HZSM-5 synthesized by montmorillonite and its application for production of hydrogen via steam reforming of dimethyl ether
-
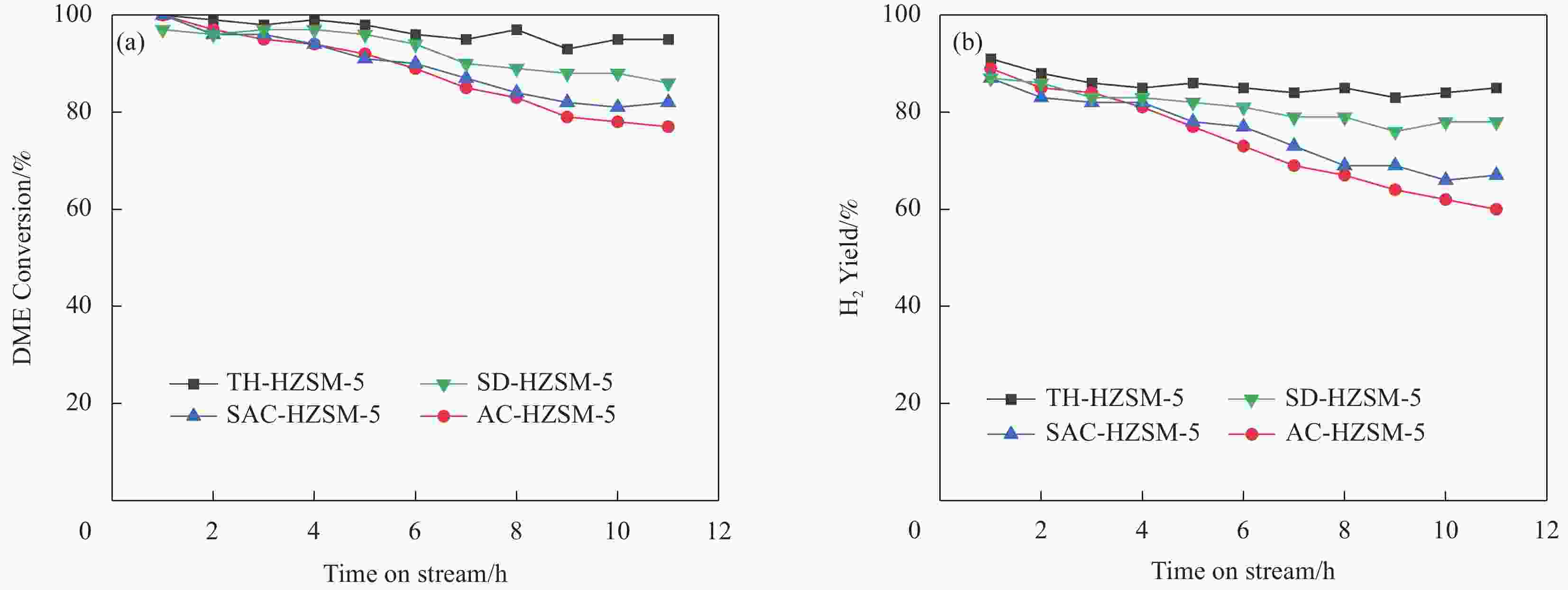
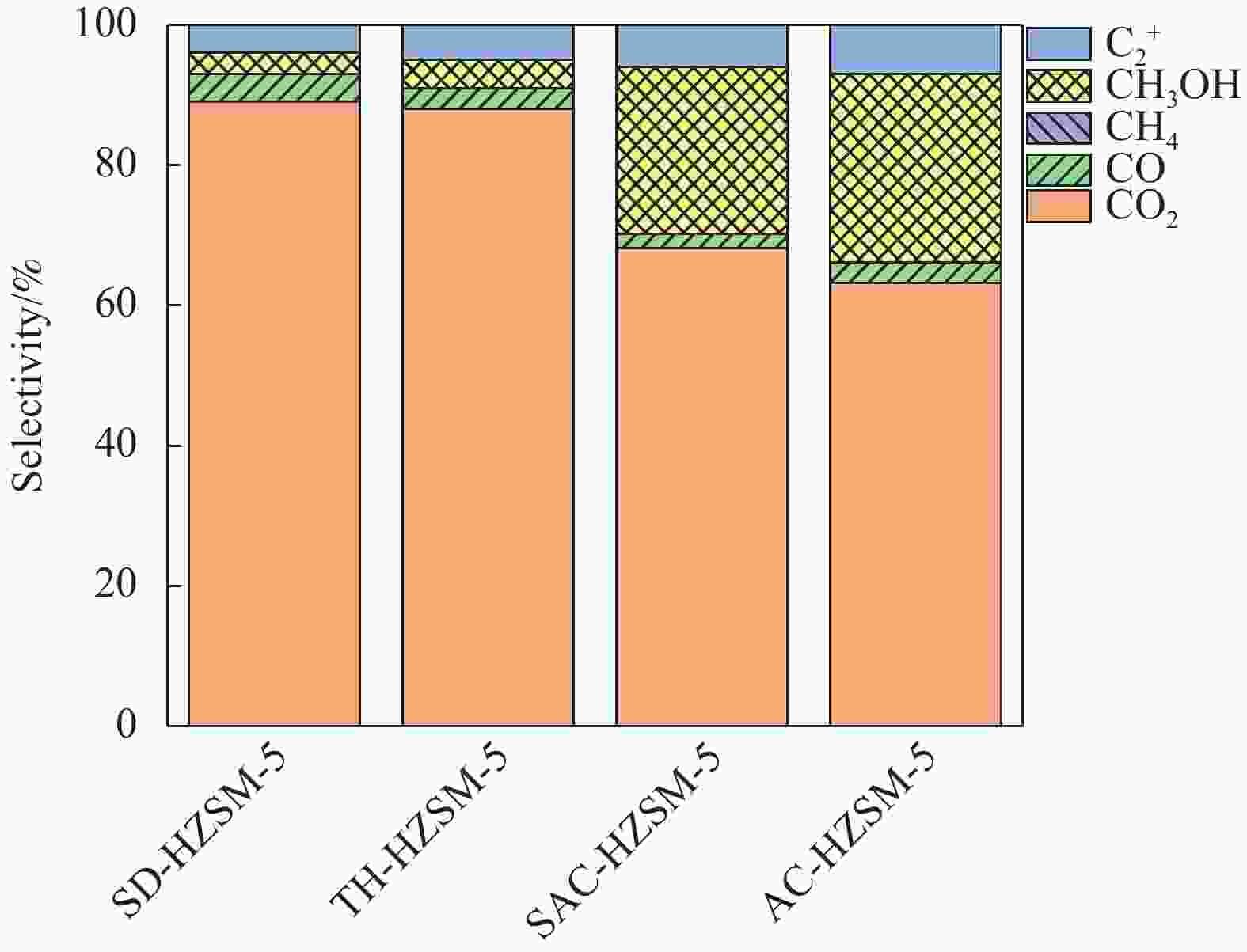
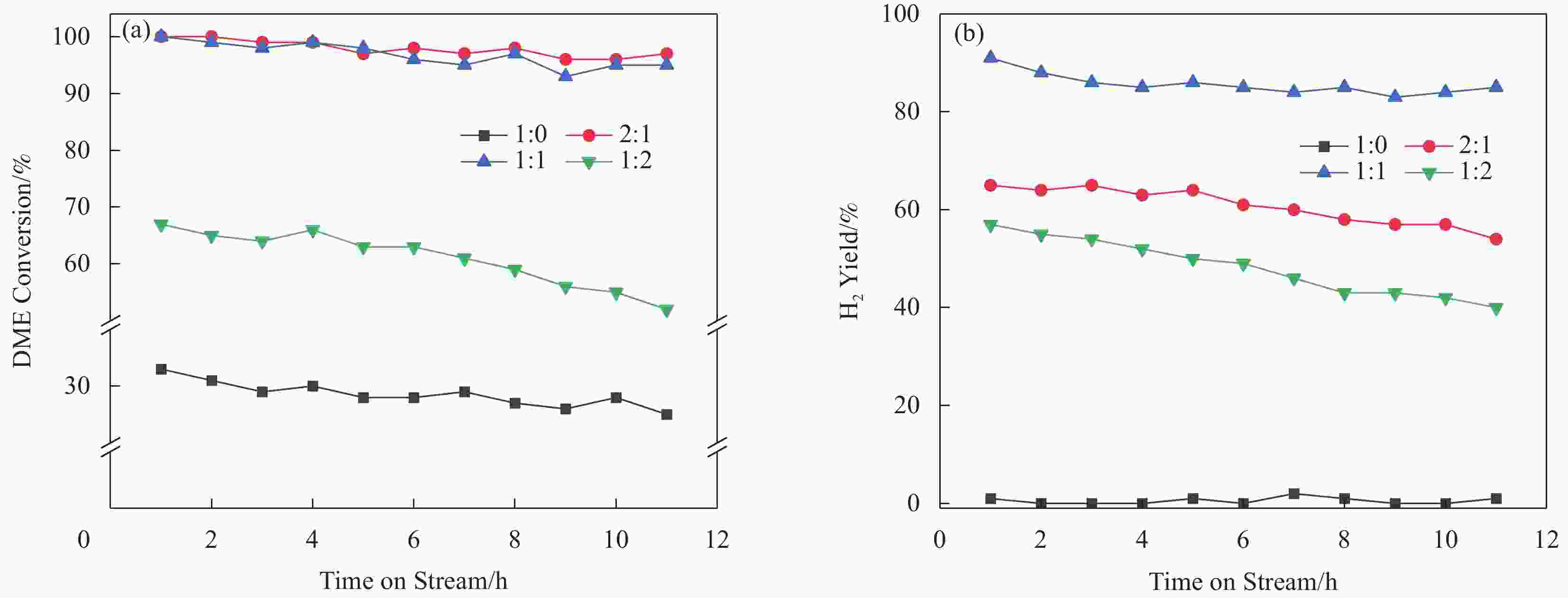
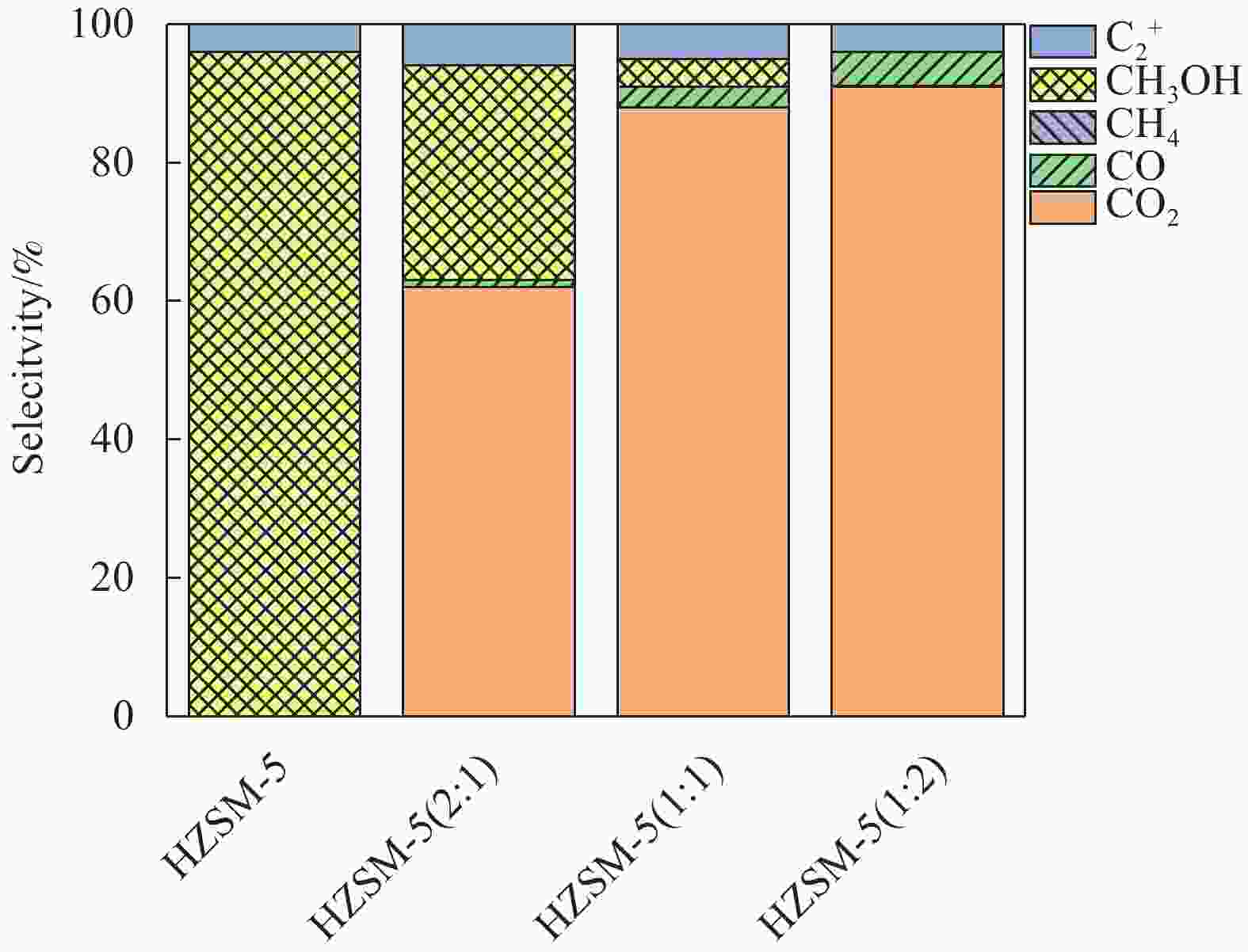
摘要: 以亚熔岩活化蒙脱土为原料,采用传统水热法、晶种导向法、蒸汽辅助晶化法和气溶胶晶化法制备了具有不同织构和酸性的HZSM-5,并以其为固体酸与商品化的Cu/ZnO/Al2O3物理混合组成双功能催化剂,并考察其二甲醚水蒸气重整(SRD)反应性能。通过XRD、FT-IR、SEM、N2低温吸附-脱附和NH3-TPD对样品进行表征。结果表明,合成方法影响了HZSM-5的晶体尺寸、织构及酸性等,进而影响了以其为固体酸的双功能催化剂的SRD性能。传统水热法合成的TH-HZSM-5因其具有适宜的酸量以及相对大的表面积、孔容和孔径,以其为固体酸与Cu/ZnO/Al2O3以质量比为1∶1组成的双功能催化剂表现出较好的SRD反应性能,在压力为0.1 MPa、温度为300 ℃、空速为
5000 mL/(g·h)的反应条件下,二甲醚初始转化率和氢气收率分别达到了100%和91%。Abstract: HZSM-5 with different texture and acidity was prepared using sub-molten salt activated montmorillonite as raw material by traditional hydrothermal method, seed-directed method, steam-assisted crystallization method and aerosol crystallization method. And the HZSM-5 used as solid acid was physically mixed with commercial Cu/ZnO/Al2O3 to obtain bifunctional catalysts for steam reforming of dimethyl ether (SRD) reaction. The samples were systematically characterized by XRD, FT-IR, SEM, N2 adsorption-desorption at low temperature, and NH3-TPD techniques. The results showed that the crystal size, textural and acidity properties of HZSM-5 could be effectively regulated by the synthesis method, and then affecting the SRD performance of the corresponding bifunctional catalyst. TH-HZSM-5 synthesized by the traditional hydrothermal method exhibited suitable acid content and relatively large surface area, pore volume and pore size, and thus bifunctional catalyst composed of TH-HZSM-5 as solid acid and Cu/ZnO/Al2O3 with a mass ratio of 1∶1 showed better SRD reaction performance, and the dimethyl ether conversion and H2 yield reached 100% and 91% under the conditions of reaction temperature 300 ℃, pressure 0.1 MPa, space velocity5000 mL/(g·h), respectively.-
Key words:
- dimethyl ether /
- steam reforming /
- hydrogen production /
- HZSM-5 /
- montmorillonite
-
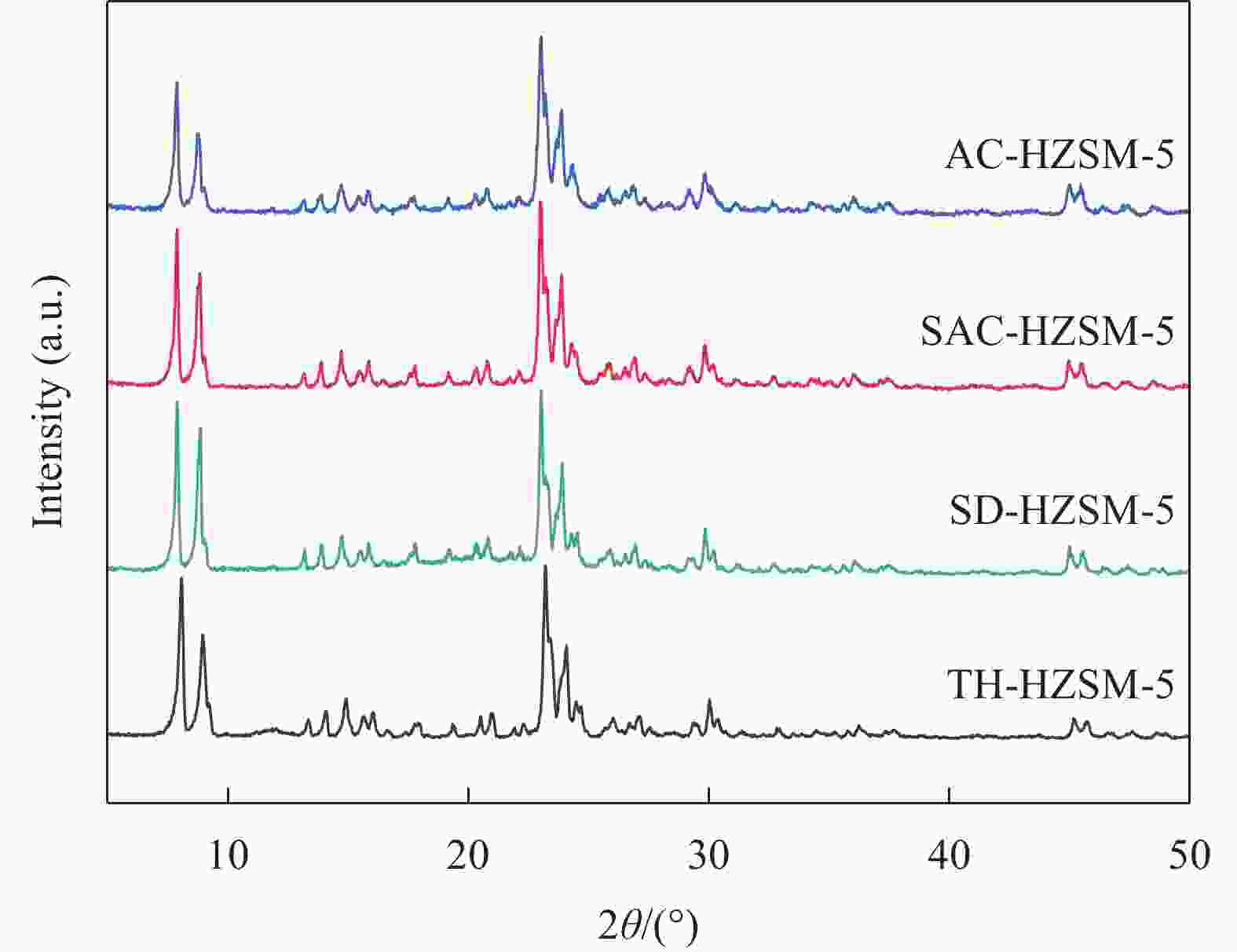
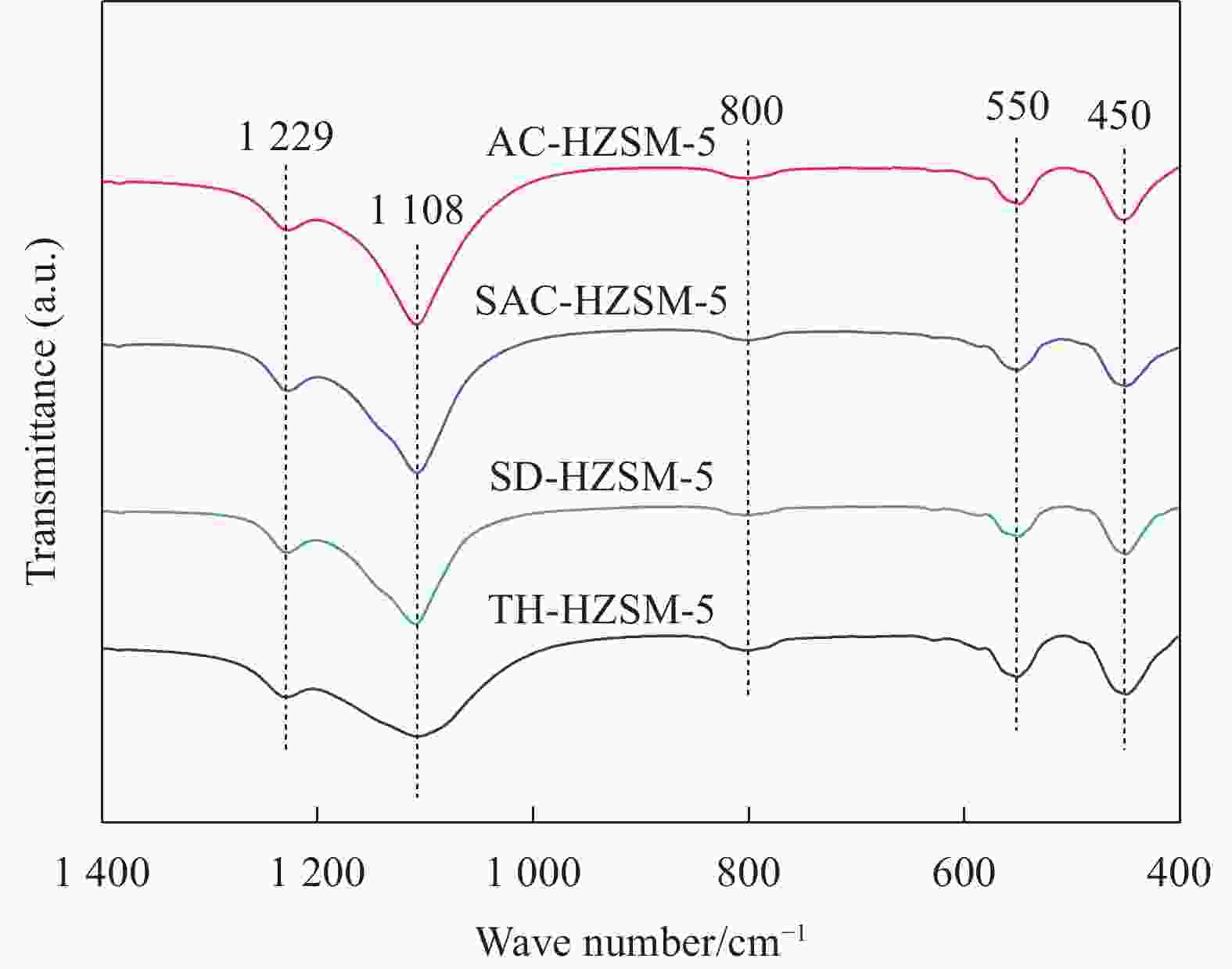
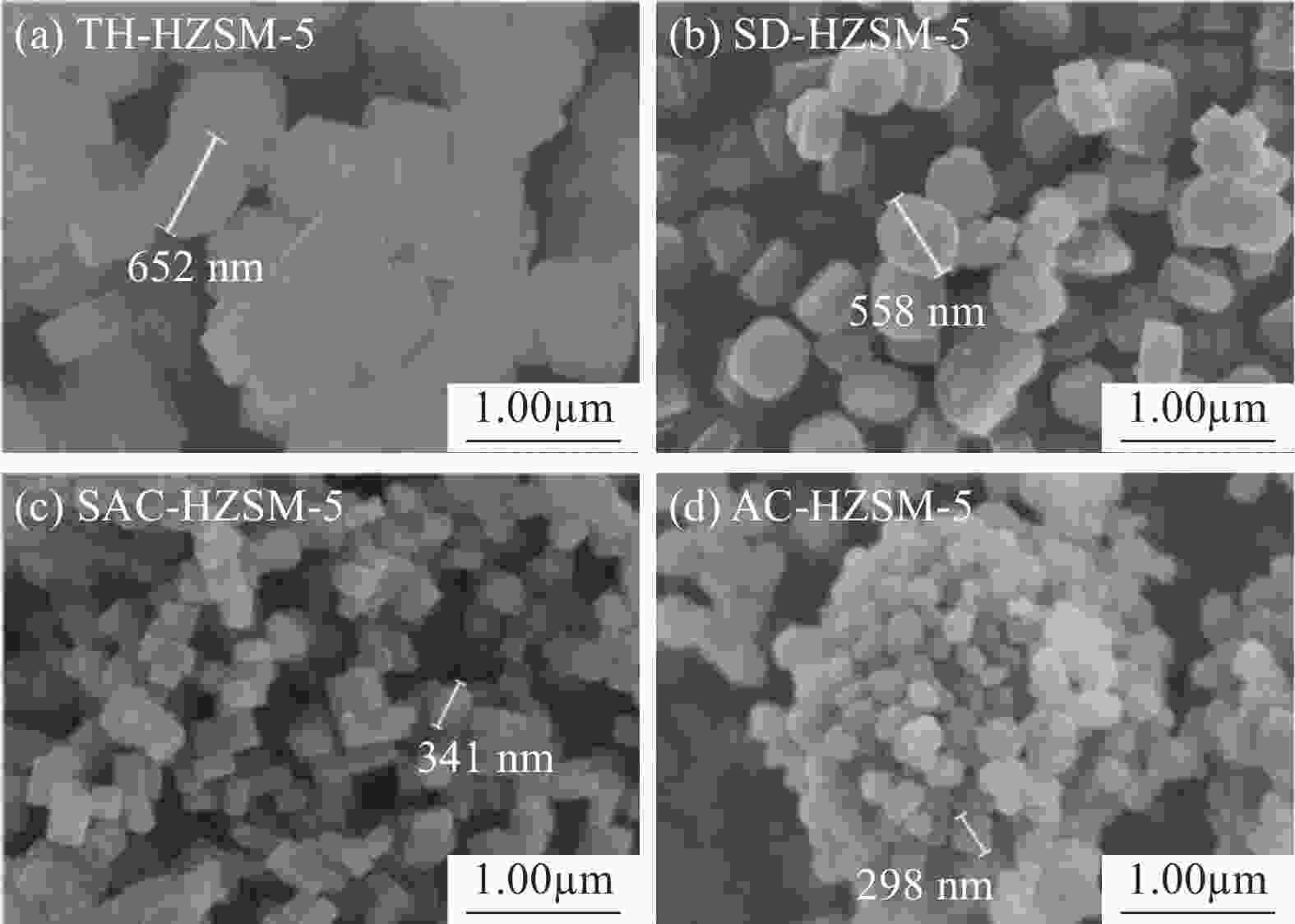
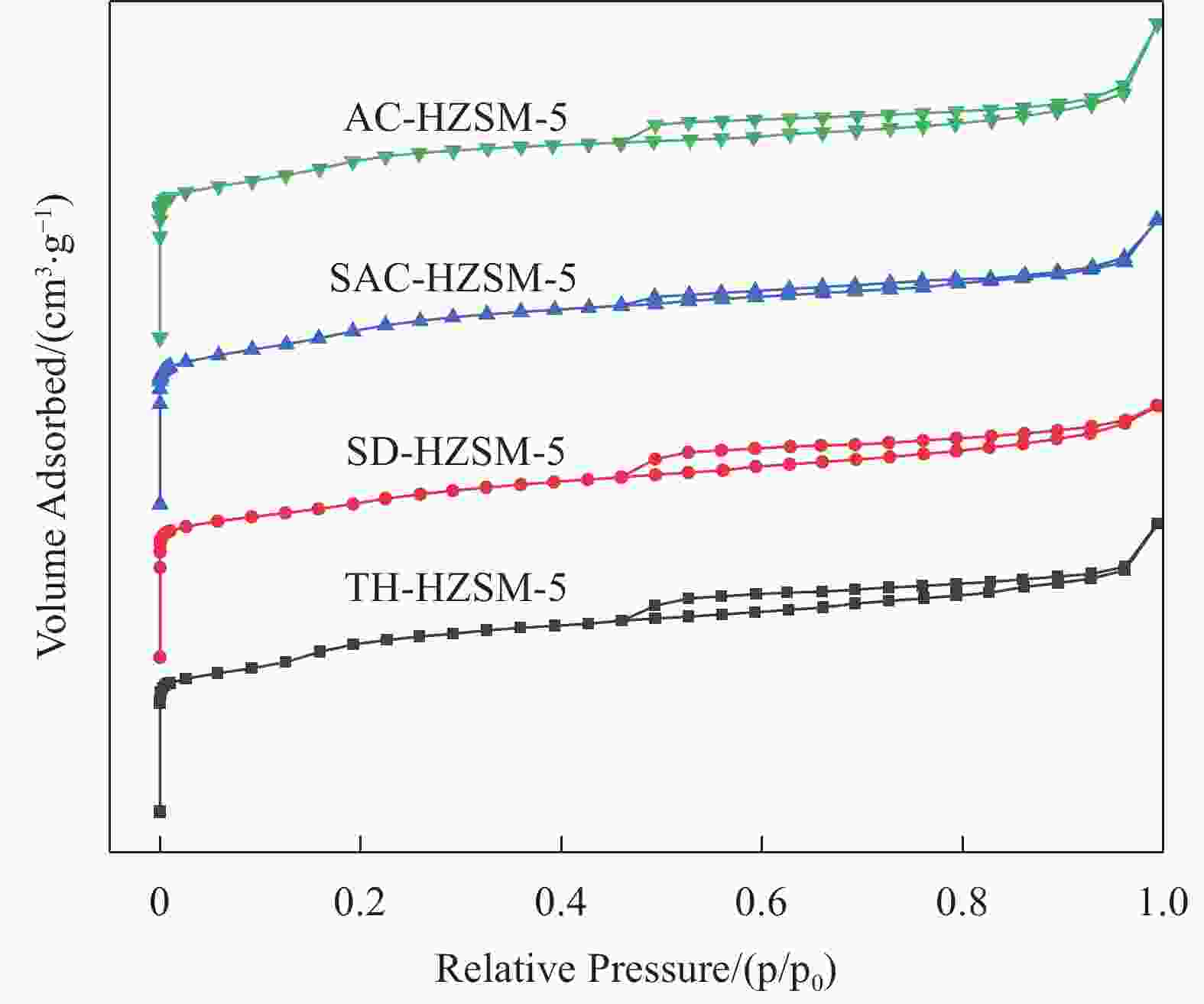
表 1 不同方法合成的HZSM-5分子筛的织构特征
Table 1. Summary of the textural properties of HZSM-5 synthesized by different methods
Sample SBET/(m2·g−1) Vtotal/(cm3·g−1) dave/nm Smicro/(m2·g−1) Vmicro/(cm3·g−1) TH-HZSM-5 399 0.30 3.03 315 0.15 SD-HZSM-5 380 0.26 2.77 288 0.13 SAC-HZSM-5 416 0.30 2.86 335 0.16 AC-HZSM-5 422 0.33 3.11 371 0.17 Notes: SBET—BET(Brunner-Emmet-Teller) specific surface area; Vtotal—total pore volume; dave—average pore size; Smicro—microporous surface area; Vmicro—microporous volume; TH-HZSM-5, SD-HZSM-5, SAC-HZSM-5, AC-HZSM-5—HZSM-5 synthesized by traditional hydrothermal, seed-directed, steam-assisted crystallization and aerosol crystallization method, respectively. 表 2 不同方法合成的HZSM-5的组成及硅铝比
Table 2. The composition and molar ratio of SiO2/Al2O3 of HZSM-5 synthesized by different methods
Sample SiO2/wt% Al2O3/wt% Na2O/wt% Other impurity/wt% SiO2/Al2O3 molar ratio TH-HZSM-5 89.86 3.63 0.66 5.85 42 SD-HZSM-5 93.20 3.43 0.92 2.45 46 SAC-HZSM-5 92.30 3.42 0.74 3.54 46 AC-HZSM-5 92.49 3.40 0.82 3.29 46 表 3 不同方法合成HZSM-5的酸量
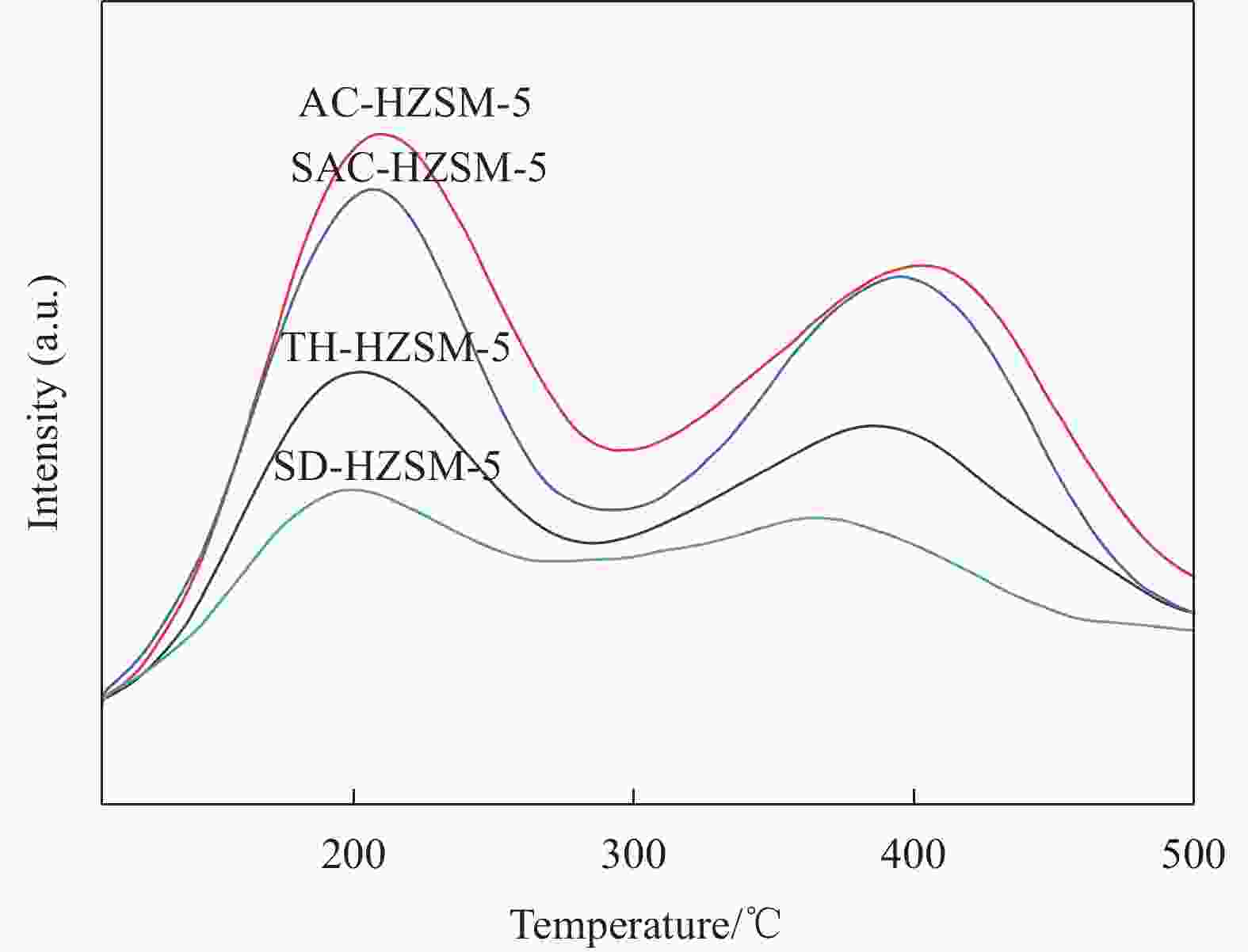
Table 3. The amount of acid site of HZSM-5 synthesized by different methods
Sample Amount of desorbed NH3/(mmol·g−1) Weak acid Strong acid Total acid TH-HZSM-5 0.13 0.09 0.22 SD-HZSM-5 0.08 0.04 0.12 SAC-HZSM-5 0.22 0.18 0.40 AC-HZSM-5 0.24 0.20 0.44 -
[1] LI J, WU T, CHENG C, et al. A review of the research progress and application of key components in the hydrogen fuel cell system[J]. Processes, 2024, 12(2): 249-277. doi: 10.3390/pr12020249 [2] YANG M, MEN Y, LI S L, et al. Enhancement of catalytic activity over TiO2-modifed Al2O3 and ZnO-Cr2O3 composite catalyst for hydrogen production via dimethyl ether steam reforming[J]. Appl Catal A: Gen, 2012, 433-434: 26-34. doi: 10.1016/j.apcata.2012.04.032 [3] 高天宇, 赵永华, 郑择, 等. 酸活化蒙脱土在二甲醚水蒸气重整制氢中的应用[J]. 燃料化学学报, 2021, 49(10): 1498-1503.GAO Tianyu, ZHAO Yonghua, ZHENG Ze, et al. Acid activation of montmorillonite and its application for production of hydrogen via steam reforming of dimethyl ether[J]. J Fuel Chem Technol, 2021, 49(10): 1498-1503 (in Chinese). [4] WEI S, LI C, REN H, et al. Design and optimization of hydrogen production system model for dimethyl ether self-heating steam reforming[J]. International Journal of Hydrogen Energy, 2024, 49: 1450-1467. doi: 10.1016/j.ijhydene.2023.08.369 [5] YANG W W, MA X, TANG X Y, et al. Review on developments of catalytic system for methanol steam reforming from the perspective of energy-mass conversion[J]. Fuel, 2023, 345: 128234. doi: 10.1016/j.fuel.2023.128234 [6] LI J, ZHANG Q J, LONG X, et al. Hydrogen production for fuel cells via steam reforming of dimethyl ether over commercial Cu/ZnO/Al2O3 and zeolite[J]. Chem Eng J, 2012, 187: 299-305. doi: 10.1016/j.cej.2012.01.126 [7] GAO T Y, ZHAO Y H, ZHANG Q J, et al. Zinc oxide modified HZSM-5 as an efficient acidic catalyst for hydrogen production by steam reforming of dimethyl ether[J]. React Kinet Mech Catal, 2019, 128: 235-249. doi: 10.1007/s11144-019-01642-5 [8] LONG X, SONG Y H, LIU Z T, et al. Insights into the long-term stability of the magnesia modified H-ZSM-5 as an efficient solid acid for steam reforming of dimethyl ether[J]. Int J Hydrog Energy, 2019, 44: 21481-21494. doi: 10.1016/j.ijhydene.2019.06.177 [9] NISHIGUCHI T, OKA K, MATSUMOTO T, et al. Durability of WO3/ZrO2-CuO/CeO2 catalysts for steam reforming of dimethyl ether[J]. Appl Catal A: Gen, 2006, 301: 66-74. doi: 10.1016/j.apcata.2005.11.011 [10] LUO H X, ZHAO Y H, ZHANG Q J, et al. The role of promoters in Cu/Acid-MMT catalysts for production of hydrogen via steam reforming of dimethyl ether[J]. J Chem Technol Biotechnol, 2023, 98: 718-725. doi: 10.1002/jctb.7275 [11] FAUNGNAWAKIJ K, KIKUCHI R, SHIMODA N, et al. Effect of thermal treatment on activity and durability of CuFe2O4-Al2O3 composite catalysts for steam re-forming of dimethyl ether[J]. Angew Chem Int Ed, 2008, 47: 9314-9317. doi: 10.1002/anie.200802809 [12] LIU Y, HAN S Y, GUAN D D, et al. Rapid green synthesis of ZSM-5 zeolite from leached illite clay[J]. Micropor Mesopor Mat, 2019, 280: 324-330. doi: 10.1016/j.micromeso.2019.02.027 [13] HAN S Y, LIU Y, YIN C R, et al. Fast synthesis of submicron ZSM-5 zeolite from leached illite clay using a seed-assisted method[J]. Micropor Mesopor Mat, 2019, 275: 223-228. doi: 10.1016/j.micromeso.2018.08.028 [14] LIU H Y, YUE Y Y, SHEN T, et al. Transformation and crystallization behaviors of titanium species in synthesizing Ti-ZSM-5 zeolites from natural rectorite mineral[J]. Ind Eng Chem Res, 2019, 58(27): 11861-11870. doi: 10.1021/acs.iecr.9b01826 [15] PAN F, LU X C, YAN Y, et al. Synthesis of nano/micro scale ZSM-5 from kaolin and its catalytic performance[J]. Kinet Catal, 2017, 58(5): 541-548. doi: 10.1134/S0023158417050184 [16] 蔡玉福, 周艳军, 路君凤, 等. 碱活化蒙脱土负 载铁类芬顿体系去除亚甲基蓝[J]. 复合材料学报, 2023, 40(8): 4601-4612.CAI Yufu, ZHOU Yanjun, LU Junfeng, et al. Removal of methylene blue by Fenton-like system with alkali-activated montmorillonite supported iron catalyst[J]. Acta Materiae Compositae Sinica, 2023, 40(8): 4601-4612(in Chinese). [17] ZHAO Y H, GAO T Y, WANG Y J, et al. Zinc supported on alkaline activated HZSM-5 for aromatization reaction[J]. React Kinet Mech Catal, 2018, 125: 1085-1098. doi: 10.1007/s11144-018-1426-9 [18] 王荧光, 桂建舟, 张晓彤, 等. 纳米ZSM-5分子筛的合成与表征[J]. 光谱实验室, 2005, 22(2): 225-229. doi: 10.3969/j.issn.1004-8138.2005.02.001WANG Yingguang, GUI Jianzhou, ZHANG Xiaotong, et al. Synthesis and characterization of nanosized ZSM-5 zeolite[J]. Chinese J Spectrosc Lab, 2005, 22(2): 225-229 (in Chinese). doi: 10.3969/j.issn.1004-8138.2005.02.001 [19] XIAO W Y, WANG F, Xiao G M. Performance of hierarchical HZSM-5 zeolites prepared by NaOH treatments in the aromatization of glycerol[J]. RSC Adv, 2015, 5: 63697-63704. doi: 10.1039/C5RA07593A [20] LUO C W, HUANG C, LI A, et al. Influence of reaction parameters on the catalytic performance of alkaline-treated zeolites in the novel synthesis of pyridine bases from glycerol and ammonia[J]. Ind Eng Chem Res, 2016, 55: 893-911. doi: 10.1021/ie504934n [21] SHIRAZI L, JAMSHIDI E, GHASEMI M R. The effect of Si/Al ratio of ZSM-5 zeolite on its morphology, acidity and crystal size[J]. Cryst Res Technol, 2008, 43: 1300-1306. doi: 10.1002/crat.200800149 [22] ZHANG S G, HIGASHIMOTO S, YAMASHITA H, et al. Characterization of vanadium oxide/ZSM-5 zeolite catalysts prepared by the solid-state reaction and their photocatalytic reactivity. In situ photoluminescence, XAFS, ESR, FT-IR, and UV-Vis Investigations[J]. J Phys Chem B, 1998, 102: 5590-5594. doi: 10.1021/jp981230r [23] DUMITRIU D, BÂRJEGA R, FRUNZA L, et al. BiOx clusters occluded in a ZSM-5 matrix: preparation, characterization, and catalytic behavior in liquid-phase oxidation of hydrocarbons[J]. J Catal, 2003, 219: 337-351. doi: 10.1016/S0021-9517(03)00216-1 [24] THOMMES M, KANEKO K, NEIMARK A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure Appl Chem, 2015, 87: 1051-1069. doi: 10.1515/pac-2014-1117 [25] FAUNGNAWAKIJ K, TANAKA Y, SHIMODA N, et al. Influence of solid-acid catalysts on steam reforming and hydrolysis of dimethyl ether for hydrogen production[J]. Appl Catal A: Gen, 2006, 304: 40-48. doi: 10.1016/j.apcata.2006.02.021 [26] SEMELSBERGER T A, OTT K C, BORUP R L, et al. Generating hydrogen-rich fuel-cell feeds from dimethyl ether (DME) using physical mixtures of a commercial Cu/Zn/Al2O3 catalyst and several solid-acid catalysts[J]. Appl Catal B: Environ, 2006, 65(3-4): 291-300. doi: 10.1016/j.apcatb.2006.02.015 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 48
- HTML全文浏览量: 30
- 被引次数: 0




 下载:
下载: