Synthesis and photocatalytic hydrogen production performance of nickel-iron hydrotalcite/poly(dibenzothiophene-S,S-dioxide)composites
-
摘要: 镍铁水滑石(LDH)(Ni7Fe1)易于合成、来源丰富、价格低廉,是目前催化体系中性能优良的催化剂之一,可望用于替代成本高昂的贵金属催化剂。采用原位聚合法将二维层状结构的Ni7Fe1与聚S,S-二氧-二苯并噻吩(PDBTSO)复合,制备了无机/有机复合材料Ni7Fe1/PDBTSO,并验证了其光催化性能。实验得到:复合材料15-Ni7Fe1/PDBTSO相较于添加3wt% Pt助催化剂PDBTSO的光催化产氢效率提高了22.6%,其产氢效率达到36.8 mmol·g−1·h−1且具有良好的循环稳定性,表明Ni7Fe1为替代光催化制氢反应中贵金属助催化剂理想的候选材料之一。结合XRD、FTIR、TEM和XPS等手段进一步讨论了复合材料光催化产氢的机制。Ni7Fe1/PDBTSO高效的光催化制氢性能及低成本的制备方法为光催化制氢领域提供了新的思路。
-
关键词:
- 光催化 /
- 聚S,S-二氧-二苯并噻吩 /
- 氢气 /
- 水滑石 /
- 复合材料
Abstract: Two-dimensional layered double hydroxide (LDH) of Ni7Fe1 is the most excellent catalyst in the catalytic system for its facile preparation, abundant sources, and low-cost, which is also an ideal substitute for noble metal catalyst in photocatalytic production. In this study, we prepared composites of Ni7Fe1/PDBTSO by in-situ polymerization of Ni7Fe1 and poly(dibenzothiophene-S,S-dioxide) (PDBTSO). Furthermore, we investigated their catalytic performance. The experimental results show that 15-Ni7Fe1/PDBTSO exhibites the hydrogen generation rate of 36.8 mmol·g−1·h−1, which is 22.6% higher than that of PDBTSO with 3wt% Pt as co-catalyst. Besides, 15-Ni7Fe1/PDBTSO shows good photocatalytic stability, making it an ideal candidate for photocatalytic hydrogen production. XRD, FTIR, TEM and XPS were used to explore the mechanism of photocatalytic hydrogen production performance. The high photocatalytic efficiency and low cost of Ni7Fe1/PDBTSO provide a new idea for the field of photocatalytic hydrogen production.-
Key words:
- photocatalysis /
- poly(dibenzothiophene-S,S-dioxide) /
- hydrogen /
- layered double hydroxide /
- composite
-
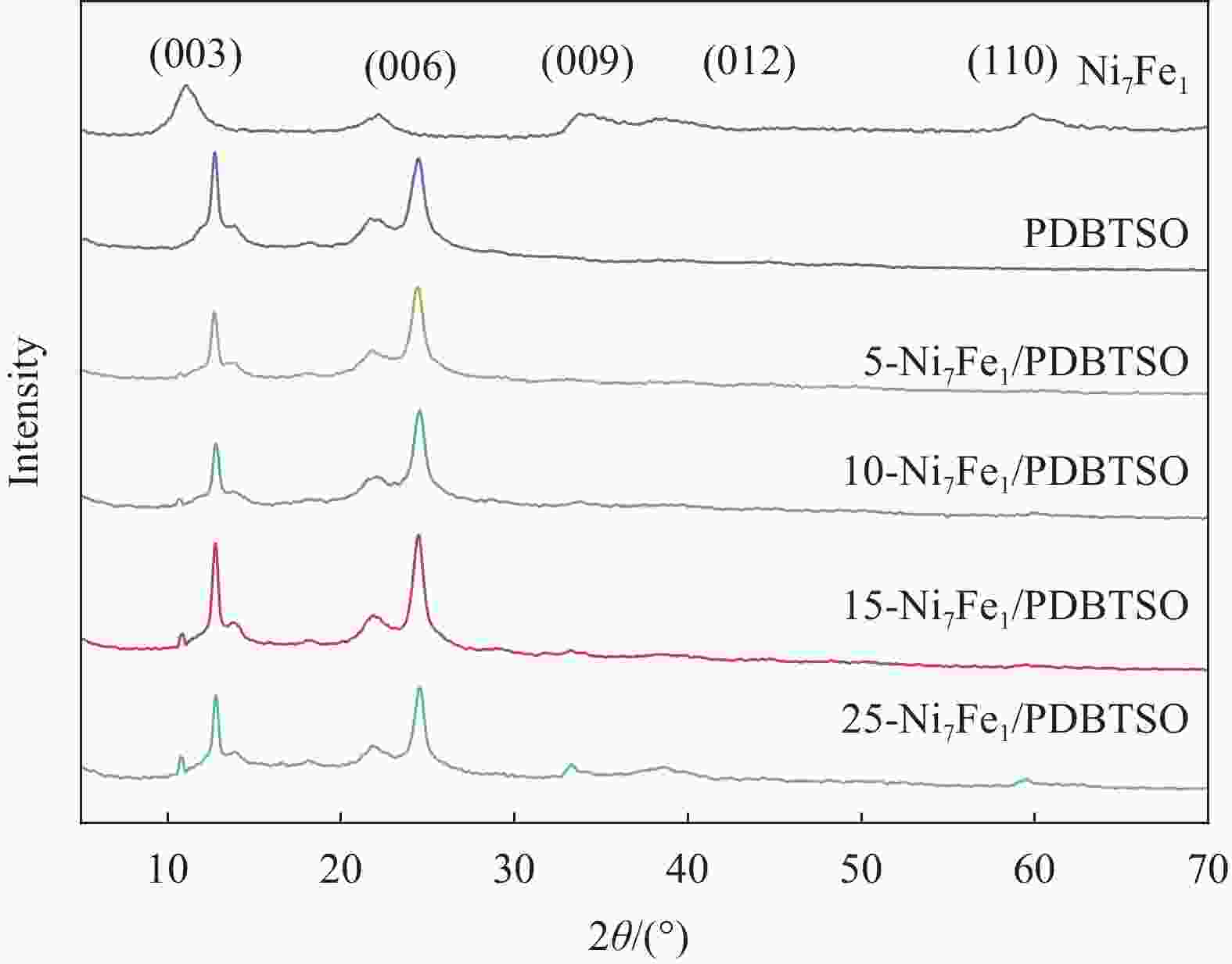
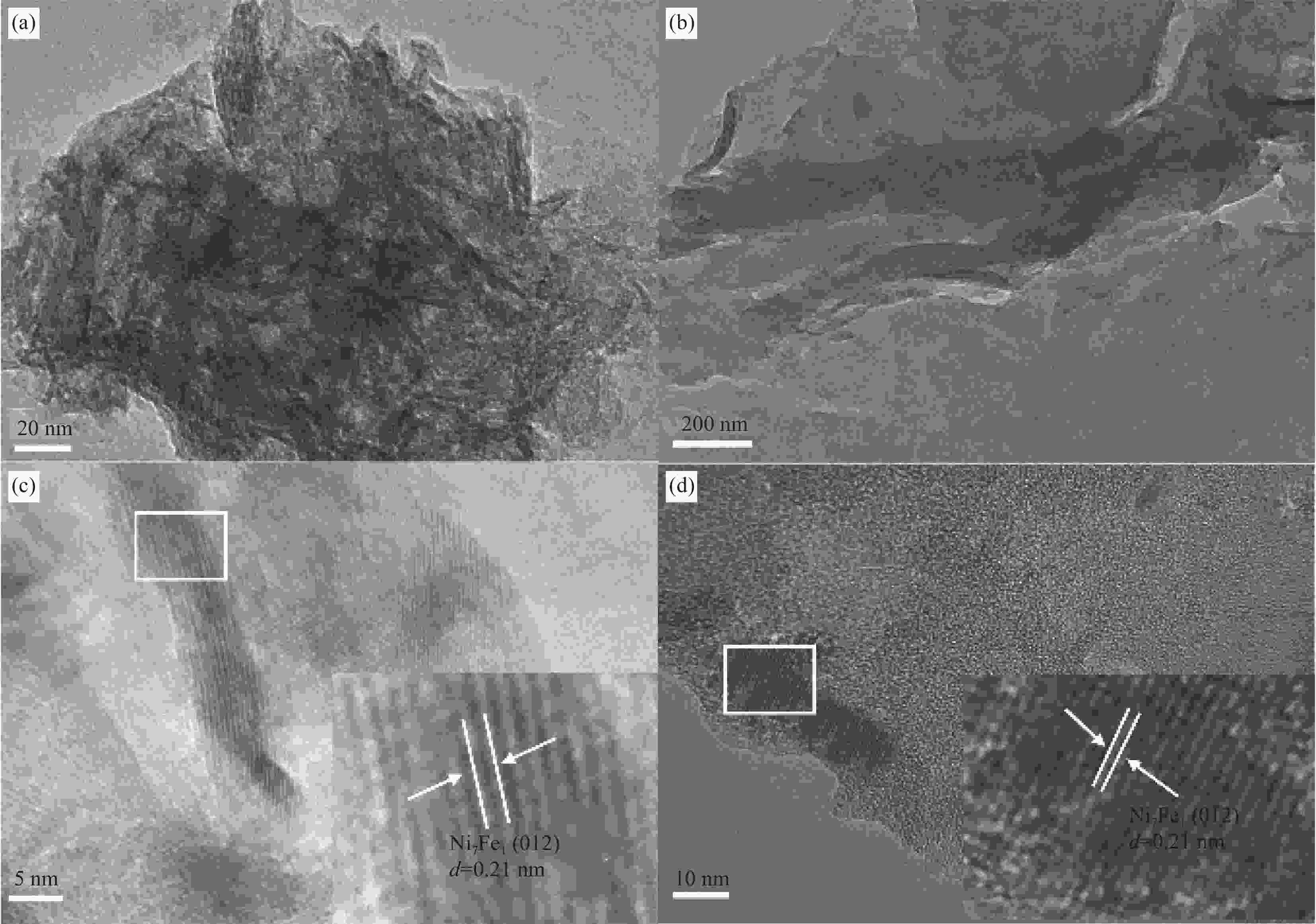
图 4 Ni7Fe1 (a)、PDBTSO (b)和15-Ni7Fe1/PDBTSO (c)的SEM图像;(d) 15-Ni7Fe1/PDBTSO的EDX图谱;((e)~(i)) C、O、S、Ni和Fe元素分布图
Figure 4. SEM images of PDBTSO (a), Ni7Fe1 (b) and 15-Ni7Fe1/PDBTSO(c); (d) EDX image of a single 15-Ni7Fe1/PDBTSO and multi-elemental image of C (e), O (f), S (g), Ni (h), Fe (i) elemental maps
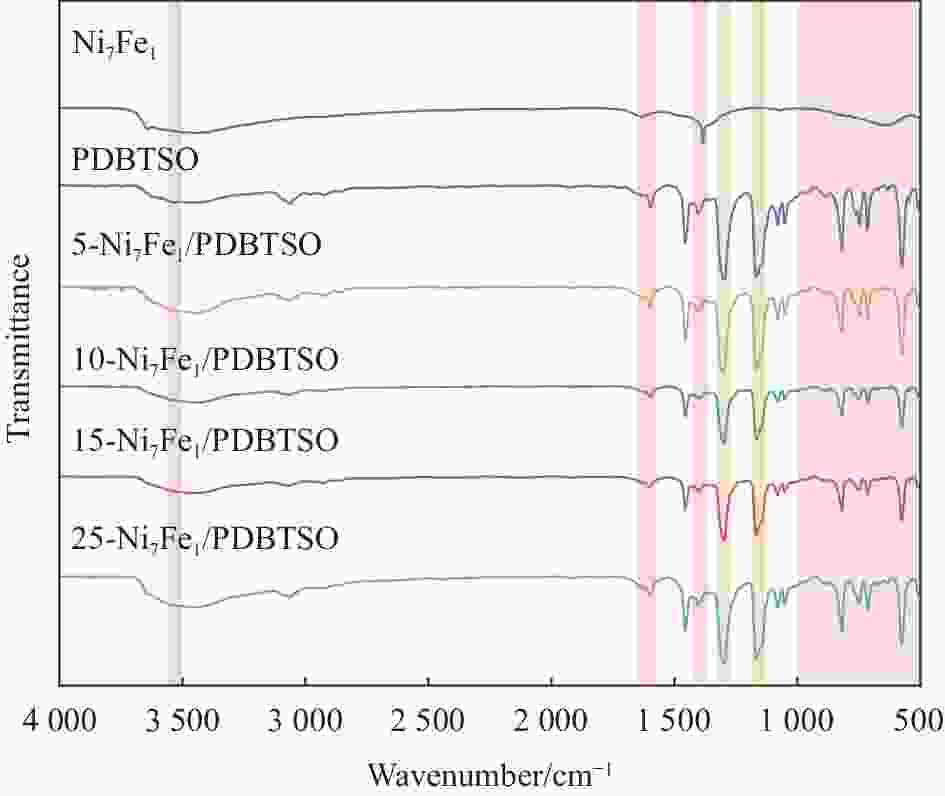
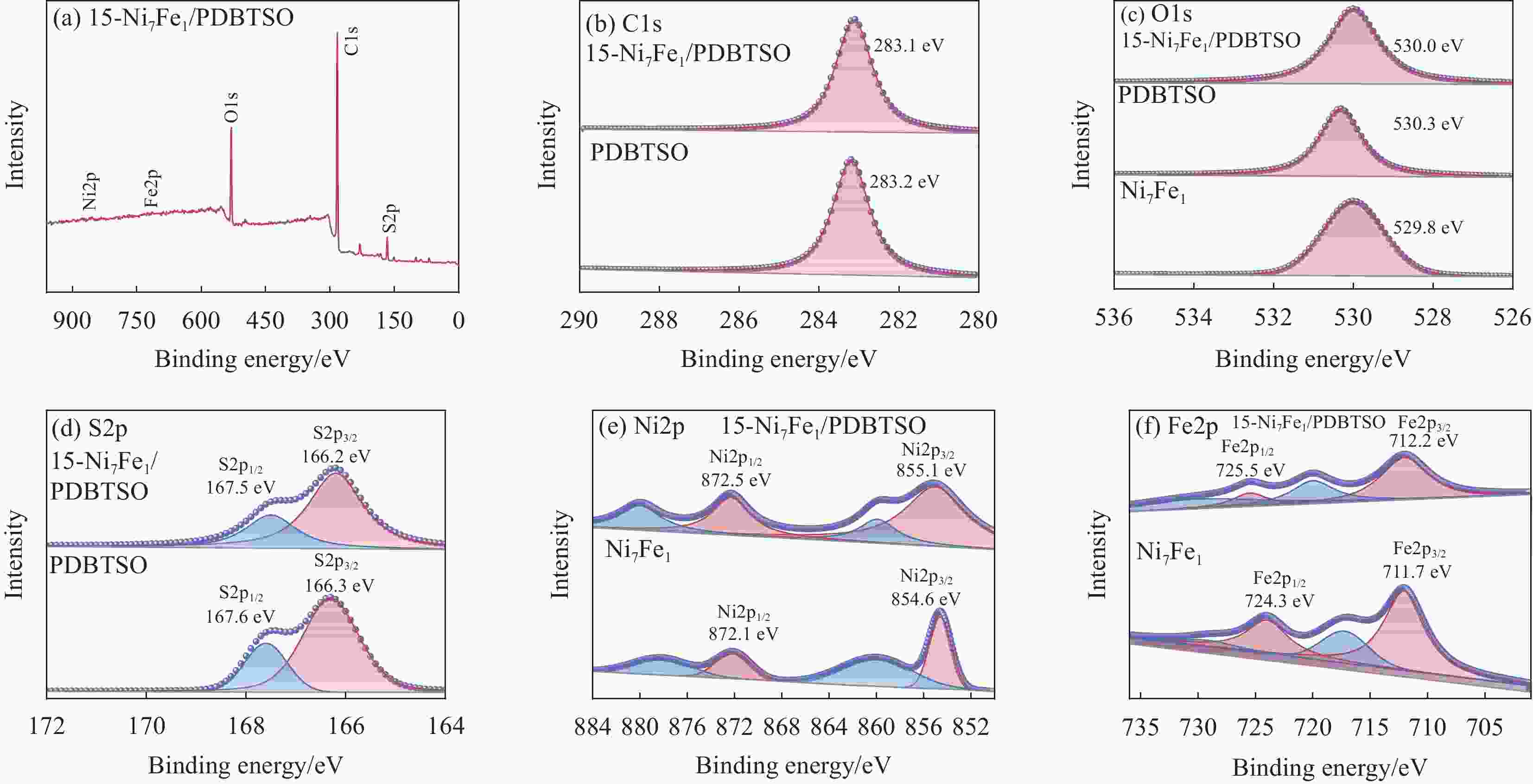
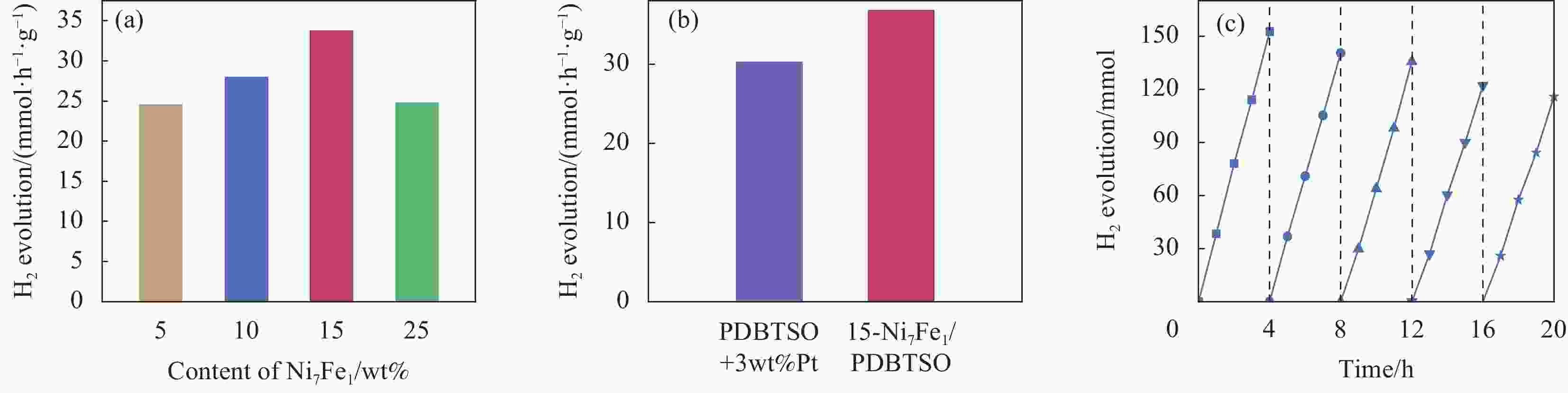
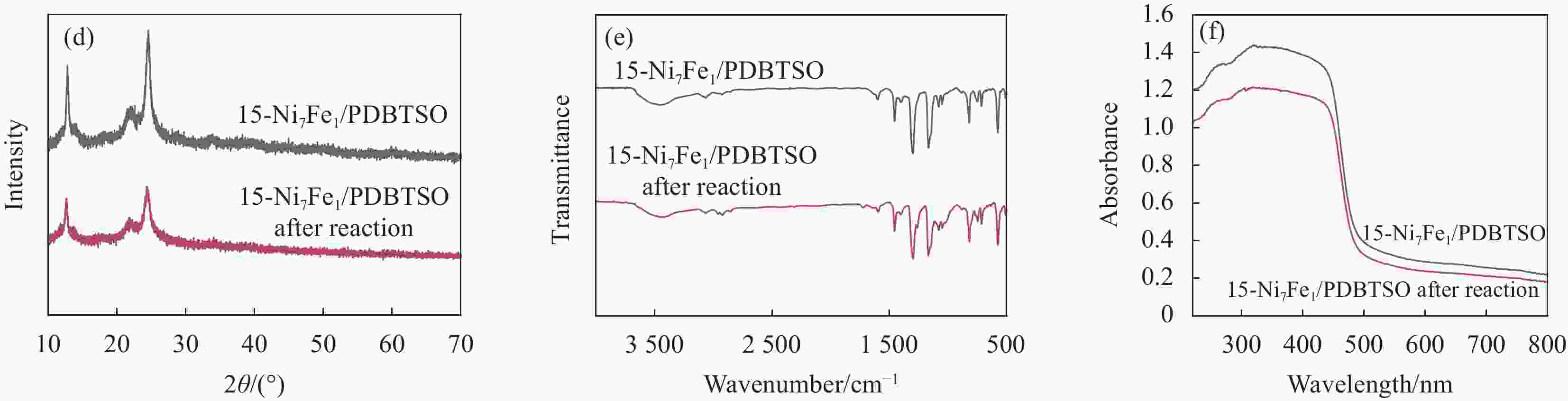
图 8 Ni7Fe1/PDBTSO (a)、PDBTSO添加3wt%Pt助催化剂和15-Ni7Fe1/PDBTSO (b) 的光催化产氢性能;(c) 15-Ni7Fe1/PDBTSO光催化制氢稳定性测试;光催化反应前后15-Ni7Fe1/PDBTSO的XRD图谱 (d)、FTIR图谱 (e) 和UV-DRS图谱(f)
Figure 8. Hydrogen evolution reactions of Ni7Fe1/PDBTSO (a), PDBTSO with 3wt%Pt and 15-Ni7Fe1/PDBTSO (b); (c) Stability test of 15-Ni7Fe1/PDBTSO photocatalytic reaction; XRD patterns (d), FTIR spectra (e) and UV-DRS spectra (f) of 15-Ni7Fe1/PDBTSO before and after photocatalytic reaction
图 9 PDBTSO和15-Ni7Fe1/PDBTSO的稳态荧光光谱图 (a) 和荧光寿命图 (b);(c) Ni7Fe1、PDBTSO和15-Ni7Fe1/PDBTSO的EIS图谱;(d) PDBTSO和15-Ni7Fe1/PDBTSO的光电流响应图谱
Figure 9. Photoluminescence spectroscopy spectra (a) and time-resolved PL spectra (b) of PDBTSO and 15-Ni7Fe1/PDBTSO; (c) EIS spectra of Ni7Fe1, PDBTSO and 15-Ni7Fe1/PDBTSO; (d) Photoelectrode transient photocurrent response image of PDBTSO and 15-Ni7Fe1/PDBTSO
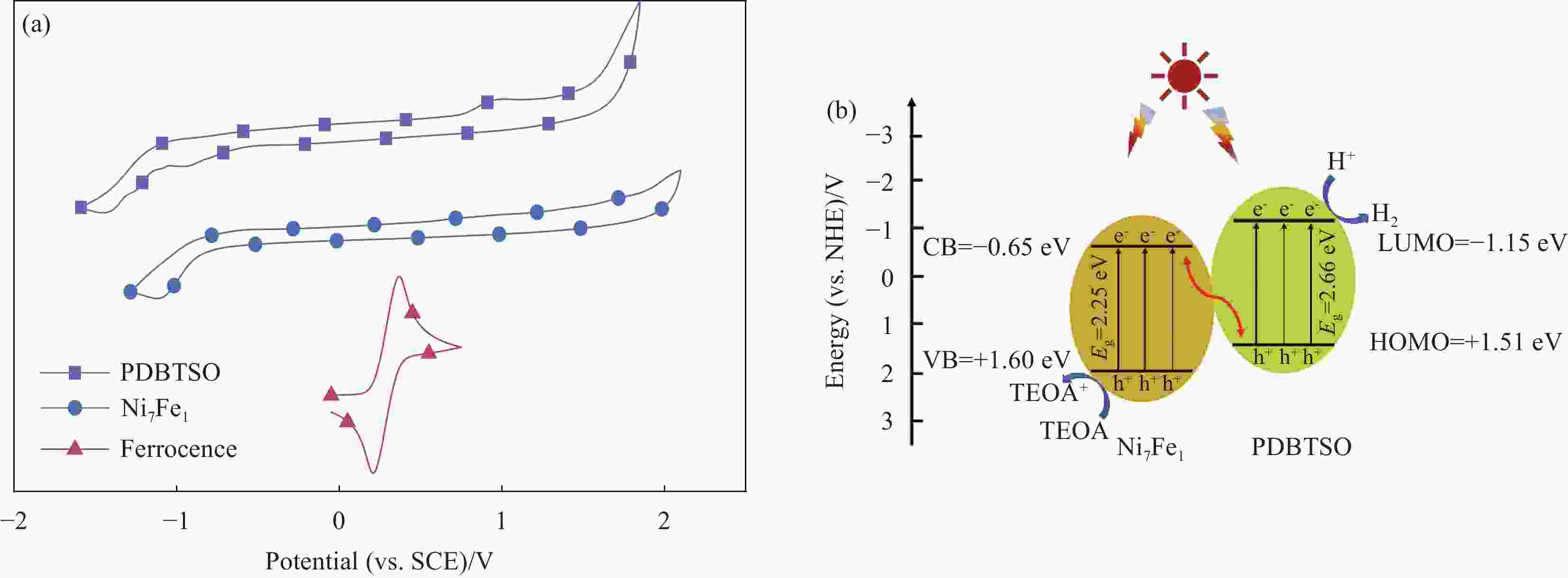
图 10 (a) Ni7Fe1和PDBTSO的伏安(CV)曲线; (b) Ni7Fe1/PDBTSO的产氢机制图
Figure 10. (a) Current-vlotage (CV) curves of Ni7Fe1 and PDBTSO; (b) Proposed mechanism for photocatalytic hydrogen evolution at the Ni7Fe1/PDBTSO composite
CB—Conduction band; VB—Valence band; Eg—Optical band gaps; TEOA—Triethanolamine; LUMO—Lowest unoccupied molecular orbital; HOMO—Highest occupied molecular orbital
表 1 PDBTSO和15-Ni7Fe1/PDBTSO荧光寿命
Table 1. Fitted decay time of the PDBTSO and 15-Ni7Fe1/PDBTSO
Sample τ1/ns Rel/% τ2/ns Rel/% τ3/ns Rel/% τ/ns PDBTSO 0.291 43.68 0.767 31.62 2.946 24.70 0.502 15-Ni7Fe1/PDBTSO 0.468 53.54 2.122 37.30 8.252 9.16 0.751 Notes: τ1, τ2, τ3—Fitted fluorescence lifetime value; τ—Average lifetime; Rel—Related function. 表 2 Ni7Fe1和PDBTSO的光化学性能
Table 2. Optical and electrochemical properties for the PDBTSO and Ni7Fe1
Sample Reduction potential/eV Oxidation potential/eV ELUMO(CB)/eV EHOMO(VB)/eV Ega/eV Egb/eV PDBTSO −1.15 1.51 −3.35 −6.01 2.66 2.55 Ni7Fe1 −0.65 1.60 −3.86 −6.11 2.25 2.20 Notes: ELUMO(CB)—Conduction band potential; EHOMO(VB)—Valence band potential; Ega—Band gaps calculated from ELUMO(CB)-EHOMO(VB); Egb—Optical band gaps; Egb=1240/λ. -
[1] WANG X C, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials,2009,8(1):76-80. doi: 10.1038/nmat2317 [2] CHENG C, WANG X C, LIN Y Y, et al. The effect of molecular structure and fluorination on the properties of pyrene-benzothiadiazole-based conjugated polymers for visible-light-driven hydrogen evolution[J]. Polymer Chemistry,2018,9(35):4468-4475. doi: 10.1039/C8PY00722E [3] LIU L J, KOCHMAN M A, XU Y J, et al. Acetylene-linked conjugated polymers for sacrificial photocatalytic hydrogen evolution from water[J]. Journal of Materials Chemistry A,2021,9(32):17242-17248. doi: 10.1039/D1TA04288B [4] DAI C H, LIU B. Conjugated polymers for visible-light-driven photocatalysis[J]. Energy & Environmental Science,2020,13(1):24-52. [5] WANG Z J, YANG X Y, YANG T J, et al. Dibenzothiophene dioxide based conjugated microporous polymers for visible-light-driven hydrogen production[J]. ACS Catalysis,2018,8(9):8590-8596. doi: 10.1021/acscatal.8b02607 [6] FU J W, YU J G, JIANG C J, et al. g-C3N4-based heterostructured photocatalysts[J]. Advanced Energy Materials,2018,8(3):1701503. doi: 10.1002/aenm.201701503 [7] YANG C, MA B C, ZHANG L Z, et al. Molecular engineering of conjugated polybenzothiadiazoles for enhanced hydrogen production by photosynthesis[J]. Angewandte Chemie International Edition,2016,128(32):9348-9352. [8] DAMAS G, MARCHIORI C F N, ARAUJO C M. On the design of donor–acceptor conjugated polymers for photocatalytic hydrogen evolution reaction: First-principles theory-based assessment[J]. The Journal of Physical Chemistry C,2018,122(47):26876-26888. doi: 10.1021/acs.jpcc.8b09408 [9] 王晓爽, 李育珍, 易思远, 等. Bi2MoS2O4改性g-C3N4光催化降解罗丹明B[J]. 复合材料学报, 2022, 39(8):3845-3851.WANG Xiaoshuang, LI Yuzhen, YI Siyuan, et al. Bi2MoS2O4 modified g-C3N4 photocatalytic degradation of Rhodamine B[J]. Acta Materiae Compositae Sinica,2022,39(8):3845-3851(in Chinese). [10] 胡新军, 胡勇, 谢文玲, 等. ZnS/还原氧化石墨烯复合材料的制备及光催化性能[J]. 复合材料学报, 2019, 36(1):207-212. doi: 10.13801/j.cnki.fhclxb.20180509.001HU Xinjun, HUong, XIE Wenling, et al. Preparation and photocatalytic properties of ZnS/reduced graphene oxide composite[J]. Acta Materiae Compositae Sinica,2019,36(1):207-212(in Chinese). doi: 10.13801/j.cnki.fhclxb.20180509.001 [11] 黄有鹏, 吴福礼, 李兵, 等. WO3/g-C3N4复合光催化剂制备及其可见光催化性能[J]. 复合材料学报, 2021, 38(12):4287-4294. doi: 10.13801/j.cnki.fhclxb.20210303.001HUANG Youpeng, WU Fuli, LI Bing, et al. Preparation and visible light catalytic performance of WO3/g-C3N4 composite photocatalyst[J]. Acta Materiae Compositae Sinica,2021,38(12):4287-4294(in Chinese). doi: 10.13801/j.cnki.fhclxb.20210303.001 [12] ZHANG X, SHEN F, HU Z C, et al. Biomass nanomicelles assist conjugated polymers/Pt cocatalysts to achieve high photocatalytic hydrogen evolution[J]. ACS Sustainable Chemistry & Engineering,2019,7(4):4128-4135. [13] SUN D R, LIU W J, FU Y H, et al. Noble metals can have different effects on photocatalysis over metal–organic frameworks (MOFs): A case study on M/NH2-MIL-125(Ti) (M=Pt and Au)[J]. Chemistry-A European Journal,2014,20(16):4780-4788. doi: 10.1002/chem.201304067 [14] LIU J Z, LI Y H, ZHOU X D, et al. Positively charged Pt-based cocatalysts: An orientation for achieving efficient photocatalytic water splitting[J]. Journal of Materials Chemistry A,2020,8(1):17-26. doi: 10.1039/C9TA10568A [15] XIAO N, LI S, LI X, et al. The roles and mechanism of cocatalysts in photocatalytic water splitting to produce hydrogen[J]. Chinese Journal of Catalysis,2020,41(4):642-671. doi: 10.1016/S1872-2067(19)63469-8 [16] LI X G, BI W T, ZHANG L, et al. Single-atom Pt as Co-catalyst for enhanced photocatalytic H2 evolution[J]. Advanced Materials,2016,28(12):2427-2431. doi: 10.1002/adma.201505281 [17] MENG X Y, YANG Y S, CHEN L F, et al. A control over hydrogenation selectivity of furfural via tuning exposed facet of Ni catalysts[J]. ACS Catalysis,2019,9(5):4226-4235. doi: 10.1021/acscatal.9b00238 [18] GAO Z, LIU F Q, WANG L, et al. Hierarchical Ni2P@NiFeAlOx nanosheet arrays as bifunctional catalysts for superior overall water splitting[J]. Inorganic Chemistry,2019,58(5):3247-3255. doi: 10.1021/acs.inorgchem.8b03327 [19] GAO Y, DOU L G, ZHANG S, et al. Coupling bimetallic Ni-Fe catalysts and nanosecond pulsed plasma for synergistic low-temperature CO2 methanation[J]. Chemical Engineering Journal,2021,420:127693. doi: 10.1016/j.cej.2020.127693 [20] NAYAK S, MOHAPATRA L, PARIDA K. Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction[J]. Journal of Materials Chemistry A,2015,3(36):18622-18635. doi: 10.1039/C5TA05002B [21] NAYAK S, PARIDA K M. Deciphering Z-scheme charge transfer dynamics in heterostructure NiFe-LDH/N-rGO/g-C3N4 nanocomposite for photocatalytic pollutant removal and water splitting reactions[J]. Scientific Reports,2019,9(1):2458. doi: 10.1038/s41598-019-39009-4 [22] SAHOO D P, PATNAIK S, PARIDA K. Construction of a Z-scheme dictated WO3-X/Ag/ZnCr LDH synergistically visible light-induced photocatalyst towards tetracycline degradation and H2 evolution[J]. ACS Omega,2019,4(12):14721-14741. doi: 10.1021/acsomega.9b01146 [23] SPRICK R S, BONILLO B, CLOWES R, et al. Visible-light-driven hydrogen evolution using planarized conjugated polymer photocatalysts[J]. Angewandte Chemie-International Edition,2016,55(5):1792-1796. doi: 10.1002/anie.201510542 [24] DAI C H, XU S D, LIU W, et al. Dibenzothiophene-S, S-dioxide-based conjugated polymers: Highly efficient photocatalyts for hydrogen production from water under visible light[J]. Small,2018,14(34):1801839. doi: 10.1002/smll.201801839 [25] SACHS M, SPRICK R S, PEARCE D, et al. Understanding structure-activity relationships in linear polymer photocatalysts for hydrogen evolution[J]. Nature Communications,2018,9(1):4968. doi: 10.1038/s41467-018-07420-6 [26] SHU G, LI Y D, WANG Z, et al. Poly(dibenzothiophene-S, S-dioxide) with visible light-induced hydrogen evolution rate up to 44.2 mmol·h−1·g−1 promoted by K2HPO4[J]. Applied Catalysis B: Environmental,2020,261:118230. doi: 10.1016/j.apcatb.2019.118230 [27] XU K L, CHEN G M, SHEN J Q, et al. Exfoliation and dispersion of micrometer-sized LDH particles in poly(ethylene terephthalate) and their nanocomposite thermal stability[J]. Applied Clay Science,2013,75-76:114-119. doi: 10.1016/j.clay.2013.02.004 [28] SAIAH F B D, SU B L, BETTAHAR N. Nickel-iron layered double hydroxide (LDH): Textural properties upon hydrothermal treatments and application on dye sorption[J]. Journal of Hazardous Materials,2009,165(1):206-217. [29] XU Q L, ZHANG L Y, YU J G, et al. Direct Z-scheme photocatalysts: Principles, synthesis, and applications[J]. Materials Today,2018,21(10):1042-1063. doi: 10.1016/j.mattod.2018.04.008 [30] ZHANG X Z, XIAO J, HOU M, et al. Robust visible/near-infrared light driven hydrogen generation over Z-scheme conjugated polymer/CdS hybrid[J]. Applied Catalysis B: Environmental,2018,224:871-876. doi: 10.1016/j.apcatb.2017.11.038 [31] MOHAPATRA L, PARIDA K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts[J]. Journal of Materials Chemistry A,2016,4(28):10744-10766. doi: 10.1039/C6TA01668E [32] WANG Z, LI C, DOMEN K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting[J]. Chemical Society Reviews,2019,48(7):2109-2125. doi: 10.1039/C8CS00542G [33] SHU G, WANG Y, LI Y D, et al. A high performance and low cost poly(dibenzothiophene-S, S-dioxide)@TiO2 compo-site with hydrogen evolution rate up to 51.5 mmol·h−1·g−1[J]. Journal of Materials Chemistry A,2020,8(35):18292-18301. doi: 10.1039/D0TA06159J [34] WAGEH S, AL-GHAMDI A A, JAFER R, et al. A new heterojunction in photocatalysis: S-scheme heterojunction[J]. Chinese Journal of Catalysis,2021,42(5):667-669. doi: 10.1016/S1872-2067(20)63705-6 [35] XU Q, ZHANG L, CHENG B, et al. S-scheme heterojunction photocatalyst[J]. Chemistry,2020,6(7):1543-1559. doi: 10.1016/j.chempr.2020.06.010 [36] ZHEN L, DAN J, WANG Z H. ZnO/CdSe-diethylenetriamine nanocomposite as a step-scheme photocatalyst for photocatalytic hydrogen evolution[J]. Applied Surface Science,2020,529:147071. doi: 10.1016/j.apsusc.2020.147071 -






 下载:
下载: