Ru sensitized MOF electronic structure for efficient water electrolysis
-
摘要: 金属有机框架(MOF)材料因其强大的结构和功能可调性已成为极具潜力的电解水催化剂。然而,MOF催化剂由于自身较弱的电荷转移能力及有限的稳定性,其固有活性较低。因此,设计出兼具高活性与高稳定性的MOF催化剂仍是具有挑战性的难题。本文通过两步水热法成功合成了CoRu-BDC/NF(BDC:对苯二甲酸、NF:泡沫镍),Ru的引入调节了负载在NF上Co-BDC的内在活性,赋予了CoRu-BDC/NF丰富的活性位点、较快的电荷传输能力,有利于电催化性能的提升。结果表明,CoRu-BDC/NF在酸性环境中电流密度达到10 mA·cm−2时,HER过电位为34 mV,Tafel斜率为33 mV·dec−1。值得注意的是,在碱性条件下,当电流密度为10 mA·cm−2,HER和OER所需的过电位也仅为32 mV和280 mV,Tafel斜率分别为39 mV·dec−1 、49 mV·dec−1,均表现出优异的电催化性能。Ru的引入导致Co的电子结构和配位环境发生变化,使原始Co-MOF的表面积增加,为电化学反应提供了更好的电子转移平台,加快了电子在电极和电解液界面间的传递,进一步提升了电催化性能。Abstract: Metal-organic framework (MOF) materials have become potential catalysts for water electrolysis due to their strong structural and functional adjustability. However, the intrinsic activity of MOF catalyst is low due to its weak charge transfer ability and limited stability. Therefore, it is still a challenging problem to design MOF catalysts with high activity and high stability. In this paper, CoRu-BDC/NF(BDC: terylene acid, NF: nickel foam) was successfully synthesized by two-step hydrothermal method. The introduction of Ru regulated the intrinsic activity of Co-BDC loaded on NF, endowed CoRu-BDC/NF with abundant active sites and fast charge transport capacity, which was conducive to the improvement of electrocatalytic performance. The results show that when the current density of CoRu-BDC/NF reaches 10 mA·cm−2 in acidic environment, HER overpotential is 34 mV and Tafel slope is 33 mV·dec−1. It is worth noting that under alkaline conditions, when the current density is 10 mA·cm−2, the required overpotential of HER and OER is only 32 mV and 280 mV, and the slope of Tafel is 39 mV·dec−1 and 49 mV·dec−1, showing excellent electrocatalytic performance. The introduction of Ru leads to changes in the electronic structure and coordination environment of Co, increasing the surface area of the original Co-MOF, providing a better electron transfer platform for the electrochemical reaction, speeding up the transfer of electrons between the electrode and the electrolyte interface, and further improving the electrocatalytic performance.
-
Keywords:
- CoRu-BDC /
- Ru introduction /
- HER /
- OER /
- coordinated environment
-
化石燃料消耗的增加和生活环境的恶化促使人们探索环境友好型和可持续型能源,作为传统化石燃料的替代品[1-4]。其中,氢能因其能量密度高和二氧化碳零排放而被认为是最有潜力的替代品[5,6]。在这方面,电化学水分解是实现可再生能源转化为氢能的一种很有前途的策略[7,8]。在水电解过程中,水分子通过析氧反应(OER)和析氢反应(HER)分解为氧和氢[9,10]。然而,OER和HER缓慢的动力学限制了电解水技术大规模的应用。因此,开发高效的电催化剂已迫在眉睫[11,12]。到目前为止,虽然已经发展了多种催化剂,但由于催化机理的巨大差异,只有少数电极能够同时对OER和HER表现出良好的活性[9]。为此,通过制造具有低成本效益的双功能电催化剂来代替复杂制备和高消耗能的催化剂是非常有利的。
金属有机框架(MOFs)是一类新兴的多孔晶体材料[13,14],由各种有机配体和金属中心组成,在水分解[15]、气体存储[16]和金属空气电池[14]等方面具有一定的应用价值。由于其灵活的可调控性和良好的结构,可以通过基本的分子结构进行化学性能优化[17,18]。这使得MOFs成为在分子水平上研究催化剂设计的很有前途的模型催化剂。缺陷MOF[19]和晶格应变MOF[20]已经被报道来设计先进的OER电催化剂。因此,开发性能优异的双功能MOF材料用于全解水仍是具有挑战性的课题。
目前,金属掺杂策略因其优异的活性在电催化领域引起了广泛的关注[21]。值得注意的是,其催化活性主要来自于金属原子独特的电子结构和金属原子与载体之间的相互作用[22,23]。金属原子除了作为反应活性位点,还可以将金属原子结合到催化剂中通过电子相互作用调制初始催化剂的局部电子结构[24,25],同时保留原始材料的结构特征[6,26,27]。这为引入金属原子来提高MOFs的电催化性能提供了巨大的发展潜力。
因此,本实验通过水热法成功将微量Ru元素引入到Co-BDC/NF MOF材料上。金属Ru的引入调节了Co-BDC/NF电子结构,同时使原始Co-MOF的表面积增加,为电化学反应提供了更好的电子转移平台,加快了电子在电极和电解液界面间的传递,进一步优化催化效率。值得注意的是,本实验所合成的CoRu-BDC/NF在酸性和碱性均具有优异的HER性能。在酸性条件下,仅需34 mV的过电位就可以驱动10 mA·cm−2的HER,Tafel斜率为33 mV·dec−1;碱性条件下,仅需32 mV的过电位就可以驱动10 mA·cm−2的HER,Tafel斜率为39 mV·dec−1。此外,CoRu-BDC/NF在碱性溶液中也表现出优异的OER活性。
1. 实验部分
1.1 材料的制备与表征
1.1.1 试剂
实验中使用的主要试剂包括:氯化钴(CoCl2· 6H2O,分析纯)、氯化钌(RuCl3,分析纯)和对苯二甲酸(H2BDC,分析纯),购自阿拉丁试剂有限公司;N,N-二甲基甲酰胺(DMF,分析纯)和无水乙醇(C2H6O,分析纯),均购自国药集团化学试剂有限公司。
1.1.2 实验方法
把裁剪好的泡沫镍(2×3 cm)依次放入丙酮、盐酸、乙醇中超声清洗10分钟,然后将其放在60℃真空烘箱中6小时烘干备用。
将1 mmol CoCl2·6H2O加入到15 ml DMF中记为溶液A,再将1 mmol H2BDC加入到14 mL DMF和1 mL 0.4 M NaOH的混合溶液中记为溶液B。将溶液A和溶液B分别搅拌均匀后,混合继续搅拌30分钟,将该混合溶液和处理好的泡沫镍转移到50 mL反应釜中,在120℃烘箱中加热12小时,得到的样品依次用DMF、乙醇清洗数次后在真空烘箱60℃烘干得到Co-BDC/NF。
称取50 mg RuCl3溶解在25 mL乙醇中搅拌10分钟后,将Co-BDC/NF样品和该溶液转移至50 mL反应釜中,120℃加热6小时,得到的样品用乙醇清洗数次后在真空烘箱60℃烘干,得到样品CoRu-BDC/NF。
1.1.3 表征仪器
通过X射线衍射仪 (XRD,DX-2700)表征样品晶相结构,Cu Kα 辐射(波长为 0.154 18 nm),加速电压和电流分别为45 kV和40 mA,扫描范围 2θ=5°~ 90°,扫描速率5 °·min−1;使用场发射扫描电子显微镜 (FE-SEM,SU-8020,加速电压为10 kV)和透射电子显微镜 (TEM,JEM-F200,加速电压为200 kV)观察样品形貌结构;使用X射线光电子能谱仪 (XPS,Kratos Analytical Ltd.)对样品进行元素分析,以C1s 结合能 (284.8 eV)校准峰位置。
1.2 电极的制备与性能测试
将制备好的CoRu-BDC/NF裁剪成(1×0.5 cm)小片,夹在夹片电极上制作成工作电极。
电化学测试是三电极配置的(Admiral Squidstat Plus 1554型)电化学工作站进行的。三电极分别为饱和甘汞电极(SCE)(参比电极)、铂丝(对电极)、纳米材料(工作电极)。电势通过能斯特方程转换为可逆氢电极(E(vs.RHE)=E(Vs.SCE)+0.241+0.059×pH),在可逆氢电极(RHE)上对参比电极进行了标定。
2. 结果与分析
2.1 CoRu-BDC微观结构分析
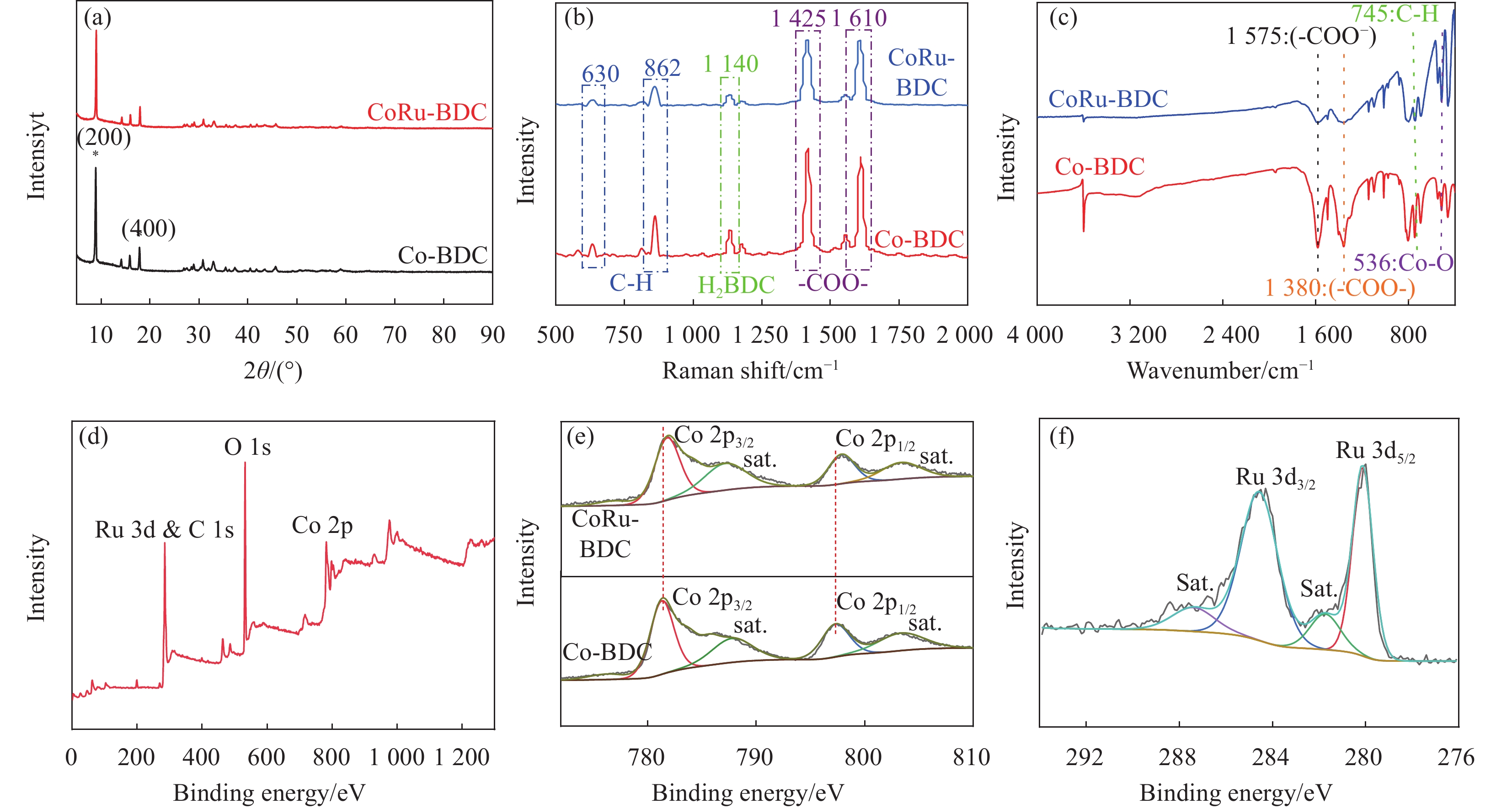
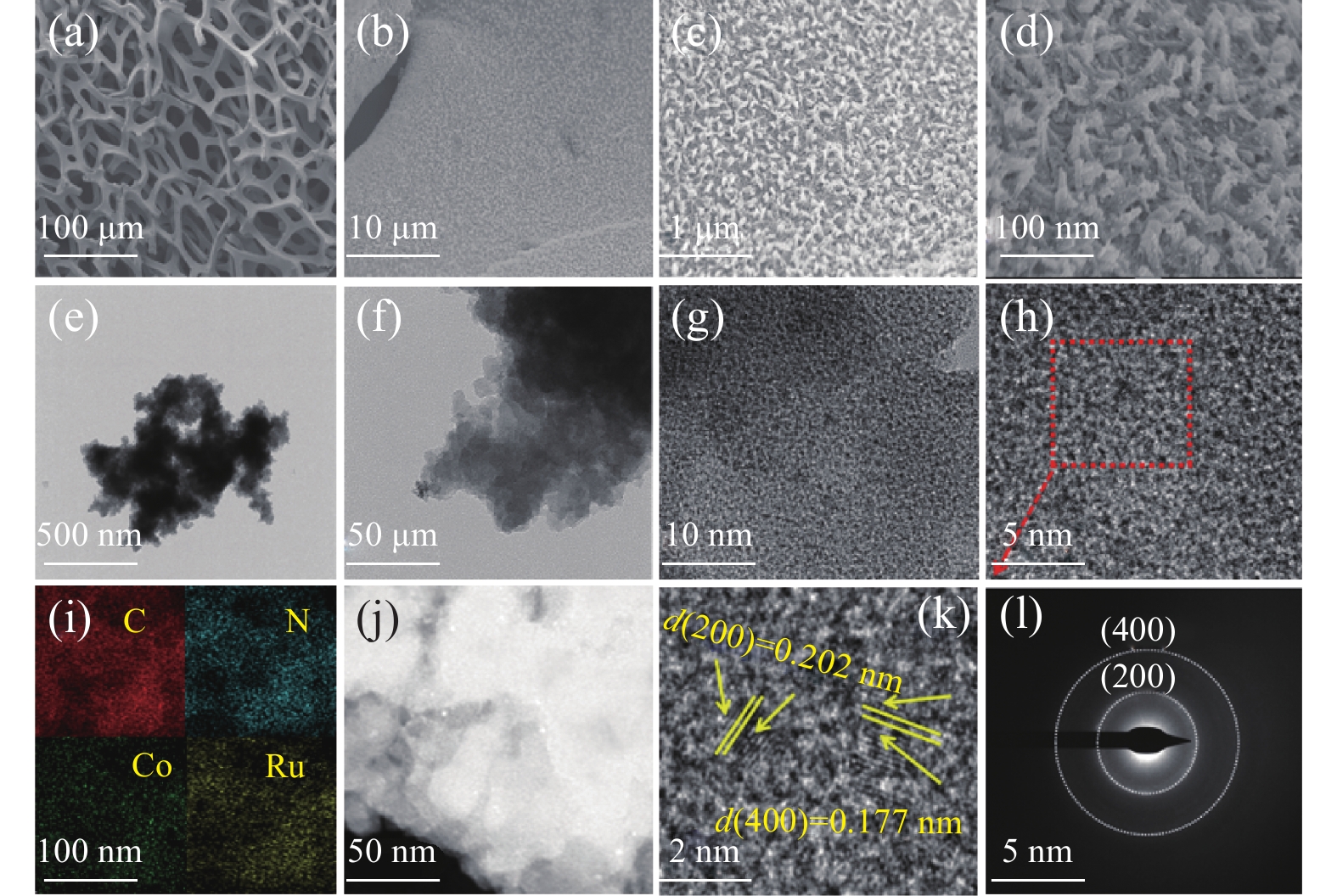
采用场发射扫描电镜 (FE-SEM)和透射电子显微镜 (TEM)对所制备样品的形貌和结构进行了表征,如图1所示。图1(a)-1(d)为CoRu-BDC/NF的扫描电镜图。图1(c)表明CoRu-BDC以不规则颗粒的形貌成功生长在NF上。通过TEM进一步观察了CoRu-BDC的形态,图1(e)-1(g)可以清晰的看到不规则的CoRu-BDC颗粒堆积在泡沫镍上。图1(i)-(j)为CoRu-BDC的TEM-EDX(能量色散 X射线谱)图,显示C、N、Co、Ru元素的均匀分布,表明我们成功的引入了Ru元素。对图1(h)选定区域进行晶格条纹测量,如图1(k)所示晶格间距为 0.177 和 0.202 nm,分别对应Co-BDC的(400)和(200)晶面[28,29]。所选区域的电子衍射(SAED,图1(l))呈现出一些明显的衍射环。从内到外的明亮环可以与Co-BDC的(400)和(200)晶面相匹配,与XRD分析结果一致[30]。
![]() 图 1 (a-d)分别CoRu-BDC/NF纳米复合材料的FE-SEM图像;(e-h,k)为CoRu-BDC的TEM图;(i-j)为CoRu-BDC的TEM-EDX图;(l)为CoRu-BDC电子衍射图Figure 1. Fig 1(a-d) are the FE-SEM images of CoRu-BDC/NF nanocomposites, respectively. (e-h,k-i) indicates the TEM image of CoRu-BDC, (i-j) is the TEM-EDX image of CoRu-BDCBDC—Terylene acid; NF—NAickel foam
图 1 (a-d)分别CoRu-BDC/NF纳米复合材料的FE-SEM图像;(e-h,k)为CoRu-BDC的TEM图;(i-j)为CoRu-BDC的TEM-EDX图;(l)为CoRu-BDC电子衍射图Figure 1. Fig 1(a-d) are the FE-SEM images of CoRu-BDC/NF nanocomposites, respectively. (e-h,k-i) indicates the TEM image of CoRu-BDC, (i-j) is the TEM-EDX image of CoRu-BDCBDC—Terylene acid; NF—NAickel foam通过XRD对合成的粉末状Co-BDC、CoRu-BDC的晶体结构进行了表征。如图2(a)所示,对于CoRu-BDC样品,在8.9°和17.9°处,可以清晰地观察到Co-BDC的(200)和(400)晶面对应的特征峰,表明CoRu-BDC仍然保持着Co-BDC的晶体结构,其分散峰由于Ru元素的掺入而变钝。这种变化表明,Ru元素引入会破坏Co与有机配体之间的配位作用,从而诱导不饱和配位Co原子,这种不饱和配位金属原子可以作为水电解的高活性位点[19,31,32]。
![]() 图 2 Co-BDC和CoRu-BDC(a)XRD图;(b)拉曼图;(c)傅里叶变换红外光谱;(d) XPS全谱图;(e) Co 2p轨道的高分辨XPS图;(f) Ru 3d轨道的高分辨XPS图Figure 2. Co-BDC and CoRu-BDC (a) XRD; (b) Raman map; (c) Fourier transform infrared spectrum; (d) XPS full spectrum; (e) high resolution XPS map of Co 2p orbit; (f) high resolution XPS map of Ru 3d orbit
图 2 Co-BDC和CoRu-BDC(a)XRD图;(b)拉曼图;(c)傅里叶变换红外光谱;(d) XPS全谱图;(e) Co 2p轨道的高分辨XPS图;(f) Ru 3d轨道的高分辨XPS图Figure 2. Co-BDC and CoRu-BDC (a) XRD; (b) Raman map; (c) Fourier transform infrared spectrum; (d) XPS full spectrum; (e) high resolution XPS map of Co 2p orbit; (f) high resolution XPS map of Ru 3d orbit通过拉曼和傅里叶变换红外(FT-IR)光谱,研究了MOF结构的官能团。如图2(b)所示,在630和862 cm−1周围的振动带为苯环上C-H的拉伸区域[33]。在
1425 和1609 cm−1处的特征峰归属于羧酸基的面内和面外的拉伸[34]。图2(c)所示,Co-BDC和CoRu-BDC在745 cm−1处呈现出条带,是由苯环上C—H键振动引起的。在1575 cm−1和1380 cm−1处的峰可以归属于有机配体的Vasym (—COO—)和Vsym 上(—COO—),这意味着暴露的羧酸基团锚定在MOF结构的表面上[35]。在539 cm−1处存在的峰可能是由于金属原子(Co)与H2BDC的羧基之间的配位键的振动,表明金属离子与H2BDC有机配体成功配位。2.2 CoRu-BDC XPS图谱分析
通过X射线光电子能谱(XPS)进一步研究了CoRu-BDC的化学组成和化学键价态。从图2(d)可以看出,CoRu-BDC的全谱表明C、O、Co、Ru元素存在。在图2(f)中281.9 eV和284.9 eV的峰,分别归属为Ru 3 d5/2和Ru 3 d3/2,表明Ru3+成功的掺入到Co-BDC中[36-43]。Co-BDC和CoRu-BDC的Co 2 p高分辨率光谱如图2(e)所示,在Co-BDC的Co 2 p谱图中,位于781.4 eV和797.3 eV的两个峰为Co2+ 2 p3/2和Co2+ 2 p1/2的特征峰[44,45]。与Ru3+结合后,CoRu-BDC的Co 2 p比Co-BDC明显向更高的能量区域转移,表明Ru和Co结合之后使得Co-BDC产生大量的不饱和Co原子,导致Co原子上电子密度降低,配位环境发生改变[19,32,46]。
2.3 CoRu-BDC析氢及析氧性能的究
2.3.1 酸性条件下HER
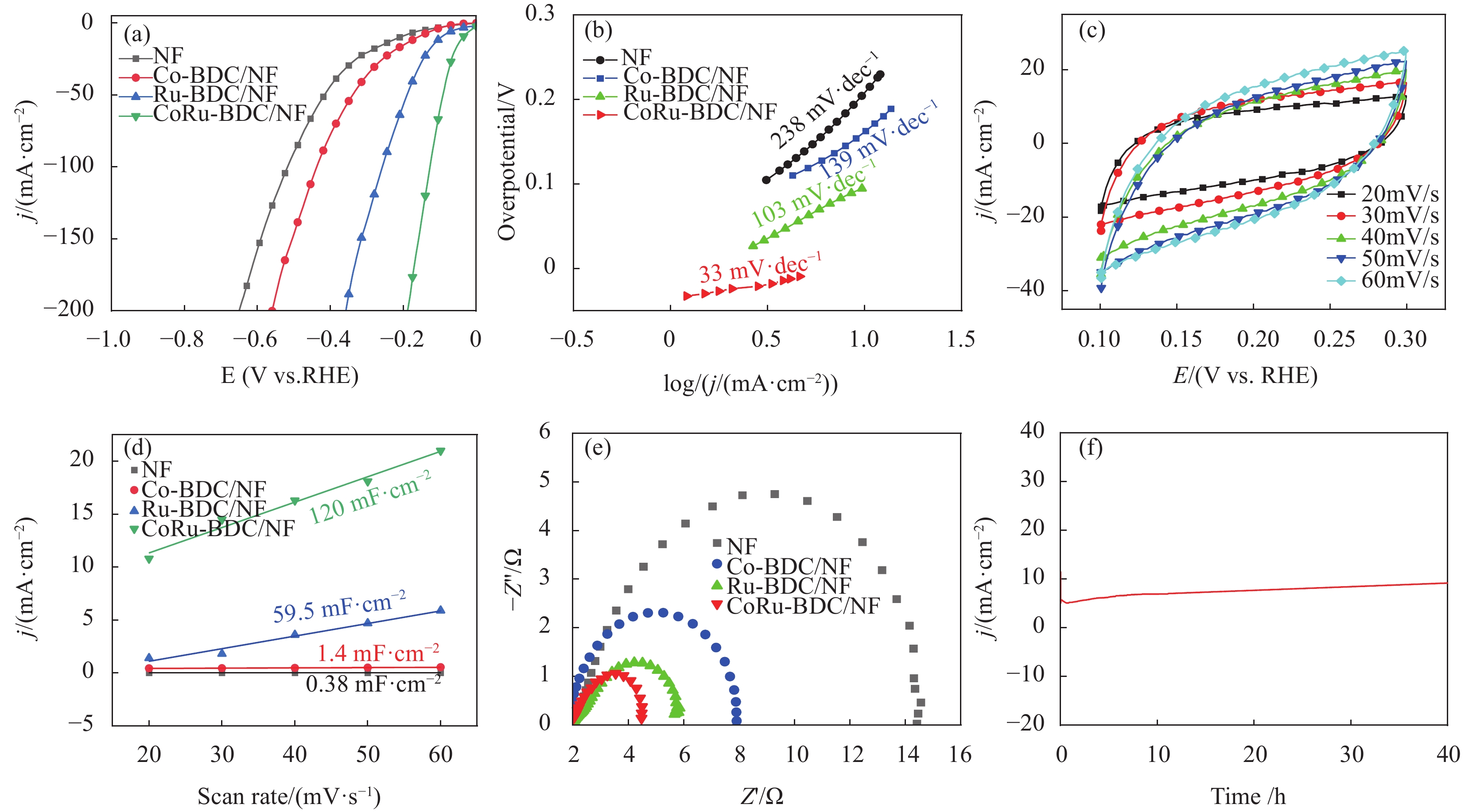
首先在室温下用三电极体系在0.5 mol/L H2SO4溶液中测定了催化剂的催化性能。为了评价这些材料的HER活性,我们使用商业Pt/C作为基准。如图3(a)所示为iR补偿后的极化曲线,Co-BDC/NF的电催化活性较差,电流密度为10 mA·cm−2时过电位为163 mV。Co-BDC/NF中引入Ru原子后,催化剂对HER具有较高的电催化性能。值得注意的是,在0.5 mol/L H2SO4溶液中,CoRu-BDC/NF在10 mV·cm−2时表现出较高的HER活性,具有较低的过电位(34 mV),远低于Ru/C(115 mV),甚至可与商业Pt/C(22 mV)相媲美[47]。此外,CoRu- BDC/NF只需要127 mV的过电位就能达到100 mA·cm−2的高电流密度,低于Pt/C(139 mV)。CoRu-BDC/NF显著的电催化性能也优于之前报道的其他HER电催化剂。CoRu-BDC/NF的Tafel斜率(图3(b))仅为33 mV·dec−1,均低于Co-BDC/NF(139 mV·dec−1)、Ru-BDC/NF(103 mV·dec−1)以及NF(238 mV·dec−1),表明CoRu-BDC/NF为最佳催化剂。
![]() 图 3 NF、Co-BDC/NF、Ru-BDC/NF、CoRu-BDC/NF在0.5 mol/L H2SO4中的电化学HER测量:(a) 极化曲线;(b) Tafel斜率; (c) CoRU-BDC/NF不同扫速下的CV曲线; (d) 0.25 V (vs.RHE)处电流密度与扫速的差值; (e) 阻抗图;(f)HER过程中CoRu-BDC/NF的稳定性Figure 3. Electrochemical HER measurements of NF, Co-BDC/NF, Ru-BDC/NF and CoRu-BDC/NF in 0.5 M H2SO4 . (a) Polarization curves, (b) Tafel plots,(c) CV curves at different sweeps of CoRu-BDC/NF speed, (d) Difference of current density at 0.25 V( vs.RHE) as a function of scan rate, (e) Nyquist plots, (f) Stability of the CoRu-BDC/NF during the HER processj—Current density; E —Overpotentials; Z’ —Impedance the real part; Z’’ —Impedance virtual part
图 3 NF、Co-BDC/NF、Ru-BDC/NF、CoRu-BDC/NF在0.5 mol/L H2SO4中的电化学HER测量:(a) 极化曲线;(b) Tafel斜率; (c) CoRU-BDC/NF不同扫速下的CV曲线; (d) 0.25 V (vs.RHE)处电流密度与扫速的差值; (e) 阻抗图;(f)HER过程中CoRu-BDC/NF的稳定性Figure 3. Electrochemical HER measurements of NF, Co-BDC/NF, Ru-BDC/NF and CoRu-BDC/NF in 0.5 M H2SO4 . (a) Polarization curves, (b) Tafel plots,(c) CV curves at different sweeps of CoRu-BDC/NF speed, (d) Difference of current density at 0.25 V( vs.RHE) as a function of scan rate, (e) Nyquist plots, (f) Stability of the CoRu-BDC/NF during the HER processj—Current density; E —Overpotentials; Z’ —Impedance the real part; Z’’ —Impedance virtual part为了证明CoRu-BDC/NF电催化剂活性增强的原因,进行了双层电容(Cdl)测量。Cdl是用来评估电化学活性表面积(ECSA)的一个重要指标,ECSA一般与催化剂的Cdl成正比[48],在0.1-0.3 V(vs.RHE)的电位下获得了在不同扫描速率下的CV曲线,如图3(c)所示。在0.25 V(vs.RHE)处,以电流密度的差值为纵坐标,扫描速率为横坐标作图,所得曲线斜率即为2 Cdl值,如图3(d)所示。CoRu-BDC/NF的Cdl值为120 mF·cm−2,远高于Co-BDC/NF、Ru-BDC/NF、NF的Cdl值。可见,CoRu-BDC/NF暴露出更多的电活性位点。采用电化学阻抗谱(EIS)对电荷转移机制进行了研究,如图3(e)所示。由图可知,纯的NF,Co-BDC/NF、Ru-BDC/NF、CoRu-BDC/NF的Rct值依次减小,这意味着CoRu-BDC/NF电荷转移更快。Co原子配位环境的改变,使得材料的导电性能增加,有助于电子在电极和电解液界面间的传递,从而促进电化学反应的发生[49]。当过电位为-0.2 V(vs RHE)时,在0.5 mol/L H2SO4溶液中测量了CoRu-BDC/NF的可持续稳定性。如图3(f)所示。经过长达40小时的测试,电流密度基本没发生变化,表明CoRu-BDC/NF具有优异的稳定性。
2.3.2 碱性条件下HER
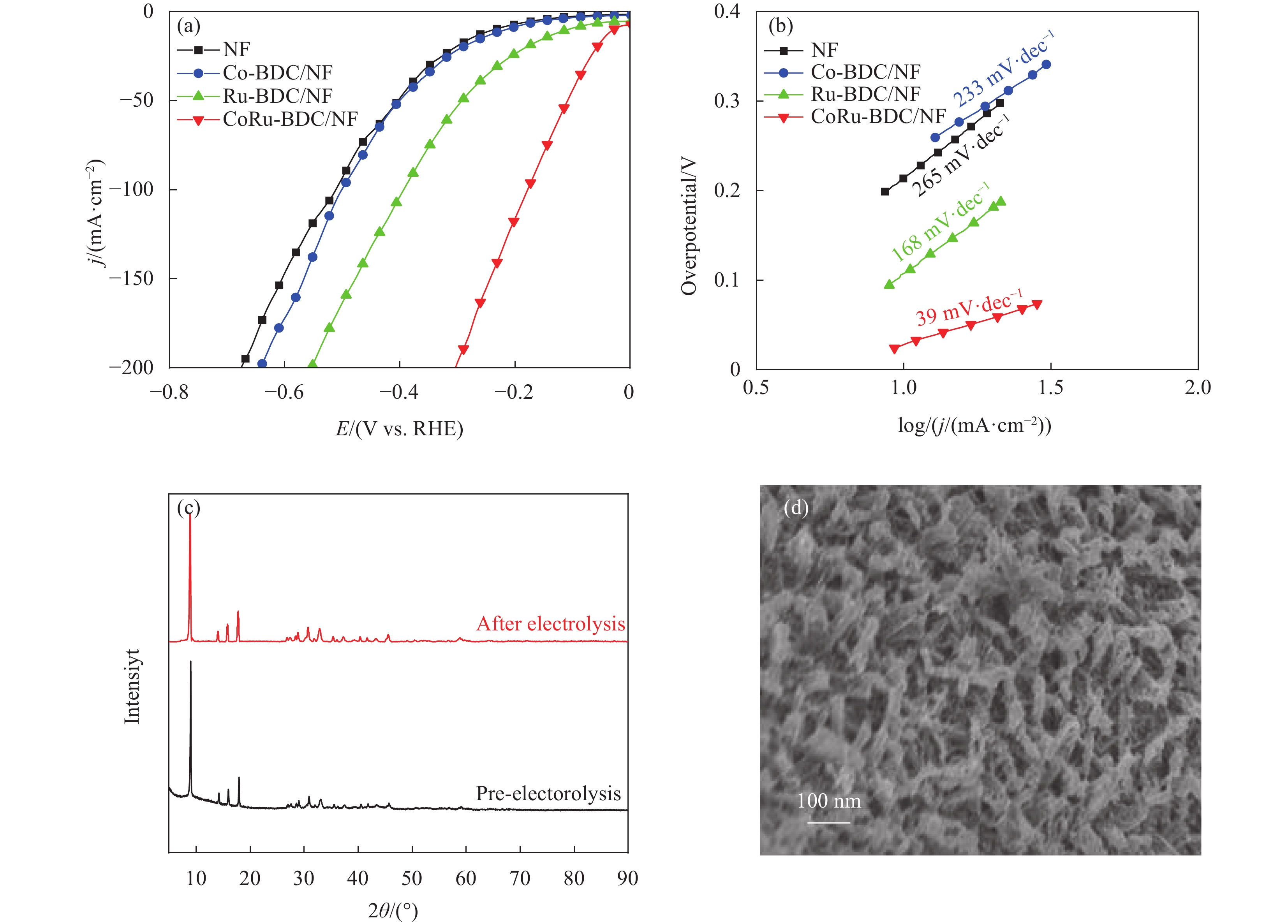
采用相同的三电极系统研究了NF、Co-BDC/NF、Ru-BDC/NF、CoRu-BDC/NF在1 mol/L NaOH中的HER性能。图4(a)为iR补偿后催化剂的LSV曲线。值得注意的是,NF的催化活性较低,Ru的引入可以极大地提高Co-BDC/NF的HER性能。在电流密度为10 mA·cm−2条件下通过过电位(η)直观地比较了不同催化剂的HER活性,如图4(a)所示。CoRu-BDC/NF的η10(电流密度为10 mA·cm−2时的过电位)值仅为32 mV,而Co-BDC/NF具有较大的过电位(215 mV),Ru的引入提高了电化学活性。图4(b)显示,NF、Co-BDC/NF、Ru-BDC/NF、CoRu-BDC/NF的Tafel斜率值分别为265、233、168和39 mV·dec−1。同样,CoRu-BDC/NF的Tafel值最小,表明CoRu-BDC/NF电极之间的反应动力学最有效。为了探究材料在碱性溶液中的稳定性,对测试前后的XRD和SEM进行了对比,图4(c)和4(d)显示,我们发现CoRu-BDC/NF材料的形貌和晶形未发生改变,证明该材料在碱性条件下也有良好的稳定性。
2.3.3 OER性能评价
在1 M KOH中使用相同的三电极系统研究NF、Co-BDC/NF、Ru-BDC/NF、CoRu-BDC/NF的OER性能。图5(a)为iR补偿后的极化曲线,三种催化剂均表现出明显的OER活性,在电流密度为10 mA·cm−2时,Co-BDC/NF过电位为390 mV。Ru的引入使得Co-BDC/NF获得了更好的OER活性,在电流密度为10 mA·cm−2时,CoRu-BDC/NF过电位为280 mV,这优于商用的IrO2 (316 mV)[50]。可见,Ru的引入显著提高了Co-BDC/NF的OER活性。在所有样品中,CoRu-BDC/NF拥有最小的Tafel斜率(约49 mV·dec−1),表明其具有最快的OER动力学(图5(b))。从阻抗图(图5(c))可以看出,CoRu-BDC/NF比Co-BDC/NF和Ru-BDC/NF的OER反应要快得多。如图5(e)所示,CoRu-BDC/NF(34.9 mF·cm−2)的双层电容(Cdl)远高于Co-BDC(5.9 mF·cm−2)和Ru-BDC(11.6 mF·cm−2),这一结果表明,该催化剂(CoRu-BDC/NF)具有较多的反应活位位点。
![]() 图 5 NF、Co-BDC/NF、Ru-BDC/NF和CoRu-BDC/NF:(a)在1 mol/L KOH中的电化学OER测试的极化曲线;(b) Tafel斜率; (c)阻抗图;(d) CoRU-BDC/NF不同扫速下的CV曲线; (e) 0.25 V (vs.RHE)处电流密度与扫速的差值;(f) CoRu-BDC/NF||CoRu-BDC/NF以5 mV·s−1在1 mol/L KOH整体水分的极化曲线。Figure 5. NF, Co-BDC/NF, Ru-BDC/NF and CoRu-BDC/NF. (a) Polarization curves for the electrochemical OER tests in 1 M KOH, (b)Tafel plots, (c) Nyquist plots, (d) CV curves at different sweeps of CoRu-BDC/NF speed, (e) Difference of current density at 0.25 V (vs.RHE) as a function of scan rate, (e) Polarization curves for CoRu-BDC/NF||CoRu-BDC/NF couples toward overall water splitting recorded at a scan rate of 5 mV s −1 in 1 M KOH.
图 5 NF、Co-BDC/NF、Ru-BDC/NF和CoRu-BDC/NF:(a)在1 mol/L KOH中的电化学OER测试的极化曲线;(b) Tafel斜率; (c)阻抗图;(d) CoRU-BDC/NF不同扫速下的CV曲线; (e) 0.25 V (vs.RHE)处电流密度与扫速的差值;(f) CoRu-BDC/NF||CoRu-BDC/NF以5 mV·s−1在1 mol/L KOH整体水分的极化曲线。Figure 5. NF, Co-BDC/NF, Ru-BDC/NF and CoRu-BDC/NF. (a) Polarization curves for the electrochemical OER tests in 1 M KOH, (b)Tafel plots, (c) Nyquist plots, (d) CV curves at different sweeps of CoRu-BDC/NF speed, (e) Difference of current density at 0.25 V (vs.RHE) as a function of scan rate, (e) Polarization curves for CoRu-BDC/NF||CoRu-BDC/NF couples toward overall water splitting recorded at a scan rate of 5 mV s −1 in 1 M KOH.由于CoRu-BDC/NF对OER和HER均具有良好的电催化活性,使用双电极碱性电解槽进一步研究了整体的水电解性能,图5(f)为电催化剂的LSV曲线。采用CoRu-BDC/NF||CoRu-BDC/NF双电极电解水时,当电流密度达到10 mA·cm−2,仅需要1.47 V的电压。通过对比相关材料作为全解水催化剂时的电催化性能(如表1所示),表明CoRu-BDC/NF催化剂在全解水方面有着广泛的应用前景。
表 1 近期报道的相关材料全解水性能的比较Table 1. Comparison of the recent reported overall water solution properties of related materialsElectrocatalyst Acid conditions
HER η10/mVAlkaline conditions
HER η10/mVAlkaline conditions
OER η10/mVOverall Water/V Reference CoRu-BDC/NF 34 32 280 1.47 This work Co-Co2C/CC / 96 261 1.63 [51] CoFeP-N / 64 219 1.56 [52] CoCu@NC / 199 301 1.87 [53] Co-CN@NiFe / 87 233 1.55 [54] Notes:η10—Overpotentials with a current density of 10 mA·cm−2 3. 结 论
本文采用溶剂热法成功在泡沫镍(NF)上制备了Co-对苯二甲酸(BDC) MOF材料,以RuCl3为Ru源,通过水热法合成了电催化性能优异的CoRu-BDC/NF。表征及测试结构表明:
(1) Ru的引入导致Co的电子结构和配位环境发生变化,使原始Co-BDC产生了额外的不饱和配位金属原子,暴露出更多的活性位点,从而促进电化学反应的发生。
(2) Co和Ru之间的协同作用改变了金属的晶格结构,使得材料的导电性能增加,有助于电子在电极和电解液界面间的传递,提高了电催化效率。
(3) 在酸性条件下CoRu-BDC/NF电流密度达到10 mA·cm−2时,HER过电位仅为34 mV。在碱性条件下,电流密度为10 mA·cm−2,HER和OER所需的过电位分别为32 mV和280 mV。采用CoRu-BDC/NF||CoRu-BDC/NF作为双电极电解水时,当电流密度达到10 mA·cm−2,仅需要1.47 V的电压。综上所述,Ru的引入有效的提高了Co-BDC的电催化性能,且在全解水方面有着广泛的应用前景,为构建高效MOFs电催化剂提供了新的思路。
-
图 1 (a-d)分别CoRu-BDC/NF纳米复合材料的FE-SEM图像;(e-h,k)为CoRu-BDC的TEM图;(i-j)为CoRu-BDC的TEM-EDX图;(l)为CoRu-BDC电子衍射图
Figure 1. Fig 1(a-d) are the FE-SEM images of CoRu-BDC/NF nanocomposites, respectively. (e-h,k-i) indicates the TEM image of CoRu-BDC, (i-j) is the TEM-EDX image of CoRu-BDC
BDC—Terylene acid; NF—NAickel foam
图 2 Co-BDC和CoRu-BDC(a)XRD图;(b)拉曼图;(c)傅里叶变换红外光谱;(d) XPS全谱图;(e) Co 2p轨道的高分辨XPS图;(f) Ru 3d轨道的高分辨XPS图
Figure 2. Co-BDC and CoRu-BDC (a) XRD; (b) Raman map; (c) Fourier transform infrared spectrum; (d) XPS full spectrum; (e) high resolution XPS map of Co 2p orbit; (f) high resolution XPS map of Ru 3d orbit
图 3 NF、Co-BDC/NF、Ru-BDC/NF、CoRu-BDC/NF在0.5 mol/L H2SO4中的电化学HER测量:(a) 极化曲线;(b) Tafel斜率; (c) CoRU-BDC/NF不同扫速下的CV曲线; (d) 0.25 V (vs.RHE)处电流密度与扫速的差值; (e) 阻抗图;(f)HER过程中CoRu-BDC/NF的稳定性
Figure 3. Electrochemical HER measurements of NF, Co-BDC/NF, Ru-BDC/NF and CoRu-BDC/NF in 0.5 M H2SO4 . (a) Polarization curves, (b) Tafel plots,(c) CV curves at different sweeps of CoRu-BDC/NF speed, (d) Difference of current density at 0.25 V( vs.RHE) as a function of scan rate, (e) Nyquist plots, (f) Stability of the CoRu-BDC/NF during the HER process
j—Current density; E —Overpotentials; Z’ —Impedance the real part; Z’’ —Impedance virtual part
图 5 NF、Co-BDC/NF、Ru-BDC/NF和CoRu-BDC/NF:(a)在1 mol/L KOH中的电化学OER测试的极化曲线;(b) Tafel斜率; (c)阻抗图;(d) CoRU-BDC/NF不同扫速下的CV曲线; (e) 0.25 V (vs.RHE)处电流密度与扫速的差值;(f) CoRu-BDC/NF||CoRu-BDC/NF以5 mV·s−1在1 mol/L KOH整体水分的极化曲线。
Figure 5. NF, Co-BDC/NF, Ru-BDC/NF and CoRu-BDC/NF. (a) Polarization curves for the electrochemical OER tests in 1 M KOH, (b)Tafel plots, (c) Nyquist plots, (d) CV curves at different sweeps of CoRu-BDC/NF speed, (e) Difference of current density at 0.25 V (vs.RHE) as a function of scan rate, (e) Polarization curves for CoRu-BDC/NF||CoRu-BDC/NF couples toward overall water splitting recorded at a scan rate of 5 mV s −1 in 1 M KOH.
表 1 近期报道的相关材料全解水性能的比较
Table 1 Comparison of the recent reported overall water solution properties of related materials
Electrocatalyst Acid conditions
HER η10/mVAlkaline conditions
HER η10/mVAlkaline conditions
OER η10/mVOverall Water/V Reference CoRu-BDC/NF 34 32 280 1.47 This work Co-Co2C/CC / 96 261 1.63 [51] CoFeP-N / 64 219 1.56 [52] CoCu@NC / 199 301 1.87 [53] Co-CN@NiFe / 87 233 1.55 [54] Notes:η10—Overpotentials with a current density of 10 mA·cm−2 -
[1] MENG C, CAO Y, LUO Y, et al. A Ni-MOF nanosheet array for efficient oxygen evolution electrocatalysis in alkaline media[J]. Inorganic Chemistry Frontiers, 2021, 8(12): 3007-3011. DOI: 10.1039/D1QI00345C
[2] JIAO L, ZHU J, ZHANG Y, et al. Non-Bonding Interaction of Neighboring Fe and Ni Single-Atom Pairs on MOF-Derived N-Doped Carbon for Enhanced CO(2) Electroreduction[J]. J Am Chem Soc, 2021, 143(46): 19417-19424. DOI: 10.1021/jacs.1c08050
[3] CHEN C, SUN M, ZHANG F, et al. Adjacent Fe Site boosts electrocatalytic oxygen evolution at Co site in single-atom-catalyst through a dual-metal-site design[J]. Energy & Environmental Science, 2023, 16(4): 1685-1696.
[4] YAO Y, LIAO G, CUI H, et al. Monodispersed In2O3@ZIF-8 core-shell nanocomposite with selectivity and enhanced photocatalytic activity[J]. Journal of Materials Science: Materials in Electronics, 2023, 34(18): 1445. DOI: 10.1007/s10854-023-10870-4
[5] YANG X, LI Q X, CHI S Y, et al. Hydrophobic perfluoroalkane modified metal-organic frameworks for the enhanced electrocatalytic reduction of CO2[J]. SmartMat, 2022, 3(1): 163-172. DOI: 10.1002/smm2.1086
[6] ZHU W, SONG X, LIAO F, et al. Stable and oxidative charged Ru enhance the acidic oxygen evolution reaction activity in two-dimensional ruthenium-iridium oxide[J]. Nat Commun, 2023, 14(1): 5365. DOI: 10.1038/s41467-023-41036-9
[7] SONG F, LI W, YANG J, et al. Interfacing nickel nitride and nickel boosts both electrocatalytic hydrogen evolution and oxidation reactions[J]. Nat Commun, 2018, 9(1): 4531. DOI: 10.1038/s41467-018-06728-7
[8] YAO M, WANG B, SUN B, et al. Rational design of self-supported Cu@WC core-shell mesoporous nanowires for pH-universal hydrogen evolution reaction[J]. Applied Catalysis B: Environmental, 2021, 280: 119451. DOI: 10.1016/j.apcatb.2020.119451
[9] INAMDAR A I, CHAVAN H S, HOU B, et al. A Robust Nonprecious CuFe Composite as a Highly Efficient Bifunctional Catalyst for Overall Electrochemical Water Splitting[J]. Small, 2020, 16(2): e1905884. DOI: 10.1002/smll.201905884
[10] LI L, WANG B, ZHANG G, et al. Electrochemically Modifying the Electronic Structure of IrO2 Nanoparticles for Overall Electrochemical Water Splitting with Extensive Adaptability[J]. Advanced Energy Materials, 2020, 10(30): 2001600. DOI: 10.1002/aenm.202001600
[11] YU L, WU L, MCELHENNY B, et al. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting[J]. Energy & Environmental Science, 2020, 13(10): 3439-3446.
[12] WANG P, PU Z, LI W, et al. Coupling NiSe2-Ni2P heterostructure nanowrinkles for highly efficient overall water splitting[J]. Journal of Catalysis, 2019, 377: 600-608. DOI: 10.1016/j.jcat.2019.08.005
[13] GONG Y N, JIAO L, QIAN Y, et al. Regulating the Coordination Environment of MOF-Templated Single-Atom Nickel Electrocatalysts for Boosting CO2 Reduction[J]. Angewandte Chemie International Edition, 2020, 59(7): 2705-2709. DOI: 10.1002/anie.201914977
[14] ZHANG Y, HAN Y, DENG F, et al. Enhancement of the performance of Ge-air batteries under high temperatures using conductive MOF-modified Ge anodes[J]. Carbon Energy, 2024: e580.
[15] MADHU R, KARMAKAR A, BERA K, et al. Recent developments in transition metal-based MOFs for electrocatalytic water splitting emphasizing fundamental and structural aspects[J]. Materials Chemistry Frontiers, 2023, 7(11): 2120-2152. DOI: 10.1039/D3QM00089C
[16] LIM Y, KIM B, KIM J. Data-Driven Design of Flexible Metal-Organic Frameworks for Gas Storage[J]. Chemistry of Materials, 2024, 36(11): 5465-5473. DOI: 10.1021/acs.chemmater.4c00398
[17] YU M-H, GENG L, CHANG Z, et al. Coordination Bonding Directed Molecular Assembly toward Functional Metal-Organic Frameworks: From Structural Regulation to Properties Modulation[J]. Accounts of Materials Research, 2023, 4(10): 839-853. DOI: 10.1021/accountsmr.3c00097
[18] DONG P, ZHANG X, HISCOX W, et al. Toward High-Performance Metal-Organic-Framework-Based Quasi-Solid-State Electrolytes: Tunable Structures and Electrochemical Properties[J]. Adv Mater, 2023, 35(32): e2211841. DOI: 10.1002/adma.202211841
[19] XUE Z, LIU K, LIU Q, et al. Missing-linker metal-organic frameworks for oxygen evolution reaction[J]. Nat Commun, 2019, 10(1): 5048. DOI: 10.1038/s41467-019-13051-2
[20] CHENG W. Lattice-strained metal-organic-framework arrays for bifunctional oxygen electrocatalysis[J]. Nat. Energy, 2019, 4(2): 115-122. DOI: 10.1038/s41560-018-0308-8
[21] MA Y, LENG D, ZHANG X, et al. Enhanced Activities in Alkaline Hydrogen and Oxygen Evolution Reactions on MoS2 Electrocatalysts by In-Plane Sulfur Defects Coupled with Transition Metal Doping[J]. Small, 2022, 18(39): 2203173. DOI: 10.1002/smll.202203173
[22] HUANG Y, HAN J, WANG H, et al. Multiple metallic dopants in nickel nanoparticles for electrocatalytic oxygen evolution[J]. Progress in Natural Science: Materials International, 2023, 33(1): 67-73. DOI: 10.1016/j.pnsc.2023.03.002
[23] ZHANG J, CHENG C, XIAO L, et al. Construction of Co-Se-W at Interfaces of Phase-Mixed Cobalt Selenide via Spontaneous Phase Transition for Platinum-Like Hydrogen Evolution Activity and Long-Term Durability in Alkaline and Acidic Media[J]. Adv Mater, 2024, 36(28): e2401880. DOI: 10.1002/adma.202401880
[24] SUN P, QIAO Z, DONG X, et al. Designing 3d Transition Metal Cation-Doped MRuO(x) As Durable Acidic Oxygen Evolution Electrocatalysts for PEM Water Electrolyzers[J]. J Am Chem Soc, 2024, 146(22): 15515-15524. DOI: 10.1021/jacs.4c04096
[25] LIU X, WEI S, CAO S, et al. Lattice Strain with Stabilized Oxygen Vacancies Boosts Ceria for Robust Alkaline Hydrogen Evolution Outperforming Benchmark Pt[J]. Adv Mater, 2024, 36(33): e2405970. DOI: 10.1002/adma.202405970
[26] Zhou L, Shao Y, Yin F, et al. Stabilizing non-iridium active sites by non-stoichiometric oxide for acidic water oxidation at high current density[J]. Nature Communications, 2023, 14(1): 7644. DOI: 10.1038/s41467-023-43466-x
[27] CHEN X, XU X, CHENG Y, et al. Achieving High-Performance Electrocatalytic Water Oxidation on Ni(OH)(2) with Optimized Intermediate Binding Energy Enabled by S-Doping and CeO(2) -Interfacing[J]. Small, 2024, 20(8): e2303169. DOI: 10.1002/smll.202303169
[28] GUO X, WAN X, LIU Q, et al. Phosphated IrMo bimetallic cluster for efficient hydrogen evolution reaction[J]. eScience, 2022, 2(3): 304-310. DOI: 10.1016/j.esci.2022.04.002
[29] QI Y, XIAO X, MEI Y, et al. Modulation of Brønsted and Lewis Acid Centers for NixCo3-xO4 Spinel Catalysts: Towards Efficient Catalytic Conversion of Lignin[J]. Advanced Functional Materials, 2022, 32(15): 2111615. DOI: 10.1002/adfm.202111615
[30] ZHOU J, QIAO F, REN Z, et al. Amorphization Engineering of Bimetallic Metal-Organic Frameworks to Identify Volcano-Type Trend toward Oxygen Evolution Reaction[J]. Advanced Functional Materials, 2023, 34(1): 2304380.
[31] ZHAO S, WANG Y, DONG J, et al. Ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution[J]. Nature Energy, 2016, 1(12): 1-10.
[32] HUANG L, GAO G, ZHANG H, et al. Self-dissociation-assembly of ultrathin metal-organic framework nanosheet arrays for efficient oxygen evolution[J]. Nano Energy, 2020, 68: 104296. DOI: 10.1016/j.nanoen.2019.104296
[33] SUN F, WANG G, DING Y, et al. NiFe-Based Metal-Organic Framework Nanosheets Directly Supported on Nickel Foam Acting as Robust Electrodes for Electrochemical Oxygen Evolution Reaction[J]. Advanced Energy Materials, 2018, 8(21): 1800584. DOI: 10.1002/aenm.201800584
[34] BORDIGA S, LAMBERTI C, RICCHIARDI G, et al. Electronic and vibrational properties of a MOF-5 metal-organic framework: ZnO quantum dot behaviour[J]. Chem Commun (Camb), 2004, (20): 2300-1. DOI: 10.1039/B407246D
[35] WANG C-P, FENG Y, SUN H, et al. Self-Optimized Metal-Organic Framework Electrocatalysts with Structural Stability and High Current Tolerance for Water Oxidation[J]. ACS Catalysis, 2021, 11(12): 7132-7143. DOI: 10.1021/acscatal.1c01447
[36] CHEN G, WANG T, ZHANG J, et al. Accelerated Hydrogen Evolution Kinetics on NiFe-Layered Double Hydroxide Electrocatalysts by Tailoring Water Dissociation Active Sites[J]. Adv Mater, 2018, 30(10): 1706279. DOI: 10.1002/adma.201706279
[37] ZHANG L-N, LANG Z-L, WANG Y-H, et al. Cable-like Ru/WNO@C nanowires for simultaneous high-efficiency hydrogen evolution and low-energy consumption chlor-alkali electrolysis[J]. Energy & Environmental Science, 2019, 12(8): 2569-2580.
[38] PENG Y, LU B, CHEN L, et al. Hydrogen evolution reaction catalyzed by ruthenium ion-complexed graphitic carbon nitride nanosheets[J]. Journal of Materials Chemistry A, 2017, 5(34): 18261-18269. DOI: 10.1039/C7TA03826G
[39] LU B, GUO L, WU F, et al. Ruthenium atomically dispersed in carbon outperforms platinum toward hydrogen evolution in alkaline media[J]. Nat Commun, 2019, 10(1): 631. DOI: 10.1038/s41467-019-08419-3
[40] WANG Z-L, SUN K, HENZIE J, et al. Spatially Confined Assembly of Monodisperse Ruthenium Nanoclusters in a Hierarchically Ordered Carbon Electrode for Efficient Hydrogen Evolution[J]. Angewandte Chemie International Edition, 2018, 57(20): 5848-5852. DOI: 10.1002/anie.201801467
[41] LI W, ZHANG H, ZHANG K, et al. Monodispersed ruthenium nanoparticles interfacially bonded with defective nitrogen-and-phosphorus-doped carbon nanosheets enable pH-universal hydrogen evolution reaction[J]. Applied Catalysis B: Environmental, 2022, 306: 121095. DOI: 10.1016/j.apcatb.2022.121095
[42] LI W, ZHANG H, HONG M, et al. Defective RuO2/TiO2 nano-heterostructure advances hydrogen production by electrochemical water splitting[J]. Chemical Engineering Journal, 2022, 431: 134072. DOI: 10.1016/j.cej.2021.134072
[43] ZHANG M, CHEN J, LI H, et al. Ru-RuO2/CNT hybrids as high-activity pH-universal electrocatalysts for water splitting within 0.73 V in an asymmetric-electrolyte electrolyzer[J]. Nano Energy, 2019, 61: 576-583. DOI: 10.1016/j.nanoen.2019.04.050
[44] FAN R, MU Q, WEI Z, et al. Atomic Ir-doped NiCo layered double hydroxide as a bifunctional electrocatalyst for highly efficient and durable water splitting[J]. Journal of Materials Chemistry A, 2020, 8(19): 9871-9881. DOI: 10.1039/D0TA03272G
[45] LI Z, WU Y, LU G. Highly efficient hydrogen evolution over Co(OH)2 nanoparticles modified g-C3N4 co-sensitized by Eosin Y and Rose Bengal under Visible Light Irradiation[J]. Applied Catalysis B: Environmental, 2016, 188: 56-64. DOI: 10.1016/j.apcatb.2016.01.057
[46] LI G, LI J, LIU X, et al. Ir-doped Co-BDC MOF as efficient bifunctional catalyst for overall electrochemical water splitting[J]. Ionics, 2023, 29(5): 1963-1973. DOI: 10.1007/s11581-023-04928-w
[47] SUN Y, XUE Z, LIU Q, et al. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution[J]. Nat Commun, 2021, 12(1): 1369. DOI: 10.1038/s41467-021-21595-5
[48] CAO C, MA D D, XU Q, et al. Semisacrificial Template Growth of Self-Supporting MOF Nanocomposite Electrode for Efficient Electrocatalytic Water Oxidation[J]. Advanced Functional Materials, 2018, 29(6): 1970033.
[49] WANG Y, ZHU R, WANG Z, et al. Cu induced formation of dendritic CoFeCu ternary alloys on Ni foam for efficient oxygen evolution reaction[J]. Journal of Alloys and Compounds, 2021, 880: 160523. DOI: 10.1016/j.jallcom.2021.160523
[50] LI W, ZHANG H, ZHANG K, et al. Altered electronic structure of trimetallic FeNiCo-MOF nanosheets for efficient oxygen evolution[J]. Chem Commun (Camb), 2023, 59(32): 4750-4753. DOI: 10.1039/D2CC06727G
[51] WANG P, ZHU J, PU Z, et al. Interfacial engineering of Co nanoparticles/Co2C nanowires boosts overall water splitting kinetics[J]. Applied Catalysis B: Environmental, 2021, 296: 120334. DOI: 10.1016/j.apcatb.2021.120334
[52] WANG R, SUN X, ZHONG J, et al. Low-temperature plasma-assisted synthesis of iron and nitrogen co-doped CoFeP-N nanowires for high-efficiency electrocatalytic water splitting[J]. Applied Catalysis B: Environment and Energy, 2024, 352: 124027. DOI: 10.1016/j.apcatb.2024.124027
[53] ZHANG Z, WANG H, LI Y, et al. Confined Pyrolysis Synthesis of Well-dispersed Cobalt Copper Bimetallic Three-dimensional N-Doped Carbon Framework as Efficient Water Splitting Electrocatalyst[J]. Chemical Research in Chinese Universities, 2022, 38(3): 750-757. DOI: 10.1007/s40242-022-1504-4
[54] GUO T, CHEN L, LI Y, et al. Controllable Synthesis of Ultrathin Defect-Rich LDH Nanoarrays Coupled with MOF-Derived Co-NC Microarrays for Efficient Overall Water Splitting[J]. Small, 2022, 18(29): e2107739. DOI: 10.1002/smll.202107739
-
目的
化石燃料消耗的增加和生活环境的恶化促使人们探索环境友好型和可持续型能源,作为传统化石燃料的替代品。其中,氢能因其能量密度高和二氧化碳零排放而被认为是最有潜力的替代品。在这方面,电化学水分解是实现可再生能源转化为氢能的一种很有前途的策略。
方法为了得到了高稳定性且高活性的电催化剂,通过水热法成功将微量Ru元素引入到Co-BDC/NF MOF材料上。金属Ru的引入调节了Co-BDC/NF电子结构,同时使原始Co-MOF的表面积增加,为电化学反应提供了更好的电子转移平台,加快了电子在电极和电解液界面间的传递,进一步优化催化效率。
结果经过一系列表征测试,所合成的CoRu-BDC/NF在酸性和碱性均具有优异的HER性能。在酸性条件下,仅需34 mV的过电位就可以驱动10 mA·cm的HER,Tafel斜率为33 mV·dec;碱性条件下,仅需32 mV的过电位就可以驱动10 mA·cm的HER,Tafel斜率为39 mV·dec。此外,CoRu-BDC/NF在碱性溶液中也表现出优异的OER活性。
结论研究表明,CoRu-BDC/NF之所以具有更好的电催化活性,这归因于金属有机框架的灵活可调控性和金属掺杂优异的活性。金属原子除了作为反应活性位点,还可以将金属原子结合到催化剂中通过电子相互作用调制初始催化剂的局部电子结构,同时保留原始材料的结构特征。而Ru的引入导致Co的电子结构和配位环境发生变化,使原始Co-BDC产生了额外的不饱和配位金属原子,暴露出更多的活性位点,从而促进电化学反应的发生。Co和Ru之间的协同作用改变了金属的晶格结构,使得材料的导电性能增加,有助于电子在电极和电解液界面间的传递,提高了电催化效率。
-
本文成功制备了CoRu-BDC/NF,通过引入Ru活性位点来调节Co-BDC/NF的内在活性,赋予了CoRu-BDC/NF优异的催化能力和较快的电荷传输能力,从而设计出了兼具高活性与高稳定性的MOF电催化剂。CoRu-BDC/NF具有极佳的全解水性能,在碱性条件下,其 HER和OER所需的过电位分别为仅32 mV和280 mV。同时具有较强的抗酸能力,在酸性条件下也能达到34 mV的HER电催化效率,为构建高效MOFs电催化剂提供了新的思路。

NF、Co-BDC/NF、Ru-BDC/NF、CoRu-BDC/NF:(a)在0.5 mol/L H2SO4中的电化学HER测量极化曲线(b)在1 mol/L KOH中的电化学HER测试的极化曲线;(c)在1 mol/L KOH中的电化学OER测试的极化曲线;(d)CoRu-BDC/NF||CoRu-BDC/NF在1mol/L KOH整体水分的的极化曲线




 下载:
下载: