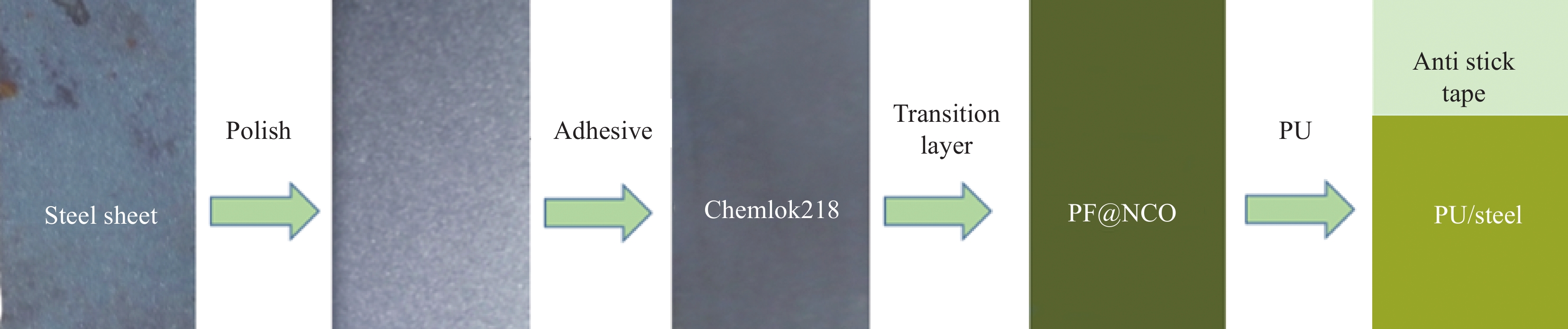
Modification of phenolic resin and its effect on adhesive properties of polyurethane/metal
-
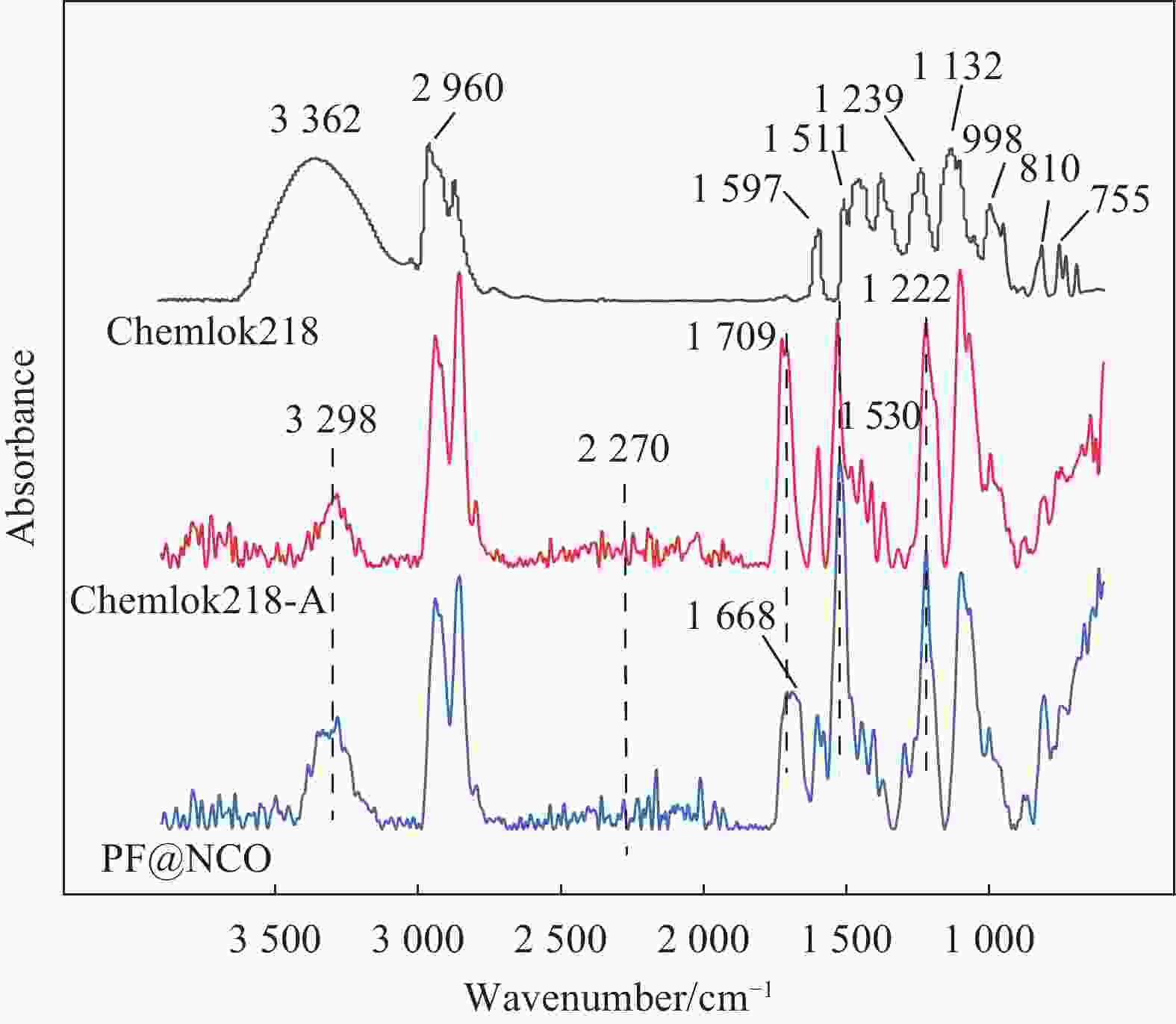
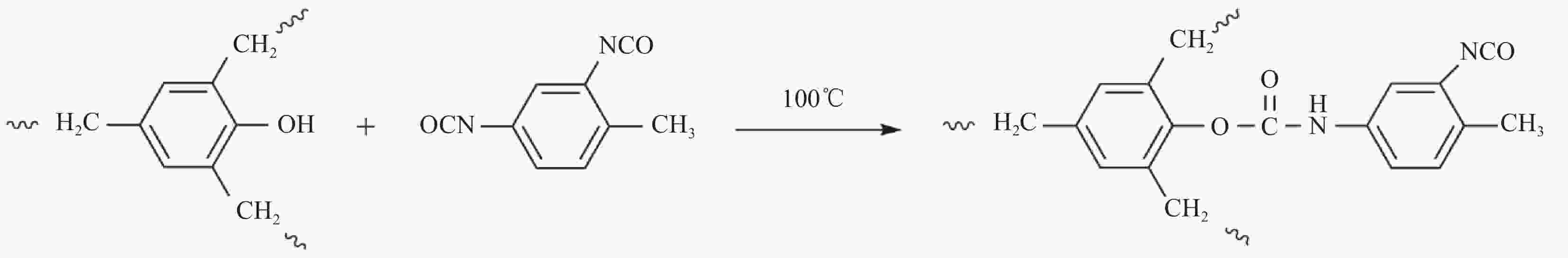
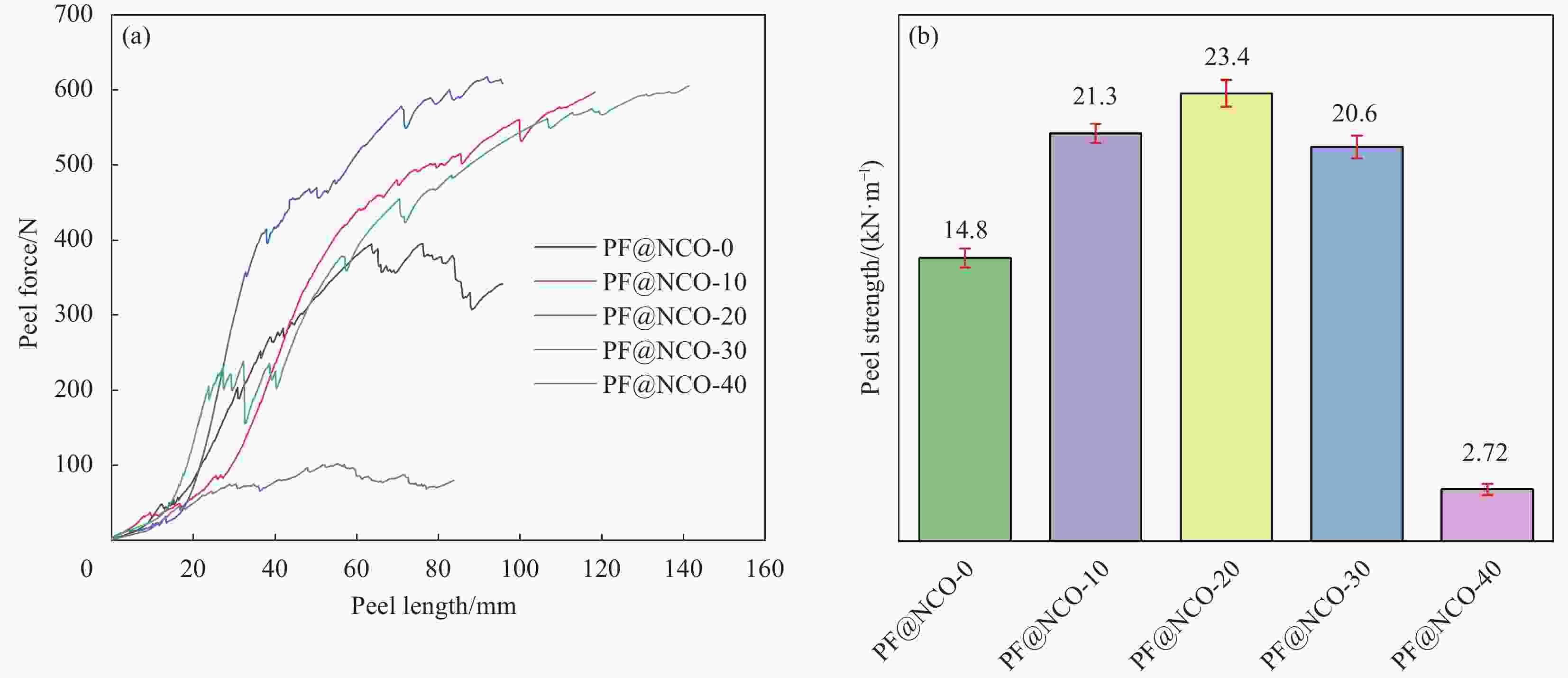
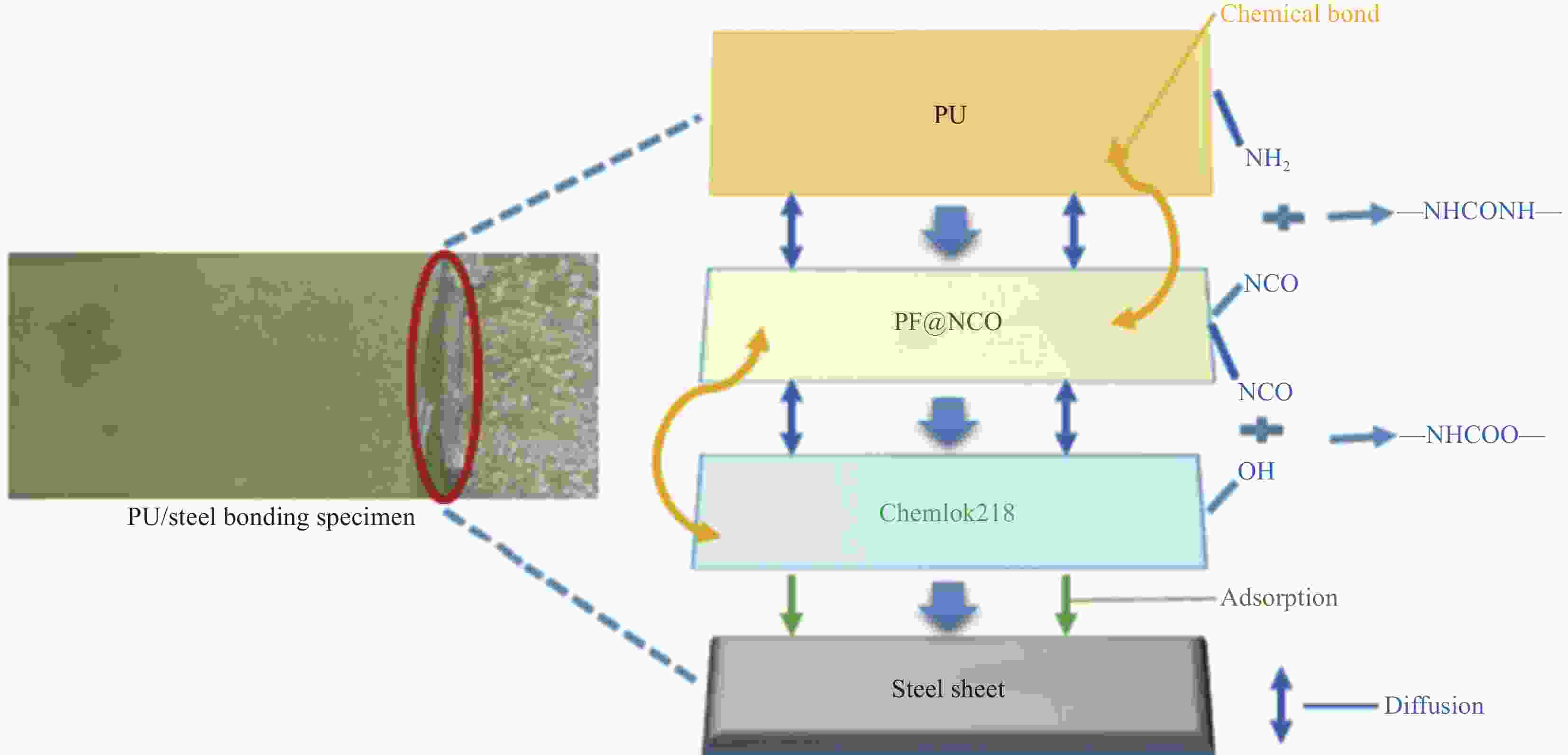
摘要: 为了解决Chemlok218为粘接剂时,室温下聚氨酯(PU)/金属粘接效果差的问题,采用一种高活性的PU改性剂,对Chemlok218中的酚醛树脂(PF)进行改性获得PF@NCO,在Chemlok218涂层与PU间形成一层过渡层, FTIR、TG分析表明:PU改性剂中NCO基团与PF中的羟基反应生成氨基甲酸酯基团。与Chemlok218涂层相比,PF@NCO过渡层表面能提高,与PU相容性增加。当Chemlok218与PU改性剂的质量比为80∶20时,PF@NCO-20的剥离强度达到23.4 kN·m−1,比纯Chemlok218提高了58.1%,整个PU/金属粘接试样无粘接薄弱点及缺陷。为解决室温下PU/金属的粘接强度问题提供借鉴。Abstract: To solve the problem of poor bonding effect of polyurethane (PU)/metal at room temperature when Chemlok218 was used as adhesive, a highly active PU modifier was used to modify phenolic resin (PF) in Chemlok218 to form a PF@NCO transition layer between Chemlok218 coating and PU. FTIR and TG analysis showed that the NCO group in PU modifier reacted with the hydroxyl group of PF to form carbamate group, and the surface energy of PF@NCO could be improved. The compatibility with PU had increased. When the mass ratio of Chemlok218 to PU modifier is 80∶20, the peel strength of PF@NCO-20 reaches 23.4 kN·m−1, which is 58.1% higher than that of pure Chemlok218, and the whole PU/metal bonding sample had no bonding weaknesses and defects. The purpose of this paper is to provide a reference for solving the problem of bonding strength of PU/metal at room temperature.
-
Key words:
- modification /
- phenolic resin /
- polyurethane /
- metal bonding /
- bonding property /
- Chemlok218
-
表 1 酚醛树脂(PF)@异氰酸酯基(NCO)的命名
Table 1. Naming of phenolic resin (PF)@ isocyanate group (NCO)
Sample Mass ratio of Chemlok218 :PU PF@NCO-0 100∶0 PF@NCO-10 90∶10 PF@NCO-20 80∶20 PF@NCO-30 70∶30 PF@NCO-40 60∶40 Note: PU—Polyurethane. 表 2 Chemlok218和PF@NCO的接触角与表面能
Table 2. Contact angle and surface energy of Chemlok218 and PF@NCO
Sample Contact angle θ/(°) Surface energy/(mJ·m−2) Water Ethylene glycol γS d γS p γS Chemlock218 70.1(1.2) 60.7(1.1) 5.1 12.9 18.0 PF@NCO 73.7(0.9) 55.0(1.4) 13.9 20.2 34.1 Notes: γS, γS d and γS p—Surface energy, non-polar part of surface energy and polar part of surface energy respectively. The data in parentheses refers to the standard deviation. -
[1] NIKOUKALAM M T, SIDERIS P. Resilient bridge rocking columns with polyurethane damage-resistant end segments and replaceable energy-dissipating links[J]. Journal of Bridge Engineering,2017,22(10):04017064. doi: 10.1061/(ASCE)BE.1943-5592.0001069 [2] VIJAYAKUMAR K R, JAYASEELAN J, ETHIRAJ N, et al. Investigation on aluminium/mild steel plates bonded polyurethane sheets to control vibration[J]. Materials Today: Proceedings,2020,45:5860-5867. [3] 易玉华, 陈智兴, 周鑫. 混合扩链剂对聚氨酯弹性体氢键和阻尼性能的影响[J]. 湖南大学学报(自然科学版), 2021, 49(12):142-147.YI Yuhua, CHEN Zhixing, ZHOU Xin. Effect of mixed chain extender on hydrogen bond and damping properties of polyurethane elastomer[J]. Journal of Hunan University (Natural Sciences),2021,49(12):142-147(in Chinese). [4] 郭睿, 李秀环, 何观伟, 等. HDI-BPA酚醛树脂的合成及应用性能[J]. 精细化工, 2019, 36(12):2503-2511.GUO Rui, LI Xiuhuan, HE Guanwei, et al. Synthesis and application properties of HDI-BPA phenolic resin[J]. Fine Chemicals,2019,36(12):2503-2511(in Chinese). [5] ABDULLAHI T, AHMAD N, WAHIT M U, et al. Adhesive bonding of thermoplastic polyurethane with metallic wire[J]. Advanced Science Letters,2016,24:4045-4049. [6] 张海, 易玉华, 马铁军. 聚氨酯-橡胶复合轮胎的制造方法, CN100415477 C[P]. 2008-09-03.ZHANG Hai, YI Yuhua, MA Tiejun. Manufacturing method of polyurethane-rubber composite tires: CN100415477 C[P]. 2008-09-03(in Chinese). [7] SHEIKHY H, SHAHIDZADEH M, RAMEZANZADEH B, et al. Studying the effects of chain extenders chemical structures on the adhesion and mechanical properties of a polyurethane adhesive[J]. Journal of Industrial and Engineering Chemistry,2013,19(6):1949-1955. doi: 10.1016/j.jiec.2013.03.008 [8] 罗筱烈, 刘瑾, 胡克良, 等. MDI与TDI封端聚酯预聚物和扩链剂间反应速率的研究[J]. 高等学校化学学报, 1995, 16(5):799, 801-803. doi: 10.3321/j.issn:0251-0790.1995.05.027LUO Xiaolie, LIU Jin, HU Keliang, et al. The study on the chain extension reaction of MDI and TDI terminated prepolymers with extenders[J]. Chemical Journal of Chinese Univertities,1995,16(5):799, 801-803(in Chinese). doi: 10.3321/j.issn:0251-0790.1995.05.027 [9] ZHANG Z Y, WANG W L, KORPACZ A N, et al. Binary liquid mixture contact-angle measurements for precise estimation of surface free energy[J]. Langmuir,2019,35(38):12317-12325. doi: 10.1021/acs.langmuir.9b01252 [10] RYTLEWSKI P, ZENKIEWICZ M. Effects of laser irradiation on surface properties of poly(ethylene terephthalate)[J]. Journal of Adhesion Science and Technology,2010,24(4):685-697. doi: 10.1163/016942409X12517106974741 [11] 中国国家标准化管理委员会. 硫化橡胶与金属粘接180°剥离试验: GB/T 15254—2014[S]. 北京: 中国标准出版社, 2014.Standardization Administration of the People's Republic of China. Rubber, vulcanized—Determination of adhesion to metal—180° peel test methods: GB/T 15254—2014[S]. Beijing: China Standards Press, 2014(in Chinese). [12] 牛永安, 王超, 陈泽明, 等. 一种高耐热酚醛树脂的合成及表征[J]. 中国胶粘剂, 2008, 17(1):8-11. doi: 10.3969/j.issn.1004-2849.2008.01.003NIU Yongan, WANG Chao, CHEN Zeming, et al. Synthesis and characterize a new type highly- heat- resistant phenolic resin[J]. China Adhesives,2008,17(1):8-11(in Chinese). doi: 10.3969/j.issn.1004-2849.2008.01.003 [13] OHTSUKA K, MATSUMOTO A, KIMURA H. Preparation and cured properties of diallyl phthalate resin modified with epoxy resin and allyl ester compound having carboxylic acid[J]. Journal of Applied Polymer Science,2010,116(2):913-919. [14] KOTB Y, CAGNARD A, HOUSTON K R, et al. What makes epoxy-phenolic coatings on metals ubiquitous: Surface energetics and molecular adhesion characteristics[J]. Journal of Colloid and Interface Science,2022,608:634-643. doi: 10.1016/j.jcis.2021.09.091 [15] LIU X M, WU Y Q, SHMULSKY R, et al. Developing a renewable hybrid resin system. Part I: Characterization of Co-polymers of isocyanate with different molecular weights of phenolic resins[J]. Bioresources,2016,11(2):5299-5311. [16] KALAMI S, AREFMANESH M, MASTER E, et al. Replacing 100% of phenol in phenolic adhesive formulations with lignin[J]. Journal of Applied Polymer Science,2017,134(30):520-528. [17] LIN Y M, YAN R Y, ZHANG Y, et al. Synthesis of biobased polyphenols for preparing phenolic polyurethanes with self-healing properties[J]. Polymer Testing,2022,112:107644. doi: 10.1016/j.polymertesting.2022.107644 [18] FUENSANTA M, MARTN-MARTNEZ J M. Influence of the hard segments content on the structure, viscoelastic and adhesion properties of thermoplastic polyurethane pressure sensitive adhesives[J]. Journal of Adhesion Science and Technology,2020,34(24):2652-2671. doi: 10.1080/01694243.2020.1780774 [19] RIMDUSIT S, SUDJIDJUNE M, JUBSILP C, et al. Enhanced film forming ability of benzoxazine-urethane hybrid polymer network by sequential cure method[J]. Journal of Applied Polymer Science,2014,131(13):40502. [20] ARSHAD N, ZIA K M, JABEEN F, et al. Synthesis, characterization of novel chitosan based water dispersible polyurethanes and their potential deployment as antibacterial textile finish[J]. International Journal of Biological Macromolecules,2018,111:485-492. doi: 10.1016/j.ijbiomac.2018.01.032 [21] DOMINGUEZ J C, OLIET M, ALONSO M V, et al. Structural, thermal and rheological behavior of a bio-based phenolic resin in relation to a commercial resol resin[J]. Industrial Crops and Products,2013,42:308-314. doi: 10.1016/j.indcrop.2012.06.004 [22] 柳云钊, 师建军, 王筠, 等. PICA中的酚醛树脂热分解机制[J]. 宇航材料工艺, 2016, 46(6):68-73, 78.LIU Yunzhao, SHI Jianjun, WANG Yun, et al. Pyrolysis mechanism of PICA phenolics[J]. Aerospace Materials & Technology,2016,46(6):68-73, 78(in Chinese). [23] ZOU X, CHEN K, YAO H N, et al. Chemical reaction and bonding mechanism at the polymer-metal interface[J]. ACS Applied Materials & Interfaces,2022,14(23):27383-27396. doi: 10.1021/acsami.2c04971 [24] LIU J Y, ZHANG Q D, ZHANG B Y, et al. The bonding mechanism of the micro-interface of polymer coated steel[J]. Polymers,2020,12(12):3052. doi: 10.3390/polym12123052 [25] ZHANG L, JI C, WANG X, et al. Strengthening and converse strengthening effects of polyurea layer on polyurea-steel composite structure subjected to combined actions of blast and fragments[J]. Thin-Walled Structures,2022,178:109527. doi: 10.1016/j.tws.2022.109527 [26] ARUNKUMAR T, RAMACHANDRAN S. Investigation of morphological and mechanical features of polyurea[J]. Applied Mechanics & Materials,2015,766-767:606-611. doi: 10.4028/www.scientific.net/AMM.766-767.606 -





 下载:
下载: