Preparation and photocatalytic hydrogen production performance of N-g-C3N4/CoS2 composites
-
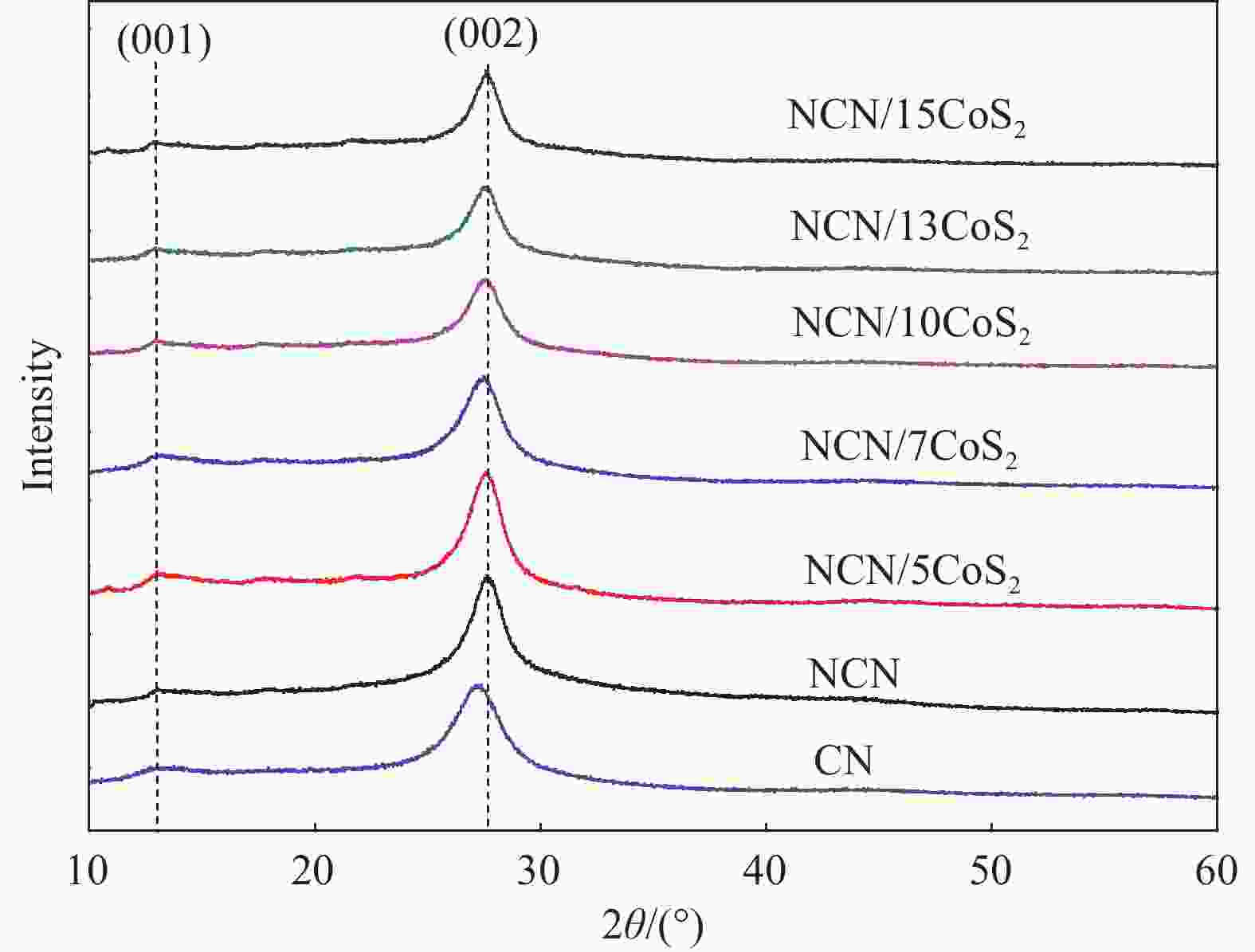
摘要: 氮化碳(CN)是目前最具发展前景的非金属催化剂之一,但由于其特殊的电子结构导致分子内载流子分离效率较差,其光催化活性不理想。为了改善其光催化性能,首先以尿素和柠檬酸为原料,利用高温缩合方法得到N掺杂的g-C3N4(NCN)。然后通过光沉积的方法成功制备了CoS2修饰的NCN光催化复合材料(NCN/CoS2)。CoS2的引入有效增强的氮化碳的光生载流子分离效率。同时N的掺杂有效调控氮化碳的带隙,拓宽了氮化碳的可见光响应范围。光催化产氢结果显示,在可见光照射(λ > 420 nm)下,NCN/10CoS2复合材料具有最佳的光催化产氢性能(73.8 μmol·g−1·h−1),分别为NCN(15.0 μmol·g−1· h−1和CN/10CoS2(7.1 μmol·g−1· h−1)的4.9和10.4倍。Abstract: Carbon nitride (CN) is one of the most promising non-metal catalysts currently, but its photocatalytic activity is not ideal due to its poor carrier separation efficiency within molecules caused by its unique electronic structure. In order to improve its photocatalytic performance, N-doped g-C3N4(NCN) was firstly prepared by high temperature condensation method using urea and citric acid as raw materials. Then, the CoS2-modified NCN composites (NCN/CoS2) were successfully prepared by photodeposition. The introduction of CoS2 effectively enhances the photogenerated carrier separation efficiency of carbon nitride. At the same time, N doping effectively regulates the band gap of carbon nitride and broadens the visible light response range of carbon nitride. The photocatalytic hydrogen production results show that under visible light irradiation (λ > 420 nm), the NCN/10CoS2 presents the best photocatalytic hydrogen production performance (73.8 μmol·g−1·h−1), which is 4.9 and 10.4 times higher than that of NCN (15.0 μmol·g−1·h−1) and g-C3N4/10CoS2 (7.1 μmol·g−1·h−1), respectively.
-
Key words:
- g-C3N4 /
- N-doping /
- CoS2 /
- photocatalysis /
- hydrogen production
-
表 1 CN和NCN/10CoS2中的C/N原子比
Table 1. C/N atomic ratio of CN and NCN/10CoS2
Samples C/at% N/at% C/N CN 42.77 57.23 0.75 NCN/10 CoS2 39.07 60.83 0.64 表 2 NCN和NCN/10 CoS2的比表面积和孔体积
Table 2. Specific surface areas and pore volumes of the NCN and NCN/10 CoS2
Samples Surface area/(m2·g−1) Pore volume/(cm3·g−1) NCN 61.51 0.23 NCN/10 CoS2 65.90 0.25 -
[1] ZHAO B, ZHONG W, CHEN F, et al. High-crystalline g-C3N4 photo-catalysts: synthesis, structure modulation, and H2-evolution applica-tion[J]. Chinese Journal of Catalysis, 2023, 52: 127-143. doi: 10.1016/S1872-2067(23)64491-2 [2] BIE C, WANG L, YU J. Challenges for photo-catalytic overall water splitting[J]. Chem, 2022, 8: 1567-1574 doi: 10.1016/j.chempr.2022.04.013 [3] FU J, Xu Q, Low J, et al. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photoca-talyst[J]. Applied Catalysis B: Environment and Energy, 2019, 243: 556-565. doi: 10.1016/j.apcatb.2018.11.011 [4] LU N, ZHANG M, JING X, et al. Electrospun semiconductor-based nano-heterostructures for photocatalytic energy conversion and environmental remediation: opportunities and challenges[J]. Energy & Environmental Materials, 2023, 6: e12338. [5] LU N, JING X, ZHANG M, et al. Effective cascade modulation of charge-carrier kinetics in the well-designed multi-component nanofiber system for highly-efficient photocatalytichydrogen generation[J]. Acta Physico-chimica Sinica, 2023, 39(4): 2207045. [6] YU Q, HUANG J, LI A, et al. Engineering semireversed quantum well photocatalysts for highly-efficient solar-to-fuels conversion[J]. Advanced Materials, 2024, 36(16): 2311764. doi: 10.1002/adma.202311764 [7] JIANG X, HUANG J, BI Z, et al. Plasmonic active “hot spots”-confined photocatalytic CO2 reduction with high selectivity for CH4 production[J]. Advanced Materials, 2022, 34(14): 2109330. doi: 10.1002/adma.202109330 [8] LIANG X, Xue S, Yang C, et al. The directional crystallization process of poly (triazine imide) single crystals in molten salts[J]. Angewandte Chemie International Edition, 2023, 62(14): e202216434. doi: 10.1002/anie.202216434 [9] ZHANG G, XU Y, ZHU J, et al. Enhanced phto catalytic H2 production independent of exciton dissociation in crystalline carbon nitride[J]. Applied Catalysis B: Environment and Energy, 2023, 338: 123049. doi: 10.1016/j.apcatb.2023.123049 [10] LIU M H, WEI C G, ZHOU J M, et al. Fully condensed poly (triazine imide) crystals: extended π-conjugation and structural defects for overall water splitting[J]. Angewandte Chemie International Edition, 2021, 61(2): e202113389. [11] YANG M, XIAO W; ZENG Y, et al. Homogeneous carbon/potassium-incorporation strategy for syn-thesizing red polymeric carbon nitride capable of near-infrared photocatalytic H2 production[J]. Advanced Materials, 2021, 39(33): 2101455. [12] CUI L, SONG J, Fang X, et al. Constructing highly uniform onionring-like graphitic carbon nitride for efficient visible-light-driven photocatalytic hydrogen evolution[J]. ACS Nano 2018, 12(6): 5551-555. [13] ZHU D, ZHOU Q. Nitrogen doped g-C3N4 with the extremely narrow band gap for excellent photocatalytic activities under visible light[J]. Applied Catalysis B: Environment and Energy, 2021, 281: 119474.8. [14] JIANG L B, YUAN X Z, ZENG G M, et al. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation[J]. Journal of Colloid and Interface Science, 2019, 536: 17-29. doi: 10.1016/j.jcis.2018.10.033 [15] KAVITHA R, NITHYA M. Noble metal deposited graphitic carbon nitride based heterojunction photocatalysts[J]. Applied Surface Science, 2020, 4: 145142. [16] 牛凤延, 何齐升, 武世然, 等. 光沉积Pt复合石墨相氮化碳实现高效光催化产氢[J]. 复合材料学报, 2024, 41(1): 219-226.NIU Fengyan, HE Qisheng, WU Shiran, et al. Photodeposition Pt composite graphitic carbon nitride realizes efficient photocatalytic hydrogenproduction[J]. Acta Materiae Compositae Sinica, 2024, 41(1): 219-226(in Chinese). [17] XU X, WANG S, GUO S, et al. Cobalt phos phor sulfide nanoparticles encapsulated into heteroatom-doped carbon as bifunctional electrocatalyst for Zn-air battery[J]. Advanced Powder Materials, 2022, 1(3): 100027. doi: 10.1016/j.apmate.2021.12.003 [18] YANG S, GUO X, LIU K, et al. Size effect of CoS2 cocatalyst on photocatalytic hydrogen evolution performance of g-C3N4[J]. Journal of Colloid and Interface Science, 2023, 635: 305-315. doi: 10.1016/j.jcis.2022.12.149 [19] VINOTH S, RAJAITHA P M. In-situ pyrolytic processed zinc stannate incorporated graphitic carbon nitride nanocomposite for selective and sensitive electrochemical determination of nitrobenzene[J]. Composites Science And Technology, 2020, 195: 08192 [20] VINOTH S, RAJAITHA P M. Nickel sulfide-incorporated sulfur-doped graphitic carbon nitride nanohybrid interface for non-enzymatic electrochemical sensing of glucose[J]. Nanoscale Advances, 2020, 2: 4242-4250. doi: 10.1039/D0NA00172D [21] XIANG Q J, YU J G, Jaroniec M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites[J]. Journal of Physical Chemistry C, 2011, 115(15): 7355-7363. doi: 10.1021/jp200953k [22] YU J G, WANG S H, CHENG B, et al. Noble metal-free Ni(OH)2-g-C3N4 composite photocatalyst with enhanced visible-light photocatalytic H2-production activity[J]. Catalysis Science and Technology, 2013, 3: 1782-1789. doi: 10.1039/c3cy20878h [23] DONG G H, ZHANG L Z. Porous structure dependent photoreactivity of graphitic carbon nitride under visible light[J]. Journal of Materials Chemistry A, 2012, 22(3): 1160-1166. doi: 10.1039/C1JM14312C [24] ZHANG Y Z, SHI J W, HUANG Z X, et al. Synchronous construction of CoS2 in-situ loading and S doping for g-C3N4: enhanced photocatalytic H2-evolution activity and mechanism insight[J]. Chemical Engineering Journal, 2020, 7: 126435. [25] Xu Y F, Sun J L, He Y N, et al. Construction of CoS2 nanoparticles embedded in well-structured carbon nanocubes for high-performance potassium-ion half/full batteries[J]. Science China Chemistry, 2021, 64: 1401-1409. doi: 10.1007/s11426-021-1057-3 [26] MA J L, LI X Z, JIAO G J, et al. Single-atom zinc catalyst for co-production of hydrogen and fine chemicals over soluble biomass solution[J]. Advance Powder Materials, 2022, 1: 100058. doi: 10.1016/j.apmate.2022.100058 [27] JIAO Y Y, HUANG Q Z, WANG J S, et al. A novel MoS2 quantum dots (QDs) decorated Z-scheme g-C3N4 nanosheet/N-doped carbon dots heterostructure photocatalyst for photocatalytic hydrogen evolution[J]. Applied Catalysis B: Environment and Energy, 2019, 247: 124-132. doi: 10.1016/j.apcatb.2019.01.073 [28] QI W L, LIU S Q, ZHAO S L, et al. Prussian blue derived Fe2N for efficiently improving photocatalytic hydrogen evolution activity of g-C3N4 nanosheets[J]. Catalysis Science and Technology, 2019, 9: 2571-2577. doi: 10.1039/C9CY00198K [29] LU Y T, CHU D M, ZHU M S, et al. Exfoliated carbon nitride nanosheets decorated with NiS as an efficient noble-metal-free visible-light-driven photocatalyst for hydrogen evolution[J]. Physical Chemistry Chemical Physics, 2015, 26(17): 17355-17361 [30] ZENG D Q, ONG W J, CHEN Y Z, et al. Co2P nanorods as an efficient cocatalyst decorated porous g-C3N4 nanosheets for photocatalytic hydrogen production under visible light irradiation[J]. Particle and Particle Systems Characterization, 2018, 35(1): 1700251 doi: 10.1002/ppsc.201700251 [31] YE P, LIU X L, IOCOZZIA J, et al. A highly sta ble non-noble metal Ni2P co-catalyst for increased H2 generation by g-C3N4 under visible light irradiation[J]. Journal of Materials Chemistry A, 2017, 18(5): 8493-8498. -

 点击查看大图
点击查看大图
计量
- 文章访问数: 42
- HTML全文浏览量: 22
- 被引次数: 0




 下载:
下载:











