Preparation and disproportionation properties of NaAlCl4/ZSM-5@γ-Al2O3 core-shell catalyst
-
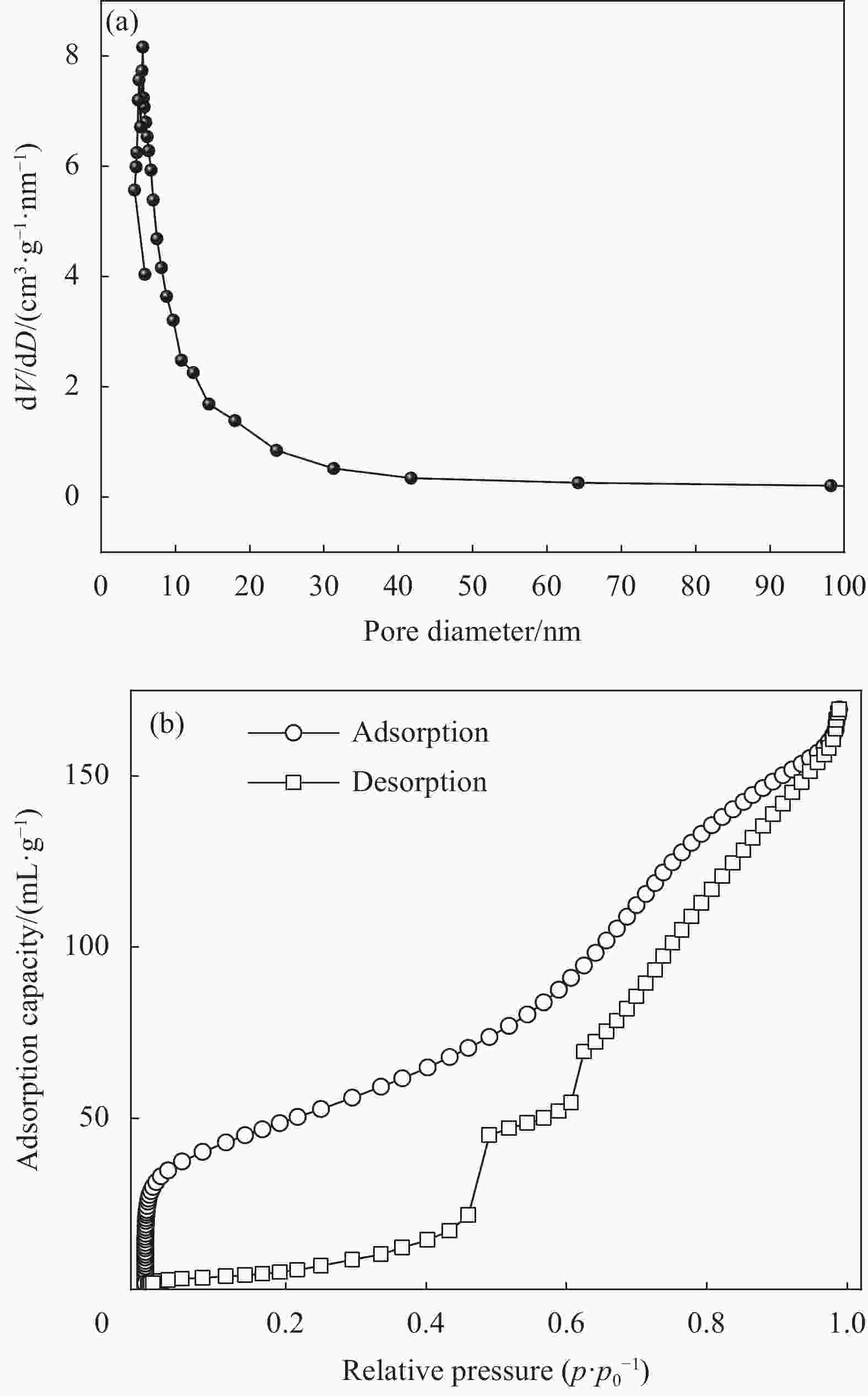
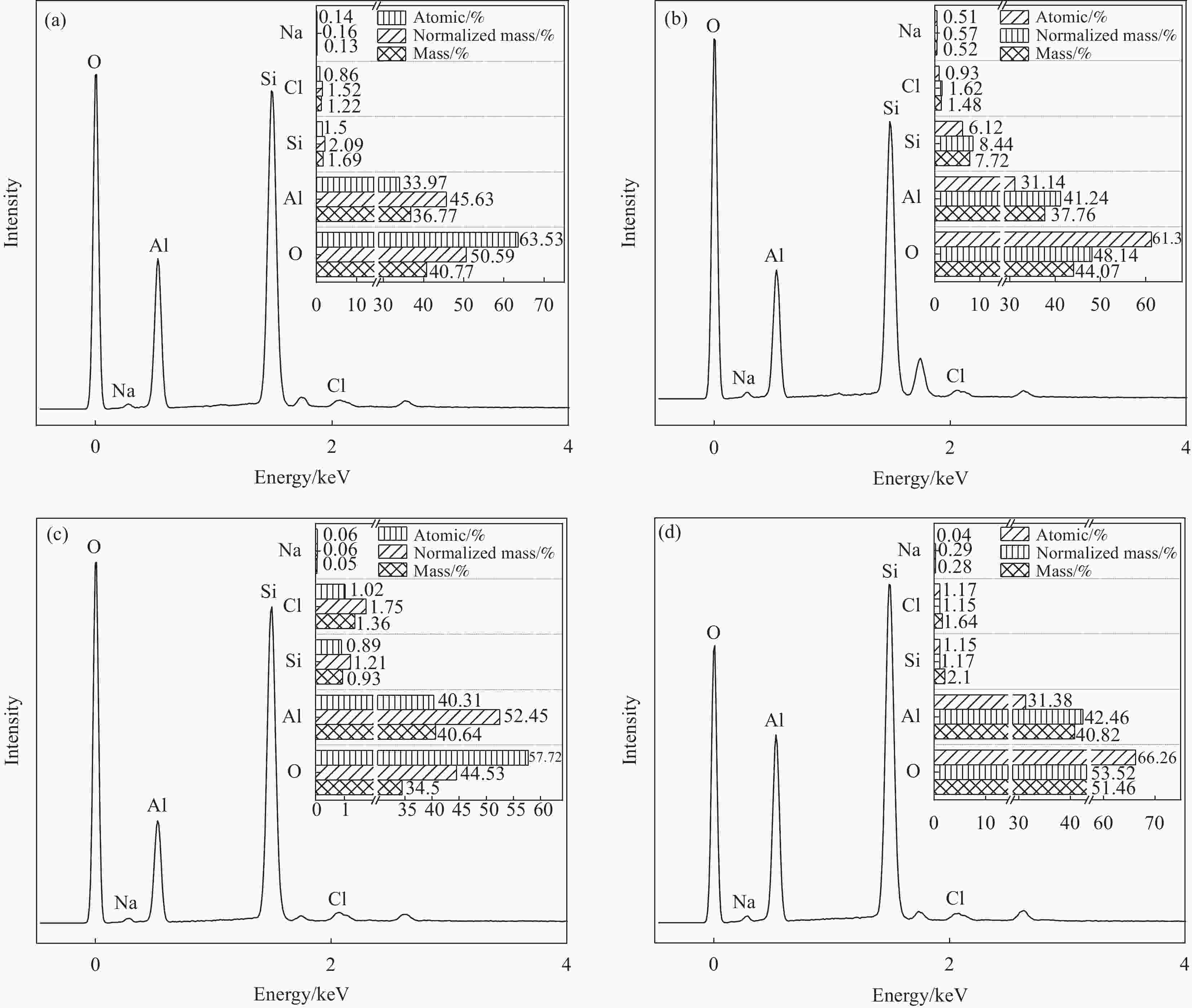
摘要: 针对氯硅烷残留物的危害性和资源化利用,通过歧化反应将副产物制备成经济效益更高的二甲基二氯硅烷。由田菁胶为粘结剂,以γ-Al2O3为壳,对ZSM-5分子筛表面进行修饰,构筑ZSM-5@γ-Al2O3核壳载体;然后,通过高温浸渍负载法,将NaAlCl4负载在ZSM-5@γ-Al2O3核壳载体表面。综合研究了不同Si/Al摩尔比的ZSM-5分子筛、不同NaAlCl4负载比例和AlCl3溶液浸渍时间对歧化制备反应二甲基二氯硅烷的影响。采用XRD、SEM、SEM-EDS、BET和FTIR对样品进行表征分析,结果表明,当温度为200℃,硅铝摩尔比为50,复盐NaAlCl4比例为8wt%,AlCl3浸渍时间为3 h时,催化剂活性达到最佳,产率为71.81%。通过NaAlCl4复盐负载ZSM-5@γ-Al2O3核壳表面,改善单一催化成分性能不稳定,提高催化效率,再分配氯硅烷副产品甲基三氯硅烷(M1)和三甲基氯硅烷(M3),得到商业价值较高的二甲基二氯硅烷(M2) ,实现变废为宝。Abstract: In view of the harmness and resource utilization of chlorosilane residues, the by-products are prepared into dimethyldichlorosilane with higher economic benefit through disproportionation reaction. The ZSM-5@γ-Al2O3 core-shell carrier was constructed by modifying the surface of ZSM-5 molecular sieve with Tianjing gum as the binder and γ-Al2O3 as the shell. NaAlCl4 was loaded on the surface of ZSM-5@γ-Al2O3 core-shell carrier by high-temperature impregnation loading method. The effect of ZSM-5 molecular sieve with different Si/Al molar ratios on the disproportionation preparation of dimethyldichlorosilane, the effect of different NaAlCl4 loading ratios and the impregnation time of AlCl3 solution on the reaction of disproportionation preparation of dimethyldichlorosilane were investigated comprehensively, and the samples were characterized by XRD, SEM, SEM-EDS, BET and FTIR. The results show that the catalyst activity reach the optimum with 71.81% yield when the temperature is 200°C, the silica-alumina molar ratio is 50, the compound salt NaAlCl4 ratio is 8wt%, and the AlCl3 impregnation time is 3 h. The NaAlCl4 compound salt loading on the surface of the ZSM-5@γ-Al2O3 core-shell catalyst shows the improved catalytic efficiency and enhanced performance instability compared with single catalytic component. Further redistribution chlorosilane by-products methyl trichlorosilane (M1) and trimethylchlorosilane (M3) synthesis can obtain high commercial value dimethyl dichlorosilane (M2), so as to realize the transformation of waste into treasure.
-
Key words:
- NaAlCl4 /
- ZSM-5 /
- γ-Al2O3 /
- core-shell /
- disproportionation /
- dimethyldichlorosilane /
- catalyst
-
图 2 NaAlCl4/ZSM-5@γ-Al2O3催化剂负载不同质量比例的NaAlCl4复盐对M2产率的影响(Na/Al摩尔比1,AlCl3溶液的浸渍时间2 h,M1/M3的体积比为1vol%,催化剂的用量为0.6 g)
Figure 2. Effect of NaAlCl4/ZSM-5@γ-Al2O3 catalyst loaded with different mass ratios of NaAlCl4 double salt on M2 yield (Molar ratio of Na/Al is 1, the impregnation time of AlCl3 solution is 2 h, the volume ratio of M1/M3 is 1vol% and the amount of catalysts is 0.6 g)
图 3 不同AlCl3溶液浸渍时间的NaAlCl4/ZSM-5@γ-Al2O3催化剂对M2产率的影响(Na/Al摩尔比1,复盐NaAlCl4负载比例8wt%,M1/M3的体积比为1vol%,催化剂的用量为0.6 g)
Figure 3. Effect of NaAlCl4/ZSM-5@γ-Al2O3 catalysts on the yield of M2 with different impregnation time of AlCl3 solution (Molar ratio of Na/Al is 1, the loading ratio of compound salt NaAlCl4 is 8wt%, the volume ratio of M1/M3 is 1vol%, and the amount of catalyst is 0.6 g)
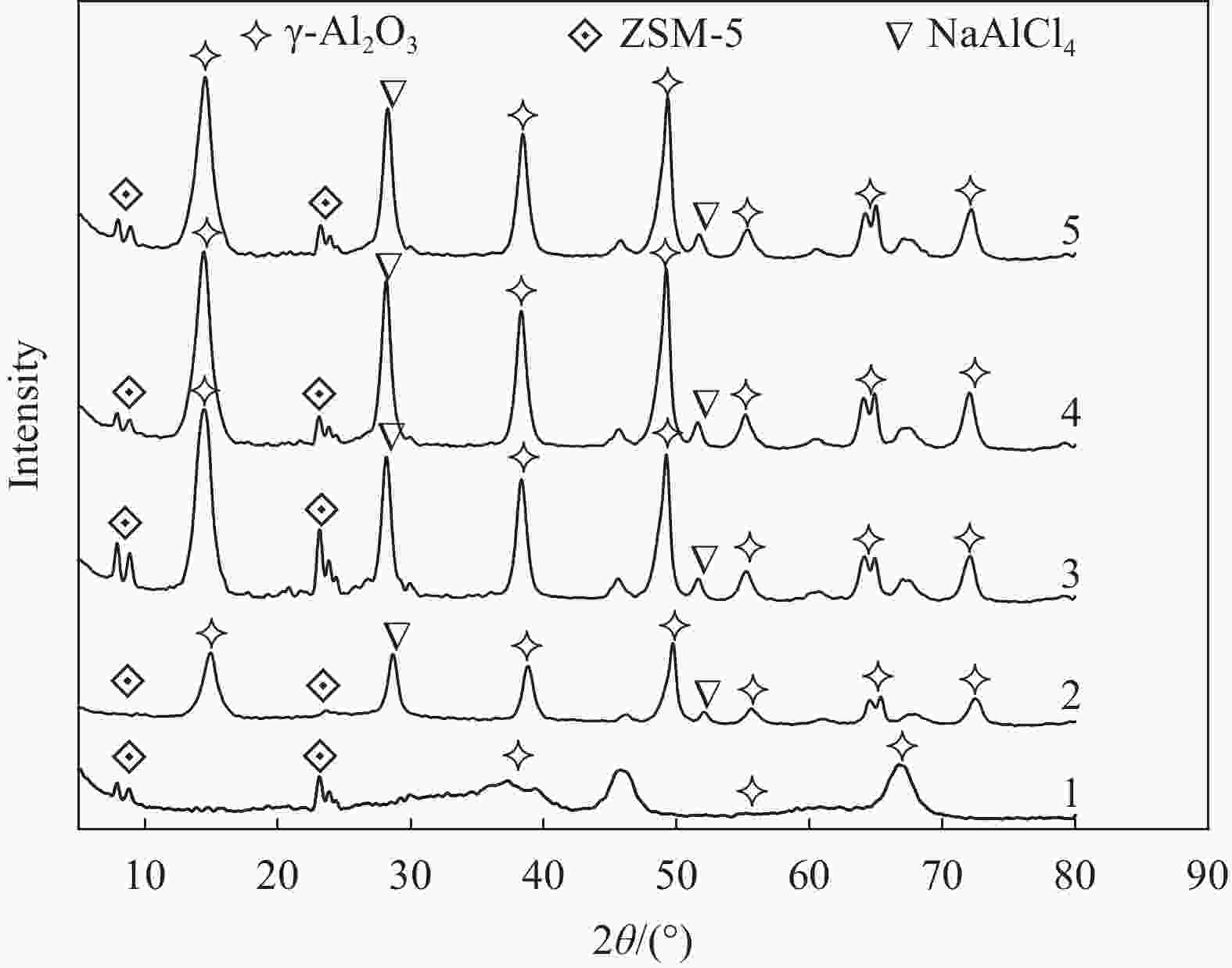
图 4 ZSM-5@γ-Al2O3载体及NaAlCl4/ZSM-5@γ-Al2O3催化剂负载不同比例NaAlCl4复盐的XRD图谱
Figure 4. XRD patterns of ZSM-5@γ-Al2O3 support and NaAlCl4/ZSM-5@γ -Al2O3 catalysts supported with different proportions of NaAlCl4 compound salts
1—ZSM-5@γ-Al2O3; 2—4wt%NaAlCl4/ZSM-5@γ-Al2O3; 3—8wt%NaAlCl4/ZSM-5@γ-Al2O3; 4—12wt%NaAlCl4/ZSM-5@γ-Al2O3; 5—16wt%NaAlCl4/ZSM-5@γ-Al2O3
-
[1] SETIADJI S, SUMIYANTO E, FITRILAWATI, et al. Synthesis of polydimethylsiloxane and its monomer from hydrolysis of dichlorodimethylsilane[J]. Key Engineering Materials,2020,860:234-238. doi: 10.4028/www.scientific.net/KEM.860.234 [2] PALAPRAT G, GANACHAUD F. Synthesis of polydimethylsiloxane microemulsions by self-catalyzed hydrolysis/condensation of dichlorodimethylsilane[J]. Comptes Rendus Chimie,2003,6(11-12):1385-1392. doi: 10.1016/j.crci.2003.09.002 [3] WANG Y F, LI Z, HUBER L, et al. Reducing the thermal hazard of hydrophobic silica aerogels by using dimethyldichlorosilane as modifier[J]. Journal of Sol-Gel Science and Technology,2020,93(1):111-122. doi: 10.1007/s10971-019-05170-5 [4] 赵建波, 张宁. 有机硅高沸物裂解歧化制备二甲基二氯硅烷的研究进展[J]. 工业催化, 2003(11):37-41.ZHAO Jianbo, ZHANG Ning. Catalytic conversion of high-boiling components in silicone to dimethyldichloro-silane[J]. Industrial Catalysis,2003(11):37-41(in Chinese). [5] ZHANG P, DUAN J H, CHEN G H, et al. Effect of bed characters on the direct synthesis of dimethyldichlorosilane in fluidized bed reactor[J]. Scientific Reports,2015,5:8827. doi: 10.1038/srep08827 [6] JIANG Y Q, CHEN W G, LIU Y J, et al. Synthesis of tri-methylchlorosilane by [BMIM]Cl−nAlCl3 ionic liquids-catalyzed redistribution between methyltrichlorosilane and low-boiling products from the direct synthesis of methylchlorosilanes[J]. Industrial & Engineering Chemistry Research,2011,50(4):1893-1898. doi: 10.1021/ie1022207 [7] LI J, NI Z B, JI Y J, et al. ZnO supported on Cu2O{1 0 0} enhances charge transfer in dimethyldichlorosilane synthesis[J]. Journal of Catalysis,2019,374:284-296. doi: 10.1016/j.jcat.2019.02.029 [8] ZHANG Y, LI J, LIU H Z, et al. Recent advances in Rochow-Müller process research: Driving to molecular catalysis and to a more sustainable silicone industry[J]. ChemCatChem,2019,11(12):2757-2779. doi: 10.1002/cctc.201900385 [9] PACHALY B, SCHINABECK A. Separation of methylchlorosilanes from high boiling residues of methylchlorosilane synthesis: USA, 5288892 A[P]. 1994-02-24. [10] WANG A L, JIANG Y Q, CHEN W G, et al. [BMIM]Cl-nAlCl3 ionic liquid-catalyzed redistribution reaction between methyltrichlorosilane and low-boiling residue to dimethyldichlorosilane[J]. Journal of Industrial and Engineering Chemistry,2012,18(1):237-242. doi: 10.1016/j.jiec.2011.11.023 [11] KOSRI C, KIATPHUENGPORN S, BUTBUREE T, et al. Selective conversion of xylose to lactic acid over metal-based Lewis acid supported on γ-Al2O3 catalysts[J]. Catalysis Today,2021,367:205-212. doi: 10.1016/j.cattod.2020.04.061 [12] BADMAEV S, SOBYANIN V. Production of hydrogen-rich gas by oxidative steam reforming of dimethoxymethane over CuO-CeO2/γ-Al2O3 catalyst[J]. Energies,2020,13(14):3684-3693. doi: 10.3390/en13143684 [13] 颜曦明, 王宝宇, 柯明, 等. La/Hβ-Al2O3复合材料改善轻汽油醚化活性[J]. 复合材料学报, 2018, 35(12):3466-3473. doi: 10.13801/j.cnki.fhclxb.20180316.005YAN Ximing, WANG Baoyu, KE Ming, et al. La/Hβ-Al3O2composite materials improve light gasoline etherification activity[J]. Acta Material Composite Sinica,2018,35(12):3466-3473(in Chinese). doi: 10.13801/j.cnki.fhclxb.20180316.005 [14] XU W Y, YANG M, LI X Y, et al. Study on the mechanism of methylchlorosilanes disproportionation catalyzed by AlCl3/(AlCl2)zz+-γ-Al2O3[J]. Russian Journal of Physical Chemistry A,2019,92(13):2634-2639. [15] XU W Y, LIU Y P, ZHOU J X, et al. Transforming Brønsted acid to lewis acid on ZSM-5 disproportionation catalyst before and after loading AlCl3[J]. Asian Journal of Chemistry,2015,27(3):1147-1152. doi: 10.14233/ajchem.2015.18493 [16] XU W Y, LI X Y, YANG M, et al. Redistribution mechanism of chloromethylsilanes catalyzed by HZSM-5 with big and small apertures[J]. Chinese Journal of Structural Chemistry,2018,37(4):543-550. doi: 10.14102/j.cnki.0254-5861.2011-1808 [17] XU W Y, YAN F, YANG S M, et al. Mechanism on the disproportionating synthesis of dichlorodimethylsilane by ZSM-5(5T)@γ-Al2O3 series core-shell catalysts[J]. Applied Organometallic Chemistry,2020,34(3):e5419. doi: 10.1002/aoc.5419 [18] LU R E, XU M W, FU B, et al. Single capillary electrospinning of magnetic core-shell nanofibers[J]. ChemistrySelect,2016,1(7):1510-1514. doi: 10.1002/slct.201600321 [19] JIANG S, DU Y, MARCELLO M, et al. Core-shell crystals of porous organic cages[J]. Angewandte Chemie International Edition,2018,57(35):11228-11232. doi: 10.1002/anie.201803244 [20] ABDALLA A, ARUDRA P, AL-KHATTAF S S. Catalytic cracking of 1-butene to propylene using modified H-ZSM-5 catalyst: A comparative study of surface modification and core-shell synthesis[J]. Applied Catalysis A: General,2017,533:109-120. doi: 10.1016/j.apcata.2017.01.003 [21] CHENG Y B, WANG Y, LI S Y, et al. Mechanism on redistribution synthesis of dichlorodimethylsilane by AlCl3/ZSM-5(3T)@γ-Al2O3 core-shell catalyst[J]. Journal of Molecular Modeling,2021,27(9):255-267. doi: 10.1007/s00894-021-04859-1 [22] XU W Y, KUANG X, YAN F, et al. Active center changed: Disproportionation mechanism for preparing dimethyldichlorosilane catalyzed by core(4T)-shell catalyst[J]. Chinese Journal of Structural Chemistry,2020,39(6):1146-1156. doi: 10.14102/j.cnki.0254-5861.2011-2538 [23] 徐文媛, 王利伟, 万欢欢, 等. NaAlCl4/ZSM-5催化甲基三氯硅烷歧化反应性能[J]. 郑州大学学报, 2015, 36(5):25-29.XU Wenyuan, WANG Liwei, WAN Huanhuan, et al. Study on the NaAlCl4/ZSM-5 catalysts by redistributing methyl-trichlorosilane[J]. Journal of Zhengzhou University,2015,36(5):25-29(in Chinese). [24] MASALSKA A, GRZECHOWIAK J R, JAROSZEWSKA K. Effect of metal-support interactions in Ni/ZSM-5+Al2O3 catalysts on the transformation of n-paraffins[J]. Topics in Catalysis,2013,56(11):981-994. doi: 10.1007/s11244-013-0062-x [25] YU H C, LI F W, HE W, et al. Synthesis of micro-mesoporous ZSM-5 zeolite with microcrystalline cellulose as co-template and catalytic cracking of polyolefin plastics[J]. RSC Advances,2020,10(37):22126-22136. doi: 10.1039/D0RA03082A [26] LI H S, HE S C, MA K, et al. Micro-mesoporous composite molecular sieves H-ZSM-5/MCM-41 for methanol dehydration to dimethyl ether: Effect of SiO2/Al2O3 ratio in H-ZSM-5[J]. Applied Catalysis A: General,2013,450:152-159. doi: 10.1016/j.apcata.2012.10.014 [27] DAUDA I B, YUSUF M, GBADAMASI S, et al. Highly selective hierarchical ZnO/ZSM-5 catalysts for propane aromatization[J]. ACS Omega,2020,5(6):2725-2733. doi: 10.1021/acsomega.9b03343 -





 下载:
下载: