Study on preparation and properties of graphene/Ca(OH)2 nanocomposite in different temperatures
-
摘要: 纳米Ca(OH)2对风化后的大理岩石质文物有良好的加固效果,在较低温度下得到性能优异的纳米颗粒对其成本降低和推广应用有重要意义。本研究通过调节反应温度,引入石墨烯量子点,制备得到了一系列石墨烯/Ca(OH)2纳米复合材料,并采用TEM、激光粒度仪、Raman、FTIR、UV-Vis、XRD、SEM、分光测色仪、压汞仪、硬度计、超声波测速仪等对材料形貌组成、相对动力学稳定性、碳酸化反应和模拟样品加固性能进行分析研究。结果表明反应温度的适当升高有利于石墨烯与Ca(OH)2的复合,以及纳米颗粒粒径的减小,在80℃下得到的产物相对动力学稳定性、碳酸化速率和加固性能较好;随着温度继续升高,90℃及以上所制备的材料转变为球状结晶,而且碳酸化后部分会保持为亚稳态球霰石物相,并未表现出更好的加固性能。Abstract: Graphene/Ca(OH)2 nanocomposite has a good consolidation effect on weathered marble artifacts. Preparation of this kind of material with excellent performance at lower temperatures plays an important role on its cost reduction and popularization. In this study, a series of graphene/Ca(OH)2 nanocomposites were prepared by adjusting the reaction temperature and addition of graphene quantum dot. The morphology, composition, relative kinetic stability, carbonation reaction and reinforcement property were studied by transmission electron microscope, laser particle analyzer, Raman spectroscopy, Fourier-transform infrared spectroscopy, UV-visible spectrometer, X-ray diffractometer, scanning electron microscope, colorimeter, mercury injection apparatus, leeb hardness tester and ultrasonic tester. The results showed that the appropriate increase of reaction temperature was conducive to the improvement of combination and the reduction of nanoparticle size. The products obtained at 80℃ exhibited good relative kinetic stability, fast carbonation rate and excellent reinforcement property, while the materials prepared at 90℃ and above grew into spherical crystals and did not show better properties, may due to the influence of calcium hydroxide transform to vaterite.
-
Key words:
- graphene /
- nano material /
- calcium hydroxide /
- consolidation /
- graphene quantum dots
-
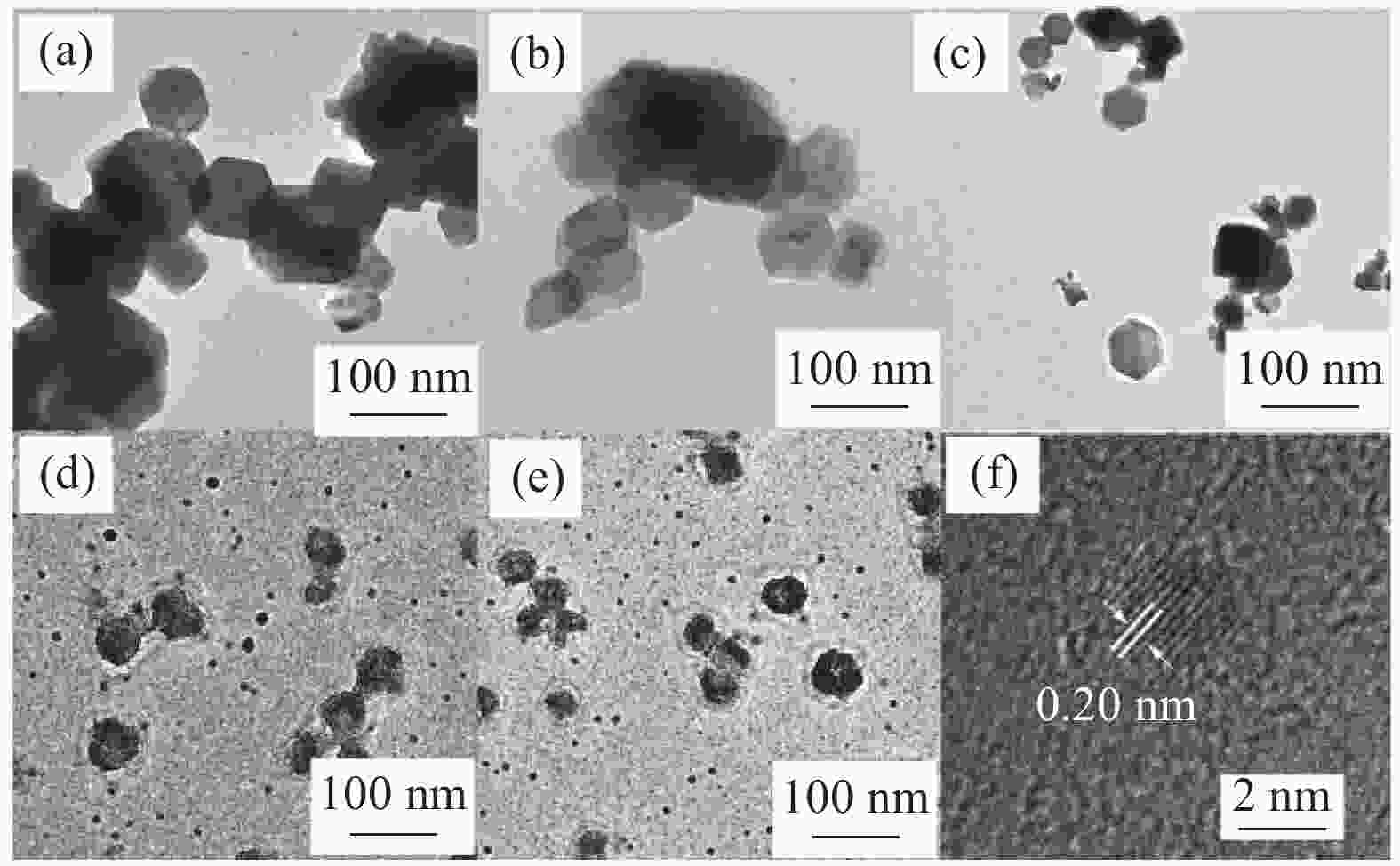
图 1 石墨烯量子点(GQDs)/Ca(OH)2-60℃至95℃(a-e)及GQDs/Ca(OH)2-60℃中散落微小颗粒的TEM图像(f)
Figure 1. TEM images of samples graphene quantum dots (GQDs)/Ca(OH)2-60℃ to 95℃ (a-e) and the tiny particles in Ca(OH)2-60℃ (f) (Notes:image of GQDs/Ca(OH)2-80℃ is same with the result in ammoniated solution[14])
-
[1] DU Yucheng, MENG Qi, HOU Ruiqin, et al. Fabrication of nano-sized Ca(OH)2 with excellent adsorption ability for N2O4[J]. Particuology, 2012, 10(6): 737-743. doi: 10.1016/j.partic.2012.03.010 [2] NARAYAN R B, GOUTHAM R, SRIKANTH B, et al. A novel nano-sized calcium hydroxide catalyst prepared from clam shells for the photodegradation of methyl red dye[J]. Journal of Environmental Chemical Engineering, 2018, 6(3): 3640-3647. doi: 10.1016/j.jece.2016.12.004 [3] CANER E, CANER-SALTIK E N. A practical method for preparing Ca(OH)2 nanodispersions for the consolidation of archaeological calcareous stones[J]. Mediterranean Archaeology and Archaeometry, 2018, 18(3): 63-70. [4] 郭青林, 李平, 张博等. 微纳米Ca(OH)2加固遗址土室内试验研究[J]. 岩土力学, 2023, 44(8): 2221-2228.GUO Qing-lin, LI Ping, ZHANG Bo, et al. Laboratory test of micro-nano Ca(OH)2 reinforced earthen sites[J]. Rock and Soil Mechanics, 2023, 44(8): 2221-2228 (in Chinese). [5] CHELAZZI D, POGGI G, JAIDAR Y, et al. Hydroxide nanoparticles for cultural heritage: consolidation and protection of wall paintings and carbonate materials[J]. J Colloid Interface Sci, 2013, 392: 42-49. doi: 10.1016/j.jcis.2012.09.069 [6] ROY A, BHATTACHARYA J. Synthesis of Ca(OH)2 nanoparticles by wet chemical method[J]. Micro & Nano Letters, 2010, 5: 131-134. [7] RODRIGUEZ−NAVARRO C, SUZUKI A, RUIZ−AGUDO E. Alcohol Dispersions of Calcium Hydroxide Nanoparticles for Stone Conservation[J]. Langmuir, 2013, 29(36): 11457-11470. doi: 10.1021/la4017728 [8] DELFORT B, BORN M, CHIVE A, et al. Colloidal calcium hydroxide in organic medium: synthesis and analysis[J]. Journal of Colloid & Interface Science, 1997, 189: 151-157. [9] POGGI G, TOCCAFONDI N, CHELAZZI D, et al. Calcium hydroxide nanoparticles from solvothermal reaction for the deacidification of degraded waterlogged wood[J]. Journal of Colloid and Interface Science, 2016, 473: 1-8. doi: 10.1016/j.jcis.2016.03.038 [10] 0] RODORICO G, DEI L, PIERO B. A New Method for Consolidating Wall Paintings Based on Dispersions of Lime in Alcohol[J]. Studies in Conservation, 2000, 3: 154-161. [11] SONG T, GAO F, GUO S, et al. A review of the role and mechanism of surfactants in the morphology control of metal nanoparticles[J]. Nanoscale, 2021, 13(7): 3895-3910. doi: 10.1039/D0NR07339C [12] ZHU JM, LI X, ZHANG Y, et al. Graphene-Enhanced Nanomaterials for Wall Painting Protection[J]. Advanced functional materials, 2018, 28(44): 1803872. doi: 10.1002/adfm.201803872 [13] 3] YU S, OGUCHI C T. Complex relationships between salt type and rock properties in a durability experiment of multiple salt–rock treatments[J]. Earth Surface Processes and Landforms, 2009, 34(15): 2096-2110. doi: 10.1002/esp.1904 [14] WANG Feng, GU Yaoqi, ZHA Jianrui, et al. Synthesis of Graphene Quantum Dots Enhanced Nano Ca(OH)2 from Ammoniated CaCl2[J]. Materials, 2023, 16(4): 1568. doi: 10.3390/ma16041568 [15] LÓPEZ−ARCE P, GOMEZ−VILLALBA L S, PINHO L, et al. Influence of porosity and relative humidity on consolidation of dolostone with calcium hydroxide nanoparticles: effectiveness assessment with non−destructive techniques[J]. Materials Characterization, 2010, 61(2): 168-184. doi: 10.1016/j.matchar.2009.11.007 [16] 6] GIORGI R, DEI L, BAGLIONI P. A new method for consolidating wall paintings based on dispersions of lime in alcohol[J]. Studies in conservation, 2000, 45(3): 154-161. doi: 10.1179/sic.2000.45.3.154 [17] 中国电力企业联合会. 工程岩体试验方法标准: GB/T 50266−2013[S]. 北京: 中国计划出版社, 2013.CHINA Electricity Council. Standard For Test Methods Of Engineering Rock Mass: GB/T 50266−2013[S]. Beijing: China Planning Press, 2013(in Chinese). [18] 中交第二公路勘察设计研究院. 公路工程岩石试验规程: JTG E41-2005[S]. 北京: 人民交通出版社, 2005.CHINA Communications Second Highway Consultants Co., Ltd. Test methods of rock for highway engineering: JTG E41-2005[S]. Beijing: China communications press, 2005(in Chinese). [19] 湖北省地质实验研究所. 岩石物理力学性质试验规程: DZ/T 0276.9−2015[S]. 北京: 中国标准出版社, 2015.HUBEI Geological Research Laboratory. Regulation for testing the physical and mechanical properties of rock: DZ/T 0276.9−2015[S]. Beijing: Standards press of China, 2015(in Chinese). [20] SHARMA G. Color Fundamentals for Digital lmaging, Digital Color lmagining Handbook[M]. CRC Press, Boca Raton, FL, USA, 2003, 44. [21] 冯怡, 马天翼, 刘蕾, 等. 无机纳米晶的形貌调控及生长机理研究[J]. 中国科学(B辑: 化学), 2009, 39(9): 864-886.FENG Yi, MA TianYi, LIU Lei, et al. Insights into shape control and growth mechanism of inorganic nanocrystals[J]. Science in China Series B: Chemistry, 2009, 39(9): 864-886(in Chinese). [22] PADANYI Z V. The Raman spectrum of Ca(OH)2[J]. Solid State Communications, 1970, 8(7): 541-543. doi: 10.1016/0038-1098(70)90300-5 [23] FERRARI A C, ROBERTSON J. Interpretation of Raman spectra of disordered and amorphous carbon[J]. Physical review B, 2000, 61(20): 14095. doi: 10.1103/PhysRevB.61.14095 [24] LI Y, LIU H, LIU X, et al. Free-radical-assisted rapid synthesis of graphene quantum dots and their oxidizability studies[J]. Langmuir, 2016, 32(34): 8641-8649. doi: 10.1021/acs.langmuir.6b02422 [25] WANG L, WANG Y, XU T, et al. Gram−scale synthesis of single−crystalline graphene quantum dots with superior optical properties[J]. Nature communications, 2014, 5(1): 1-9. [26] CLARKSON J R, PRICE T J, ADAMS C J. Role of metastable phases in the spontaneous precipitation of calcium carbonate[J]. Journal of the Chemical Society, Faraday Transactions, 1992, 88(2): 243-249. doi: 10.1039/ft9928800243 [27] 叶嘉成, 张中俭. 北京大理岩物理力学参数的相关性研究[J]. 工程地质学报, 2019, 27(3): 532-538.YE Jiacheng, ZHANG Zhongjian. Estimation of uniaxial compressive strength and modulus of elasticity of Beijing marbles based on Schmidt hardness and leeb hardness and p-wave velocity[J]. Journal of Engineering Geology, 2019, 27(3): 532-538(in Chinese). [28] 吴刚, 何国梁, 张磊, 等. 大理岩循环冻融试验研究[J]. 岩石力学与工程学报, 2006, 25(1): 2930-2938. doi: 10.3321/j.issn:1000-6915.2006.z1.050WU Gang, HE Guoliang, ZHANG Lei, et al. Experimental study on cycles of freeze-thaw of marble[J]. Chinese Journal of Rock Mechanics and Engineering, 2006, 25(1): 2930-2938(in Chinese). doi: 10.3321/j.issn:1000-6915.2006.z1.050 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 65
- HTML全文浏览量: 32
- 被引次数: 0




 下载:
下载:








