,人为破坏薄膜表面造成裂纹进行SEM测试。如图8(a)所示,裂纹均非直线传播,而是随着主裂纹的扩展出现了明显的微裂纹偏转(图8(a)中白色折线),说明MMT、PCNC、PVA之间存在强烈的相互作用,与之前分析一致。存在于片层之间的PCNC通过静电以及氢键作用将PVA和MMT相互连接,当应力施加时充当桥连剂,阻止裂纹扩展,随着应力增大,裂纹将沿着纳米纤维发生偏转,直至最终断裂,这一过程将导致裂纹路径增大,消耗更多的能量,实现复合材料的强韧化[26, 39]。
![]() 图 8 1MMT-2PCNC/PVA复合膜断裂形态的SEM图像和断裂示意图:(a) 断裂过程中裂纹扩展的SEM图像(白色折线表示裂纹偏转);(b) 断裂模型示意图Figure 8. SEM image of fracture morphologies of 1MMT-2PCNC/PVA hybrid films and the proposed fracture model: (a) SEM image of crack propagation occurred during fracture (White line indicate crack propagation); (b) Schematic illustration of the fracture model
图 8 1MMT-2PCNC/PVA复合膜断裂形态的SEM图像和断裂示意图:(a) 断裂过程中裂纹扩展的SEM图像(白色折线表示裂纹偏转);(b) 断裂模型示意图Figure 8. SEM image of fracture morphologies of 1MMT-2PCNC/PVA hybrid films and the proposed fracture model: (a) SEM image of crack propagation occurred during fracture (White line indicate crack propagation); (b) Schematic illustration of the fracture model通过观察组装体中MMT与PCNC的不同比例组装体对薄膜力学性能的影响发现,随着组装体中PCNC含量的增加,拉伸强度逐渐减弱,当MMT与PCNC比例为1∶4时,断裂伸长率也明显下降(图6),原因可能是随着PCNC含量的增加,组装体质量加重,更易发生聚沉,且当其分布于聚合物基体中时,不易分散均匀,导致复合膜力学性能下降[40-41]。
3. 结 论
(1) 组装体1MMT(蒙脱土)-1PCNC(纤维素纳米晶)和1MMT-2PCNC对PVA(聚乙烯醇)力学性能增强较为明显,拉伸强度分别增加196%、142%;断裂伸长率增幅分别为79%、175%;韧性增幅分别为800%、900%;复合膜杨氏模量略有下降。
(2) MMT-PCNC/PVA复合膜中存在多种弱相互作用(静电相互作用、氢键),给应力的传递提供了有效的途径,同时引起裂纹偏转,达到消耗能量的目的,使其在拉伸强度提高的同时断裂伸长率也得到提升。本文提出了一种新的合理设计构筑先进复合纳米材料的思路,即用自组装的方法制备组装体,并利用组装体中存在的大量弱相互作用提升聚合物薄膜的力学性质,从而拓展其应用。
-
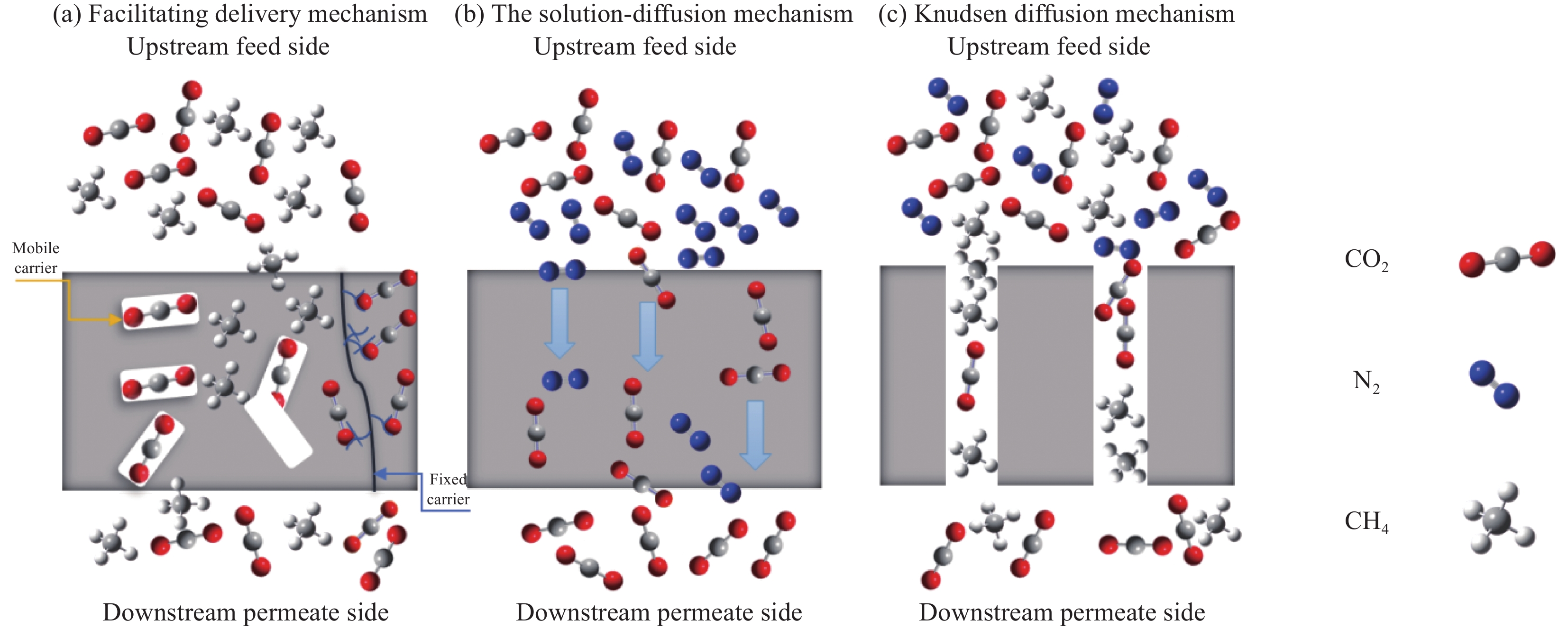
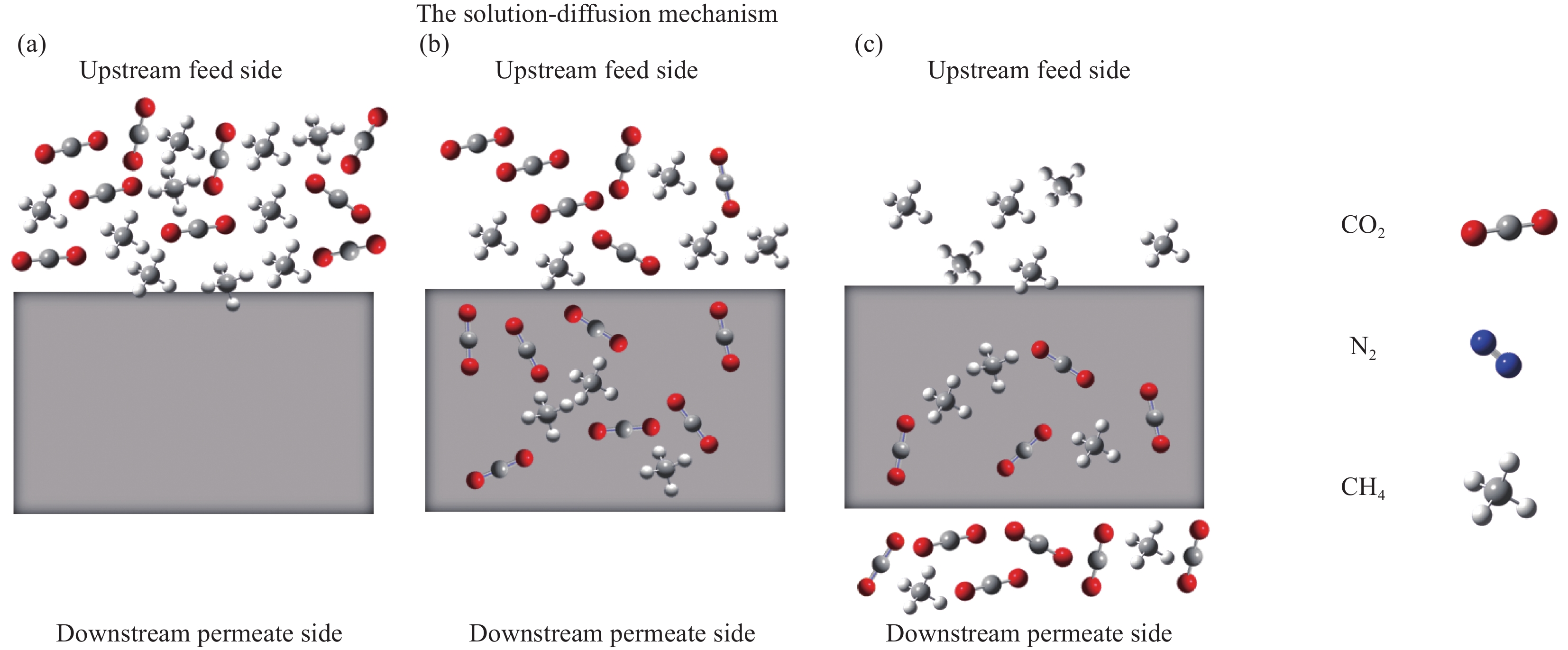
图 2 溶解-扩散过程示意图(a) 与膜接触的上游气体溶解到膜表面;(b) 由于浓度梯度,气体分子通过膜扩散;(c)气体分子通过膜以线性浓度梯度达到扩散平衡。
Figure 2. Dissolved - diffusion process diagram (a) contact with the membrane of upstream gas dissolves in the membrane surface; (b) Diffusion of gas molecules through membranes due to concentration gradients; (c) The diffusion equilibrium of gas molecules through the membrane is achieved in a linear concentration gradient.
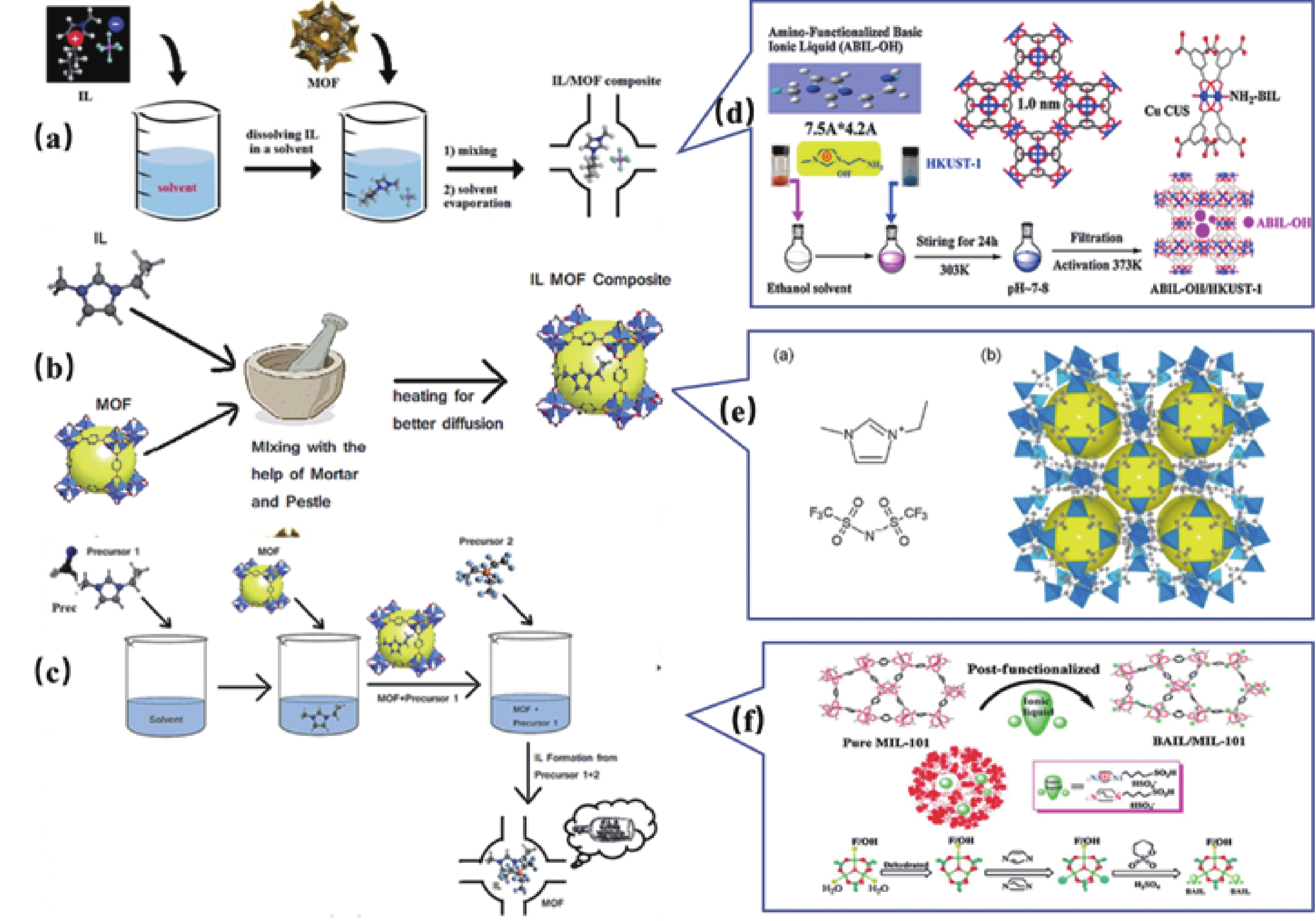
图 5 离子液体(IL)/MOF复合材料合成中使用的不同的合成后浸渍[34]方法的示意图:(a)湿法浸渍法[35],(b)毛细管作用法[36],(c)瓶中船法[37],(d)采用湿法浸渍合成 ABIL-OH@HKUST-1[35],(e)采用毛细管作用法合成的EMI-TFSA的结构[41],(f)合成限制在Cr-MIL-101纳米笼中的BAIL的示意图[42]。
Figure 5. Schematic diagram of different post-synthetic impregnation methods used in Ionic Liquid (IL)/MOF composites synthesis[34] : (a) wet impregnation method [35], (b) capillary action method [36], (c) ship in a bottle method [37], (d) wet impregnation method for ABIL-OH@HKUST-1[35], (e) Structure of EMI-TFSA prepared by capillary action [41], (f) Schematic diagram of synthesized BAIL confined to Cr-MIL-101 nanoccages [42].
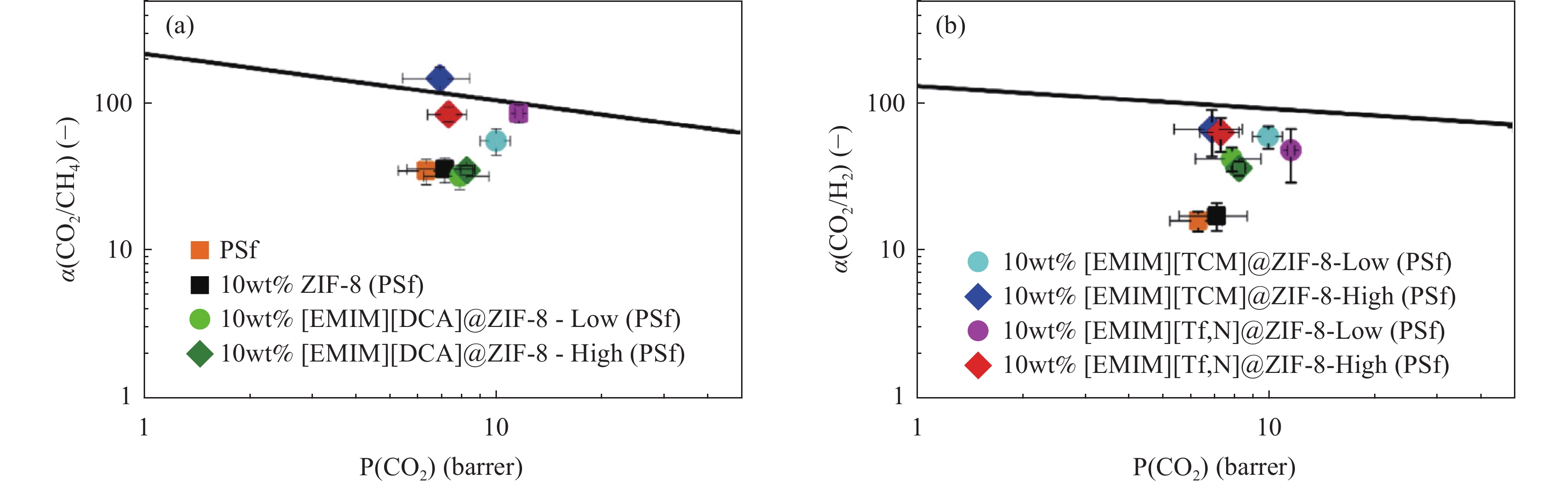
图 8 CO2与Robeson上限(2008年)相比,不同含量的PSf和几种固定10 wt%填料的MMM在303.15 K时的纯气体渗透结果:(a) CO2/CH4及(b) CO2/N2理想的选择性[64]。
Figure 8. CO2 vs. Robeson upper limit (2008) Pure gas permeation results at 303.15 K for PSf with different contents and MMM of several fixed 10 wt% fillers: (a) Ideal selectivity of CO2/CH4 and (b) CO2/N2[64].
表 1 通过不同合成方法合成IL/MOF复合材料的相关研究
Table 1 Studies on the synthesis of IL/MOF composites by different synthesis methods
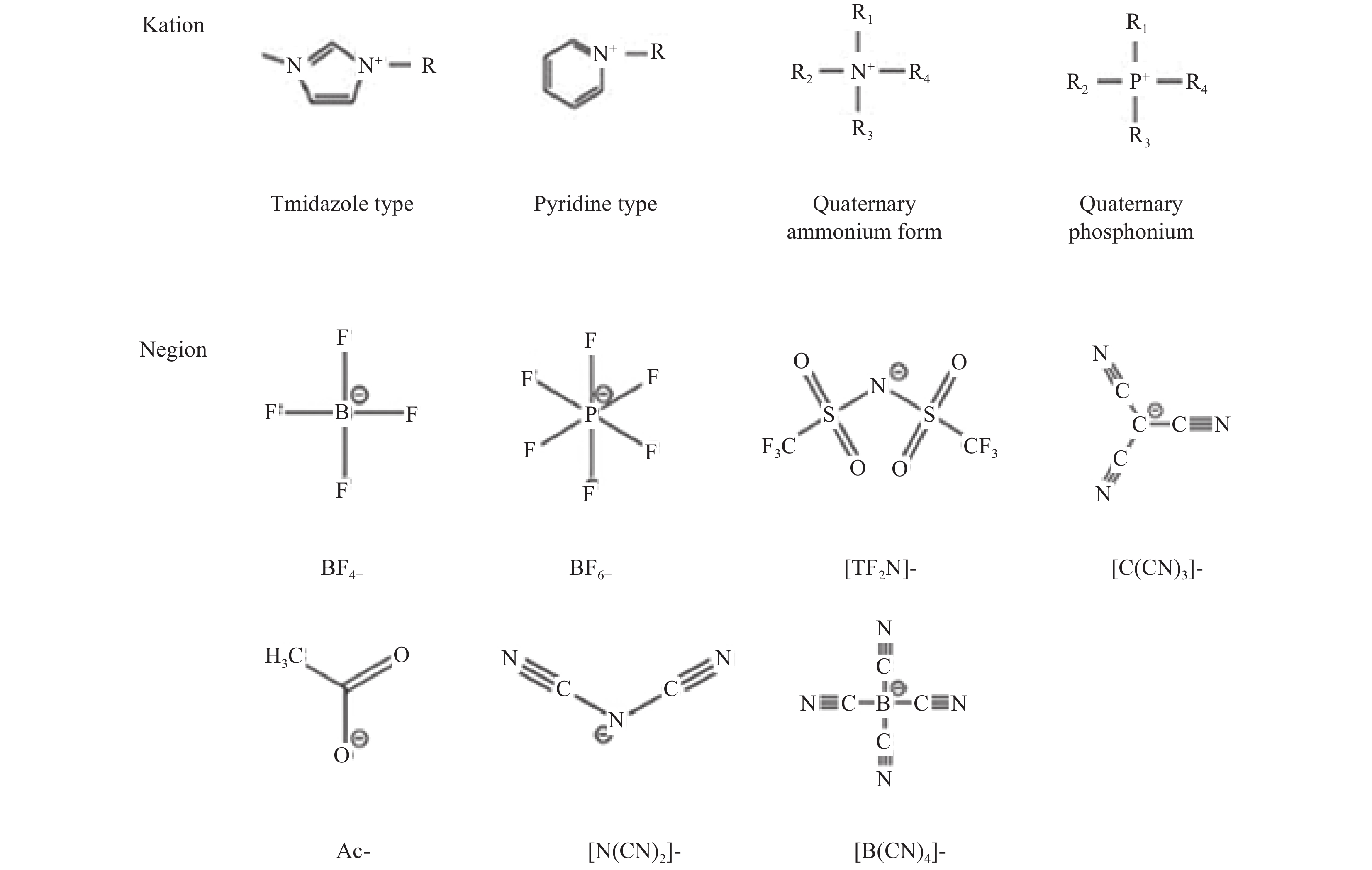
IL MOF IL/MOF
Method of preparationReference [HMIM]Br ZIF-8 Solvothermal synthesis [32] [BMIM]Br MOF-5 Solvothermal synthesis [33] [rmi]X Mn-MOF Solvothermal synthesis [43] [EMIM][HBDC], [EMIM]2[BDC] UiO-66 Solvothermal synthesis [44] [AMI]Br Co-MOF Solvothermal synthesis [45] [BMI]Cl Zn-MOF Solvothermal synthesis [46] [BMIM]Otf ZIF-8 Solvothermal synthesis [47] [EMIM][Tf2N] HKUST-1 Wet dipping method [47] [HEMIM] [DCA] ZIF-8 Wet dipping method [48] C8H15ClN2 MIL-101 (Cr) Wet dipping method [49] [SO3H-(CH2)3-HIM][HSO4] MIL-100(Fe) Wet dipping method [50] [BMIM]Cl MIL-101 Wet dipping method [51] [mim(CH2)3COOH]Cl Uio-66 Wet dipping method [52] [OMIM] Br MIL-100 (Fe) Wet dipping method [53] [BMIM][Tf2N]、[Emim][Tf2N]、[BMIM][BF4] ZIF-67 Wet dipping method [54] [DPP-NC(3)bim] [PMO] MIL-101 (Al) Ship in a bottle method [37] MBIAIL MIL-101 (Cr) Ship in a bottle method [55] BMIMOAc MIL-101-NH2 Ship in a bottle method [40] BAIL MIL-101 Ship in a bottle method [42] N(n-Bu)3Br、P(n-Bu)3Br MIL-101 Ship in a bottle method [56] AmPyI ZIF-90 Ship in a bottle method [57] EMIMCl Uio-67(Zr) Capillary action method [36] EIMS MIL-101 Capillary action method [58] EMI-TFSI ZIF-8 Capillary action method [41] EIMS-HTFSI MIL-101(Cr) Capillary action method [58] EMI-TFSI ZIF-8 Capillary action method [26] [EMIM][DCN]、[EMIM][TCB] MIL-100
(Al)Capillary action method [59] Notes: [HMIM]Br is 1-Hexyl-3-methylimidazolium bromide ;
[BMIM]Br is 1-Butyl-3-methylimidazolium bromide;
[rmi]X is rmi = 1-alkyl-3-methylimidazolium; r = ethyl or propyl, X = Cl, Br, or I(As a template agent); [EMIM][HBDC] is 1-Ethyl-3-methylimidazolium hydrogen bis(2-ethylhexyl) phosphate; [EMIM]2[BDC] is 1-Ethyl-3-methylimidazolium bis(2-ethylhexyl) phosphate;
[AMI]Br is 1-Allyl-3-methylimidazolium chloride;
[BMI]Cl is 1-Butyl-3-methylimidazolium chloride;
[BMIM]Otf is 1-Butyl-3-methylimidazolium trifluoromethansulfonate;
[HEMIM] [DCA] is 1-Hexyl-3-methylimidazolium dicyanamide;
C8H15ClN2 is 1-Butyl-3-methylimidazolium chloride;
[SO3H-(CH2)3-HIM][HSO4] is 3-Sulfonic acid propylimidazolium hydrogen sulfate;
[BMIM]Cl is 1-Butyl-3-methylimidazolium chloride;
[mim(CH2)3COOH]Cl is 1-Methyl-3-(3-carboxypropyl)imidazolium chloride;
[OMIM] Br is 1-Octyl-3-methylimidazolium bromide;
[BMIM][Tf2N] is 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide;
[Emim][Tf2N] is 1-Ethylimidazolium bis(trifluoromethylsulfonyl)imide ;
[BMIM][BF4] is 1-Butyl-3-methylimidazolium tetrafluoroborate;
[DPP-NC(3)bim] [PMO] is Di(3,3'-dipyridyl)methane di(3-cyanomethyl)imidazolium phosphate;
MBIAIL is Methylbenzimidazolium ionic liquid;
BMIMOAc is 1-Butyl-3-methylimidazolium acetate;
BAIL is Amine-based ionic liquid; N(n-Bu)3Br is Tributylamine bromide;
P(n-Bu)3Br is Tributylphosphine bromide;
AmPyI is 1-Aminopyridinium iodide;
EMIMCl is 1-Ethyl-3-methylimidazolium chloride;
EIMS is 1-Ethyl-3-methylimidazolium ethyl sulfate;
EMI-TFSI is 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide;
EIMS-HTFSI is 1-Ethyl-3-methylimidazolium bis(heptafluorobutylsulfonyl)imide;
EMI-TFSI is 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide;
[EMIM][DCN] is 1-Ethyl-3-methylimidazolium dicyanamide;
[EMIM][TCB] is 1-Ethyl-3-methylimidazolium trichlorobenzene.表 2 不同IL/MOF复合材料的制备方法和MOF纳米负载的聚合物基MMM的CO2捕获能力渗透性的比较,膜的CO2/CH4和CO2/N2选择性。
Table 2 Preparation methods of different IL/MOF composites and comparison of CO2 capture capacity of MOF NPS-supported polymer based MMM permeability, CO2/CH4 and CO2/N2 selectivity of the membranes.
MOF IL Polymer Preparation method Filler content/% CO2 permeability/
barrerCO2/CH4 select-ivity CO2/N2
selec-tivityReference ZIF-8 [BMIM][Tf2N] Pebax 1657 Blending method 16.8–18.3% IL in ZIF-8; 0–25% composite 104.9 34 83.9 [27] ZIF-8 [BMIM][Tf2N] PSf Blending method 6 307 26 53 [27] ZIF-8 [BMIM][Tf2N] Pebax Blending method 5 80 21 58 [27] ZIF-8 [BMIM][Tf2N] PSf Pre-modified by ship in bottle method Wide range 310 45.7 130 [30] ZIF-8 [BMIm]Otf PVIM/PI Pre-modified by ship in bottle method 570 37.5 39 [30] ZIF-8 [EMIM][DCA] PSf Blending method 10%/30% (composite—3–30% IL) 8.3 35.51 36.83 [64] ZIF-8 [EMIM][TCM] PSf 10.05 56.29 60.43 [64] ZIF-8 [EMIM][Tf2N] PSf 7.36 85.3 64.25 [64] ZIF-8 DnBMCl Pebax 1657 Blending method 40% IL in PX—0–32% filler 260 37 70 [65] ZIF-8 [EMIM][BF4] PIL
P[vbim][Tf2N]Wet impregnation 33% IL in PIL—0-25% filler 340 16.59 29.06 [66] ZIF-8 [EMIM][Tf2N] 693.6 12.1 19.65 [66] ZIF-8 [EMIM][B(CN)4] 1062.4 12.34 24.2 [66] ZIF-67 [BMIM][BF4] PIM-1
6 FDA-
durene
Wet impregnation
Wet impregnation
10 % ZIF-67/PIM-1
5% IL in composite 20% in MMM1300 17 27 [54] ZIF-67 TSIL 9971.3 7.6 [67] ZIF-67 [EMIM][Tf2N] 1200 25 25 [54] ZIF-67 [BMIM][Tf2N] 900 28 27.5 [54] ZIF-67 [BMIM][BF4] PI Pre-modified by ship in bottle method 20 1250 24 25 [54] HKUST-1/Cu3(BTC)2 [EMIM][BF4] Matrimid
5218Blending method 10 32.5 46.7 18.69 [68] HKUST-1/Cu3(BTC)2 [EMIM][OTF] Matrimid
5218Blending method 10 37.78 97 24.4 [68] HKUST-1 [EMIM][Tf2N] PI Pre-modified by ship in bottle method 10 1101.6 29.3 27.1 [69] NH2-MIL-101(Cr) [NH2bim][Tf2N] PIM-1 Solvothermal synthesis 5 2979 - 37 [70] UiO-66 IL-ClO4 PU Solvothermal synthesis 30 - 15.3 24.4 [71] UiO-66 IL-ClO4 PU Solvothermal synthesis 50 - 32.3 18.3 [71] Notes: Pebax 1657 is Poly (ether-block-amide) resin-1657 ;
PSf is Polysulfone;
Pebax is Poly(ether-block-amide);
PVIM is Poly(N-vinylimidazole) ;
PI is Polyimide;
PILis Poly(N-isopropylacrylamide);
P[vbim][Tf2N] is Poly(vinylimidazole) bis(trifluoromethanesulfonyl)imide;
PIM-1 is Prolyl Isomerase of Mammalian-1;
Matrimid5218 is Polyamide-imide;
PU is Polyurethane. -
[1] HAN Y, YANG Y, HO W S W. Recent Progress in the Engineering of Polymeric Membranes for CO2 Capture from Flue Gas[J]. Membranes (Basel), 2020, 10(11): 365. DOI: 10.3390/membranes10110365
[2] ROBESON L M. The upper bound revisited[J]. Journal of Membrane Science, 2008, 320(1-2): 390-400. DOI: 10.1016/j.memsci.2008.04.030
[3] ADATOZ E, AVCI A K, KESKIN S. Opportunities and challenges of MOF-based membranes in gas separations[J]. Separation and Purification Technology, 2015, 152: 207-237. DOI: 10.1016/j.seppur.2015.08.020
[4] QIU S, XUE M, ZHU G. Metal–organic framework membranes: from synthesis to separation application[J]. Chem Soc Rev, 2014, 43: 6116-6140. DOI: 10.1039/C4CS00159A
[5] SIAGIAN U W R, RAKSAJATI A, HIMMA N F, et al. Membrane-based carbon capture technologies: Membrane gas separation vs. membrane contactor[J]. Journal of Natural Gas Science and Engineering, 2019, 67: 172-195. DOI: 10.1016/j.jngse.2019.04.008
[6] CHUNG T-S, JIANG L Y, LI Y, et al. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation[J]. Progress in Polymer Science, 2007, 32(4): 483-507. DOI: 10.1016/j.progpolymsci.2007.01.008
[7] PRASETYA N, HIMMA N F, SUTRISNA P D, et al. A review on emerging organic-containing microporous material membranes for carbon capture and separation[J]. Chemical Engineering Journal, 2020, 391.123575
[8] LI J R, SCULLEY J, ZHOU H C. Metal-organic frameworks for separations[J]. Chem Reviews, 2012, 112(2): 869-932. DOI: 10.1021/cr200190s
[9] AN J, ROSI N L. Tuning MOF CO2 Adsorption Properties via Cation Exchange[J]. Journal of the American Chemical Society, 2010, 132(16): 5575-5579.
[10] KRISHNA R, VAN BATEN J M. In silico screening of metal–organic frameworks in separation applications[J]. Physical Chemistry Chemical Physics, 2011, 13(22): .10593-10616. DOI: 10.1039/c1cp20282k
[11] KRISHNA R, VAN BATEN J M. In silico screening of zeolite membranes for CO2 capture[J]. Journal of Membrane Science, 2010, 360(1-2): 323-333. DOI: 10.1016/j.memsci.2010.05.032
[12] WANG Q, DAI Y, RUAN X, et al. ZIF-8 hollow nanotubes based mixed matrix membranes with high-speed gas transmission channel to promote CO2/N2 separation[J]. Journal of Membrane Science, 2021, 630: 119323 DOI: 10.1016/j.memsci.2021.119323
[13] 文桂林, 李莹, 张红星, 等. 离子液体/金属-有机骨架复合材料制备方法、理论计算及应用研究进展[J]. 复合材料学报, 2021, 38(2): 298-314. WEN Guilin, LI Ying, ZHANG Hongxing. et al. Research progress on preparation methods, theoretical calculation and application of ionic liquid/metal-organic matrix composites[J]. Journal of Composite Materials, 2021, 38(2): 298-314(in Chinese).
[14] PANDYA I, EL SEOUD O A, ASSIRI M A, et al. Ionic liquid/ metal organic framework composites as a new class of materials for CO2 capture: Present scenario and future perspective[J]. Journal of Molecular Liquids, 2024, 395.
[15] 赵亚梅, 曹婷婷, 丁思奇, 等. 基于离子液体修饰的金属有机骨架材料在CO2分离与转化方面的研究进展[J]. 化学研究与应用, 2021, 33(9): 1633-1641. ZHAO Yamei, CAO Tingting, DING Siqi, et al. Research progress on CO2 separation and conversion of metal-organic matrix materials modified by ionic liquids[J]. Chemical research and application, 2021, 33(9): 1633-1641(in Chinese).
[16] KOYUTURK B, ALTINTAS C, KINIK F P, et al. Improving Gas Separation Performance of ZIF-8 by [BMIM][BF4] Incorporation: Interactions and Their Consequences on Performance[J]. The Journal of Physical Chemistry C, 2017, 121(19): 10370-10381.
[17] 俞江南, 李康, 陈飞, 等. 面向CO2分离的混合基质膜研究进展[J]. 化学工业与工程, 2023, 40(3): 74-83. YU Jiangnan, LI Kang. CHEN Fei. et al. Research progress of hybrid matrix membrane for CO2 separation[J]. Chemical Industry and Engineering, 2023, 40(3): 74-83(in Chinese).
[18] QIN Z, MA Y, WEI J, et al. Recent progress in ternary mixed matrix membranes for CO2 separation[J]. Green Energy & Environment, 2024, 9(5): 831-858.
[19] WIJMANS J G, BAKER R W. The solution-diffusion model: a review[J]. Journal of Membrane Science, 1995, 107(1): 1-21.
[20] XU X, WANG J, ZHOU A, et al. High-efficiency CO2 separation using hybrid LDH-polymer membranes[J]. Nature Communications, 2021, 12(1): 3039. DOI: 10.1038/s41467-021-23171-3
[21] KRISHNA R, VAN BATEN J M. A comparison of the CO2 capture characteristics of zeolites and metal–organic frameworks[J]. Separation and Purification Technology, 2012, 87: 120-126. DOI: 10.1016/j.seppur.2011.11.031
[22] Hou Q, Wu Y, Zhou S, et al. Ultra-tuning of the aperture size in stiffened ZIF-8 cm frameworks with mixed-linker strategy for enhanced CO2/CH4 separation[J]. Angewandte Chemie International Edition, 2019, 58(1): 327-331 DOI: 10.1002/anie.201811638
[23] NIU Z, HE N, YAO Y, et al. Mixed matrix membranes for gas separations: A review[J]. Chemical Engineering Journal, 2024, 494: 152912. DOI: 10.1016/j.cej.2024.152912
[24] THORNTON A W, AHMED A, KANNAM S K, et al. Analytical Diffusion Mechanism model combining specular, Knudsen and surface diffusion[J]. Journal of Membrane Science, 2015, 485: 1-9. DOI: 10.1016/j.memsci.2015.03.004
[25] AHMAD N N R, LEO C P, MOHAMMAD A W, et al. Re-cent progress in the development of ionic liquid-based mixed matrix membrane for CO2 separation: A review[J]. International Journal of Energy Research, 2021, 45(7): 9800-9830. DOI: 10.1002/er.6518
[26] FUJIE K, YAMADA T, IKEDA R, et al. Introduction of an Ionic Liquid into the Micropores of a Metal–Organic Framework and Its Anomalous Phase Behavior[J]. Angewandte Chemie International Edition, 2014, 53(42): 11302-11305. DOI: 10.1002/anie.201406011
[27] DAI Z, ANSALONI L, DENG L. Recent advances in multi-layer composite polymeric membranes for CO2 separation: A review[J]. Green Energy & Environment, 2016, 1(2): 102-208.
[28] KINIK F P, UZUN A, KESKIN S. Ionic Liquid/Metal–Organic Framework Composites: From Synthesis to Applications[J]. ChemSusChem, 2017, 10(14): 2842-2863 DOI: 10.1002/cssc.201700716
[29] SOO X Y D, LEE J J C, WU W-Y, et al. Advancements in CO2 capture by absorption and adsorption: A comprehensive review[J]. Journal of CO2 Utilization, 2024, 81. 102727.
[30] BAN Y, LI Z, LI Y, et al. Confinement of Ionic Liquids in Nanocages: Tailoring the Molecular Sieving Properties of ZIF-8 for Membrane-Based CO2 Capture[J]. Angewandte Chemie International Edition, 2015, 54(51): 15483-15487. DOI: 10.1002/anie.201505508
[31] KATAYAMA Y, BENTZ K C, COHEN S M. Defect-Free MOF-Based Mixed-Matrix Membranes Obtained by Corona Cross-Linking[J]. ACS Applied Materials & Interfaces, 2019, 11(13): 13029-13037.
[32] LIU C, ZHANG G, ZHAO C, et al. MOFs synthesized by the ionothermal method addressing the leaching problem of IL–polymer composite membranes[J]. Chemical Communications, 2014, 50(91): 14121-14124. DOI: 10.1039/C4CC05526H
[33] YANG H M, SONG X L, YANG T L, et al. Electrochemical synthesis of flower shaped morphology MOFs in an ionic liquid system and their electrocatalytic application to the hydrogen evolution reaction[J]. RSC advances, 2014, 4(30): 15720-15726. DOI: 10.1039/C3RA47744D
[34] GU J. Experimental Study on CO2 Capture by Impregnating ZIF-67 with Ionic Liquid[J]. Hans Journal of Chemical Engineering and Technology, 2022, 12(3): 234-239. DOI: 10.12677/HJCET.2022.123031
[35] LUO Q-X, SONG X-D, JI M, et al. Molecular-size and shape-selective Knoevenagel condensation over microporous Cu3(BTC)2 immobilized amino-functionalized basic ionic liquid catalyst[J]. Applied Catalysis A: General, 2014, 478: 81-90. DOI: 10.1016/j.apcata.2014.03.041
[36] CHEN H, HAN S-Y, LIU R-H, et al. High conductive, long-term durable, anhydrous proton conductive solid-state electrolyte based on a metal-organic framework impregnated with binary ionic liquids: Synthesis, characteristic and effect of anion[J]. Journal of Power Sources, 2018, 376: 168-176. DOI: 10.1016/j.jpowsour.2017.11.089
[37] HUANG R, GUO X, MA S, et al. Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin[J]. Polymers, 2020, 12(1): 108. DOI: 10.3390/polym12010108
[38] MOHAMEDALI M, IBRAHIM H, HENNI A. Incorporation of acetate-based ionic liquids into a zeolitic imidazolate framework (ZIF-8) as efficient sorbents for carbon dioxide capture[J]. Chemical Engineering Journal, 2018, 334: 817-828. DOI: 10.1016/j.cej.2017.10.104
[39] HUSSAIN S, DONG H, ZHANG Y, et al. Impregnation of 1-n-Butyl-3-methylimidazolium Dicyanide [BMIM][DCA] into ZIF-8 as a Versatile Sorbent for Efficient and Selective Separation of CO2[J]. Industrial & Engineering Chemistry Research, 2022, 61(1): 706-715.
[40] CHONG S Y, WANG T T, CHENG L C, et al. Metal–Organic Framework MIL-101-NH2-Supported Acetate-Based Butylimidazolium Ionic Liquid as a Highly Efficient Heterogeneous Catalyst for the Synthesis of 3-Aryl-2-oxazolidinones[J]. Langmuir, 2018, 35(2): 495-503.
[41] FUJIE K, OTSUBO K, IKEDA R, et al. Low temperature ionic conductor: ionic liquid incorporated within a metal–organic framework[J]. Chemical Science, 2015, 6(7): 4306-4310. DOI: 10.1039/C5SC01398D
[42] LUO Q-X, SONG X-D, JI M, et al. Molecular size- and shape-selective Knoevenagel condensation over microporous Cu3(BTC)2 immobilized amino-functionalized basic ionic liquid catalyst[J]. Applied Catalysis A: General, 2014, 478: 81-90. DOI: 10.1016/j.apcata.2014.03.041
[43] XU L, KWON Y-U, DE CASTRO B, et al. Novel Mn(II)-Based Metal–Organic Frameworks Isolated in Ionic Liquids[J]. Crystal Growth & Design, 2013, 13(3): 1260-6.
[44] ERMER M, MEHLER J, KRIESTEN M, et al. Synthesis of the novel MOF hcp UiO-66 employing ionic liquids as a linker precursor[J]. Dalton Transactions, 2018, 47(41): 14426-14430. DOI: 10.1039/C8DT02999G
[45] XU L, LIU B, LIU S-X, et al. The influence of 1-alkyl-3-methyl imidazolium ionic liquids on a series of cobalt-1, 4-benzenedicarboxylate metal–organic frameworks[J]. Crystal Engineering Communications, 2014, 16(46): 10649-10657. DOI: 10.1039/C4CE01722F
[46] ZHANG Z-H, LIU B, XU L, et al. Combination effect of ionic liquid components on the structure and properties in 1, 4-benzenedicarboxylate based zinc metal–organic frameworks[J]. Dalton Transactions, 2015, 44(41): 17980-17989. DOI: 10.1039/C5DT02672E
[47] LIN R, GE L, DIAO H, et al. Ionic Liquids as the MOFs/Polymer Interfacial Binder for Efficient Membrane Separation[J]. ACS Applied Materials & Interfaces, 2016, 8(46): 32041-32049.
[48] ZEESHAN M, NOZARI V, YAGCI M B, et al. Core–Shell Type Ionic Liquid/Metal Organic Framework Composite: An Exceptionally High CO2/CH4 Selectivity[J]. Journal of the American Chemical Society, 2018, 140(32): 10113-10116. DOI: 10.1021/jacs.8b05802
[49] KHAN N A, HASAN Z, JHUNG S H. Ionic Liquids Supported on Metal-Organic Frameworks: Remarkable Adsorbents for Adsorptive Desulfurization[J]. Chemistry – A European Journal, 2013, 20(2): 376-380.
[50] WAN H, CHEN C, WU Z, et al. Encapsulation of Heteropolyanion-Based Ionic Liquid within the Metal–Organic Framework MIL-100(Fe) for Biodiesel Production[J]. ChemCatChem, 2014, 7(3): 441-449.
[51] BAHADORI M, TANGESTANINEJAD S, BERTMER M, et al. Task-Specific Ionic Liquid Functionalized–MIL–101(Cr) as a Heterogeneous and Efficient Catalyst for the Cycloaddition of CO2 with Epoxides Under Solvent Free Conditions[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(4): 3962-3973.
[52] QI Z, QIU T, WANG H, et al. Synthesis of ionic-liquid-functionalized UiO-66 framework by post-synthetic ligand exchange for the ultra-deep desulfurization[J]. Fuel, 2020, 268. 117336.
[53] JIN M, NIU Q, LIU G, et al. Encapsulation of ionic liquids into POMs-based metal–organic frameworks: screening of POMs-ILs@MOF catalysts for efficient cycloolefins epoxidation[J]. Journal of Materials Science, 2020, 55(19): 8199-8210. DOI: 10.1007/s10853-020-04611-9
[54] VU M-T, LIN R, DIAO H, et al. Effect of ionic liquids (ILs) on MOFs/polymer interfacial enhancement in mixed matrix membranes[J]. Journal of Membrane Science, 2019, 587: 117157. DOI: 10.1016/j.memsci.2019.05.081
[55] HAN M, LI Y, GU Z, et al. Immobilization of thiol-functionalized ionic liquids onto the surface of MIL-101(Cr) frameworks by S Cr coordination bond for biodiesel production[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 553: 593-600.
[56] MA D, LI B, LIU K, et al. Bifunctional MOF heterogeneous catalysts based on the synergy of dual functional sites for efficient conversion of CO2 under mild and co-catalyst free conditions[J]. Journal of Materials Chemistry A, 2015, 3(46): 23136-23142. DOI: 10.1039/C5TA07026K
[57] THARUN J, BHIN K-M, ROSHAN R, et al. Ionic liquid tethered post functionalized ZIF-90 framework for the cycloaddition of propylene oxide and CO2[J]. Green Chemistry, 2016, 18(8): 2479-2487. DOI: 10.1039/C5GC02153G
[58] SUN X L, DENG W H, CHEN H, et al. A Metal–Organic Framework Impregnated with a Binary Ionic Liquid for Safe Proton Conduction above 100 °C[J]. Chemistry – A European Journal, 2016, 23(6): 1248-1252.
[59] AIJAZ A, AKITA T, YANG H, et al. From ionic-liquid@metal–organic framework composites to heteroatom-decorated large-surface area carbons: superior CO2 and H2 uptake[J]. Chemical Communications, 2014, 50(49): 6498-6501. DOI: 10.1039/c4cc02459a
[60] LI J-R, KUPPLER R J, ZHOU H-C. Selective gas adsorption and separation in metal–organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. DOI: 10.1039/b802426j
[61] LI M D, ZHANG X P, ZENG S J, et al. Pebax-based composite membranes with high gas transport properties enhanced by ionic liquids for CO2 separation[J]. Rsc Advances, 2017, 7(11): 6422-6431. DOI: 10.1039/C6RA27221E
[62] CASADO-COTERILLO C, FERNáNDEZ-BARQUíN A, ZORNOZA B, et al. Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2 separation[J]. Royal Society of Chemistry Advances, 2015, 5(124): 102350-102361.
[63] 时飞, 李奕帆. 混合基质膜在碳捕集领域的研究进展[J]. 化工进展, 2020, 39(06): 2453-2462. SHI Fei, LI Yifan. Research progress of mixed matrix membranes in carbon capture[J]. Advances in Chemical Industry, 2019, 39(06): 2453-2462 (in Chinese).
[64] ORTIZ-ALBO P, FERREIRA T J, MARTINS C F, et al. Impact of Ionic Liquid Structure and Loading on Gas Sorption and Permeation for ZIF-8-Based Composites and Mixed Matrix Membranes[J]. Membranes, 2021, 12(1): 13. DOI: 10.3390/membranes12010013
[65] JOMEKIAN A, BAZOOYAR B, BEHBAHANI R M, et al. Ionic liquid-modified Pebax® 1657 membrane filled by ZIF-8 particles for separation of CO2 from CH4, N2 and H2[J]. Journal of Membrane Science, 2017, 524: 652-662. DOI: 10.1016/j.memsci.2016.11.065
[66] HAO L, LI P, YANG T, et al. Room temperature ionic liquid/ZIF-8 mixed-matrix membranes for natural gas sweetening and post-combustion CO2 capture[J]. Journal of Membrane Science, 2013, 436: 221-231. DOI: 10.1016/j.memsci.2013.02.034
[67] HAN J, BAI L, JIANG H, et al. Task-Specific Ionic Liquids Tuning ZIF-67/PIM-1 Mixed Matrix Membranes for Efficient CO2 Separation[J]. Industrial & Engineering Chemistry Research, 2020, 60(1): 593-603.
[68] MONTEIRO B, NABAIS A, CASIMIRO M, et al. Impact on CO2/N2 and CO2/CH4 Separation Performance Using Cu-BTC with Supported Ionic Liquids-Based Mixed Matrix Membranes[J]. Membranes, 2018, 8(4): 93. DOI: 10.3390/membranes8040093
[69] LING R J, GE L, DIAO H, et al. Ionic Liquids as the MOFs/Polymer Interfacial Binder for Efficient Membrane Separation[J]. ACS Applied Materials & Interfaces, 2016, 8(46): 32041-32049.
[70] MA J, YING Y, GUO X, et al. Fabrication of mixed-matrix membrane containing metal–organic framework composite with task-specific ionic liquid for efficient CO2 separation[J]. Journal of Materials Chemistry A, 2016, 4(19): 7281-7288. DOI: 10.1039/C6TA02611G
[71] YAO B-J, DING L-G, LI F, et al. Chemically Cross-Linked MOF Membrane Generated from Imidazolium-Based Ionic Liquid-Decorated UiO-66 Type NMOF and Its Application toward CO2 Separation and Conversion[J]. ACS Applied Materials & Interfaces, 2017, 9(44): 38919-38930.
[72] LEE W G, KANG S W. Highly selective poly(ethylene oxide)/ionic liquid electrolyte membranes containing CrO3 for CO2/N2 separation[J]. Chemical Engineering Journal, 2019, 356: 312-327. DOI: 10.1016/j.cej.2018.09.049
[73] ROGERS R D, SEDDON K R. Ionic Liquids--Solvents of the Future?[J]. Science, 2003, 302(5646): 792-793.
[74] 李孟盈, 吕春捷, 徐立华, 等. 离子液体-醇胺水溶液捕集CO2研究进展[J]. 现代化工, 2021, 41(2): 70-74. LI Mengying, LV Chunjie, Xu Lihua, et al. Research progress of CO2 capture in ionic liquid-alcohol amine aqueous solution[J]. Modern Chemical Industry, 2021, 41(2): 70-74(in Chinese).
[75] 王雪莉, 杨卫亚, 张会成, 等. MOF基混合基质膜界面改性方法及其气体分离性能[J]. 化工进展: 1-15. WANG Xueli, YANG Weiya, ZHANG Huicheng. et al. Interfacial modification of MOF-based hybrid matrix membrane and its gas separation performance[J]. Chemical Industry Progress: 1-15(in Chinese).
[76] Y YASMEEN I, ILYAS A, SHAMAIR Z, et al. Synergistic effects of highly selective ionic liquid confined in nanocages: Exploiting the three component mixed matrix membranes for CO2 capture[J]. Chemical Engineering Research & Design, 2020, 155: 123-132.
[77] DA SILVA F W M, MAGALHAES G M, JARDIM E O, et al. CO2 Adsorption on Ionic Liquid-Modified Cu-BTC: Experimental and Simulation Study[J]. Adsorption Science & Technology, 2015, 33(2): 223-242.
-
目的
探讨离子液体(Ionic Liquid, IL)与金属有机框架(Metal-Organic Framework, MOF)作为高效填料共同用于三元相MMM的制备过程,深入分析了IL与MOF整合入MMM后所产生的协同效应,尤其是它们对提升膜材料机械稳定性和气体吸附性能方面的显著贡献。
方法利用表格罗列和文献总结归纳了IL与MOF作为高效填料共同用于三元相MMM的制备过程、产生的协同效应、对性能的多维度贡献等。同时详细介绍了MMM中气体分离的传输机制,总结了IL/MOF复合材料的结构和制备工艺,并对三元相MMM应用于CO分离的现状进行了分析和综述,以及在CO分离领域的应用效果和最新发展。
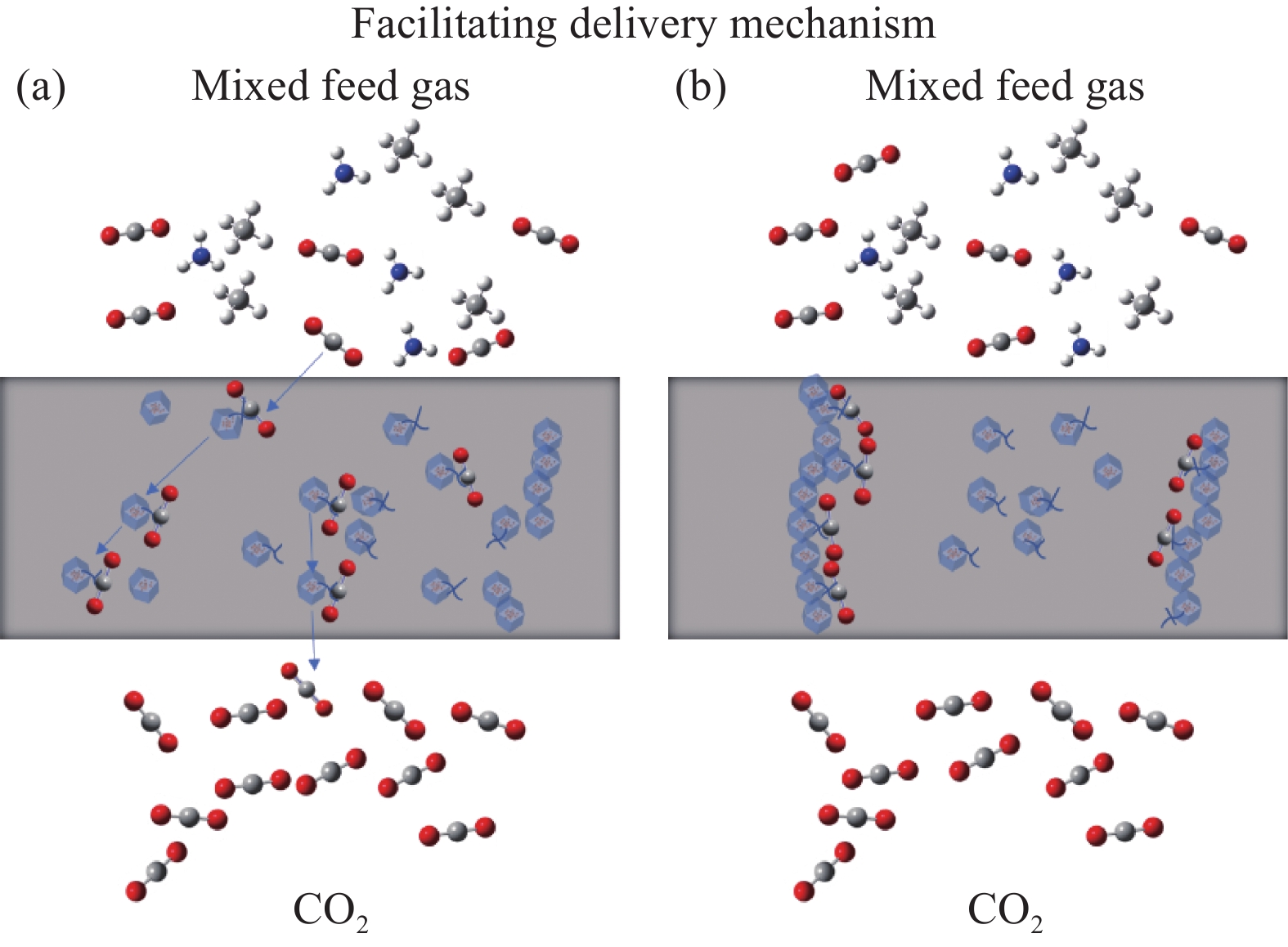
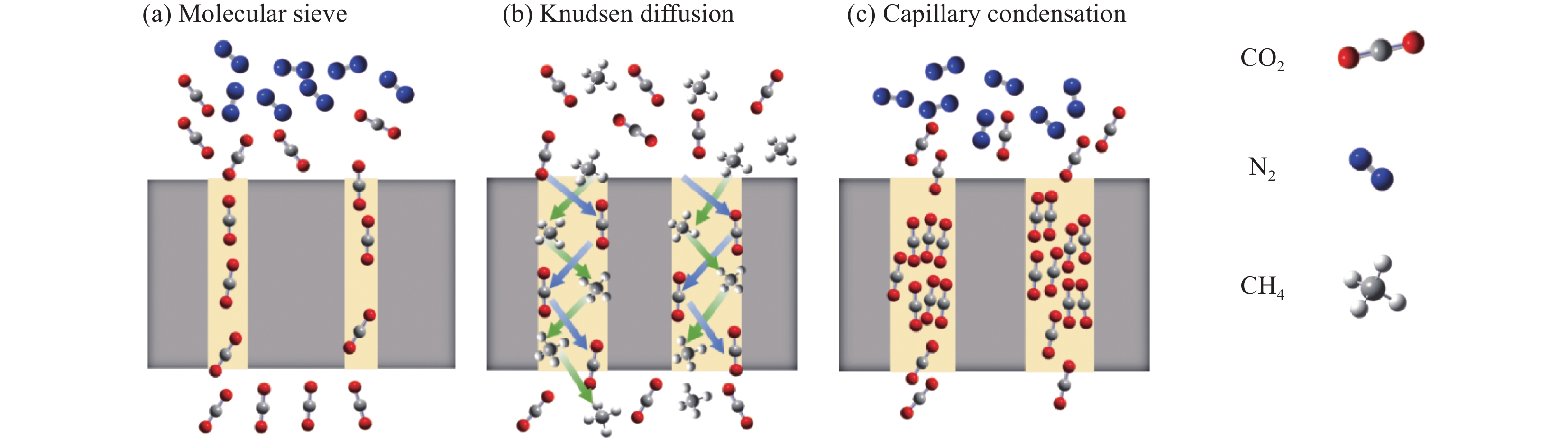
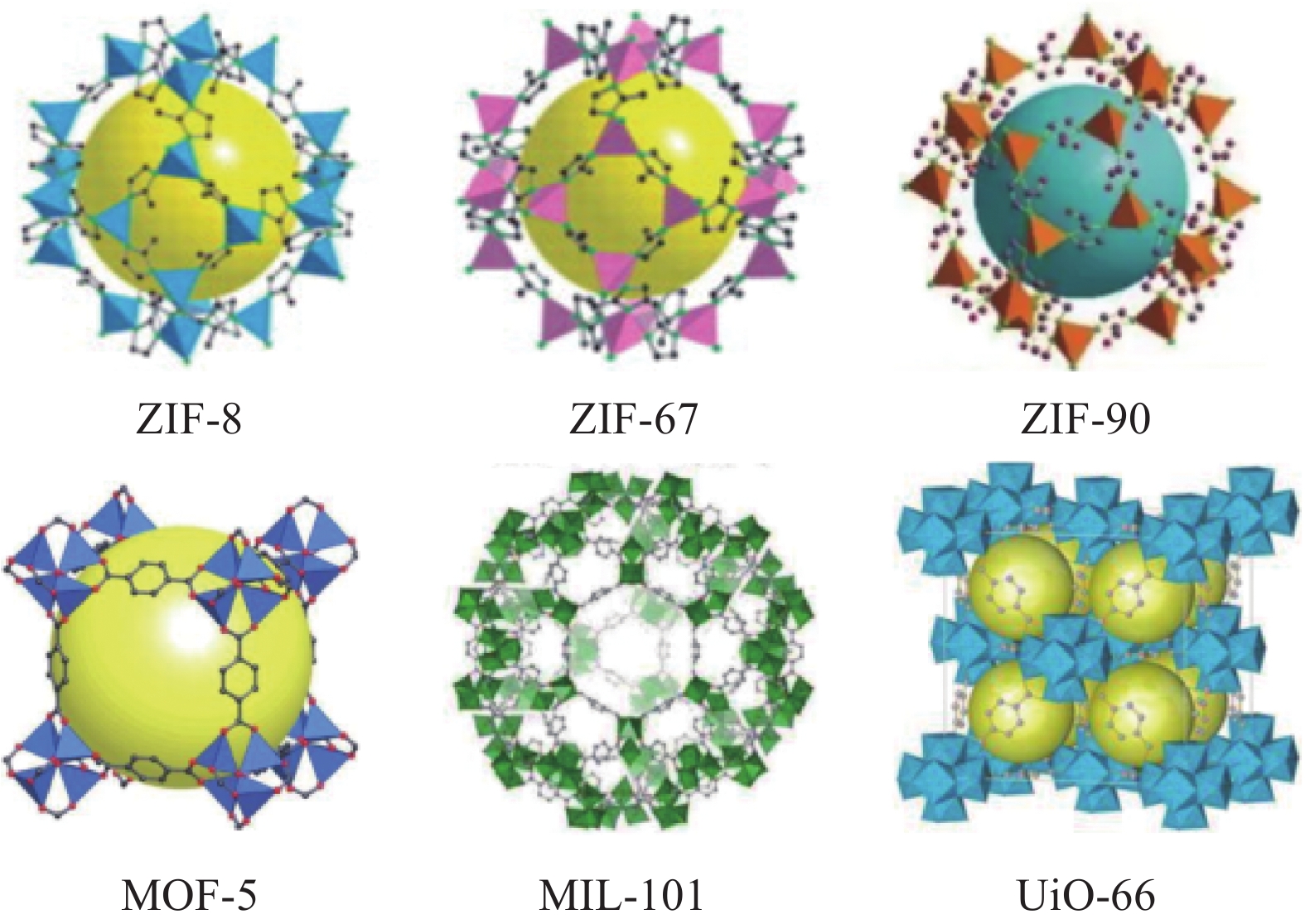
结果在应用二元MMM进行气体分离时,由于有机聚合物与无机填料之间的不相容性,以及它们之间的粘附性差异,实现理想的填料分散和填料/聚合物界面仍然具有挑战性。而IL可以填补填料与聚合物相之间的空隙,并改善填料的分散性和填料/聚合物界面的结合性,向MOF中添加IL并融入聚合物基质中,不仅可提升膜材料的机械强度,还能增强对某些气体的吸收能力。利用IL进行功能化处理,可以调节填料的孔道结构和化学特性,改善三元MMM对CO的捕获能力。通常,IL与聚合物和无机填料结合形成IL/填料/聚合物的三元MMM,制备IL/MOF三元MMM的主流方法有共混法、IL对二元MMM后处理、IL改性聚合物等方法,而制备方法的选择会影响膜的孔隙率、孔径分布和表面粗糙度等特性,膜的渗透性、选择性、机械强度等与膜的孔结构直接相关,改变制备条件与策略从而影响膜的气体分离性能;另外,在三元MMM中,MOF、IL种类和气体成分是影响CO2去除效率的关键因素:在MOF的选择方面,高比表面积和可调控的孔道结构以及热/化学稳定性是关键,例如ZIF-8、UiO-66和MIL-101等在气体分离膜应用广泛的填料都表现出较好的CO吸附性能。在IL的选择方面,IL与MOF的相容性和稳定性是首要考虑的因素,而在常见的IL中,咪唑类IL由于其独特的化学结构和物理性质,例如[BMIM][TfN]、[EMIM][TfN]等都具有极高的CO溶解度,能够显著提高膜的渗透性和选择性,在三元MMM分离CO的研究中表现出显著优势。而聚合物基质如PSf、Pebax和PIM-1为膜提供了必要的机械支撑和分离基础。在实际应用中,混合气体的影响也不能忽视,由于气体混合物中分子间相互作用改变了单一气体的渗透行为。在气体分离过程中,常见的现象包括竞争吸附、浓差极化以及渗透剂诱导的塑化效应等。而研究发现,通过综合考虑材料设计中的关键因素,如MOF的类型、IL的选择以及改性策略,三元MMM有望在混合气体条件下依然表现出优异的分离性能。
结论MOF在结构和组分上的高度可调节性和多样性等优势在三元MMM领域尚未得到充分体现,未来可以以具体的分离体系为导向,设计具有特定吸附位点和孔道结构的MOF,以提高膜材料的选择性和渗透性。同时,可以通过引入不同的金属节点和有机配体,以实现对MOF性能的精细调控。此外,结合离子液体的特性,可以探索新的合成策略,以制备出具有优异分离性能的IL/MOF复合膜材料。




 下载:
下载: