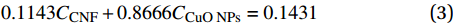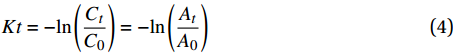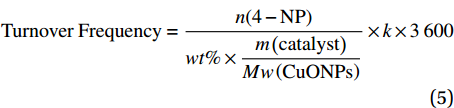Preparation of catalytic composite copper oxide nanoparticles (CuO NPs)@cellulose nanofiber (CNF)-Si-N(OH)2 and its catalytic reduction of 4-nitrophenol
-
摘要: 针对目前降解工业废水中4-硝基苯酚(4-NP)的催化剂效率低,催化活性差等问题,以桉木漂白化学浆为原料,通过超微粒研磨机和高压均质机处理制备得到直径50~100 nm和长度1500~2000 nm的纤维素纳米纤丝(CNF),在其表面原位负载纳米氧化铜颗粒(CuO NPs),并通过3-氯丙基三甲氧基硅烷(CPTES)与二乙醇胺(DEA)进行接枝反应制备得到复合催化材料-CuO NPs@CNF-Si-N(OH)2。探讨了DEA添加量对CuO NPs@CNF–Si–N(OH)2的性能影响,采用Zeta电位、FTIR、XRD、XPS、热重分析和形貌分析等方法对复合材料进行了表征。结果表明,CuO NPs被原位还原并成功负载在CNF表面,其直径约为3.84 nm,负载量为3.83wt%,通过硅烷化改性及接枝胺基可提高CuO NPs在复合材料表面的分散性及稳定性,进而增强了其催化活性。CNF基复合催化材料对4-NP的催化还原结果显示,DEA添加量为20wt%下的CuO NPs@CNF-Si-N(OH)2对4-NP催化还原性能最佳,在180 s内可催化还原98.39%的4-NP,且反应符合伪一级动力学模型,表观速率常数为5.50×10−3 s−1,转化效率为1723.41 h−1,研究结果可为高性能催化复合材料的制备提供新思路和新途径。Abstract: In view of the low efficiency and poor catalytic activity of 4-nitrophenol (4-NP) catalysts for degradation of industrial wastewater, eucalyptus wood bleaching chemical pulp was used as the raw material and treated with ultrafine grinder and high pressure homogenizer to produce cellulose nanofiber (CNF) with the diameter of 50-100 nm and the length of 1500-2000 nm. Then, copper oxide nanoparticles (CuO NPs) were in-situ loaded onto CNF, 3-chloropropyltrimethoxysilane (CPTES) and diethanolamine (DEA) were added for grafting reaction to obtain the catalytic composite of CuO NPs@CNF-Si-N(OH)2. The catalytic composite was characterized with Zeta potential, FTIR, XRD, XPS, thermal gravimetric analysis and morphology analysis. The results show that CuO NPs are in-situ loaded on CNF, and the grafting of amine groups can make the loading of CuO NPs more uniform and stable. In addition, it is also found that CuO NPs@CNF-Si-N(OH)2 show the optimal catalytic performance with 20wt% DEA. Furthermore, 98.39% of 4-NP is catalytically reduced after 180 s, and the reaction fit the pseudo-first order kinetics equation, in which the reacting constant reaches 5.50×10−3 s−1 and the turnover frequency achieves 1723.24 h−1. The composite catalysts of CuO NPs@CNF-Si-N(OH)2 exhibite excellent recycling performance, and 94.42% of 4-NP can be catalytically reduced after a recycling time of 8. The results can provide a new idea and approach for the preparation of high-performance catalytic composite.
-
Keywords:
- cellulose nanofibers /
- 3-chloropropyltriethoxysilane /
- diethanolamine /
- 4-nitrophenol /
- catalytic /
- reduction
-
4-硝基苯酚(4-NP)作为一种常见的酚类有机污染物,具有溶解度高、稳定性强、毒性大等特点,4-NP通常存在于工业废水中,排放后进入环境中并在土壤中聚集,部分4-NP渗透会进入地下水中,污染水资源[1]。4-NP与其他难降解有机物类似,具有较高的毒性以及致畸、致突变性特点,因此,土壤和水体被4-NP污染后会严重威胁人类及其他动植物的正常发育,4-NP已被美国国家环保局(U.S. Environmental Protection Agency,USEPA)列为当前129种需要优先治理的污染物之一,并要求其在水体中浓度必须小于10 ng/L[2]。因此如何高效环保地对4-NP进行处理是目前亟待解决的问题。
近年来,有研究表明在化学法中利用催化剂可高效环保地对4-NP进行催化还原。其中,由于金属纳米颗粒具有较大的比表面积和高催化活性,是目前常用于还原4-NP的催化剂[3,4]。但是由于金属纳米粒子的热力学不稳定性和较高的表面能,使其极易团聚,导致催化活性大幅降低,并且大多数金属纳米粒子催化剂是由贵金属化合物制备而来,价格昂贵。纳米氧化铜颗粒(CuO NPs)作为一种多功能无机材料,具有较好的电、磁、催化特性,因此近年来被广泛用于催化剂、载体和电极活性材料制备等领域。Zhou等[5]通过硼氢化钠还原法将平均尺寸为7 nm的CuO NPs原位负载于纳米纤维素晶(Cellulose nanocrystal,CNC)表面制备得到CNC-CuO NPs,结果发现CNC-CuO NPs对4-NP的催化还原活性显著提高,说明将金属纳米颗粒有效负载于碳材料、有机高分子聚合物和金属氧化物等载体上表面制备纳米复合材料,可有效解决其团聚问题,并提高其催化活性。
纤维素纳米纤丝(Cellulose nanofibers,CNF)作为一种天然的、丰富的生物材料,具有杨氏模量高、比表面积大、聚合度高、稳定性好等优点,其表面有大量的羟基且比表面积大,利于金属纳米粒子的均匀并可通过静电吸附和氢键间结合力与CNF进行有效结合[6],此外,其特殊的结构特点使其利于改性或接枝某些基团,可赋予其特殊的性能。而桉木漂白化学浆由于原料来源广、价格低、生产工艺成熟、白度稳定性高,是制备CNF的一种常见的原料[7]。
本文利用CNF丰富的表面基团、高聚合度及高比表面积等优点,通过在其表面原位负载CuO NPs,并添加3-氯丙基三甲氧基硅烷(CPTES)和二乙醇胺(DEA)接枝胺基,制得可高效催化还原4-硝基苯酚的CuO NPs@CNF-Si-N(OH)2。探究并分析了制备CuO NPs@CNF-Si-N(OH)2过程中DEA添加量对催化还原4-NP的影响,并探讨了不同反应时间下CuO NPs@CNF-Si-N(OH)2对4-NP的催化还原效果。
1. 实验原料及方法
1.1 原材料
巴西产商用桉木漂白浆版、3-氯丙基三甲氧基硅烷(CPTES,分析纯,上海麦克林生化科技有限公司)、二乙醇胺(DEA,分析纯,国药集团化学试剂有限公司)、NaOH(分析纯,国药集团化学试剂有限公司)、无水乙醇(分析纯,国药集团化学试剂有限公司)、硼氢化钠(NaBH4,分析纯,天津市永大化学试剂有限公司)、4-硝基苯酚(4-NP,分析纯,上海麦克林生化科技有限公司)。
1.2 纤维素纳米纤丝的制备
取适量桉木漂白浆版,将其剪成5 cm×5 cm大小,转移至去离子水中浸泡12 h至纤维润胀后进行疏解,控制浆料浓度为10wt%,并转移至超微粒研磨机(MKCA6-5 J, Kawaguchi公司,日本)中以−900 μm磨盘间距研磨5次,研磨完毕后加入去离子水使浓度保持在0.5wt%,转移至高压均质机(M-110 EH-30, Microfluidizer公司,美国)中,通过200 μm筛网高压均质10遍,再通过87 μm筛网均质20遍,即得CNF,将其置于4℃冷藏室中保存待用。
1.3 纳米纤维素的活化
取适量CNF于三颈烧瓶中,加入一定量体积比为1∶1的NH3·H2O(质量分数为37wt%)和H2O2(质量分数为30wt%)混合液,室温搅拌反应1 h。反应结束后加入去离子水终止反应,离心洗涤至上清液为中性为止,取下层沉淀平衡水分,于4℃冷藏室中冷藏备用。
1.4 CuO NPs@CNF复合材料的制备
取一定质量的活化CNF于三颈烧瓶中,向其中加入适量2 mmol/L的CuSO4·5H2O并在室温下搅拌持续5 min,然后将温度提高至75℃,待温度达到平衡时加入20 mmol/L的NaBH4,并持续搅拌30 min,溶液由白色变为浅棕色后加入去离子水终止反应,离心洗涤至上清液为中性为止,取下层沉淀即为CuO NPs@CNF,并置于4℃中冷藏备用。CuO NPs的制备条件如下:取一定质量的去离子水于三颈烧瓶中,向其中加入适量2 mmol/L的CuSO4·5H2O并在室温下持续搅拌5 min,然后将温度提高至75℃,待温度达到平衡时加入20 mmol/L的NaBH4,并持续搅拌30 min,溶液由白色变为浅棕色后加入去离子水终止反应,离心洗涤至上清液为中性为止,取下层沉淀即为CuO NPs,并置于4℃冷藏室中冷藏备用。
1.5 CuO NPs@CNF复合材料的功能化改性
将CuO NPs@CNF与一定量水乙醇(体积比V乙醇∶V水=3∶1)置于三颈烧瓶中,持续搅拌30 min使CuO NPs@CNF分散均匀,再加入10wt%的CPTES(相对于绝干的CuO NPs@CNF),并在70℃油浴条件下反应6 h以进行接枝反应。反应结束后,用水乙醇离心洗涤3次以去除未反应的硅烷偶联剂,得到的下层沉淀为CuO NPs@CNF-Si,并置于4℃冷藏室中冷藏备用。
将CuO NPs@CNF-Si与一定量水乙醇于三颈烧瓶中充分搅拌后,分别加入5wt%、10wt%、15wt%、20wt%的DEA并持续通入氮气,于50℃油浴中反应12 h。反应结束后离心洗涤至上清液无氯离子为止(0.1 moL/L硝酸银检验)。将离心产物置于冷冻干燥机中干燥至恒重,即得CuO NPs@CNF-Si-N(OH)2。
1.6 4-NP溶液的配制
称取69.6 mg对硝基苯酚标准品溶于适量去离子水中,转移至1000 mL容量瓶中定容。反复摇晃容量瓶并于超声波反应器中超声30 min,转移至棕色瓶中待用,其浓度为0.5 mmol/L。
1.7 性能表征
1.7.1 Zeta电位分析
将CNF与CNF基复合催化材料配成浓度为0.3wt%的溶液,充分搅拌后超声10 min,将样品转移至DTS1070比色皿中,用粒径及Zeta电位分析仪(Nano ZS-90,马尔文公司,英国)进行测试,测试条件为:扫描温度为25℃,平衡稳定时间为10 s,单次测量20次,循环3次。
1.7.2 官能团分析
取适量CNF与CNF基复合催化材料与烘干至恒重的溴化钾混合均匀并进行压片处理,用傅里叶变换红外光谱仪(IR Prestige-21型,岛津公司,日本)进行测试,测试条件为:扫描速度32次/s,分辨率4 cm−1,测试波长4000~500 cm−1。
1.7.3 元素及价态分析
为了得到样品表面元素组成及价态分布信息,通过X射线光电子能谱仪(ESCALAB Xi+,赛默飞世尔科技有限公司,美国)对样品进行XPS表征。具体的制样方法为:取少量的样品用双面胶带粘在铝箔纸的哑光面,用压力机压到6 MPa,维持20~30 s,将带有样品的铝箔纸剪下来附在样品台上进行测试。C1s峰校正位置为284.8 eV,校正之后进行高斯分峰拟合,即可得到样品表面元素的相关信息。
1.7.4 晶体结构分析
取适量完全干燥的待测样品于X射线衍射仪(SmartLabSE, Rigaku理学公司,日本)中的射线衍射槽内,采用铜靶X光管,设置扫描速率为10°/min,在5°~80°衍射角范围内扫描。
1.7.5 热稳定性分析
称取5~10 mg待测样品于坩埚中,然后转移至同步热分析仪(耐驰STA449 F4, Jupiter公司,德国)中进行测试,测试条件为:氮气流速为50 mL/min,升温范围为30~800℃,升温速率为10℃/min。
1.7.6 微观形貌分析
将CNF与CNF基复合催化材料配制成一定浓度的悬浮液,并转移至样品瓶中超声振荡进行分散。将悬浮液用50 μL移液枪滴至铜网表面,再用0.01wt%的磷钨酸避光染色10 min,用洁净的滤纸将多余的染色剂吸除,再将铜网转移至40℃烘箱中干燥12 h。然后将干燥后的铜网固定于透射电子显微镜(JEM 2100, JEOL公司,日本)样品台上进行AFM表征,每个样品选取多点进行观察。
1.7.7 亲疏水性分析
将CNF与CNF基复合催化材料的浓度统一调整至0.3wt%,并对其进行超声消泡0.5 h(以防止其中存在气泡),然后以0.18 g/cm2的成膜量在聚四氟乙烯模具中流延成膜,并在40℃条件下干燥备用。将干燥完成后的CNF膜与改性CNF膜裁剪为1 cm×10 cm大小,并置于接触角分析仪(OCA50,奥德利诺公司,德国)样品台上,通过摄像装置观察CNF膜与水滴的接触照片,测量液滴与CNF膜所形成的接触角大小。
1.7.8 催化性能分析
催化性测试反应中,可通过紫外可见光谱(UV-vis,UV2600,岛津公司,日本)观察4-NP的还原过程。4-NP水溶液在UV-vis中λ=317 nm处有最大吸收波长,当加入足量的NaBH4,4-NP的峰值发生偏移,转移至400 nm处。若4-NP被还原,则λ=400 nm处的峰值会发生下降,此时4-NP的还原产物对氨基苯酚(4-AP)产生,相应的,λ=300 nm处会产生4-AP的吸收峰。
(1)不同催化剂种类对4-NP催化还原的影响
将相同浓度(0.3wt%)的CNF与CNF基复合催化材料分别加入至0.5 mmol/L的4-NP溶液中,机械搅拌均匀,然后加入30 mL 20 mmol/L的 NaBH4,并在室温下搅拌反应180 s后结束反应,取3 mL液体过滤并转移至比色皿中,然后通过UV-vis进行分析,筛选出最佳催化剂种类。
(2)不同DEA添加量对4-NP催化还原的影响
将不同DEA添加量(5wt%、10wt%、15wt%、20wt%)的CuO NPs@CNF-N(OH)2分别加入至0.5 mmol/L的4-NP溶液中,机械搅拌均匀,然后加入30 mL 20 mmol/L的 NaBH4,并在室温下搅拌反应180 s后结束反应,取3 mL液体过滤并转移至比色皿中,然后通过UV-vis进行分析,筛选出最佳DEA添加量。
(3)不同反应时间对4-NP催化还原的影响
称取7组一定质量的最佳的催化剂加入至0.5 mmol/L的4-NP溶液中,机械搅拌均匀,然后分别加入30 mL 20 mmol/L的 NaBH4,并在室温下搅拌反应0 s、30 s、60 s、90 s、120 s、150 s、180 s后结束反应,分别取3 mL液体过滤并转移至比色皿中,然后通过UV-vis进行分析,筛选出最佳反应时间。
1.7.9 回用性测试
称取3组滤膜,置于恒温恒湿箱中,测量其水分质量分数为w。此外量取50 mL 15 mmol/L 的4-NP于烧杯中,向其中加入300 mL 0.6 mol/L的NaBH4,可见混合液由浅黄色逐渐加深,此时向其中加入30 g上述最优条件下制得的催化剂,反应180 s后结束反应,将液体通过砂芯漏斗过滤,并将过滤后的液体转移至比色皿中,然后通过UV-vis进行分析。此外将滤膜取出并用0.6 mol/L的NaBH4反复冲洗,洗涤完成后将滤膜上的催化剂取出重复进行上述操作。重复8次后将初始质量为m1滤膜置于60℃烘箱中烘干至恒重并称取其质量,记作m2。催化剂回用率M的计算公式如下:
M=m2−m1×(1−w)30×0.3%×100% (1) 2. 结果与讨论
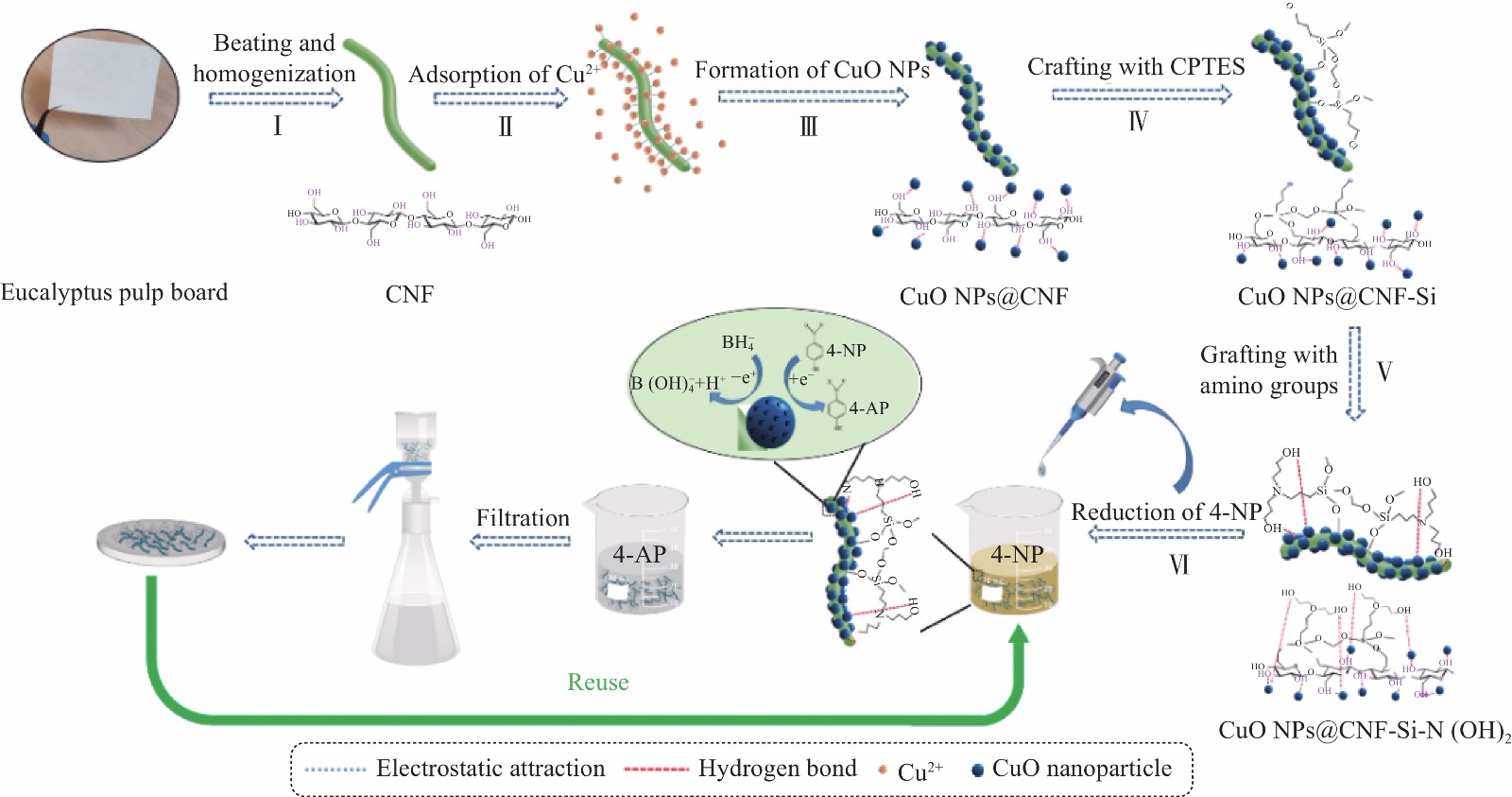
CNF基复合材料的制备及其降解4-NP的过程和回用过程如图1所示。通过超微粒研磨和纳米高压均质法制备得到分散均匀的CNF。然后,将Cu2+通过静电吸引力吸附在CNF表面。其次,通过还原剂NaBH4的还原性将负载在CNF表面的Cu2+原位还原为CuO NPs,制得CuO NPs@CNF。然后,将CuO NPs@CNF与CPTES与DEA进行化学接枝反应,最终制得具有多胺基和多羟基的纳米复合材料CuO NPs@CNF-Si-N(OH)2。最后,此复合材料可与NaBH4将毒性较高的4-NP还原为毒性较低且附加值高的4-AP。
2.1 CNF基复合催化材料的Zeta电位分析
图2为CNF基复合催化材料的Zeta电位图。可知,CNF呈电负性,因此带正电荷的Cu2+可通过静电吸引力吸附于其表面[8],并在还原剂(NaBH4)的作用下形成Cu纳米团簇[9],进而被氧化为CuO纳米团簇,CuO纳米团簇作为成核中心,催化周围其他Cu2+的还原[10],最终形成电负性降低的CuO NPs@CNF,Zeta电位为−22.0 mV。CuO NPs@CNF电负性降低的原因主要是CNF表面带负电荷的羟基与带正电荷的Cu2+结合,导致带负电荷基团减少;此外,经硅烷偶联剂改性得到CuO NPs@CNF-Si的Zeta电位为−20.6 mV,这是由于3-氯丙基三甲氧基硅烷末端羟基与CNF表面羟基脱水缩合,导致复合体表面带负电荷羟基数量减少,从而电负性降低;经DEA改性得到的CuO NPs@CNF-Si-N(OH)2的Zeta电位减小至−22.2 mV,主要是DEA末端有大量羟基的引入,使电负性增强。此外,上述4种材料的Zeta电位均在−20 mV以下,说明其分散性能较好。
2.2 CNF基复合催化材料的表面元素及价态分析
图3(a)为CNF基复合催化材料的XPS图谱。可以看出,CNF中主要是C、O元素,而原位负载CuO NPs的CuO NPs@CNF、CuO NPs@CNF-Si及CuO NPs@CNF-Si-N(OH)2宽谱图中均出现了Cu2p[11],说明其表面含有铜元素;此外,在CuO NPs@CNF表面接枝末端基为—Cl的硅烷偶联剂后形成的CuO NPs@CNF-Si宽谱图中发现了Cl2p,说明硅烷偶联剂已成功接枝;同时在进行二乙醇胺改性的CuO NPs@CNF-Si-N(OH)2XPS图谱中发现N1s,说明其表面成功接枝胺基。对CuO NPs@CNF进行Cu2p高分辨XPS图谱分析(图3(b)),其中,933.5 eV处对应Cu2+—O[12-13],说明CNF存在CuO。
2.3 CNF基复合催化材料的官能团分析
图4为CNF基复合催化材料的FTIR图谱。对CuO NPs@CNF的FTIR图谱分析发现,560 cm−1和860 cm−1处出现了两个新的吸收峰,分别是CuO的单斜相的伸缩振动[14]和Cu—O—Cu晶格振动[15]引起的,并且CuO NPs@CNF在1461 cm−1处出现了新吸收峰,这是CuO NPs表面羟基的弯曲和拉伸振动导致的[16];此外,CuO NPs@CNF-Si和CuO NPs@CNF-Si-N(OH)2均在1200 cm−1处出现了新的峰值,这是由于Si-C-R振动引起的;1674 cm−1处的特征峰是CuO NPs@CNF-Si-N(OH)2的N—H弯曲振动峰。以上结果可说明,CuO NPs已成功地负载到CNF表面,硅烷偶联剂也成功接枝至CuO NPs@CNF表面,并且DEA的N—H键取代了CPTES的中—Cl键。
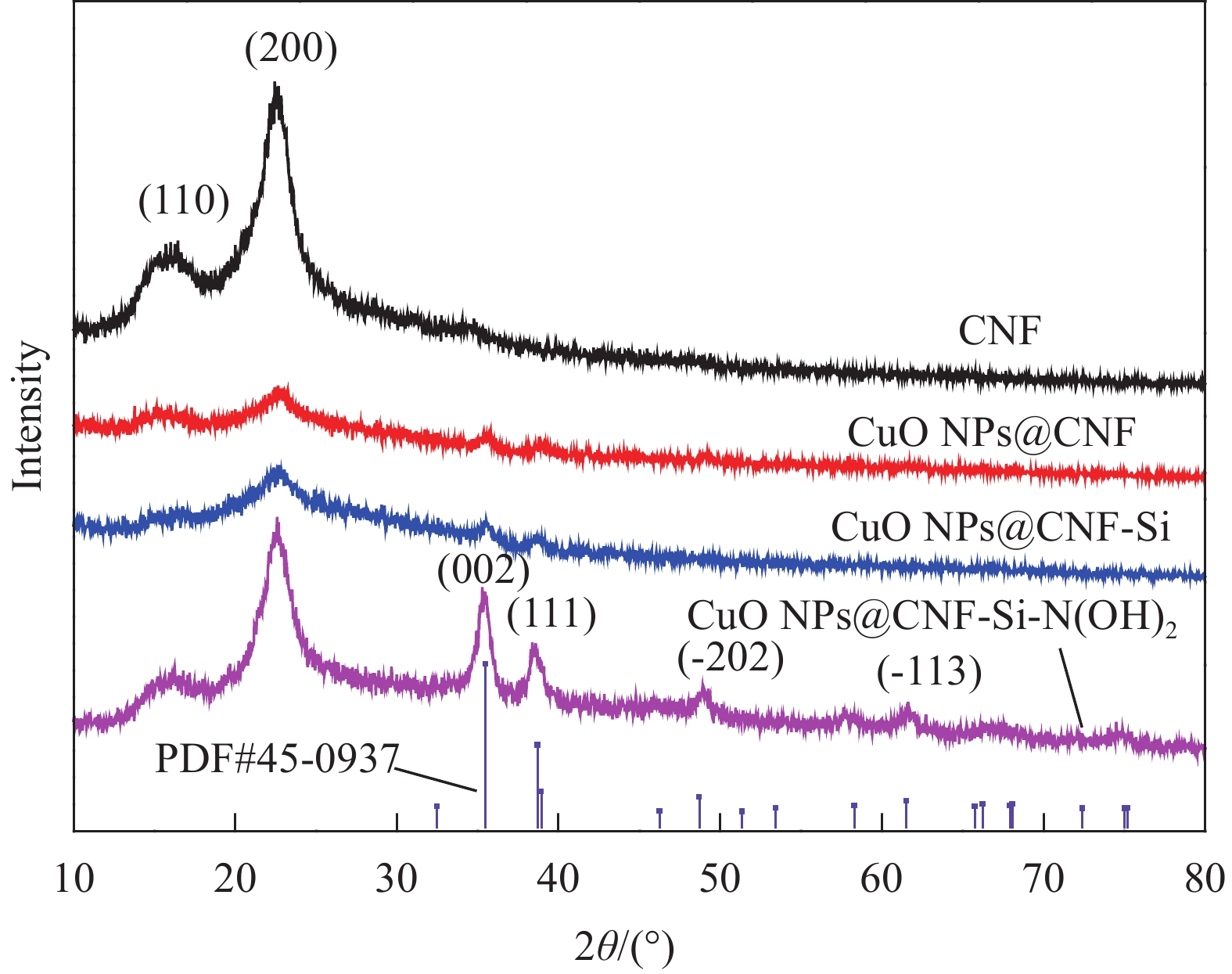
2.4 CNF基复合催化材料的晶体结构分析
图5为CNF基复合催化材料的XRD谱图,分析可知,CNF及CNF基复合催化材料均在衍射角2θ为15.97°、22.58°处有较强的衍射峰,分别对应纤维素结晶区衍射峰(110)面与(200)面[17-18];CuO NPs@CNF和CuO NPs@CNF-Si的XRD谱图均在2θ为35.50°和38.73°出现新的峰值,分别对应CuO的(002)晶面、(111)晶面,对照标准卡片PDF NO.45-0937发现为结晶良好的的纯相CuO,说明CuO已成功生成并负载于CNF表面。此外,CuO NPs@CNF与CuO NPs@CNF-Si,CuO NPs@CNF-Si-N(OH)2在2θ为49.17°、53.51°、57.96°、61.64°、66.45°出现了衍射峰,经过对比发现,这些衍射峰为CuO的衍射峰,但是CuO的衍射峰的强度在CuO NPs@CNF-Si-N(OH)2中明显升高,说明接枝胺基可使CuO NPs@CNF-Si-N(OH)2有更好的结晶倾向[19]。
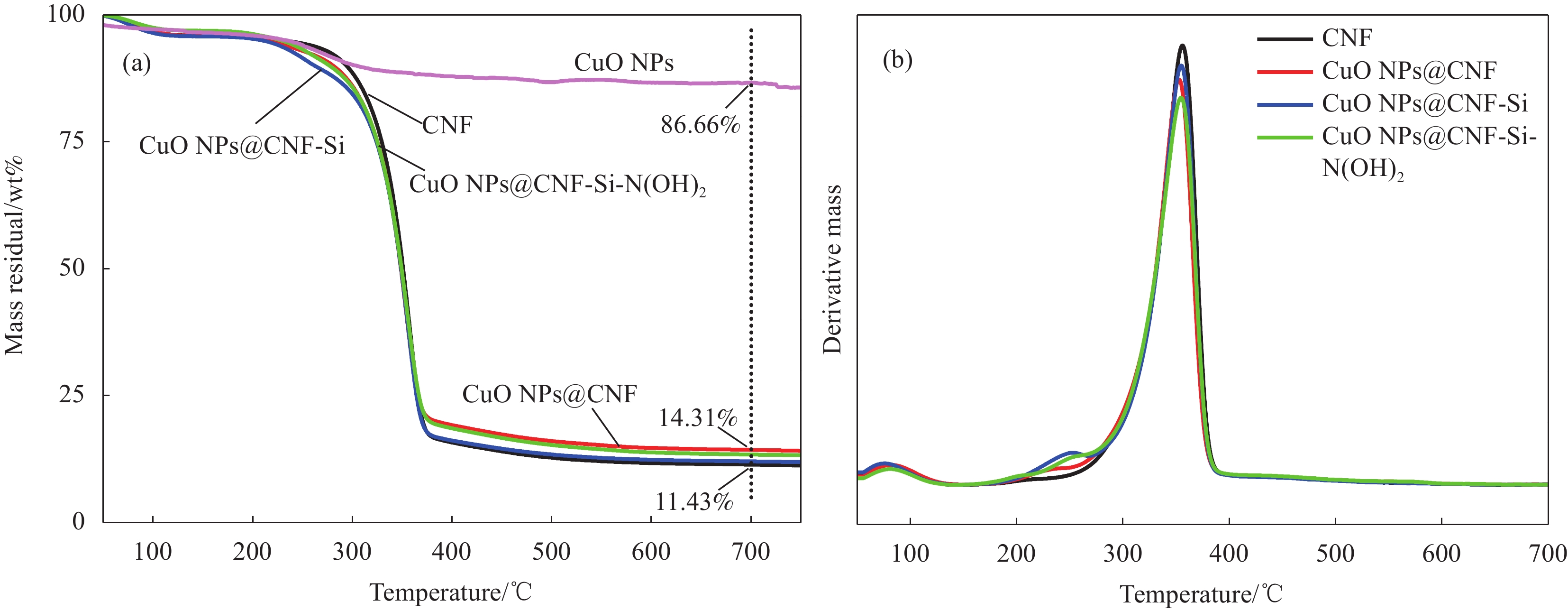
2.5 CNF基复合催化材料的热稳定性分析
图6和表1为CNF基复合催化材料的热稳定性分析。由图6(a)分析发现,CNF及CNF基复合催化材料均在50℃至105℃区间内有轻微失重,主要是由于样品中自由水和乙醇的蒸发。此外,CuO NPs@CNF在130℃至180℃区间有轻微失重,可能是由于脱羟基作用所致。由图6(b)与表1结合分析可知,CuO NPs@CNF、CuO NPs@CNF-Si与CuO NPs@CNF-Si-N(OH)2在240℃至360℃范围内失重更多,且其失重起始温度均低于CNF的失重起始温度;此外,其达到最大降解速率时的温度也低于CNF。综上,说明CNF基复合催化材料在此温度范围内的热稳定性略低于CNF,主要是CNF基复合催化材料中部分有机成分如硅烷化后形成的丙基解聚的解聚、氧化所致[20]。此外,CuO NPs@CNF-Si-N(OH)2的热稳定性最低,主要是由于其表面的CuO NPs更加稳定,且CuO NPs为具有催化活性的金属纳米颗粒,会与复合催化材料表面的胺基发生螯合作用,使反应活化能降低,解聚反应速率增快[21]。当降解温度高于370℃时,CNF逐渐被碳化,残余质量百分数仅为11.43%,低于CNF基复合催化材料。此外,可通过热稳定性测试计算CuO NPs的负载率,计算公式如下[22]:
表 1 CNF及CNF基复合催化材料的热重特征温度表Table 1. The characteristic temperature of TG thermograms of CNF and CNF based catalytic compositeSample T1/°C T2/°C Residual mass/% Mass loss/% CNF 324.76 356.00 11.43 84.56 CuO NP@CNF 321.67 352.67 14.31 81.59 CuO NP@CNF-Si 322.19 354.84 12.06 83.74 CuO NP@CNF-Si-N(OH)2 318.68 354.59 13.41 83.54 Notes: T1—Initial temperature of extrapolation; T2—Temperature at maximum rate of mass change. CCNF+CCuONPs=1 (2) 0.1143CCNF+0.8666CCuONPs=0.1431 (3) 其中,CCNF与CCuO NPs分别为CuO NPs@CNF中CNF与CuO NPs的质量分数。0.1143、0.8666与0.1431分别为700℃时热稳定测试中CNF、CuO NPs与CuO NPs@CNF的质量分数。综上,计算可知,CuO NPs在CuO NPs@CNF中的负载率为3.83wt%。
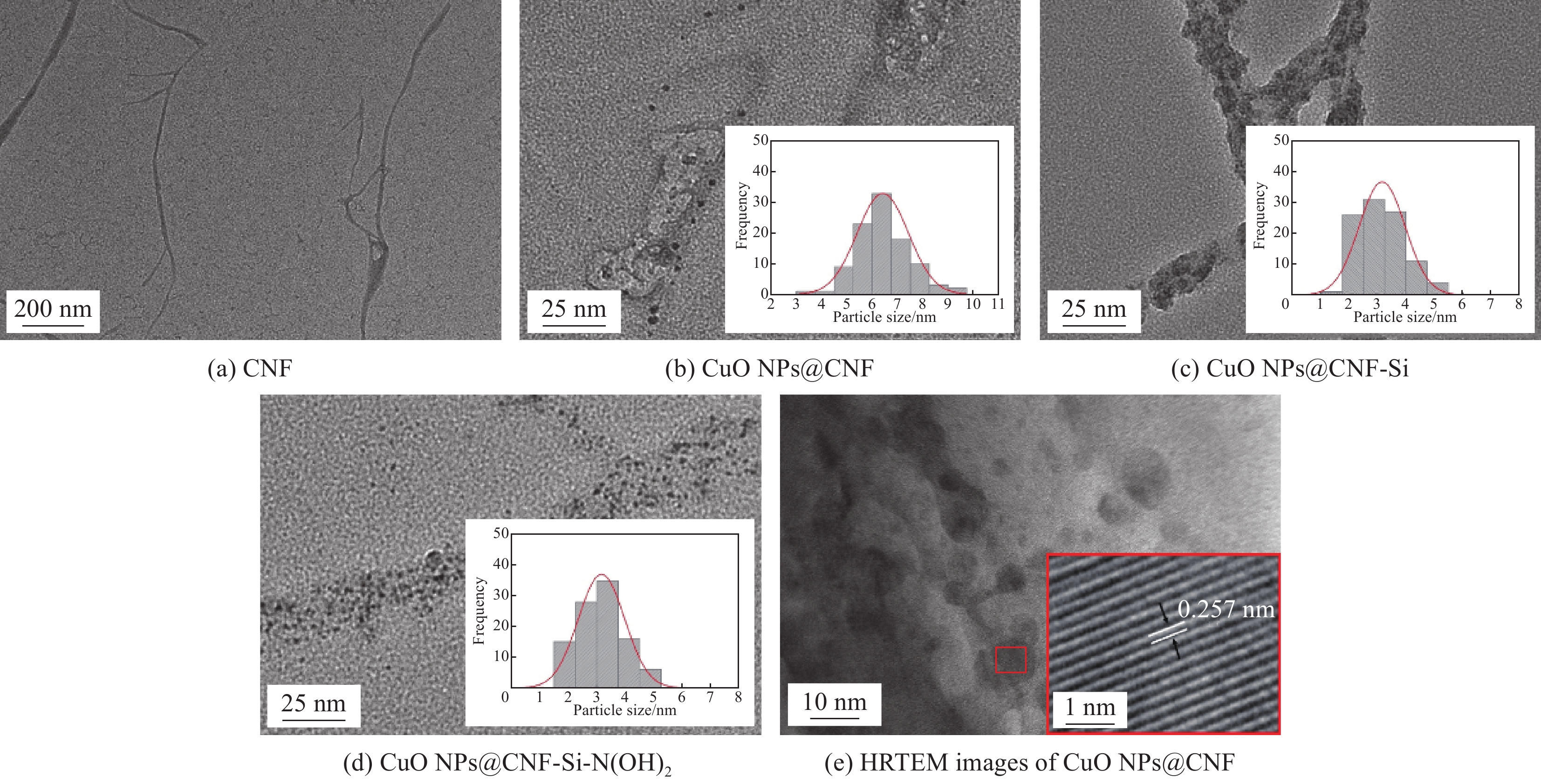
2.6 CNF基复合催化材料的微观形貌分析
图7为CNF基复合催化材料的表面形貌图。由图7(a)可看出CNF表面光滑,宽度约50~100 nm,长度约为1500~2000 nm。而由图7(b)可看出,有较多小颗粒分布在CNF表面,且呈规则圆球状,进行放大后发现其具有晶格条纹(图7(e)),且原子晶格条纹间距为0.257 nm,为CuO(002)面的特征间距,且颗粒大小均在100 nm以下,可证明其为CuO NPs。此外,在图7(b)中可观察到有部分直径超过20 nm的颗粒,主要是由体积较小的CuO NPs团聚而成。由图7(c)中可发现CNF表面较粗糙,这是由于硅烷偶联剂化学接枝造成的。图7(d)中可发现CuO NPs颗粒尺寸明显变小,平均粒径大小为3.84 nm左右,且从直方图图中可发现其粒径分布较均匀,这是由于经过硅烷化改性后,CuO NPs团聚作用减弱,分散性增强导致的[23-24],接枝DEA后,胺基会与复合材料表面的CuO NPs产生螯合作用,使其稳定性更强[25-26]。
2.7 CNF基复合催化材料对4-NP的催化还原性能分析
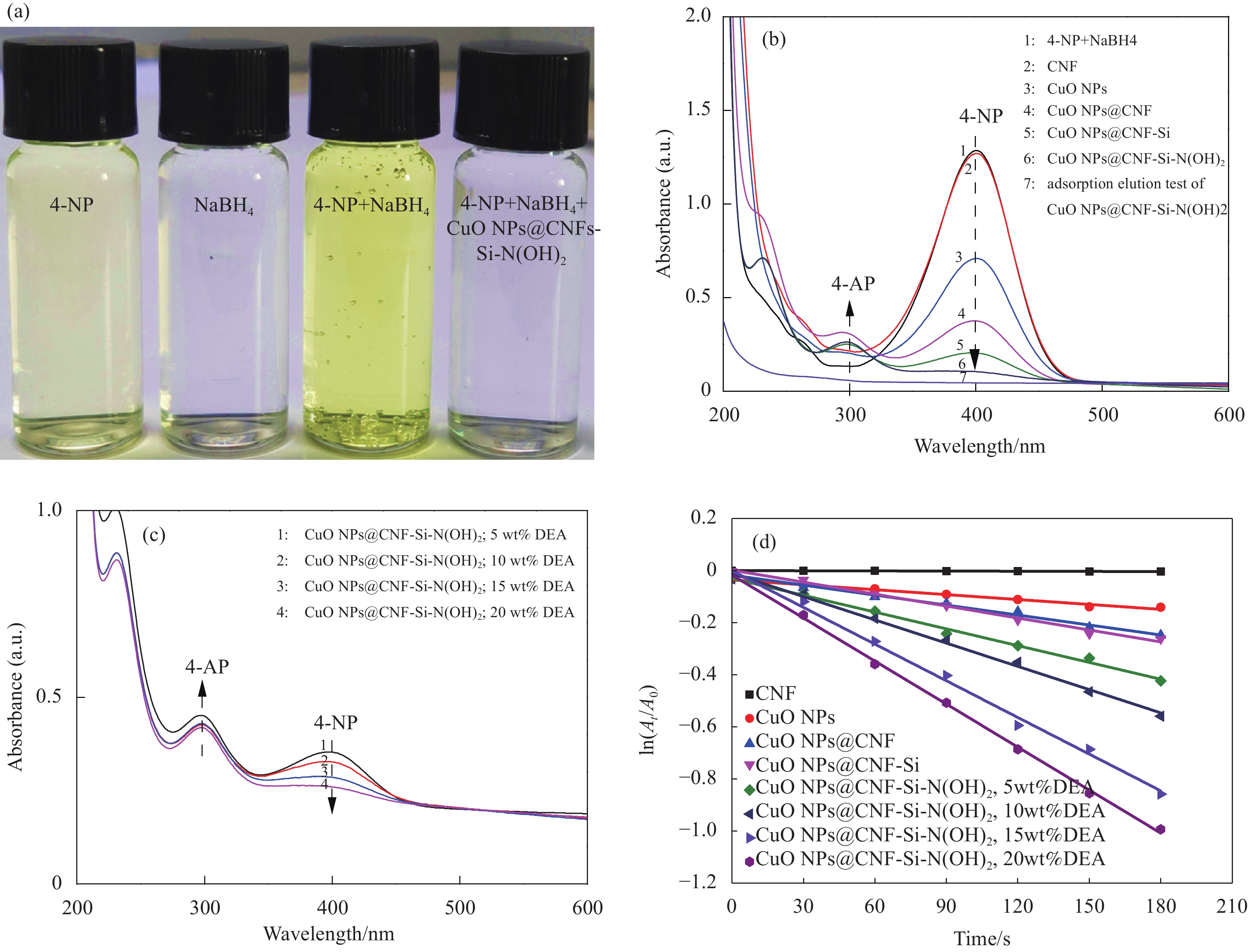
以4-NP为模型化合物对CNF基复合催化材料的催化性能进行研究,在4-NP的还原过程中,NaBH4作为还原剂,CNF基复合催化材料作为催化剂进行催化反应。其中,NaBH4会与水反应生成大量可作为电子供体的和具有还原性的H+[27]。在此还原反应中,加入过量的NaBH4使其在催化反应作为一个确定因素,以排除其在不同催化反应中产生不同的影响。如图8(a)所示,4-NP常温下为黄色溶液,且紫外特征吸收峰在317 nm处,而将无色NaBH4加入至4-NP中,混合液颜色加深,其紫外特征吸收峰立即转移至400 nm处,主要是由于在碱性条件下生成大量的对硝基苯酚离子所导致[28]。将CNF基复合催化材料加入至4-NP和NaBH4混合液中后,混合液的颜色由黄色逐渐变为无色,且通过紫外-可见分光光谱图中可发现400 nm处的吸收峰逐渐降低,在300 nm处(4-氨基苯酚,4-AP)出现了新的吸收峰,说明了4-NP逐渐被还原,并且会产生4-AP。
![]() 图 8 (a) 4-NP还原过程中各阶段的光学图片;(b) CNF及CNF基复合催化材料在180 s内还原4-NP的紫外-可见分光光谱图;(c) 不同DEA含量(5wt%~20wt%)的CuO NPs@CNF-Si-N(OH)2 180 s内还原4-NP的紫外-可见分光光谱图;(d) CNF、CuO NPs及CNF基复合催化材料的动力学曲线Figure 8. (a) Color changes of 4-NP solution after adding CuO NPs@CNF-Si-N(OH)2; (b) UV-vis absorption spectra after adding CNF and CNF based catalytic composite for 180 s; (c) UV-vis absorption spectra after adding CuO NPs@CNF-Si-N(OH)2 grafted with different DEA contents (5wt%-20wt%) for 180 s; (d) Kinetic curves of CNF, CuO NPs and CNF based catalytic compositeA0−Absorbance of the mixture at the initial moment of reaction; At−Absorbance at 400 nm (4-NP) after reaction time t
图 8 (a) 4-NP还原过程中各阶段的光学图片;(b) CNF及CNF基复合催化材料在180 s内还原4-NP的紫外-可见分光光谱图;(c) 不同DEA含量(5wt%~20wt%)的CuO NPs@CNF-Si-N(OH)2 180 s内还原4-NP的紫外-可见分光光谱图;(d) CNF、CuO NPs及CNF基复合催化材料的动力学曲线Figure 8. (a) Color changes of 4-NP solution after adding CuO NPs@CNF-Si-N(OH)2; (b) UV-vis absorption spectra after adding CNF and CNF based catalytic composite for 180 s; (c) UV-vis absorption spectra after adding CuO NPs@CNF-Si-N(OH)2 grafted with different DEA contents (5wt%-20wt%) for 180 s; (d) Kinetic curves of CNF, CuO NPs and CNF based catalytic compositeA0−Absorbance of the mixture at the initial moment of reaction; At−Absorbance at 400 nm (4-NP) after reaction time t图8(b)为CNF、CuO NPs及CNF基复合催化材料在180 s内还原4-NP的紫外-可见分光光谱图。可知,NaBH4与4-NP的混合液在400 nm处有很强的吸收峰,加入CNF后紫外特征吸收峰峰值未见明显变化,说明CNF对4-NP的还原并没有催化作用;而当加入CuO NPs@CNF后,其紫外特征吸收峰峰值出现明显下降,同时4-AP的紫外特征吸收峰峰值上升,说明CuO NPs@CNF对4-NP的还原有一定催化作用;在此过程中,NaBH4遇水会释放大量电子,而CuO NPs@CNF表面的CuO NPs可加速电子向4-硝基苯酚离子移动,加速4-NP的还原[29]。此外,CuO NPs@CNF-Si对4-NP的催化还原作用比CuO NPs@CNF稍强,主要是由于进行硅烷化改性后,复合催化材料表面的CuO NPs分散性增强导致的。CuO NPs@CNF-Si-N(OH)2对4-NP催化还原作用最佳,分析原因是由于复合催化材料进行DEA接枝后,CuO NPs在其表面的稳定性得到明显提高,暴露了更多的活性位点,加速反应进行[30]。此外,为了考察DEA在CuO NPs@CNF-Si-N(OH)2催化性能中的影响,制备了不同DEA添加量(5wt%、10wt%、15wt%、20wt%)的CuO NPs@CNF-Si-N(OH)2。由图8(c)分析可知,随着CuO NPs@CNF-Si-N(OH)2中DEA含量的增加,4-NP的紫外特征吸收峰峰值逐渐降低,主要是由于接枝DEA后可提高CuO NPs的稳定性,使其在反应中不易脱落,进一步验证了接枝DEA可提高复合催化材料对4-NP的催化效率。
此外,为了更加直观地表征材料的催化活性,可通过伪一级动力学模型对实验数据进行拟合,其模型式如下[31]:
Kt=−ln(CtC0)=−ln(AtA0) (4) 式中:K为表观数率常数(s−1);t为催化反应时间(s);Ct为当反应时间为t时4-NP的浓度(moL/L);C0为初始反应时4-NP的浓度(moL/L);At为t时刻时4-NP在400 nm处的紫外吸收峰值;A0为初始时刻时4-NP在400 nm处的紫外吸收峰值。
图8(d)为4-NP降解过程中的动力学曲线。结果发现实验数据能够很好地符合伪一级动力学模型,表2为其具体数据。对图8(d)与表2分析可知,在相同反应条件下,CuO NPs的表观数率常数较低,仅有0.60×10−3 s−1,且4-NP的催化还原率也仅有46.14%,这可能是由于CuO NPs存在团聚现象,导致其在4-NP还原反应中的催化活性并不高;而CuO NPs@CNF的表观数率常数则达到1.30×10−3 s−1,4-NP的催化还原率也提升至71.82%;随着硅烷化改性和接枝胺基后,其表观速率常数和对4-NP的催化活性得到进一步提升,DEA添加量为5wt%的CuO NPs@CNF-Si-N(OH)2的表观速率常数达到1.30×10−3 s−1,可催化86.71%的4-NP还原。而随着DEA添加量的增大,其催化活性逐渐增强,当DEA添加量为20wt%时,其催化活性最高,加速反应体系内电子移动速度,表观速率常数高达5.50×10−3 s−1。
表 2 不同催化剂对4-NP的催化还原性能分析Table 2. Catalytic performance of the CuO NPs and CNF based catalytic compositeSample Temperature/K Molar ratio of
4-NP/catalystTime Apparent rate
constant K/s−1Conversion
yield/%CuO NPs 298 87∶1 180 s 0.60×10−3 46.14 CuO NPs@CNF 298 87∶1 180 s 1.30×10−3 71.82 CuO NPs@CNF-Si 298 87∶1 180 s 1.50×10−3 84.46 CuO NPs@CNF-Si-N(OH)2 (5 wt% DEA) 298 87∶1 180 s 2.20×10−3 86.71 CuO NPs@CNF-Si-N(OH)2 (10 wt% DEA) 298 87∶1 180 s 3.00×10−3 87.92 CuO NPs@CNF-Si-N(OH)2 (15 wt% DEA) 298 87∶1 180 s 4.70×10−3 90.90 CuO NPs@CNF-Si-N(OH)2 (20 wt% DEA) 298 87∶1 180 s 5.50×10−3 98.39 综上所述,DEA添加量为20wt%的CuO NPs@CNF-Si-N(OH)2对4-NP具有良好的催化还原性能,主要原因如下:(1) CuO NPs作为优异的催化剂,因其表面能较大而易发生团聚,而CNF作为良好的载体,可较好地负载CuO NPs,减少其聚集[5]。(2) 对CuO NPs@CNF进行硅烷化改性,有利于提高CuO NPs的分散性,使其分布更均匀。(3) DEA接枝后可提高CuO NPs在CNF表面的稳定性,并可加速体系内电子转移速度[25]。
此外,通过TOF(Turnover frequency,转换效率,每摩尔催化剂每小时转换4-NP的摩尔数)可进一步研究CNF基纳米复合催化材料的催化活性,其计算公式如下[31]:
TurnoverFrequency=n(4−NP)wt%×m(catalyst)Mw(CuONPs)×k×3600 (5) 式中:n为4-NP的摩尔数(moL);wt%为CNF基复合催化材料中CuO NPs的含量;Mw为CuO NPs的分子量(80 g/moL);k为CNF基复合催化材料催化还原4-NP时的表观速率常数(s−1)。
表3为CNF基复合催化材料与其他纳米纤维素基复合催化材料在特定条件下对4-NP的转换效率。分析发现,CuO NPs@CNF的转换效率为407.31 h−1,而DEA添加量为20wt%的CuO NPs@CNF-Si-N(OH)2对4-NP的转换效率则高达1723.41 h−1,说明改性后的复合催化材料的催化性能得到明显提高,且其转换效率比其他已报道的负载CuO NPs的复合催化材料和纳米纤维素负载贵金属的复合催化材料的催化效率高。
表 3 CNF基复合催化材料与其他纳米纤维素基复合催化材料的转化效率分析Table 3. Comparison of catalytic performance of the CNF based catalytic composite with other reported metal nanoparticles based catalystsSample Temp/K Molar ratio of4-NP/ catalyst TOF/h−1 Reference CuO NPs@CNF 298 87∶1 407.31 This work CuO NPs@CNF-Si-N(OH)2 (20 wt% DEA) 298 87∶1 1723.24 This work CuO NPs@GO 298 150∶1 816.2 [5] Au NPs@CNF 298 150∶1 563 [32] Au NPs@PDDA/NCC 298 37∶1 212 [7] Au NPs@CNCs 298 30∶1 109 [33] Ag NPs@CNCs/CTAB 298 97.2∶1 1077.3 [34] Notes: TOF—Turnover Frequency; CCF—Carboxymethylated cellulose fibers; GO—Graphene oxide; PDDA—Poly(diallyldimethylammonium chloride); NCC—Nanocellulose crystal; CNC—Cellulose nanocrystal; CTAB—Hexadecyl-trimethylammonium bromide. 2.8 CuO NPs@CNF-Si-N(OH)2的回用性分析
图9为CuO NPs@CNF-Si-N(OH)2在不同回用次数下催化4-NP的还原转化率。分析可知,当重复使用8次后,CuO NPs@CNF-Si-N(OH)2的回用率为96.61%(由公式1计算可得),对4-NP的转化率为94.42%,略低于首次使用时的转化率(98.39%),但与其他纤维素基材料负载贵金属复合催化材料相比(表4),DEA添加量为20wt%的CuO NPs@CNF-Si-N(OH)2在重复使用后对4-NP仍能保持较高的催化活性,说明此复合催化材料在重复使用后稳定性仍能保持在一个较高的水平。
3. 结 论
(1) 利用机械法制备的桉木浆CNF,通过在其表面原位负载CuO NPs制得了CuO NPs@CNF,然后将其进行硅烷偶联剂和二乙醇胺化学改性,成功制备出不同DEA添加量下的复合催化材料CuO NPs@CNF-Si-N(OH)2,并用制备的复合催化材料对4-NP的进行催化还原。
(2) CNF的电负性有利于吸附大量Cu2+,从而原位负载上CuO NPs,CuO NPs负载量为3.83wt%,且CuO NPs@CNF具有较好的分散性与稳定性。
(3) CuO NPs@CNF通过硅烷偶联剂和二乙醇胺改性成功,表面接枝上了胺基,制得了CuO NPs@CNF-Si-N(OH)2,其表面的CuO NPs分布更均匀、稳定,提高了复合催化材料的催化效率。
(4) 20wt%DEA添加量下的CuO NPs@CNF-Si-N(OH)2对4-NP的催化还原效果最佳,在180 s内可催化还原98.39%的4-NP,且反应符合伪一级动力学模型,表观速率常数为5.50×10−3 s−1,转换效率高达1723.41 h−1。重复使用8次后其对4-NP的催化还原率仍为94.42%。研究结果可为工业废水的处理、纳米纤维素基催化复合材料的制备和应用提供一定的理论依据和技术支持。
表 4 CuO NPs@CNF-Si-N(OH)2与其他纤维素基复合催化材料的回用效果分析Table 4. Comparison of the conversion of the CNF based nanohybrids with other reported cellulose based compositeSample Catalyst Carrier Repeated times Conversion yield Reference CuO NPs@CNF-Si-N(OH)2 (20 wt% DEA) CuO NPs Cellulose nanofibers 8 94.42% This work Ag NPs@ MOF‐199 s/CCFs Ag NPs Cellulose fibers 5 91.0% [35] Ag NPs@CFP Ag NPs Cellulose filter paper 4 90.0% [36] Ag NPs@CMFs Ag NPs Cellulose microfibers 6 87.0% [37] Fe3O4/Ag@NFC Ag NPs Nanofibrillated cellulose 7 81.8% [38] Notes: CFP—cellulose filter paper; CMF—cellulose microfiber; NFC—Nanofibrillated cellulose. -
图 8 (a) 4-NP还原过程中各阶段的光学图片;(b) CNF及CNF基复合催化材料在180 s内还原4-NP的紫外-可见分光光谱图;(c) 不同DEA含量(5wt%~20wt%)的CuO NPs@CNF-Si-N(OH)2 180 s内还原4-NP的紫外-可见分光光谱图;(d) CNF、CuO NPs及CNF基复合催化材料的动力学曲线
Figure 8. (a) Color changes of 4-NP solution after adding CuO NPs@CNF-Si-N(OH)2; (b) UV-vis absorption spectra after adding CNF and CNF based catalytic composite for 180 s; (c) UV-vis absorption spectra after adding CuO NPs@CNF-Si-N(OH)2 grafted with different DEA contents (5wt%-20wt%) for 180 s; (d) Kinetic curves of CNF, CuO NPs and CNF based catalytic composite
A0−Absorbance of the mixture at the initial moment of reaction; At−Absorbance at 400 nm (4-NP) after reaction time t
表 1 CNF及CNF基复合催化材料的热重特征温度表
Table 1 The characteristic temperature of TG thermograms of CNF and CNF based catalytic composite
Sample T1/°C T2/°C Residual mass/% Mass loss/% CNF 324.76 356.00 11.43 84.56 CuO NP@CNF 321.67 352.67 14.31 81.59 CuO NP@CNF-Si 322.19 354.84 12.06 83.74 CuO NP@CNF-Si-N(OH)2 318.68 354.59 13.41 83.54 Notes: T1—Initial temperature of extrapolation; T2—Temperature at maximum rate of mass change. 表 2 不同催化剂对4-NP的催化还原性能分析
Table 2 Catalytic performance of the CuO NPs and CNF based catalytic composite
Sample Temperature/K Molar ratio of
4-NP/catalystTime Apparent rate
constant K/s−1Conversion
yield/%CuO NPs 298 87∶1 180 s 0.60×10−3 46.14 CuO NPs@CNF 298 87∶1 180 s 1.30×10−3 71.82 CuO NPs@CNF-Si 298 87∶1 180 s 1.50×10−3 84.46 CuO NPs@CNF-Si-N(OH)2 (5 wt% DEA) 298 87∶1 180 s 2.20×10−3 86.71 CuO NPs@CNF-Si-N(OH)2 (10 wt% DEA) 298 87∶1 180 s 3.00×10−3 87.92 CuO NPs@CNF-Si-N(OH)2 (15 wt% DEA) 298 87∶1 180 s 4.70×10−3 90.90 CuO NPs@CNF-Si-N(OH)2 (20 wt% DEA) 298 87∶1 180 s 5.50×10−3 98.39 表 3 CNF基复合催化材料与其他纳米纤维素基复合催化材料的转化效率分析
Table 3 Comparison of catalytic performance of the CNF based catalytic composite with other reported metal nanoparticles based catalysts
Sample Temp/K Molar ratio of4-NP/ catalyst TOF/h−1 Reference CuO NPs@CNF 298 87∶1 407.31 This work CuO NPs@CNF-Si-N(OH)2 (20 wt% DEA) 298 87∶1 1723.24 This work CuO NPs@GO 298 150∶1 816.2 [5] Au NPs@CNF 298 150∶1 563 [32] Au NPs@PDDA/NCC 298 37∶1 212 [7] Au NPs@CNCs 298 30∶1 109 [33] Ag NPs@CNCs/CTAB 298 97.2∶1 1077.3 [34] Notes: TOF—Turnover Frequency; CCF—Carboxymethylated cellulose fibers; GO—Graphene oxide; PDDA—Poly(diallyldimethylammonium chloride); NCC—Nanocellulose crystal; CNC—Cellulose nanocrystal; CTAB—Hexadecyl-trimethylammonium bromide. 表 4 CuO NPs@CNF-Si-N(OH)2与其他纤维素基复合催化材料的回用效果分析
Table 4 Comparison of the conversion of the CNF based nanohybrids with other reported cellulose based composite
Sample Catalyst Carrier Repeated times Conversion yield Reference CuO NPs@CNF-Si-N(OH)2 (20 wt% DEA) CuO NPs Cellulose nanofibers 8 94.42% This work Ag NPs@ MOF‐199 s/CCFs Ag NPs Cellulose fibers 5 91.0% [35] Ag NPs@CFP Ag NPs Cellulose filter paper 4 90.0% [36] Ag NPs@CMFs Ag NPs Cellulose microfibers 6 87.0% [37] Fe3O4/Ag@NFC Ag NPs Nanofibrillated cellulose 7 81.8% [38] Notes: CFP—cellulose filter paper; CMF—cellulose microfiber; NFC—Nanofibrillated cellulose. -
[1] 杨晓闪. SiO2@C负载Ni、Cu催化剂的制备及性能研究[D]. 郑州: 郑州大学, 2019. YANG Xiaoshan. Preparation and properties of SiO2@C supported Ni and Cu catalysts[D]. Zhengzhou: Zhengzhou University, 2019(in Chinese).
[2] WAN N, GU J D, YAN Y. Degradation of p-nitrophenol by chromobacter xylosoxidans Ns isolated from wetland sediment[J]. International Biodeterioration & Biodegradation,2007(59):90-96.
[3] 梁艳莉, 马剑琪, 郭少波. CoFe2O4@PDA@Pt核壳型磁性复合材料的制备及催化性能[J]. 复合材料学报, 2021, 38(5):1551-1557. LIANG Yanli, MA Jianqi, GUO Shaobo. Preparation and catalytic properties of CoFe2 O4@PDA@Pt magnetic composite with core shell structure[J]. Acta Materiae Compositae Sinica,2021,38(5):1551-1557(in Chinese).
[4] 林兆云, 戢德贤, 杨桂花, 等. 纤维素基金属纳米粒子复合催化剂的制备与应用[J]. 复合材料学报, 2022, 39(3):977-988. LIN Zhaoyun, JI Dexian, YANG Guihua, et al. Preparation and application of cellulose-based metal nanoparticles composite catalysts[J]. Acta Materiae Compositae Sinica,2022,39(3):977-988(in Chinese).
[5] ZHOU Z, LU C, WU X, et al. Cellulose nanocrystals as a novel support for CuO nanoparticles catalysts: Facile synthesis and their application to 4-nitrophenol reduction[J]. RSC Advances,2013,3(48):26066-26073. DOI: 10.1039/c3ra43006e
[6] CHEN L, CAO W J, QUINLAN P, et al. Sustainable catalysts from gold-loaded polyamidoamine dendrimer-cellulose nanocrystals[J]. ACS Sustainable Chemistry & Engineering,2015,3(5):978-985.
[7] LAM E, HRAPOVIC S, MAJID E, et al. Catalysis using gold nanoparticles decorated on nanocrystalline cellulose[J]. Nanoscale,2012,4(3):997-1002. DOI: 10.1039/c2nr11558a
[8] 王海英, 刘志明, 毕晓欣, 等. 桉木浆纳米纤维素制备优化条件初探[J]. 江苏农业科学, 2012, 40(7):242-245. DOI: 10.3969/j.issn.1002-1302.2012.07.092 WANG Haiying, LIU Zhiming, BI Xiaoxin, et al. Preliminary study on optimum preparation conditions of nanocrystalline cellulose from Eucalyptus pulp[J]. Jiangsu Agricultural Sciences,2012,40(7):242-245(in Chinese). DOI: 10.3969/j.issn.1002-1302.2012.07.092
[9] PINTO R J B, MÁRCIA C, NETO C P, et al. Growth and chemical stability of copper nanostructures on cellulosic fibers[J]. European Journal of Inorganic Chemistry,2012,2012(31):5043-5049. DOI: 10.1002/ejic.201200605
[10] GOY-LÓPEZ S, TABOADA P, CAMBON A, et al. Modulation of size and shape of Au nanoparticles using amino-X-shaped poly(ethylene oxide)-poly(propylene oxide) Block Copolymers[J]. The Journal of Physical Chemistry B,2010,114(1):66-76. DOI: 10.1021/jp908569z
[11] 倪镜博, 刘如一, 张明, 等. 原位聚合法制备纳米核-壳型PS-CHO@RGO复合微球及其催化活化过硫酸氢钾降解亚甲基蓝[J]. 复合材料学报, 2021, 38(7):2132-2139. NI Jingbo, LIU Ruyi, ZHANG Ming, et al. Preparation of nano core-shell PS-CHO@RGO composite microspheres by in-situ polymerization as a potassium hydrogen persulfate catalytic activator for methylene blue degradation[J]. Acta Materiae Compositae Sinica,2021,38(7):2132-2139(in Chinese).
[12] ARMELAO L, BARRECA D, BERTAPELLE M, et al. A sol-gel approach to nanophasic copper oxide thin films[J]. Thin Solid Films,2003,442(1):48-52.
[13] VAINIO U, PIRKKALAINEN K, KISKO K, et al. Copper and copper oxide nanoparticles in a cellulose support studied using anomalous small-angle X-ray scattering[J]. The European Physical Journal D,2007,42(1):93-101. DOI: 10.1140/epjd/e2007-00015-y
[14] FAN J C, XIE Z. Effects of substrate temperature on structural, electrical and optical properties of As-doped ZnO films[J]. Materials Science & Engineering B,2008,150(1):61-65.
[15] BHATTACHARJEE A, AHMARUZZAMAN M. Green synthesis of 2 D CuO nanoleaves (NLs) and its application for the reduction of pnitrophenol[J]. Materials Letters,2015,161:79-82. DOI: 10.1016/j.matlet.2015.08.061
[16] REDDY K R. Green synthesis, morphological and optical studies of CuO nanoparticles[J]. Journal of Molecular Structure,2017,1150:553-557. DOI: 10.1016/j.molstruc.2017.09.005
[17] SAITO T, KIMURA S, NISHIYAMA Y, et al. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose[J]. Biomacromolecules,2007,8(8):2485-2491. DOI: 10.1021/bm0703970
[18] ISOGAI A, ATALLA R H. Dissolution of cellulose in aqueous NaOH solutions[J]. Cellulose,1998,5(4):309-319. DOI: 10.1023/A:1009272632367
[19] 叶瑞荣, 汪朝阳, 杨凯, 等. 二乙醇胺改性聚乳酸的直接熔融聚合法合成及其表征[J]. 化学通报, 2009, 72(7):637-643. YE Ruirong, WANG Chaoyang, YANG Kai, et al. Direct melt polycondensation and characterization of polylactic acid modified by diethanolamine[J]. Chemistry,2009,72(7):637-643(in Chinese).
[20] LIONEL M, ALEXIS C, HEINRICH H. Polymer adsorption on iron oxide nanoparticles for one-step amino-functionalized silica encapsulation[J]. Journal of Nanomaterials,2015,16(1):239.
[21] KHAN S B, ALAMRY K A, BIFARI E N, et al. Assessment of antibacterial cellulose nanocomposite for water permeability and salt rejection[J]. Journal of Industrial & Engineering Chemistry,2015,24:266-275.
[22] TANG J, SHI Z, BERRY R M, et al. Mussel-inspired green metallization of silver nanoparticles on cellulose nanocrystals and their enhanced catalytic reduction of 4-nitrophenol in the presence of β-cyclodextrin[J]. Industrial & Engineering Chemistry Research,2015,54(13):3299-3308.
[23] CAO M, HU C, WANG Y, ET AL. A controllable synthetic route to Cu, Cu2O, and CuO nanotubes and nanorods[J]. Chemical Communications,2003(15):1884-1885. DOI: 10.1039/b304505f
[24] MARÍNPAREJA N, CANTINI M, GONZALEZGARCIA C, et al. Different organization of type I collagen immobilized on silanized and nonsilanized titanium surfaces affects fibroblast adhesion and fibronectin secretion[J]. ACS Applied Materials & Interfaces,2015,7(37):20667-20677.
[25] BOUAZIZI N, BARGOUGUI R, THEBAULT P, ET AL. Development of a novel functional core-shell-shell nanoparticles: From design to anti-bacterial applications[J]. Journal of Colloid & Interface Science,2017,513:726-735.
[26] WANG C, WANG H, YU H. Preparation and application of biomimetic superhydrophobic silica and polyurethane composite coating[J]. International Journal of Surface Science and Engineering,2015,9(6):510-519. DOI: 10.1504/IJSURFSE.2015.072832
[27] LIU B H, LI Z P. A review: Hydrogen generation from borohydride hydrolysis reaction[J]. Journal of Power Sources,2009,187(2):527-534. DOI: 10.1016/j.jpowsour.2008.11.032
[28] YANG Y, CHEN Z, WU X, et al. Nanoporous cellulose membrane doped with silver for continuous catalytic decolorization of organic dyes[J]. Cellulose,2018,25(4):2547-2558. DOI: 10.1007/s10570-018-1710-x
[29] BAE S, GIM S, KIM H, et al. Effect of NaBH4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of p-nitrophenol[J]. Applied Catalysis B: Environmental,2016,182:541-549. DOI: 10.1016/j.apcatb.2015.10.006
[30] LI J, LIU C Y, LIU Y. Au/graphene hydrogel: Synthesis, characterization and its use for catalytic reduction of 4-nitrophenol[J]. Journal of Materials Chemistry,2012,22(17):8426-8430. DOI: 10.1039/c2jm16386a
[31] WU X, LU C, ZHOU Z, et al. Strategy for synthesizing porous cellulose nanocrystal supported metal nanocatalysts[J]. ACS Sustainable Chemistry & Engineering,2016,1(1):71-79.
[32] KOGA H, TOKUNAGA E, HIDAKA M, et al. Topochemical synthesis and catalysis of metal nanoparticles exposed on crystalline cellulose nanofibers[J]. Chemical Communications,2010,46(45):8567-8569. DOI: 10.1039/c0cc02754e
[33] WU X, LU C, ZHOU Z, et al. Green synthesis and formation mechanism of cellulose nanocrystal-supported gold nanoparticles with enhanced catalytic performance[J]. Environmental Science Nano,2014,1(1):71-79. DOI: 10.1039/c3en00066d
[34] AN X, LONG Y, NI Y. Cellulose nanocrystal/hexadecyltrimethylammonium bromide/silver nanoparticle composite as a catalyst for reduction of 4-nitrophenol[J]. Carbohydrate Polymers[J],2017,156:253-258. DOI: 10.1016/j.carbpol.2016.08.099
[35] DUAN C, LIU C, MENG X, et al. Fabrication of carboxymethylated cellulose fibers supporting Ag NPs@MOF-199 s nanocatalysts for catalytic reduction of 4-nitrophenol[J]. Applied Organometallic Chemistry,2019,33(5):4865. DOI: 10.1002/aoc.4865
[36] AHMAD I, KAMAL T, KHAN S B, et al. An efficient and easily retrievable dip catalyst based on silver nanoparticles/chitosan-coated cellulose filter paper[J]. Cellulose,2016,23(6):3577-3588.
[37] XU P, CEN C, CHEN N, et al. Facile fabrication of silver nanoparticles deposited cellulose microfiber nanocompo-site for catalytic application[J]. Journal of Colloid and Interface Science,2018,526:194-200. DOI: 10.1016/j.jcis.2018.04.045
[38] XIONG R, LU C, WANG Y, et al. Nanofibrillated cellulose as the support and reductant for the facile synthesis of Fe3O4/Ag nanocomposite with catalytic and antibacterial activity[J]. Journal of Materials Chemistry A,2013,1(47):14910-14918. DOI: 10.1039/c3ta13314a
-
期刊类型引用(1)
1. 姜姗姗,王恩通. 磁性Fe_3O_4纳米粒子催化剂的制备及催化性能研究. 兵器材料科学与工程. 2024(03): 58-62 .  百度学术
百度学术
其他类型引用(1)
-





 下载:
下载: