Synthesis of Fe-doped Co3O4 and investigation of the effect of trace Fe in electrolyte on its OER performance
-
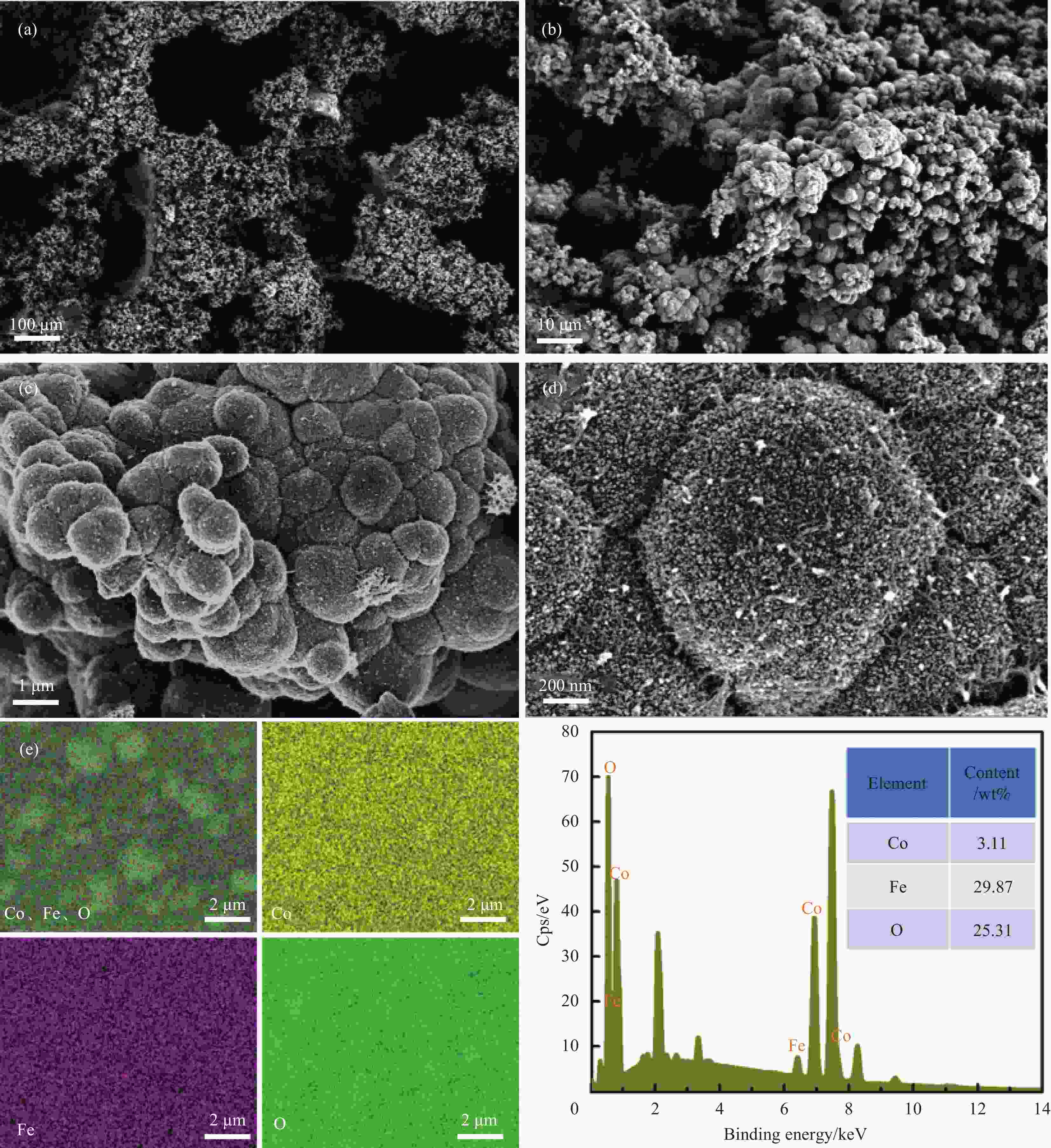
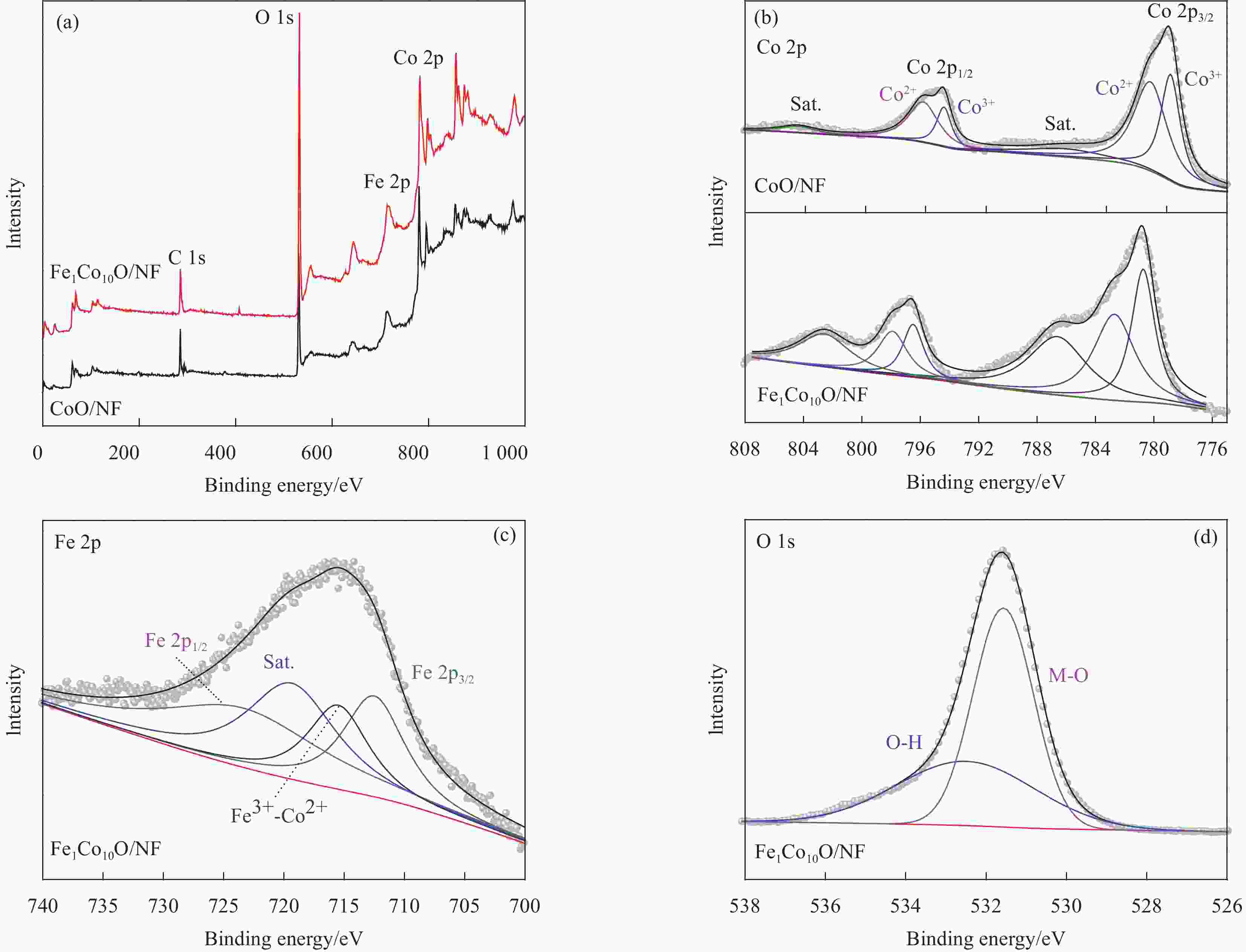
摘要: 高活性和耐久性强的析氧反应(OER)电催化剂的开发在可持续能源储存和转化系统中备受关注。研究表明,电催化剂中的Fe被认为是最具活性的位点,而CoOOH则扮演着导电性、高表面积和化学稳定性的宿主角色。在此,本文通过简单的溶剂热法,在泡沫镍基底(NF)上合成了铁掺杂Co3O4微球薄膜。优化后的Fe1Co10O/NF复合电极在1 mol·L−1 KOH中表现出较强的OER催化性能,仅需243 mV的超低电位便可达到50 mA·cm−2的电流密度。同时兼具优异的长期稳定性。此外,在去铁KOH电解液中证明了Co3O4电催化剂的高活性对Fe的依赖性。本研究提供了一种快捷、经济的策略用以制备性能高、耐用性强的廉价双金属制氢电催化剂。Abstract: The development of highly active and durable oxygen evolution reaction (OER) electrocatalysts is of great interest in sustainable energy storage and conversion systems. It has been shown that Fe in electrocatalysts is considered to be the most active site, while CoOOH plays the role of a host for electrical conductivity, high surface area and chemical stability. In this paper, Fe-doped Co3O4 microsphere films were synthesized on nickel foam substrate (NF) by a simple solvothermal method. The optimized Fe1Co10O/NF composite electrode exhibits strong OER catalytic performance in 1 mol·L−1 KOH, and achieves a current density of 50 mA·cm−2 at an ultra-low potential of only 243 mV. It also combines excellent long-term stability. In addition, the present study demonstrated the Fe dependence of the high activity of Co3O4 electrocatalysts in Fe-depleted KOH electrolyte.
-
Key words:
- electrocatalytic /
- OER /
- iron-doped /
- Co3O4 /
- Fe-Free KOH
-
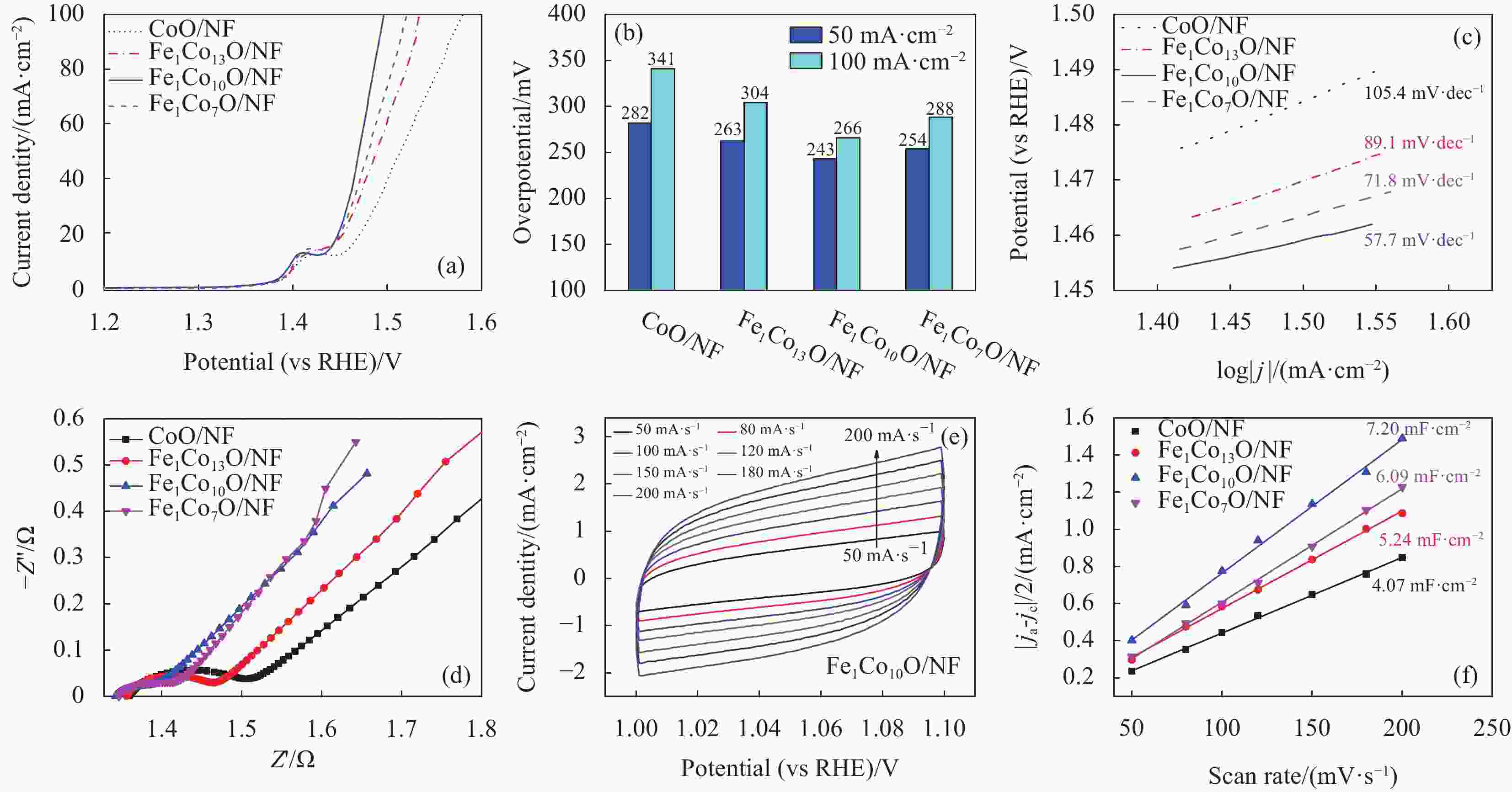
图 4 (a) CoO/NF、 Fe1Co13O/NF、 Fe1Co10O/NF和Fe1Co7O/NF样品的90% iR补偿后的LSV极化曲线图,(b) 50 mA·cm−2、100 mA·cm−2下的过电位性能图,(c)由LSV极化曲线拟合得到的Tafel斜率图,(d)电化学阻抗图,(e) Fe1Co10O/NF在不同扫速下的CV图和(f)所有样品的Cdl图
Figure 4. (a) LSV polarization curves after 90% iR compensation, (b) overpotential at 50 and 100 mA·cm−2, (c) corresponding Tafel slope, (d) electrochemical impedance test of CoO/NF、 Fe1Co13O/NF、 Fe1Co10O/NF and Fe1Co7O/NF, (e) CV curves of Fe1Co10O/NF at different scan rates, and (f) Cdl plot of all samples
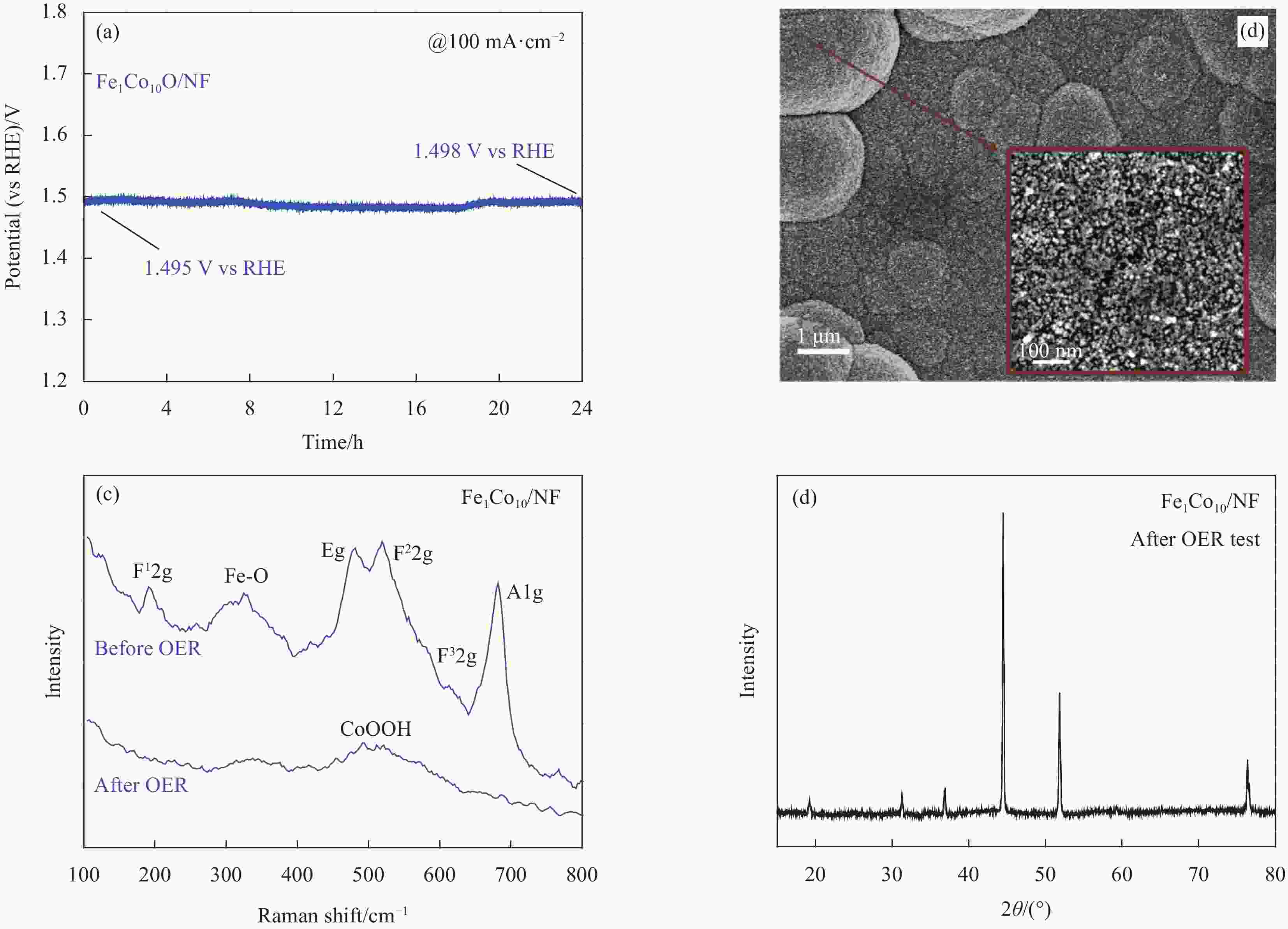
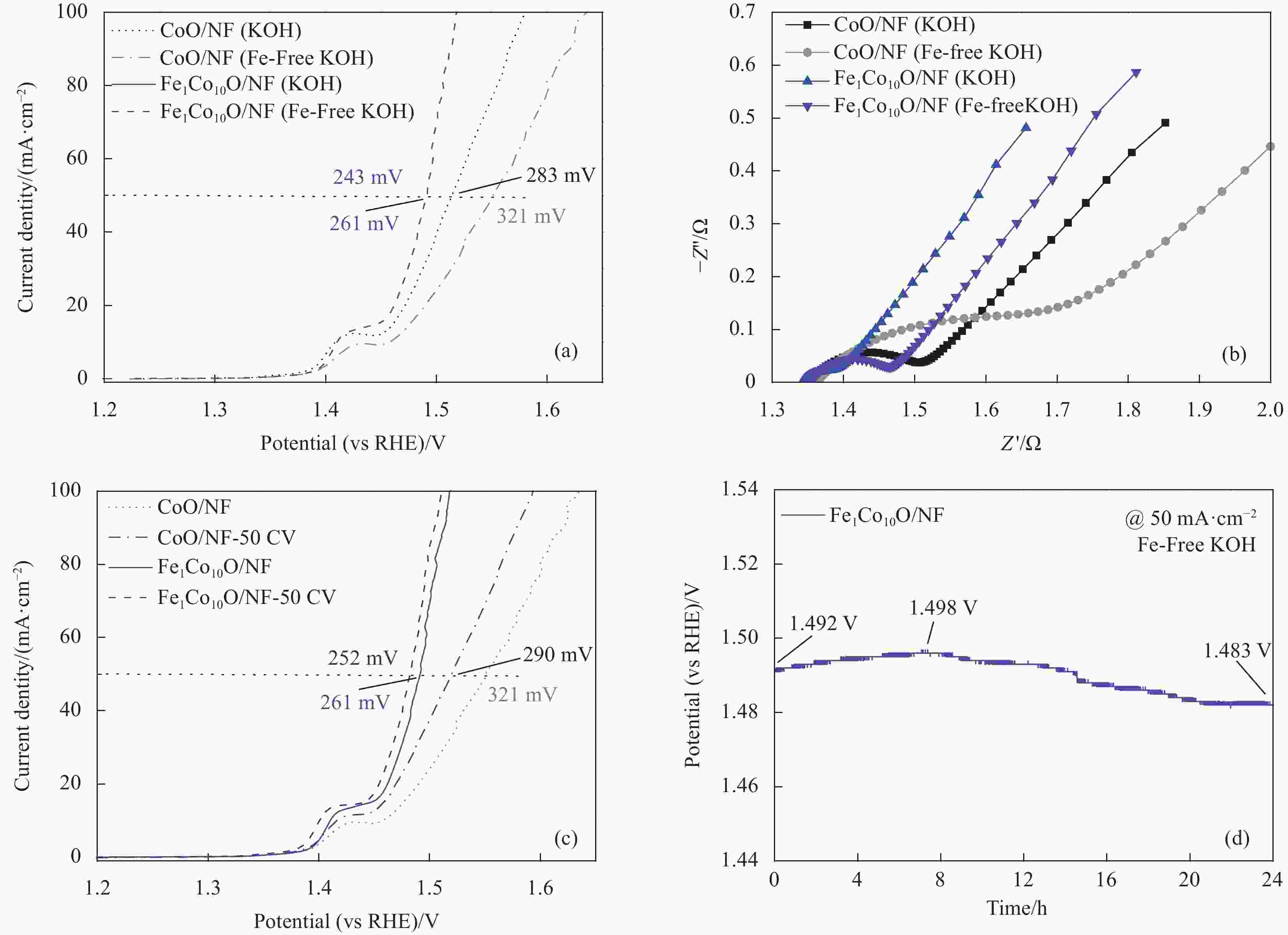
图 6 去铁KOH电解液中Fe1Co10O/NF和CoO/NF的(a) LSV极化曲线,(b)电化学阻抗测试,(c) 50次CV后的LSV极化曲线和(d) Fe1Co10O/NF在铁KOH电解液中稳定性测试
Figure 6. (a) LSV polarization curves, (b) electrochemical impedance test, (c) LSV polarization curves after 50 CV of Fe1Co10O/NF and CoO/NF in Fe removal KOH electrolyte, and stability test of Fe1Co10O/NF in Fe KOH electrolyte
表 1 六水合硝酸钴、九水合硝酸铁的添加量
Table 1. Added amount of cobalt nitrate hexahydrate and ferric nitrate ninahydrate
CoO/
NFFe1Co13O/
NFFe1Co10O/
NFFe1Co7O/
NFx g Co(NO3)2·6H2O 2.91 2.70 2.68 2.59 y g Fe(NO3)2·9H2O 0 0.29 0.37 0.51 -
[1] 涂言言, 赵子涵, 孙一强. FeOOH-Ni(OH)2复合材料的制备及其电催化析氧性能[J]. 复合材料学报, 2020, 37(8): 1944-1950.TU Yanyan, ZHAO Zihan, SUN Yiqiang. FeOOH-Ni(OH)2 composites preparation and the electrocatalytic oxygen precipitation performance[J]. Acta Materiae Compositae Sinica, 2020, 37(8): 1944-1950(in Chinese). [2] LIU G, WANG M, WU Y, et al. 3D porous network heterostructure NiCe@NiFe electrocatalyst for efficient oxygen evolution reaction at large current densities[J]. Applied Catalysis B: Environmental, 2020, 260: 118199. doi: 10.1016/j.apcatb.2019.118199 [3] YU Y, CHEN X, LI J, et al. Ni-based heterostructure with protective phosphide layer to enhance the oxygen evolution reaction for the seawater electrolysis[J]. International Journal of Hydrogen Energy, 2024, 51: 1373-1380. doi: 10.1016/j.ijhydene.2023.07.282 [4] 张海成, 杨邦志, 张娇, 等. 钴掺杂铜基复合材料的制备及其电催化析氧性能[J]. 复合材料学报, 2024, 41(4): 1923-1933.ZHANG Haicheng, YANG Bangjie, ZHANG Jiao, et al. Preparation of cobalt-doped copper matrix composites and their electrocatalytic oxygen precipitation properties[J]. Acta Materiae Compositae Sinica, 2024, 41(4): 1923-1933(in Chinese). [5] GU W, HU L, ZHU X, et al. Rapid synthesis of Co3O4 nanosheet arrays on Ni foam by in situ electrochemical oxidization of air-plasma engraved Co(OH)2 for efficient oxygen evolution[J]. Chemical Communications, 2018, 54(90): 12698-12701. doi: 10.1039/C8CC06399K [6] YIN D, JIN Z, LIU M, et al. Microwave-assisted synthesis of the cobalt-iron phosphates nanosheets as an efficient electrocatalyst for water oxidation[J]. Electrochimica Acta, 2018, 260: 420-429. doi: 10.1016/j.electacta.2017.12.007 [7] LI Y, ZHAO C. Iron-doped nickel phosphate as synergistic electrocatalyst for water oxidation[J]. Chemistry of materials, 2016, 28(16): 5659-5666. doi: 10.1021/acs.chemmater.6b01522 [8] DUAN D, GUO D, GAO J, et al. Electrodeposition of cobalt-iron bimetal phosphide on Ni foam as a bifunctional electrocatalyst for efficient overall water splitting[J]. Journal of Colloid and Interface Science, 2022, 622: 250-260. doi: 10.1016/j.jcis.2022.04.127 [9] SIDHUREDDY B, DONDAPATI JS, CHEN A. Shape-controlled synthesis of Co3O4 for enhanced electrocatalysis of the oxygen evolution reaction[J]. Chemical communications, 2019, 55(25): 3626-3629. doi: 10.1039/C8CC10194A [10] LIU TT, LIANG YH, LIU Q, et al. Electrodeposition of cobalt-sulfide nanosheets film as an efficient electrocatalyst for oxygen evolution reaction[J]. Electrochemistry Communications, 2015, 60: 92-96. doi: 10.1016/j.elecom.2015.08.011 [11] 李创, 王宇, 张亚男, 等. 氮掺杂碳负载表面部分暴露的CoF2O4用于高性能催化析氧反应[J]. 复合材料学报, 2023, 40(3): 1552-1559.LI Chuang, WANG Yu, ZHAO Yanan, et al. Nitrogen-doped carbon loaded surface partially exposed CoF2O4 for high performance catalytic oxygen precipitation reaction[J]. Acta Materiae Compositae Sinica, 2023, 40(3): 1552-1559(in Chinese). [12] CHENG W, ZHAO X, SU H, et al. Lattice-strained metal–organic-framework arrays for bifunctional oxygen electrocatalysis[J]. Nature Energy, 2019, 4(2): 115-122. doi: 10.1038/s41560-018-0308-8 [13] YI L, NIU Y, FENG B, et al. Simultaneous phase transformation and doping via a unique photochemical–electrochemical strategy to achieve a highly active Fe-doped Ni oxyhydroxide oxygen evolution catalyst[J]. Journal of Materials Chemistry A, 2021, 9(7): 4213-4220. doi: 10.1039/D0TA09617B [14] BAI L, HSU C-S, ALEXANDER DT, et al. A cobalt–iron double-atom catalyst for the oxygen evolution reaction[J]. Journal of the American Chemical Society, 2019, 141(36): 14190-14199. doi: 10.1021/jacs.9b05268 [15] LI J, LI G, WANG J, et al. A novel core–double shell heterostructure derived from a metal–organic framework for efficient HER, OER and ORR electrocatalysis[J]. Inorganic Chemistry Frontiers, 2020, 7(1): 191-197. doi: 10.1039/C9QI01080G [16] ANANTHARAJ S, EDE SR, SAKTHIKUMAR K, et al. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe, Co, and Ni: a review[J]. Acs Catalysis, 2016, 6(12): 8069-8097. doi: 10.1021/acscatal.6b02479 [17] LIU Y, HAN N, JIANG J, et al. Boosting the oxygen evolution electrocatalysis of layered nickel hydroxidenitrate nanosheets by iron doping[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10627-10636. doi: 10.1016/j.ijhydene.2019.03.010 [18] HE L, LI Z, ZHANG Z. Rapid, low-temperature synthesis of single-crystalline Co3O4 nanorods on silicon substrates on a large scale[J]. Nanotechnology, 2008, 19(15): 155606. doi: 10.1088/0957-4484/19/15/155606 [19] YANG C, HE T, ZHOU W, et al. Iron-tuned 3D cobalt–phosphate catalysts for efficient hydrogen and oxygen evolution reactions over a wide pH range[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(36): 13793-13804. [20] FU S, SONG J, ZHU C, et al. Ultrafine and highly disordered Ni2Fe1 nanofoams enabled highly efficient oxygen evolution reaction in alkaline electrolyte[J]. Nano Energy, 2018, 44: 319-326. doi: 10.1016/j.nanoen.2017.12.010 [21] LIU J, HE X, WANG Y, et al. Deep reconstruction of highly disordered iron/nickel nitrate hydroxide nanoplates for high-performance oxygen evolution reaction in alkaline media[J]. Journal of Alloys and Compounds, 2022, 927: 167060. doi: 10.1016/j.jallcom.2022.167060 [22] SU Y, LIU H, LI C, et al. Hydrothermal-assisted defect engineering in spinel Co3O4 nanostructures as bifunctional catalysts for oxygen electrode[J]. Journal of Alloys and Compounds, 2019, 799: 160-168. doi: 10.1016/j.jallcom.2019.05.331 [23] BANDAL HA, JADHAV AR, TAMBOLI AH, et al. Bimetallic iron cobalt oxide self-supported on Ni-Foam: An efficient bifunctional electrocatalyst for oxygen and hydrogen evolution reaction[J]. Electrochimica Acta, 2017, 249: 253-262. doi: 10.1016/j.electacta.2017.07.178 [24] GRAU-CRESPO R, AL-BAITAI AY, SAADOUNE I, et al. Vacancy ordering and electronic structure of γ-Fe2O3 (maghemite): a theoretical investigation[J]. Journal of Physics: Condensed Matter, 2010, 22(25): 255401. doi: 10.1088/0953-8984/22/25/255401 [25] FENG C, CHEN M, ZHOU Y, et al. High-entropy NiFeCoV disulfides for enhanced alkaline water/seawater electrolysis[J]. Journal of Colloid and Interface Science, 2023, 645: 724-734. doi: 10.1016/j.jcis.2023.04.172 [26] XU J, GAO P, ZHAO T. Non-precious Co3O4 nano-rod electrocatalyst for oxygen reduction reaction in anion-exchange membrane fuel cells[J]. Energy & Environmental Science, 2012, 5(1): 5333-5339. [27] GU Z, XIANG X, FAN G, et al. Facile synthesis and characterization of cobalt ferrite nanocrystals via a simple reduction−oxidation route[J]. The Journal of Physical Chemistry C, 2008, 112(47): 18459-18466. doi: 10.1021/jp806682q [28] KITIPHATPIBOON N, CHEN M, FENG C, et al. Modification of spinel MnCo2O4 nanowire with NiFe-layered double hydroxide nanoflakes for stable seawater oxidation[J]. Journal of Colloid and Interface Science, 2023, 632: 54-64. doi: 10.1016/j.jcis.2022.11.044 [29] LI L, HU Z, TAO L, et al. Efficient electronic transport in partially disordered Co3O4 nanosheets for electrocatalytic oxygen evolution reaction[J]. ACS Applied Energy Materials, 2020, 3(3): 3071-3081. doi: 10.1021/acsaem.0c00190 [30] NANDAN R, GAUTAM A, NANDA KK. Anthocephalus cadamba shaped FeNi encapsulated carbon nanostructures for metal–air batteries as a resilient bifunctional oxygen electrocatalyst[J]. Journal of Materials Chemistry A, 2018, 6(41): 20411-20420. doi: 10.1039/C8TA05822A [31] BAO F, KEMPPAINEN E, DORBANDT I, et al. Host, suppressor, and promoter—the roles of Ni and Fe on oxygen evolution reaction activity and stability of NiFe alloy thin films in alkaline media[J]. ACS Catalysis, 2021, 11(16): 10537-10552. doi: 10.1021/acscatal.1c01190 [32] CHUNG DY, LOPES PP, FARINAZZO BERGAMO DIAS MARTINS P, et al. Dynamic stability of active sites in hydr (oxy) oxides for the oxygen evolution reaction[J]. Nature Energy, 2020, 5(3): 222-230. doi: 10.1038/s41560-020-0576-y -

 点击查看大图
点击查看大图
计量
- 文章访问数: 51
- HTML全文浏览量: 18
- 被引次数: 0





 下载:
下载: