Properties of polylactic acid/maleic anhydride grafted polypropylene composites regulated by modified hydrotalcite
-
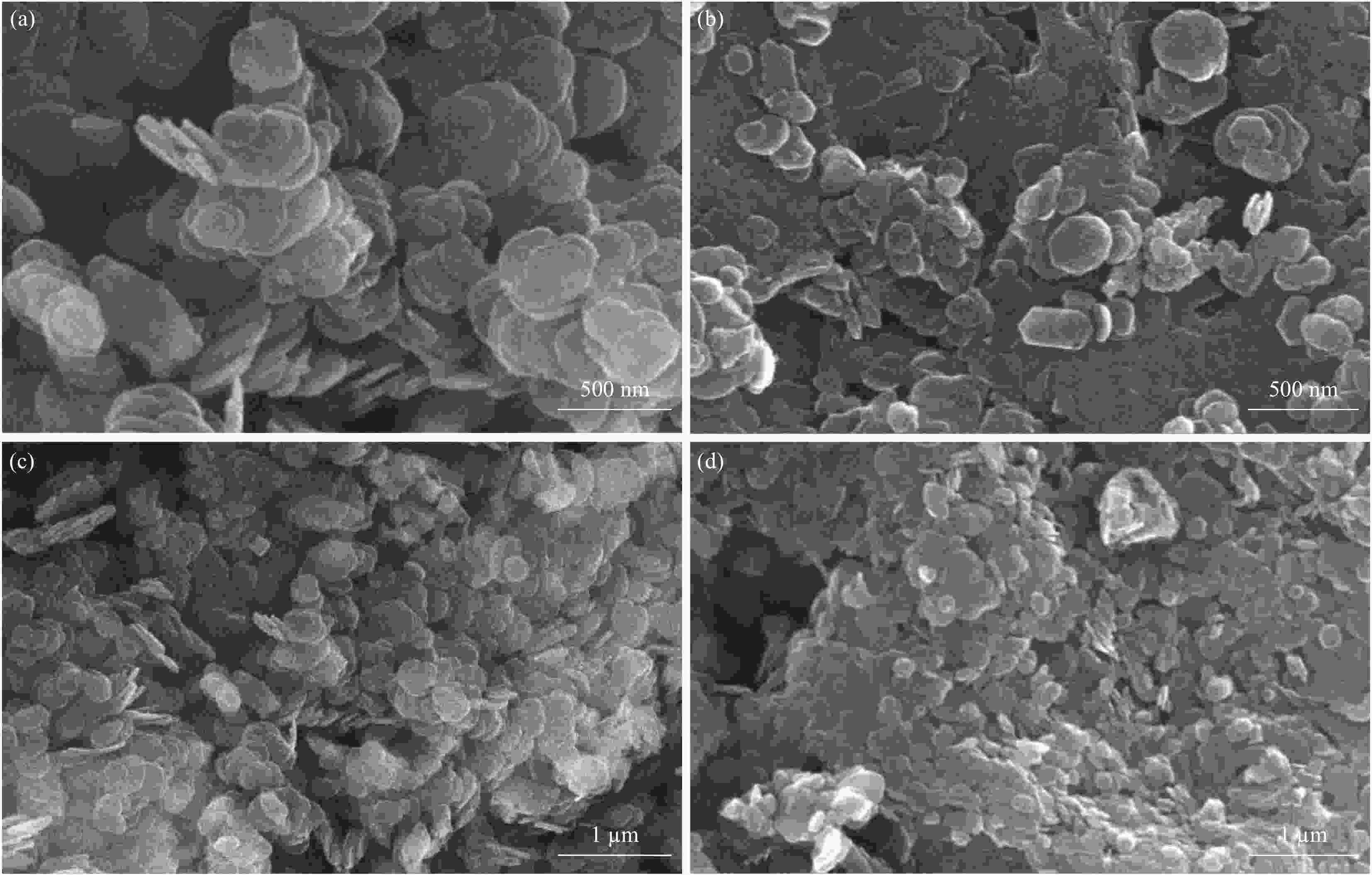
摘要: 为了研究水滑石复合材料的力学性能、热稳定性、动态热机械性能、结晶行为和界面相容性等,通过共沉淀法制备镁铝水滑石,再将水滑石进行焙烧,得到焙烧镁铝水滑石,以控制变量法考察温度和时间对水滑石层间结构的影响,利用TG、SEM、XRD分别表征测试了300℃下焙烧后的水滑石(LDHs(300))的形貌和热稳定性,证实其层间水已经大部分除去,且保持层状结构而不损害其原有的性能。利用FTIR、XRD表征测试了十二烷基苯磺酸钠(SDBS)能成功插层改性焙烧后的水滑石。再采用熔融共混法,将不同表面改性的水滑石颗粒添加到聚乳酸-马来酸酐接枝聚丙烯(PLA-PP-g-MAH)得到不同的复合材料。结果表明,焙烧改性后的水滑石复合材料的性能最优。通过SEM进行的形态分析表明,随着水滑石添加到PLA-PP-g-MAH中,PP-g-MAH颗粒相畴尺寸显著减小,PP-g-MAH相具有更好的润湿性,从而产生更高的冲击强度,冲击性能与纯PLA相比提高了37.72%。从流变行为发现,焙烧改性后的水滑石复合材料的流变性能比PLA显著增加。DMA表明,在PLA-PP-g-MAH共混物中添加焙烧改性后的水滑石,PLA的玻璃化转变温度
$ \left({T}_{\mathrm{g}}\right) $ 升高和冷结晶温度$ {(T}_{\mathrm{c}\mathrm{c}}) $ 降低。从DSC和POM表明,焙烧改性后的水滑石复合材料提高了结晶速率,结晶度比PLA提高了62.8%。Abstract: In order to study the mechanical properties, thermal stability, dynamic thermomechanical properties, crystallization behavior and interfacial compatibility of hydrotalcite composites, Mg-Al hydrotalcite was prepared by coprecipitation method, and then calcined to obtain calcined Mg-Al hydrotalcite. The effects of temperature and time on the interlayer structure of hydrotalcite were investigated by variable control method. The hydrotalcite calcined at 300℃ (LDHs(300)) was characterized and tested by TG, SEM and XRD. The morphology and thermal stability confirmed that most of the interlayer water had been removed, and the layered structure was maintained without damaging its original properties. The calcined hydrotalcite was successfully intercalated with sodium dodecylbenzene sulfonate (SDBS) by FTIR and XRD. Then different surface modified hydrotalcite particles were added to polylactic acid/maleic anhydride grafted polypropylene (PLA-PP-g-MAH) by melt blending to obtain different composites. The results show that the properties of hydrotalcite composites modified by calcination are the best. The morphological analysis by SEM shows that with the addition of hydrotalcite to PLA-PP-g-MAH, the domain size of PP-g-MAH particles decreases significantly, and the PP-g-MAH phase has better wettability, resulting in higher impact strength, compared with pure PLA, it increased by 37.72%. From the rheological behavior, it is found that the rheological properties of calcined hydrotalcite composites are significantly higher than those of PLA. DMA shows that when calcined hydrotalcite is added to PLA-PP-g-MAH blend, the glass transition temperature (Tg) of PLA increased and the cold crystallization temperature (Tcc) decreased. DSC and POM show that the crystallization rate and crystallinity of calcined hydrotalcite composites are higher than those of PLA by 62.8%. -
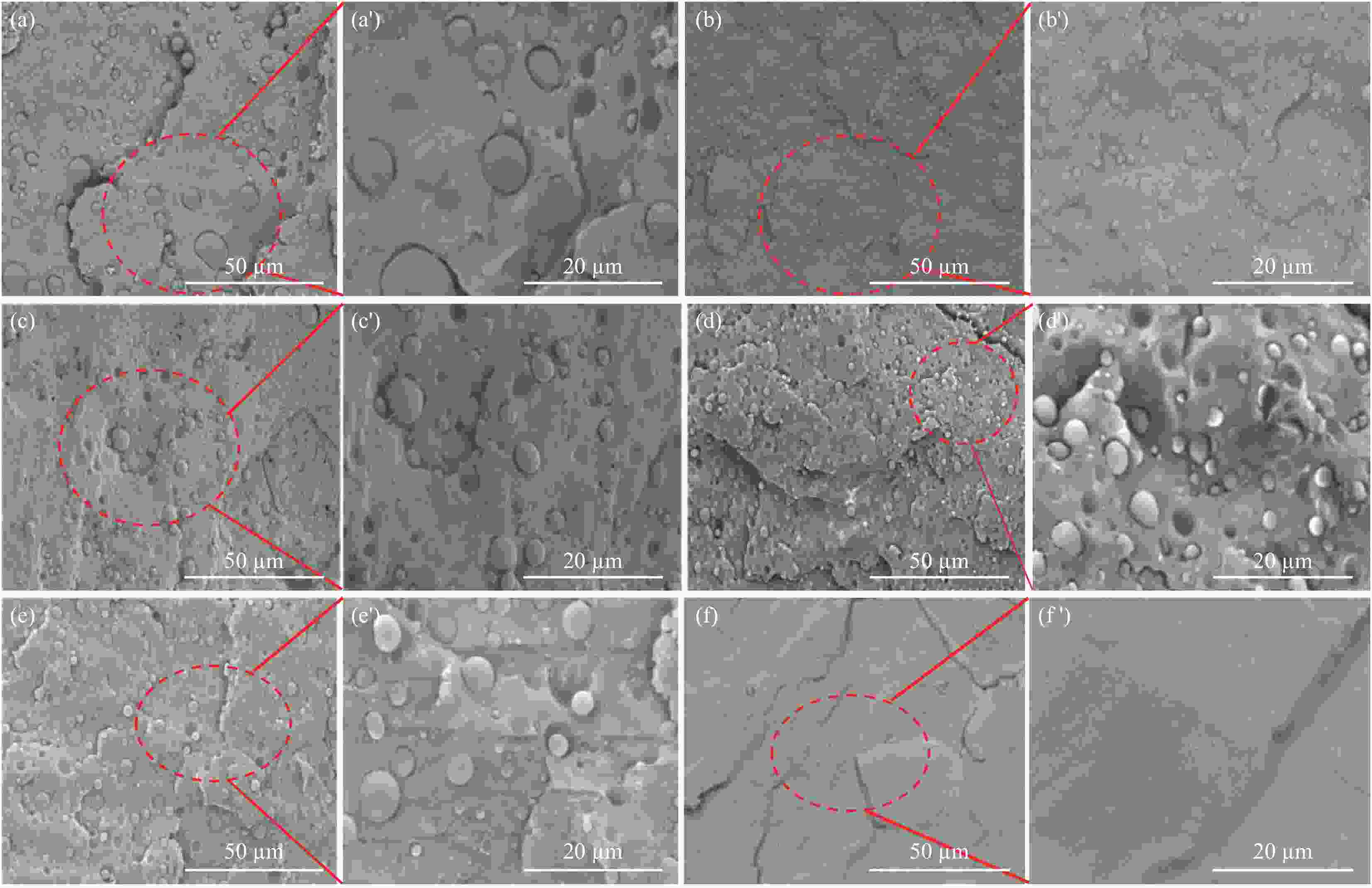
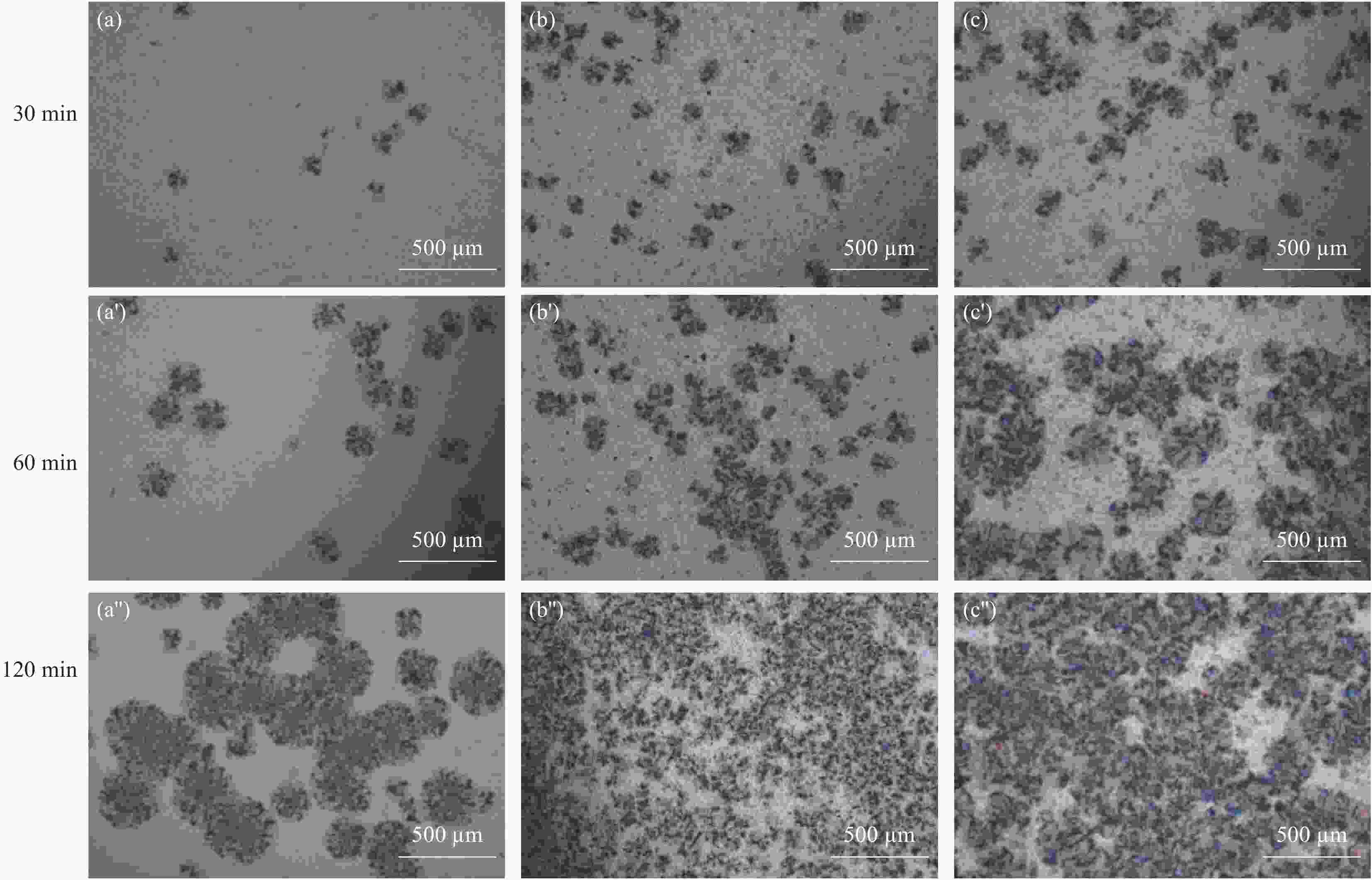
图 7 水滑石复合材料的纯样品及共混物的SEM图像:(a) PLA-PP-g-MAH;(b) LDHs(300)-SDBS/PLA-PP-g-MAH;(c) LDHs(300)/PLA-PP-g-MAH;(d) LDHs-SDBS/PLA-PP-g-MAH;(e) LDHs/PLA-PP-g-MAH;(f) Neat PLA;((a′)~(f′)) 对应的局部放大图
Figure 7. SEM images of the pure samples and blends of hydrotalcite composites: (a) PLA-PP-g-MAH; (b) LDHs(300)-SDBS/PLA-PP-g-MAH; (c) LDHs(300)/PLA-PP-g-MAH; (d) LDHs-SDBS/PLA-PP-g-MAH; (e) LDHs/PLA-PP-g-MAH; (f) Neat PLA; ((a′)~(f′)) Corresponding local enlarged view
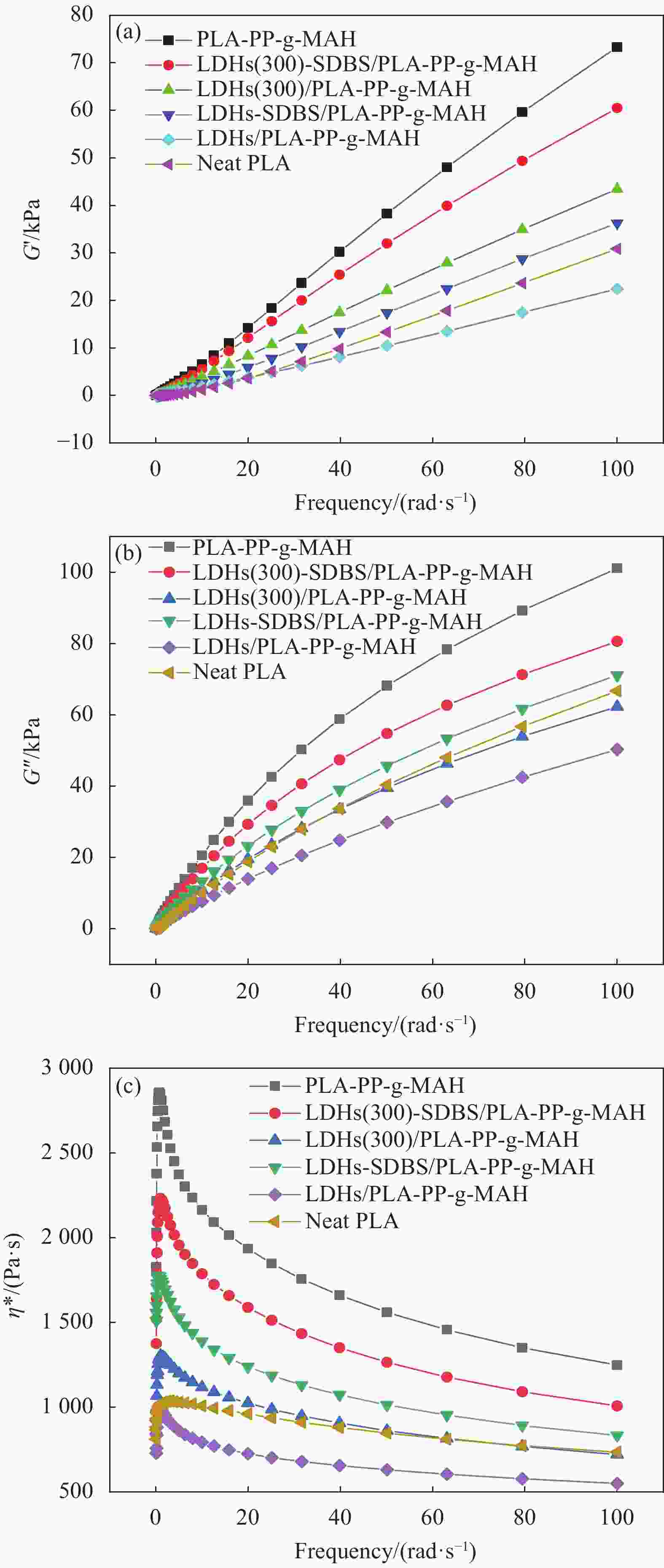
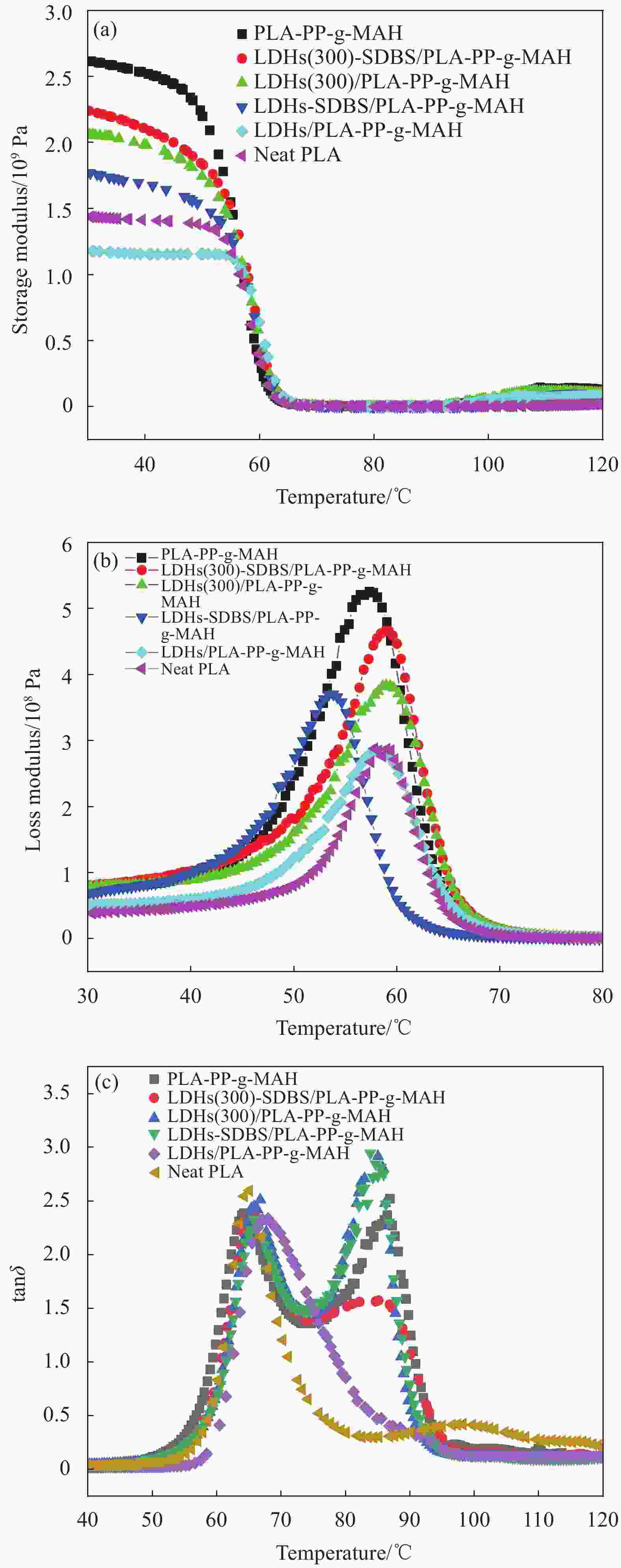
图 9 不同频率下PLA-PP-g-MAH、LDHs(300)-SDBS/PLA-PP-g-MAH、LDHs(300)/PLA-PP-g-MAH、LDHs-SDBS/PLA-PP-g-MAH、LDHs/PLA-PP-g-MAH和纯PLA的储能模量G′(a)、损耗模量G′′ (b) 和复数黏度η* (c)
Figure 9. Storage modulus G′ (a), loss modulus G′′ (b) and complex viscosity η* (c) of PLA-PP-g-MAH, LDHs(300)-SDBS/PLA-PP-g-MAH, LDHs(300)/PLA-PP-g-MAH, LDHs-SDBS/PLA-PP-g-MAH, LDHs/PLA-PP-g-MAH and neat PLA at different frequencies
图 13 PLA-PP-g-MAH、LDHs(300)-SDBS/PLA-PP-g-MAH、LDHs(300)/PLA-PP-g-MAH、LDHs-SDBS/PLA-PP-g-MAH、LDHs/PLA-PP-g-MAH和纯PLA的力学性能:(a) 冲击性能;(b) 拉伸强度
Figure 13. Mechanical properties of PLA-PP-g-MAH, LDHs(300)-SDBS/PLA-PP-g-MAH, LDHs(300)/PLA-PP-g-MAH, LDHs-SDBS/PLA-PP-g-MAH, LDHs/PLA-PP-g-MAH and neat PLA: (a) Impact strength; (b) Tensile strength
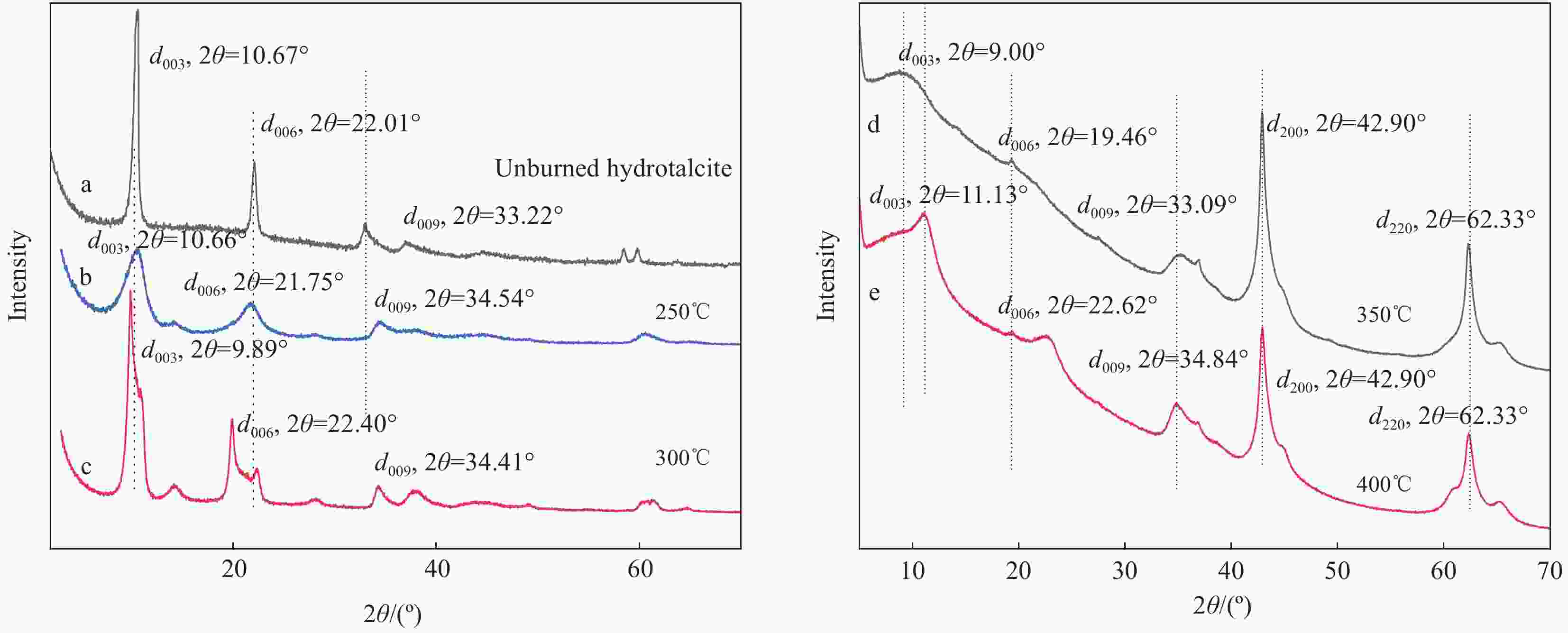
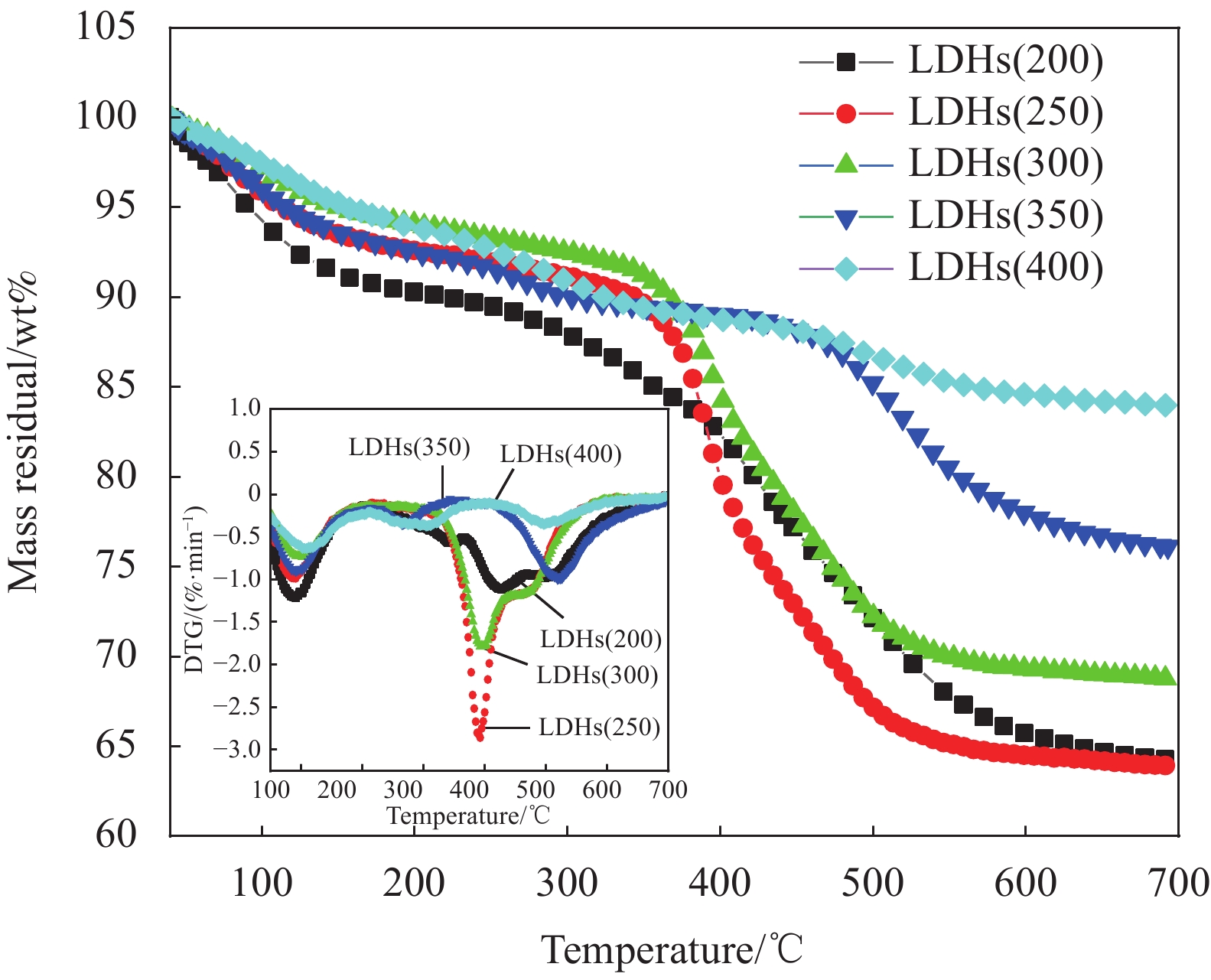
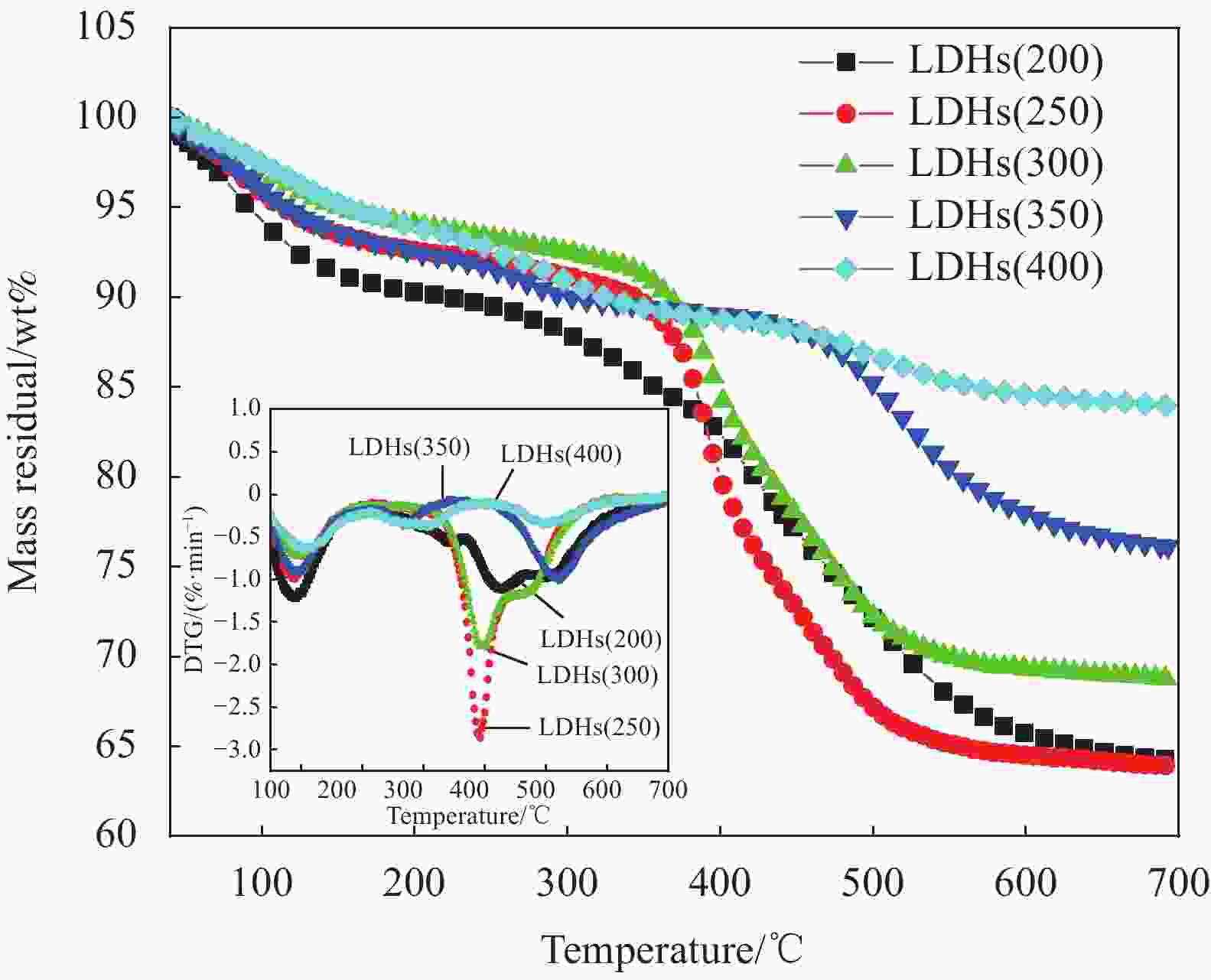
表 1 不同温度焙烧后的水滑石(LDHs)
Table 1. Hydrotalcite (LDHs) calcined at different temperatures
Sample Calcination temperature/℃ LDHs(200) 200 LDHs(250) 250 LDHs(300) 300 LDHs(350) 350 LDHs(400) 400 表 2 不同温度下焙烧水滑石24 h的集中热分解温度
Table 2. Concentrated thermal decomposition temperature of hydrotalcite calcined at different temperatures for 24 h
Sample T1max of stage 1/℃ T2max of stage 2/℃ ∆Tmax of stage 1/℃ Residual quantity/wt% LDHs(200) 87.5 453.4 — 64.20 LDHs(250) 90.0 391.3 2.5 63.76 LDHs(300) 93.7 523.8 6.2 68.96 LDHs(350) 96.2 397.7 8.7 76.00 LDHs(400) 108.8 495.9 21.3 84.01 Notes: T1max and T2max—Maximum decomposition temperature in the first and second stage; ∆Tmax—T1max–T1max (LDHs(200)). 表 3 水滑石复合材料的纯样及共混物的集中热分解温度
Table 3. Temperature of concentrated thermal decomposition of the pure samples and blends of hydrotalcite composites
Sample Tmax of stage 1/℃ ∆Tmax of stage 1/℃ Char residue at 600℃/wt% PLA-PP-g-MAH 364.5 −21.9 0.52 LDHs(300)-SDBS/PLA-PP-g-MAH 342.6 — 1.96 LDHs(300)/PLA-PP-g-MAH 327.3 15.3 1.46 LDHs-SDBS/PLA-PP-g-MAH 308.6 34.1 6.94 LDHs/PLA-PP-g-MAH 338.9 3.7 1.46 Neat PLA 363.9 −21.3 0.52 Notes: Tmax—Maximum decomposition temperature in the first stage. 表 4 水滑石复合材料纯样及共混物的玻璃转化温度Tg
Table 4. Glass transition temperature Tg of pure samples and blends of hydrotalcite composites
Sample $ {T}_{\mathrm{g}} $(PLA)/
℃$ {T}_{\mathrm{g}} $(PP-g-MAH)/
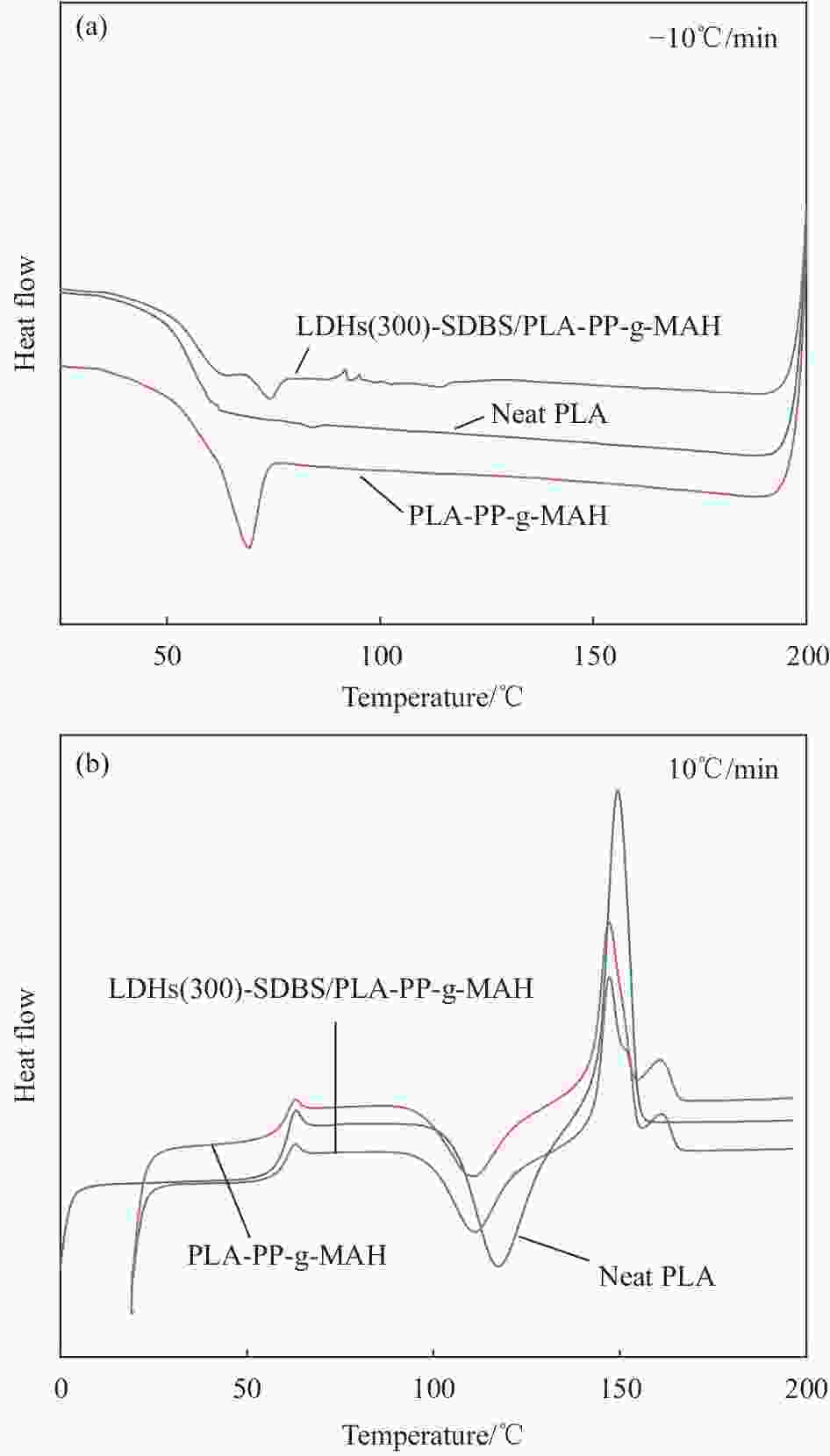
℃PLA-PP-g-MAH 64.3 86.9 LDHs(300)-SDBS/PLA-PP-g-MAH 65.2 83.1 LDHs(300)/PLA-PP-g-MAH 65.5 83.9 LDHs-SDBS/PLA-PP-g-MAH 65.8 85.0 LDHs/PLA-PP-g-MAH 67.6 89.7 Neat PLA 64.9 — 表 5 纯PLA、PLA-PP-g-MAH及LDHs(300)-SDBS/PLA-PP-g-MAH复合材料的DSC所得参数
Table 5. DSC parameters of neat PLA, PLA-PP-g-MAH and LDHs(300)-SDBS/PLA-PP-g-MAH composites
Sample $ {T}_{\mathrm{g}}/{}^\circ \text{C} $ $ {T}_{\mathrm{c}\mathrm{c}} /{}^\circ \text{C}$ $ {T}_{\mathrm{m}}/{}^\circ \text{C} $ $ {\mathrm{\Delta }H}_{\mathrm{c}\mathrm{c}}\text{/}\left( \text{kJ}\cdot \text{mo}{{\text{l}}^{{-1}}} \right) $ $ {\mathrm{\Delta }H}_{\mathrm{m}} \text{/}\left( \text{kJ}\cdot \text{mo}{{\text{l}}^{{-1}}} \right) $ ${\chi }_{\mathrm{c} }$/% Neat PLA 56.6 117.4 149.4 −22.52 23.54 1.09 PLA-PP-g-MAH 59.4 110.7 147.1 −22.13 24.87 1.24 LDHs(300)-SDBS/PLA-PP-g-MAH 59.4 110.0 147.1 −25.94 28.41 2.93 Notes: $ {T}_{\mathrm{c}\mathrm{c}} $—Cold crystallization peak; $ {\mathrm{\Delta }H}_{\mathrm{c}\mathrm{c}} $—Cold crystallization enthalpy; $ {\mathrm{\Delta }H}_{\mathrm{m}} $—Melt crystallization; $ {\chi }_{\mathrm{c}} $—Degree of crystallinity; Tm—Meliling temperature. -
[1] HASHIMA K, NISHITSUJI S, INOUE T. Structure-properties of super-tough PLA alloywith excellent heat resistance[J]. Polymer,2010,51(17):3934-3939. doi: 10.1016/j.polymer.2010.06.045 [2] OYAMA H T. Super tough poly (lactic acid)mat prials: Reactive blending with ethylene copolymer[J]. Polymer,2009,50(3):747-751. doi: 10.1016/j.polymer.2008.12.025 [3] LI Y J, SHIMIZU H. Improvement in toughness of poly (L-lac-tide) (PLLA) through reactive blending with acrylonitrile-bu-tadiene styrene copolymer (ABS): Morphology and properties[J]. European Polymer Journal,2009,45(3):738-746. doi: 10.1016/j.eurpolymj.2008.12.010 [4] YU T, LI Y, REN J, et al. Preparation and properties of short natural fiber reinforced poly(lactic acid) composites[J]. Transactions of Nonferrous Metals Society of China,2009,19(s3):s651-s655. [5] RAHIMIPOUR S, BAHRI-LALEH N, EHSANI M, et al. Preparation and properties of enhanced bio-based PLA/PA6/graphene nanocomposites in the presence of an ester–amide exchange catalyst[J]. Journal of Polymers and the Environment,2021,29(7):2302-2309. [6] HUDA M S, DRZAL L T, MOHANTY A K, et al. Effect of fiber surface-treatments on the properties of laminated biocomposites from poly(lactic acid) (PLA) and kenaf[J]. Composites Science and Technology,2008,68(2):424-432. doi: 10.1016/j.compscitech.2007.06.022 [7] PAPAGEORGIOU G Z, ACHILIASD S, NANAKI S, et al. PLA nanocomposites: Effect of filler type on non-isothermal crystallization[J]. Thermochimica Acta,2010,511(1-2):129-139. doi: 10.1016/j.tca.2010.08.004 [8] BAI H, HUANG C, XIU H, et al. Enhancing mechanical performance of polylactide by tailoring crystal morphology and lamellae orientation with the aid of nucleating agent[J]. Polymer,2014,55(26):6924-6934. doi: 10.1016/j.polymer.2014.10.059 [9] XU Y, DELGADO P, TODD A D, et al. Lightweight micro-cellular plastics from polylactide/polyolefin hybrids[J]. Polymer,2016,102:73-83. doi: 10.1016/j.polymer.2016.08.102 [10] ZHAO P, LIU W, WU Q, et al. Preparation, mechanical, and thermal properties of biodegradable polyesters/poly (lactic acid) blends[J]. Journal of Nanomaterials,2010,2010:287082. [11] MOMENI S, REZVANI G E, SHAKIBA M, et al. The effect of poly (ethylene glycol) emulation on the degradation of PLA/starch composites[J]. Polymers,2021,13(7):1019. doi: 10.3390/polym13071019 [12] ROBLES E, URRUZOLA I, LABIDI J, et al. Surface-modified nano-cellulose as reinforcement in poly (lactic acid) to conform new composites[J]. Industrial Crops and Products,2015,71:44-53. doi: 10.1016/j.indcrop.2015.03.075 [13] MAD DESA M S Z, HASSAN A, ARSAD A, et al. The effect of natural rubber toughening on mechanical properties of poly (lactic acid)/multiwalled carbon nanotube nanocomposite[J]. Advanced Materials Research,2013,747:639-642. [14] YU W, WANG X, FERRARIS E, et al. Melt crystallization of PLA/talc in fused filament fabrication[J]. Materials & Design,2019,182:108013. [15] AGRAWAL P, ARAUJO A P M, BRITO G F, et al. Rheological and mechanical properties of poly (lactic acid)/bio-based polyethylene/clay biocomposites containing montmorillonite and vermiculite clays[J]. Journal of Polymers and the Environment,2021,29(6):1777-1788. doi: 10.1007/s10924-020-02015-z [16] LI S, LIAO X, XIAO W, et al. The improved foaming behavior of PLA caused by the enhanced rheology properties and crystallization behavior via synergistic effect of carbon nanotubes and graphene[J]. Journal of Applied Polymer Science,2022,139(13):51874. [17] SABZI M, JIANG L, ATAI M, et al. PLA/sepiolite and PLA/calcium carbonate nanocomposites: A comparison study[J]. Journal of Applied Polymer Science,2013,129(4):1734-1744. doi: 10.1002/app.38866 [18] NUNEZ K, ROSALES C, PERERA R, et al. Nanocomposites of PLA/PP blends based on sepiolite[J]. Polymer Bulletin,2011,67(9):1991-2016. doi: 10.1007/s00289-011-0616-7 [19] AYRILMIS N. Effect of layer thickness on surface properties of 3D printed materials produced from wood flour/PLA filament[J]. Polymer Testing,2018,71:163-166. doi: 10.1016/j.polymertesting.2018.09.009 [20] KOMAL U K, LILA M K, SINGH I, et al. PLA/banana fiber based sustainable biocomposites: A manufacturing perspective[J]. Composites Part B: Engineering,2020,180:107535. doi: 10.1016/j.compositesb.2019.107535 [21] WU G, LIU S, JIA H, et al. Preparation and properties of heat resistant polylactic acid (PLA)/nano-SiO2 composite filament[J]. Journal of Wuhan University of Technology Materials Science Edition,2016,31(1):164-171. doi: 10.1007/s11595-016-1347-2 [22] HUANG S M, HWANG J J, LIU H J, et al. A characteristic study of polylactic acid/organic modified montmorillonite (PLA/OMMT) nanocomposite materials after hydrolyzing[J]. Crystals,2021,11(4):376. doi: 10.3390/cryst11040376 [23] 黄博文, 吕荥宾, 陈建钧, 等. 镁铝水滑石的合成及其在废水脱磷中的应用研究[J]. 高校化学工程学报, 2018, 32(3):683-689.HUANG Bowen, LV Xingbin, C Jianjun, et al. Synthesis of Mg Al hydrotalcite and its application in wastewater dephosphorization[J]. Journal of Chemical Engineering,2018,32(3):683-689(in Chinese). [24] 张永, 张延武, 朱艳青, 等. 水滑石类化合物的研究进展[J]. 河南化工, 2007, 24(12):9-12.ZHANG Yong, ZHANG Yanwu, ZHU Yanqing, et al. Research progress of hydrotalcite compounds[J]. Henan Chemical Industry,2007,24(12):9-12(in Chinese). [25] 郭志强, 倪哲明, 方彩萍, 等. 铜钴作为二价金属的类水滑石对NOx吸附性能研究[J]. 浙江工业大学学报, 2005(1):102-104.GUO Zhiqiang, NI Zheming, FANG Caiping, et al. Study on NOx adsorption performance of Cu Co hydrotalcite like compounds as divalent metals[J]. Journal of Zhejiang University of Technology,2005(1):102-104(in Chinese). [26] MEILI L, LINS P V, ZANTA C, et al. MgAl-LDH/biochar composites for methylene blue removal by adsorption[J]. Applied Clay Science,2019,168:11-20. doi: 10.1016/j.clay.2018.10.012 [27] DU J Z, JIN L, ZENG H Y, et al. Facile preparation of an efficient flame retardant and its application in ethylene vinyl acetate[J]. Applied Clay Science,2019,168:96-105. doi: 10.1016/j.clay.2018.11.004 [28] 荆秋叶, 彭冬, 袁小亚, 等. 煅烧型锌铝水滑石光还原Cr(Ⅵ)[J]. 材料导报, 2018, 32(14):2345-2350. doi: 10.11896/j.issn.1005-023X.2018.14.004JING Qiuye, PENG Dong, YUAN Xiaoya, et al. Photoreduction of Cr(Ⅵ) by calcined zinc aluminum hydrotalcite[J]. Materials Guide,2018,32(14):2345-2350(in Chinese). doi: 10.11896/j.issn.1005-023X.2018.14.004 [29] ZHANG L, LV S, SUN C, et al. Effect of MAH-g-PLA on the properties of wood fiber/polylactic acid composites[J]. Polymers,2017,9(11):591. doi: 10.3390/polym9110591 [30] 何飞雄, 卞军, 蔺海兰, 等. 功能化纳米石墨烯片/PP-PP-g-MAH复合材料的制备与表征[J]. 复合材料学报, 2015, 32(1):47-53.HE Feixiong, BIAN Jun, LIN Hailan, et al. Preparation and characterization of functionalized nano graphene sheet/PP-PP-g-MAH composites[J]. Acta Materiae Compositea Sinica,2015,32(1):47-53(in Chinese). [31] WANG W, ZHANG W, LIANG B. The influences of multiple factors for flexural performance of polypropylene: Crystallization, crystal evolution, nanoparticles[J]. Journal of Materials Science,2021,56(28):15667-15683. doi: 10.1007/s10853-021-06243-z [32] 中国国家标准化管理委员会. 塑料薄膜拉伸性能试验方法: GB 13022—91[S]. 北京: 中国标准出版社, 1991.Standardization Administration of the People’s Republic of China. Test method for tensile properties of plastic films: GB 13022—91[S]. Beijing: China Standards Press, 1991(in Chinese). [33] 中国国家标准化管理委员会. 塑料薄膜抗摆锤冲击试验方法: GB/T 8809—2015[S]. 北京: 中国标准出版社, 2015.Standardization Administration of the People’s Republic of China. Test method for pendulum impact resistance of plastic films: GB/T 8809—2015[S]. Beijing: China Standards Press, 2005(in Chinese). [34] CHAILLOT D, BENNICI S, BRENDLE J, et al. Layered double hydroxides and LDH-derived materials in chosen environmental applications: A review[J]. Environmental Science and Pollution Research,2021,28(19):24375-24405. doi: 10.1007/s11356-020-08498-6 [35] WANG L, SU S, CHEN D, et al. Variation of anions in layered double hydroxides: effects on dispersion and fire properties[J]. Polymer Degradation and Stability,2009,94(5):770-781. doi: 10.1016/j.polymdegradstab.2009.02.003 [36] HONG J S, NAMKUNG H, AHN K H, et al. The role of organically modified layered silicate in the breakup and coalescence of droplets in PBT/PE blends[J]. Polymer,2006,47(11):3967-3975. doi: 10.1016/j.polymer.2006.03.077 [37] DHARAIYA D P, JANA S C. Nanoclay-induced morphology development in chaotic mixing of immiscible polymers[J]. Journal of Polymer Science Part B: Polymer Physics,2005,43(24):3638-3651. doi: 10.1002/polb.20657 [38] KIM Y F, CHANG N C, KIM Y D, et al. Compatibilization of immiscible poly(L-lactide) and low density polyethylene blends[J]. Fibers & Polymers, 2004, 5(4): 270-274. [39] DELPOUVE N, SAITERR-FOURCIN A, COIAI S, et al. Effects of organo-LDH dispersion on thermal stability, crystallinity and mechanical features of PLA[J]. Polymer,2020,208:122952. doi: 10.1016/j.polymer.2020.122952 [40] CAM D, MARUCCI M. Influence of residual monomers and metals on poly (L-lactide)thermal stability[J]. Polymer,1997,38:1879-1884. doi: 10.1016/S0032-3861(96)00711-2 [41] WEI B, CHEN D, WANG H, et al. In-situ grafting of carboxylic acid terminated poly (methyl methacrylate) onto ethylene-glycidyl methacrylate copolymers: One-pot strategy to compatibilize immiscible poly (vinylidene fluoride)/low density polyethylene blends[J]. Polymer,2019,160:162-169. doi: 10.1016/j.polymer.2018.11.042 [42] WANG Q, ZHANG X, WANG C J, et al. Polypropylene/layered double hydroxide nanocomposites[J]. Journal of Materials Chemistry,2012,22(36):19113-19121. doi: 10.1039/c2jm33493c [43] MANGIACAPRA P, RAIMONDO M, TAMMARO L, et al. Nanometric dispersion of a Mg/Al layered double hydroxide into a chemically modified polycaprolactone[J]. Biomacromolecules,2007,8(3):773-779. doi: 10.1021/bm0605964 -







 下载:
下载: