Defluoridation performance of electrospun La2O3-CeO2 nanofibers
-
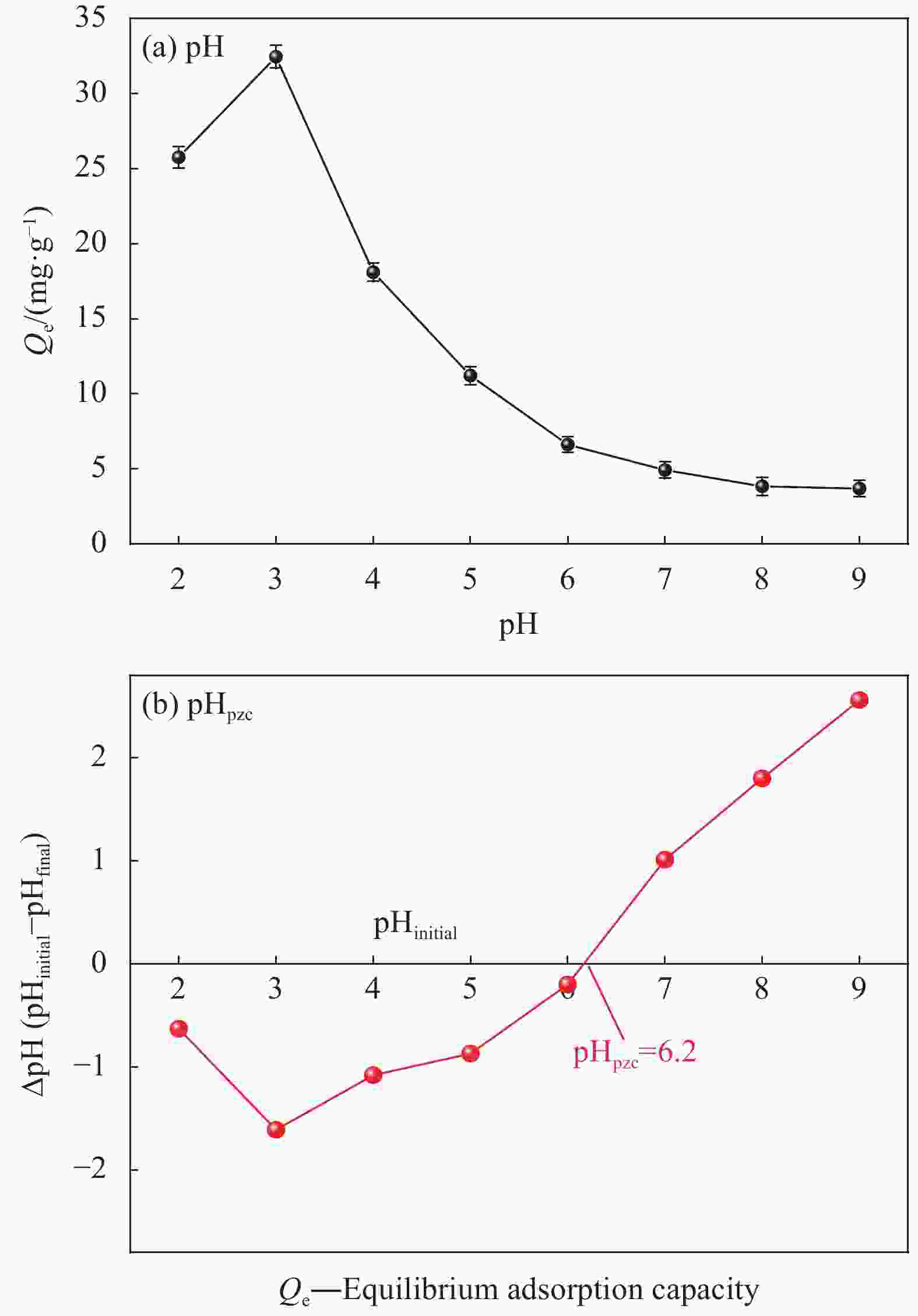
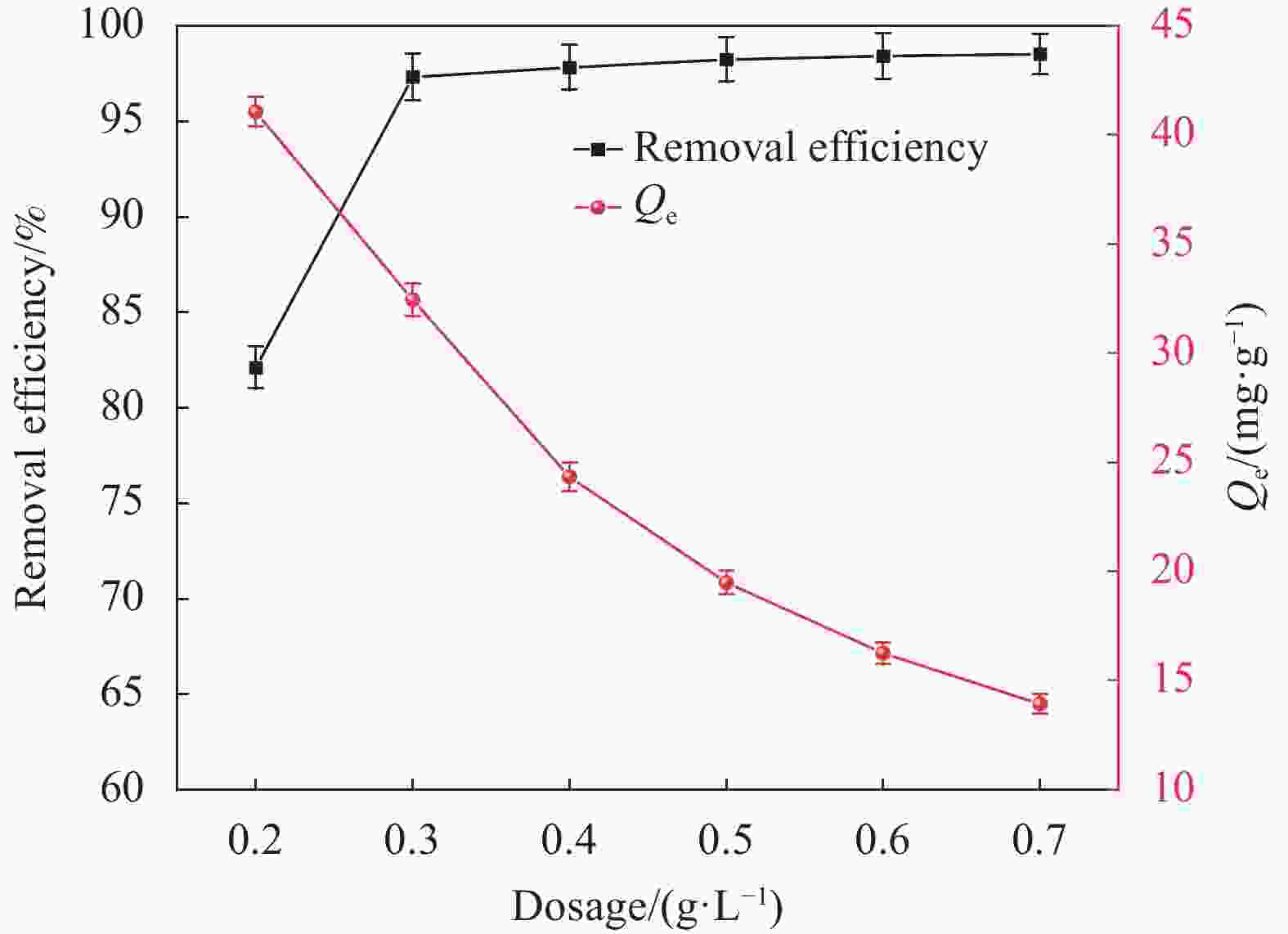
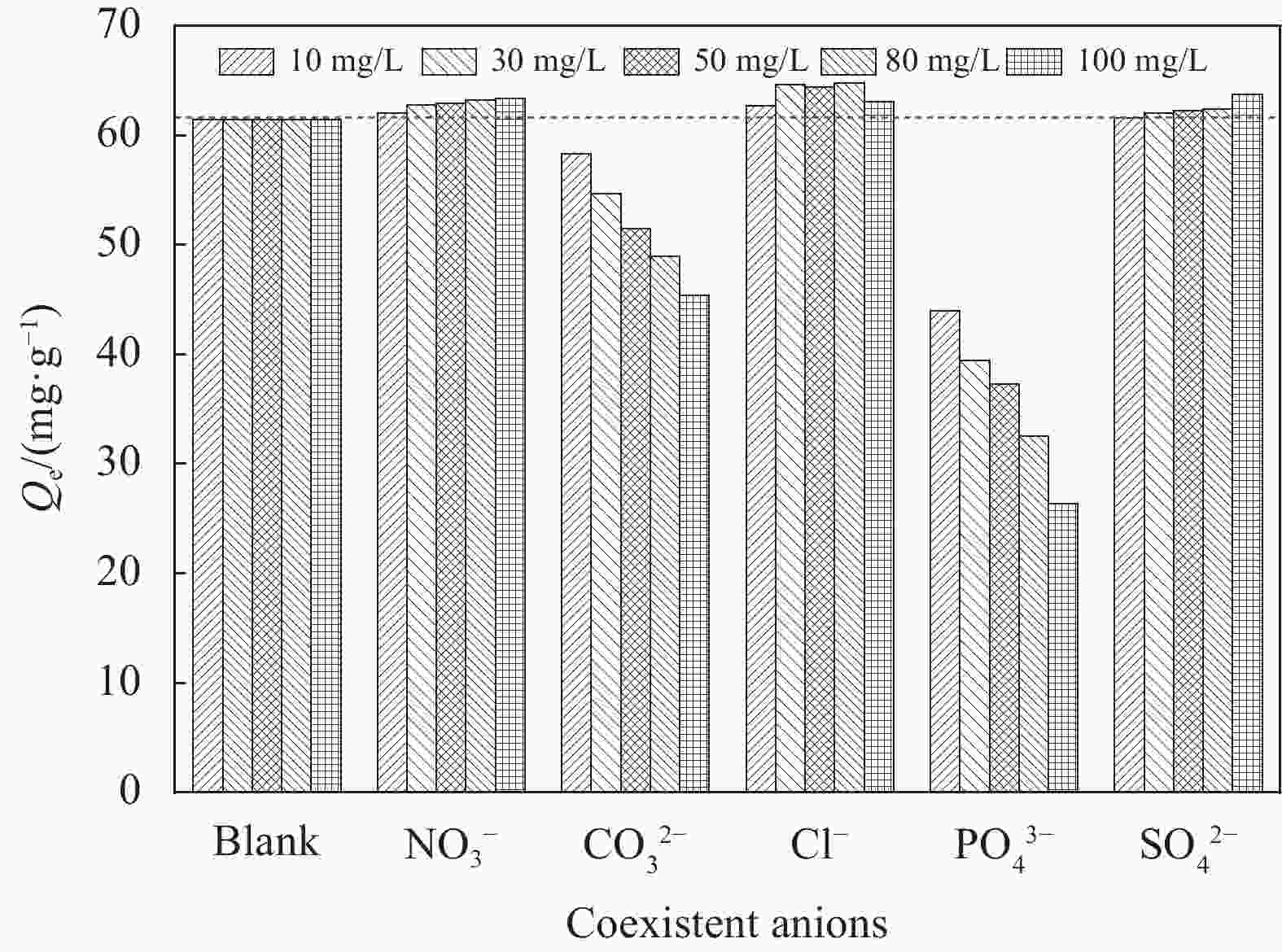
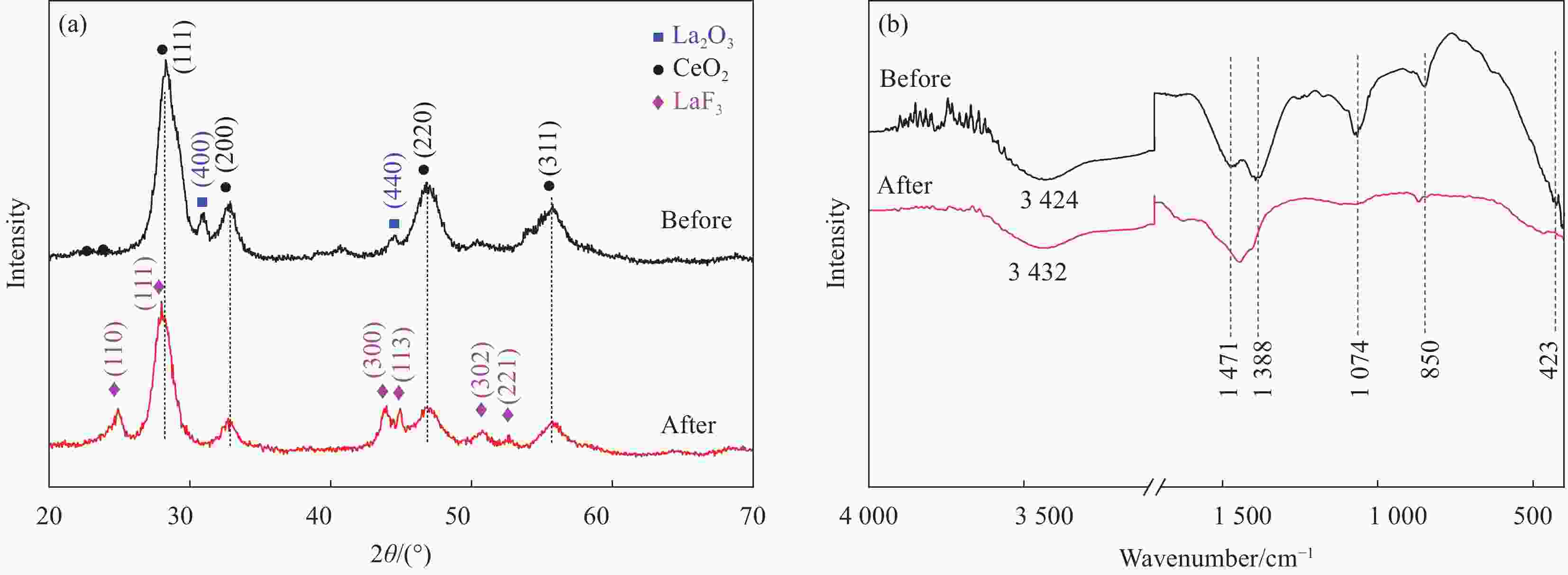
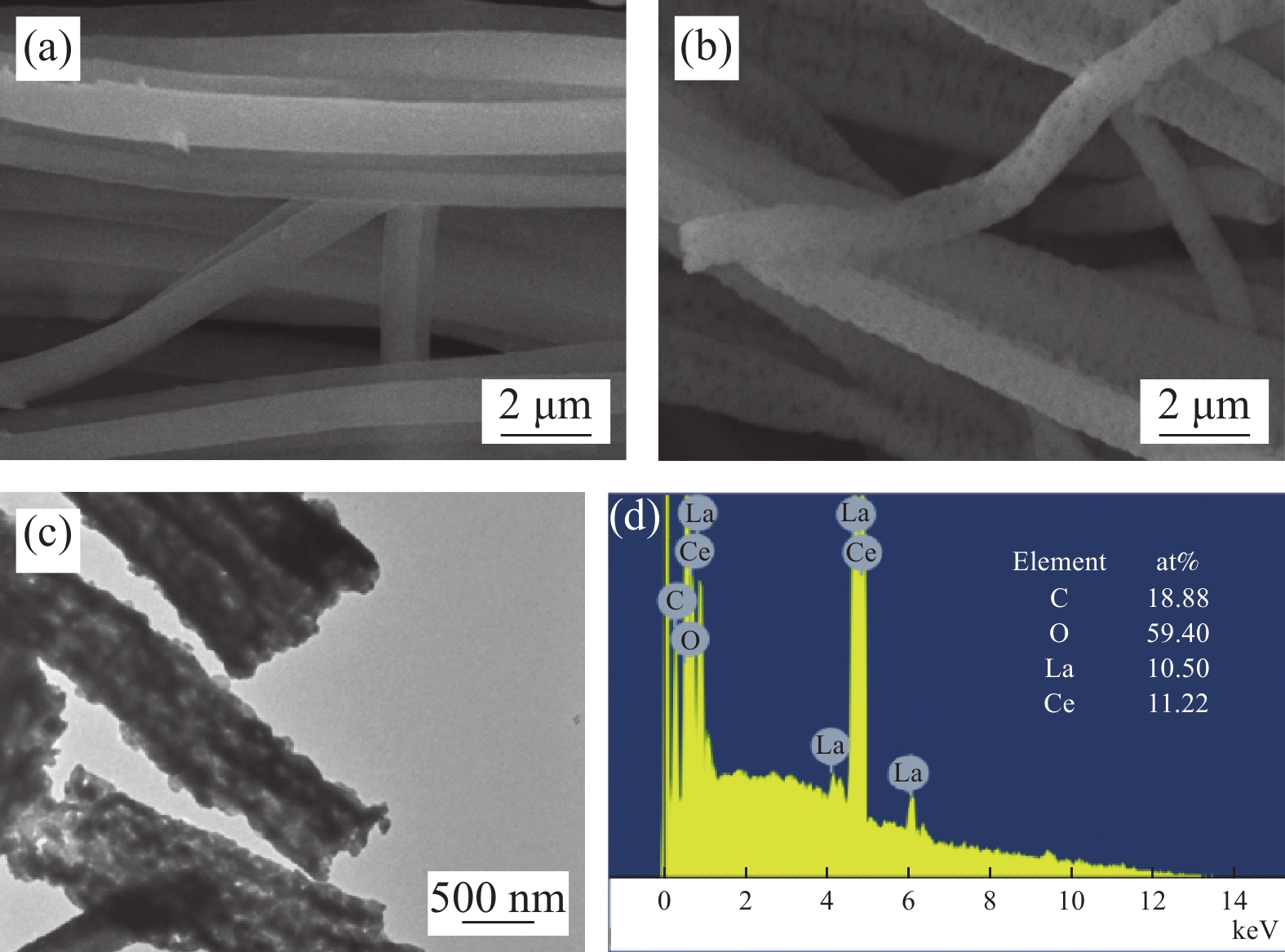
摘要: 纳米双金属氧化物作为除氟剂具有广泛的应用前景。以六水合硝酸铈和六水合硝酸镧为原料,聚丙烯腈(PAN)为模板,通过静电纺丝技术与煅烧相结合制备La2O3-CeO2纳米纤维,利用TEM、SEM-EDS、BET、FTIR和XRD对La2O3-CeO2纳米纤维的形貌和结构进行表征。探究了La2O3-CeO2纳米纤维对氟离子吸附性能,研究了pH、吸附质(F−)初始浓度、吸附时间、La2O3-CeO2纳米纤维投加量和共存阴离子等对除氟效率的影响。研究结果表明,La2O3-CeO2纳米纤维的比表面积为31.04 m2·g−1。pH为3时,La2O3-CeO2纳米纤维的除氟性能最佳。La2O3-CeO2的吸附效率随着F−初始浓度的增大而上升。准二级动力学和Langmuir等温线模型(R2>0.99)能够很好描述La2O3-CeO2纳米纤维的除氟过程,最大吸附量可达111.98 mg·g−1。热力学研究结果表明,La2O3-CeO2纳米纤维的除氟过程是自发的(ΔG0<0)、熵增(ΔS0=56.63 J·mol−1·K−1)和吸热(ΔH0=9.90 kJ·mol−1)的反应。Abstract: Nano bimetallic oxides have broad application prospects as fluoride removal agents. La2O3-CeO2 nanofibers were fabricated via electrospinning-calcination method using Ce(NO3)3·6H2O and La(NO3)3·6H2O as raw materials and polyacrylonitrile (PAN) as template. TEM, SEM-EDS, BET, FTIR and XRD were employed to verify the morphology and structure of La2O3-CeO2 nanofibers. The defluoridation properties of La2O3-CeO2 nanofibers were discussed under batch mode, and the effects of adsorbate (F−) initial concentration, pH, contact time, La2O3-CeO2 nanofibers dose and coexisting anions on the defluoridation were explored. The results illustrate that the specific surface area of La2O3-CeO2 adsorbent is 31.04 m2·g−1. The La2O3-CeO2 nanofibers exhibit the best defluoridation performance at pH of 3, and the adsorption capacity of La2O3-CeO2 nanofibers climbs up with rise of the initial concentration of F−. The pseudo-second-order kinetic and Langmuir isotherm model (R2>0.99) simulate the defluoridation process of La2O3-CeO2 nanofibers better, and the maximum uptake of F− by La2O3-CeO2 nanofibers is 111.98 mg·g−1 at 45℃. Thermodynamic studies suggest that the defluoridation process of La2O3-CeO2 nanofibers is a spontaneous (ΔG0<0), entropy increase (ΔS0=56.63 J·mol−1·K−1) and endothermic (ΔH0=9.90 kJ·mol−1) process.
-
Key words:
- CeO2 /
- La2O3 /
- fluoride /
- adsorption /
- nanofibers /
- nano bimetallic oxides
-
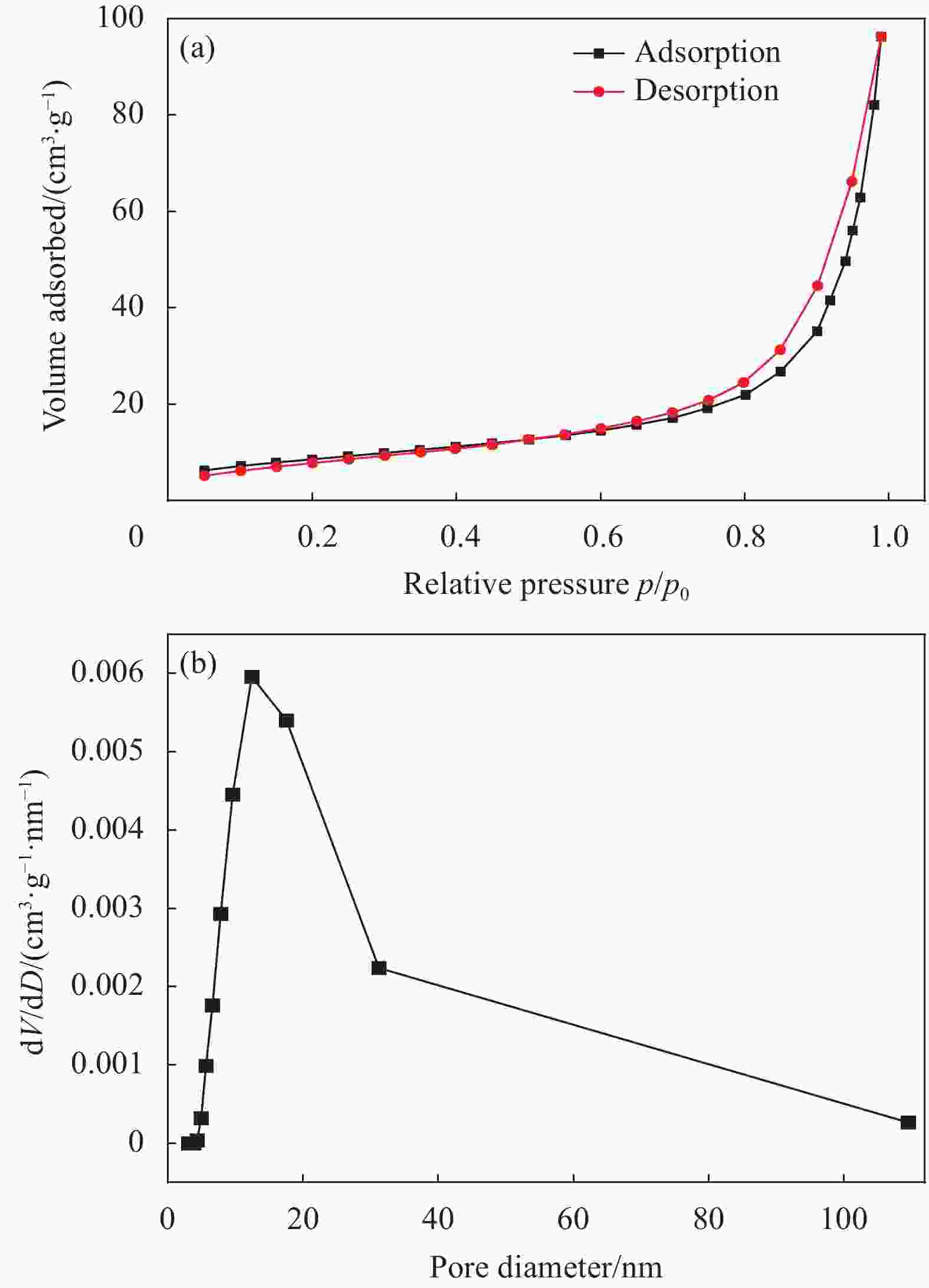
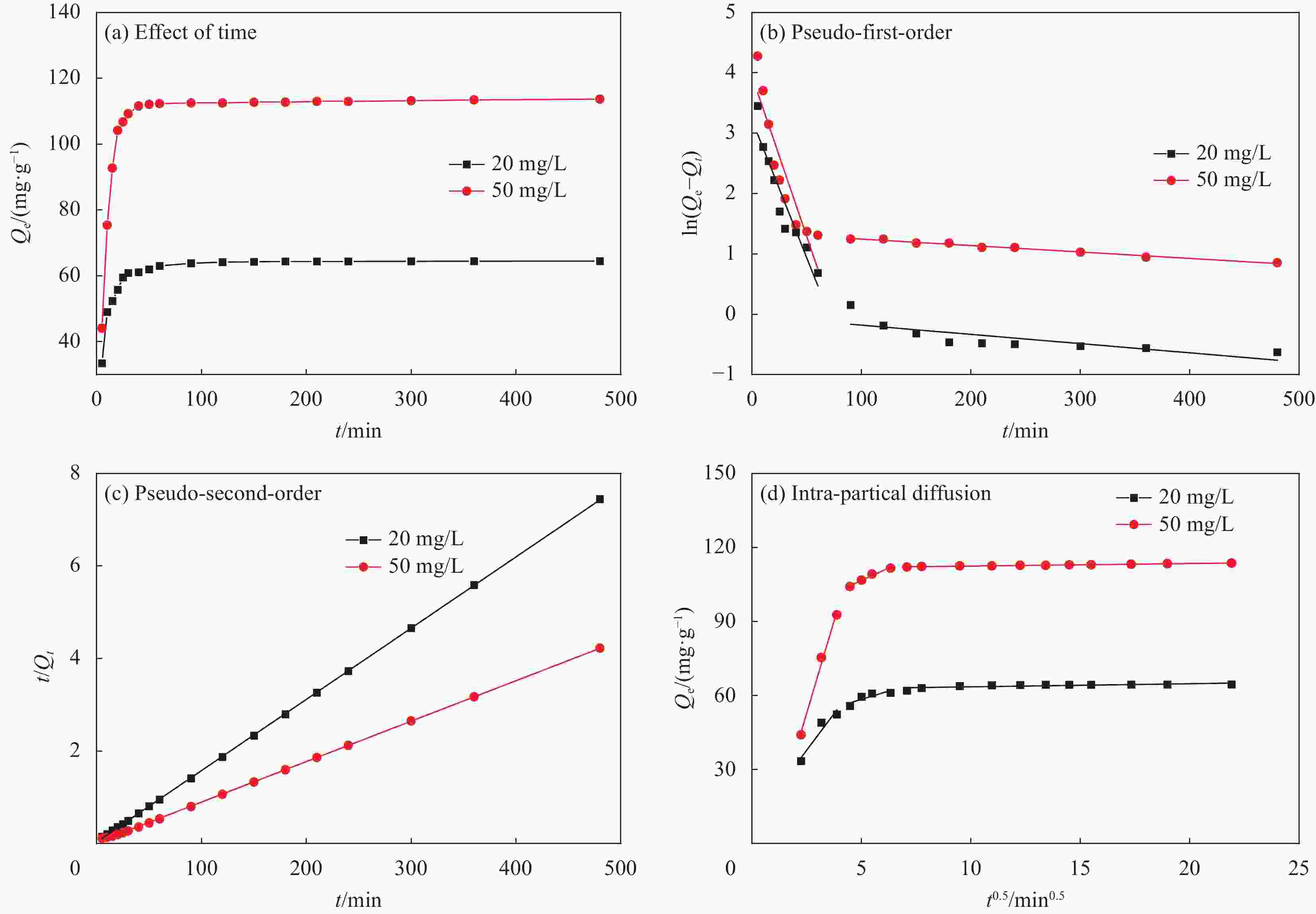
表 1 La2O3-CeO2纳米纤维的吸附动力学拟合参数(T=25℃)
Table 1. Adsorption kinetics fitting parameters of La2O3-CeO2 nanofibers (T=25℃)
Model parameters C0/(mg·L−1) 20 50 Pseudo-first-order k1×102 1.626 1.640 Qe 12.33 33.06 R2 0.8888 0.8268 Pseudo-second-order k2×102 0.532 0.147 Qe 64.98 114.42 R2 0.9999 0.9999 Weber-Morris model kid1 11.812 29.903 R2 0.8463 0.9844 kid2 2.6920 4.0108 R2 0.6032 0.9652 kid3 0.5101 0.9792 R2 0.1105 0.1113 Notes: k1―Pseudo-first-order kinetic constant; k2―Pseudo-second-order kinetic constant; kid―Intra-particle diffusion rate constant; R2—Fit coefficient; C0—Solution F− concentration before adsorption. 表 2 La2O3-CeO2纳米纤维的吸附等温线拟合参数
Table 2. Adsorption isotherm fitting parameters of La2O3-CeO2 nanofibers
T/℃ Langmuir Freundlich b/(L·mg−1) Qm/(mg·g−1) R2 kF/(L1/n·mg1−1/n·g−1) n R2 25 0.3944 93.02 0.9999 48.37 0.2218 0.7012 35 0.6636 101.32 0.9966 53.37 0.2187 0.6953 45 0.6439 111.98 0.9990 62.84 0.2018 0.5756 Notes: Qm―Maximum adsorption capacity; b, kF, n―Isotherm constants. 表 3 La2O3-CeO2纳米纤维与其他相关吸附剂除氟性能的比较
Table 3. Comparison of defluoridation performance of other related adsorbents with La2O3-CeO2 nanofibers
Adsorbents pH Qm/(mg·g−1) Ref. Lanthanum modified mesoporous alumina (La/MA) 6 26.45 [25] Cerium modified mesoporous alumina (Ce/MA) 6 13.06 [25] La-Ce-Fe3O4 3 19.78(45℃) [26] La2O3-CeO2/laterite − 58.02(RT) [15] Bx-Ce-La@500 3 88.13(25℃) [27] Ce-AlOOH 3 62.77(30℃) [28] ZCPC films 7 12.88(30℃) [29] ZLPC films 7 11.57(30℃) [29] La-Al-Fe trioxide composite 8.25 28.06(25℃) [30] CeO2 micro-nanofibers 3 21.45(35℃) [14] La2O3-CeO2 nanofibers 3 111.98(45℃) This sthdy Note: RT—Room temperature; ZCPC—Zirconium-cerium; ZLPC—Zirconium-lanthanum. 表 4 La2O3-CeO2纳米纤维吸附F−的热力学常数
Table 4. Thermodynamic parameters of defluoridation by La2O3-CeO2 nanofibers
T/℃ lnKD ΔG0
/(kJ·mol−1)ΔH0
/(kJ·mol−1)ΔS0
/(J·mol−1·K−1)15 4.77 −11.43 9.90 56.63 25 5.22 −12.94 35 5.59 −14.32 45 6.23 −16.49 Note: ΔG0, ΔH0 and ΔS0—Gibbs free energy change, the enthalpy change and the entropy change, respectively. -
[1] WAN K, HUANG L, YAN J, et al. Removal of fluoride from industrial wastewater by using different adsorbents: A review[J]. Science of the Total Environment,2021,773:145535. doi: 10.1016/j.scitotenv.2021.145535 [2] 张海阳, 高柏, 樊骅, 等. XRD和FTIR对Ce/γ-Al2O3除氟除砷的机理研究[J]. 光谱学与光谱分析, 2020, 40(9):2869-2874.ZHANG H Y, GAO B, FAN H, et al. Mechanism of fluoride and arsenic removal by Ce/γ-Al2O3 based on XRD and FTIR[J]. Spectroscopy and Spectral Analysis,2020,40(9):2869-2874(in Chinese). [3] HE X, LI P, WU J, et al. Poor groundwater quality and high potential health risks in the Datong Basin, Northern China: Research from published data[J]. Environmental Geochemistry and Health,2021,43:791-812. doi: 10.1007/s10653-020-00520-7 [4] JEIHANIPOUR A, SHEN J J, ABBT-BRAUN G, et al. Seasonal variation of organic matter characteristics and fluoride concentration in the Maji ya Chai River (Tanzania): Impact on treatability by nanofiltration/reverse osmosis[J]. Science of the Total Environment,2018,637:1209-1220. [5] DUBEY S, AGRAWAL M, GUPTA A B. Advances in coagulation technique for treatment of fluoride-contaminated water: A critical review[J]. Review Chemical Engineering,2018,35(1):109-137. doi: 10.1515/revce-2017-0043 [6] RMA C, SPA C, DVBB C, et al. Preparation of polyvinylidene fluoride blend anion exchange membranes via non-solvent induced phase inversion for desalination and fluoride removal[J]. Desalination,2018,445:85-94. doi: 10.1016/j.desal.2018.07.032 [7] FAROOQI A, MASUDA H, KUSAK A E M, et al. Distribution of highly arsenic and fluoride contaminated groundwater from east punjab, pakistan, and the controlling role of anthropogenic pollutants in the natural hydrological cycle[J]. Geochemical Journal,2007,41(4):213-234. doi: 10.2343/geochemj.41.213 [8] ZHANG Y X, JIA Y. Fluoride adsorption on manganese carbonate: Ion-exchange based on the surface carbonate-like groups and hydroxyl groups[J]. Journal of Colloid & Interface Science,2017,510:407-417. [9] TANDEKAR S, SARAVANAN D, JUGADE R. Zirconia-chitosan beads as highly efficient adsorbent for defluoridation of water[J]. Indian Journal of Chemistry,2020,59A:1067-1075. [10] ZHANG J, CHEN N, SU P, et al. Fluoride removal from aqueous solution by zirconium-chitosan/graphene oxide membrane[J]. Reactive and Functional Polymers,2017,114:127-135. doi: 10.1016/j.reactfunctpolym.2017.03.008 [11] CHIGONDO M, KAMDEM P H, BHAUMIK M, et al. Hydrous CeO2-Fe3O4 decorated polyaniline fibers nanocomposite for effective defluoridation of drinking water[J]. Journal of Colloid and Interface Science,2018,532:500-516. doi: 10.1016/j.jcis.2018.07.134 [12] KONG L, TIAN Y, PANG Z, et al. Synchronous phosphate and fluoride removal from water by 3D rice-like lanthanum-doped La@MgAl nanocomposites[J]. Chemical Engineering Journal,2019,71:893-902. [13] ZHANG Y, QIAN Y, LI W, et al. Fluoride uptake by three lanthanum based nanomaterials: behavior and mechanism dependent upon lanthanum species[J]. Science of the Total Environment,2019,683:609-616. doi: 10.1016/j.scitotenv.2019.05.185 [14] 简绍菊, 程意婷, 洪慧芳, 等. 静电纺丝法制备CeO2微纳米纤维及其除氟性能[J]. 人工晶体学报, 2022, 51(2):316-323.JIAN S J, CHENG Y T, HONG H F, et al. Fabrication of CeO2 micro-nanofibers by electrospinning and its fluoride removal performance[J]. Journal of Synthetic Crystals,2022,51(2):316-323(in Chinese). [15] LIM D T, TUYEN T N, NHIEM D N, et al. Fluoride and arsenite removal by adsorption on La2O3-CeO2/laterite[J]. Journal of Nanomaterials,2021,2021:1-13. [16] 王智辉, 徐开蒙, 张钰禄, 等. LiCl/DMAc溶剂中活化处理对不同类型纤维素静电纺丝效果的影响[J]. 林业工程学报, 2020, 5(4):108-113.WANG Z H, XU K M, ZHANG Y L, et al. Study on electrospin performance of different types of cellulose by activation in the solvent of LiCl/DMAc[J]. Journal of Forestry Engineering,2020,5(4):108-113(in Chinese). [17] JIAN S, CHENG Y, MA X, et al. Excellent fluoride removal performance by electrospun La-Mn bimetal oxide nanofibers[J]. New Journal of Chemistry,2022,46:490-497. doi: 10.1039/D1NJ04976C [18] HU J, WU D, RA R, et al. Adsorption kinetics of fluoride on bone char and its regeneration[J]. Environment Protection Engineering,2017,43:93-112. [19] 韦冬芳, 韦仲华, 金城凤鹤, 等. 竹炭陶的制备及其气体吸附和调湿性能[J]. 林业工程学报, 2020, 5(1):109-113.WEI D F, WEI Z H, KANESHIRO H, et al. Preparation of bamboo charcoal pottery and its gas adsorption and humidity regulation performance[J]. Journal of Forestry Engineering,2020,5(1):109-113(in Chinese). [20] 冯江涛, 王睎, 赵旭阳, 等. 改性聚吡咯材料去除水中氟离子的性能[J]. 化工进展, 2021, 40(7):4036-4046. doi: 10.16085/j.issn.1000-6613.2020-1551FENG J T, WANG X, ZHAO X Y, et al. Removal of fluoride from water by modified polypyrrole[J]. Chemical Industry and Engineering Progress,2021,40(7):4036-4046(in Chinese). doi: 10.16085/j.issn.1000-6613.2020-1551 [21] YAO G, ZHU X, WANG M, et al. Controlled fabrication of the biomass cellulose-CeO2 nanocomposite membrane as efficient and recyclable adsorbents for fluoride removal[J]. Industrial & Engineering Chemistry Research,2021,60:5914-5923. [22] LI W, ZHANG T, LV L, et al. Room-temperature synthesis of MIL-100(Fe) and its adsorption performance for fluoride removal from water[J]. Colloids and Surfaces A Physicochemical and Engineering Aspects,2021,624:126791. doi: 10.1016/j.colsurfa.2021.126791 [23] TANG D, ZHANG G. Efficient removal of fluoride by hierarchical Ce-Fe bimetal oxides adsorbent: Thermodynamics, kinetics and mechanism[J]. Chemical Engineering Journal,2016,283:721-729. doi: 10.1016/j.cej.2015.08.019 [24] 王姿轮, 胡传双, 涂登云, 等. 油茶果壳活性炭的制备及其吸附性能[J]. 林业工程学报, 2020, 5(5):96-102.WANG Z L, HU C S, TU D Y, et al. Preparation and adsorption property of activated carbon made from Camellia olerea shells[J]. Journal of Forestry Engineering,2020,5(5):96-102(in Chinese). [25] HE Y, ZHANG L, AN X, et al. Enhanced fluoride removal from water by rare earth (La and Ce) modified alumina: Adsorption isotherms, kinetics, thermodynamics and mechanism[J]. Science of the Total Environment,2019,688:184-198. doi: 10.1016/j.scitotenv.2019.06.175 [26] 赵瑨云, 苏丽鳗, 徐婕, 等. Ce-La@Fe3O4吸附剂制备及其除氟性能研究[J]. 人工晶体学报, 2020, 49(9):1705-1710. doi: 10.3969/j.issn.1000-985X.2020.09.023ZHAO J Y, SU L M, XU J, et al. Fabrication of Ce-La@Fe3O4 adsorbents and its property for defluorination[J]. Journal of Synthetic Crystals,2020,49(9):1705-1710(in Chinese). doi: 10.3969/j.issn.1000-985X.2020.09.023 [27] ALHASSAN S I, WANG H, HE Y, et al. Fluoride remediation from on-site wastewater using optimized bauxite nanocomposite (Bx-Ce-La@500): Synthesis maximization, and mechanism of F− removal[J]. Journal of Hazardous Materials,2022,430:128401. doi: 10.1016/j.jhazmat.2022.128401 [28] TAO W, ZHONG H, PAN X, et al. Removal of fluoride from wastewater solution using Ce-AlOOH with oxalic acid as modification[J]. Journal of Hazardous Materials,2020,384:121373. doi: 10.1016/j.jhazmat.2019.121373 [29] KIRAN S M, BHANDARI R, NEHRA A, et al. Zirconium-cerium and zirconium-lanthanum complexed polyvinyl alcohol films for efficient fluoride removal from aqueous solution[J]. Journal of Dispersion Science and Technology,2020,42(10):1-16. [30] GASPAROTTO J M, ROTH D, PERILLI A, et al. A novel Fe-Al-La trioxide composite: Synthesis, characterization, and application for fluoride ions removal from the water supply[J]. Journal of Environmental Chemical Engineering,2021,9:106350. doi: 10.1016/j.jece.2021.106350 [31] 蒋珊, 周淼, 邓稳, 等. 木质纤维纸基吸附材料及其重金属离子吸附性能[J]. 林业工程学报, 2020, 5(3):101-107.JIANG S, ZHOU M, DENG W, et al. High-wet-strength paper-based lignocellulosic adsorbents and its heavy metal ion adsorption properties[J]. Journal of Forestry Engineering,2020,5(3):101-107(in Chinese). [32] KHATUN H, MOU S S, MORTUZA A A, et al. Investigation on LaF3/porous silicon system for photonic application[J]. Chinese Optics Letters,2010,3:306-308. [33] 马雪慧, 赵彦保, 吴志申. 表面修饰中空LaF3纳米微粒的制备及其摩擦学性能[J]. 物理化学学报, 2008, 24(11):2037-2041. doi: 10.3866/PKU.WHXB20081117MA X H, ZHAO Y B, WU Z S. Preparation of surface modified hollow LaF3 nanoparticles and their tribological performances[J]. Acta Physico-Chimica Sinica,2008,24(11):2037-2041(in Chinese). doi: 10.3866/PKU.WHXB20081117 [34] DONG C, WU X, GAO Z, et al. A novel and efficient metal oxide fluoride absorbent for drinking water safety and sustainable development[J]. Sustainability,2021,13(2):883. doi: 10.3390/su13020883 [35] XIANG W, ZHANG G, ZHANG Y, et al. Synthesis and characterization of cotton-like Ca-Al-La composite as an adsorbent for fluoride removal[J]. Chemical Engineering Journal,2014,250:423-430. doi: 10.1016/j.cej.2014.03.118 [36] THATHSARA T, COORAY P, MUDIYANSELAGE T K, et al. A novel Fe-La-Ce tri-metallic composite for the removal of fluoride ions from aqueous media[J]. Journal of Environmental Management,2018,207:387-395. [37] REGMI C, ASHITIANI S, SOFTER Z, et al. CeO2-blended cellulose triacetate mixed-matrix membranes for selective CO2 separation[J]. Membranes,2021,11(8):632. doi: 10.3390/membranes11080632 [38] MA H Y, ZHOU D, LIU J M, et al. Preparation and spectral characteristics of SO42−/CeO2-TiO2 photocatalyst[J]. Spectroscopy and Spectral Analysis,2017,37(10):3315-3320. -





 下载:
下载: