Preparation and characterization of a new pH-responsive hydrogel with bacteriostatic properties
-
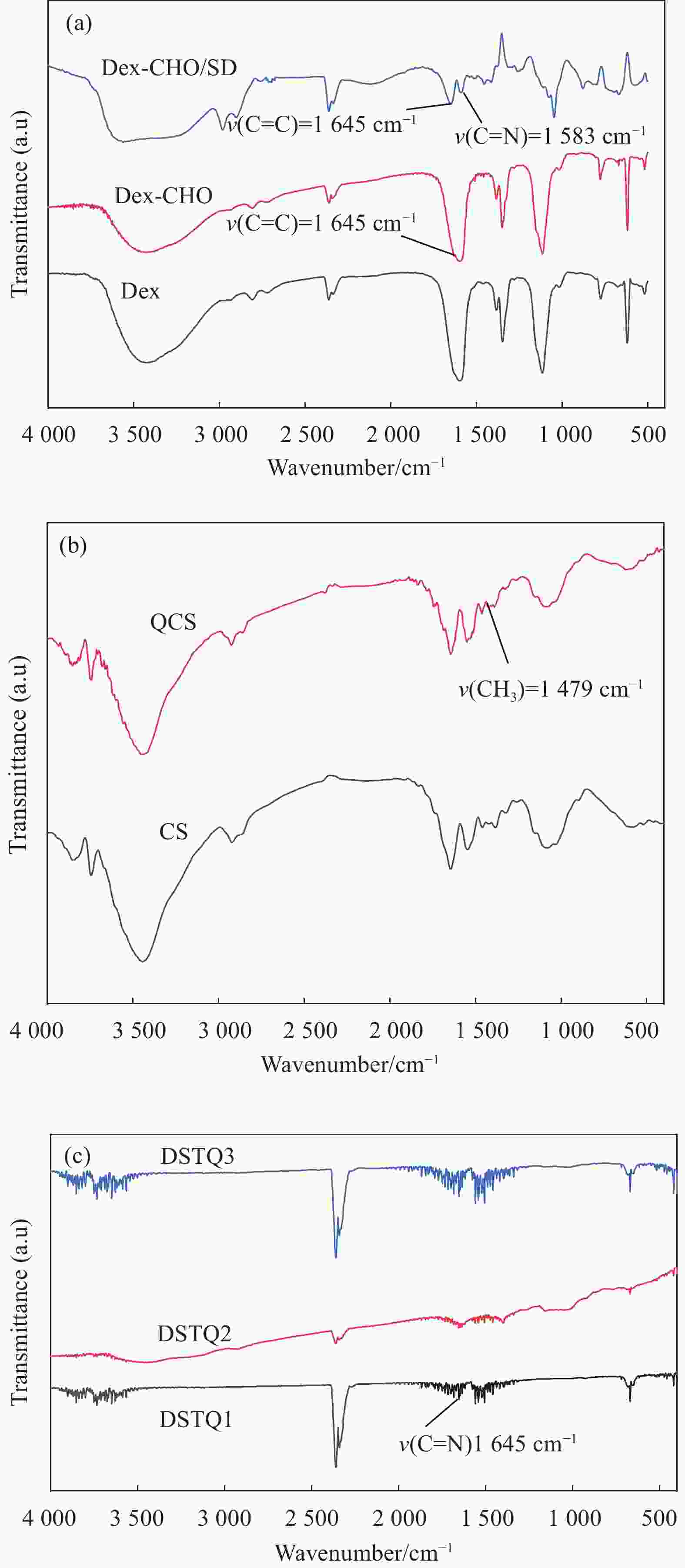
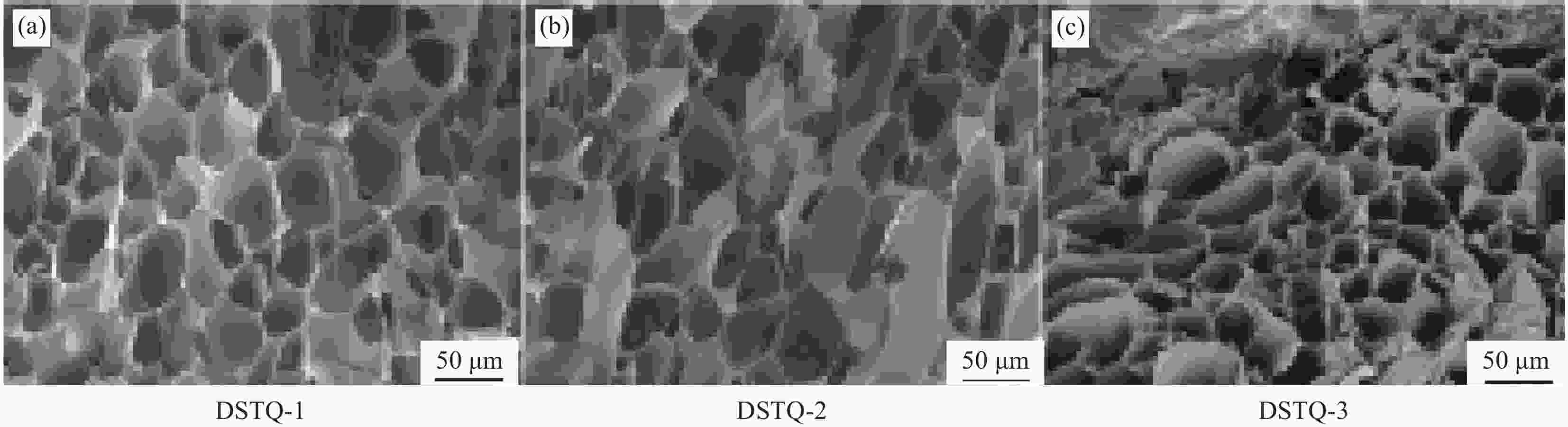
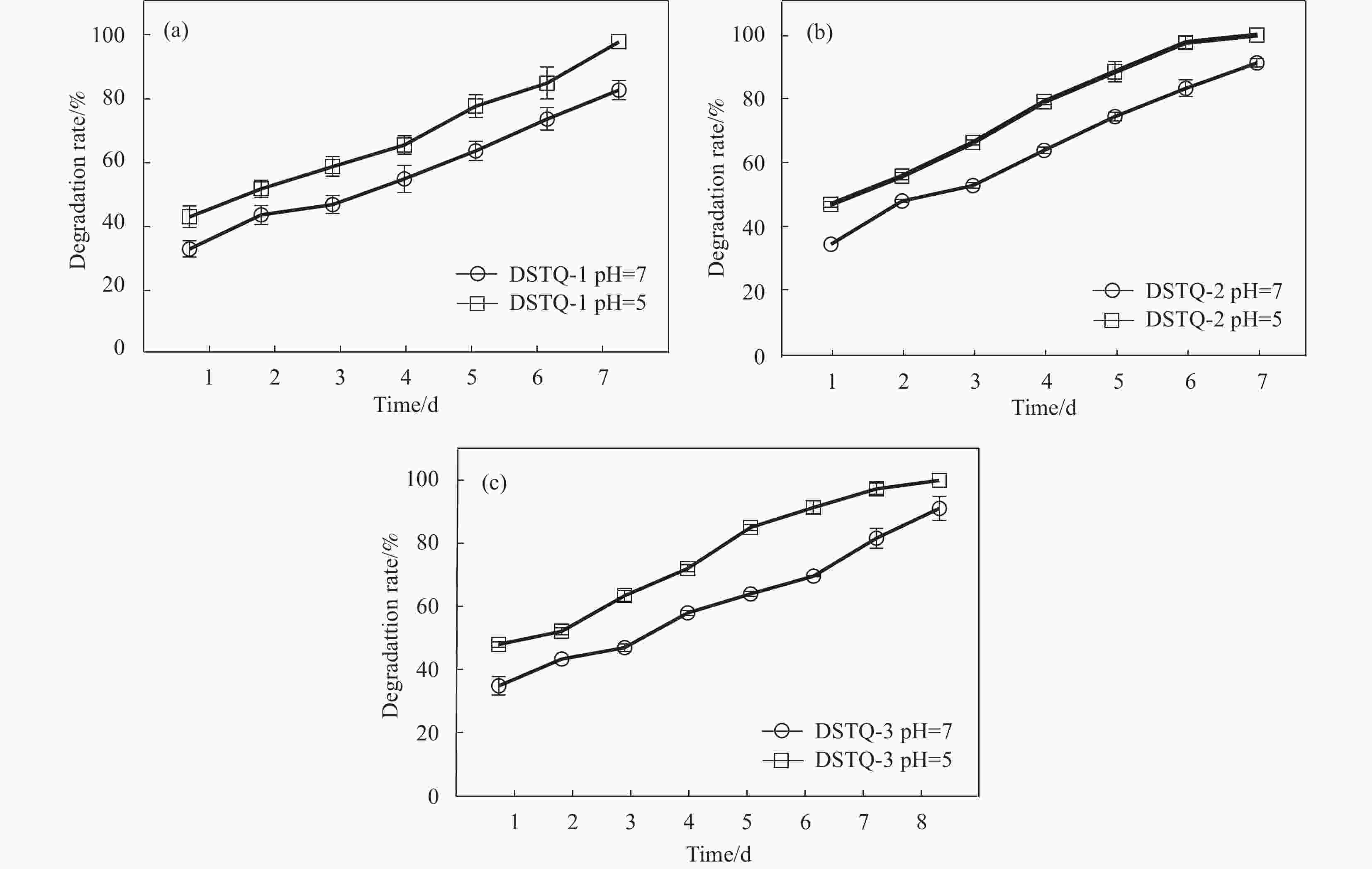
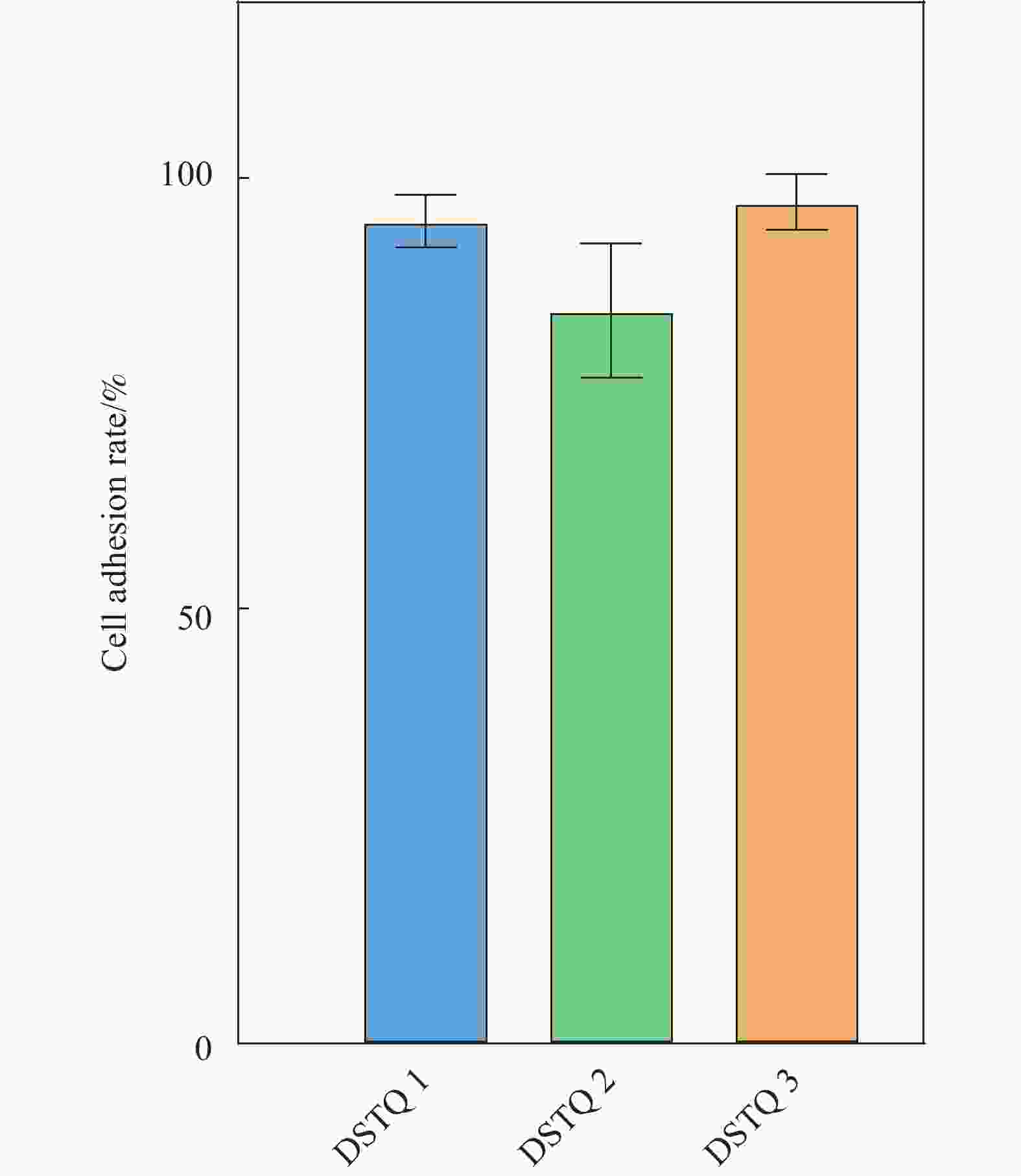
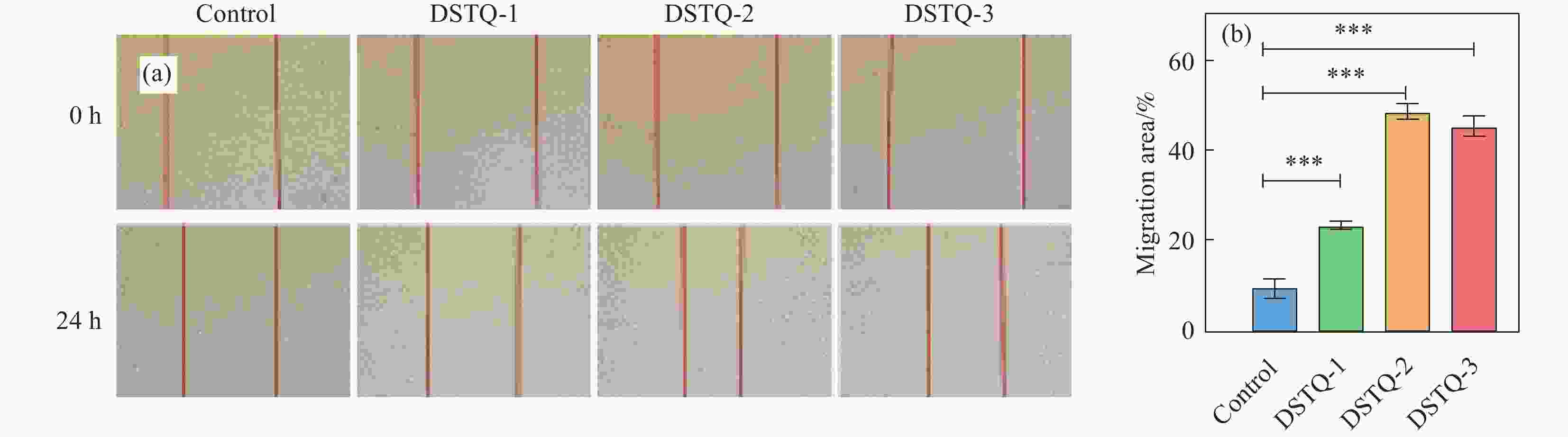
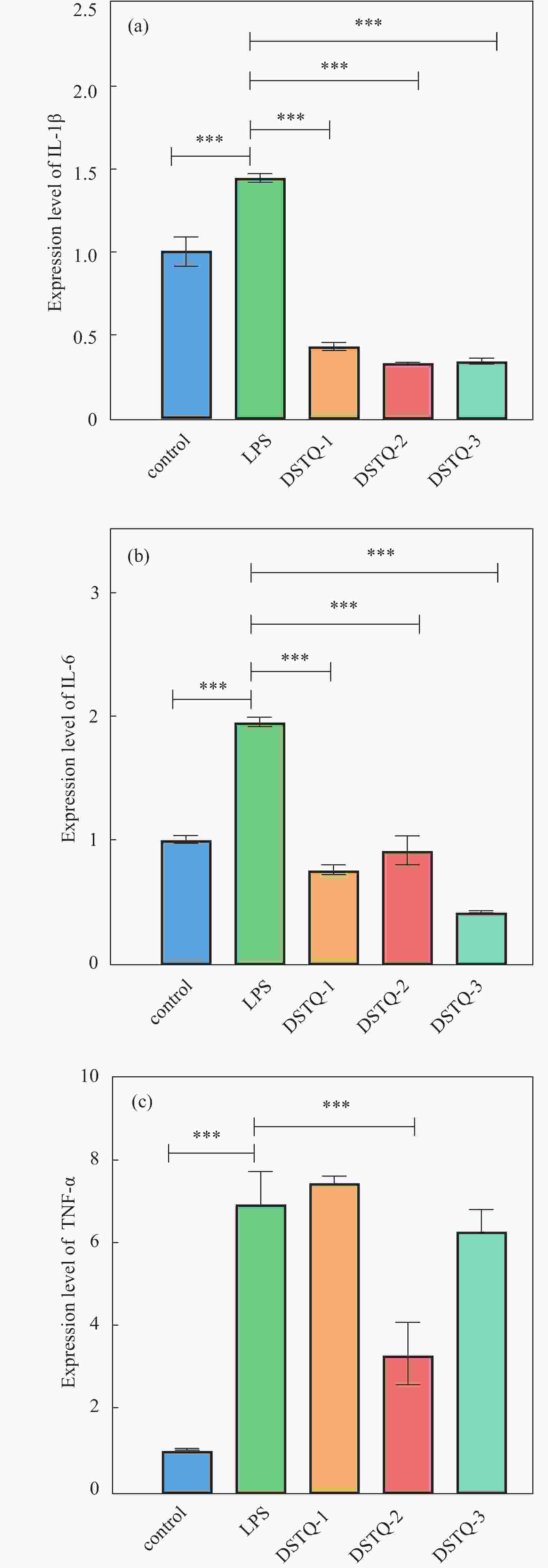
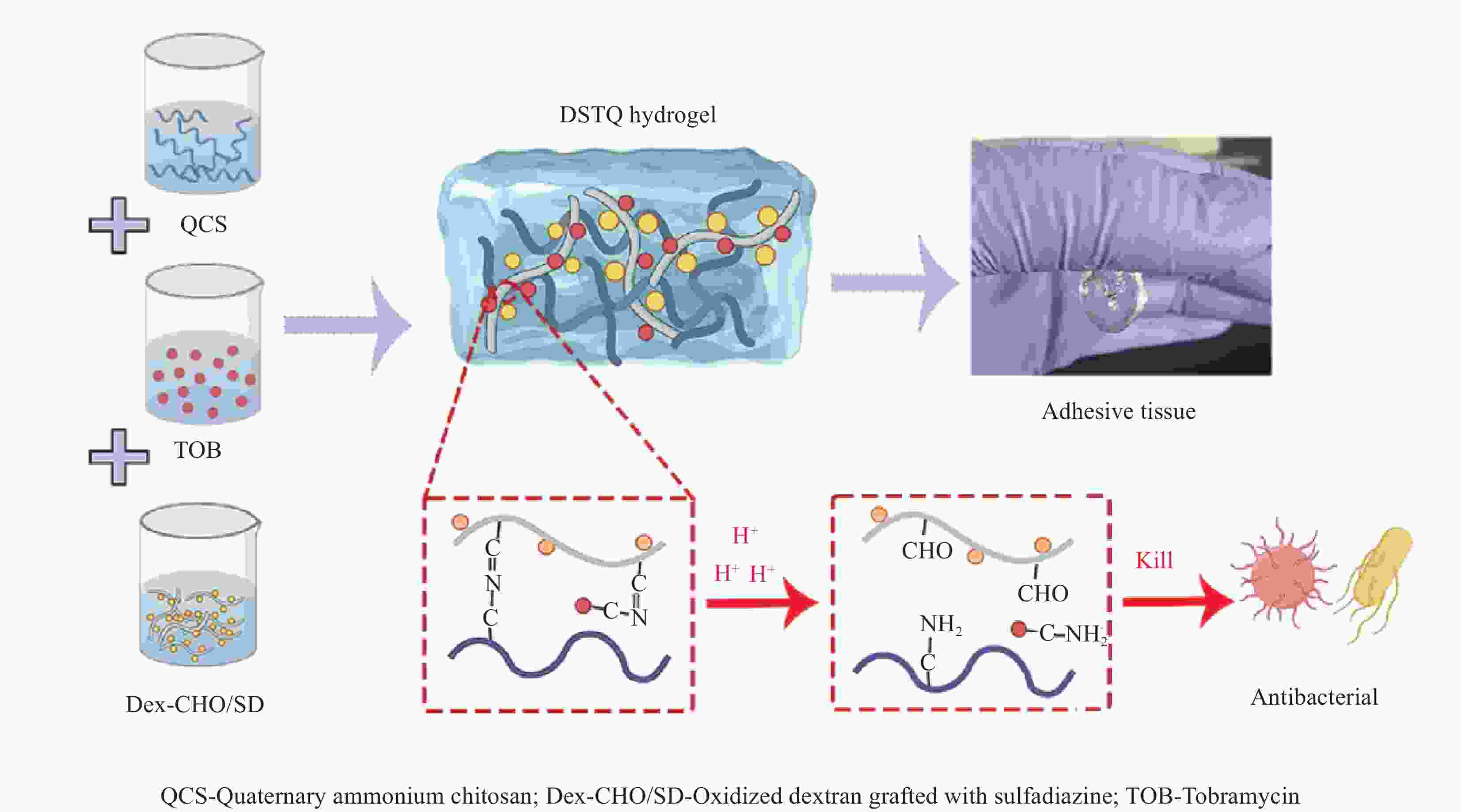
摘要: 为设计一种能够响应pH的、新型多功能创面敷料,使用季铵化壳聚糖(QCS)、接枝了磺胺嘧啶(SD)的氧化葡聚糖(Dex-CHO/SD)和妥布霉素(TOB),通过席夫碱键和氢键反应形成DSTQ水凝胶。对水凝胶的形貌、降解性、抑菌性、细胞相容性、血液相容性等性能进行研究。结果表明:具有三维孔状结构的DSTQ水凝胶可以响应感染造成的pH降低,进而发生降解,释放药物;DSTQ水凝胶对铜绿假单胞菌的抑制率显著高于金黄色葡萄球菌;DSTQ水凝胶毒性低,生物安全性高,与L929细胞共培养3天后,细胞活力高于95%;DSTQ水凝胶具有良好的血液相容性,溶血率远小于5%;细胞在DSTQ水凝胶表面能够快速迁移,有利于伤口愈合;当DSTQ水凝胶负载生长因子后具有良好的抗炎效果。研究结果表明,DSTQ水凝胶可以作为新型创面敷料,有望应用于临床促伤口愈合。Abstract: To develop a novel wound dressing with multiple functionalities and pH-responsiveness, a combination of quaternary ammonium chitosan (QCS), oxidized dextran grafted with sulfadiazine (Dex-CHO/SD), and tobramycin(TOB) was employed to fabricate the DSTQ hydrogel through Schiff base bonding and hydrogen bonding. The hydrogel formation relied on Schiff base bonding and hydrogen bonding. The morphology, degradation, bacteriostatic, cytocompatibility, and hemocompatibility properties of the hydrogels were analyzed. The results indicated that the 3D pore structure of DSTQ hydrogel could respond to pH drops caused by infections and degrade, facilitating drug release. The inhibition rate of DSTQ hydrogel against Pseudomonas aeruginosa was significantly higher than that of Staphylococcus aureus. DSTQ hydrogel exhibited low toxicity, high biosafety, and cell viability above 95% after three days of co-culture with L929 cells. Additionally, it demonstrated excellent hemocompatibility, with a hemolysis rate below 5%. Furthermore, cells exhibited rapid migration on the material's surface, promoting wound healing. When loaded with growth factors, DSTQ hydrogel demonstrated good anti-inflammatory effects. These findings suggest that DSTQ hydrogel can be an effective wound-dressing option in clinical settings.
-
Key words:
- hydrogel /
- wound healing /
- pH-responsive /
- antibacterial properties /
- wound dressing
-
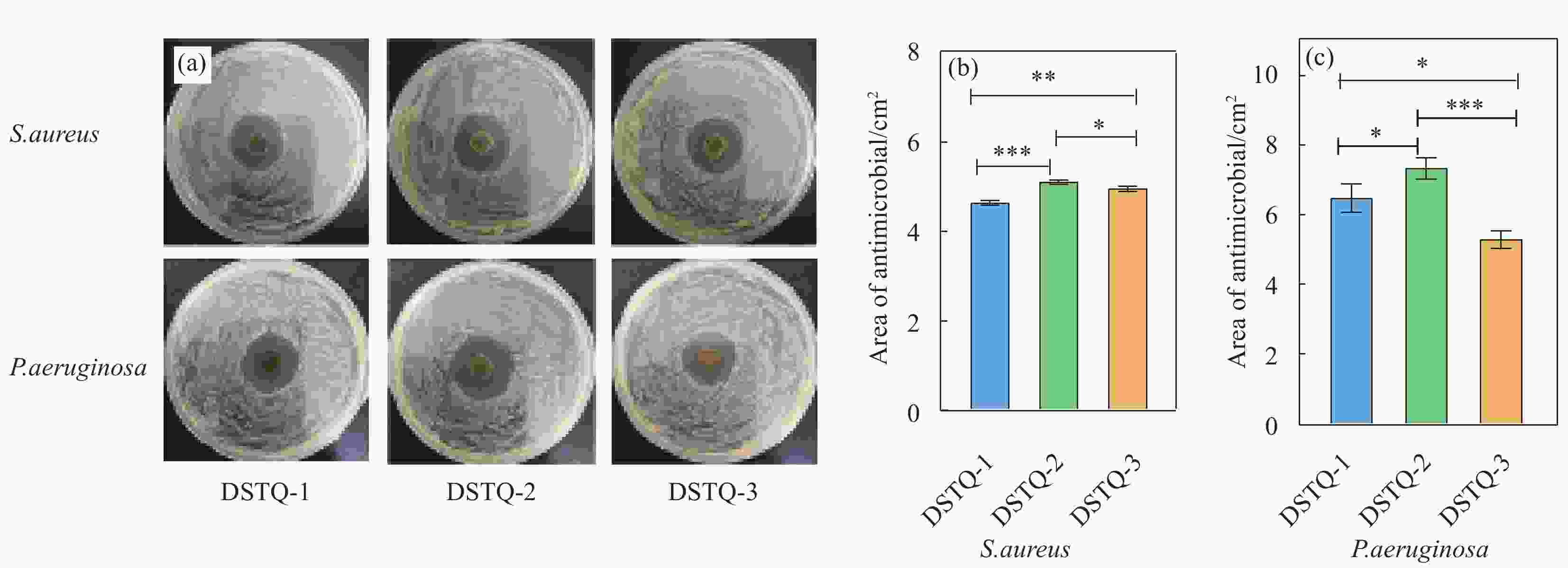
图 6 (a)DSTQ水凝胶对金黄色葡萄球菌和铜绿假单胞菌的抑菌图像及(b)金黄色葡萄球菌和(c)铜绿假单胞菌的定量统计结果。***代表有显著性差异(P<0.001,n=3)
Figure 6. (a) Antibacterial images of Staphylococcus aureus and Pseudomonas aeruginosa and (b) Staphylococcus aureus and (c) Pseudomonas aeruginosa in DSTQ hydrogels. *** The asterisk indicates a significant difference (P<0.001,n=3)
-
[1] NGUYEN A V, SOULIKA A M. The Dynamics of the Skin’s Immune System[J]. International Journal of Molecular Sciences, 2019, 20(8): 1811. doi: 10.3390/ijms20081811 [2] RODRIGUES M, KOSARIC N, BONHAM C A, et al. Wound Healing: A Cellular Perspective[J]. Physiological Reviews, 2019, 99(1): 665-706. doi: 10.1152/physrev.00067.2017 [3] OLSSON M, JäRBRINK K, DIVAKAR U, et al. The humanistic and economic burden of chronic wounds: A systematic review[J]. Wound Repair and Regeneration, 2018, 27(1): 114-125. [4] GARG S S, DUBEY R, SHARMA S, et al. Biological macromolecules-based nanoformulation in improving wound healing and bacterial biofilm-associated infection: A review[J]. International Journal of Biological Macromolecules, 2023, 247: 125636. doi: 10.1016/j.ijbiomac.2023.125636 [5] YANG H, WANG W-S, TAN Y, et al. Investigation and analysis of the characteristics and drug sensitivity of bacteria in skin ulcer infections[J]. Chinese Journal of Traumatology, 2017, 20(4): 194-7. doi: 10.1016/j.cjtee.2016.09.005 [6] 李兢思, 李俊欣, 王思炜, 等. 浅析生物医用材料中伤口敷料的研究现状[J]. 生物医学工程与临床: 1-6.LI J S, LI J X, WANG S W, et al. Brief analysis on the research status of wound dressing in biomedical materials[J]. Biomedical Engineering and Clinical Medicine, 1-6. (in Chinese) [7] HE Y, LI Y, SUN Y, et al. A double-network polysaccharide-based composite hydrogel for skin wound healing[J]. Carbohydrate Polymers, 2021, 261(1): 117870. [8] GRAçA M F P, MIGUEL S P, CABRAL C S D, et al. Hyaluronic acid—Based wound dressings: A review[J]. Carbohydrate Polymers, 2020, 241: 116314. doi: 10.1016/j.carbpol.2020.116314 [9] TAN M, ZENG J, ZHANG F-Z, et al. Double-Layer Hydrogel with Glucose-Activated Two-Stage ROS Regulating Properties for Programmed Diabetic Wound Healing[J]. ACS Applied Materials & Interfaces, 2023, 15(44): 50809-50820. [10] KANG D-S, YANG S-Y, LEE C-Y. Fabrication of innocuous hydrogel scaffolds based on modified dextran for biotissues[J]. Carbohydrate Research, 2022, 522: 108699. doi: 10.1016/j.carres.2022.108699 [11] ZHAO C, ZHOU L, CHIAO M, et al. Antibacterial hydrogel coating: Strategies in surface chemistry[J]. Advances in Colloid and Interface Science, 2020, 285: 102280. doi: 10.1016/j.cis.2020.102280 [12] CHENG H, SHI Z, YUE K, et al. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities[J]. Acta Biomaterialia, 2021, 124: 219-232. doi: 10.1016/j.actbio.2021.02.002 [13] YANG Z, HUANG R, ZHENG B, et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing[J]. Advanced Science, 2021, 8(8): 2003627. doi: 10.1002/advs.202003627 [14] DING Q, SUN T, SU W, et al. Bioinspired Multifunctional Black Phosphorus Hydrogel with Antibacterial and Antioxidant Properties: A Stepwise Countermeasure for Diabetic Skin Wound Healing[J]. Advanced Healthcare Materials, 2022, 11(12): 2102791. doi: 10.1002/adhm.202102791 [15] WANG Z, ZHANG Y, YIN Y, et al. High-strength and Injectable Supramolecular Hydrogel Self-Assembled by Monomeric Nucleoside for Tooth-Extraction Wound Healing[J]. Advanced Materials, 2022, 34(13): 2108300. doi: 10.1002/adma.202108300 [16] QIN X H, WANG X, ROTTMAR M, et al. Near-infrared light-sensitive Polyvinyl Alcohol Hydrogel Photoresist for Spatiotemporal Control of Cell-Instructive 3D Microenvironments[J]. Advanced Materials, 2018, 30(10): 1705564. doi: 10.1002/adma.201705564 [17] HU J, LIU X, GAO Q, et al. Thermosensitive PNIPAM-Based Hydrogel Crosslinked by Composite Nanoparticles as Rapid Wound-Healing Dressings[J]. Biomacromolecules, 2023, 24(3): 1345-1354. doi: 10.1021/acs.biomac.2c01380 [18] HENDI A, UMAIR HASSAN M, ELSHERIF M, et al. Healthcare Applications of pH-Sensitive Hydrogel-Based Devices: A Review[J]. International Journal of Nanomedicine, 2020, 15: 3887-3901. doi: 10.2147/IJN.S245743 [19] GUO S, REN Y, CHANG R, et al. Injectable Self-Healing Adhesive Chitosan Hydrogel with Antioxidative, Antibacterial, and Hemostatic Activities for Rapid Hemostasis and Skin Wound Healing[J]. ACS Applied Materials & Interfaces, 2022, 14(30): 34455-34469. [20] LIANG Y, LI Z, HUANG Y, et al. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing[J]. ACS Nano, 2021, 15(4): 7078-7093. doi: 10.1021/acsnano.1c00204 [21] QU J, ZHAO X, LIANG Y, et al. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing[J]. Biomaterials, 2018, 183: 185-199. doi: 10.1016/j.biomaterials.2018.08.044 [22] XUE C, XU X, ZHANG L, et al. Self-healing/pH-responsive/inherently antibacterial polysaccharide-based hydrogel for a photothermal strengthened wound dressing[J]. Colloids and Surfaces B: Biointerfaces, 2022, 218: 112738. doi: 10.1016/j.colsurfb.2022.112738 [23] HILL M, TWIGG M, SHERIDAN E A, et al. Alginate/Chitosan Particle-Based Drug Delivery Systems for Pulmonary Applications[J]. Pharmaceutics, 2019, 11(8): 379. doi: 10.3390/pharmaceutics11080379 [24] CUI Z-K, KIM S, BALJON J J, et al. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering[J]. Nature Communications, 2019, 10(1): 3523. doi: 10.1038/s41467-019-11511-3 [25] 中国国家标准化管理委员会(标准制定单位). 医疗器械生物学评价: GB/T 16886.5—2022[S]. 北京: 中国标准出版社, 2022.Standardization Administration of the People’s Republic of China. Biological evaluation of medical devices: GB/T 16886.4—2022[S]. Beijing: China Standards Press, 2022(in Chinese). [26] 中国国家标准化管理委员会(标准制定单位). 医疗器械生物学评价: GB/T 16886.4—2017[S]. 北京: 中国标准出版社, 2017.Standardization Administration of the People’s Republic of China. Biological evaluation of medical devices: GB/T 16886.5—2017[S]. Beijing: China Standards Press, 2017(in Chinese). [27] TORRES P, CASTRO M, REYES M, et al. Histatins, wound healing, and cell migration[J]. Oral Diseases, 2018, 24(7): 1150-1160. doi: 10.1111/odi.12816 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 157
- HTML全文浏览量: 89
- 被引次数: 0




 下载:
下载: