Preparation of nano core-shell PS-CHO@RGO composite microspheres by in-situ polymerization as a potassium hydrogen persulfate catalytic activator for methylene blue degradation
-
摘要: 还原氧化石墨烯(RGO)具有比表面积大、电子传输效率高、吸附速率快等优点,在处理油污、重金属离子、有机染料等领域均有应用,但由于自团聚而造成的分散性差等问题限制了其进一步应用。采用原位聚合法制备纳米核-壳型聚苯乙烯醛基微球(PS-CHO)@RGO复合微球。利用TEM、Raman、XRD、XPS及绝缘电阻测试仪对PS-CHO@RGO复合微球的形貌及理化性能进行表征。以亚甲基蓝(MB)为目标污染物,探究了PS-CHO@RGO复合微球在少量过硫酸氢钾(PMPS)存在下的氧化活性,并提出了降解机制。结果表明,RGO片层均匀包覆于PS-CHO微球表面,有效改善了分散性。制备所得PS-CHO@RGO复合微球的渗透阈值低,导电网络完善。降解实验中,PS-CHO@RGO复合微球可以激发PMPS生成硫酸根自由基(SO4−•),MB的氧化降解率显著提高,60 min内可达98%以上。PS-CHO@RGO复合微球同时表现出良好的稳定性,通过高速离心的方式实现循环利用。Abstract: The reduced graphene oxide (RGO) has been widely used in the treatment of oil, heavy metal ions, organic dyes and other fields due to the large specific surface area, high electron transport efficiency and fast adsorption rate. However, the poor dispersion caused by agglomeration limited the further research. In-situ polymerization was used to prepare nano core-shell polystyrene aldehyde microspheres (PS-CHO)@RGO composite microspheres. The morphology and physicochemical properties of the PS-CHO@RGO composite microspheres were characterized by TEM, Raman, XRD, XPS and insulation resistance tester. The methylene blue (MB) was selected as the target pollutant, the oxidation activity of PS-CHO@RGO composite microspheres in the presence of potassium hydrogen persulfate (PMPS) was investigated, and the degradation mechanism was proposed. The results indicate that RGO layer is uniformly coated on the surface of PS-CHO microspheres, which effectively improved the dispersion. The prepared PS-CHO@RGO composite microspheres are described as low penetration threshold and perfect conductive network. In the degradation experiment, PS-CHO@RGO composite microspheres can stimulate PMPS to generate sulfate radicals (SO4−•), over 98% of MB is catalytically degraded within 60 minutes. Meanwhile, the PS-CHO@RGO composite microspheres also show good stability and can be recycled by high-speed centrifugation.
-
Keywords:
- composite microspheres /
- reduced graphene oxide (RGO) /
- core-shell type /
- methylene blue /
- catalytic /
- degradation
-
石墨烯是由碳原子的sp2杂化组成的蜂窝状2D晶体结构物质[1-2],是制备其他石墨烯基复合材料的基础。由于其高导热率、出众的机械强度、柔韧性和较大的比表面积,被广泛应用于超高速晶体管、生物传感器和电动机械[3-5]等领域。还原氧化石墨烯(RGO)是由氧化石墨烯(GO)经强还原剂还原后形成的。由于sp2碳原子网络的恢复,重新引入了强烈的π-π相互作用破坏了原有的微观结构[6],在吸附、离子迁移、催化等方面带来了不良影响。因此,必须要解决RGO片层之间的堆叠问题[7]。
染料废水是当今世界上最常见、污染范围最广的工业废水之一[8-10],是环境中水污染的重要来源。最近的研究表明,由于硫酸根自由基(SO4−•)较高的氧化电位(2.5~3.1 eV)而展现出比羟基自由基(OH−• ,氧化电位为2.7 eV)更优异的降解活性[11-12],是一种处理染料废水的新选择。Ling等[12]研究证明,均相Co(Ⅱ)可以激发过硫酸盐(PMS)从而生成强氧化性的SO4−•,室温下90 min内对亚甲基蓝(MB)的降解率可达70.54%。但这一体系的弊端在于反应前Co(Ⅱ)几乎不可避免的浸出,对水体和环境造成新的污染。鉴于绿色和可持续发展的新要求,急需研发出一种环境友好型的无金属催化剂。而石墨烯基复合材料在催化降解染料废水领域的报告尚不多见。
本文依据GO表面基团的电离π电子与聚合物表面π-π共轭自组装的原理,在硼氢化钠的还原作用下,原位聚合法制备聚苯乙烯醛基(PS-CHO)微球为核、表面包覆RGO的纳米核-壳型PS-CHO@RGO复合微球。PS-CHO微球作为阻隔物有效阻止了RGO片层之间的堆叠,改善了其分散性。研究了PS-CHO@RGO复合微球催化过硫酸氢钾(2KHSO5•KHSO4•K2HSO4)降解MB染料的性能,并探讨了其机制。
1. 实验材料及方法
1.1 原材料
苯乙烯(ST)、丙烯醛(C3H4O)、聚乙烯吡咯烷酮(PVP)、偶氮二异丁腈(AIBN),中国医疗药品集团总公司。其中,ST使用前需在70℃下减压蒸馏,以去除阻聚剂。石墨粉(GP)、硼氢化钠、过硫酸氢钾(2KHSO5•KHSO4•K2HSO4)、无水乙醇,阿拉丁石化有限公司。亚甲基蓝(MB)溶液由去离子水配制。
1.2 聚苯乙烯醛基(PS-CHO)微球的制备
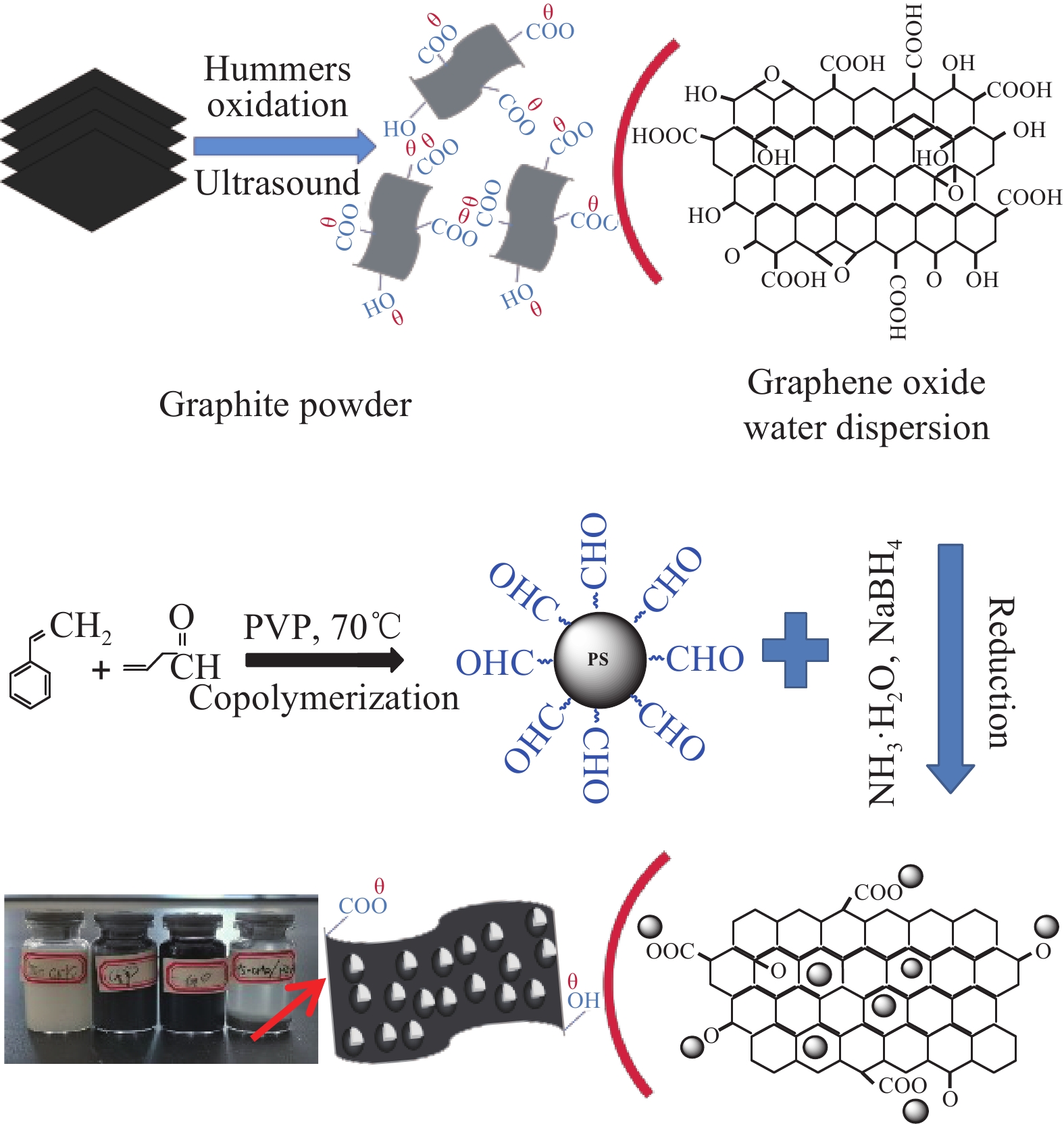
通过共聚合[13-14]的方式制备表面连接醛基的PS-CHO微球。在超声作用下,准确称取2.5 g PVP、0.1 g AIBN分散在40 mL去离子水中;然后将6 mL ST和20 mL无水乙醇依次加入上述分散液中,充分搅拌30 min,待混合液呈乳白色时快速加入3 mL C3H4O,稀硝酸(2 mol/L)调节pH值至中性。将混合液置入恒温水浴中,70℃反应8 h,自然冷却至室温后将所得混合液过滤、洗涤,并在65℃的真空烘箱中干燥12 h,即制得PS-CHO微球。
1.3 PS-CHO@还原氧化石墨烯(RGO)复合微球的制备
氧化石墨(GO)由改良的Hummer法[15]制备。称取5 mg干燥后的GO,通过超声辅助溶解分散在20 mL去离子水中。称取0.2 g PS-CHO微球分散在40 mL无水乙醇中,室温下将两溶液进行混合搅拌。将混合液置入恒温70℃水浴中,持续搅拌条件下向反应体系中缓慢滴加硼氢化钠/水溶液(10 g/L)。滴加完成后水浴升温至85℃,反应3 h,即得到PS-CHO@RGO复合微球。石墨烯的堆积密度定为2.5 g/cm3,计算可知,PS-CHO@RGO复合微球中RGO的体积分数为0.98vol%,记为PS-CHO@RGO(0.98vol%)。图1为PS-CHO@RGO复合微球的制备流程及样品照片。可知,混合液下层出现褐色絮状沉淀,上层为透明溶液,取下层沉淀离心即可得到PS-CHO@RGO复合微球,真空烘箱中40℃烘干后备用。
1.4 测试与表征
复合微球选用PS-CHO@RGO(0.98vol%)作为基准样。采用JEM-2000型TEM表征复合微球的微观形貌;采用TH-268A型绝缘电阻测试仪测试复合微球的电阻;采用Escalab 250Xi型XPS进行元素分析;采用Renishaw 2000型Raman光谱仪进行化学结构分析;采用德国Bruke D8型XRD进行晶体结构分析,CuKα靶(λ=0.154 nm),电压为40 kV,电流为35 mA。
1.5 催化降解实验
PS-CHO@RGO复合微球的催化活性通过MB的降解来评估。MB的水溶液在紫外-可见光有响应,在特定吸收波长为664 nm处出现吸收峰,且峰的强弱和溶液浓度呈线性相关[16-17],因此通过紫外-可见分光光度计对降解进程进行监测。取0.02 g PS-CHO@RGO复合微球超声分散在80 mL去离子水中,向其中加入100 mg/L的MB/水溶液20 mL,充分混合均匀后加入0.03 g过硫酸氢钾。将混合液置入50℃恒温水浴反应。每隔5 min从体系中取样4 mL。在10000 r/min的速率下离心。底部沉积的复合微球洗净烘干后投入下一次催化实验,取上层清液检测溶度。降解率计算如下:
R=(1−CtC0)×100% 式中:R为降解率;C0为初始浓度;Ct为实时浓度。
2. 结果与讨论
2.1 PS-CHO@RGO复合微球的微观形貌
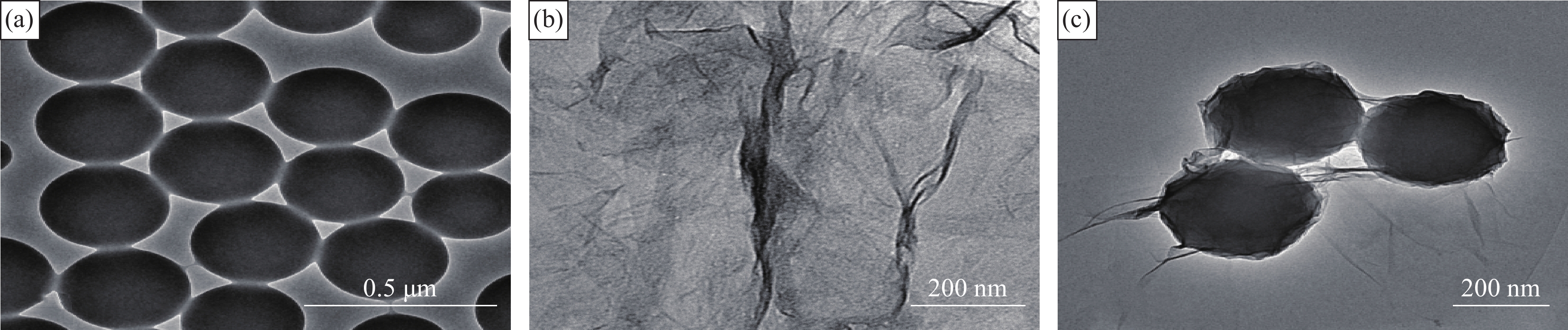
图2为PS-CHO微球、GO和PS-CHO@RGO复合微球的TEM图像。由图2(a)可知,共聚合制备的PS-CHO微球粒径均匀,分散性良好。由图2(b)可知,GO呈片层状褶皱。由图2(c)可见,原本光滑的微球表面出现石墨烯的特征褶皱,PS-CHO@RGO复合微球具有清晰的纳米核-壳结构,其粒径为(210±5) nm。还原过程没有破坏核-壳结构。RGO全部黏附在PS-CHO微球表面,没有散落团聚在溶液体系中,证明结构具有稳定性,PS-CHO微球的存在改善了RGO的分散性。
2.2 PS-CHO@RGO复合微球的微观结构
2.2.1 PS-CHO@RGO复合微球的化学结构
图3为PS-CHO微球、GO和PS-CHO@RGO复合微球的拉曼图谱。可知,1340 cm−1处为D峰,1580 cm−1处为G峰,分别代表石墨烯材料的无序、有序状态和C原子的堆叠方式[18]。GO及PS-CHO@RGO复合微球均有一个突出的G峰及D峰,这归因于结构缺陷和E2g振动模式的一阶散射。G峰与D峰的强度比(ID/IG)可以用来衡量石墨晶的无序程度[19]。GO的G峰和D峰大小基本相同,且ID/IG=0.87,这是由于GO片层中插入的含氧官能团大大增加了其无序状态,材料的规整程度下降,缺陷增大。与GO相比,PS-CHO@RGO复合微球的ID/IG提高到1.07。这是由于PS链的苯环与RGO基面之间形成非共价π-π堆积。将PS链接枝到RGO骨架上后,部分sp2 C原子转换为sp3 C原子,导致ID/IG增大。说明GO在被还原过程中sp2区域平均尺寸减小,多芳族结构域数量增加及碳晶格的缺陷提高。
2.2.2 PS-CHO@RGO复合微球的晶体结构
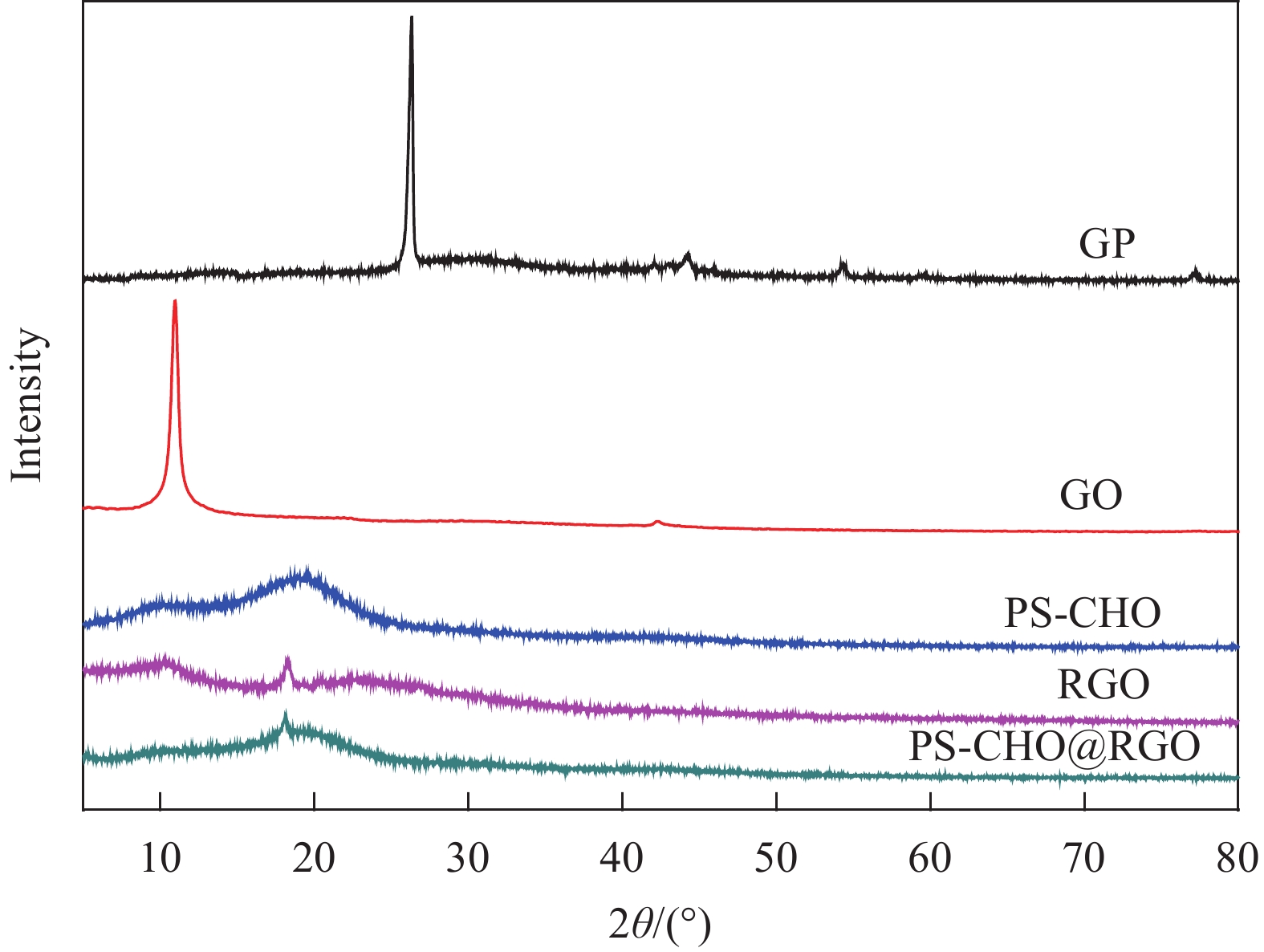
图4为PS-CHO微球、GP、GO和PS-CHO@RGO复合微球的XRD图谱。可知,GP和GO的图谱中出现尖锐的X射线衍射峰,说明GP和GO具有良好的晶型。GP在2θ=26.38°处出现特征峰,GO在2θ=11.15°处有一个显著的特征峰,布拉格公式如下:
d=nλ2sinθ 式中:d为晶面间距(nm);n为任何正整数,又称衍射级数,取1;λ为X射线的波长,为0.154 nm;θ为入射角的余角。由式(2)计算可知,dGP=0.152 nm,dGO=0.303 nm。说明在氧化过程中GP的层间被插入了大量含氧官能团,使产物GO的层间距扩大[20]。PS-CHO微球在2θ=19°附近出现的无定型峰为聚苯乙烯的特征峰。RGO在2θ=18.05°处出现特征衍射峰,对应RGO的(002)晶面[18],证明GO被成功还原。PS-CHO@RGO复合微球在2θ=18.05°处也出现了特征衍射峰,证明RGO已经包覆于PS-CHO微球表面。
2.3 PS-CHO@RGO复合微球的化学元素
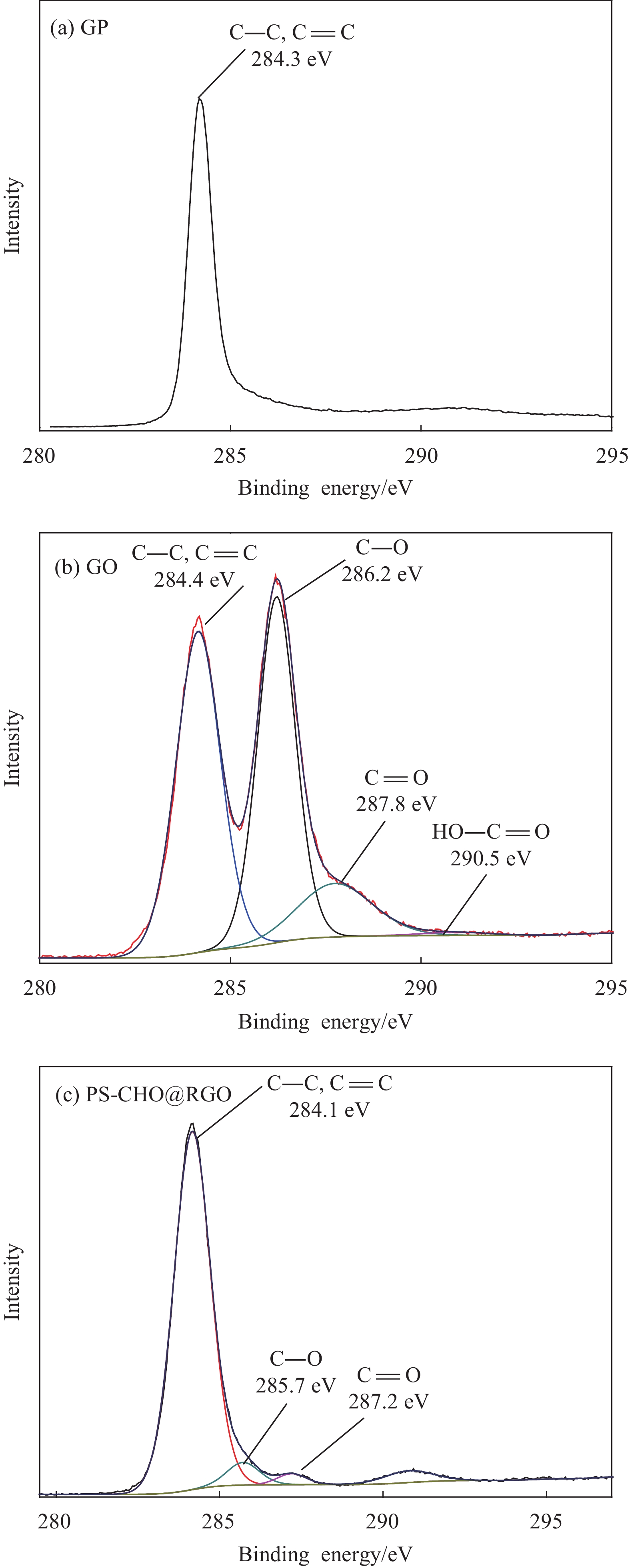
图5为GP、GO和PS-CHO@RGO复合微球的C1s XPS图谱。由图5(a)可知,GP在284.3 eV处的主峰对应石墨的C—C或(C=C)键[21]。由图5(b)可知,GO在四种不同的C环境下具有相当程度的氧化。GO在284.4、286.2、287.8、290.5 eV处的四个峰分别为C—H (或C—C、C=C)、C—OH (或环氧化物)、C=O (羰基碳)和O=C—OH (羰酸碳)官能团[22]。其中,在286.2 eV处的sp3强峰表明GO中大多数含氧官能团为羟基(C—OH)和环氧化物(C—O—C)。由图5(c)可以看出,PS-CHO@RGO复合微球归属于C—OH(或环氧化物)的峰强明显减弱,强度降低,表明通过硼氢化钠还原可有效除去氧基团,尤其是可大量除去C—OH(或环氧化物),大多数共轭碳网络已恢复。表1为GP、GO和PS-CHO@RGO复合微球的化学元素含量。可知,GP中的O元素含量随着其氧化程度的差异而产生变化。GO中O元素含量提高,同时也证明在氧化过程中大量含氧基团被引入石墨。这些含氧基团破坏了石墨sp2的键合碳网络。PS-CHO@RGO复合微球中C元素和O元素原子比的提高证明PS-CHO成功与RGO复合。
表 1 GP、GO和PS-CHO@RGO复合微球的化学元素含量Table 1. Chemical element contents of GP, GO and PS-CHO@RGO composite microspheresSample C/at% O/at% C/O ratio GP 98.05 1.03 93.38 GO 60.23 39.67 1.51 PS-CHO@RGO 90.34 9.66 9.35 2.4 PS-CHO@RGO复合微球的导电性能
首先,PS-CHO@RGO复合微球在10 MPa压力下压片,通过双电测四探针测试仪测量表面电阻[23]。计算公式如下:
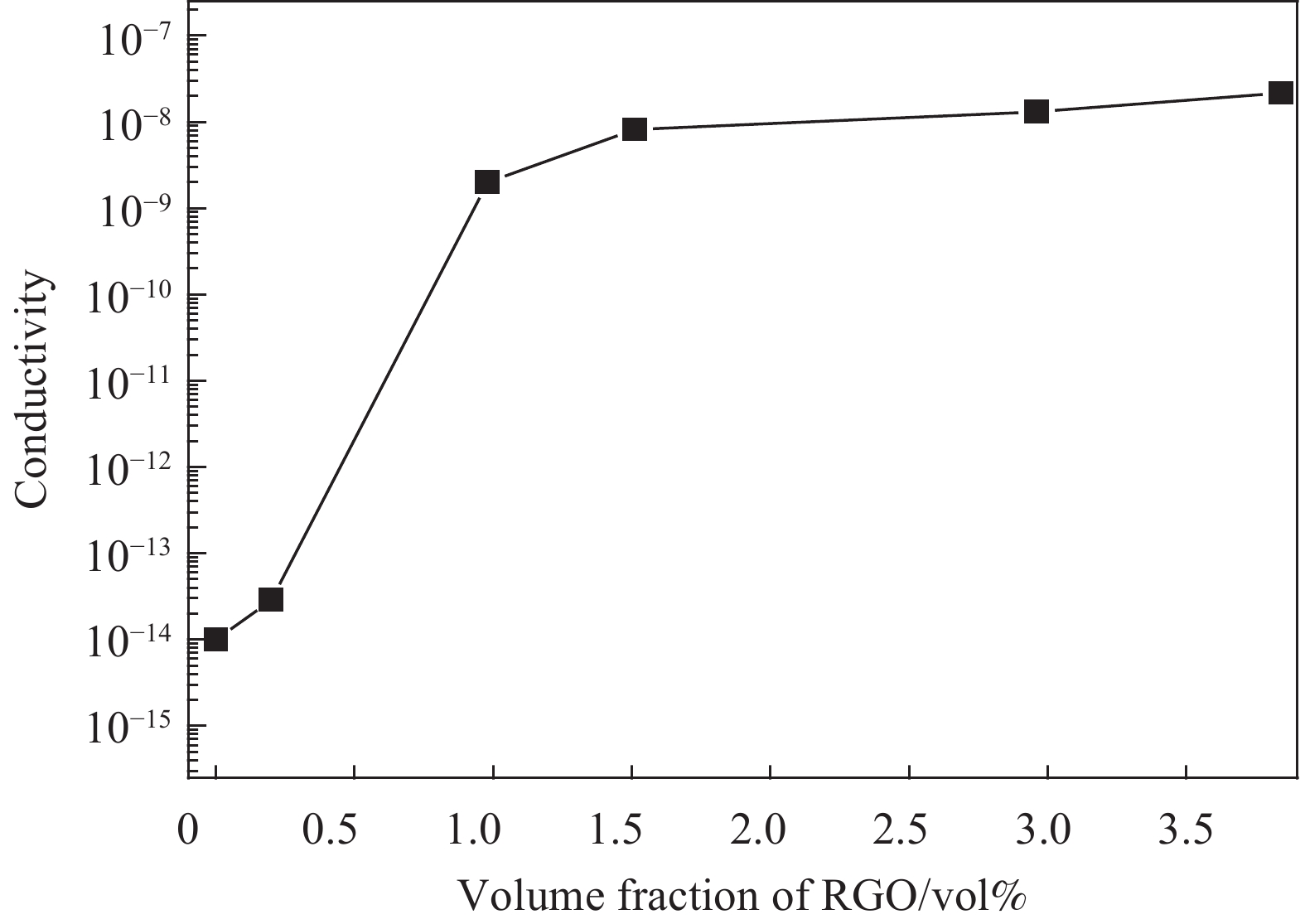
ρ=RSAL=(RT−RB)AL 式中:ρ为电阻率;A为截面面积;L为试样厚度;RT为总电阻;RS为样品电阻;RB为参比电阻。电导率σ为电阻率ρ的倒数。图6为不同RGO体积分数的PS-CHO@RGO复合微球的电导率。可知,PS-CHO微球电阻为1×1014 Ω,属于完全不导电的绝缘体。随着RGO体积分数的增加,PS-CHO@RGO复合微球电导率也随之提高。当RGO体积分数达到0.98vol%时,PS-CHO@RGO复合微球的电导率出现五个数量级的梯度式提高,证明渗透阈值出现,PS-CHO@RGO复合微球出现渗虑,此时其电导率已达到半导体水平。极低的渗透阈值也意味着复合材料具有良好的导电性和完善的导电网络。
2.5 PS-CHO@RGO复合微球的催化活性
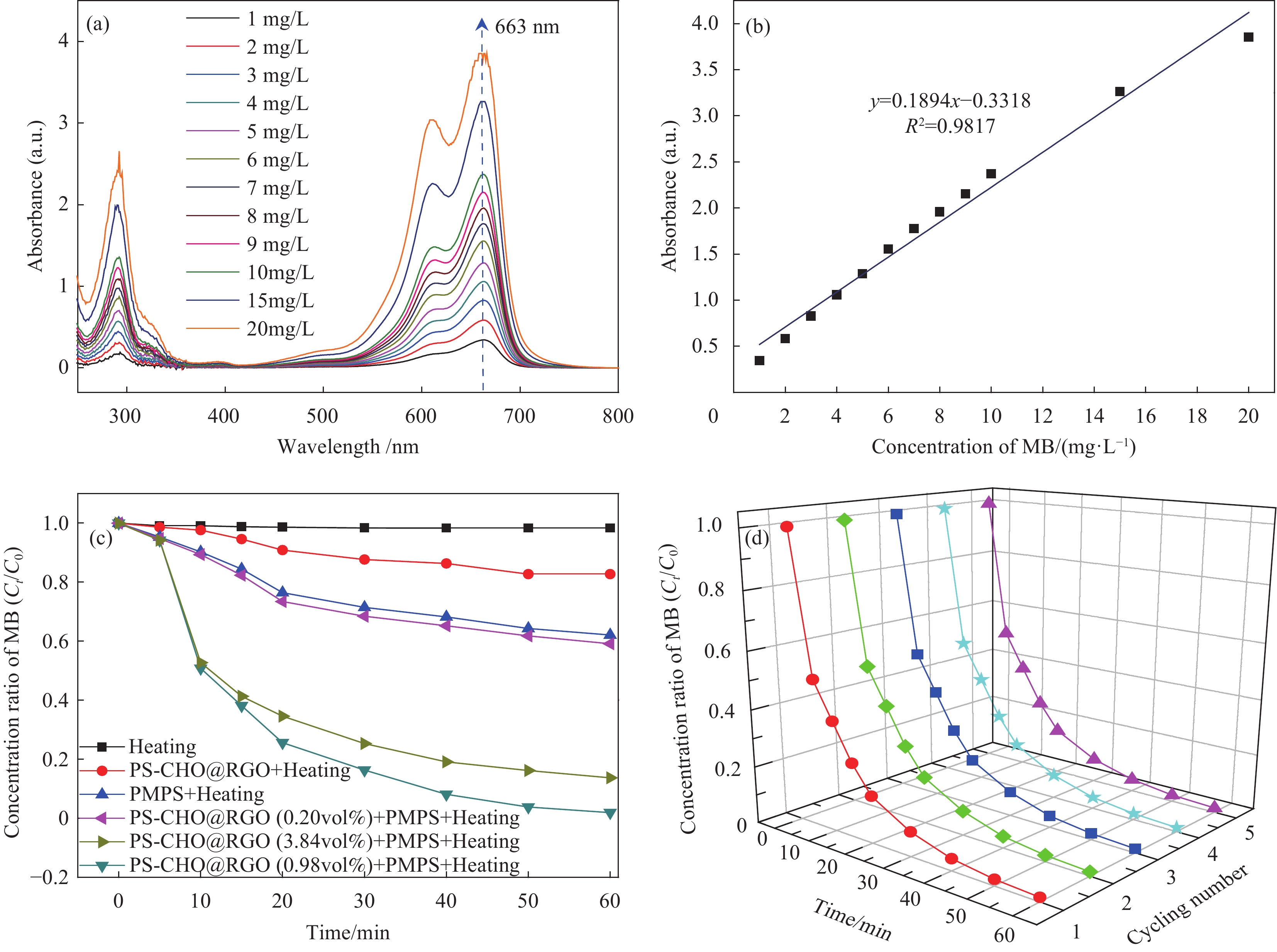
图7(a)和图7(b)分别为不同浓度MB的吸光度及MB的吸光度与浓度线性拟合曲线。可知,MB吸光度随着其浓度的增加而呈线性增长。拟合后的线性方程为y=0.1894x−0.3318,R2=0.9817,拟合度较高,展现出较好的线性关系。图7(c)为不同条件下PS-CHO@RGO复合微球降解MB的浓度随反应时间的变化曲线。可知,MB结构稳定,在50℃水浴条件下几乎不发生降解。PS-CHO@RGO复合微球对降解MB的效果也十分微弱,60 min内降解率仅有17.3%。过硫酸氢钾具有一定氧化性,能缓慢降解MB,60 min内降解率为37.6%。PS-CHO@RGO(0.98vol%)复合微球为催化剂,可以有效加速降解过程的反应速率,且高效彻底,60 min内基本实现完全降解。由文献[8]可知,在相同反应条件下,相较于单一片层状RGO作催化剂的反应体系,MB的降解速率得到显著提高,降解率提高了1.8倍,可达到98.54%。可知,当RGO体积分数为0.20vol%时,PS-CHO@RGO复合微球的催化活性微弱。这是由于导电网络尚未成型,PS-CHO@RGO复合微球的电导率较低。当RGO体积分数增加至PS-CHO@RGO复合微球出现渗透阈值时,期催化活性得到大幅提高,验证了PS-CHO@RGO复合微球电导率曲线的突变。当RGO体积分数为3.84 vol%时,PS-CHO@RGO复合微球的催化活性有所降低。这是引用大量RGO片层无规则堆叠在PS-CHO微球表面,导电能力小幅提高的同时掩蔽了暴露的活性位点。图7(d)为PS-CHO@RGO复合微球降解MB的循环催化曲线。可知,在经过5次重复实验后,PS-CHO@RGO复合微球依然可以保持良好的催化活性,降解率仅在第5次的40 min后出现少量下降,证明PS-CHO@RGO复合微球的结构稳定,RGO被牢牢固定在其表面。
![]() 图 7 PS-CHO@RGO复合微球的催化活性: (a)不同浓度亚甲基蓝(MB)的吸光度; (b)吸光度与浓度线性方程;(c)不同反应条件下MB浓度的变化曲线; (d)循环催化曲线Figure 7. Catalytic activities of PS-CHO@RGO composite microspheres: (a) Absorbance of different concentrations of methylene blue (MB); (b) Linear equation of absorbance and concentration of MB; (c) Concentration rate curves of MB under different reaction conditions; (d) Cyclic catalysis plotsPMPS—Potassium hydrogen persulfate
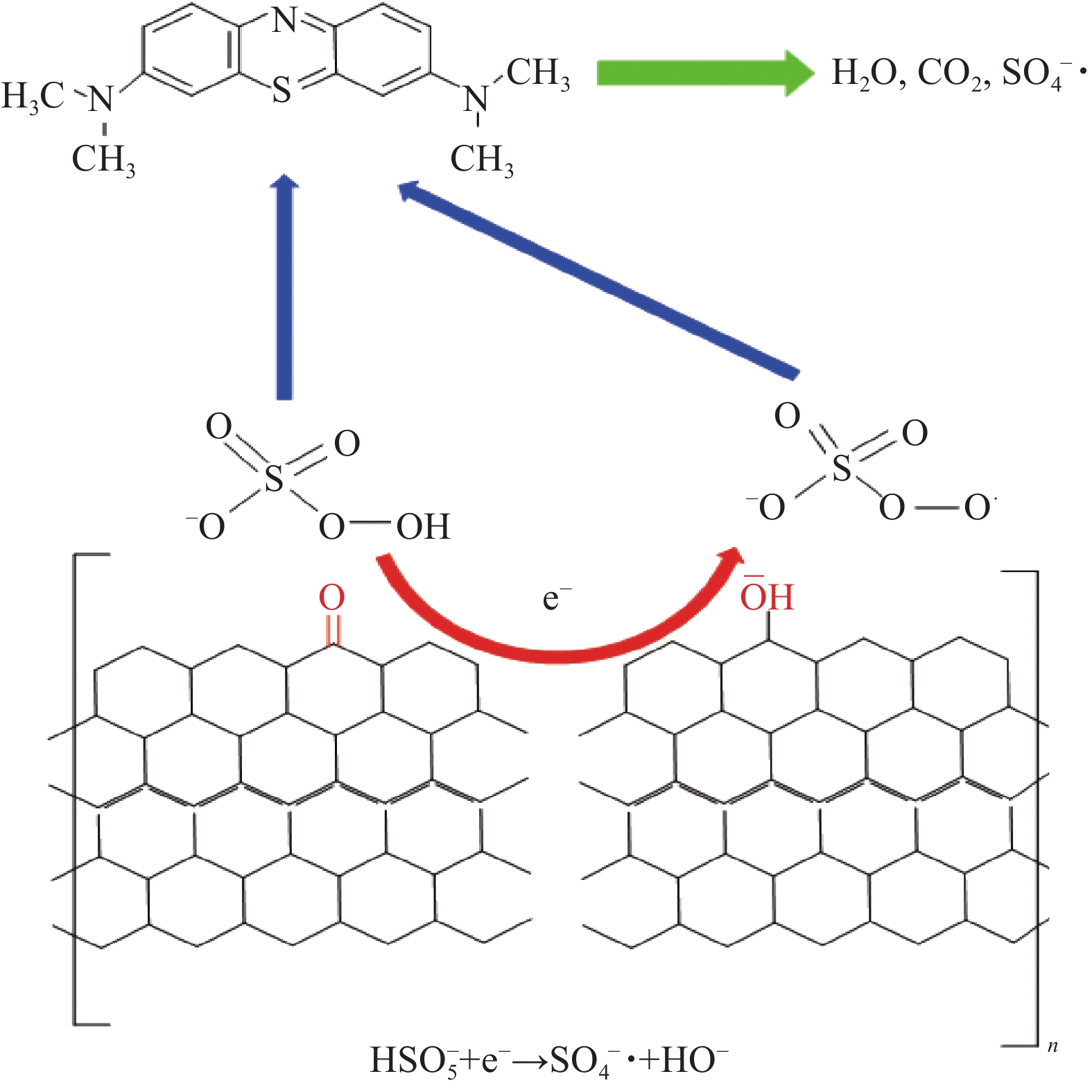
图 7 PS-CHO@RGO复合微球的催化活性: (a)不同浓度亚甲基蓝(MB)的吸光度; (b)吸光度与浓度线性方程;(c)不同反应条件下MB浓度的变化曲线; (d)循环催化曲线Figure 7. Catalytic activities of PS-CHO@RGO composite microspheres: (a) Absorbance of different concentrations of methylene blue (MB); (b) Linear equation of absorbance and concentration of MB; (c) Concentration rate curves of MB under different reaction conditions; (d) Cyclic catalysis plotsPMPS—Potassium hydrogen persulfate图8为PS-CHO@RGO复合微球催化降解MB的机制。可知,受界面张力影响,RGO的边缘缺陷和曲率会产生非六元碳环[24],引发C—C σ键的断裂,产生之字型边缘。边缘处的非稳态使π电子不再受边缘的限制[25-26]。RGO将在锯齿形边缘保留少量富含电子的含氧官能团,如酮基团(—C=O—)作为催化活性位点,提供游离态电子(e−),激发过硫酸根(HSO5−)生成强氧化性的硫酸根自由基(SO4−•),氧化MB生成CO2和水,实现染料废水的绿色降解。
3. 结 论
(1)原位聚合法制备了聚苯乙烯醛基微球负载还原氧化石墨烯(PS-CHO@RGO)复合微球。通过TEM、XRD、Raman及XPS等表征,证明PS-CHO@RGO复合微球粒径均匀,分散性良好。PS-CHO微球的引入可以有效改善RGO片层间的堆叠,提高分散性。RGO体积分数达到0.98vol%时,PS-CHO@RGO复合微球的电导率出现渗透阈值,导电能力显著提高,且导电网络完善。
(2) PS-CHO@RGO复合微球具有特殊的纳米核-壳结构,RGO可以包覆于微球表面使层状边缘产生断裂,从而产生催化活性。
(3) PS-CHO@RGO复合微球作为催化剂,可以激发过硫酸氢钾(2KHSO5•KHSO4•K2HSO4)产生强氧化性的硫酸根自由基(SO4−•),实现对亚甲基蓝染料废水的高效降解,60 min降解率可达到98.54%,催化活性显著优于无序片状RGO。同时,PS-CHO@RGO复合微球结构稳定,可以循环使用5次以上,因此有望成为催化降解有机污染物的新材料。
-
图 7 PS-CHO@RGO复合微球的催化活性: (a)不同浓度亚甲基蓝(MB)的吸光度; (b)吸光度与浓度线性方程;(c)不同反应条件下MB浓度的变化曲线; (d)循环催化曲线
Figure 7. Catalytic activities of PS-CHO@RGO composite microspheres: (a) Absorbance of different concentrations of methylene blue (MB); (b) Linear equation of absorbance and concentration of MB; (c) Concentration rate curves of MB under different reaction conditions; (d) Cyclic catalysis plots
PMPS—Potassium hydrogen persulfate
表 1 GP、GO和PS-CHO@RGO复合微球的化学元素含量
Table 1 Chemical element contents of GP, GO and PS-CHO@RGO composite microspheres
Sample C/at% O/at% C/O ratio GP 98.05 1.03 93.38 GO 60.23 39.67 1.51 PS-CHO@RGO 90.34 9.66 9.35 -
[1] WU J M, JING G J, LU X L, et al. The effect of sulfonated graphene on the rheological properties of cement paste[J]. Journal of Nanoscience and Nanotechnology,2020,20(12):7495-7505. DOI: 10.1166/jnn.2020.18871
[2] LI D, KANER R B. Graphene-based materials[J]. Nature Nanotechnology,2008,3:101-105. DOI: 10.1038/nnano.2007.451
[3] WEI X, MENG Z, RUIZ L, et al. Recoverable slippage mechanism in multilayer graphene leads to repeatable energy dissipation[J]. ACS Nano,2016,10(2):1820-1828. DOI: 10.1021/acsnano.5b04939
[4] LI C, XUE Z, QIN J, et al. Synthesis of nickel hydroxide/delaminated-Ti3C2 MXene nanosheets as promising anode material for high performance lithium ion battery[J]. Journal of Alloys and Compounds,2020,842:155812.
[5] ANSARI N, PAYAMI Z. Synthesis of magnetic graphene-Fe3O4 nanocomposites by electrochemical exfoliation method[J]. Journal of Nanostructures,2020,10(1):39-43.
[6] CHI F, CHEN P, MAO C. Highly efficient photocatalytic disinfection of Escherichia coli by rose bengal-functionalized graphene oxide nanosheets[J]. Journal of Nanoscience and Nanotechnology,2020,20(12):7558-7568. DOI: 10.1166/jnn.2020.18615
[7] RAMANATHAN T, ABDALA A A, STANKOVICH S, et al. Functionalized graphene sheets for polymer nanocomposites[J]. Nature Nanotechnology,2008,3:327-331. DOI: 10.1038/nnano.2008.96
[8] SUN H, LIU S, ZHOU G, et al. Reduced graphene-oxide for catalytic oxidation of aqueous organic pollutants[J]. ACS Applied Materials & Interfaces,2012,4(10):5466-5471.
[9] SHUKLA P, SUN H, WANG S, et al. Co-SBA-15 for heterogeneous oxidation of phenol with sulfate radical for wastewater treatment[J]. Catalysis Today,2011,175(1):380-385. DOI: 10.1016/j.cattod.2011.03.005
[10] JAYANTHI S, LAVANYA T, DUTTA M, et al. Fabrication and characterization of graphene nanofibers by electrospinning technique and its electrochemical properties[J]. Journal of Nanoscience and Nanotechnology,2020,20(12):7659-7664. DOI: 10.1166/jnn.2020.18625
[11] ANIPSITAKIS G P, STATHATOS E, DIONYSIOU D D. Heterogeneous activation of oxone using Co3O4[J]. The Journal of Physical Chemistry B,2005,109(27):13052-13055. DOI: 10.1021/jp052166y
[12] LING S K, WANG S, PENG Y. Oxidative degradation of dyes in water using Co2+/H2O2 and Co2+/peroxymonosulfate[J]. Journal of Hazardous Materials,2010,178(1-3):385-389. DOI: 10.1016/j.jhazmat.2010.01.091
[13] BAI F, YANG X L, ZHAO Y Z, et al. Synthesis of core-shell microspheres with active hydroxyl groups by two-stage precipitation polymerization[J]. Polymer International, 2005, 54(1): 1 68-174.
[14] OMI S, SAITO M, HASHIMOTO T, et al. Preparation of monodisperse polystyrene spheres incorporating polyimide prepolymer by dispersion polymerization in the presence of L-ascorbic acid[J]. Journal of Applied Polymer Science,1998,68(6):897-907. DOI: 10.1002/(SICI)1097-4628(19980509)68:6<897::AID-APP4>3.0.CO;2-C
[15] RIAHI K Z, SDIRI N, ENNIGROU D J, et al. Investigations on electrical conductivity and dielectric properties of graphene oxide nanosheets synthetized from modified Hummer’s method[J]. Journal of Molecular Structure,2020,1216:128304.
[16] HOUAS A, LACHHEB H, KSIBI M, et al. Photocatalytic degradation pathway of methylene blue in water[J]. Applied Catalysis B: Environmental,2001,31(2):145-157. DOI: 10.1016/S0926-3373(00)00276-9
[17] UMEBAYASHI T, YAMAKI T, TANAKA S, et al. Visible light-induced degradation of methylene blue on S-doped TiO2[J]. Chemistry Letters,2003,32(4):330-331. DOI: 10.1246/cl.2003.330
[18] WU H, ZHAO W, HU H, et al. One-step in situ ball milling synthesis of polymer-functionalized graphene nanocomposites[J]. Journal of Materials Chemistry,2011,21(24):8626-8632. DOI: 10.1039/c1jm10819k
[19] LIU Y T, YANG J M, XIE X M, et al. Polystyrene-grafted graphene with improved solubility in organic solvents and its compatibility with polymers[J]. Materials Chemistry and Physics,2011,130(1-2):794-799. DOI: 10.1016/j.matchemphys.2011.07.067
[20] FAN Z, WANG K, WEI T, et al. An environmentally friendly and efficient route for the reduction of graphene oxide by aluminum powder[J]. Carbon,2010,48(5):1686-1689. DOI: 10.1016/j.carbon.2009.12.063
[21] ZHANG H, LIU S. Electrochemical biosensors based on nitrogen-doped reduced graphene oxide for the simultaneous detection of ascorbic acid, dopamine and uric acid[J]. Journal of Alloys and Compounds,2020,842:155873.
[22] KWON O S, PARK S J, HONG J Y, et al. Flexible FET-type VEGF aptasensor based on nitrogen-doped graphene converted from conducting polymer[J]. ACS Nano,2012,6(2):1486-1493. DOI: 10.1021/nn204395n
[23] QI X Y, YAN D, JIANG Z G, et al. Enhanced electrical conductivity in polystyrene nanocomposites at ultra-low graphene content[J]. ACS Applied Materials & Interfaces,2011,8(3):3130-3133.
[24] LUO Y, ZHAO P, YANG Q, et al. Fabrication of conductive elastic nanocomposites via framing intact interconnected graphene networks[J]. Composites Science and Technology,2014,100:143-151. DOI: 10.1016/j.compscitech.2014.05.037
[25] JU S A, KIM K, KIM J H, et al. Graphene-wrapped hybrid spheres of electrical conductivity[J]. ACS Applied Materials & Interfaces,2011,3(8):2904-2911.
[26] KAVITHA K, URADE A R, KAUR G, et al. Low-temperature chemical vapor deposition growth of graphene layers on copper substrate using camphor precursor[J]. Journal of Nanoscience and Nanotechnology,2020,20(12):7698-7704. DOI: 10.1166/jnn.2020.18862
-




 下载:
下载: