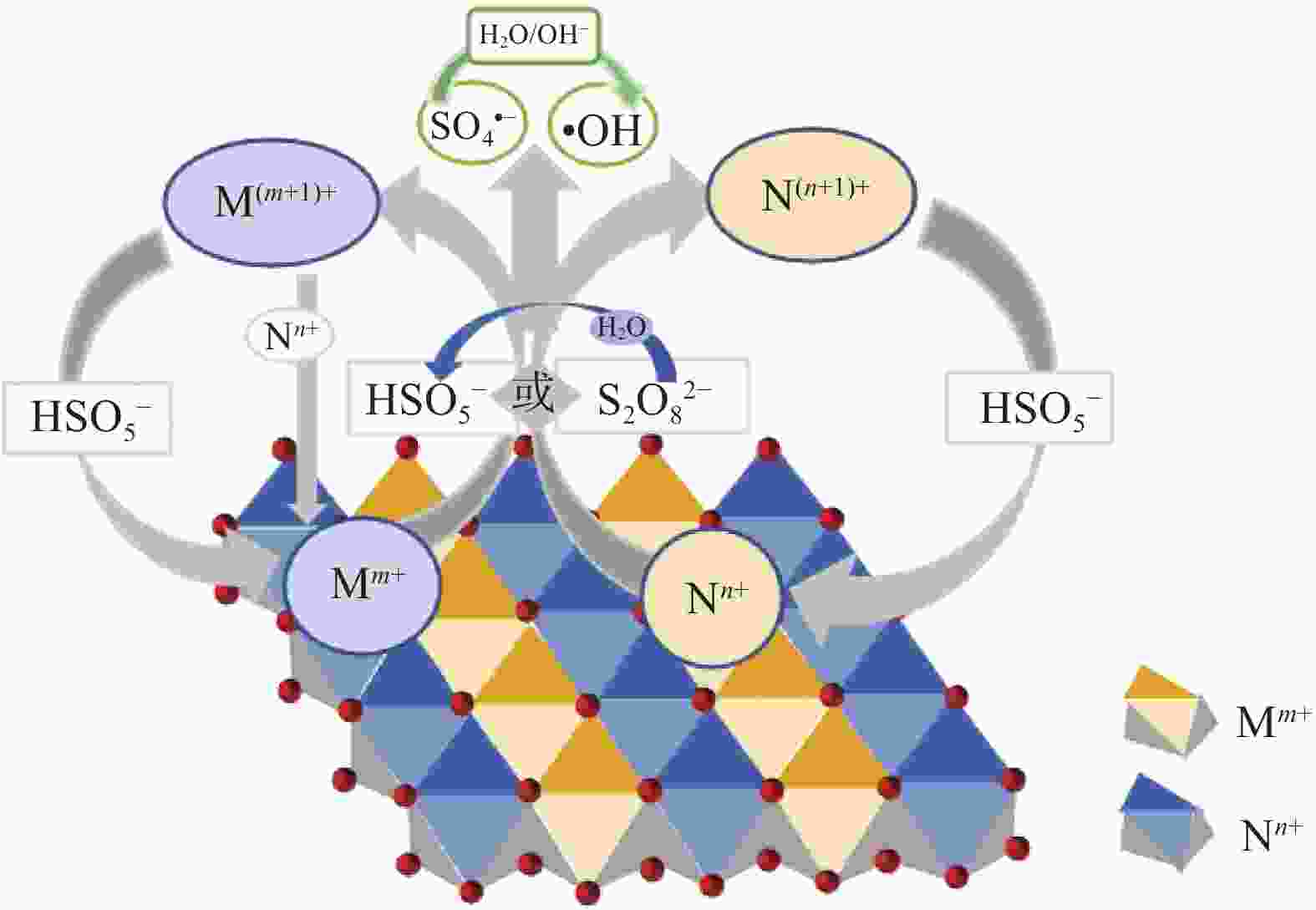
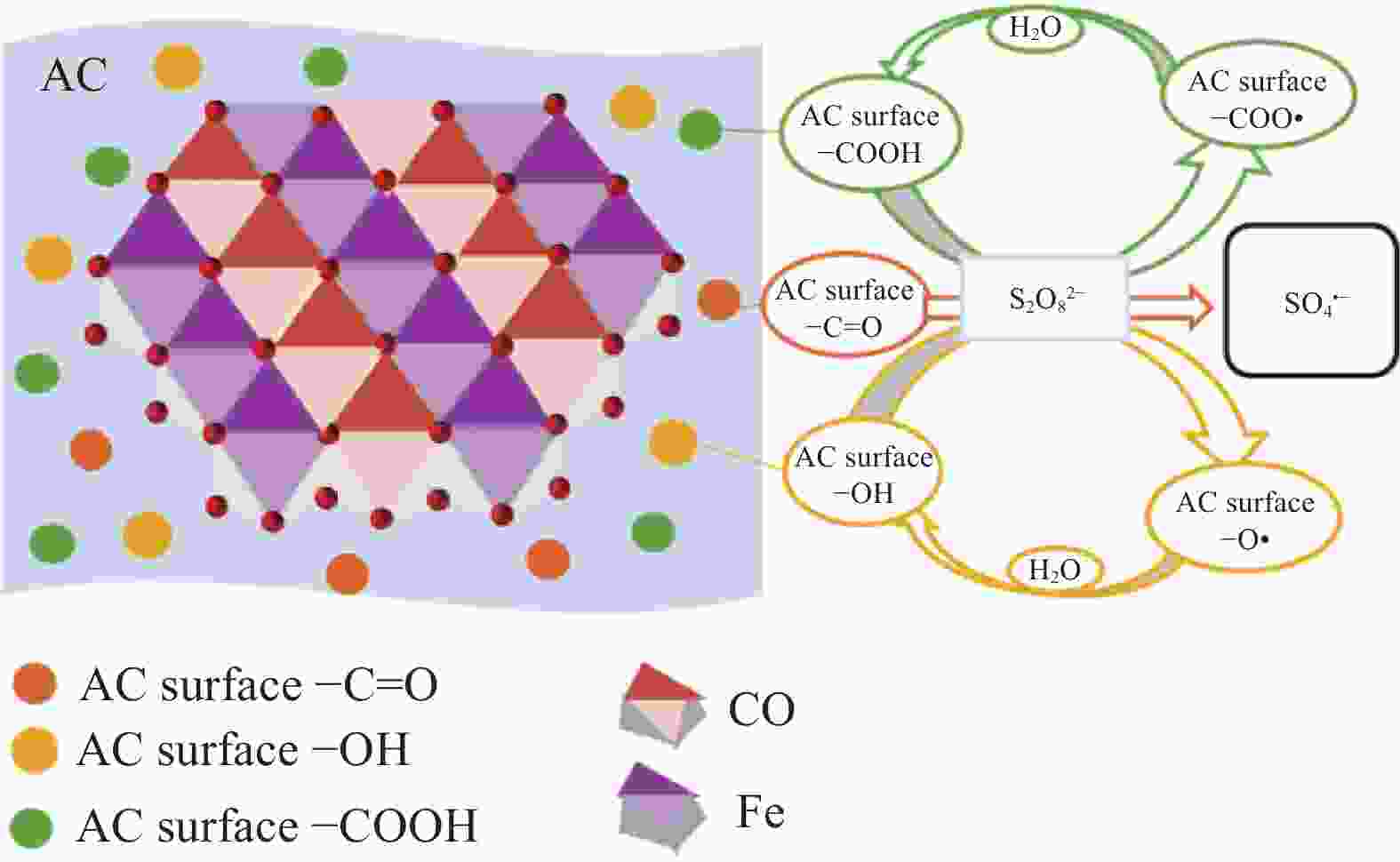
Layered double hydroxides mediated persulfate activation for organic pollutants degradation: A review
-
摘要: 近代工业的快速发展造成大量难降解的新型有机污染物进入水体,亟需经济、高效的难降解有机污染物污染控制和削减技术。近年来,基于硫酸根自由基(SO4•–)的高级氧化技术(SR-AOPs)具有强氧化性、宽pH耐受性以及方便操作性等优势而备受关注。不同种类的金属氧/硫化物、碳基材料、金属-非金属复合材料以及有机金属材料等被用来活化过硫酸盐产生活性氧,从而实现对有机污染物的氧化降解和进一步矿化。其中,层状双金属氢氧化物(Layered double hydroxides, LDHs)因其独特的层状结构优势、阴离子可交换性和客体分子可调节性,在活化过硫酸盐方面表现出优良的反应活性和催化优势。本论文从催化剂类型、催化性能与机制以及降解体系影响因素等方面,综述了LDHs及其复合材料作为非均相催化剂活化过硫酸盐的研究现状,并对催化体系持续改进以及未来发展提出相关展望。
-
关键词:
- 层状双金属氢氧化物(LDHs) /
- 非均相活化 /
- 过硫酸盐 /
- 硫酸根自由基(SO4•–) /
- 有机污染物
Abstract: The rapid development of modern industry has caused a large number of refractory organic pollutants to enter the water body, there is an urgent need for economic and efficient pollution control and reduction technologies for refractory organic pollutants. In recent years, advanced oxidation technologies (SR-AOPs) based on sulfate radicals (SO4•–) have attracted much attention because of their strong oxidizing properties, wide pH tolerance, and ease of operation. Different types of metal oxygen/sulfide, carbon-based materials, metal-non-metal composite materials and organic metal materials are used to activate persulfate to generate active oxygen, thereby achieving oxidative degradation and further mineralization of organic pollutants. Among them, layered double hydroxides (LDHs) show excellent reactivity and catalytic advantages in activating persulfate due to their unique layered structure advantages, anion exchangeability and guest molecule adjustability. This article reviews the current research status of LDHs and their composites as heterogeneous catalysts to activate persulfate from the aspects of catalyst type, catalytic performance and mechanism, and degradation system influencing factors, and proposes relevant prospects for the continuous improvement of the catalytic system and future development. -
表 1 不同单一型LDHs催化剂非均相活化过硫酸盐体系去除有机污染物效能对比
Table 1. Comparison of the removal efficiency of different single-type LDHs catalysts in heterogeneously activated persulfate systems
Catalyst Contaminants Reaction condition Removal efficiency TOC removal Dominant
ROSIon
leachingRef. FeCo-LDH RhB c(RhB)=20 mg/L,
c(Catalyst)=0.2 g/L,
c(PMS)= 0.15 g/L,
pH=3.42, T=25℃10 min,
100%— SO4•− — [36] Fe2Co-LDH BPA c(BPA)=30 mg/L,
c(Catalyst)=0.3 g/L,
c(PS)=4 mmol/L,
pH=7, T=25℃60 min,
99.38%58.99% SO4•−, •OH — [37] MgMnCo-LDH SMX c(SMX)=0.05 mmol/L,
c(Catalyst)=0.1 g/L,
c(PMS)=0.40 mmol/L,
pH=5.010 min,
99%67.8% SO4•−, 1O2 — [38] CuFe-LDH MV c(Catalyst)=0.2 g/L,
c(MV)=20 mg/L
c(PS)=0.2 g/L,
Visible light>420 nm,
T=25℃18 min,
100%— SO4•−, •OH c(Fe)≤0.1 mg/L [46] CoFeLa-LDH TC c(TC)=30 mg/L,
c(Catalyst)= 0.05 g/L,
c(PMS)=1.0 mmol/L,
pH=5.4, T=25℃10 min,
81.6%-90.1%— SO4•−, •OH,
1O2— [47] CoMn-LDH AOG c(AOG)=0.05 g/L,
c(Catalyst)=0.025 g/L,
c(PMS)=0.1 g/L,
pH=6.87, T=25℃120 s,
99.8%50.5%
(30 min)SO4•− c(Co)=0.53 mg/L, c(Mn)=0.16 mg/L (After 4 cycles) [48] FeMn-LDH ODA c(Catalyst)=0.4 g/L,
c(ODA)=0.01 g/L,
c(PMS)=0.4 g/L,
pH=1.8, T=25℃25 min,
85%— SO4•−, •OH c(Mn)=772 µg/L, c(Fe)=13 µg/L [49] CoFeNi-LDH CR/RhB c(Catalyst)=0.2 g/L,
c(CR)=20 mg/L or
c(RhB)=20 mg/L,
c(PMS)=0.15 m g/L,
pH=7, T=25℃6 min(CR), 100%;
10 min(RhB), 100%— SO4•− c(Co)=0.68 mg/L [50] CuCoFe-LDH NB c(Catalyst)=0.1 g/L,
c(NB)=2.0 mg/L,
c(PMS)=0.5 mmol/L,
pH=6.5, T=25℃6 min,
>99%— •OH c(Co)=34 μg/L,
c(Cu)=92 μg/L[51] MgCuFe-LDH Acetaminophen c(Catalyst)=0.3 g/L,
c(Acetaminophen)=5 g/L,
c(PMS)=0.5 mmol/L,
pH=6.0, T=25℃20 min,
93%64.5%
(2 h)SO4•−, •OH c(Cu)=0.77 mg/L,
c(Mg)=4.98 mg/L
(30 min)[52] Notes: RhB—Rhodamine B; BPA—Bisphenol A; MV—Methyl violet; TC—Tetracycline; AOG—Acid orange G; ODA—Octadecylamine; CR—Congo red; NB—Nitrobenzene; PMS—Peroxymonosulfate; PS—Persulfate; SMX—Sulfamethoxazole; TOC—Total organic carbon;ROS—Reactive oxygen species. 表 2 不同单一型层状复合氧化物 (LDOs) 催化剂非均相活化过硫酸盐体系去除有机污染物效能对比
Table 2. Comparison of the removal efficiency of different single-type layered double oxides (LDOs) catalysts in heterogeneously activated persulfate
Catalyst Contaminants Reaction condition Removal efficiency TOC removal Dominant ROS Ion leaching Ref. CuMgAl-LDO SMD c(SMD)=10 mg/L,
c(Catalyst)=0.3 g/L,
c(PS)=0.7 mmol/L,
pH=6.4, T=25℃120 min,
99.49%— SO4•− c(Cu)=0.89 mg/L
(After 5 cycles)[39] MgMn-LDO TC c(TC)=44.4 mg/L,
c(Catalyst)=0.1 g/L,
c(PMS)=0.50 mmol/L,
pH=5.020 min,
97.1%47% 1O2 — [61] CuCo-LDO LOM c(LOM)=10 mg/L,
c(Catalyst)=0.04 g/L,
c(PMS)=0.15 g/L,
pH=6.67, T=25℃30 min,
96.19%— SO4•−, 1O2,
•OH— [62] Co2FeAl-LDO CBZ c(CBZ)=10 mg/L,
c(Catalyst)=60 mg/L,
c(PMS)=0.2 mmol/L,
pH=6.130 min,
>99%30% SO4•− c(Co)=0.4 mg/L;
c(Fe)≈0
(After 5 cycles)[63] CoMgFe-LDO CBZ c(CBZ)=5 mg/L,
c(Catalyst)=20 mg/L,
c(PMS)=0.2 mmol/L,
pH=5.8, T=30℃20 min,
100%21.3% SO4•− — [64] CoCuAl-LDO AO7 c(AO7)=20 mg/L,
c(Catalyst)=0.1 g/L,
c(PMS)=0.1 g/L,
pH=6.7, T=25℃30 min,
>99%— SO4•− c(Co)<0.03 mg/L; c(Cu)<0.3 mg/L [65] CuMgFe-LDO Phenol c(Phenol)=0.1 mmol/L,
c(Catalyst)=0.5 g/L,
c(PS)=0.5 mmol/L,
pH=6.4, T=25℃30 min,
59.3%— SO4•−, •OH c(Cu)=0.19 mg/L
(After 3 cycles)[66] CoMgAl-LDO ATZ c(ATZ)=10 mg/L, c(Catalyst)=75 mg/L,
c(PMS)=0.4 mmol/L,
pH=6.3, T=30℃15 min,
98.7%26.5% SO4•− c(Cu)=0.35 mg/L
(After 4 cycles)[67] Notes: SMD—Sulfa p-methoxypyrimidine; LOM—Lomefloxacin; CBZ—Carbamazepine; AO7—Acid orange 7; ATZ—Atrazine; LDO—Layered double oxides. 表 3 不同碳复合型LDHs催化剂非均相活化过硫酸盐体系去除有机污染物效能对比
Table 3. Comparison of the removal efficiency of different carbon composite type LDHs catalysts in heterogeneously activated persulfate
Catalyst Contaminants Reaction condition Removal
efficiencyTOC removal Dominant ROS Ion leaching Ref. AC@CoFe-LDH LMF c(Catalyst)=0.2 g/L,
c(Catalyst)=0.2 g/L,
c(PS)=1.0 g/L,
pH=4.5, T=25℃60 min,
93.2%— SO4•− — [40] LDH-GO GAT c(GAT)=38 mg/L,
c(Catalyst)=40 mg/L,
c(PMS)=400 mg/L,
pH=7.0, T=25℃45 min,
100%5 h,
55.5%SO4•− — [76] DOM-FeAl-LDH BPA c(BPA)=20 mg/L,
c(Catalyst)=200 mg/L,
c(PMS)=200 mg/L, pH=5.58, T=25℃6 min,
93%— •OH c(Fe)=50 μmol/L [77] LDH@PVDF SMX c(SMX)=10 mg/L,
c(Catalyst)=17.5 mg/L,
c(PMS)= 25 mg/L,
T=25℃60 min,
92.8%— — c(Co)=0.044 mg/L;
c(Cu)=0.026 mg/L[78] CuCo-LDH@PAN SMX c(SMX)=10 mg/L,
c(Catalyst)=60 m/L,
c(PMS)=0.24 mmol/L,
pH=5.77, T=25℃5 min,
83.9%59.40% SO4•−,
•OHc(Co)=0.014 mg/L; c(Cu)=0.010 mg/L [79] CMK-LDH SMX c(SMX)=25 mg/L,
c(Catalyst)=0.15 g/L,
c(PS)=0.5 g/L,
pH=5, T=25℃150 min,
84.9%— •OH — [80] Co7Fe3/CoFe2O4@C RhB;
p-Nitrophenolc(Pollutant)=50 mg/L,
c(Catalyst)=0.1 g/L,
c(PMS)=0.5 g/L,
pH=7, T=25℃,RhB: 10 min,
100%;
p-Nitropheno:
30 min, 99%p-Nitropheno:
180 min, 72%SO4•−,
•OHc(Fe)=0.068 mg/L;
c(Co)=0.0592 mg/L[81] CoAl-LDH/g-C3N4 SDZ c(SDZ)=10 μmol/L,
c(Catalyst)=0.1 g/L,
c(PMS)=0.5 mmol/L,
pH=6.015 min,
87.1%— h+ c(Co)≈0 [82] Notes: AC—Activate carbon; LMF—Lomefloxacin; GO—Graphene oxide; DOM—Dissolved organic matter; BPA—Bisphenol A; PVDF—Polyvinylidene fluoride; PAN—Polyacrylonitrile; CMK—Mesoporous carbon; GAT—Gatifloxacin; SDZ—Sulfadiazine. -
[1] CHEN D F, SHENY, WANG S J, et al. Efficient removal of various coexisting organic pollutants in water based on β-cyclodextrin polymer modified flower-like Fe3O4 particles[J]. Journal of Colloid and Interface Science,2021,589:217-228. doi: 10.1016/j.jcis.2020.12.109 [2] NATARAJAN T S, THOMAS M, NATARAJAN K, et al. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye[J]. Chemical Engineering Journal,2011,169(1/3):126-134. doi: 10.1016/j.cej.2011.02.066 [3] GOEL M, DAS A. A review on treatment of pharmaceuti-cals and personal care products (PPCPs) in water and waste water[J]. Pergamon,1998,36(10):2149-2173. [4] LIU C Y, JIANG X, MA Y C, et al. Pollutant and soil types influence effectiveness of soil-applied absorbents in reducing rice plant uptake of persistent organic pollutants[J]. Pedosphere,2017,27(3):537-547. doi: 10.1016/S1002-0160(17)60349-7 [5] GIANNAKIS S, LIN K Y, GHANBARI F. A review of the recent advances on the treatment of industrial wastewaters by sulfate radical-based advanced oxidation processes (SR-AOPs)[J]. Chemical Engineering Journal,2020,406:127083. [6] HU P D, LONG M C. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications[J]. Applied Catalysis B: Environmental,2016,181:103-117. doi: 10.1016/j.apcatb.2015.07.024 [7] LUCA A D, DANTAS R F, ESPLUGAS S. Assessment of iron chelates efficiency for photo-Fenton at neutral pH[J]. Water Research,2014,61(15):232-242. [8] DHAKSHINAMOORTHY A, NAVALON S, ALVARO M, et al. Metal nanoparticles as heterogeneous Fenton catalysts[J]. Chemsuschem,2012,5(1):46-64. doi: 10.1002/cssc.201100517 [9] OH W D, DONG Z L, LIM T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects[J]. Applied Catalysis B: Environmental,2016,194:169-201. doi: 10.1016/j.apcatb.2016.04.003 [10] LI H C, SHAN C, PAN B C. Fe(III)-doped g-C3N4 mediated activation of peroxymonosulfate for selective degradation of phenolic compounds via high valent iron-oxo species[J]. Environmental Science and Technology,2018,52:2197-2205. doi: 10.1021/acs.est.7b05563 [11] QIN X, FANG S W, ZHAO L, et al. Cobalt super-microparticles anchored on nitrogen-doped graphene for aniline oxidation based on sulfate radicals[J]. Science of the Total Environment,2017(601/602):99-108. [12] ANTONIOU M G, CRUZ A A, DIONYSIOU D. Degradation of microcystin-LR using sulfate radicals generated though photolysis thermolysis and e-transfer mechanisms[J]. Applied Catalysis B: Environmental,2010,96(3/4):290-298. doi: 10.1016/j.apcatb.2010.02.013 [13] PENG Y T, TANG H M, YAO B, et al. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of pollutants: Review[J]. Chemical Engi-neering Journal,2021,414:128800. doi: 10.1016/j.cej.2021.128800 [14] LIU C G, WU B, CHEN X E. Sulfate radical-based oxidation for sludge treatment: A review[J]. Chemical Engineering Journal,2017,335:865-875. [15] WACLAWEK S, LUTZE H V, GRUBEL K, et al. Chemistry of persulfates in water and wastewater treatment: A review[J]. Chemical Engineering Journal,2017(330):44-62. [16] LIU C W, MA J, JIANG J, et al. Simulation and comparative study on the oxidation kinetics of atrazine by UV/H2O2[J]. Water Research,2015,80:99-108. doi: 10.1016/j.watres.2015.05.019 [17] KANAYO L O, QIAO M, ZHAO X, et al. Oxidative degradation of bisphenol A in aqueous solution using cobalt ion-activated peroxymonosulfate[J]. Journal of Molecular Liquids,2020,313:113569. doi: 10.1016/j.molliq.2020.113569 [18] LI X N, HUANG X, XI S B, et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis[J]. Journal of the American Chemical Society,2018,140:12469-12475. doi: 10.1021/jacs.8b05992 [19] AHAMAD T, NAUSHAD M, ALZAHARANI M, et al. Photocatalytic degradation of bisphenol-A with g-C3N4/MoS2-PANI nanocomposite: Kinetics, main active species, intermediates and pathways[J]. Journal of Molecular Liquids,2020,311:113339. doi: 10.1016/j.molliq.2020.113339 [20] 武利园, 郭朋朋, 李海燕, 等. 二硫化钼催化活化单过硫酸盐降解有机污染物研究现状[J]. 复合材料学报, 2021, 38(5):1348-1357.WU Liyuan, GUO Pengpeng, LI Haiyan, et al. MoS2 catalyzed peroxymonosulfate activation for organic pol lutants degradation: A review[J]. Acta Materiae Compositae Sinica,2021,38(5):1348-1357(in Chinese). [21] YANG Q, CHOI H, AL-ABED S R, et al. Iron-cobalt mixed oxide nanocatalysts: Heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications[J]. Applied Catalysis B,2009,88(3/4):462-469. [22] YANG Q, CHOI H, CHEN Y, et al. Heterogeneous activation of peroxymonosulfate by supported cobaltcatalysts for the degradation of 2, 4-dichlorophenol in water: The effect of support, cobalt precursor, and UV radiation[J]. Applied Catalysis B,2008,77(3/4):300-307. [23] LONG X X, YANG S J, QIU X J, et al. Heterogeneous activation of peroxymonosulfate for bisphenol A degradation using CoFe2O4 derived by hybrid cobalt-ion hexacyanoferrate nanoparticles[J]. Chemical Engineering Journal,2021,404(39):127052. [24] YANG Y, LI Y L, HONG P D, et al. Surface-active MnFeO@C cubes as enhanced peroxymonosulfate activators for efficient degradation of bisphenol A[J]. Applied Surface Science,2020,538:148008. [25] WANG Y, GAO C Y, ZHANG Y Z, et al. Bimetal-organic framework derived CoFe/NC porous hybrid nanorods as high-performance persulfate activators for bisphenol A degradation[J]. Chemical Engineering Journal,2021,404:127800. [26] MA R Z, LIU Z P, TAKADA K, et al. Synthesis and exfoliation of Co2+-Fe3+ layered double hydroxides: An innova-tive topochemical approach[J]. Journal of the American Chemical Society,2007,129(16):5257. doi: 10.1021/ja0693035 [27] YANG Q, ZHONG Y, LI X M, et al. Adsorption-coupled reduction of bromate by Fe(II)-Al(III) layered double hydroxide in fixed-bed column: Experimental and breakthrough curves analysis[J]. Journal of Industrial & Engi-neering Chemistry,2015,28:54-59. [28] HAN X, WANG S B, HANG H W, et al. Hydroxyl radicals and sulfate radicals synergistically boosting the photocatalytic and mineralization ability of 1D-2D Bi5O7I/NiFe-LDH heterojunction[J]. Applied Surface Science,2020,540:148237. doi: 10.1016/j.apsusc.2020.148237 [29] YANG Q, WANG S N, CHEN F, et al. Enhanced visible-light-driven photocatalytic removal of refractory pollutants by Zn/Fe mixed metal oxide derived from layered double hydroxide[J]. Catalysis Communications,2017,99:15-19. [30] NAYAK S, MOHAPATRA L, PARIDA K. Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction[J]. Journal of Materials Chemistry A,2015,3:18622-18635. doi: 10.1039/C5TA05002B [31] YU L, ZHAO H Q, SUN J Y, et al. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting[J]. Energy & Environmental Science,2017,10(8):1820-1827. [32] PAN S, LI B, YU J, et al. Composition controllable fabrication of ultrathin 2D CoMn layered double hydroxides for highly efficient electrocatalytic oxygen evolution[J]. Applied Surface Science,2021,539:148305. doi: 10.1016/j.apsusc.2020.148305 [33] ZOU Y D, WANG X X, WU F, et al. Controllable synthesis of Ca-Mg-Al layered double hydroxides and calcined layered double oxides for the efficient removal of U(VI) from wastewater solutions[J]. Sustainable Chemistry & Engi-neering,2016,5(1):1173-1185. [34] ZHANG M L, YAO Q F, LIU C, et al. Layered double hydro-xide-carbon dot composite: High-performance adsorbent for removal of anionic organic dye[J]. ACS Applied Materials& Interfaces,2014,6(22):20225-20233. [35] HOU T L, YAN L G, LI J, et al. Adsorption performance and mechanistic study of heavy metals by facile synthesized magnetic layered double oxide/carbon composite from spent adsorbent[J]. Chemical Engineering Journal,2019,384:123331. [36] GONG C, CHEN F, YANG Q, et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B[J]. Chemical Engineering Journal,2017,321:222-232. doi: 10.1016/j.cej.2017.03.117 [37] MA Y H, CHEN F, YANG Q, et al. Sulfate radical induced degradation of methyl violet azo dye with CuFe layered doubled hydroxide as heterogeneous photoactivator of persulfate[J]. Journal of Environmental Management, 2018, 227(1): 406-414. [38] LI X G, HOU T L, YAN L G, et al. Efficient degradation of tetracycline by CoFeLa-layered double oxides catalyzed peroxymonosulfate: Synergistic effect of radical and nonradical pathways[J]. Journal of Hazardous Materials, 2020, 398: 122884. [39] ZHANG H M, JIA Q Z, YAN F Y, et al. Heterogeneous activation of persulfate by CuMgAl layered double oxide for catalytic degradation of sulfameter[J]. Green Energy & Environment, 2022, 1(7): 105-115 [40] MA Q L, NENGZI L C, LI B, et al. Heterogeneously catalyzed persulfate with activated carbon coated with CoFe layered double hydroxide (AC@CoFe-LDH) for the degradation of lomefloxacin[J]. Separation and Purification Technology, 2020, 235: 116204. [41] ZENG H X, DENG L, ZHANG H J, et al. Development of oxygen vacancies enriched CoAl hydroxide@hydroxysulfide hollow flowers for peroxymonosulfate activation: A highly efficient singlet oxygen-dominated oxidation process for sulfamethoxazole degradation[J]. Journal of Hazardous Materials, 2020, 400: 123297. [42] ANIPSITAKIS G P, DIONYSIOU D D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology, 2004, 38(13): 3705-3712. [43] VIGNESHWARAN S, JUNC B M, PRABHUB S M, et al. Enhanced sonophotocatalytic degradation of bisphenol A using bimetal sulfide-intercalated MXenes, 2D/2D nanocomposite[J]. Separation and Purification Technology, 2020, 250(1): 117-178. [44] HU L M, ZHANG G S, LIU M, et al. Application of nickel foam-supported Co3O4-Bi2O3 as a heterogeneous catalyst for BPA removal by peroxymonosulfate activation[J]. Science of the Total Environment, 2019, 647: 352-361. [45] REN Y M, LIN L Q, MA J, et al. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M = Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water[J]. Applied Catalysis B: Environmental, 2015, 165: 572-578. [46] WU L Y, HONG J M, ZHANG Q, et al. Deciphering highly resistant characteristics to different pHs of oxygen vacancy-rich Fe2Co1-LDH/PS system for bisphenol A degradation[J]. Chemical Engineering Journal, 2019, 385: 123620. [47] ZHAO X F, NIU C G, ZHANG L, et al. Co-Mn layered double hydroxide as an effective heterogeneous catalyst for degradation of organic dyes by activation of peroxymonosulfate[J]. Chemosphere,2018,204:11-21. doi: 10.1016/j.chemosphere.2018.04.023 [48] CHEN G, NENGZI L C, LI B, et al. Octadecylamine degradation through catalytic activation of peroxymonosulfate by FeMn layered double hydroxide[J]. Science of the Total Environment,2019,695:133963. doi: 10.1016/j.scitotenv.2019.133963 [49] ZENG H X, ZHANG W Q, DENG L, et al. Degradation of dyes by peroxymonosulfate activated by ternary CoFeNi-layered double hydroxide: Catalytic performance, mechanism and kinetic modeling[J]. Journal of Colloid and Interface Science, 2018, (515): 92-100. [50] LU H T, SUI M H, YUAN B J, et al. Efficient degradation of nitrobenzene by Cu-Co-Fe-LDH catalyzed peroxymonosulfate to produce hydroxyl radicals[J]. Chemical Engi-neering Journal,2019,357:140-149. doi: 10.1016/j.cej.2018.09.111 [51] ZHU J Y, ZHU Z L, ZHANG H, et al. Efficient degradation of organic pollutants by peroxymonosulfate activated with MgCuFe-layered double hydroxide[J]. Royal Society of Chemistry,2019,9:2284-2291. [52] CHEN M Q, HUANG Z Y, LIANG S L, et al. Immobilized Co2+ and Cu2+ induced structural change of layered double hydroxide for efficient heterogeneous degradation of antibiotic[J]. Journal of Hazardous Materials, 2020, 403: 123554. [53] ZHANG H, SONG Y, NENGZI L C, et al. Activation of persulfate by a novel magnetic CuFe2O4/Bi2O3 composite for lomefloxacin degradation[J]. Chemical Engineering Journal, 2019, 379: 122362. [54] TAN C, GAO N, DENG Y, et al. Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate[J]. Journal of Hazardous Materials, 2014(276): 452-460. [55] TU J, PENG X, WANG S, et al. Effective capture of aqueous uranium from saline lake with magnesium-based binary and ternary layered double hydroxides[J]. Science of the Total Environment, 2019, 677(10): 556-563. [56] ABASI C Y, EJIDIKE I P, DIKIO E D. Synthesis, characterisation of ternary layered double hydroxides (LDH) for sorption kinetics and thermodynamics of Cd2+[J]. International Journal of Environmental Studies, 2018, 76(3): 441–455. [57] SOLDATOVA A V, BALAKRISHNAN G, OYERINDE O F, et al. Biogenic and synthetic MnO2 nanoparticles: Size and growth probed with absorption and raman spectroscopies and dynamic light scattering[J]. Environmental Science & Technology, 2019, 53(8): 4185-4197. [58] 罗青松, 李雷, 王作新, 等. 镁铝水滑石层板与层间阴离子相互作用的理论研究[J]. 无机化学学报, 2001, 17(6): 835-842.LUO Qingsong, LI Lei, WANG Zuoxin, et al. Theoretical study of the layer-anion interactions in magnesium aluminum layered double hydroxides[J]. Chinese Journal of Inorganic Chemistry, 2001,17(6): 835-842(in Chinese). [59] 潘国样, 倪晳明, 李小年. 类水滑石主体层板与客体CO32-、H2O间的超分子作用[J]. 物理化学学报, 2007, 23(8): 1195-1200.PAN Guoxiang, NI Ximing, LI Xiaonian. Supra-molecular interaction between host layer and guest CO32-, H2O of layered double hydroxides[J]. Acta Physico-Chimica Sinica, 2007, 23(8): 1195-1200(in Chinese). [60] 孙金陆, 甄卫军, 李进. LDHs 材料的结构、性质及其应用研究进展[J]. 化工进展, 2013, 32(3): 610-616.SUN Jinlu, ZHEN Weijun, LI Jin. Structure, properties and application of LDHs[J]. Chemical Industry and Engi-neering Progress, 2013, 32(3): 610-616(in Chinese). [61] GUO R N, ZHU Y L, CHENG X W, et al. Efficient degradation of lomefloxacin by Co-Cu-LDH activating peroxymonosulfate process: Optimization, dynamics, degradation pathway and mechanism[J]. Journal of Hazardous Materials, 2020, 399: 122966. [62] SUN Q T, XU B D, YANG J, et al. Layered oxides supported Co-Fe bimetal catalyst for carbamazepine degradation via the catalytic activation of peroxymonosulfate[J]. Chemical Engineering Journal, 2020, 400: 125899. [63] HONG Y C, ZHOU H Y, XIONG Z K, et al. Heterogeneous activation of peroxymonosulfate by CoMgFe-LDO for degradation of carbamazepine: Efficiency, mechanism and degradation pathways[J]. Chemical Engineering Journal, 2020, 391: 123604. [64] LUO L, WANG Y L, ZHU M L, et al. Co-Cu-Al layered double oxides as heterogeneous catalyst for enhanced degradation of organic pollutants in wastewater by activating peroxymonosulfate: Performance and synergistic effect[J]. Industrial & Engineering Chemistry Research, 2019, 58: 8699-8711. [65] CHEN Y, YAN J C, YANG D O, et al. Heterogeneously catalyzed persulfate by CuMgFe layered double oxide for the degradation of phenol[J]. Applied Catalysis A: General, 2017, 538: 19-26. [66] HONG Y , PENG J , ZHAO X, et al. Efficient degradation of atrazine by CoMgAl layered double oxides catalyzed peroxymonosulfate: Optimization, degradation pathways and mechanism[J]. Chemical Engineering Journal, 2019, 370: 354-363. [67] CHEN M Q, WU P X, HUANG Z Y, et al. Environmental application of MgMn-layered double oxide for simultaneous efficient removal of tetracycline and Cd pollution: Perfor-mance and mechanism[J]. Journal of Environmental Management, 2019, 246: 164-173. [68] YANG Y, LI J H, SHI H H, et al. Influence of natural organic matter on horseradish peroxidase-mediated removal of 17α-ethinylestradiol: Role of molecular weight[J]. Journal of Hazardous Materials,2018,356:9-16. [69] YANG L, CHEN C S, TU Y J, et al. Heterogeneous degradation of organic pollutants by persulfate activated by CuO-Fe3O4: Mechanism, stability, and effects of pH and bicarbonate ions[J]. Environmental Science & Technology,2015,49(11):6838-6845. [70] ALI J, LANG J, LIAO Z W, et al. Activation of persulfate by CuOx@Co-LDH: A novel heterogeneous system for contami-nant degradation with broad pH window and controlled leaching[J]. Chemical Engineering Journal,2018(335):548-559. [71] PENG L, WANG C, LI C. Epoxidation of allylic alcohols on self-assembled polyoxometalates hosted in layered double hydroxides with aqueous H2O2 as oxidant[J]. Journal of Catalysis,2009,262(1):159-168. doi: 10.1016/j.jcat.2008.12.018 [72] CLARIZIA L, RUSSO R, SOMMA I D, et al. Homogeneous photo-Fenton processes at near neutral pH: A review[J]. Applied Catalysis B: Environmental,2017,209:358-371. doi: 10.1016/j.apcatb.2017.03.011 [73] QIAN L, LIU P, SHAO S, et al. An efficient graphene supported copper salen catalyst for the activation of persulfate to remove chlorophenols in aqueous solution[J]. Chemical Engineering Journal,2019,360:54-63. doi: 10.1016/j.cej.2018.11.208 [74] KOHANTIRABI M, GHOLAMI M R. AgPt nanoparticles supported on magnetic graphene oxide nanosheets for catalytic reduction of 4-nitrophenol: Studies of kinetics and mechanism[J]. Applied Organometallic Chemistry,2017,31(11):3806. [75] NOVOSELOV K S, FAL'KO VI, COLOMBO L, et al. Aroadmap for graphene[J]. Nature, 2012, 490: 192-200. [76] DENG J, XIAO L W, YUAN S J, et al. Activation of peroxymonosulfate by CoFeNi layered double hydroxide/ graphene oxide (LDH/GO) for the degradation of gatifloxacin[J]. Separation and Purification Technology,2021,255:117685. doi: 10.1016/j.seppur.2020.117685 [77] YE Q Y, WU J Y, WU P X, et al. Enhancing peroxymonosulfate activation of Fe-Al layered double hydroxide by dissolved organic matter: Performance and mechanism[J]. Water Research,2020,185:116246. doi: 10.1016/j.watres.2020.116246 [78] GUO R N, LI Y H, CHEN Y, et al. Efficient degradation of sulfamethoxazole by CoCu LDH composite membrane activating peroxymonosulfate with decreased metal ion leaching[J]. Chemical Engineering Journal,2021,417:127887. doi: 10.1016/j.cej.2020.127887 [79] GUO R N, NENGZI L C, CHEN Y, et al. Efficient degradation of sulfamethoxazole by CuCo@LDH and LDH@fibers composite membrane activating peroxymonosulfate[J]. Chemical Engineering Journal,2020,398:125676. doi: 10.1016/j.cej.2020.125676 [80] WANG Z J, LI Y H, SHEN G H, et al. Synthesis of CMK/LDH and CMK/CLDH for sulfamethoxazole degradation by PS activation: A comparative study of characterization and operating parameter, mechanism pathway[J]. Separation and Purification Technology,2021,258:118018. doi: 10.1016/j.seppur.2020.118018 [81] DENG Y M, ZOU X, LIU Z R, et al. Co7Fe3/CoFe2O4@C Lamellar composites derived from Co-Fe LDH/PVA as an effective heterogeneous activator of peroxymonosulfate[J]. Journal of Alloys and Compounds,2021,854:157244. [82] ZENG H X, ZHAO H J, DENG L, et al. Peroxymonosulfate-assisted photocatalytic degradation of sulfadiazine using self-assembled multi-layered CoAl-LDH/g-C3N4 heterostructures: Performance, mechanism and eco-toxicity evaluation[J]. Journal of Water Process Engineering,2020,33:101084. doi: 10.1016/j.jwpe.2019.101084 [83] TANG L, LIU Y, WANG J, et al. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism[J]. Applied Catalysis B: Environmental,2018,231:1-10. [84] PREMARATHNAK S D, RAJAPAKSHA A, SARKAR B, et al. Premarathnaaanushka upamali rajapakshaabinoy sarkarbiochar-based engineered composites for sorptive decontamination of water: A review[J]. Chemical Engineering Journal,2019,9(372):536-550. [85] MCNEIL K, CANONICA S. Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties[J]. Environmental Science: Processes and Impacts,2016,18(11):1381. doi: 10.1039/C6EM00408C [86] CHA J H, PARH E B, HAN S W, et al. Core-shell structured cobalt sulfide/cobalt aluminum hydroxide nanosheet arrays for pseudo capacitor application[J]. Chemistry,2019(14):446-453. [87] WU X, ZHAO W, HUANG Y, et al. A mechanistic study of amorphous CoSx cages as advanced oxidation catalysts for excellent peroxymonosulfate activation towards antibiotics degradation[J]. Chemical Engineering Journal,2020,381:122768. doi: 10.1016/j.cej.2019.122768 [88] QI C, LIU X, MA J, et al. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollu-tants[J]. Chemosphere,2016,151:280-288. doi: 10.1016/j.chemosphere.2016.02.089 [89] LIU Y, GUO H, ZHANG H, et al. Heterogeneous activation of peroxymonosulfate by sillenite Bi25FeO40: Singlet oxygen generation and degradation for aquatic levofloxacin[J]. Chemical Engineering Journal,2018,343:128-137. doi: 10.1016/j.cej.2018.02.125 [90] ZHANG T, ZHU H, CROUE J P. Production of sulfate radical from peroxymonosulfate induced by a magneti-cally separable CuFe2O4 spinel in water: Efficiency, stabi-lity, and mechanism[J]. Environmental Science & Technology,2013,47(6):2784-2791. [91] HUANG X, ZHOU X J, SU Q Q, et al. Synergic thermal activation of peroxydisulfate intercalated Mg/Al layered double hydroxide at a low temperature[J]. Chemical Engineering Journal,2019,363:133-140. doi: 10.1016/j.cej.2019.01.128 [92] JAAFARZADEH N, GHANBAR F, AHMADI M, et al. Efficient degradation of 2, 4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes[J]. Chemical Engineering Journal,2017,320:436-447. doi: 10.1016/j.cej.2017.03.036 -





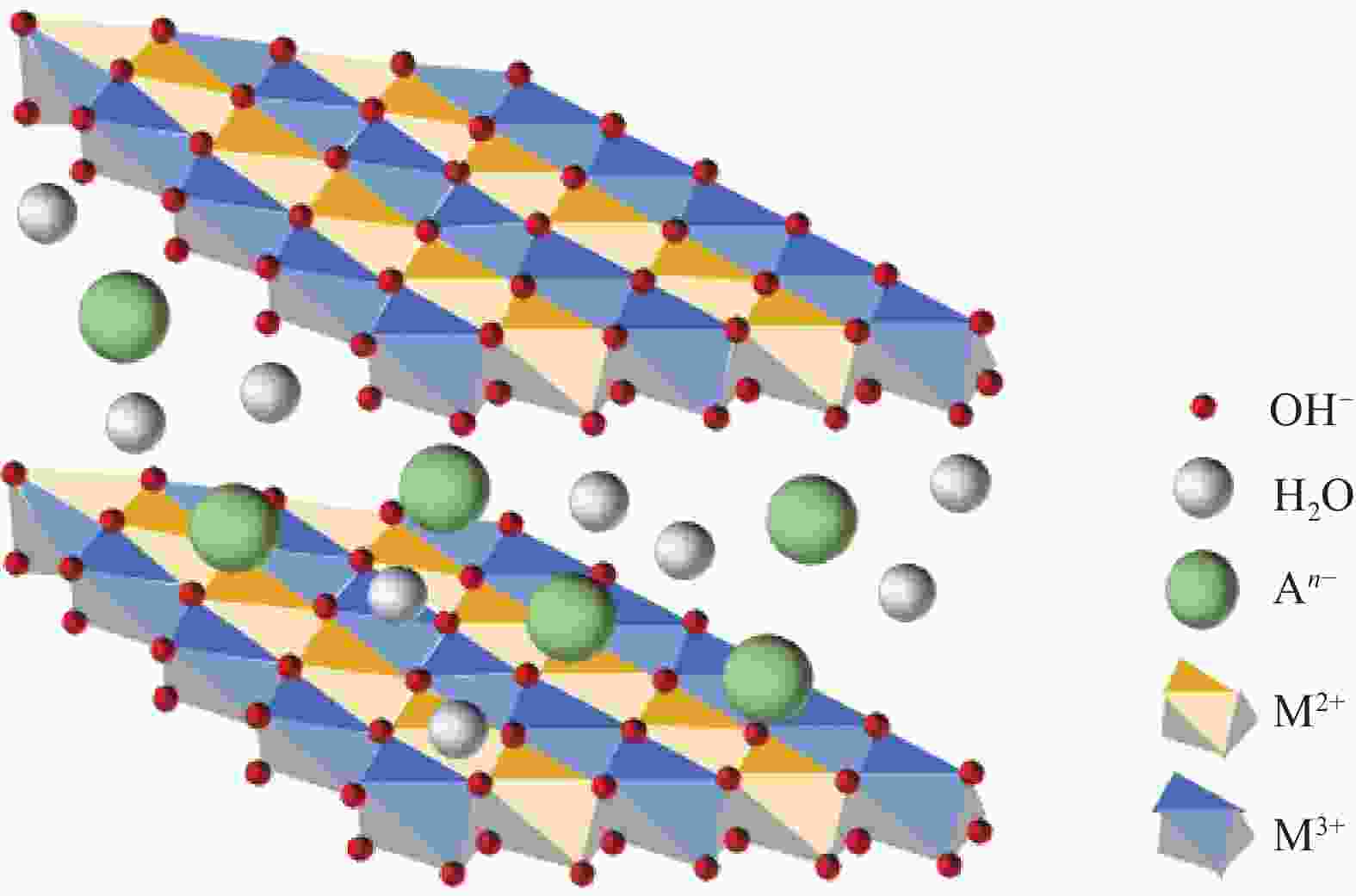
 下载:
下载: