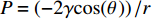Controllable preparation method and performance of three-dimensional reduced graphene oxide aerogel under mild conditions
-
摘要: 为了实现石墨烯类三维气凝胶在温和环境条件下的大面积可控制备和高性能化,本文应用水合肼作为还原剂,通过低温预冷冻结合室温自然干燥,实现了室温还原自组装法可控制备直径30 cm的大面积三维还原氧化石墨烯(3D-RGO)气凝胶。该方法制备条件温和,不需任何加热条件和特殊冷冻干燥设备。通过对气凝胶制备过程中还原时间、预冷冻时间、预冷冻温度和反应容器进行控制,可以有效调节气凝胶的形状、表面浸润性、体积收缩率等,实现3D-RGO气凝胶的可控制备。该气凝胶不会出现明显的体积收缩和结构破裂,为具有约500 μm的稳定孔径和3.8 mg/cm3的低密度的蜂窝状结构,并能够从90%的压缩应变下快速地恢复到初始状态,其干燥过程体积收缩率<5%;同时该石墨烯气凝胶展现良好稳定的导电性,在压缩应变从0%增加到90%时,其导电率从17.3 S/m增加至115.2 S/m。这种方法经济高效且易于制备出大面积的3D-RGO。Abstract: In order to realize the large-area controllable preparation and high performance of graphene-based three-dimensional aerogels under mild environmental conditions, hydrazine hydrate is used as reducing agent in this paper. Through low-temperature pre-frozen combined with natural drying at room temperature, large-area three-dimensional reduced graphene oxide (3D-RGO) aerogels with a diameter of 30 cm can be prepared effectively and controlled. The method has mild preparation conditions and does not need any heating conditions or special freeze-drying equipment. By controlling the reduction time, pre-frozen time, pre-frozen temperature and reaction vessel during the preparation of 3D-RGO aerogel, the shape, surface wettability, volume shrinkage aerogel can be effectively adjusted to achieve controllable preparation of 3D-RGO aerogel. The 3D-RGO aerogel has no obvious volume shrinkage and structural cracks. The aerogels exhibit a stable honeycomb-like structure with a stable pore size of about 500 μm and a low density of 3.8 mg/cm3, and it can quickly undergo a compression strain of 90% and return to the initial state. The volume shrinkage rate of the aerogels is <5% in the drying process. At the same time, the graphene aerogel exhibits good and stable conductivity. When the compressive strain increases from 0% to 90%, its conductivity increases from 17.3 S/m to 115.2 S/m. This method is suitable for the cost-effective preparation of large-area graphene aerogels.
-
三维还原氧化石墨烯(3D-RGO)气凝胶是一种新兴的组装结构,可以将石墨烯优良的微观性能转换成优异的宏观特性,如超低密度(<10 mg/ cm3)、高弹性(>90%)、高导电率(≈10 S/cm)及独特的热性能等[1-3]。作为近年来最有吸引力的一种碳材料,3D-RGO气凝胶在能量储备与转化、环境治理、溶剂吸附、隔热材料及太阳光热转换等领域具有巨大的应用潜力[4-8]。通过水热还原法[9]、化学气相沉积法(CVD)[10-11]、气泡衍生技术[12]、冷冻浇铸技术[13]及表面活性剂发泡溶胶-凝胶技术[14]等方法可以制备高性能和多功能的 3D-RGO气凝胶。这些方法各具优点,但过程繁琐,还需要冷冻干燥机等专用仪器,耗时耗财。譬如,CVD法的三维模板一般需要除掉,化学刻蚀法对环境污染较大,并且CVD工艺不易扩展、耗能较大、成本较高,很难实现大规模生产和应用。除CVD方法外,其他方法需要使用超临界干燥技术[15]和冷冻干燥技术[16]等去除溶剂,实现3D-RGO气凝胶不发生体积收缩和结构破裂。以低成本和简便方法开发大面积高性能3D-RGO气凝胶是其实际应用的关键挑战[17-18]。为了克服这一难题,已经报道了多种新技术,如自然干燥技术[19]、气泡衍生的定向冻结策略[20]和冷冻铸造技术[21]等。Xu等[22]用自然干燥技术制备了超弹性3D-RGO气凝胶,体积收缩率低,但是制备过程需要高温水热处理,这限制了大尺寸3D-RGO气凝胶的低成本制造。Zhang等[20]强调了气泡衍生和定向冻结策略来制造石墨烯基多孔材料,但该方法基于冷冻干燥和热退火技术,使成本增高,且规模受限。Wang等[13]报道了一种冷冻浇铸法用于制备具有中心对称结构的3D-RGO气凝胶,但该法需要冷冻干燥技术去除水。
本文以改进Hummers法制备的氧化石墨烯(GO)分散液为原材料设计了一种水合肼室温还原与预冷冻和自然干燥相结合的方法制备了3D-RGO气凝胶,通过调整工艺条件来控制3D-RGO的体积收缩。本文对该气凝胶的化学组成及微观结构等进行了表征,同时研究了气凝胶的形成机制,并探索了该室温还原自组装法制备气凝胶的性能。
1. 实验材料及方法
1.1 原材料
石墨、硝酸钠、浓硫酸、高锰酸钾,分析纯,北京化工厂。水合肼,64%,Acro试剂。双氧水,30%,北京化工厂。
1.2 3D-RGO气凝胶的制备
采用改进Hummers法制备GO水溶液[23],将其进行离心浓缩,得到GO浓缩水分散液。用去离子水将浓缩GO分散液配制成一定浓度的GO溶液,超声分散,用氨水调节溶液pH值至10.0,搅拌均匀,加入与GO质量比为3∶1的水合肼,充分搅拌,超声20 min,得到GO反应液。将GO反应液转移至相应容器在室温静置48 h,得到RGO水凝胶后转移至−18℃冰箱中冷冻6 h,取出,室温自然干燥48 h,得到3D-RGO气凝胶。
1.3 测试与表征
用德国赛默飞世尔科技有限公司NICOLET-IS10型傅里叶变换红外光谱仪对样品进行红外测试。用日本岛津公司XRD-6000型X射线衍射仪对样品进行X射线粉末衍射测试。用日本岛津公司AXIS NOVA型X射线光电子能谱仪对样品表面C元素价态进行测试。用北京赛诺飞拓科技有限公司SR-Lab1000型拉曼光谱仪对样品进行拉曼测试。用FEI公司Quanta250型扫描电子显微镜观测材料形貌。用德国 DATA PHYSICS 仪器公司的接触角测量仪对材料表面水接触角进行测试。用日本岛津公司AGS-J型万能试验机对样品进行力学性能测试。
2. 结果与讨论
2.1 3D-RGO气凝胶的形貌结构表征
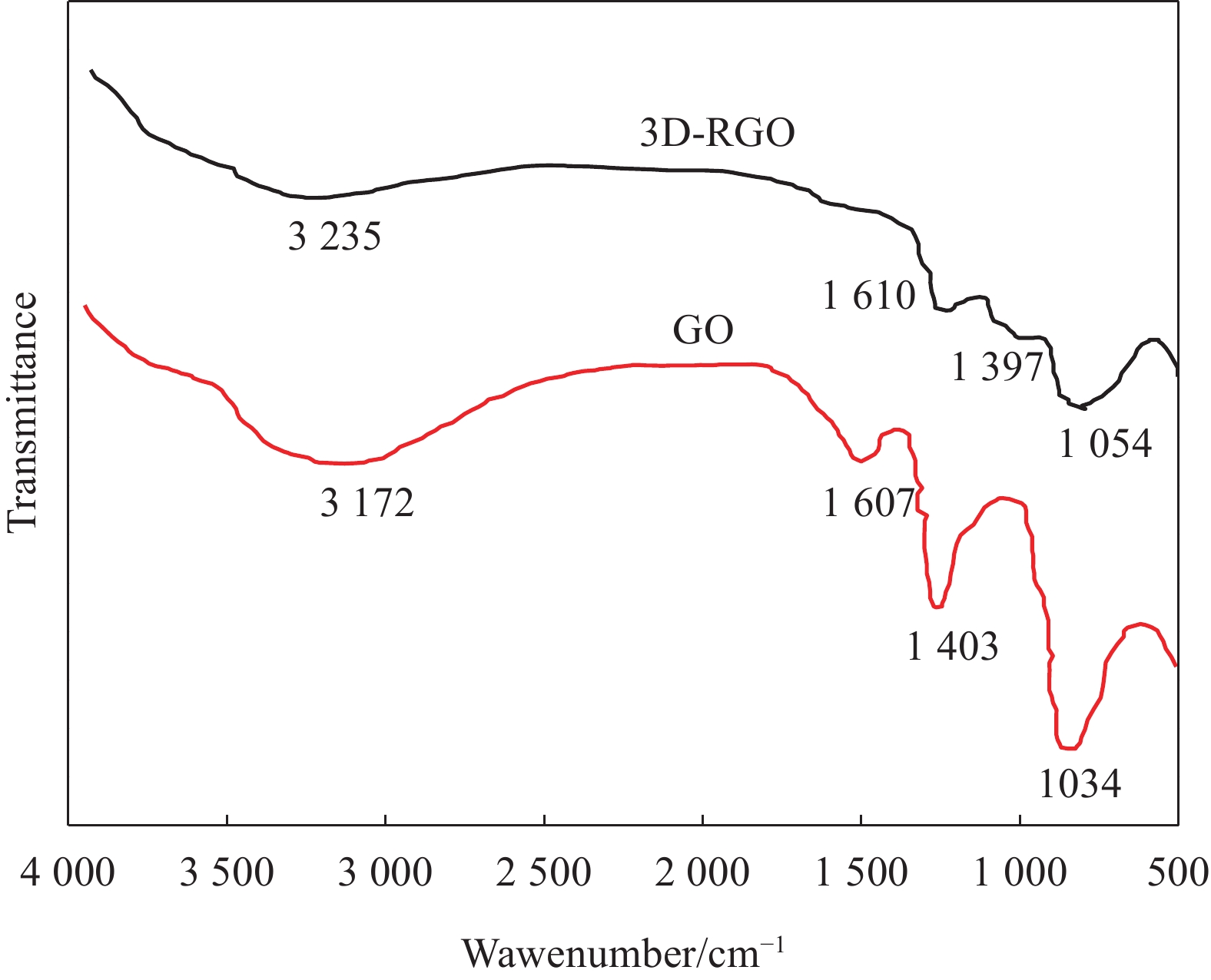
图1是GO和3D-RGO的FTIR图谱。可见,GO的FTIR图谱中,3172 cm−1是—OH的伸缩振动,1607 cm−1是C=O的伸缩振动,1034 cm−1是C—O键的伸缩振动。3D-RGO的FTIR图谱中,在3235 cm−1、1610 cm−1和1054 cm−1处出现与GO的—OH、C=O和C—O相对应的弱峰,并且特征峰的位置均出现了一定的蓝移现象,说明水合肼与GO发生了还原反应,GO得到有效还原。
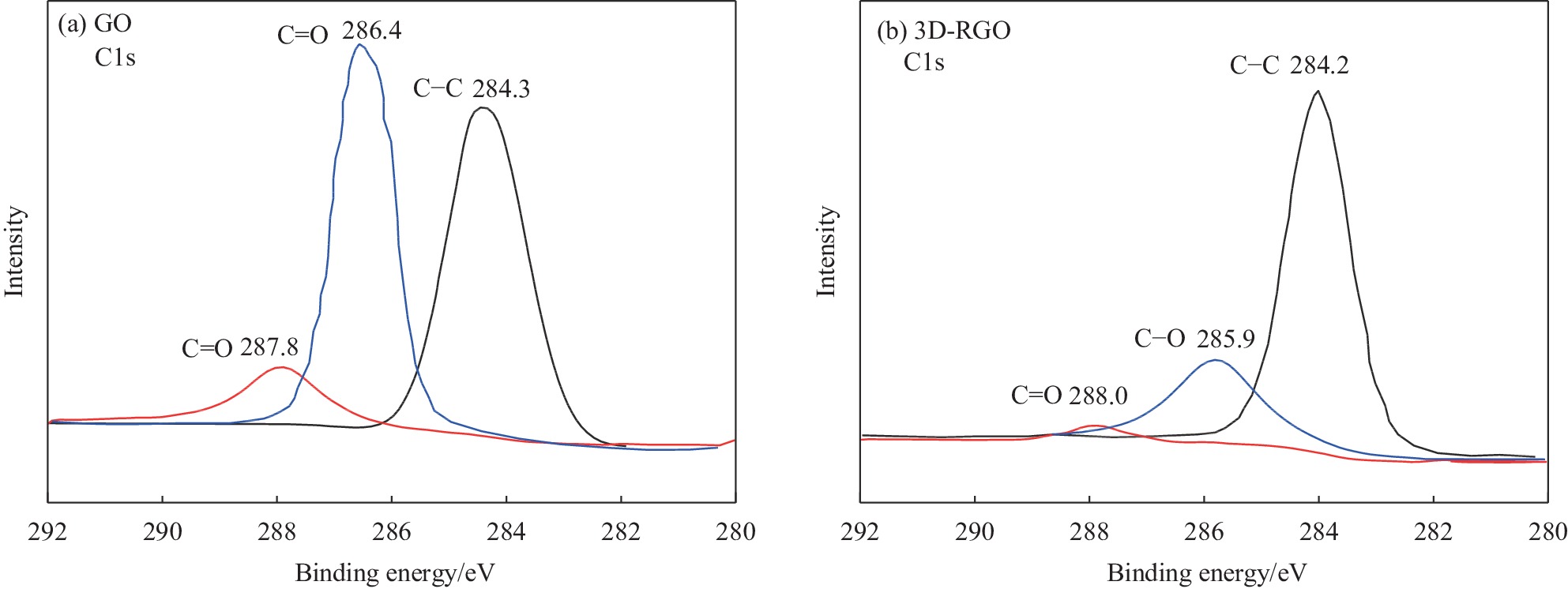
图2为GO和3D-RGO的XPS结果。可以进一步证明3D-RGO中水合肼对GO片层表面含氧官能团的还原。由GO的C1s谱可知,其具有结合能为287.8 eV、286.4 eV和284.3 eV的拟合峰,3个拟合峰分别对应于C=O、C—O和C—C键。由3D-RGO的C1s谱可知,经过水合肼还原后上述各键的峰依然存在,但是C—O和C=O的峰强度减弱,C—C键对应的峰强度基本保持不变,说明含氧官能团减少。由各峰对应的面积计算可以得到GO和RGO中C/O的元素比分别为0.95和3.7。表明在3D-RGO的制备过程中,GO片层表面的含氧官能团减少,GO得到还原。
图3是用浓度为5 mg/mL的GO溶液制备得到的3D-RGO的数码照片和SEM图像。可见,通过水合肼室温还原自组装法制备得到的气凝胶直径为30 cm,厚度约1 cm,密度为3.8 g/cm3,该气凝胶微观呈现3D蜂窝状多孔结构,气凝胶内部孔径在500 μm左右。水合肼在室温放置期间发生还原过程中会产生大量的气泡,结合具有共轭结构的GO纳米片在还原过程中的疏水性增加和π-π堆积相互作用导致的聚集,可以发生有效的自组装,干燥过程中大量的水从聚集体中排出,从而产生大量三维多孔结构,低温条件下冷冻使得凝胶的网络结构更加稳定,同时也能够起到增大孔径的作用。通过空气干燥获得的气凝胶通常由于毛细管压力会在干燥过程中出现严重的体积收缩或塌陷现象。根据拉普拉斯公式可以得知,毛细管压力(P)与接触角(θ)、孔隙液体的表面张力(γ)和孔半径(r)有关:
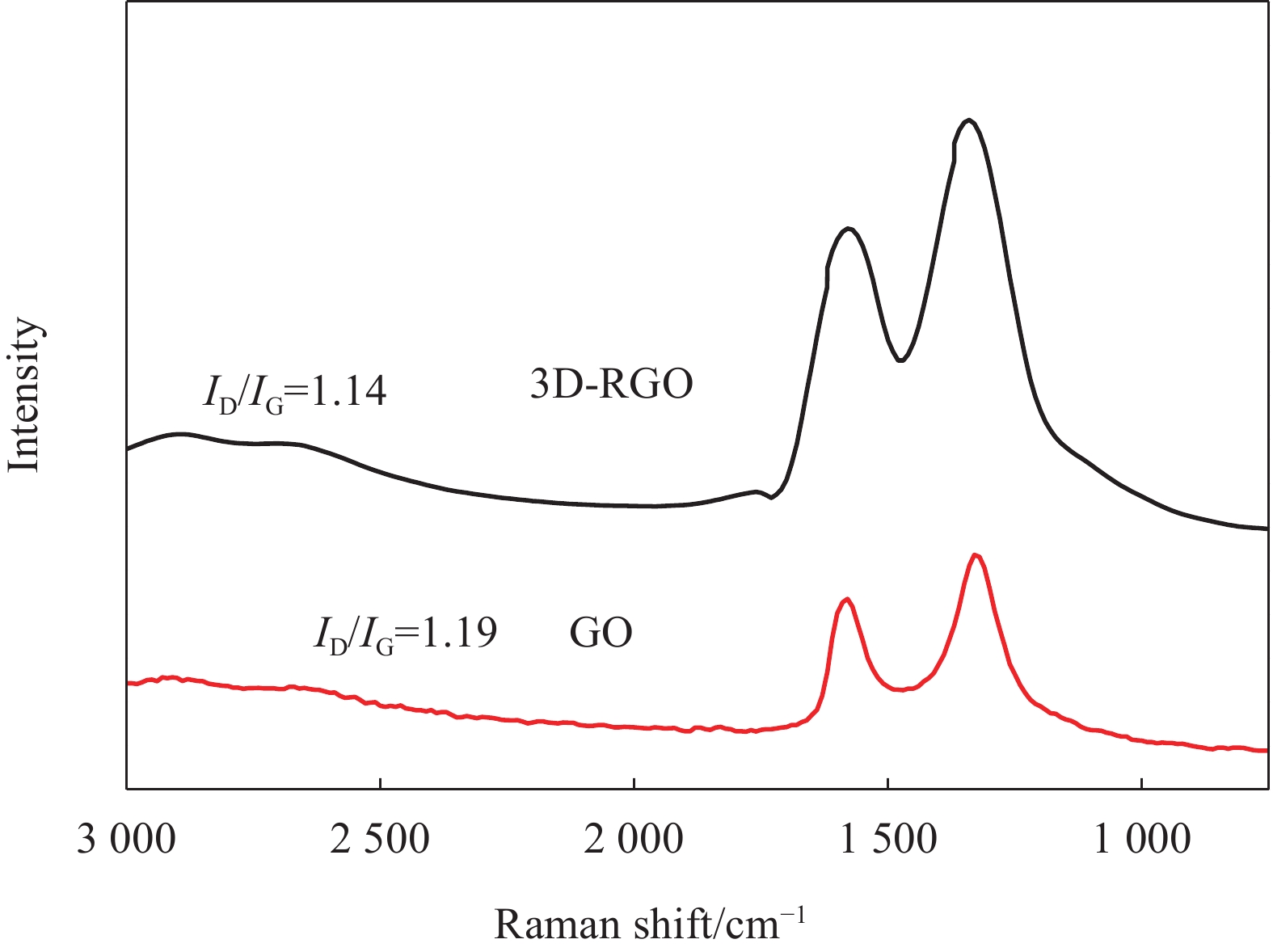
P=(−2γcos(θ))/r ,可以看出,如果θ和γ保持恒定,P将与r成反比。因此,扩大气凝胶孔径将是减小毛细管压力的一个非常有效的途径,有利于实现环境干燥。图4是GO与3D-RGO的拉曼图谱。可见,GO的Raman曲线在1325.6 cm−1和1583.7 cm−1处显示两个强峰,分别对应3D-RGO的D带和G带。D带和G带分别与二维石墨六方晶格中的sp3和sp2杂化的碳原子振动有关。从面积积分的结果来看,GO中D/G的强度比为1.19。在室温还原自组装法中由水合肼还原后3D-RGO的D/G强度比降低到1.14。这通常被认为是由于新的石墨区域的形成导致sp2杂化区域的减少及sp2簇数目的增加所引起的[24],这也说明了含氧官能团的减少。
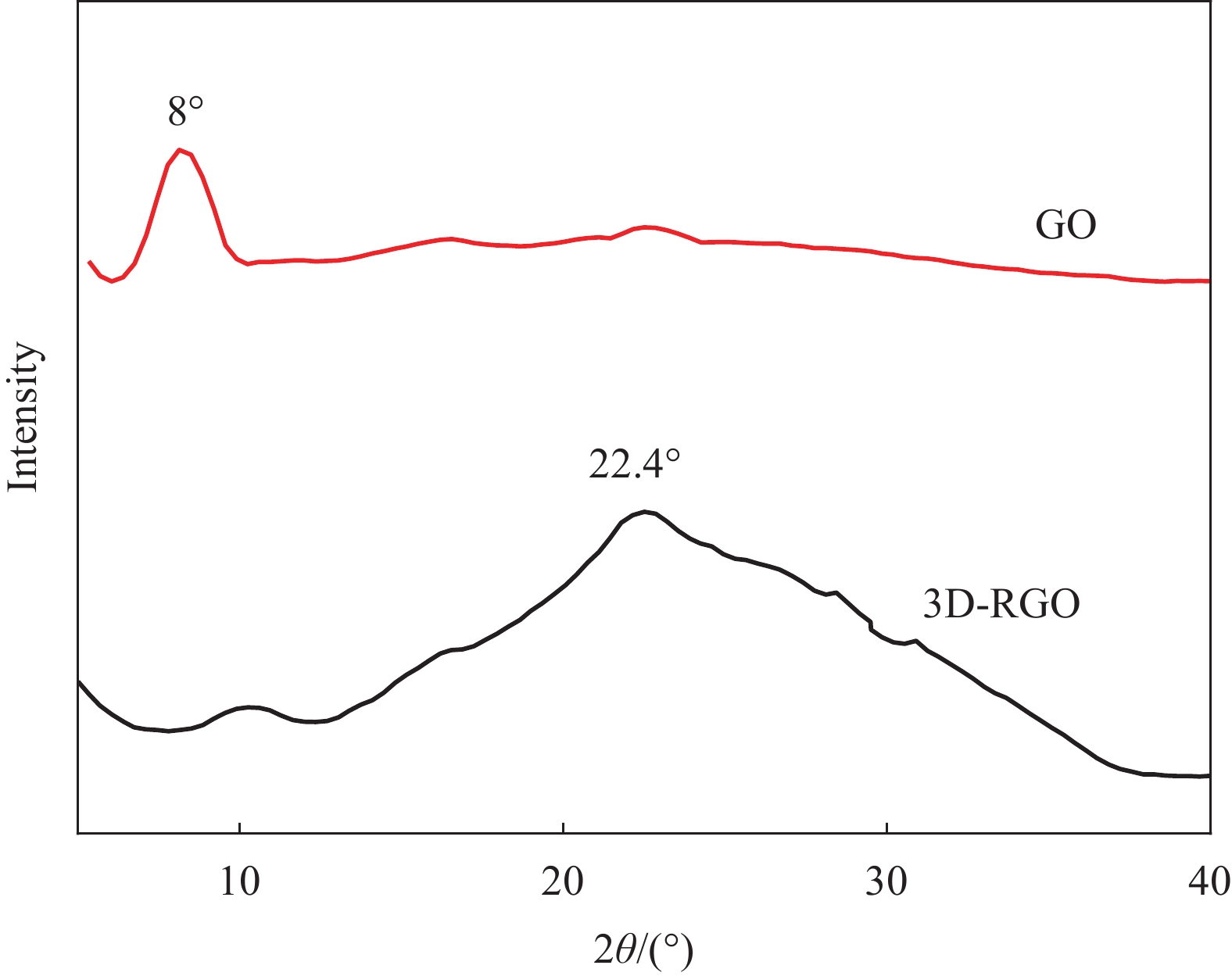
图5是GO与3D-RGO的XRD图谱。可见,GO在2θ=8.0°处显示衍射峰,对应的石墨烯片层间距为10.88 nm。3D-RGO在2θ=22.4°处出现宽衍射峰,对应石墨烯片层间距为3.93 nm。表明经过水合肼还原后,在3D-RGO中有新的石墨状结构出现。同时石墨烯片层间作用会促使在3D-RGO自组装过程中凝胶网络结构的形成。水合肼室温还原的诱发作用导致3D-RGO中石墨烯纳米片的π-π堆积作用及sp2共轭区恢复,这也是3D-RGO的形成机制[25]。
图6是根据不同类型的反应容器制备得到的气凝胶。可以看到,利用本方法制备3D-RGO不受反应容器的限制,在玻璃管、烧杯、锥形瓶等不同形状反应容器中均可得到凝胶结构,且得到凝胶形状与容器形状一致。有利于实现3D-RGO的大面积制备。
2.2 3D-RGO气凝胶的制备过程工艺优化
石墨烯气凝胶电磁波吸收方向很有应用前景,但仍存在阻抗匹配差而导致吸波性能不理想的问题,其中一个主要障碍是在凝胶化过程中体积的大幅收缩,造成密度不可控,也会引起石墨烯发生断裂等问题。因此,如何控制石墨烯气凝胶的体积收缩成为了难点。3D-RGO气凝胶体积收缩率由制备的3D-RGO水凝胶体积(V1)和经过干燥后得到的3D-RGO气凝胶的体积(V0),按照下式计算:
δ=(V1/V0)×100% 3D-RGO水凝胶在干燥过程中结构稳定,体积收缩率(δ)<5%。为了探究3D-RGO水凝胶干燥过程体积收缩率的影响因素,分别控制还原时间、预冷冻时间及预冷冻温度3个工艺条件进行试验。图7是不同实验条件下3D-RGO气凝胶积收缩率变化情况。通过控制上述3个工艺条件,图7(a)显示3D-RGO气凝胶体积收缩率随还原时间增加而减小,在还原24 h后,体积收缩率<5%,这是由于3D-RGO的还原程度随还原时间增加而变高,形成的3D网络结构更稳定。图7(b)显示3D-RGO气凝胶体积收缩率随预冷冻时间增加而减小,冷冻5 h后体积收缩率基本稳定,这是由于随冷冻时间增加,3D-RGO的3D网络结构更加稳定。通过控制冷冻温度为−5℃、−18℃和−50℃,在相同的时间内温度越低,得到的气凝胶的体积收缩率越小(图7(c)),这是由于在更低温度下冷冻能够更快速地实现3D-RGO网络结构的稳定。图7(d)是不同浓度GO溶液制备得到的3D-RGO气凝胶体积收缩率。由室温还原自组装法得到的3D-RGO气凝胶体积收缩率小于5%。RGO水凝胶的聚集主要是由水合肼引发的化学还原过程中强化的π-π堆叠相互作用来驱动的。同时,用氨水调节 pH的作用是为了提供碱性环境,促进GO纳米片之间的静电相互作用, 使GO稳定分散,从而促进GO溶液的溶胶-凝胶转变过程,有利于形成湿凝胶。冷冻后的水凝胶在室温和大气压力下水分蒸发得到3D-RGO气凝胶。
图8是在不同还原时间条件下制备的3D-RGO气凝胶的水接触角。可见,随着还原时间从12 h增加到48 h,3D-RGO气凝胶的水接触角从88.2°增加到90.3°。这表明随着处理时间的增加,3D-RGO气凝胶表面的疏水性得到提高,亲水性基团被还原。这会促进3D-RGO气凝胶体积收缩率的减小。
2.3 3D-RGO气凝胶的性能
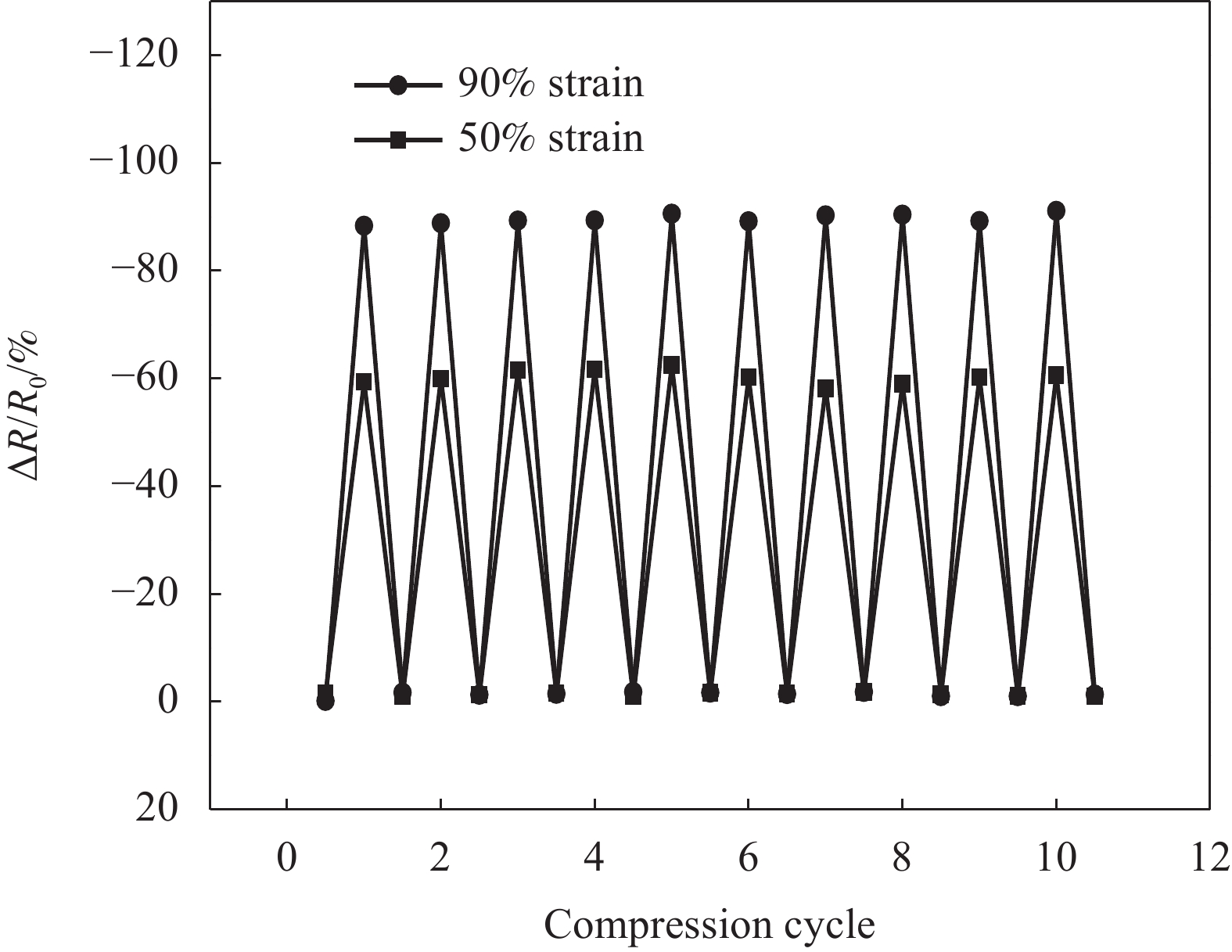
图9为3D-RGO气凝胶在不同应变下的循环压缩曲线和70%应变下的10个循环压缩曲线。从图9(a)可见,3D-RGO气凝胶在90%压缩应变下有很好的恢复性。在压力作用下,3D-RGO气凝胶会被挤压成片状,在压力释放以后,3D-RGO气凝胶能够恢复到原来的形状。图9(b)是3D-RGO气凝胶在压缩应变为70%下的10次循环压缩曲线图。在10次循环压缩过程中,压缩曲线接近重合,10次循环压缩后,最大压缩应力损失<1%。这表明3D-RGO气凝胶在压缩过程中结构稳定,并且具有优异的可逆和可重复压缩性能,在高压缩应变下能够快速恢复至初始形状。3D-RGO优异的弹性可以通过互连的3D-RGO网络大孔结构来实现[26],大孔结构主要是还原中产生的气泡和冷冻过程中水的膨胀作用导致[27]。
将3D-RGO气凝胶在蒸馏水中浸泡5 min后取出,于自然条件下干燥2天,记录其吸水-干燥前后的高度变化,同时测试其压缩性能如图10所示。在吸水-干燥过程中,样品的高度能够保持稳定,且循环压缩性能稳定。在吸水-干燥后,3D-RGO气凝胶最大应力值保持率为72.9%,最大压缩应变损失<3%,3D-RGO气凝胶表现出优异的环境稳定性和可重复利用性。由于3D-RGO的大孔径和稳定的3D网络结构使其吸水后的干燥过程中能够抵抗水分蒸发所引起的毛细管作用力,进而保持形状和结构的稳定性。
图11为由不同GO浓度制备得到的3D-RGO气凝胶的导电率。8 mg/mL的GO制备得到的3D-RGO气凝胶最高导电率为41.4 S/m。3D-RGO气凝胶的导电率随GO浓度增加而增大,这是由于GO浓度越高,形成3D-RGO气凝胶过程中石墨烯纳米片之间的堆积密度越高,导电通路更多更稳定。
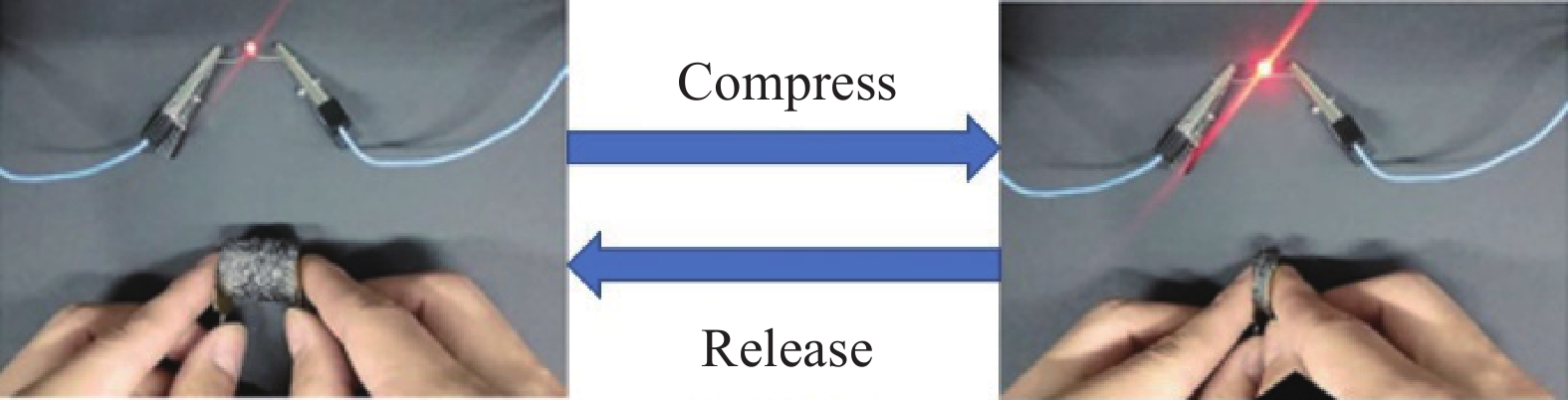
图12展示了通过3D-RGO气凝胶压缩应变控制二极管亮度。可见,将3D-RGO气凝胶连接在电路中,可以看到随着3D-RGO的不断压缩,电路中的二极管会逐渐变得更亮。图13是由5 mg/mL GO制备的3D-RGO气凝胶压缩过程导电率变化。由图13(a)可见,3D-RGO的导电率随压缩应变的增加而增大,在压缩应变从0%逐渐增加到90%时,其导电率从17.3 S/m增加至115.2 S/m。从图13(b)可见,在压缩应变达到90%时,归一化电阻(Rt/R0)值降低了81.3%。在应力释放过程中,电阻值又能够很好地恢复。在10个压缩循环过程中,Rt/R0对压缩应变的响应也比较稳定。这是由于3D-RGO气凝胶压缩过程中,石墨烯片层的弹性弯曲,会导致孔结构在保持稳定的同时,内部能量进行耗散,孔径变小,石墨烯片层之间会形成更多新的接触点,进而提供更多的电子传输通道,导电性增加[28]。载荷消除以后,3D-RGO的变形立即恢复,石墨烯片层间新建立的接触点也即断开,发光二极管(LED)灯逐渐变暗。
图14是3D-RGO的相对电阻变化(ΔR/R0)对于压缩应变的响应情况。可以看出响应非常灵敏,且具有很好的重复性能。优异的循环压缩性能及其导电性随压缩应变变化的特性为3D-RGO气凝胶在压缩传感领域的应用奠定了基础。
表1是已报道的典型3D-RGO气凝胶的制备方法、密度、压缩应变和尺寸等。可见,与本文制备的3D-RGO气凝胶相比,大部分传统方法比如冷冻干燥法制备的3D-RGO气凝胶尺寸较小。本方法在制备出大尺寸3D-RGO气凝胶同时,还保证了3.8 mg/cm3的密度和90%的高压缩应变性能。室温还原自组装法适于大规模制备3D-RGO,应用将会更广泛。获得的3D-RGO气凝胶可用于传感器和气体吸附等领域,也可作为油类或危险溶剂的高吸收性材料。
表 1 不同方法制备的3D-RGO气凝胶密度、压缩应变与尺寸比较Table 1. Density, compressive strain and size ratio of 3D-RGO aerogel prepared by various methodsSample Method Density/
(mg·cm−3)Compression strain Size/cm GFs[29] CVD ca. 5 No 17×22 Graphene aerogel[30] Supercritical drying 12-96 40% < 5 GF[31] Freeze-drying ca. 2.1 No < 5 UFAs[32] Freeze-drying 0.16 82% ca. 21 MGM[33] Freeze-drying 6.73 50% < 5 CMG-CNs[34] Freeze-drying 2.0 50% < 5 Graphene sponge [35] Freeze-drying 1.7 98% < 5 GFs [36] Sintering 11.3 10% ca. 10 Graphene aerogel [19] Vacuum-/air-drying 5.3 80% < 5 NDGA[2] Air-drying 6.7 99% ca. 10 ADGA[1] Air-drying 2.5 93% ca. 5 RGO/GNP[37] Air-drying ca. 100 50% < 5 GAB[38] Air-drying 2.8 99% >100 CNTS-GR[39] Freeze-drying 1.06 72% 5 3D-RGO
( This work)Air-drying 3.8 90% 30 Notes: GFs, GF—Graphene foams; UFAs—Ultra-flyweight aerogels; MGM—Macroporous graphene monoliths; CMG-CNs—Chemically modified graphene-cellular networks; NDGA—Naturally dried graphene aerogels; ADGA—Ambient pressure dried graphene aerogels; RGO/GNP—Reduced graphene oxide/graphene nanoplatelets; GAB—Graphene aerogel bulk; CNTS-GR—Carbon nanotubes-graphene aerogel; CVD—Chemical vapor deposition; ca.—About. 3. 结论
(1) 应用水合肼作为还原剂,通过还原自组装,室温可实现将氧化石墨烯(GO)制备得到三维还原氧化石墨烯(3D-RGO)水凝胶,结合预冷冻和室温自然干燥,可以在相对温和的条件下制备获得3D-RGO气凝胶,并可实现3D-RGO气凝胶的大尺寸制备。
(2) 通过对3D-RGO气凝胶制备过程中的工艺条件进行控制,如还原时间、预冷冻时间、预冷冻温度和反应容器等进行控制,可以有效调节3D-RGO气凝胶的形状、表面浸润性、体积收缩率等,实现3D-RGO气凝胶的可控制备。
(3) 制备的3D-RGO气凝胶具有500 μm孔径的蜂窝状结构,3.8 mg/cm3的低密度能够从90%的压缩应变下快速地恢复到初始状态。同时该石墨烯气凝胶展现良好稳定的导电性,能够被用于压缩传感器件等领域。
-
表 1 不同方法制备的3D-RGO气凝胶密度、压缩应变与尺寸比较
Table 1 Density, compressive strain and size ratio of 3D-RGO aerogel prepared by various methods
Sample Method Density/
(mg·cm−3)Compression strain Size/cm GFs[29] CVD ca. 5 No 17×22 Graphene aerogel[30] Supercritical drying 12-96 40% < 5 GF[31] Freeze-drying ca. 2.1 No < 5 UFAs[32] Freeze-drying 0.16 82% ca. 21 MGM[33] Freeze-drying 6.73 50% < 5 CMG-CNs[34] Freeze-drying 2.0 50% < 5 Graphene sponge [35] Freeze-drying 1.7 98% < 5 GFs [36] Sintering 11.3 10% ca. 10 Graphene aerogel [19] Vacuum-/air-drying 5.3 80% < 5 NDGA[2] Air-drying 6.7 99% ca. 10 ADGA[1] Air-drying 2.5 93% ca. 5 RGO/GNP[37] Air-drying ca. 100 50% < 5 GAB[38] Air-drying 2.8 99% >100 CNTS-GR[39] Freeze-drying 1.06 72% 5 3D-RGO
( This work)Air-drying 3.8 90% 30 Notes: GFs, GF—Graphene foams; UFAs—Ultra-flyweight aerogels; MGM—Macroporous graphene monoliths; CMG-CNs—Chemically modified graphene-cellular networks; NDGA—Naturally dried graphene aerogels; ADGA—Ambient pressure dried graphene aerogels; RGO/GNP—Reduced graphene oxide/graphene nanoplatelets; GAB—Graphene aerogel bulk; CNTS-GR—Carbon nanotubes-graphene aerogel; CVD—Chemical vapor deposition; ca.—About. -
[1] 郭奇, 高源, 荔栓红, 等. 石墨烯增强聚合物气密性的研究进展[J]. 复合材料学报, 2022, 39(3):896-906. GUO Qi, GAO Yuan, LI Shuanhong, et al. Research progress in the enhanced polymer airtightness of graphene[J]. Acta Materiae Compositae Sinica,2022,39(3):896-906(in Chinese).
[2] XU X, ZHANG Q, YU Y, et al. Naturally dried graphene aerogels with superelasticity and tunable Poisson's ratio[J]. Advanced Materials,2016,28(41):9223-9230. DOI: 10.1002/adma.201603079
[3] ZHAO X, YAO W, GAO W, et al. Wet-spun superelastic graphene aerogel millispheres with group effect[J]. Advanced Materials,2017,29(35):1701482. DOI: 10.1002/adma.201701482
[4] MAO J, IOCOZZIA J, HUANG J, et al. Graphene aerogels for efficient energy storage and conversion[J]. Energy & Environmental Science,2018,11(4):772-799.
[5] 朱薇, 江坤, 游峰, 等. 三维立体介孔结构的海藻酸钠/氧化石墨烯复合气凝胶的制备及其对亚甲基蓝的吸附[J]. 复合材料学报, 2022, 39(5):1696-1706. ZHU Wei, JIANG Kun, YOU Feng. Preparation of 3-dimensional mesoporous sodium alginate/graphene oxide composite aerogel for adsorption of methylene blue[J]. Acta Materiae Compositae Sinica,2022,39(5):1696-1706(in Chinese).
[6] 张宏伟, 谢鸿, 肖欣荣, 等. 不同氧化程度氧化石墨烯/聚乙烯醇气凝胶对亚甲基蓝的吸附[J]. 复合材料学报, 2021, 38(9):2795-2802. ZHANG Hongwei, XIE Hong, XIAO Xinrong, et al. Adsorption of methylene blue by graphene oxide/polyvinyl alcoholaerogels with different oxidation degrees[J]. Acta Materiae Compositae Sinica,2021,38(9):2795-2802(in Chinese).
[7] NINE M J, AYUB M, ZANDER A C, et al. Graphene oxide-based lamella network for enhanced sound absorption[J]. Advanced Functional Materials,2017,27(46):1703820. DOI: 10.1002/adfm.201703820
[8] 朱世东, 赵乾臻, 王星海. 石墨烯/高分子功能复合材料制备与应用研究进展[J]. 复合材料学报, 2022, 39(2):489-501. ZHU Shidong, ZHAO Qianzhen, WANG Xinghai. Research progress in preparation and application of graphene/polymer functional composite materials[J]. Acta Materiae Compositae Sinica,2022,39(2):489-501(in Chinese).
[9] BI H, YIN K, XIE X, et al. Low temperature casting of graphene with high compressive strength[J]. Advanced Materials, 2012, 24(37): 5124-5129.
[10] WANG Z, SHEN X, AKBARI GARAKANI M, et al. Graphene aerogel/epoxy composites with exceptional anisotropic structure and properties[J]. ACS Applied Materials & Interfaces,2015,7(9):5538-5549.
[11] BI H, CHEN I W, LIN T, et al. A new tubular graphene form of a tetrahedrally connected cellular structure[J]. Advanced Materials, 2015, 27(39): 5943-5949
[12] LV L, ZHANG P, CHENG H, et al. Solution-processed ultraelastic and strong air-bubbled graphene foams[J]. Small, 2016, 12(24): 3229-3234
[13] WANG C, CHEN X, WANG B, et al. Freeze-casting produces a graphene oxide aerogel with a radial and centrosymmetric structure[J]. ACS Nano,2018,12(6):5816-5825. DOI: 10.1021/acsnano.8b01747
[14] WANG C, HE X, SHANG Y, et al. Multifunctional graphene sheet-nanoribbon hybrid aerogels[J]. Journal of Materials Chemistry A,2014,2(36):14994-15000. DOI: 10.1039/C4TA02591A
[15] WORSLEY M A, PAUZAUSKIE P J, OLSON T Y, et al. Synthesis of graphene aerogel with high electrical conductivity[J]. Journal of the American Chemical Society,2010,132(40):14067-14069. DOI: 10.1021/ja1072299
[16] LI J, LI J, MENG H, et al. Ultra-light, compressible and fire-resistant graphene aerogel as a highly efficient and recyclable absorbent for organic liquids[J]. Journal of Materials Chemistry A,2014,2(9):2934-2941. DOI: 10.1039/c3ta14725h
[17] 周浪, 王涛. 石墨烯/功能聚合物复合材料[J]. 复合材料学报, 2020, 37(5):997-1014. ZHOU Lang, WANG Tao. Graphene/functional polymer composites[J]. Acta Materiae Compositae Sinica,2020,37(5):997-1014(in Chinese).
[18] 胡涵. 石墨烯气凝胶的控制制备、改性及性能研究[D]. 大连: 大连理工大学, 2015. HU Han. Controlled preparation, modification and performance of graphene aerogel[D]. Dalian: Dalian University of Technology, 2015(in Chinese).
[19] LI C, QIU L, ZHANG B, et al. Robust vacuum-/air-dried graphene aerogels and fast recoverable shape-memory hybrid foams[J]. Advanced Materials,2016,28(7):1510-1516. DOI: 10.1002/adma.201504317
[20] ZHANG R, HU R, LI X, et al. A bubble-derived strategy to prepare multiple graphene-based porous materials[J]. Advanced Functional Materials,2018,28(23):1705879. DOI: 10.1002/adfm.201705879
[21] XIAO J, TAN Y, SONG Y, et al. A flyweight and superelastic graphene aerogel as a high-capacity adsorbent and highly sensitive pressure sensor[J]. Journal of Materials Chemistry A,2018,6(19):9074-9080. DOI: 10.1039/C7TA11348J
[22] XU X, ZHANG Q, YU Y, et al. Naturally dried graphene aerogels with superelasticity and tunable Poisson’s ratio[J]. Advanced Materials, 2016, 28(41): 9223-9230.
[23] RUI C, TANAKA D, MENDES A. Reduced graphene oxide films as transparent counter-electrodes for dye-sensitized solar cells [J]. Solar Energy, 2012, 86 (2): 716-724.
[24] 郑晓明. 基于拉曼光谱的石墨烯研究[D]. 长沙: 国防科学技术大学, 2015. ZHENG Xiaoming. Research on graphene based on raman spectroscopy[D]. Changsha: National University of Defense Technology, 2015(in Chinese).
[25] ZHANG J, YANG H, SHEN G, et al. Reduction of graphene oxide via L-ascorbic acid[J]. Chemical Communications,2010,46(7):1112-1114. DOI: 10.1039/B917705A
[26] RHEE J H, CHUNG C C, DIAU W G. A perspective of mesoscopic solar cells based on metal chalcogenide quantum dots and organometal-halide perovskites [J]. NPG Asia Materials, 2013, 5(10): e68.
[27] MOON I K, YOON S, CHUN K Y, et al. Highly elastic and conductive N-doped monolithic graphene aerogels for multifunctional applications[J]. Advanced Functional Materials,2015,25(45):6976-6984. DOI: 10.1002/adfm.201502395
[28] LI C, DING M, ZHANG B, et al. Graphene aerogels that withstand extreme compressive stress and strain [J]. Nanoscale, 2018, 10(38): 18291-18299.
[29] CHEN Z, REN W, GAO L, et al. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition[J]. Nature Materials,2011,10(6):424-428. DOI: 10.1038/nmat3001
[30] ZHANG X, SUI Z, XU B, et al. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources[J]. Journal of Materials Chemistry,2011,21(18):6494-6497. DOI: 10.1039/c1jm10239g
[31] ZHAO Y, HU C, HU Y, et al. A versatile, ultralight, nitrogen-doped graphene framework[J]. Angewandte Chemie International Edition,2012,51(45):11371-11375. DOI: 10.1002/anie.201206554
[32] SUN H, XU Z, GAO C. Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels [J]. Advanced Materials, 2013, 25 (18): 2554-2560.
[33] LI Y, CHEN J, HUANG L, et al. Highly compressible macroporous graphene monoliths via an improved hydrothermal process[J]. Advanced Materials,2014,26(28):4789-4793. DOI: 10.1002/adma.201400657
[34] BARG S, PEREZ F M, NI N, et al. Mesoscale assembly of chemically modified graphene into complex cellular networks[J]. Nature Communications,2014,5(1):4328. DOI: 10.1038/ncomms5328
[35] WU Y, YI N, HUANG L, et al. Three-dimensionally bonded spongy graphene material with super compressive elasticity and near-zero poisson’s ratio[J]. Nature Communications,2015,6(1):6141. DOI: 10.1038/ncomms7141
[36] LI Y, ZHANG H B, ZHANG L, et al. One-pot sintering strategy for efficient fabrication of high-performance and multifunctional graphene foams[J]. ACS Applied Materials & Interfaces,2017,9(15):13323-13330.
[37] YANG J, LI X, HAN S, et al. Air-dried, high-density graphene hybrid aerogels for phase change composites with exceptional thermal conductivity and shape stability[J]. Journal of Materials Chemistry A,2016,4(46):18067-18074. DOI: 10.1039/C6TA07869A
[38] YANG H, LI Z, LU B, et al. Reconstruction of inherent graphene oxide liquid crystals for large-scale fabrication of structure-intact graphene aerogel bulk toward practical applications[J]. ACS Nano,2018,12(11):11407-11416. DOI: 10.1021/acsnano.8b06380
[39] 刘亮, 鲍瑞, 易健宏. 碳纳米管-石墨烯气凝胶的制备与性能 [J]. 复合材料学报, 2017, 34(10): 2296-2303. LIU Liang BAO Rui, YI Jianhong. Preparation and properties of CNTs-graphene aerogel[J]. Acta Materiae Compositae Sinica, 2017, 34(10): 2296-2303(in Chinese).
-
期刊类型引用(1)
1. 蔡静宇,苗晓雨,赵玉来,肖龙强. FeOOH-RGO气凝胶光催化苯制备苯酚探索性教学实验设计. 大学化学. 2024(04): 169-177 .  百度学术
百度学术
其他类型引用(1)
-




 下载:
下载: