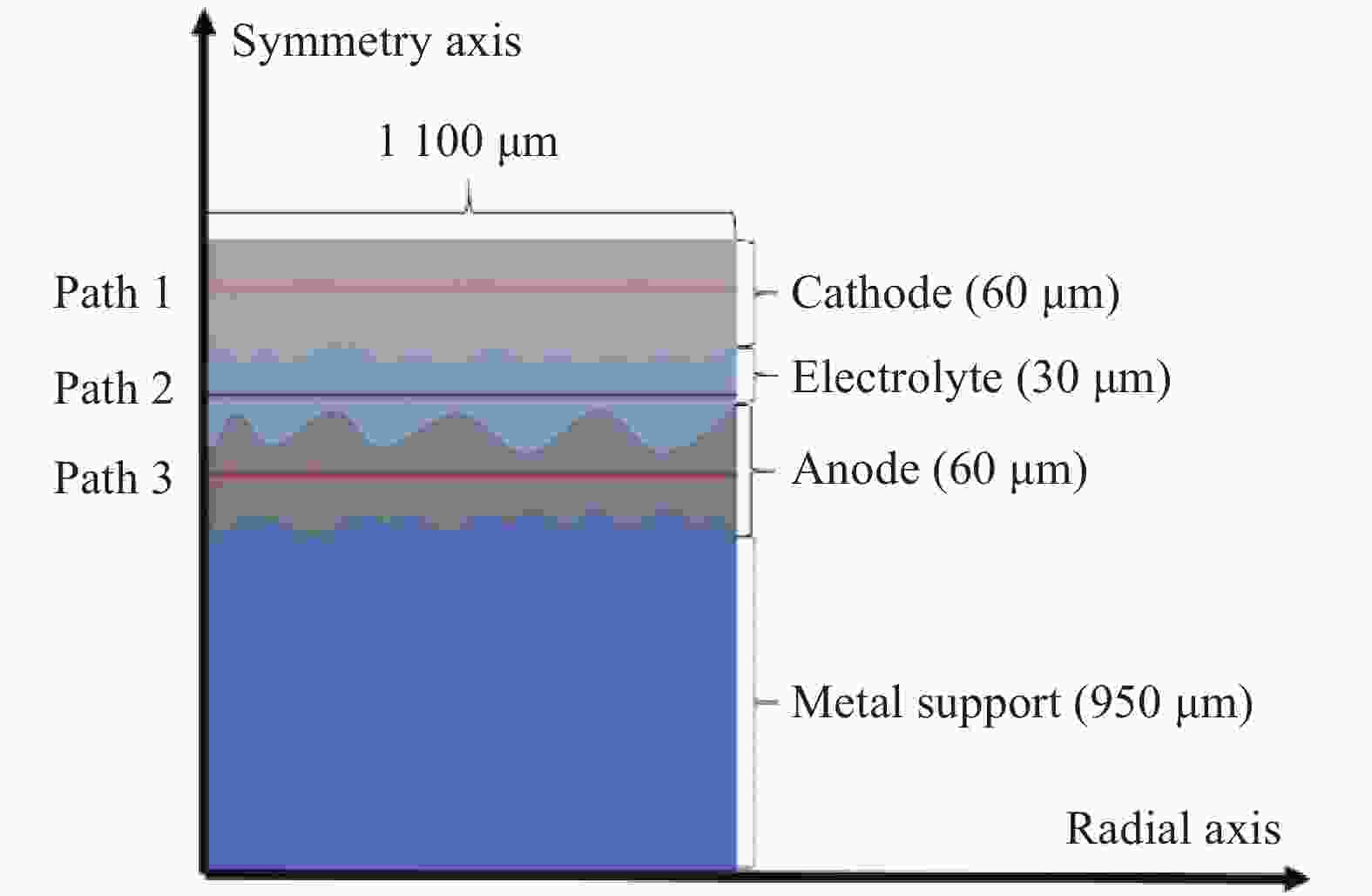
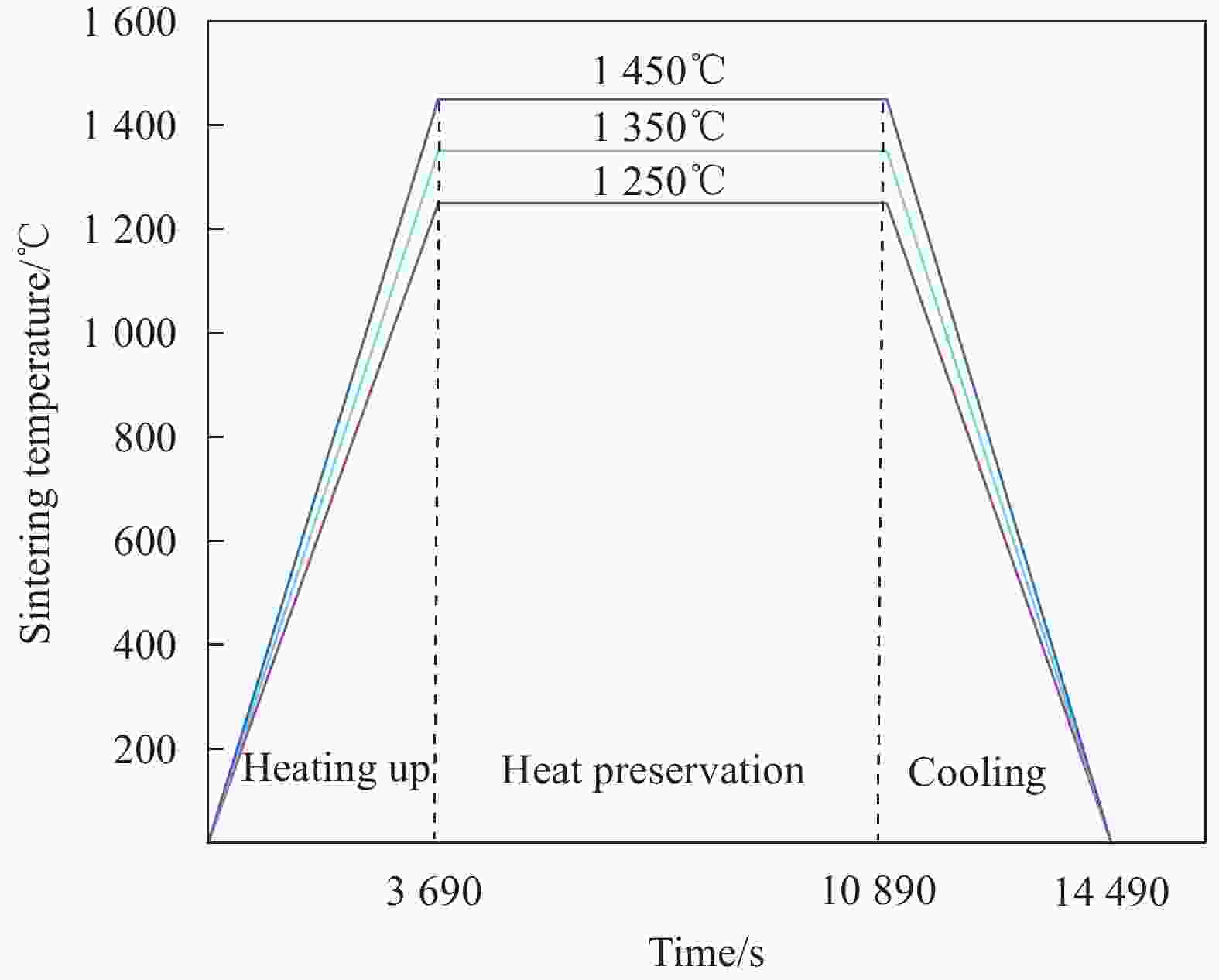
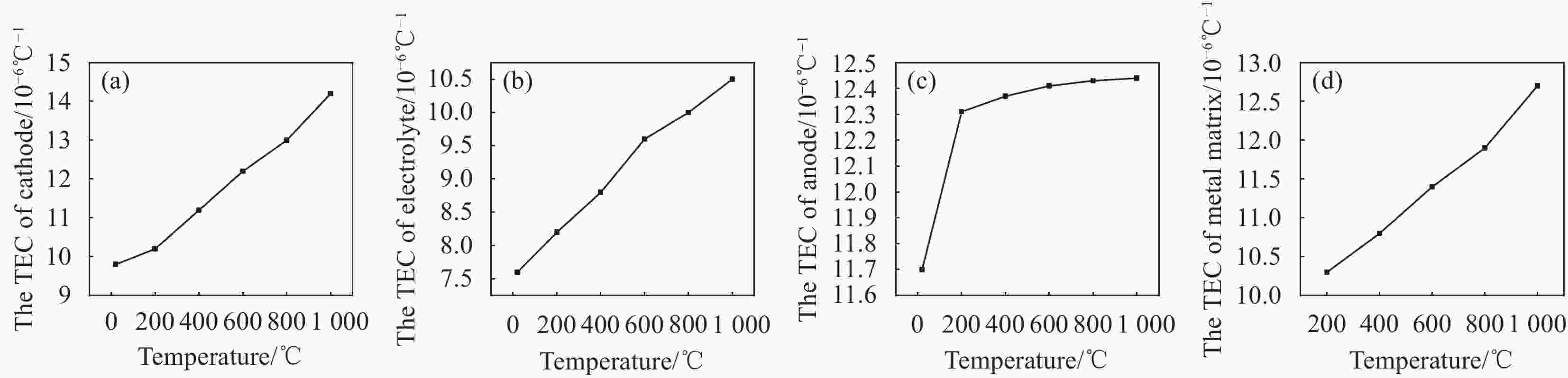
Study on co-sintering characteristics of metal supported solid oxide fuel cells
-
摘要: 在考虑电池整体热膨胀及陶瓷蠕变的情况下分析电极层和电解质层的烧结机理,阐明金属支撑固体氧化物燃料电池(MS-SOFC)在不同烧结温度及晶粒尺寸下电极和电解质层微观结构的演变、残余应力的分布及变化规律。通过建立Skorohod-Olevsky Viscous Sintering (SOVS)模型,模拟在不同烧结温度和不同晶粒尺寸下,MS-SOFC的各层和各界面的相对密度、应力的分布和演化,并通过高温烧结实验揭示异种晶粒尺寸结构烧结后微观结构形貌的变化。结果表明,电解质和电极的相对密度、各层的残余应力值和突变幅度受到烧结温度的影响。当燃料电池各层材料初始晶粒尺寸较小时,烧结导致的致密化率非常明显,随着晶粒尺寸逐渐增大,其致密化率相对较小,且电池各层的残余应力值和突变幅度逐渐减小。纳米氧化钇稳定氧化锆(YSZ)电解质层更容易烧结,且比亚微米YSZ电解质层烧结后微观缺陷降低更多。MS-SOFC烧结后,阴极和阳极的径向应力为拉伸应力,电解质的径向应力为压缩应力。轴向应力和剪切应力在拉压应力之间周期性变化。拥有微米晶的电极层能够在烧结后保持较大的孔隙率,而拥有纳米晶的电解质在提高电导率的同时还能够降低其致密化烧结温度。当晶体尺寸为纳米级时,残余应力值和分布对烧结温度很敏感。Abstract: The sintering mechanism of electrode layer and electrolyte layer was analyzed considering the whole thermal expansion of the battery and the creep of the ceramic. The evolution of the microstructure of the electrode and electrolyte layer and the distribution and change of residual stress of the metal supported solid oxide fuel cell (MS-SOFC) under different sintering temperature and grain size were expounded. The Skorohod-Olevsky Viscous Sintering (SOVS) model was established to simulate the relative density and stress distribution and evolution of different layers and different surfaces of MS-SOFC under different sintering temperatures and different grain sizes. The change of microstructure morphology of dissimilar grain size structure after sintering was revealed by high temperature sintering experiment. The results show that the relative density of electrolyte and electrode, the residual stress value of each layer and the mutation amplitude are affected by sintering temperature. When the initial grain size of each layer of fuel cell material is small, the densification rate caused by sintering is very obvious. With the gradual increase of grain size, the densification rate is relatively small, and the residual stress value and mutation amplitude of each layer of the battery are gradually reduced. Nano-yttrium oxide stabilized zirconia (YSZ) electrolyte layer is easier to sintering, and the micro-defects are reduced more than the micron YSZ electrolyte layer after sintering. After sintering of MS-SOFC, the radial stress of cathode and anode is tensile stress, and the radial stress of electrolyte is compressive stress. Axial stress and shear stress vary periodically between tensile and compressive stress. The electrode layer with micron crystals can maintain large porosity after sintering, while the electrolyte with nanocrystalline can improve the conductivity and reduce the densification sintering temperature. When the crystal size is nanometer, the residual stress value and distribution are sensitive to the sintering temperature.
-
Key words:
- MS-SOFC /
- co-sintering /
- residual stress /
- sintering temperature /
- grain size
-
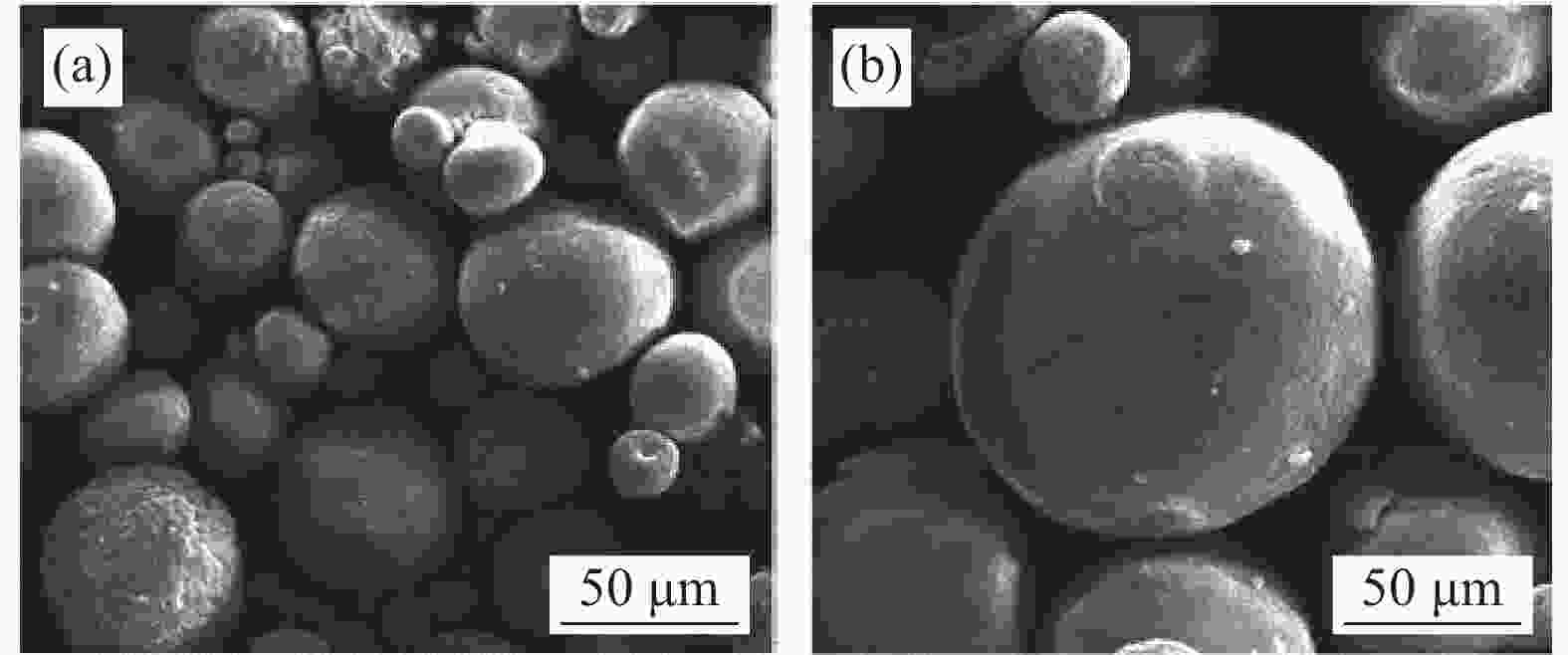
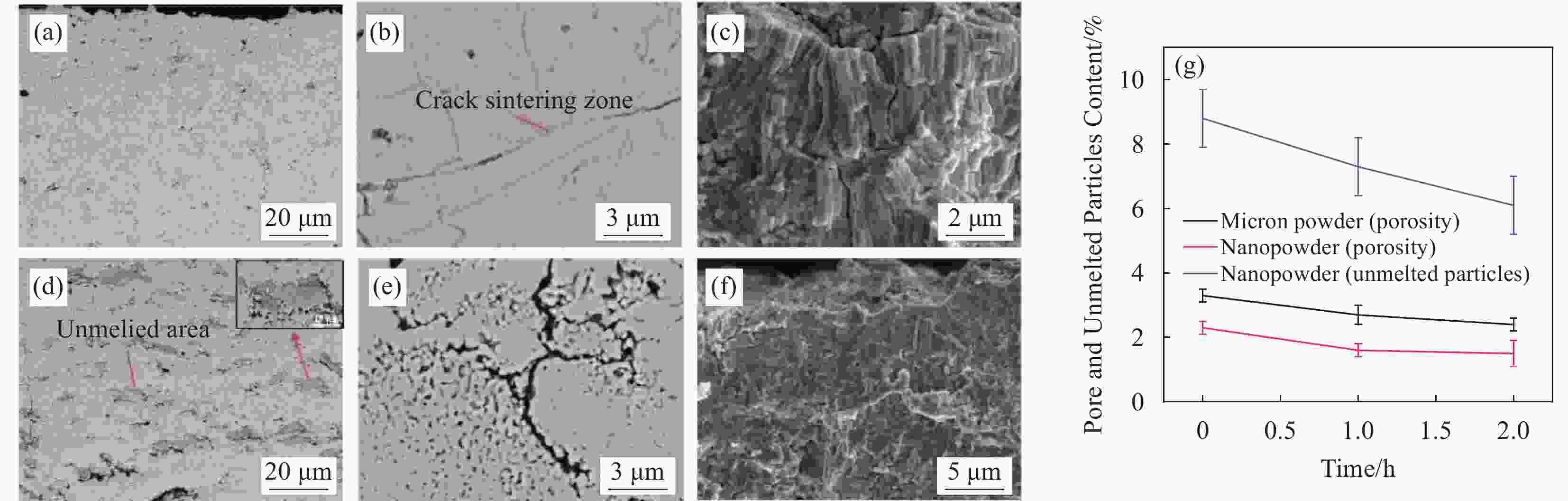
图 7 电解质涂层
1250 ℃烧结前后SEM电镜图 (a)烧结前微米涂层,(b)烧结后裂纹区域,(c)烧结后截面形貌,(d)烧结前纳米涂层,(e)烧结后未熔化区域,(f)烧结后截面形貌,(g)涂层孔隙率和未熔化颗粒含量随烧结时间的示意图Figure 7. Electrolyte coatings prepared by supersonic plasma spraying and SEM electron micrographs before and after sintering at
1250 ℃:(a) Micron coating before sintering,(b) Cracked area after sintering,(c) Cross-sectional morphology after sintering,(d) Nanocoatings before sintering,(e) Unmelted area after sintering,(f) Cross-sectional morphology after sintering,(g) Schematic diagram of coating porosity and unfused particle content with sintering time图 9 微米涂层与纳米涂层
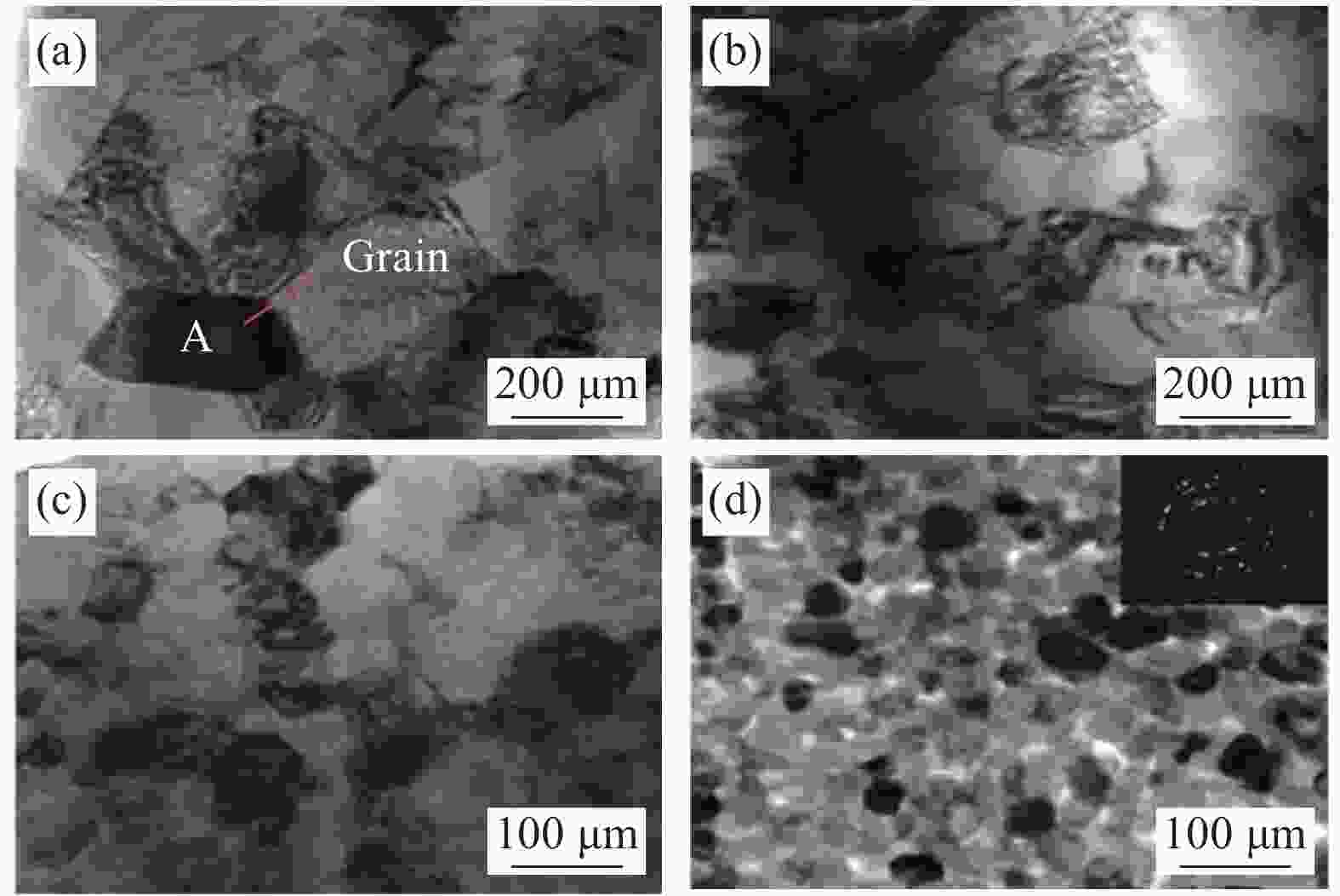
1250 ℃烧结后的透射电镜(TEM)图 (a)烧结后晶粒尺寸在200-600 nm之间的微米涂层的TEM图像,(b)烧结后晶粒尺寸在200- 500 nm之间的纳米涂层的TEM图像,(c)烧结后晶粒尺寸在20-90 nm之间的纳米涂层的TEM图像,(d)纳米涂层烧结后未熔化颗粒的晶粒尺寸在30-50 nm之间Figure 9. Transmission electron microscopy (TEM) of micron coating and nano coating after sintering at
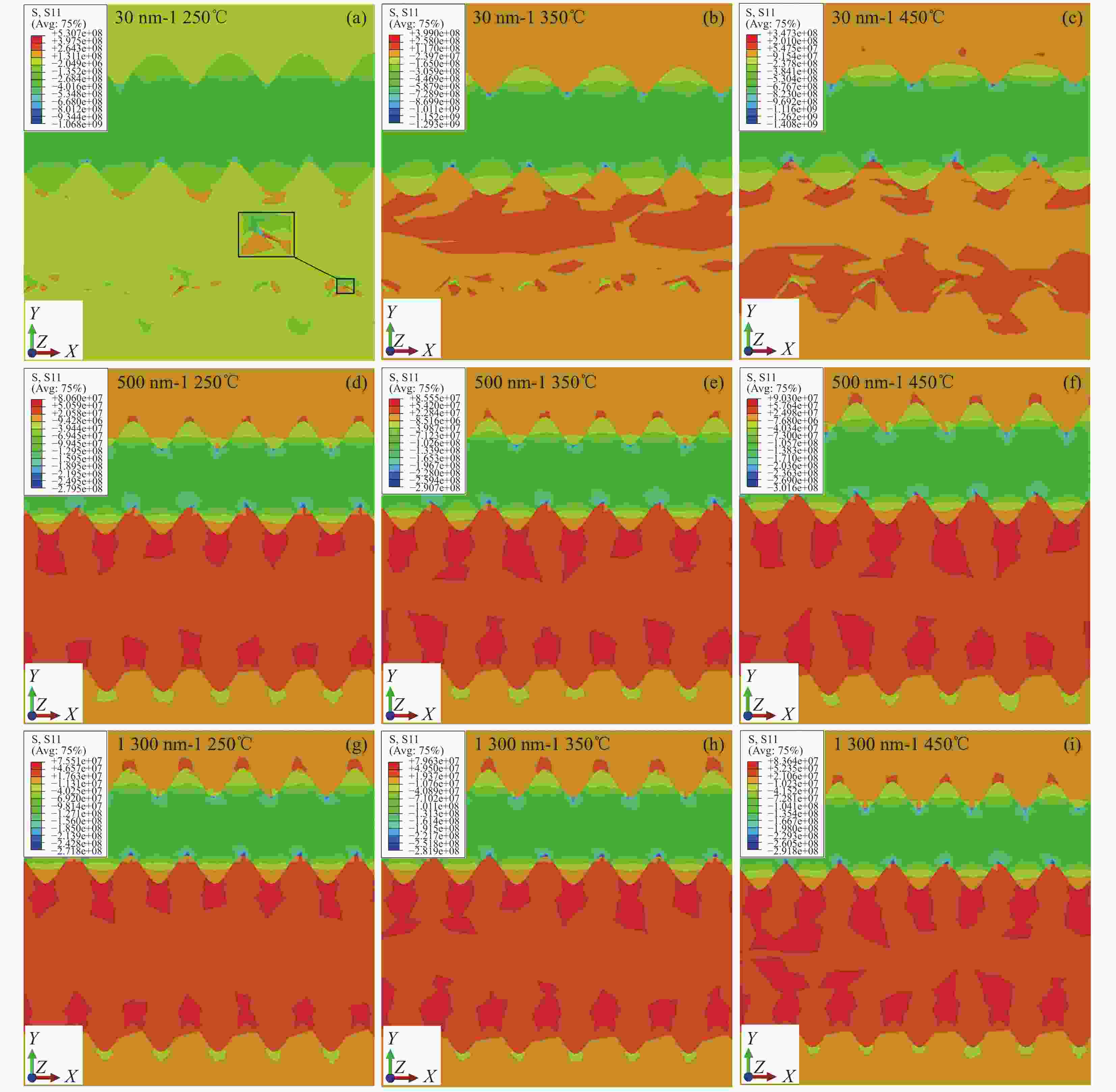
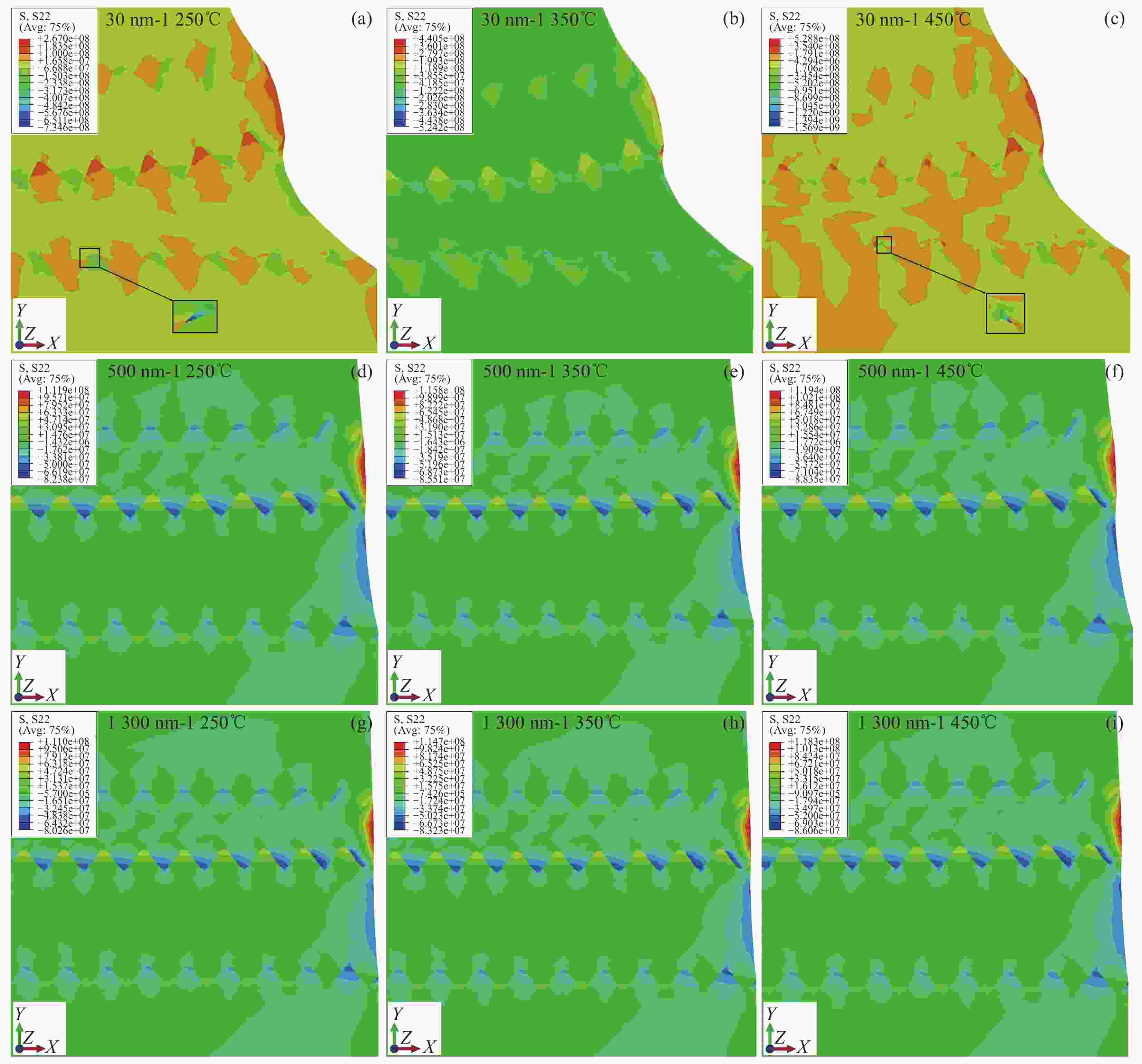
1250 ℃:(a) TEM image of the sintered micron coating with grain size between 200-600 nm, (b) TEM image of the sintered nano-coating with grain size between 200-500 nm, (c) TEM image of the sintered nano-coating with grain size between 20-90 nm,(d) The unmelted particles after sintering of the nano-coating have a grain size between 30-50 nm图 10 MS-SOFC的径向残余应力S11分布 (a)- (c)晶粒尺寸为30 nm时应力S11随温度的分布,(d)- (f)晶粒尺寸为500 nm时应力S11随温度的分布,(g)- (i)晶粒尺寸为
1300 nm时应力S11随温度的分布Figure 10. Radial residual stress S11 distribution of MS-SOFC:(a)- (c) Stress S11 distribution with temperature for grain size of 30 nm,(d)- (f) Stress S11 distribution with temperature for grain size of 500 nm,(g)- (i) Stress S11 distribution with temperature for grain size of
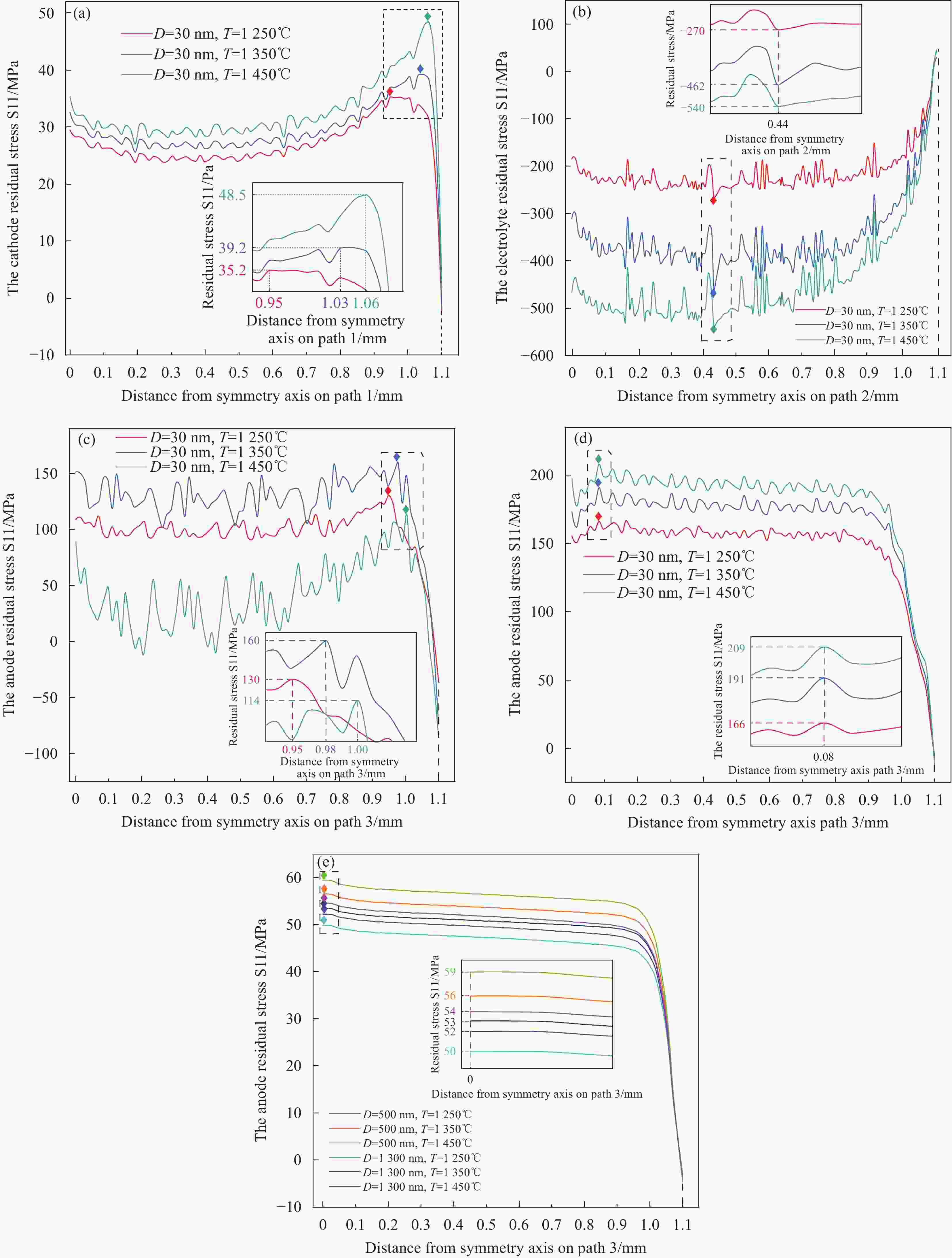
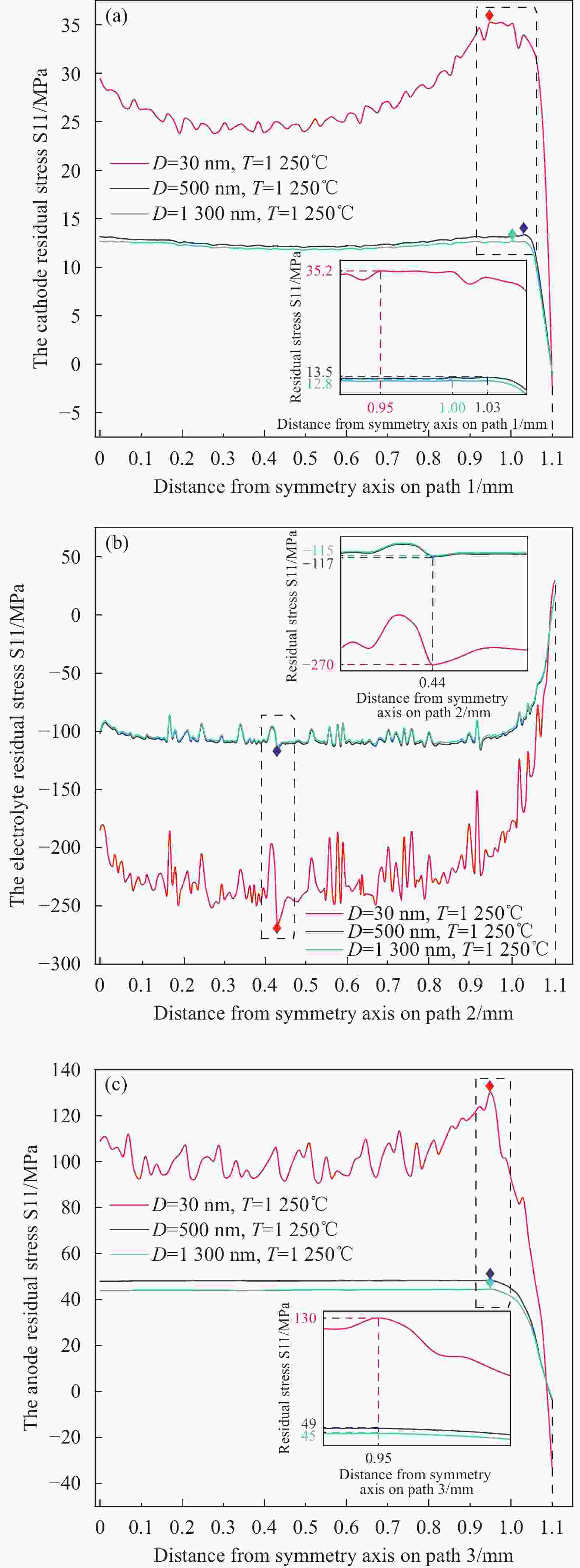
1300 nm图 11 不同烧结温度下的径向残余应力S11分布 (a)阴极-30 nm,(b)电解质-30 nm,(c)阳极-30 nm,(d)阳极(无金属基体)-30 nm,(e)阳极-500 nm和
1300 nmFigure 11. The radial residual stress S11 distribution with different sintering temperatures:(a) cathode-30 nm,(b) electrolyte-30 nm,(c) anode-30 nm,(d) anode (Without metal matrix)-30 nm,(e) anode-500 nm&
1300 nm图 13 MS-SOFC的轴向残余应力S22分布 (a)- (c)晶粒尺寸为30 nm时应力S22随温度的分布,(d)- (f)晶粒尺寸为500 nm时应力S22随温度的分布,(g)- (i)晶粒尺寸为
1300 nm时应力S22随温度的分布Figure 13. Axial residual stress S22 distribution of MS-SOFC:(a)- (c) Stress S22 distribution with temperature for grain size of 30 nm,(d)- (f) Stress S22 distribution with temperature for grain size of 500 nm,(g)- (i) Stress S22 distribution with temperature for grain size of
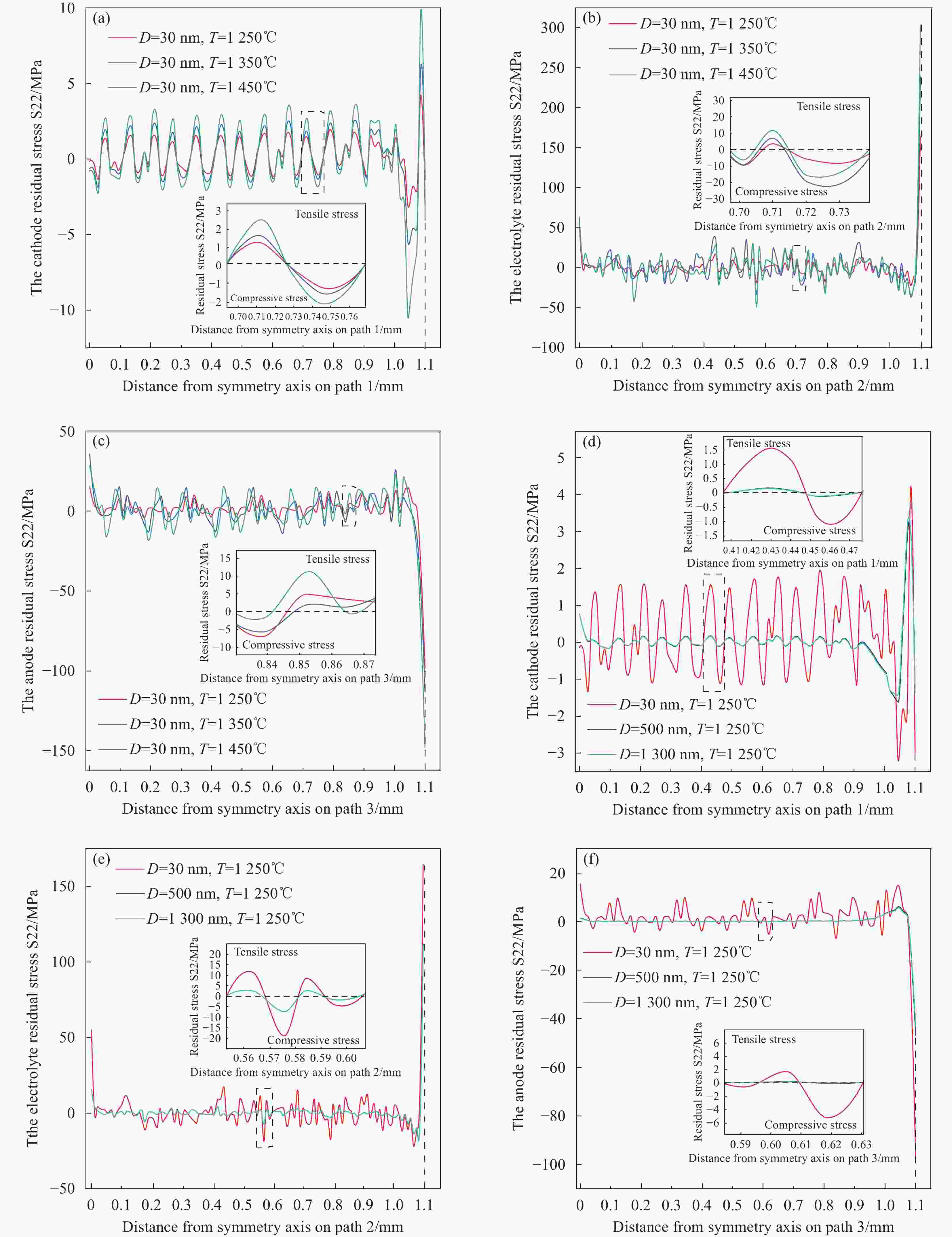
1300 nm图 14 不同烧结温度(a)阴极,(b)电解质,(c)阳极和不同晶粒尺寸(d)阴极,(e)电解质,(f)阳极下的轴向残余应力S22分布
Figure 14. The axial residual stress S22 distribution with different sintering temperatures:(a) cathode, (b) electrolyte, (c) anode;The axial residual stress S22 distribution with different crystal sizes:(d) cathode, (e) electrolyte, (f) anode
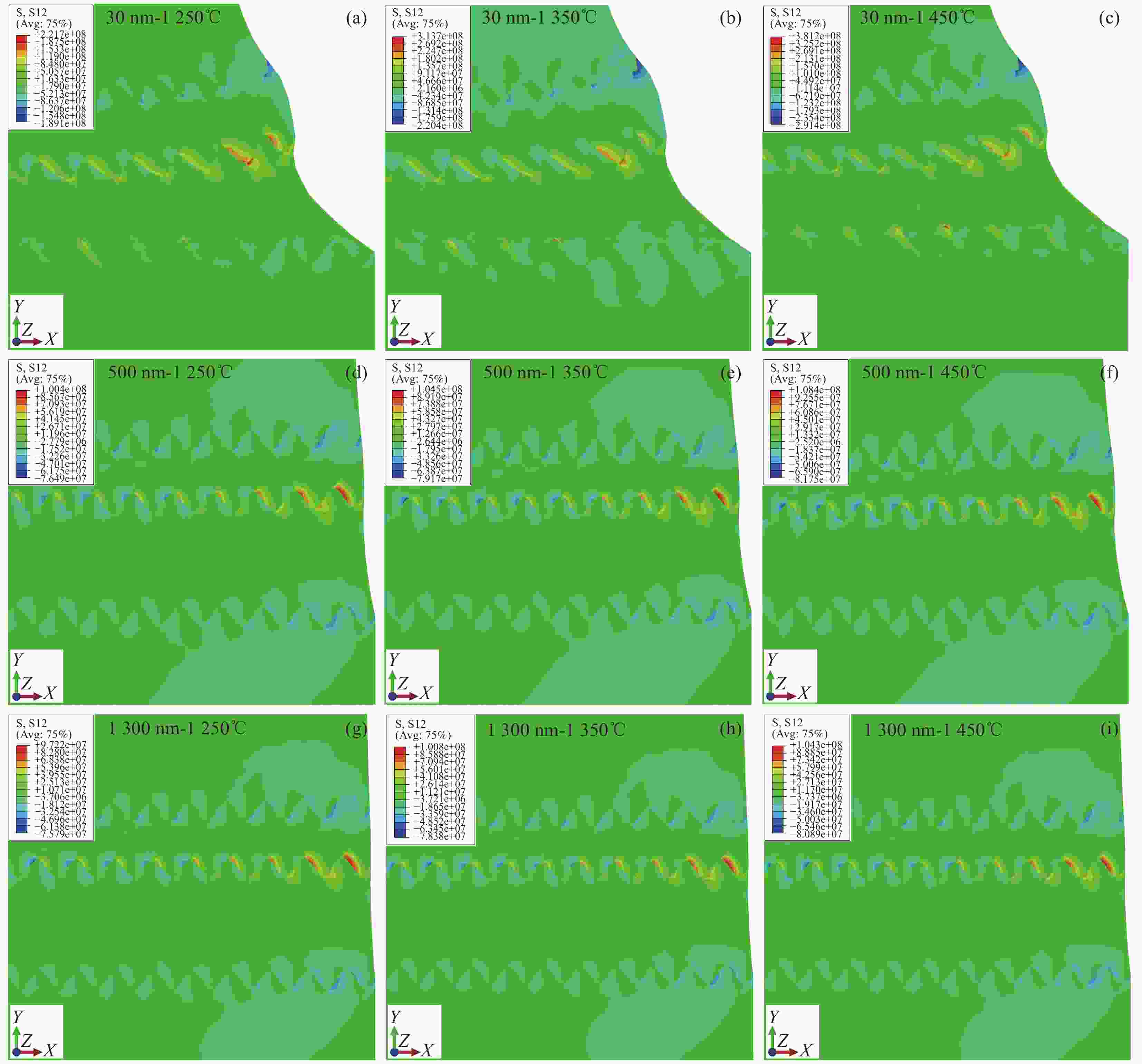
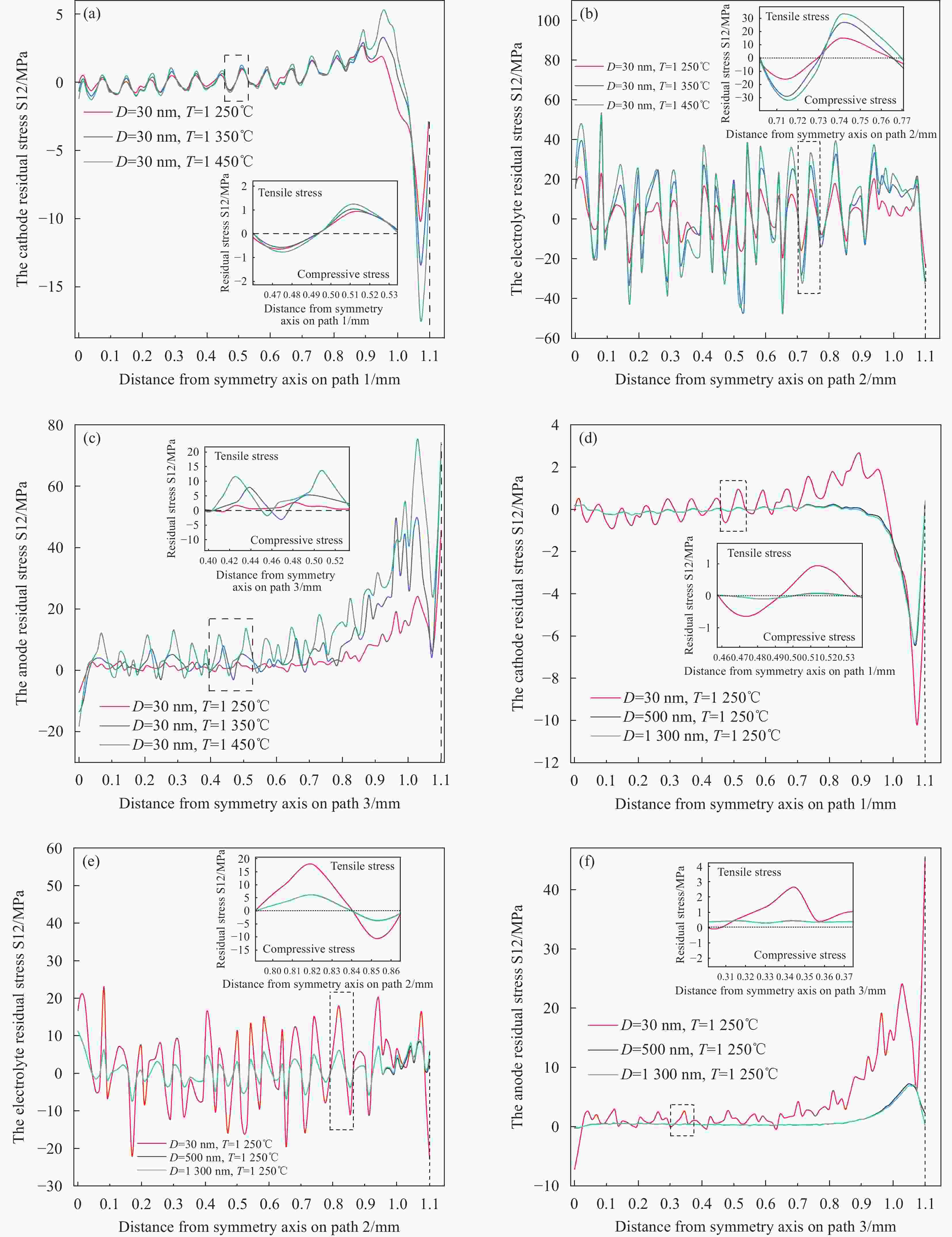
图 15 MS-SOFC的剪切残余应力S12分布 (a)- (c)晶粒尺寸为30 nm时应力S12随温度的分布,(d)- (f)晶粒尺寸为500 nm时应力S12随温度的分布,(g)- (i)晶粒尺寸为
1300 nm时应力S12随温度的分布Figure 15. Shear residual stress S12 distribution of MS-SOFC:(a)- (c) Stress S12 distribution with temperature for grain size of 30 nm,(d)- (f) Stress S12 distribution with temperature for grain size of 500 nm,(g)- (i) Stress S12 distribution with temperature for grain size of
1300 nm图 16 不同烧结温度(a)阴极,(b)电解质,(c)阳极和不同晶粒尺寸(d)阴极,(e)电解质,(f)阳极下的剪切残余应力S12分布
Figure 16. The shear residual stress S12 distribution with different sintering temperatures:(a) cathode, (b) electrolyte, (c) anode; The shear residual stress S12 distribution with different crystal sizes:(d) cathode, (e) electrolyte, (f) anode
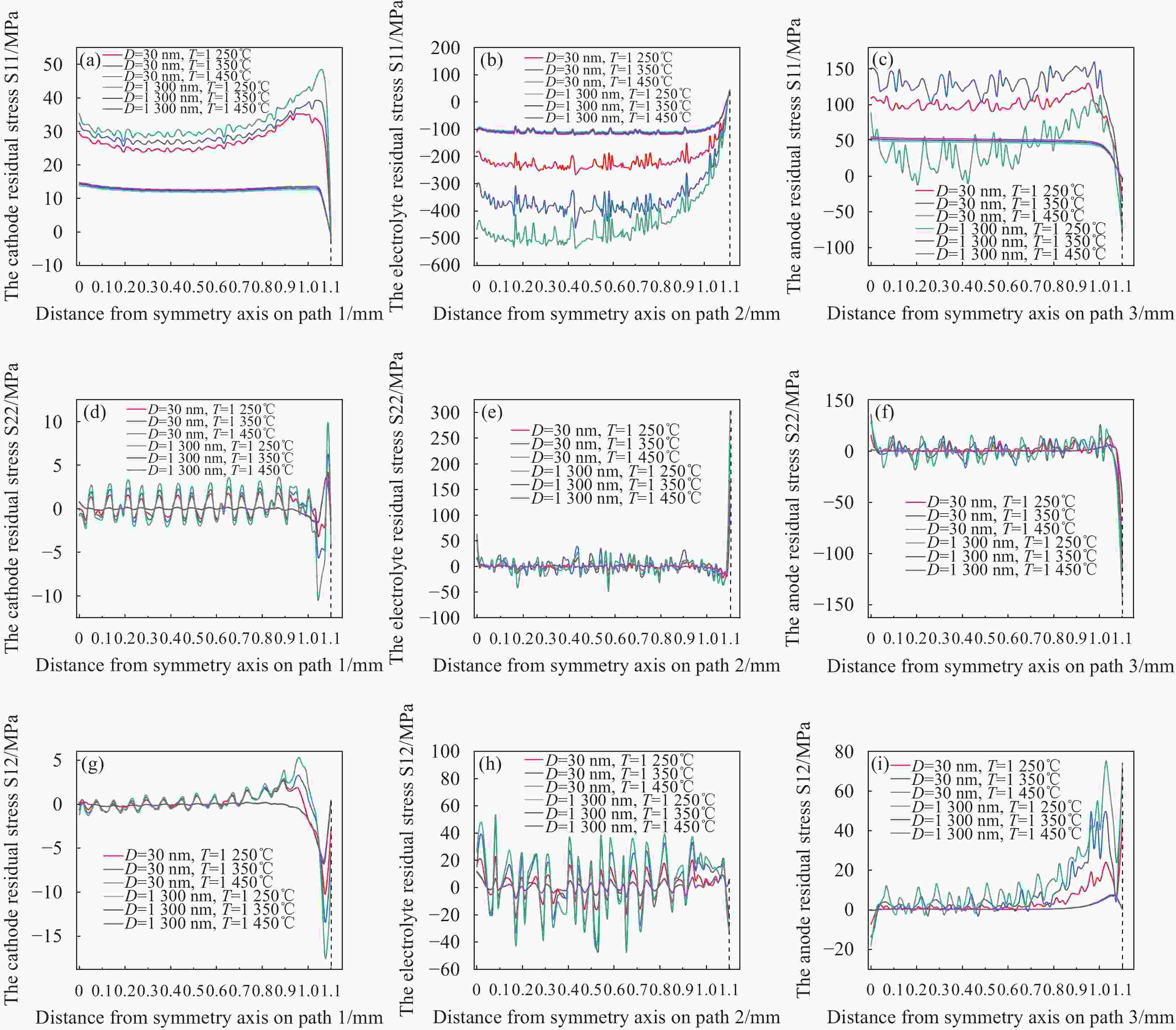
图 17 晶粒尺寸优化前后残余应力随表面温度变化的趋势 (a) s11阴极,(b) s11电解质,(c) s11阳极,(d) s22阴极,(e) s22电解质,(f) s22阳极,(g) s12阴极,(h) s12电解质,(i) s12阳极
Figure 17. Trend of residual stress with surface temperature before and after grain size optimization:(a) S11-cathode, (b) S11-electrolyte, (c) S11-anode,(d) S22-cathode, (e) S22-electrolyte, (f) S22-anode, (g) S12-cathode, (h) S12-electrolyte, (i) S12-anode
表 1 有限元分析中使用的材料特性
Table 1. Materials properties used in finite element analysis
Material properties Cathode (LSCF) Electrolyte (YSZ) Anode (NiO-YSZ) Metal matrix (Crofer 22 APU) Elasticity modulus/GPa 41.30 196.30 126.50 140 Poisson ratio 0.33 0.32 0.31 0.30 Thermal conductivity/(W·(m·℃)−1) 7.20 1.50 4.70 19.40 Specific heat/(J·(kg·℃)−1) 577 500 595 487 Density/(kg·m−3) 6070 206870 5280 7670 Notes:Lanthanum Strontium Cobalt Ferrite (LSCF) is a perovskite type oxide; YSZ is yttrium stabilized zirconia; NiO-YSZ is a mixture of nickel oxide and yttrium stabilized zirconia powder; Crofer 22 APU is a ferritic high temperature stainless steel. 表 2 电极和电解质层的蠕变参数
Table 2. Creep parameters of electrode and electrolyte layer
Parameter name Cathode (LSCF) Electrolyte (YSZ) Anode (NiO-YSZ) Viscosity parameters $ {\mu }_{0}/( $Pa·s) 6.0×1010 5.8×1010 6.2×1010 Creep temperature $ {T}_{t}/ $℃ 1150 1160 1040 Activation energy of viscous flow $ {Q}_{\mathrm{V}}/ $(kJ·mol−1) 27.85×103 25.0×103 20.0×103 Surface energy $ {\gamma }_{\mathrm{S}\mathrm{V}} $/(J·m−2) 1.80 1.27 1.50 表 3 半电池样品的YSZ电解质喷涂工艺参数
Table 3. YSZ electrolyte spraying process parameters of half battery sample
Current/
AVoltage/
VHydrogen flow
rate/slpmArgon flow
rate/slpmSpray
distance/mm540 100 15 80 100 -
[1] 宋明, 胡佳旺, 蒋文春, 等. 小冲杆试验评价固体氧化物燃料电池NiO–YSZ阳极机械强度[J]. 硅酸盐学报, 2024, 52(1): 9-18.SONG Ming, HU Jiawang, JIANG Wenchun, et al. Evaluation of mechanical strength of NiO-YSZ anode for solid oxide fuel cell by small punch test[J]. Journal of Silicates, 2024, 52(1): 9-18(in Chinese). [2] ZHANG FH, WENG QH, ZHANG YX, et al. Facile preparation of electrodes of efficient electrolyte-supported solid oxide fuel cells using a direct assembly approach[J]. Electrochimica Acta, 2022, 424: 1-9. [3] 郑云聪, 赵晋斌, 潘超, 等. 固体氧化物燃料电池的模型预测VSG调频控制策略[J/OL]. 高电压技术, 2024: 1-11.ZHENG Yuncong, ZHAO Jinbin, PAN Chao, et al. Model predictive VSG FM control strategy for solid oxide fuel cells[J/OL]. High Voltage Technology, 2024: 1-11(in Chinese). [4] 杜柯, 宋琛, 余敏, 等. 等离子喷涂制备固体氧化物燃料电池电解质涂层研究进展[J]. 中国表面工程, 2022, 35(1): 25-33. doi: 10.11933/j.issn.1007-9289.20210828001DU Ke, SONG Chen, YU Min, et al. Progress in the preparation of electrolyte coatings for solid oxide fuel cells by plasma spraying[J]. China Surface Engineering, 2022, 35(1): 25-33(in Chinese). doi: 10.11933/j.issn.1007-9289.20210828001 [5] 李晓艳, 李星, 魏甲明, 等. 固体氧化物电解池的发展及研究现状[J]. 中国有色冶金, 2024, 53(2): 1-12.LI Xiaoyan, LI Xing, WEI Jiaming, et al. Development and research status of solid oxide electrolytic cell[J]. China Nonferrous Metallurgy, 2024, 53(2): 1-12(in Chinese). [6] DING PP, LI WL, ZHAO HW, et al. Review on Ruddlesden–Popper perovskites as cathode for solid oxide fuel cells[J]. JPhys Materials, 2021, 4(2): 022002-. doi: 10.1088/2515-7639/abe392 [7] ALIPOUR S, SAGIR E, SADEGHI A. Multi-criteria decision-making approach as-sisting to select materials for low-temperature solid oxide fuel cell: Electrolyte, cathode& anode[J]. International Journal of Hydrogen Energy 2022, 47(45): 19810–19820. [8] 冯潇, 赵雪雪, 邢亚哲. 热喷涂制备固体氧化物燃料电池电解质层的研究进展[J]. 表面技术, 2019, 48(4): 10-17.FENG Xiao, ZHAO Xuexue, XING Yazhe. Research progress on the preparation of electrolyte layer for solid oxide fuel cells by thermal spraying[J]. Surface Technology, 2019, 48(4): 10-17(in Chinese). [9] NICHAREE W, SOAMWADEE C, KAZUHIRO Y, et al. Cobalt Alloying Effect on Improvement of Ni/YSZ Anode-Supported Solid Oxide Fuel Cell Operating with Dry Methane. MATERIALS TRANSACTIONS 2021, 62(10): 1541–1548. [10] 江舟, 文魁, 刘太楷, 等. 固体氧化物燃料电池金属连接体防护涂层研究进展[J]. 表面技术, 2022, 51(4): 14-23+103.JIANG Zhou, WEN Kui, LIU Taikai, et al. Research progress on protective coatings for metal connectors in solid oxide fuel cells[J]. Surface Technology, 2022, 51(4): 14-23+103(in Chinese). [11] AGARKOVA E A, BUEMISTROV I N, YALOVENKO D V , et al. Application of Yttria Stabilized Zirconia (8YSZ) and NiO Precursors for Fabrication of Composite Material for Anode-Supported SOFCs[J]. Russian Journal of Electrochemistry, 2024, 60(3): 157-161. [12] BRODNIKOVSKYI Ye M. , PODHURSKA V Ya, LYSUNENKO N O, et al. The Influence of the Anode Structure on the Electrical Properties of a Solid Oxide Fuel Cell[J]. Materials Science, 2024, 59(4): 443-450. [13] GAO Y, HUANG L, ZHANG BY, et al. Enhanced sintering ability and electrochemical performance of Gd0.1Ce0.9O1.95 composited with (Dy0.2Zr0.05Bi0.75)2O3 for low-temperature solid oxide fuel cells[J]. Electrochimica Acta, 2024, 475: 143614-. doi: 10.1016/j.electacta.2023.143614 [14] ZAINON A N, SOMALU M R, BAHRAIN A M K, et al. Influence of sintering temperature on the properties of the screen-printed anode of the LSMO4 Ruddlesden‒Popper perovskite for intermediate-temperature solid oxide fuel cells[J]. International Journal of Hydrogen Energy 2023, 48(97): 38425–38437. [15] FILONOVA E, GILEV A, MAKSIMCHUK T, et al. Development of La1.7Ca0.3Ni1−yCuyO4+δ Materials for Oxygen Permeation Membranes and Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells[J]. Membranes, 2022, 12(12): 1222-1222. doi: 10.3390/membranes12121222 [16] KRYSTIAN M, JAKUB K, ANNA N, et al. Numerical analysis of the relation between the porosity of the fuel electrode support and functional layer, and performance of solid oxide fuel cells using computational fluid dynamics[J]. International Journal of Hydrogen Energy, 2024, 52(PA): 936-951. [17] WANG ZL, YANG CC, PU J, et al. In-situ self-assembly nano-fibrous perovskite cathode excluding Sr and Co with superior performance for intermediate-temperature solid oxide fuel cells[J]. Journal of Alloys and Compounds, 2023, 947: 169470. doi: 10.1016/j.jallcom.2023.169470 [18] WATANABE S, SUKINO S, MIYASAKA T, et al. Influences of Ni content and porosity on mechanical properties of Ni–YSZ composites under solid oxide fuel cell operating conditions[J]. Journal of Materials Science, 2020, 55(4): 8679-8693. [19] HAN TT, XIE YJ, LI L, et al. Enhanced electrochemical performance of direct carbon solid oxide fuel cells by MgO-catalyzed carbon gasification: Experimental and DFT simulation studies[J]. Ceramics International, 2024, 50(9PB): 16435-16442. [20] KAUR T, KUMAR R, KHAN, S, et al. Structural and Electrical Properties of Gd Doped CeO2 (GDC) Nanoceramics for Solid Oxide Fuel Cell Applications[J]. Transactions of the Indian Ceramic Society 2022, 81(3): 127–132. [21] HARRISON C M, SARRUF B J M, KLOTZ D, et al. The effects of sintering temperature and current contacting layer on the performance of lanthan-um nickelate electrodes in Solid Oxide Fuel Cells[J]. Solid State Ionics, 2023, 403: 1-13. [22] GAO Y, HUANG L, LI QZ, et al. Highly conductive and stable ErxCe0.05Bi0.95-xO1.5+δ solid electrolytes for low-temperature solid-oxide fuel cells[J]. International Journal of Hydrogen Energy, 2024, 50(PC): 1329-1340. [23] JU Y, HA J, SONG Y, et al. Optimizing the printability and dispersibility of functionalized zirconium oxide/acrylate composites with various nano-to micro-particle ratios[J]. Ceramics International 2020, 46(17): 26903–26910. [24] PRADHAN L K, KUMARI S, MANGLAM M K, et al. Microstructure-dependent electrical properties of Bi0.5Na0.5TiO3–BaTiO3–SrTiO3 ternary solid solution[J]. Journal of Materials Science: Materials in Electronics, 2021, 32(5): 6607-6622. doi: 10.1007/s10854-021-05376-w [25] YUAN JH, GUO WM, LIU QY, et al. Influence of TiB2 and CrB2 on densification, microstructure, and mechanical properties of ZrB2 ceramics[J]. Ceramics International 2021, 47(19): 28008–28013. [26] ZHOU W, ZHANG L, WU, PT, et al. An effective meth-od for improving the permeation flux of a ceramic membrane: Single-matrix sph-erical ceramic membrane[J]. Journal of Hazardous Materials 2020, 400: 123–183. [27] RAI P, YADAV D. A first study on the current-controlled flash sintering experiments on 3YSZ-Ni composites for SOFC anode applications[J]. Materials Chemistry and Physics, 2024, 316: 129088. doi: 10.1016/j.matchemphys.2024.129088 [28] 张旭, 吴萍萍, 丁利利, 等. 基于交替浸渍法对La0.65Sr0.35MnO3氧电极的性能优化[J]. 复合材料学报, 2022, 39(12): 5736-5746.ZHANG Xu, WU Pingping, DING Lili, et al. Performance optimization of La0.65Sr0.35MnO3 oxygen electrode based on alternate infiltration method[J]. Acta Materiae Compositae Sinica, 2022, 39(12): 5736-5746 (in Chinese). [29] SHARMA S K, DAS P, MANDAL B, et al. Fabrication, characterization and optimization of industrial alpha alumina powders based ceramic membrane supports and its applicative potential for CO2/N2 separation[J]. Journal of CO2 Utilization, 2022, 63: 102-121. [30] SHI SJ, JIAN KJ, FANG MF, et al. SiO2 Modification of Silicon Carbide Membrane via an Interfacial In Situ Sol–Gel Process for Improved Filtration Performance[J]. Membranes, 2023, 13(9): 756. doi: 10.3390/membranes13090756 [31] HUANG XH DENG PH, CHEN WZ, et al. Low-temperature sintering of porous SiC ceramic membrane with industrial-grade sodium meta-aluminate as a sintering additive[J]. Ceramics International, 2023, 49(23): 39090-39098. doi: 10.1016/j.ceramint.2023.09.248 [32] 樊帅, 金天, 张山林, 等. Li2O烧结助剂对固体氧化物燃料电池LSGM电解质烧结特性及离子电导率的影响[J]. 无机材料学报, 2022, 37(10): 1087-1092. doi: 10.15541/jim20220086FAN Shuai, JIN Tian, ZHANG Shanlin, et al. Effect of Li2O sintering additives on the sintering characteristics and ionic conductivity of LSGM electrolyte for solid oxide fuel cells[J]. Journal of Inorganic Materials, 2022, 37(10): 1087-1092(in Chinese). doi: 10.15541/jim20220086 [33] SCHNEIDER M, ŠIMUNKOVA L, MICHAELIS A, et al. Study of anodic oxide films formed on solid-state sintered SiC-ceramic at high anodic potentials[J]. Ceramics International, 2021, 47(11): 15010-15016. doi: 10.1016/j.ceramint.2021.02.056 [34] ZHOU MK, CHEN HT, ZHANG X, et al. Phase composition, microstructure, and microwave dielectric properties of non-stoichiometric yttrium aluminum gar-net ceramics[J]. Journal of the European Ceramic Society, 2022, 42(2): 472-477. doi: 10.1016/j.jeurceramsoc.2021.10.040 [35] ŠIMONOVA P, PABST W, CIBULKOVA J. Crystallite size of pure tin oxide ceramics and its growth during sintering determined from XRD line broadening – A methodological case study and a practitioners’ guide[J]. Ceramics International, 2021, 47(24): 35333-35347. doi: 10.1016/j.ceramint.2021.09.076 [36] JIANG Z, SARRUF M J B, KHAROUF E A, et al. Preparation of gadolinium-doped ceria barrier layer for intermediate temperature solid oxide fuel cells by spin coating and evaluation of performance degradation by impedance analysis[J]. Ceramics International, 2024, 50(13PA): 23222-23231. [37] SHI H, GIUNTINI D, VAN DOMMELEN H, et al. Efficient modelling of ceramic sintering processes: Application to bilayers and membranes[J]. Journal of the European Ceramic Society, 2023, 43(11): 4939-4949. doi: 10.1016/j.jeurceramsoc.2023.03.053 [38] WANG J, NI Y, LIU K, et al. Numerical simulation and experimental verification of dry pressed MgTiO3 ceramic body during pressureless sintering[J]. Journal of the American Ceramic Society, 2021, 104(9): 4408-4419. doi: 10.1111/jace.17888 [39] PETROVIC V, BULJAK V, CORNAGGIA A. Skorohod-Olevsky viscous sintering model sensitivity to temperature distribution during the sintering process[J]. FME Transactions 2021, 49(3): 719–725. [40] MELEKHIN N V, BOLDIN M S, POPOV A A, et al. Dynamic Strength of a Fine-Grained Alumina Ceramic Obtained by Spark Plasma Sintering[J]. Inorganic Materials: Applied Research, 2024, 14(5-6): 1383-1394. [41] SHAKIRZYANOV R I, VOLODINA N O, KADYRZHANOV K K, et al. Study of the Aid Effect of CuO-TiOsub2/sub-Nbsub2/subOsub5/sub on the Dielectric and Structural Properties of Alumina Ceramics[J]. Materials (Basel, Switzerland), 2023, 16(14): 5018. doi: 10.3390/ma16145018 [42] MEHBOOB G, LIU Mei-jun, Xu Tong, et al. A review on failure mechanism of thermal barrier coatings and strategies to extend their lifetime[J]. Ceramics International, 2020, 46(7): 8497-8521. doi: 10.1016/j.ceramint.2019.12.200 [43] ELYN T F R, MARLON S D M, JEFFERSON B, et al. Visualization of the Final Stage of Sintering in Nanoceramics with Atomic Resolution[J]. Nano letters, 2022, (5): 22. [44] 张笑, 梁森. Al2O3透明陶瓷烧结动力学分析研究[J]. 人工晶体学报, 2018, 47(12): 2555-2560. doi: 10.3969/j.issn.1000-985X.2018.12.024ZHANG Xiao, LIANG Sen. Analysis of sintering kinetics of Al2O3 transparent ceramics[J]. Journal of Intraocular Crystals, 2018, 47(12): 2555-2560(in Chinese). doi: 10.3969/j.issn.1000-985X.2018.12.024 [45] 吴学志. 液相烧结大晶粒UO2及其烧结动力学分析[J]. 材料导报, 2023, 37(S1): 50-53.Wu Xuezhi. Analysis of large grain UO2 and its sintering kinetics in liquid phase sintering[J]. Materials Review, 2023, 37(S1): 50-53(in Chinese). [46] DANILENKO I, GORBAN O, SHYLO A, et al. Determination of the nature of the co-doping effect on the structure, mechanical properties and ionic conductivity of SOFC electrolyte based on YSZ[J]. Solid State Ionics, 2024, 412: 1-18. [47] WANG Pan, QIAN Weixing, CHEN Yuting, et al. Preparation and performance of Sr2-xPrxFe1.5Mo0.5O6-δ cathode materials for solid oxide fuel cells[J/OL]. Chinese Journal of Rare Earths, 2024: 1-8. [48] JIANG P, YANG LY, LI DJ, et al. Residual stress in air-plasma-sprayed thermal barrier coatings under long-term high-temperature oxidation[J]. Surface Coatings Technology, 2024, 484: 130827. doi: 10.1016/j.surfcoat.2024.130827 [49] LUO SY, HUANG RZ, BAI HR, et al. An indentation-based method for characterization of non-uniform triaxial residual stress in curved thermal barrier coating[J]. Measurement, 2024, 232: 5-8. [50] GAN MD, CHONG XY, LU TL, et al. Unveiling thermal stresses in RETaO4 (RE = Nd, Sm, Eu, Gd, Tb, Dy, Ho and Er) by first-principles calculations and finite element simulations[J]. Acta Materialia, 2024, 271: 4-10. [51] CHEN Q, LEI HW, WU JZ, et al. Improving the performance of LiNi0.8Co0.15Al0.05O2-δ electrode-based fuel cell through cathode modification[J]. International Journal of Hydrogen Energy, 2024, 63: 871-880. doi: 10.1016/j.ijhydene.2024.03.233 [52] WANG Y, WU ChR, DU Q, et al. Morphology and performance evolution of anode microstructure in solid oxide fuel cell: A model-based quantitative analysis[J]. Applications in Energy and Combustion Science, 2021, 5: 1-11. [53] SHIN J, PARK H J, KIM J, et al. Suppression of processing defects in large-scale anode of planar solid oxide fuel cell via multi-layer roll calendering[J]. Journal of Alloys and Compounds, 2020, 812: 152113-152113. doi: 10.1016/j.jallcom.2019.152113 [54] MUNEEB I, KHURRAM S, RIZWAN R, et al. Evaluation of densification effects on the properties of 8 mol% yttria stabilized zirconia electrolyte synthesized by cost effective coprecipitation route[J]. Ceramics International, 2020, 47(2): 2857-2863. [55] PESARAN A, HUSSIAN M A, REN Y, et al. Optimizing Bilayer Electrolyte Thickness Ratios for High-Performing Low-Temperature Solid Oxide Fuel Cells[J]. Journal of The Electrochemical Society, 2024, (5): 171. [56] MIRZAEI A, AFZALI M, KHACHATOURAIN M A, et al. Enhancing sintering behavior and conductivity of YSZ electrolyte by co-doping of ZnO and MnO2[J]. Materials Chemistry and Physics, 2024, 315: 129051-. doi: 10.1016/j.matchemphys.2024.129051 [57] ZHANG MT, SONG C, LIN KS, et al. Preparation of Plasma Sprayed GDC Electrolytes for Metal-Supported Solid Oxide Fuel Cells[J]. Journal of Thermal Spray Technology, 2024, 33(4): 964-975. doi: 10.1007/s11666-024-01751-1 [58] TUGCE U, MUEAT M, OZGE U, et al. Effect of cold sintering on the sintering shrinkage matching of NiO-GDC anode with GDC electrolytes in making anode-supported solid oxide fuel cells[J]. Solid State Ionics, 2024, 408: 1-9. [59] MUCCILLC R, FLORIO D Z, FONSECA Fa C, et al. Electric field-assisted sintering anode-supported single solid oxide fuel cell[J]. International Journal of Applied Ceramic Technology, 2021, 19(2): 906-912. [60] CHEN JP, ZHANG DY, HE YL, et al. Enhanced electrochemical performance of SOFC: GDC electrolyte grains encapsulation via DZSB film[J]. Ceramics International, 2024, 50(3PB): 5169-5178. [61] GILBILE T, PANDHARE A, MAKKI E, et al. Development and performance evaluation of (Sm1-xNdx)0.2O2-δ electrolyte for low-temperature solid oxide fuel cell application[J]. Energy Conversion and Management, 2024, 21: 100518. doi: 10.1016/j.ecmx.2023.100518 [62] CHOOLAEI M, VOSTAKOLA F M, HORRI A B. Recent Advances and Challenges in Thin-Film Fabrication Techniques for Low-Temperature Solid Oxide Fuel Cells[J]. Crystals, 2023, 13(7): 1008. doi: 10.3390/cryst13071008 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 30
- HTML全文浏览量: 34
- 被引次数: 0




 下载:
下载: