Preparation of spherical NiCo2S4 and the electrochemical behavior in KOH solution
-
摘要:
NiCo2S4中Ni和Co元素可以同时参与充放电过程,获得较高的放电比容量和能量密度。对NiCo2S4中双电层电容和赝电容的贡献率进行分析,有助于推动电极材料的深入开发。以乙酸钴为Co源、乙酸镍为Ni源、硫代乙酰胺为沉淀剂,通过水热合成法制备具有优异电化学性能的球状电极材料硫钴酸镍(NiCo2S4)。利用X射线衍射、X射线光电子能谱、扫描电子显微镜、透射电子显微镜、Mapping和N2吸附-脱附技术对NiCo2S4的物相、形貌、组成和孔结构进行分析,并对其在KOH电解液中的充放电行为进行探究。结果表明,球状NiCo2S4制备成功,并且Ni、Co和S的含量百分比为1∶2.1∶4.2。NiCo2S4为立方相多晶体,晶格常数为
0.9387 nm。Ni和Co分别以Ni2+/Ni3+和Co2+/Co3+的形式存在于NiCo2S4的晶格之中。NiCo2S4的孔体积为0.402 cm3/g,并且介孔比例为90.6%。NiCo2S4在KOH溶液中同时产生双电层电容和赝电容,两者所占比例分别为60.6%、39.4%。电流密度为0.2 A/g时,放电比容量为409.7 F/g,能量密度为14.2 W·h/kg。循环10000 次,容量保持率为90.3%。Abstract:In NiCo2S4, Ni and Co will participate in the charging-discharging process at the same time to obtain higher specific discharge capacity and energy density. Moreover, it is helpful to promote the further development of electrode materials by analyzing the contribution rates of double layer capacitance and pseudocapacitance in NiCo2S4. The spherical electrode material of nickel cobaltate (NiCo2S4) with excellent electrochemical performance was prepared through hydrothermal synthesis method using cobaltous acetate as Co source, nickel acetate as Ni source and thioacetamide as precipitant. The phase, morphology, composition and pore structure of NiCo2S4 were characterized using XRD, XPS, SEM, TEM, mapping and N2 adsorption-desorption techniques. Besides, the electrochemical behavior in KOH electrolyte was investigated. The results display that the spherical NiCo2S4 is successfully prepared and the ratio of Ni, Co and S is 1∶2.1∶4.2. NiCo2S4 is a cubic phase polycrystal and the lattice constant is
0.9387 nm. In the lattice of NiCo2S4, Ni and Co exist in the form of Ni2+/Ni3+ and Co2+/Co3+. The pore volume of NiCo2S4 is 0.402 cm3/g and the proportion of mesoporous pores is 90.6%. In KOH solution, both of double layer capacitance and pseudocapacitance are generated and the ratios are 60.6% and 39.4%. When the current density is 0.2 A/g, the specific discharge capacity is 409.7 F/g, energy density is 14.2 W·h/kg. After10000 cycles, the capacity retention is 90.3%.-
Keywords:
- NiCo2S4 /
- KOH /
- globular /
- capacitor /
- charge/discharge /
- double electrode layer /
- pseudocapacitance
-
超级电容器是重要的能量储备装置之一,已经被广泛应用于现代工业领域之中,如新能源汽车和船舶[1-2]。超级电容器的性能与电极材料有较大关系。目前,已有多种固体材料被应用于超级电容器之中,如碳材料[3-5]、导电聚合物[6-8]、MXenes[9-11]、氧化物[12-17]及相关复合材料[18-19]。邢正伟等[18]通过阳极氧化法制备了一种自支撑多孔硅/改性氧化锌复合材料(Si/ZnO)。电流密度为1 A/g时,Si/ZnO的放电比容量为15.7 F/g。胡彬等[19]在Ar氛围中通过烧结碳化法制备了一种氧化石墨烯/壳聚糖复合材料(GO/CHI)。电流密度为1 A/g时,GO/CHI复合电极材料的放电比容量为135 F/g。
电极材料之中,多孔氧化物因具有制备方法简单、制备周期较短、放电比容量和能量密度较高等优点备受青睐,如氧化镍(NiO)[12-14]、四氧化三钴(Co3O4) [15-17]等。Sethi等[13]发现,以氯化镍(NiCl2)为Ni源,通过160℃水热处理制得的多孔NiO纳米片在2 mol/L KOH电解液中具有较大的放电比容量。扫描速率为5 mV/s时,放电比容量为305 F/g。Arun等[17]通过沉淀法制得了一种纳米六边形Co3O4。电流密度为1 A/g时,Co3O4的放电比容量为718 F/g。KOH电解液中,NiO中的Ni2+和Co3O4中的Co2+均可与OH−发生反应,产生赝电容。多孔氧化物在电解液中不仅产生赝电容,其表面还产生双电层电容。然而,有关多孔电极材料的报道主要集中于材料结构、形貌、扫描速率、电流密度等因素对其充放电性能的影响,对其产生的电容类型及所占比例的报道较少。对于多孔型电极材料,须同时考虑孔结构和元素组成对其充放电性能的影响。
根据文献可知[12-17],NiO和Co3O4在KOH溶液中均具有较好的充放电性能,Ni和Co两种元素均可与OH−反应并产生赝电容。NiCo2S4中含有Ni和Co两种过渡金属元素,并且两种元素可能均显示多种价态。不同价态的Ni和Co同时存在于NiCo2S4之中,可以促使充放电顺利进行,获得较好的电化学性能。基于以上信息,本文分别以乙酸钴和乙酸镍为Co源和Ni源,采用水热合成法制备硫钴酸镍(NiCo2S4),并以其为电极材料组装对称型电容器,探究NiCo2S4在KOH中的充放电行为。
1. 实验材料及方法
1.1 原材料
蔗糖(AR)、四水乙酸镍(Ni(CH3COO)2·4H2O,AR)、四水乙酸钴(Co(CH3COO)2·4H2O,AR)、硫代乙酰胺(CH3CSNH2,AR)、N-甲基吡咯烷酮(AR)、聚偏氟乙烯(AR),购自国药集团化学试剂有限公司;导电炭黑(电池级)、泡沫镍(电池级),购自上海赛博化工有限公司;KOH(AR),购自上海阿拉丁生化科技股份有限公。
1.2 NiCo2S4的制备
将1.000 g蔗糖、1.635 g 乙酸钴、0.815 g乙酸镍溶于60 mL去离子水中,剧烈搅拌1 h,得到澄清溶液。将1.970 g硫代乙酰胺加入到上述溶液中,继续搅拌30 min。将所得溶液转移到100 mL聚四氟乙烯内衬中,加盖密封,并将其置于不锈钢反应釜中,在160℃环境中水热处理24 h。利用去离子水对所得产物进行洗涤,直至pH为7,并将所得固体产物置于100℃烘箱中干燥12 h,得到粗产物。将粗产物置于马弗炉中,以5℃/min的速率升温至350℃,保持2 h,再以相同的速率降至室温,得到NiCo2S4。
实验中,蔗糖的作用如下:蔗糖在溶液中水解为葡萄糖和果糖。葡萄糖和果糖分子中均含有较多羟基。因此葡萄糖和果糖之间可以形成氢键,形成与表面活性剂相似的小团簇,并将水分子隔开。经过350℃高温焙烧后,小团簇被移除,形成孔洞,可能获得比表面积较大、孔隙率较高的多孔电极材料NiCo2S4,促进NiCo2S4与电解液中正负离子更好地接触,获得优异的充放电性能。
为了成功制得NiCo2S4:(1) 选取0.815 g Ni(CH3COO)2·4H2O和1.635 g Co(CH3COO)2·4H2O,其中Ni和Co的摩尔比为1∶2.004,接近理论比例1∶2;(2) 温度为25℃时,硫代乙酰胺在水中的溶解度约为16.3 g/100 mL,即:0.163 g/mL。此浓度下,硫代乙酰胺不能完全水解。为了促进金属离子沉淀,本实验中加大了硫代乙酰胺的量。以Ni为基准,Ni和S的摩尔比约为1∶8。硫代乙酰胺初始浓度增大后,水解程度将有所降低,但水解后活性物种的量将明显增大,促进金属离子沉淀,获得足量的NiCo2S4 。根据实验结果,NiCo2S4的实际质量为0.958 g (理论质量为0.998 g),产率约为96%。
1.3 NiCo2S4的表征
利用北京普析XD-6型X-射线衍射仪对NiCo2S4的物相进行检测,测试角度为 10°~80°。对于面心立方结构的材料,晶面指数均为奇数或偶数时,可根据公式(1)中晶面指数和晶格常数计算电极材料的晶面间距。公式(1)中:dhkl是晶面间距,a是晶格常数,h、k、l为晶面指数。采用Thermo Scientific K-Alpha型X-射线光电子能谱仪(XPS)对NiCo2S4的结构进行表征,利用德国ZEISS Sigma 300型扫描电子显微镜(SEM)和美国FEI Tecnai F20型透射电子显微镜(TEM)对NiCo2S4的形貌和精细结构进行探究,结合XPS和FEI Tecnai F20型透射电子显微镜自带的能谱面扫(Mapping)测试结果对NiCo2S4的元素组成进行分析,通过美国Micromeritics ASAP 2460型全自动比表面及孔隙率分析仪对NiCo2S4的孔结构进行分析。
dhkl=a√h2+k2+l2 (1) 1.4 两电极组装及电化学性能测试
将聚偏二氟乙烯分散于1 g N-甲基吡咯烷酮之中,加入导电炭黑和NiCo2S4,搅拌4 h,获得黑色浆料(NiCo2S4、聚偏二氟乙烯和导电炭黑的质量比为8∶1∶1)。将浆料涂覆于直径16 mm的泡沫镍圆片上,并于80℃干燥6 h,得到NiCo2S4极片,NiCo2S4厚度约为5 μm,NiCo2S4的面密度约为0.025 mg/mm2。选取两片质量一致的极片作为正负极,6 mol/L KOH溶液为电解液组装扣式电池,探究NiCo2S4的电化学行为。
利用恒流充放电(GCD)曲线和公式(2)~(4)计算NiCo2S4扣式电池的放电比容量、能量密度和功率密度[1]。其中:Cg为NiCo2S4的恒流放电比容量(F/g);Eg为恒流放电过程的能量密度(W·h/kg);Pg为功率密度(W/kg);I为充放电电流(A);U为电压窗口(V);m为正负极极片上NiCo2S4的总质量(g);t为放电时间(s);∫Udt为放电曲线包围的面积(V·s)。为了进一步验证NiCo2S4在KOH溶液中的电化学行为,可以利用公式(5)对NiCo2S4的放电比容量进行分析[20-21],其中: Cg为总的放电比容量,v为放电时间的倒数(1/t,(1/s))。
Cg=2I∫UdtmU2 (2) Eg=I∫Udt3.6m (3) Pg=3600Egt (4) Cg=k1+k2√v (5) 2. 结果与讨论
2.1 材料表征
2.1.1 NiCo2S4的物相
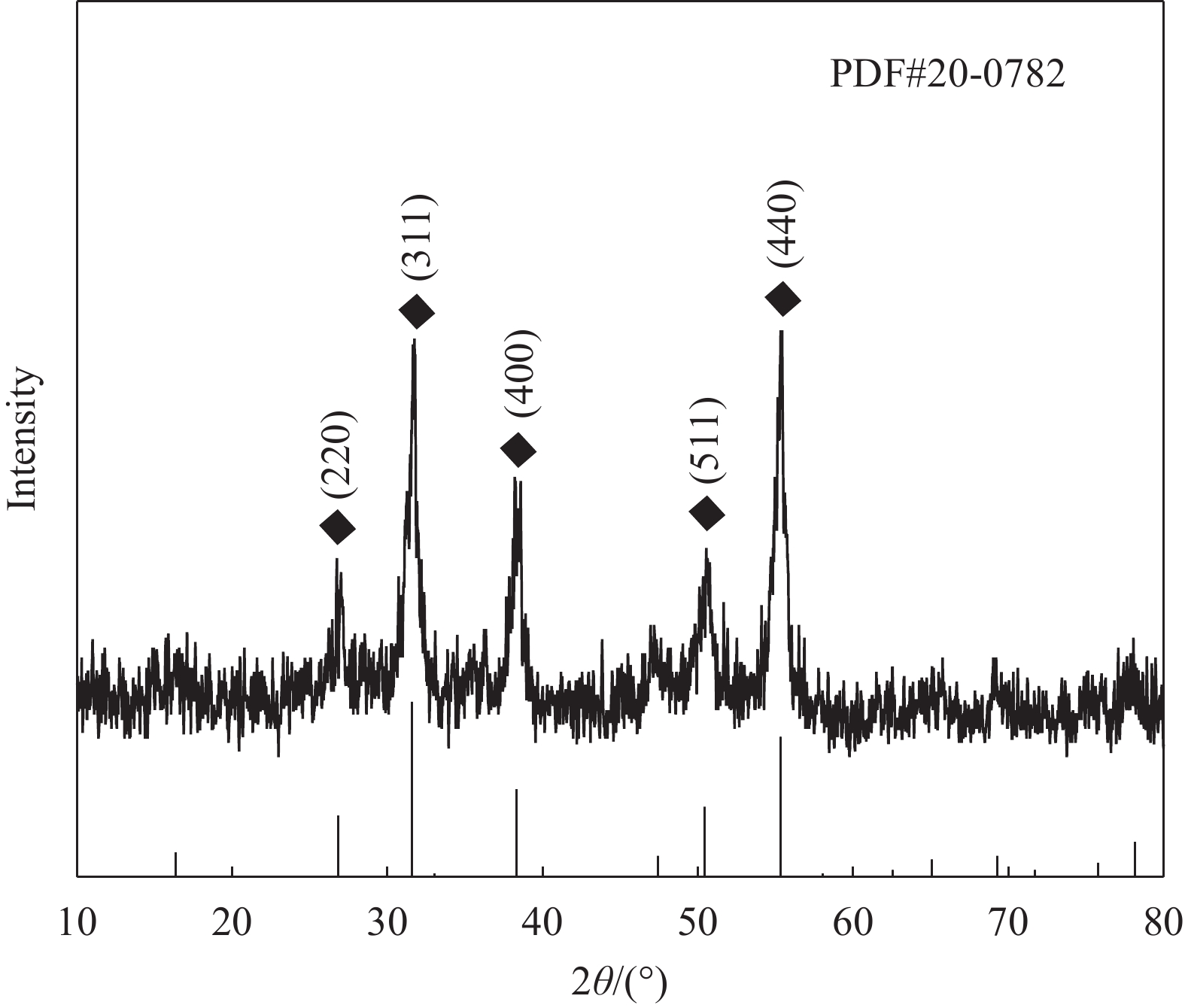
图1是样品的XRD图谱。可知,样品的XRD曲线与NiCo2S4的PDF#20-0782的信息相吻合,说明NiCo2S4晶体制备成功。根据PDF#20-0782信息可知,所制备的NiCo2S4是立方相材料,晶格常数为
0.9387 nm。2θ=26.741°、31.643°、38.267°、50.622°、55.323°分别出现(220)、(311)、(400)、(511)、(440)晶面的衍射峰,并且峰强度较强。若NiCo2S4是面心立方结构,并且晶面指数均为奇数或偶数时,可以结合公式(1)中晶面指数和晶格常数计算出所制备的NiCo2S4的晶面间距。根据计算结果可知,(220)、(311)、(400)、(511)、(440)的晶面间距分别为0.3319 nm、0.2830 nm、0.2347 nm、0.1807 nm、0.1659 nm,计算结果与PDF#20-0782的信息一致,因此NiCo2S4为面心立方晶体。2.1.2 NiCo2S4中Ni和Co的价态
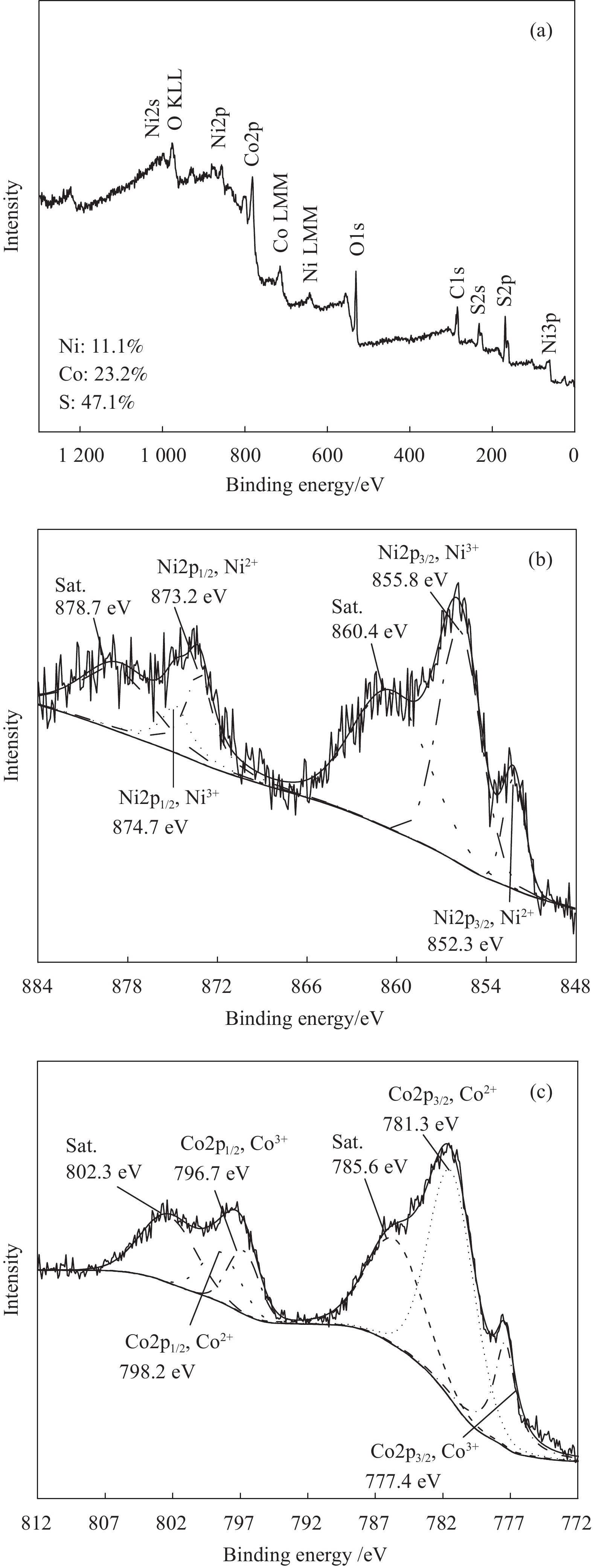
图2为NiCo2S4的XPS图谱。由全谱图(图2(a))可知,NiCo2S4样品表面检测到Ni、Co和S 3种元素,并且含量分别为11.1%、23.2%、47.1%。以Ni为基准,Ni、Co和S含量百分比为1∶2.1∶4.2,与理论值(1∶2∶4)相近。由Ni的XPS精细图谱(图2(b))可知,结合能为873.2 eV、852.3 eV处分别检测到Ni2+的2p1/2和2p3/2自旋-轨道分裂峰,874.7 eV和855.8 eV处分别检测到Ni3+的2p1/2和2p3/2自旋-轨道分裂峰[22]。因此,Ni在NiCo2S4中同时以Ni2+、Ni3+形式存在。与此相似,Co在NiCo2S4中也产生能级分裂,并且在798.2 eV、781.3 eV处出现Co2+的2p1/2和2p3/2自旋-轨道分裂峰,在796.7 eV和777.4 eV处产生Co3+的2p1/2和2p3/2自旋-轨道分裂峰[23] (图2(c))。因此,Ni和Co在NiCo2S4中以Ni2+、Ni3+、Co2+和Co3+形式存在。
2.1.3 NiCo2S4的形貌、精细结构和元素组成
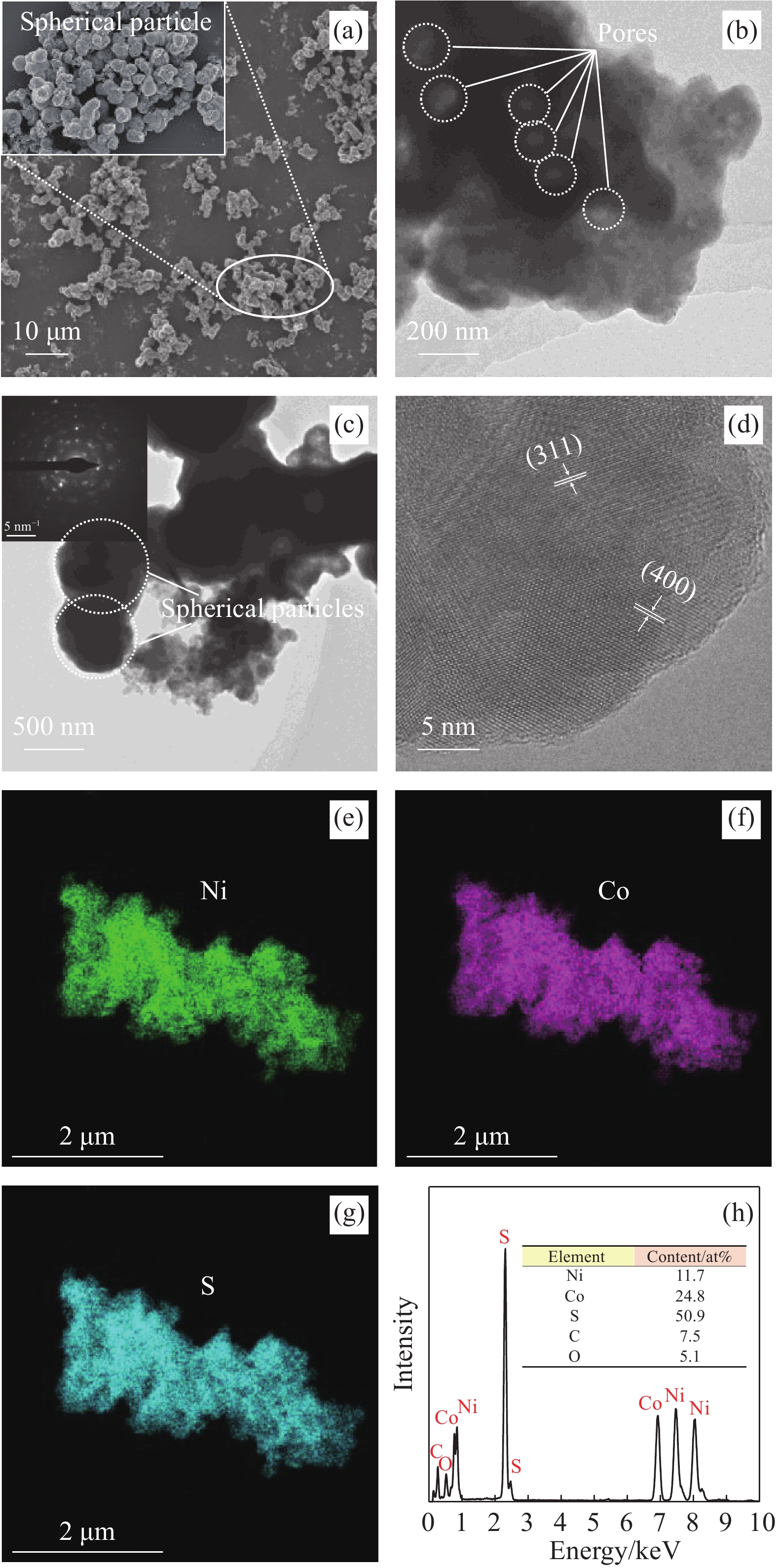
图3为NiCo2S4的SEM、TEM和Mapping测试结果。结合SEM (图3(a))和TEM (图3(b))测试结果可知,NiCo2S4微粒呈球状,有较多的孔,并且分散良好,无明显团聚。NiCo2S4这一特点有助于电解液离子在材料内部传输,获得较好的电化学性能。NiCo2S4的衍射花样中出现多个斑点,并且呈现不同半径的同心圆,说明所制备的NiCo2S4为多晶体(图3(c))。由图3(d)可知,NiCo2S4中存在明显的晶格条纹,分别归属于(311)和(400)晶面,进一步说明NiCo2S4为晶态材料,Ni、Co和S原子排列呈现长程有序的特点。由Mapping结果(图3(e)~3(h))可知,NiCo2S4中存在Ni、Co和S元素,并且元素含量分别为11.7%、24.8%、50.9%。以Ni含量为基准,Ni∶Co∶S含量百分比为1∶2.1∶4.3。Mapping结果与XPS结果一致。根据XPS和Mapping测试结果,NiCo2S4中还含有少量C和O元素,两者含量分别为7.5%、5.1% (Mapping结果)。少量C元素出现在NiCo2S4之中,可能是由于:① 蔗糖中少量C元素在焙烧过程中进入NiCo2S4体相之中;② 少量CO2吸附于多孔NiCo2S4电极材料之中。少量O元素出现在NiCo2S4之中,可能是由于少量CO2和H2O吸附于多孔NiCo2S4电极材料之中。
2.1.4 NiCo2S4的比表面积和和孔结构
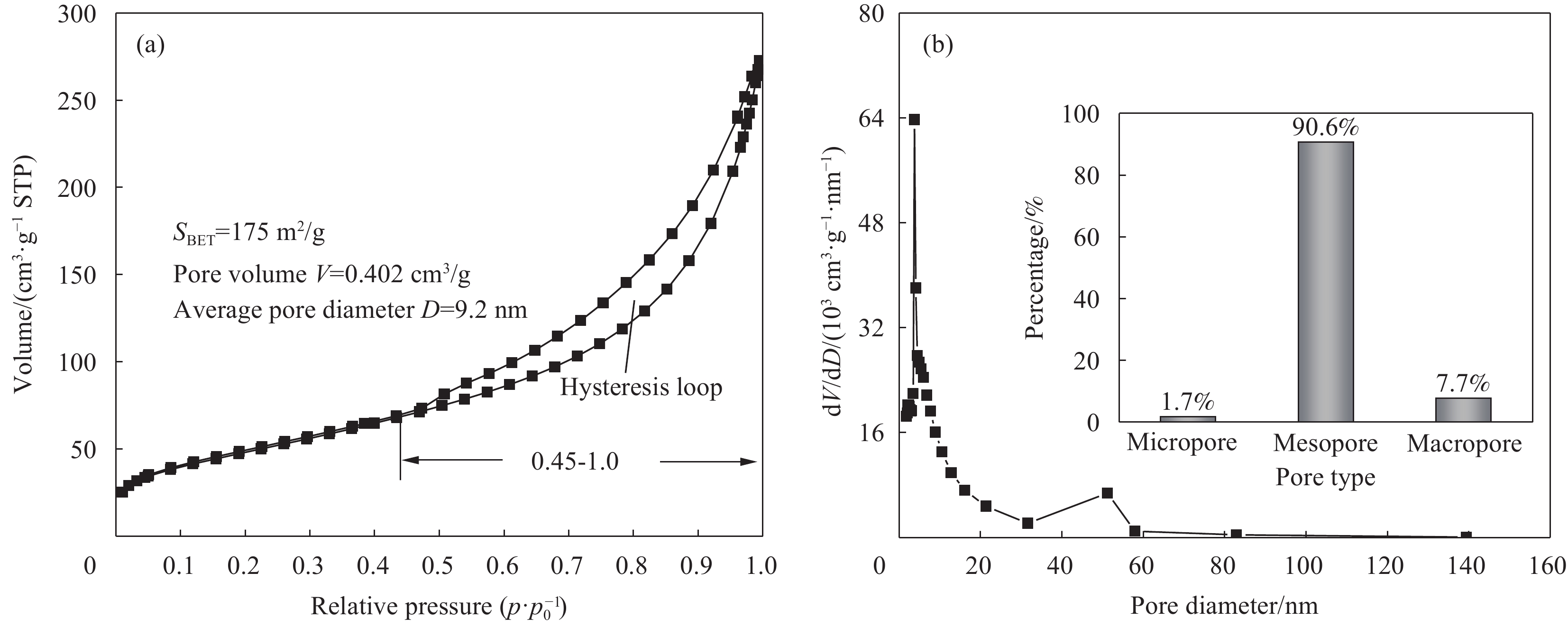
图4是NiCo2S4的N2吸附-脱附曲线。图4(a)中,NiCo2S4的N2吸附曲线为第IV型等温线,并且起点脱附曲线终点重合。相对压强p/p0处于0.45~1时,吸附曲线与脱附曲线不重合,形成滞后环,这是由于N2在NiCo2S4的孔道内产生毛细管凝聚效应所致。NiCo2S4的比表面积为175 m2/g,孔体积可达0.402 cm3/g。由图4(b)可知,NiCo2S4的孔径分布曲线从微孔延伸至大孔,但介孔所占比例最大(90.6%),并且孔径主要集中于2.1~21.3 nm之间。由以上信息可知,NiCo2S4是由介孔主导的多孔材料。
2.2 材料的电化学性能
2.2.1 循环伏安(CV)测试
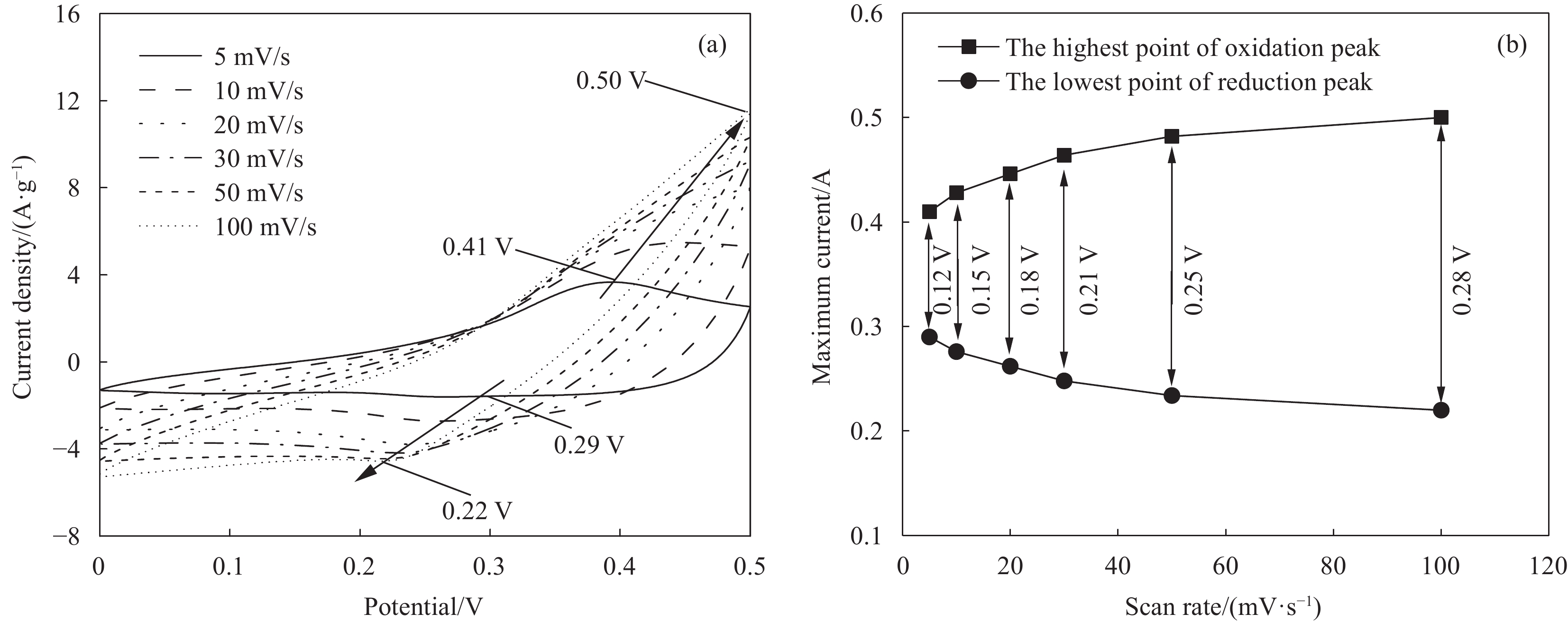
图5是球状NiCo2S4颗粒在6 mol/L KOH溶液中的CV实验结果。扫描速率为5 mV/s时,NiCo2S4的CV曲线偏离矩形,并且出现氧化还原峰,说明NiCo2S4与KOH电解液反应,产生赝电容。根据XPS结果可知,NiCo2S4中同时存在Ni2+、Ni3+、Co2+和Co3+。结合文献报道[12],Ni2+可与OH−结合,实现Ni2+/Ni3+的可逆转换。与此相似,Co2+也可与OH−结合,实现Co2+/Co3+/Co4+的可逆转换。根据方程式①和②,NiCo2S4在KOH溶液中可以同时实现Ni2+/Ni3+和Co2+/Co3+/Co4+的可逆转换,从而完成充放电并进行储能[24]。此外,NiCo2S4是多孔材料,比表面积和孔体积分别为175 m2/g、0.402 cm3/g。较大的比表面积可以为K+、OH−提供吸附位点,使较多的电解液离子发生分离并吸附于NiCo2S4表面,产生双电层电容。因此,NiCo2S4在KOH溶液中可能同时产生双电容和赝电容。随着扫描速度增大:① 氧化电位逐渐增大,还原电位逐渐减小,氧化还原电位差值(△E)逐渐增大(图5(b)),大倍率条件下充放电可逆性降低;② 电流逐渐增大,极化增强,充放电性能逐渐降低。
NiCo2S4+3OH−↔NiS4−4xOH+2CoS2xOH+3e− ①
CoSxOH+OH−↔CoSxO+H2O+e− ②
2.2.2 充放电性能(GCD)测试
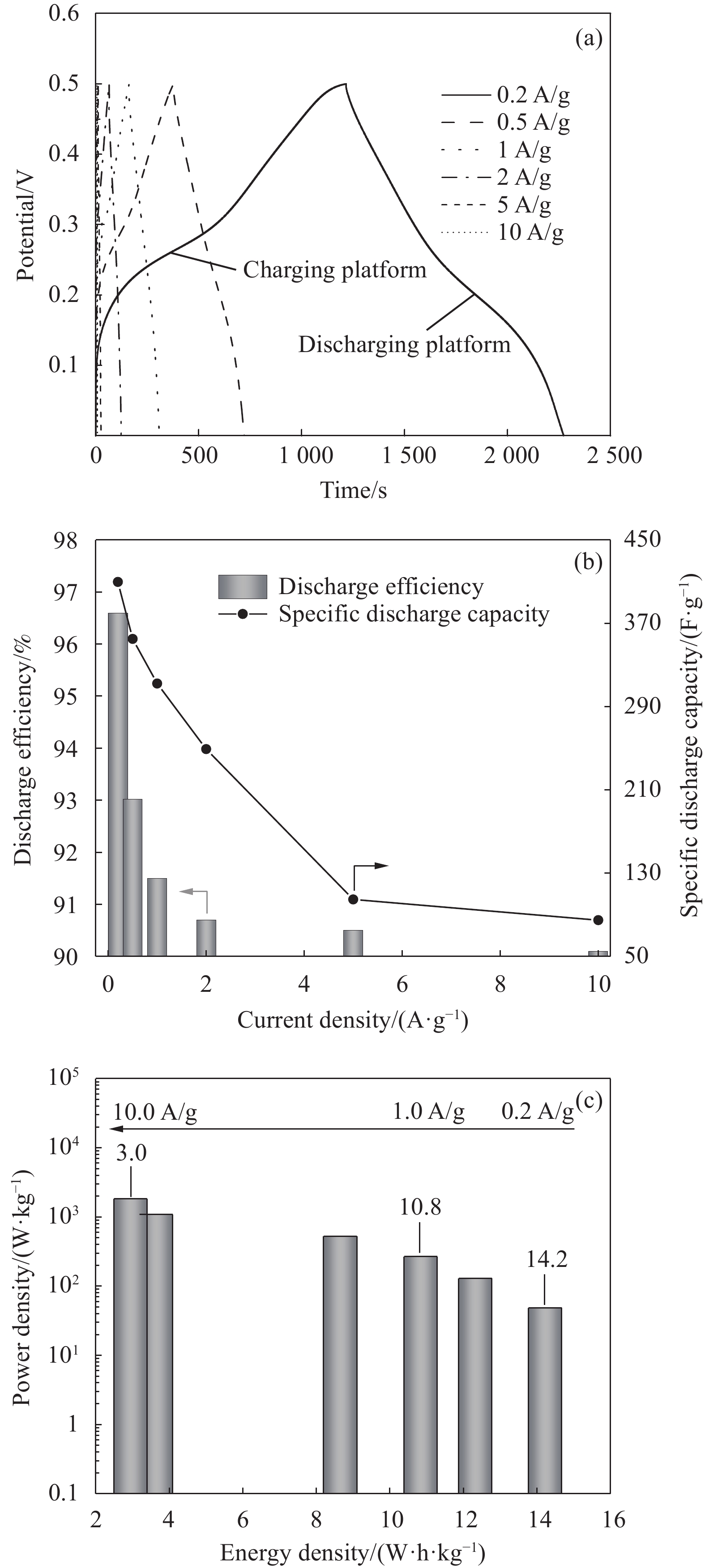
图6 是NiCo2S4在KOH电解液中的充放电性能结果。与CV曲线一致,充放电曲线为非线性曲线,并且产生充电平台和放电平台,说明NiCo2S4在KOH溶液中产生赝电容(图6(a))。随着扫描速度增大,用NiCo2S4组装的扣式电池放电效率、放电比容量和能量密度逐渐降低(图6(b)、图6(c))。电流密度为0.2 A/g时,放电效率为96.6%,放电比容量为409.7 F/g,能量密度为14.2 W·h/kg。电流密度提高至10 A/g时,放电效率、放电比容量和能量密度分别为90.1%、84.8 F/g和3.0。与0.2 A/g相比较,充放电性能降低。由CV曲线可知,扫描速率增大时,氧化还原电流增大,极化随之增大。因此,NiCo2S4的放电效率、放电比容量和能量密度逐渐减少[1, 25]。
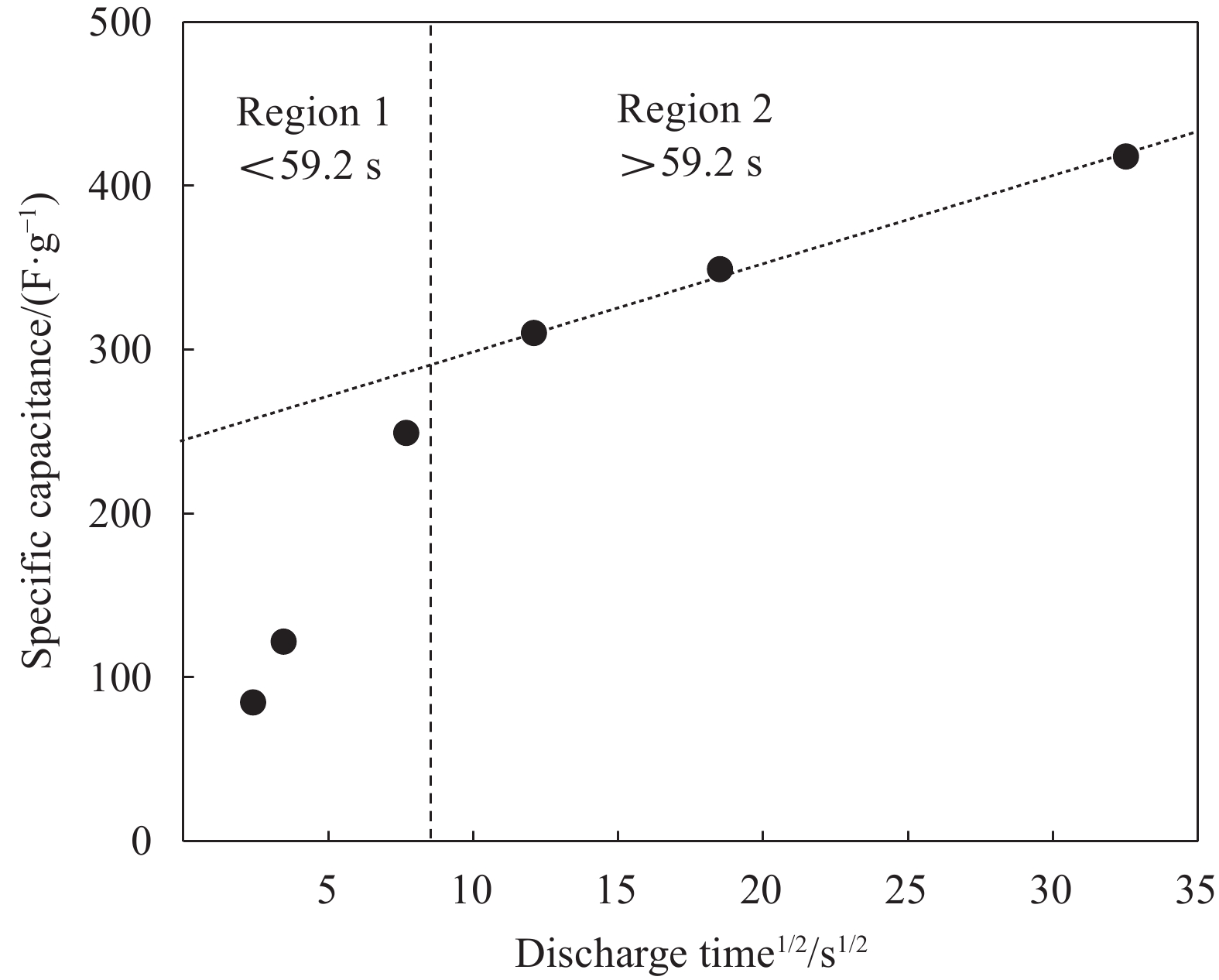
根据公式(5)对NiCo2S4的放电比容量进行分析。v趋近于∞时,放电时间t趋近于0,此时NiCo2S4表面形成双电层电容(EDLC)。v趋近于0时,放电时间t趋近于∞,NiCo2S4与KOH溶液反应完全,此时赝电容占比最大。如图7所示,y轴上的扩展截距表示t趋近于0时的放电比容量。由图7可知,截距为248 F/g,即双电层电容为248 F/g,赝电容为161.7 F/g。因此,电池的双电层电容占比为60.6%,赝电容占比为39.4%。图7进一步证明了NiCo2S4在KOH电解液中可同时产生双电层电容和赝电容。由赝电容贡献率可知,NiCo2S4的赝电容可以进一步提高。为了进一步提高NiCo2S4的充放电性能,可以考虑从以下几个方面改善NiCo2S4的结构:(1) 调节水热处理温度,增大OH−进入NiCo2S4体相的晶面间距,使OH−更容易与Ni2+/Ni3+和Co2+/Co3+接触,增大放电比容量,同时提高循环充放电性能;(2)制备纳米级别的NiCo2S4材料,缩短Ni2+/Ni3+和Co2+/Co3+在NiCo2S4体相中的迁移距离,提高NiCo2S4可逆性,进一步提高NiCo2S4的充放电性能。
2.2.3 电化学阻抗谱(EIS)
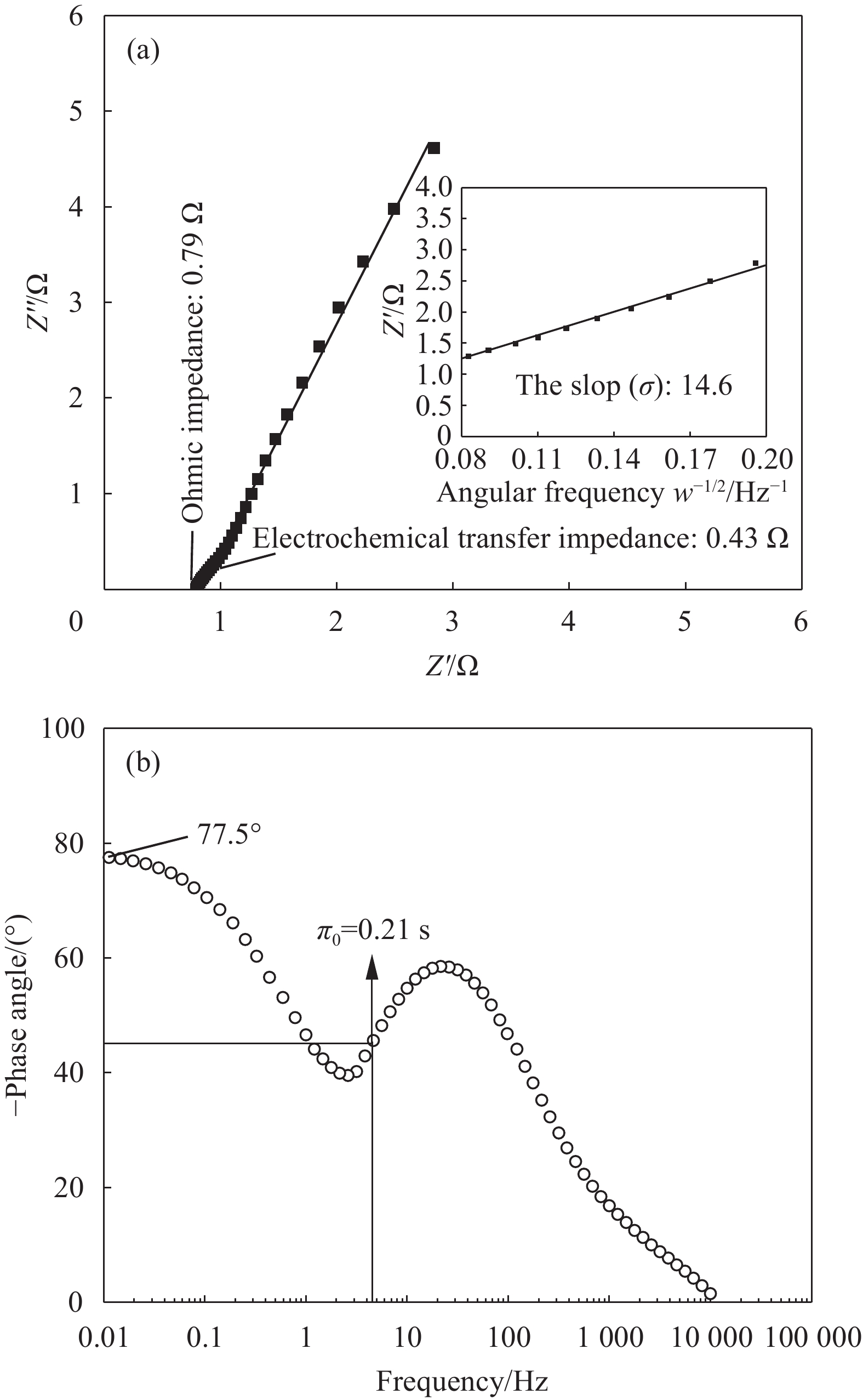
图8为NiCo2S4在KOH电解液中的EIS和Bode曲线图。由图8(a)可知,NiCo2S4的EIS曲线由中频区半圆和低频区直线组成。半圆起点为0.79 Ω,说明NiCo2S4电池中存在欧姆阻抗,即球状NiCo2S4颗粒之间及其与KOH、泡沫镍之间存在接触阻抗。中频区半圆表示电化学转移阻抗,阻抗值为0.43 Ω,由NiCo2S4与KOH发生的法拉第反应引起。低频区直线表示电解液中K+和OH−往正负极NiCo2S4表面扩散时所产生的离子迁移阻抗。根据图8(a)中插图可知,Warbuge因子为14.6。根据文献报道[26],可以根据Warbuge因子计算出离子扩散系数为1.1×10−10,即离子在NiCo2S4中具有较快的扩散速率。由Bode图可知,低频区相位角与−90°相差12.5°。这一结果与Nyquist曲线一致,即存在离子迁移阻抗。相位角为−45°时,阻抗与容抗一致。根据此时的频率可以计算出弛豫时间常数π (π=1/f0)。π值越小,可逆性越好。由图8(b)可知,π值为0.21 s,即:π值较小,充放电可逆性较好。
2.2.4 循环性能
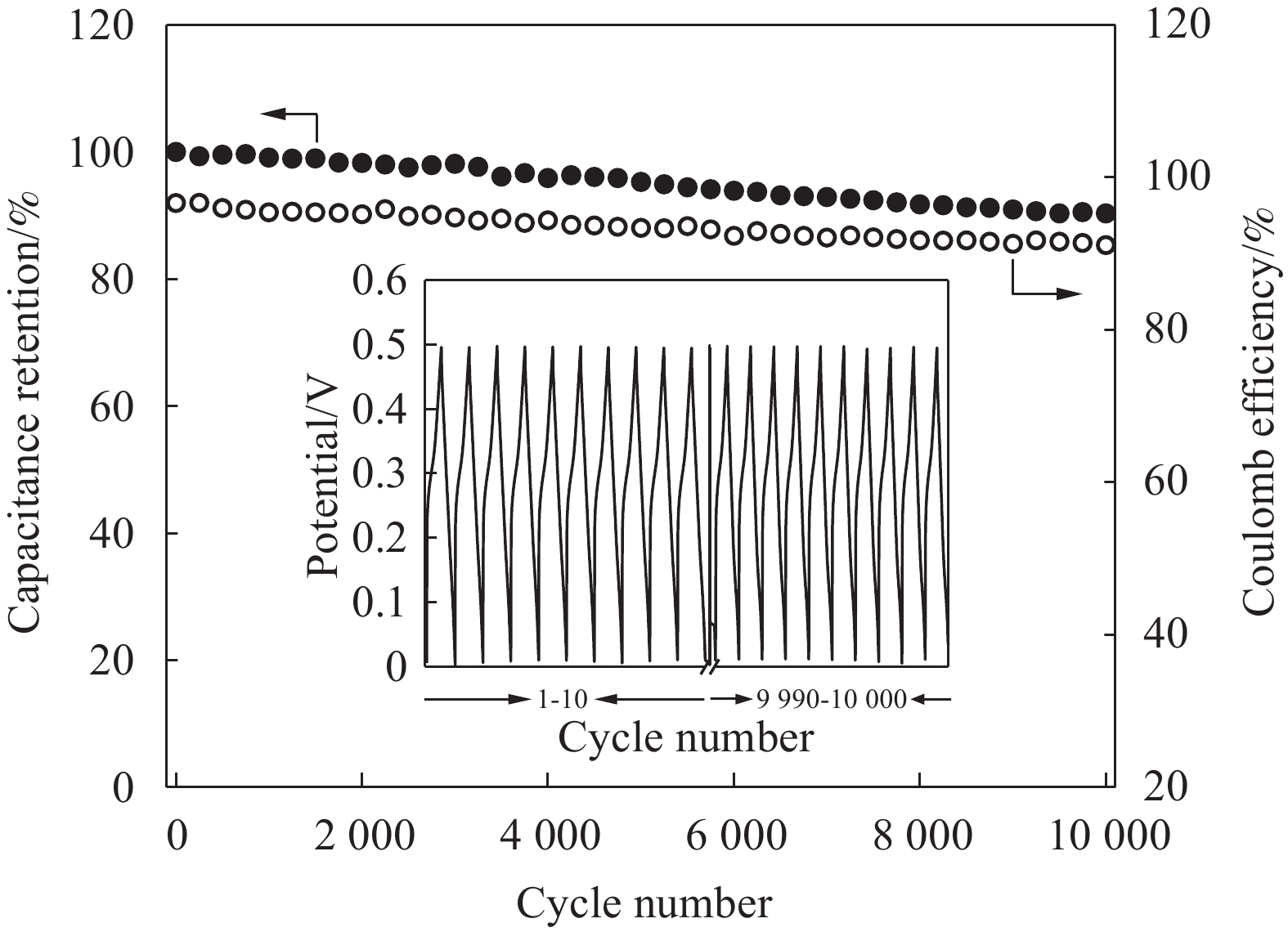
图9是NiCo2S4的循环性能测试结果。电流密度为0.2 A/g时,循环
10000 次,NiCo2S4的放电效率均大于91%,并且放电比容量保持率为90.3%。由CV、GCD和EIS结果可知,NiCo2S4在KOH电解液中充放电时的扩散系数较大,离子迁移阻抗较小。电流密度较低时,NiCo2S4充放电过程极化较小,并且放电效率较高,可逆充放电容量较大。因此,循环10000 次后,比容量保持率可达90.3%。初始库伦效率为96.6%,库伦效率较高。由CV曲线可知,扫描速率较低时,极化较小,因此库伦效率较高。尽管如此,库伦效率并未达到100%。根据EIS结果可知,两电极体系中存在内阻,导致实际输出电压低于理论电压,放出的容量稍有降低。因此,库伦效率并未达到100%。首次放电效率低于100%,导致部分活性物种无法回到立方相NiCo2S4晶格中正常位置,NiCo2S4产生晶格畸变。因此,NiCo2S4的可逆性降低,库伦效率也逐渐降低。随着库伦效率降低,放电容量保持率逐渐降低。3. 结 论
(1)通过水热合成法制备了一种NiCo2S4电极材料。NiCo2S4为球状多晶材料,晶格常数为
0.9387 nm。(2) Ni2+、Ni3+、Co2+和Co3+同时存在于NiCo2S4晶格之中,并且可以通过Ni2+/Ni3+、Co2+/Co3+的可逆转换产生赝电容。
(3)在6 mol/L KOH电解液中,NiCo2S4可以同时产生双电层电容和赝电容,并且赝电容占比39.4%。电流密度为0.2 A/g时,NiCo2S4扣式电池能量密度为14.2 W·h/kg。
-
-
[1] YANG F M, ZHOU X Y, LI X D, et al. Hollow urchin-shaped NCM811 ternary-structure for high rate charge/discharge capability and efficient CO2 adsorption[J]. Journal of Environmental Chemical Engineering, 2023, 11: 109445. DOI: 10.1016/j.jece.2023.109445
[2] YANG F M, LI X D. The preparation of rod-like porous α-Fe2O3 with large interplanar spacing for symmetric supercapacitors[J]. Australian Journal of Chemistry, 2024, 76(11): 774-783.
[3] CHENG F, YANG X P, ZHANG S P, et al. Boosting the supercapacitor performances of activated carbon with carbon nanomaterials[J]. Journal of Power Sources, 2020, 450: 227678. DOI: 10.1016/j.jpowsour.2019.227678
[4] HSU C C, TU Y H, YANG Y H, et al. Improved performance and long-term stability of activated carbon doped with nitrogen for capacitive deionization[J]. Desalination, 2020, 481: 114362. DOI: 10.1016/j.desal.2020.114362
[5] SAHIN O, YARDIM Y, BAYTAR O, et al. Enhanced electrochemical double-layer capacitive performance with CO2 plasma treatment on activated carbon prepared from pyrolysis of pistachio shells[J]. International Journal of Hydrogen Energy, 2020, 45(15): 8843-8852. DOI: 10.1016/j.ijhydene.2020.01.128
[6] ZHAO J, JI G C, LI Y, et al. Preparation of a self-healing polyaniline-based gel and its application as a healable all-in-one capacitor[J]. Chemical Engineering Journal, 2021, 420: 129790. DOI: 10.1016/j.cej.2021.129790
[7] SAHARAN P, SINGH M, GUPTA A, et al. Conducting co-polymer derived N, S co-doped metal-free hierarchical nanoporous carbon for robust electrochemical capacitor[J]. Journal of Energy Storage, 2023, 73: 108928. DOI: 10.1016/j.est.2023.108928
[8] ZHAO J, JI G C, LI Y, et al. Preparation of a self-healing polyaniline-based gel and its application as a healable all-in-one capacitor[J]. Chemical Engineering Journal, 2021, 420: 129790. DOI: 10.1016/j.cej.2021.129790
[9] CHENG W X, FU J M, HU H B, et al. Interlayer structure engineering of MXene-based capacitor-type electrode for hybrid micro-supercapacitor toward battery-level energy density[J]. Advanced Sciences, 2021, 8(16): 2100775.
[10] ANDO Y, OKUBO M, YAMADA A, et al. Capacitive versus pseudocapacitive storage in MXene[J]. Advanced Functional Materials, 2020, 30(47): 2000820. DOI: 10.1002/adfm.202000820
[11] BRADY A, LIANG K, VUONG V Q, et al. Pre-sodiated Ti3C2T x MXene structure and behavior as electrode for sodium-ion capacitors[J]. ACS Nano, 2021, 15: 2994-3003. DOI: 10.1021/acsnano.0c09301
[12] 杨泛明, 贺国文. 颗粒状NiO的制备及其电化学性能和CO2吸附性能[J]. 化工进展, 2023, 42(2): 907-916. YANG Fanming, HE Guowen. Preparation of granular NiO for the electrochemical performance and CO2 adsorption performance[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 907-916(in Chinese).
[13] SETHI M, SHENOY U S, BHAT D K. Hassle-free solvothermal synthesis of NiO nanoflakes for supercapacitor application[J]. Physica B: Physics of Condensed Matter, 2021, 611: 412959. DOI: 10.1016/j.physb.2021.412959
[14] ZHAO J S, TIAN Y, LIU A, et al. The NiO electrode materials in electrochemical capacitor: A review[J]. Materials Science in Semiconductor Processing, 2019, 96: 78-90. DOI: 10.1016/j.mssp.2019.02.024
[15] TAO Y J, WU Y T, CHEN H, et al. Synthesis of amorphous hydroxyl-rich Co3O4 for flexible high-rate supercapacitor[J]. Chemical Engineering Journal, 2020, 396: 125364. DOI: 10.1016/j.cej.2020.125364
[16] ADHIKARI S, SELVARAJ S, JI S H, et al. Encapsulation of Co3O4 nanocone arrays via ultrathin NiO for superior performance asymmetric supercapacitors[J]. Small, 2020, 16(48): 2005414. DOI: 10.1002/smll.202005414
[17] ARUN T, KUMAR T K, UAYABHASKAR R, et al. Nano hexagonal Co3O4 platelets for supercapacitor applications-synthesis and characterization[J]. Materials Research Express, 2019, 6(8): 0850b1.
[18] 邢正伟, 沈鸿烈, 唐群涛, 等. 自支撑多孔硅/ZnO复合材料的制备及其超级电容特性[J]. 复合材料学报, 2016, 33(9): 2082-2087. XING Zhengwei, SHEN Honglie, TANG Quntao, et al. Preparation of freestanding porous silicon/ZnO composites and its supercapacitor property[J]. Acta Materiae Compositae Sinica, 2016, 33(9): 2082-2087(in Chinese).
[19] 胡彬, 张红平, 姜丽丽. 碳化氧化石墨烯/壳聚糖超级电容器电极复合材料的制备及表征[J]. 复合材料学报, 2018, 35(3): 661-667. HU Bin, ZHANG Hongping, JIANG Lili. Preparation of carbonized graphene oxide/chitosan composites and their application as electrode composites for supercapacitors[J]. Acta Materiae Compositae Sinica, 2018, 35(3): 661-667(in Chinese).
[20] LI S L, ZHANG J Q, CHAO H X, et al. High energy density lithium-ion capacitor enabled by nitrogen-doped amorphous carbon linked hierarchically porous Co3O4 nanofibers anode and porous carbon polyhedron cathode[J]. Journal of Alloys and Compounds, 2022, 918: 165726. DOI: 10.1016/j.jallcom.2022.165726
[21] WANG Q, QIN B, ZHANG A, et al. Synthesis of N-doped carbon nanosheets with controllable porosity derived from bio-oil for high-performance supercapacitors[J]. Journal of Materials Chemistry A, 2018, 6: 19653-19663.
[22] BAGUS P S, NELIN C J, RICHARD C, et al. Main and satellite features in the Ni2p XPS of NiO[J]. Inorganic Chemistry, 2022, 61: 18077-18094. DOI: 10.1021/acs.inorgchem.2c02549
[23] ZENG H, OUBLA M, ZHONG X, et al. Rational defect and anion chemistries in Co3O4 for enhanced oxygen evolution reaction[J]. Applied Catalysis B: Environmental, 2021, 281: 119535.
[24] WANG F P, LI G F, ZHOU Q Q, et al. One-step hydrothermal synthesis of sandwich-type NiCo2S4@reduced graphene oxide composite as active electrode material for supercapacitors[J]. Applied Surface Science, 2017, 425: 180-187. DOI: 10.1016/j.apsusc.2017.07.016
[25] KALPANA S, BHAT V S, HEGDE G, et al. Exploring the influence of KOH electrolyte concentration on the electrochemical properties of Co3O4-GO nanocomposite[J]. Journal of Physics and Chemistry of Solids, 2024, 190: 112019. DOI: 10.1016/j.jpcs.2024.112019
[26] FU Y Q, WEI Q L, ZHANG G X, et al. High-performance reversible aqueous Zn-ion battery based on porous MnO x nanorods coated by MOF-derived N-doped carbon[J]. Advanced Energy Materials, 2018, 8(26): 1801445. DOI: 10.1002/aenm.201801445
-
其他相关附件
-
目的
超级电容器是重要的能量储备装置之一,已经被广泛应用于现代工业领域之中。超级电容器的性能与电极材料有较大关系。电极材料之中,多孔氧化物因具有制备方法简单、制备周期较短、放电比容量和能量密度较高等优点备受青睐,如氧化镍(NiO)、四氧化三钴(CoO)等。NiO中的Ni和CoO中的Co均可与OH发生反应,产生赝电容。Ni和Co存在于多孔NiCoS之中时,NiCoS可以同时产生双电层电容和多种法拉第反应,进一步提高充放电性能。然而,关于双电层电容和赝电容在多孔材料中的贡献率的报道较少。对两者的贡献率进行区分,有助于分析电极材料的开发程度,促进研究者对电极材料进行更深入的开发。
方法结合X射线衍射(XRD)和X射线光电子能谱(XPS)分析NiCoS的物相和元素价态。利用扫描电子显微镜(SEM)和透射电子显微镜(TEM)分析NiCoS的形貌和精细结构。结合XPS和Mapping技术分析NiCoS的元素组成。通过N吸附-脱附技术分析NiCoS的比表面积和孔径分布。以两片质量相等的NiCoS极片为正、负极、6 mol/L KOH溶液为电解液组装对称型扣式电池,探究NiCoS的电化学行为,并分析双电层电容和赝电容对NiCoS的放电比容量的贡献率。
结果根据材料表征
结果(1)NiCoS的XRD衍射峰与PDF#20-0782的信息一致,NiCoS制备成功。NiCoS为面心立方晶体,晶格常数为0.9387 nm。(2)NiCoS为球状多晶体,无明显团聚,并且Ni和Co以Ni、Ni、Co和Co形式存在。(3)NiCoS中,Ni、Co和S的比例为1 : 2.1 : 4.2。(4)NiCoS的孔体积为0.402 cm/g,并且介孔比例为90.6%,孔径主要集中于2.1 nm ~ 21.3 nm之间。由充放电结果可知:(1)随着扫描速度增大,氧化电位逐渐增大,还原电位逐渐减小,氧化还原电位差值逐渐增大,大倍率条件下充放电可逆性降低。(2)电流密度增大,极化增强,充放电性能逐渐降低。(3)电流密度为0.2 A/g时,放电比容量为409.7 F/g,其中:双电层电容占比为60.6%,赝电容占比为39.4%。电流密度提高至10 A/g时,放电比容量保持率为20.9%。(4)NiCoS在KOH电解液中具有较大的扩散系数,为1.1×10 m/s,OH可以在材料体相中快速扩散。(5)NiCoS在KOH电解液中充放电的弛豫时间常数为0.21 s。弛豫时间常数较小,可逆性较好。(6)0.2 A/g时,循环10000次,NiCoS的放电效率均大于91%,放电比容量保持率为90.3%。
结论(1)通过水热合成法制备了一种NiCoS电极材料。NiCoS为球状多晶材料,晶格常数为0.9387 nm。(2)Ni、Ni、Co和Co同时存在于NiCoS晶格之中,并且可以通过Ni/Ni、Co/Co的可逆转换产生赝电容。(3)在6 M KOH电解液中,NiCoS可以同时产生双电层电容和赝电容,并且赝电容占比39.4%。电流密度为0.2 A/g时,NiCoS扣式电池能量密度为14.2 Wh/kg。
-
NiO和Co3O4在KOH溶液中均具有较好的充放电性能,Ni和Co两种元素均可与OH-反应并产生赝电容。NiCo2S4中含有Ni和Co两种过渡金属元素,并且两种元素可能均显示多种价态。不同价态的Ni和Co同时存在于NiCo2S4之中,可以促使充放电顺利进行,获得较好的电化学性能。多孔氧化物因具有制备方法简单、制备周期较短、放电比容量和能量密度较高等优点备受青睐。然而,关于双电层电容和赝电容在多孔氧化物中的贡献的报道较少。对两者的贡献进行区分,有助于分析电极材料的开发程度,促进研究者对电极材料进行更深入的开发。
本文以乙酸钴为Co源、乙酸镍为Ni源、硫代乙酰胺为沉淀剂,通过水热处理制备NiCo2S4,并对其在KOH电解液中的充放电行为进行探究。结果表明,NiCo2S4为立方相球状多晶体,并且Ni和Co以Ni2+、Ni3+、Co2+、Co3+的形式存在于NiCo2S4的晶格之中。NiCo2S4中,Ni、Co和S的比例为1 : 2.1 : 4.2。NiCo2S4的孔体积为0.402 cm3/g,并且介孔的比例为90.6%。NiCo2S4在KOH溶液中同时产生双电层电容和赝电容,并且两者所占的比例分别为60.6%、39.4%。电流密度为0.2 A/g时,放电比容量为409.7 F/g,能量密度为14.2 W·h/kg。循环10000次,容量保持率为90.3%。
NiCo2S4在KOH溶液中的充放电性能
A: 放电效率与放电比容量;B: 放电比容量与放电时间关系曲线 




 下载:
下载: