Synergistic antibacterial study of Cu2O/CuO-tetracycline composites
-
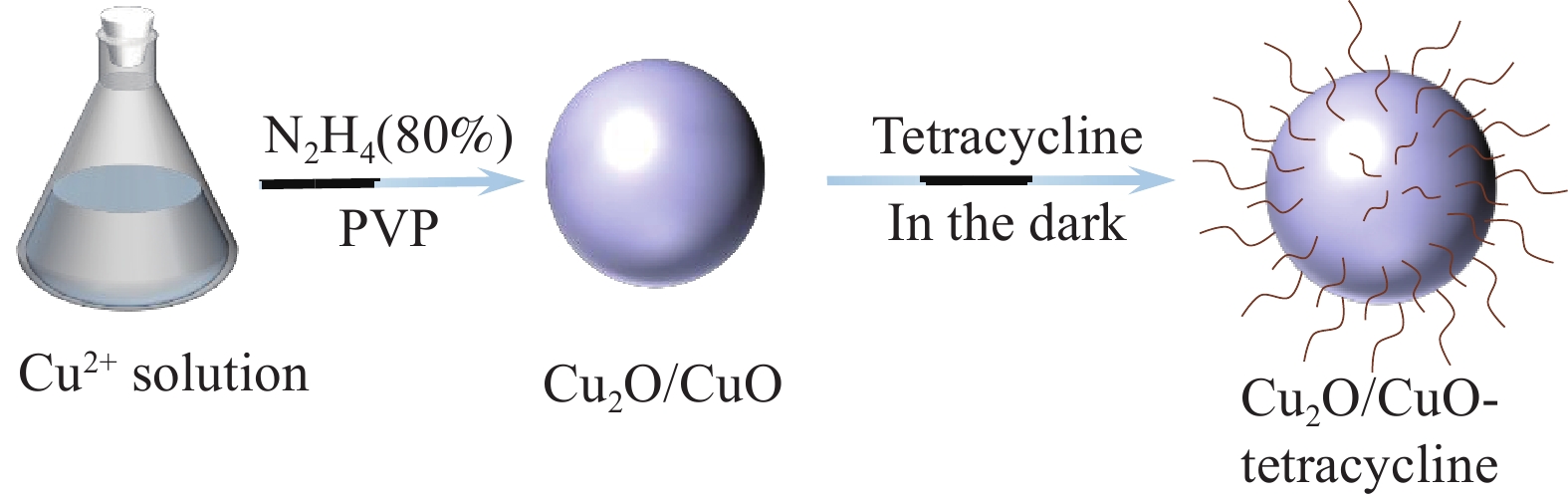
摘要: 耐药细菌快速的增长和新治疗策略的可用性越来越少,迫使人们急需研发出新型抑菌剂来解决这类难题。本文以三水硝酸铜[Cu(NO3)2·3H2O]为原料、水合肼为还原剂制备氧化亚铜(Cu2O/CuO),通过与四环素配位结合得到Cu2O/CuO-四环素复合材料。采用TEM、EDS、XRD、XPS、FTIR和UV-vis等表征技术对抑菌剂进行系统表征。探究了Cu2O/CuO-四环素复合材料对革兰氏阳性菌金黄色葡萄球菌(S. aureus)、革兰氏阴性菌大肠杆菌(E. coli)和耐药菌沙门氏菌(T-Salmonella)的抑菌性能及抑菌机制。抑菌性能结果表明:抑菌浓度为150 µg/mL的Cu2O/CuO-四环素复合材料在80 min时对E. coli、S. aureus和T-Salmonella的抑菌率均达到99.99%;与单独使用四环素和Cu2O/CuO相比,Cu2O/CuO-四环素复合材料对E. coli的抑菌效率分别提高了2.50和1.38倍,对S. aureus分别提高了1.58和1.18倍及对T-Salmonella分别提高1.26和1.12倍,总而言之,Cu2O/CuO-四环素复合材料对E. coli最敏感。抑菌机制表明,该复合材料能有效破坏细菌细胞壁,使膜通透性发生改变,最终使细菌破裂死亡。Cu2O/CuO-四环素复合材料具有优异的抑菌性能,进一步为公共卫生、生物医用等领域提供了广泛的科学依据。Abstract: The rapid growth of drug-resistant bacteria and the lack of availability of new treatment strategies make it urgent for people to develop new bacteriostatic agents to solve such problems. In this study, copper nitrate trihydrate [Cu(NO3)2·3H2O] was used as a raw material and hydrazine hydrate as a reducing agent to prepare cuprous oxide (Cu2O/CuO), then Cu2O/CuO-tetracycline composites were obtained by combining them with tetracycline. Systematic characterization of inhibitors was conducted using TEM, XRD, XPS, FTIR, and UV-vis. The inhibitory properties and mechanism of Cu2O/CuO-tetracycline on the Gram-positive bacterium Staphylococcus aureus (S. aureus), Gram-negative bacterium Escherichia coli (E. coli), and drug-resistant bacterium Salmonella (T-Salmonella) were studied. The results of antibacterial properties show that the antibacterial rate of Cu2O/CuO-tetracycline composites with antibacterial concentration of 150 µg/mL to E. coli, S. aureus, and T-Salmonella reached 99.99% at 80 min. Compared with tetracycline and Cu2O/CuO alone, the antibacterial efficiency of Cu2O/CuO-tetracycline composite increase by 2.50 and 1.38 times for E. coli, 1.58 and 1.18 times for S. aureus and 1.26 and 1.12 times for T-Salmonella, respectively. In a word, Cu2O/CuO-tetracycline composites are the most sensitive to E. coli. The antibacterial mechanism shows that the composite material can effectively destroy the bacterial cell wall, change the membrane permeability, and finally make the bacteria rupture and die. The Cu2O/CuO-tetracycline composites have excellent antibacterial properties, indicating their wide application prospects in the fields of medical devices and medical materials.
-
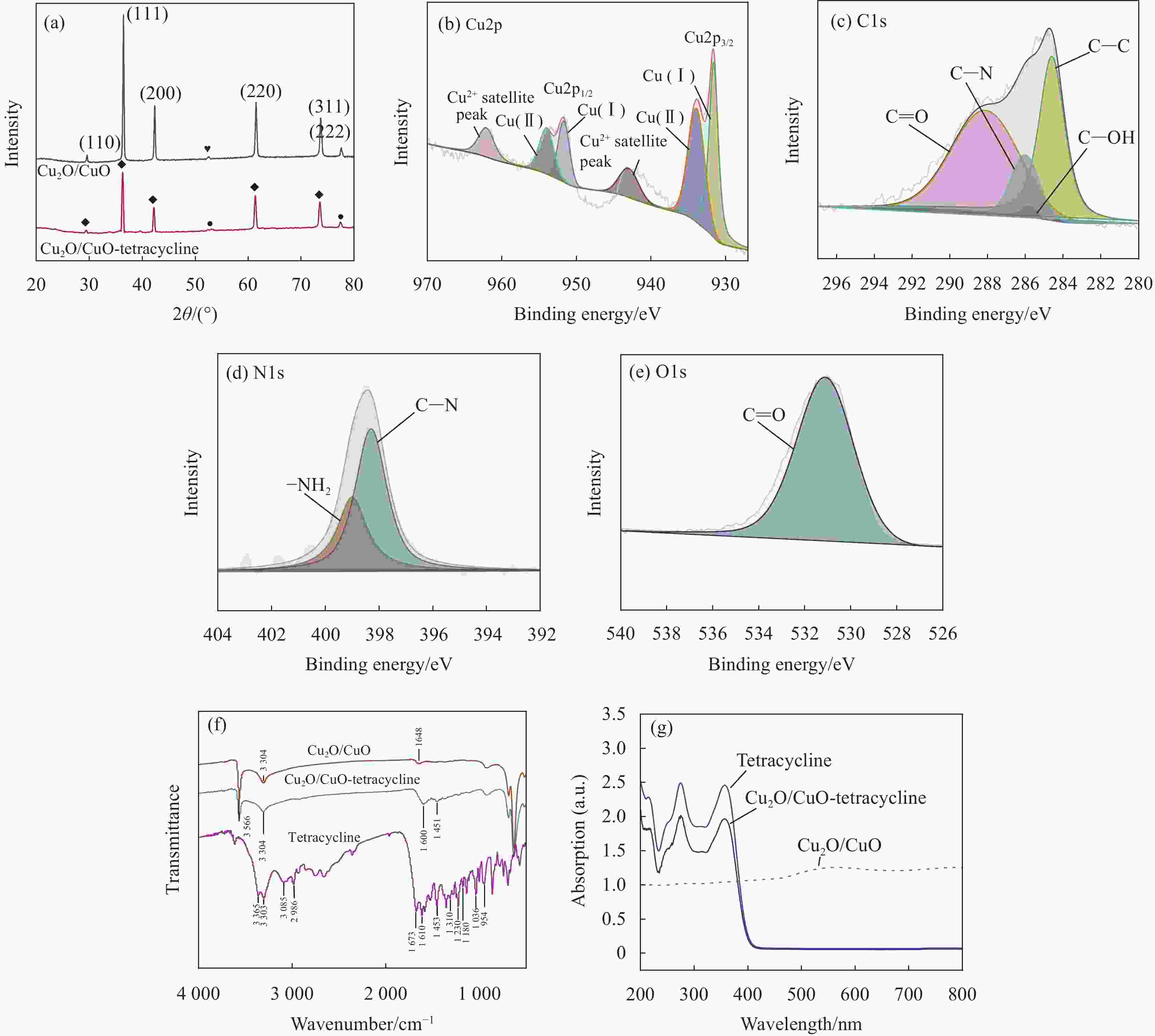
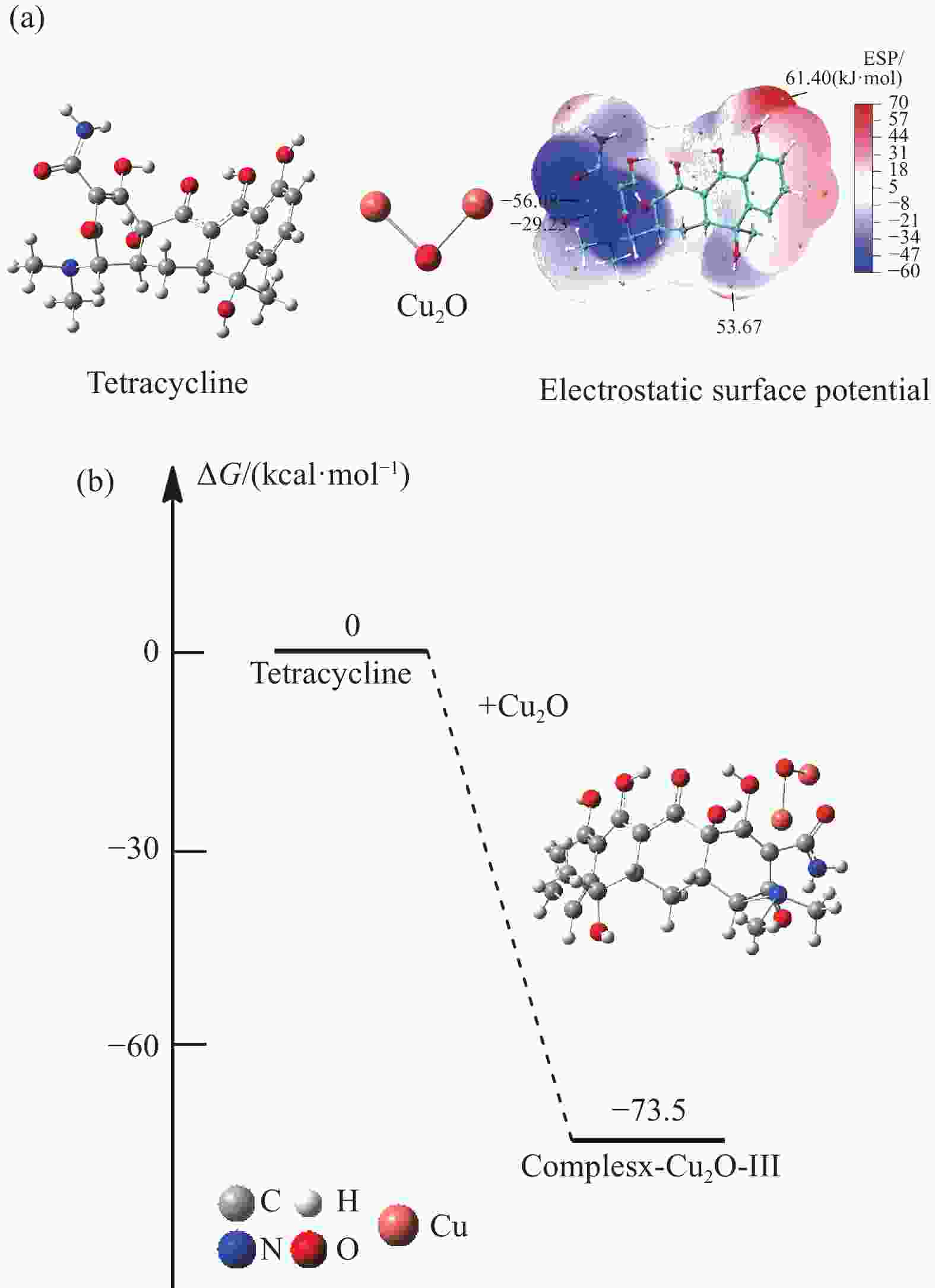
图 3 (a) Cu2O/CuO和Cu2O/CuO-四环素的XRD图谱;Cu2O/CuO-四环素复合材料中Cu2p (b)、C1s (c)、N1s (d) 和O1s (e) 的XPS图谱;(f) 四环素、Cu2O/CuO和Cu2O/CuO-四环素的FTIR图谱;(g) 光发射图谱(虚线)和Cu2O/CuO-四环素和四环素的紫外可见吸收图谱(实线)
Figure 3. (a) XRD spectra of Cu2O/CuO and Cu2O/CuO-tetracycline; XPS spectra of Cu2p (b), C1s (c), N1s (d) and O1s (e) in Cu2O/CuO-tetracycline composites; (f) FTIR spectra of tetracycline, Cu2O/CuO and Cu2O/CuO-tetracycline; (g) Photoemission spectra (dashed lines) and UV-vis absorption spectra (solid lines) of Cu2O/CuO-tetracycline and tetracycline
图 5 不同浓度下四环素、Cu2O/CuO和Cu2O/CuO-四环素复合物对E. coli (a1)、S. aureus (b1) 和T-Salmonella (c1) 的滤纸扩散结果;抑菌圈直径随E. coli (a2)、S. aureus (b2) 和T-Salmonella (c2) 浓度变化的曲线
Figure 5. Filter paper diffusion results of tetracycline, Cu2O/CuO and Cu2O/CuO-tetracycline composites on E. coli (a1), S. aureus (b1) and T-Salmonella (c1) at different concentrations; Curves of the diameter of inhibition zone changing with concentration of E. coli (a2), S. aureus (b2) and T-Salmonella (c2)
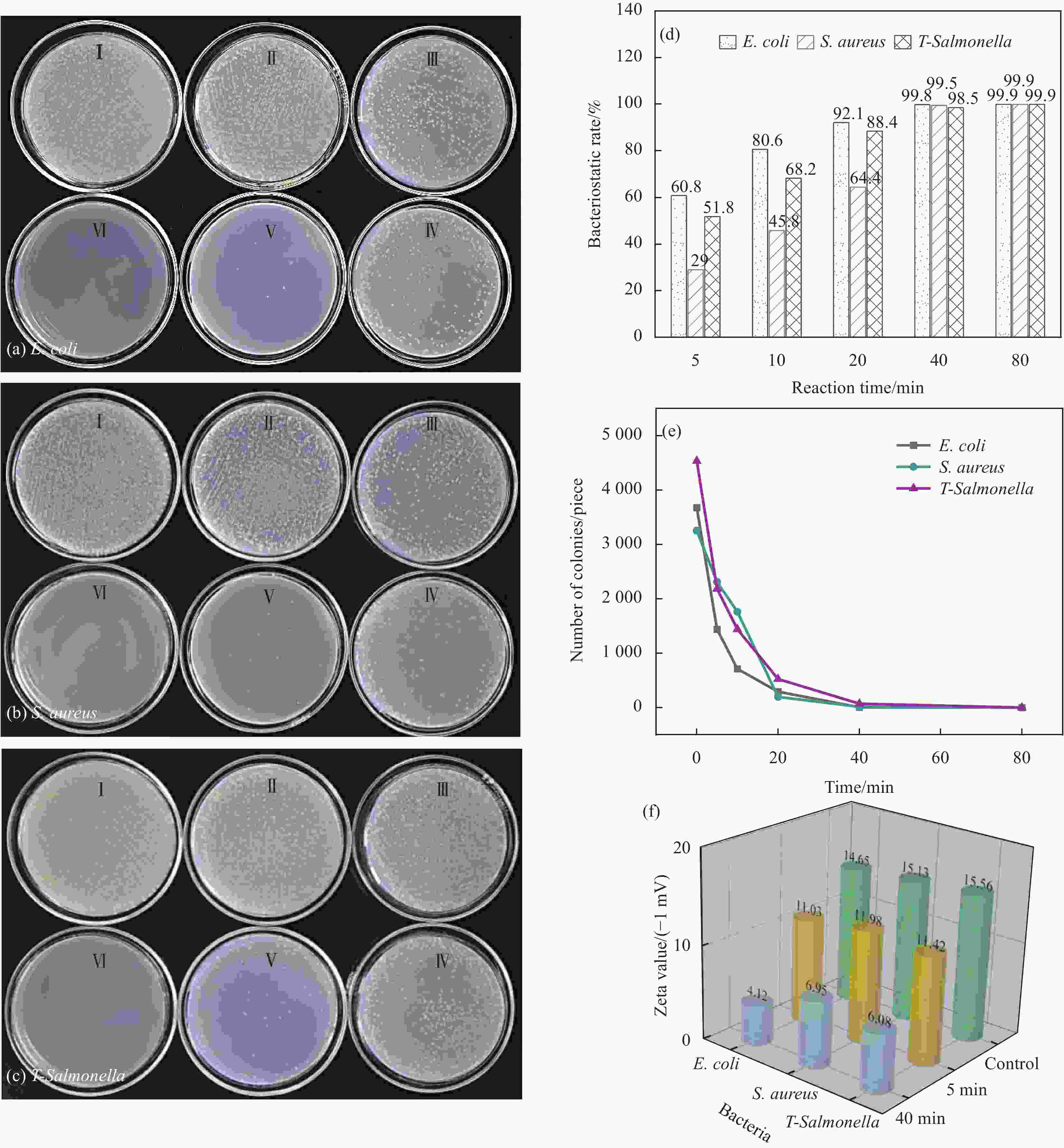
图 6 浓度为150 µg/mL的Cu2O/CuO-四环素复合材料对E. coli (a)、S. aureus (b) 和T-Salmonella (c) 的菌落计数结果;(d) Cu2O/CuO-四环素在不同时间对3种测试菌的抑菌率;(e) Cu2O/CuO-四环素在不同时间对3种测试菌的菌落数;(f) 浓度为150 µg/mL的Cu2O/CuO-四环素复合材料与3种测试菌混合5 min和40 min后的Zeta电位值
Figure 6. Colony counting results of E. coli (a), S. aureus (b) and T-Salmonella (c) by Cu2O/CuO-tetracycline composites with concentration of 150 µg/mL; (d) Antibacterial rate of Cu2O/CuO-tetracycline compound to three experimental bacteria in different time; (e) Colony number of Cu2O/CuO-tetracycline composites to 3 kinds of tested bacteria in different time; (f) Zeta potential value of Cu2O/CuO-tetracycline composites with concentration of 150 µg/mL mixed with 3 kinds of experimental bacteria for 5 min and 40 min
图 7 (a) 电感耦合等离子体发射光谱法(ICP-OES)测得铜阳离子的累积释放;((b1)~(b3)) 空白对照组纯菌E. coli、S. aureus和T-Salmonella的代表性荧光图像;((b4)~(b6)) Cu2O/CuO-四环素复合材料与E. coli、S. aureus和T-Salmonella接触2 h后的代表性荧光图像
Figure 7. (a) Cumulative release of copper cations measured by inductively coupled plasma optical emission spectrometer (ICP-OES); ((b1)-(b3)) Representative fluorescence images of pure bacteria E. coli, S. aureus and T-Salmonella in the blank control group;((b4)-(b6)) Representative fluorescence images of Cu2O/CuO-tetracycline composite after contacting with E. coli, S. aureus and T-Salmonella for 2 h
-
[1] ZHANG H X, MA J, LIU C, et al. Antibacterial activity of guanidinium-based ionic covalent organic framework anchoring Ag nanoparticles[J]. Journal of Hazardous Materials,2022,435:128965. [2] RATTHAPIT W, KANKAVEE S, NOLLAPAN N, et al. Benzoxazine monomers grafted poly(acrylic acid) as novel organic additives with fluorescence and antibacterial properties[J]. Materials Today Communications,2022,31:103500. [3] NGUYEN V N, ZHAO Z, TANG B Z, et al. Organic photosensitizers for antimicrobial phototherapy[J]. Chemical Society Reviews,2022,51(9):3324-3340. [4] VENTOLA C L. The antibiotic resistance crisis: Part 1: Causes and threats[J]. Pharmacy and Therapeutics,2015,40(4):277-283. [5] MAGIORAKOS A P, SRINIVASAN A, CAREY R B, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance[J]. Clinical Microbiology and Infection,2012,18(3):268-281. doi: 10.1111/j.1469-0691.2011.03570.x [6] SINGH R, SMITHA M S, SINGH S P. The role of nanotechnology in combating multi-drug resistant bacteria[J]. Journal of Nanoscience and Nanotechnology,2014,14(7):4745-4756. doi: 10.1166/jnn.2014.9527 [7] ZHAO D, LU Y H, WANG Z, et al. Antifouling properties of micro arc oxidation coatings containing Cu2O/ZnO nanoparticles on Ti6Al4V[J]. International Journal of Refractory Metals and Hard Materials,2016,54:417-421. doi: 10.1016/j.ijrmhm.2015.10.003 [8] RADI A, PRADHAN D, SOHN Y, et al. Nanoscale shape and size control of cubic, cuboctahedral, and octahedral Cu-Cu2O core-shell nanoparticles on Si(100) by one-step, templateless, capping-agent-free electrodeposition[J]. ACS Nano,2010,4(3):1553-1560. doi: 10.1021/nn100023h [9] ZHANG Y H, YUAN Y, CHEN W, et al. Integrated nanotechnology of synergism-sterilization and removing-residues for neomycin through nano-Cu2O[J]. Colloids and Surfaces B: Biointerfaces,2019,183:110371. doi: 10.1016/j.colsurfb.2019.110371 [10] KHURANA C, SHARMA P, PANDEY O P, et al. Synergistic effect of metal nanoparticles on the antimicrobial activi-ties of antibiotics against biorecycling microbes[J]. Jour-nal of Materials Science & Technology,2016,32(6):524-532. [11] FRISCH M J, TRUCKS G W, POPLE J A, et al. Gaussian 09, Version D. 01[CP]. Gaussian Inc: Pittsburgh, PA, 2009. [12] LU T A, CHEN F W, Multiwfn: A multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. [13] GIANNOUSI K, SARAFIDIS G, MOURDIKOUDIS S, et al. Selective synthesis of Cu2O and Cu/Cu2O NPs: Antifungal activity to yeast Saccharomyces cerevisiae and DNA interaction[J]. Inorganic Chemistry,2014,53(18):9657-9666. doi: 10.1021/ic501143z [14] KHAN A, RASHID A, YOUNAS R, et al. A chemical reduction approach to the synthesis of copper nanoparticles[J]. International Nano Letters,2016,6(1):21-26. doi: 10.1007/s40089-015-0163-6 [15] MA J Q, GUO S B, GUO X H, et al. Preparation, characterization and antibacterial activity of core-shell Cu2O@CuO@Ag composites[J]. Surface and Coatings Technology,2015,272:268-272. doi: 10.1016/j.surfcoat.2015.03.056 [16] LI J Z, MA J X, HONG L, et al. Prominent antibacterial effect of sub 5 nm Cu nanoparticles/MoS2 composite under visible light[J]. Nanotechnology,2021,33(7):075706. [17] SELVANATHAN V, AMINUZZAMAN M, TEY L H, et al. Muntingia calabura leaves mediated green synthesis of CuO nanorods: Exploiting phytochemicals for unique morphology[J]. Materials,2021,14(21):6379. doi: 10.3390/ma14216379 [18] VASQUEZ R P. CuO by XPS[J]. Surface Science Spectra,1998,5(4):262-266. doi: 10.1116/1.1247882 [19] WEI Q, WANG Y, QIN H Y, et al. Construction of rGO wrapping octahedral Ag-Cu2O heterostructure for enhanced visible light photocatalytic activity[J]. Applied Catalysis B: Environmental,2018,227:132-144. doi: 10.1016/j.apcatb.2018.01.003 [20] CHEN X N, WANG X H, FANG D. A review on C1s XPS-spectra for some kinds of carbon materials[J]. Fullerenes, Nanotubes and Carbon Nanostructures,2020,28(12):1048-1058. doi: 10.1080/1536383X.2020.1794851 [21] GUO H, CHENG J, MAO Y X, et al. Fabricating different coordination states of cobalt as magnetic acid-base bifunctional catalyst for biodiesel production from microalgal lipid[J]. Fuel,2022,322:124172. doi: 10.1016/j.fuel.2022.124172 [22] DOU H Z, XU M, ZHENG Y, et al. Bioinspired tough solid-state electrolyte for flexible ultralong-life zinc-air battery[J]. Advanced Materials,2022,34(18):2110585. doi: 10.1002/adma.202110585 [23] SIVKOV D V, PETROVA O V, NEKIPELOV S V, et al. Quantitative characterization of oxygen-containing groups on the surface of carbon materials: XPS and NEXAFS study[J]. Applied Sciences,2022,12(15):7744. doi: 10.3390/app12157744 [24] LIU S, ZHAO X R, SUN H Y, et al. The degradation of tetracycline in a photo-electro-Fenton system[J]. Chemical Engineering Journal,2013,231:441-448. doi: 10.1016/j.cej.2013.07.057 [25] TRIVEDI M K, PATIL S, SHETTIGAR H, et al. Spectroscopic characterization of chloramphenicol and tetracycline: An impact of biofield treatment[J]. Pharmaceutica Analytica Acta,2015,6(7):395. [26] JONES K H, SENFT J A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide[J]. Journal of Histochemistry & Cytochemistry,1985,33(1):77-79. [27] ROHDE M. The Gram-positive bacterial cell wall[J]. Microbiology Spectrum,2019,7(3):7.3.10. doi: 10.1128/microbiolspec.GPP3-0044-2018 [28] ROJAS E R, BILLINGS G, ODERMATT P D, et al. The outer membrane is an essential load-bearing element in Gram-negative bacteria[J]. Nature,2018,559(7715):617-621. doi: 10.1038/s41586-018-0344-3 [29] CHOPRA I. Tetracycline analogs whose primary target is not the bacterial ribosome[J]. Antimicrobial Agents and Chemotherapy,1994,38(4):637-640. doi: 10.1128/AAC.38.4.637 [30] GASPARRINI A J, MARKLEY J L, KUMAR H, et al. Tetracycline-inactivating enzymes from environmental, human commensal, and pathogenic bacteria cause broad-spectrum tetracycline resistance[J]. Communications Biology,2020,3(1):1-12. doi: 10.1038/s42003-019-0734-6 [31] LI W R, XIE X B, SHI Q S, et al. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli[J]. Applied Microbiology and Biotechnology,2010,85(4):1115-1122. doi: 10.1007/s00253-009-2159-5 -





 下载:
下载: