Preparation of WO3-x/CFs triphase catalyst and visible-light photocatalytic conversion of methane to methanol
-
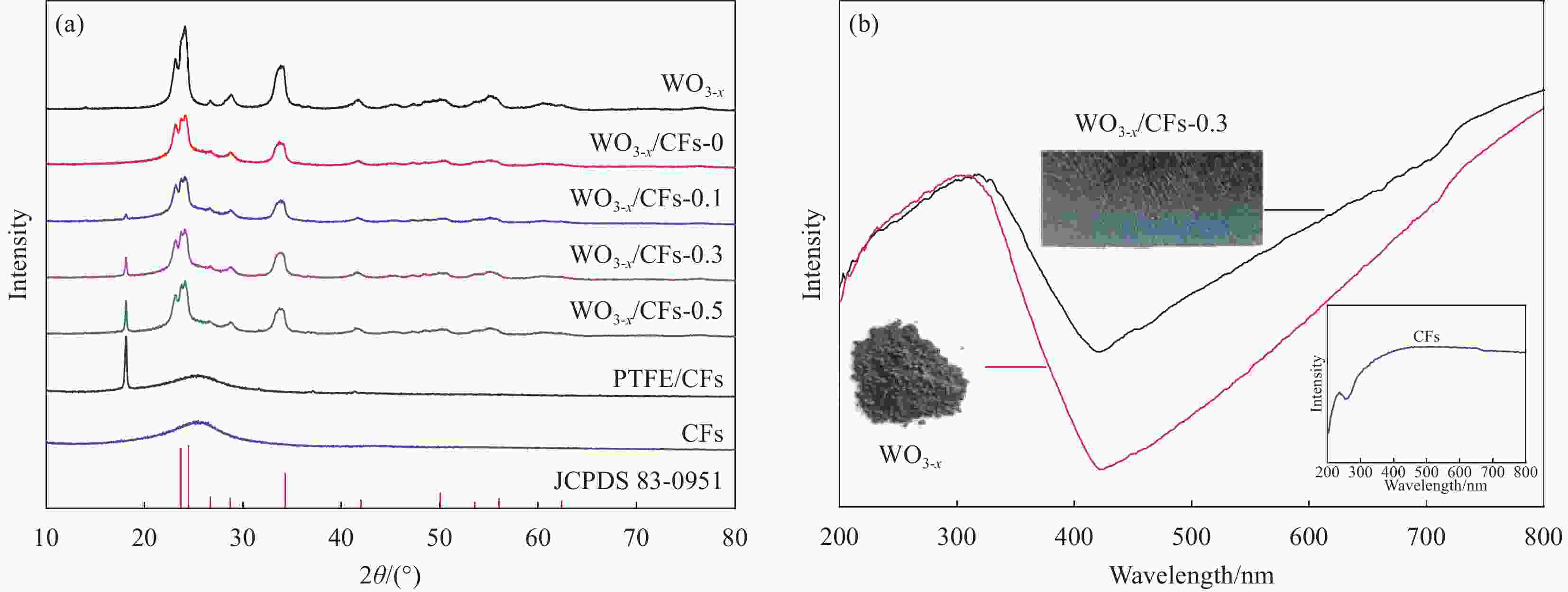
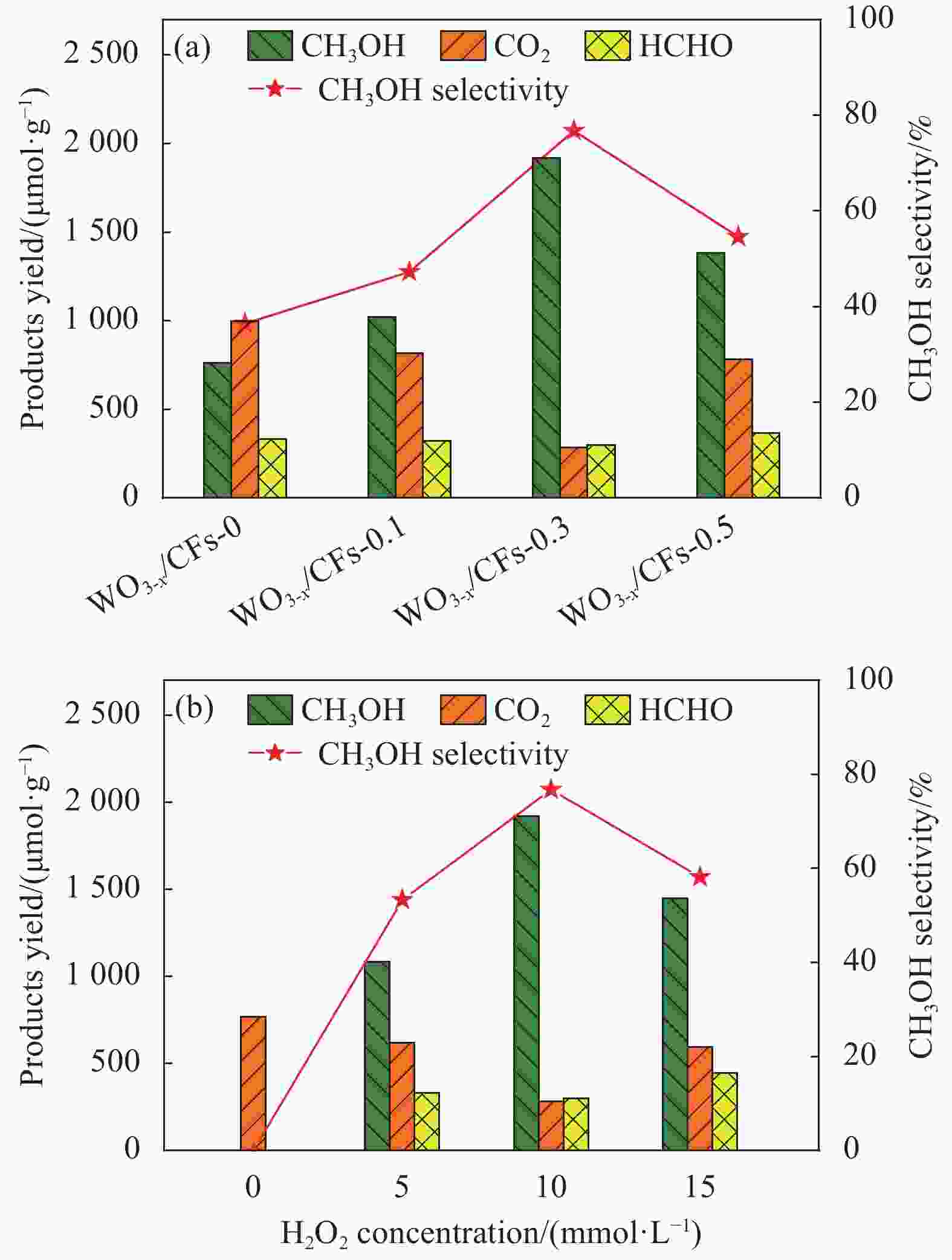
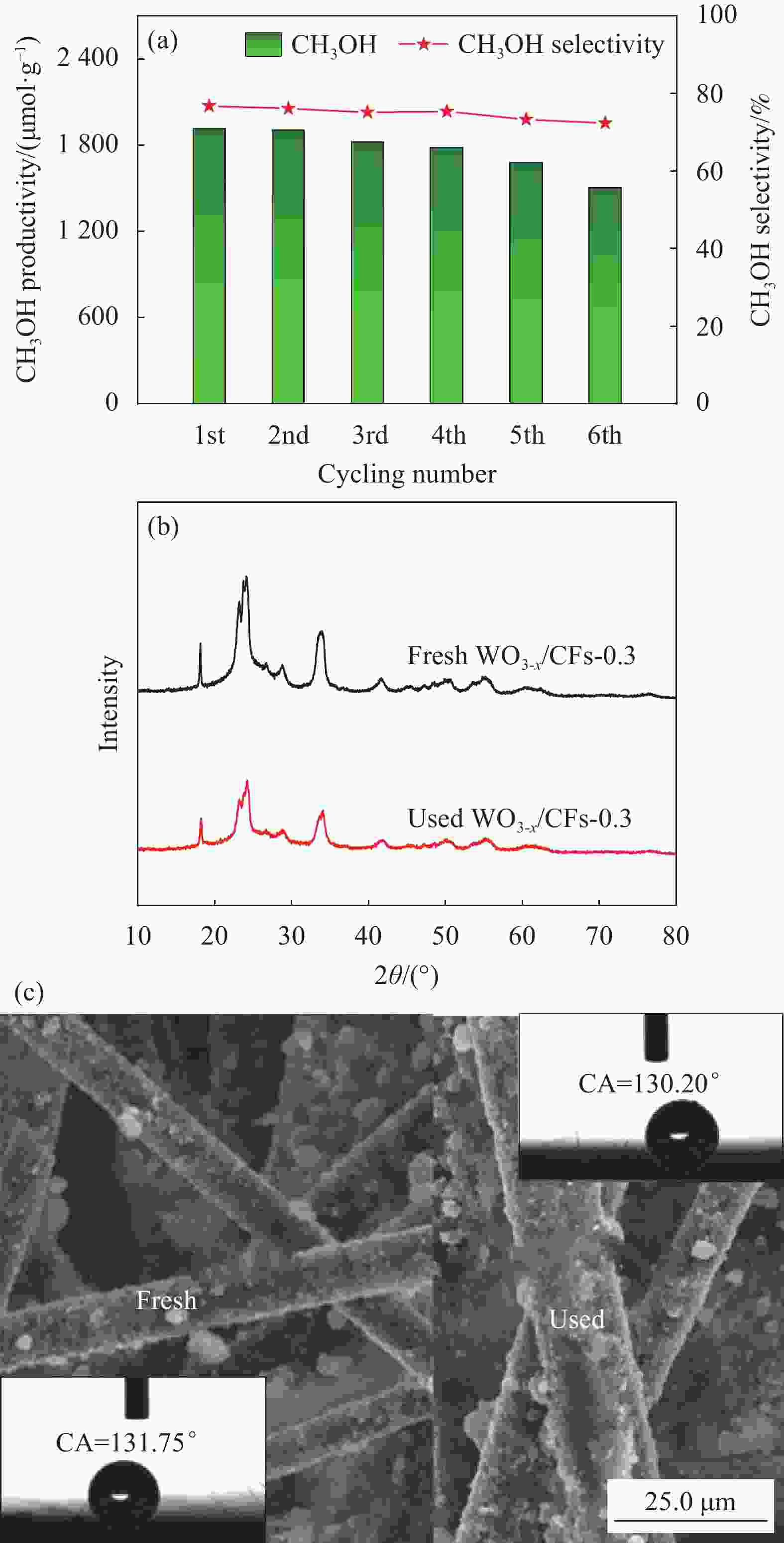
摘要: 将甲烷选择性转化为平台分子甲醇是有效利用天然气资源的理想途径之一,低碳排放的光催化技术可在室温常压下活化与转化甲烷,但水相光催化体系的甲烷转化性能仍较低。采用水热法首先合成富含氧缺陷的氧化钨(WO3-x),借助聚四氟乙烯浓缩液(PTFE)将WO3-x负载至碳纤维(CFs)表面制备WO3-x/CFs三相光催化剂,改变PTFE添加量可调控WO3-x/CFs的表面浸润性,通过XRD、SEM、水接触角、电子顺磁共振波谱仪(EPR)和低温氮吸脱附等测试技术对催化剂的形貌、结构与表面特性进行系统表征。可见光催化实验结果表明:WO3-x/CFs三相体系可显著提升甲烷至甲醇的转化性能,最优催化剂WO3-x/CFs-0.3的甲烷转化量为2522.20 μmol·g−1,分别为WO3-x/氧化铟锡导电玻璃(Glas)与粉末WO3-x两相体系的1.76倍和2.48倍;相应的甲醇产生量为1918.83 μmol·g−1,分别为WO3-x/Glas与粉末WO3-x体系的2.81倍和4.69倍,同时三相体系的甲醇选择性高达76.76%。WO3-x/CFs光催化性能增强主要源于疏水性催化剂形成的气-液-固三相界面,消耗的甲烷可经CFs气体传输通道直接传质至催化界面,促进甲烷分子活化与转化。此外,三相光催化体系循环稳定性优异,WO3-x/CFs-0.3经6次循环后甲醇产生量仍可达1506.98 μmol·g−1。Abstract: Selective conversion of methane into platform molecule methanol is one of the ideal ways to effectively utilize natural gas resources. Photocatalytic technology with low carbon emission can activate and transform methane at room temperature and atmospheric pressure, whereas the methane conversion performance of aqueous photocatalytic system is still low. Oxygen defects-rich WO3-x was firstly synthesized through hydrothermal method and then triphase photocatalyst WO3-x/CFs was constructed by loading WO3-x on carbon fiber (CFs) with polytetrafluoroethylene concentrate (PTFE). Changing the addition amount of PTFE, the surface wettability of WO3-x/CFs can be regulated. The morphology, structure and surface properties of triphase catalysts were systematically characterized by XRD, SEM, water contact angle, electron paramagnetic resonance (EPR) and low temperature nitrogen adsorption-desorption. The results of visible-light photocatalytic experiments show that WO3-x/CFs triphase system can significantly improve the conversion performance of methane into methanol. Methane conversion amount of the optimal WO3-x/CFs-0.3 catalyst is 2522.20 μmol·g−1, which is 1.76 times and 2.48 times of WO3-x/Indium tin oxide conducting glass (Glas) and powder WO3-x diphase systems, respectively. Methanol yield of WO3-x/CFs-0.3 triphase system is 1918.83 μmol·g−1, which is 2.81 times and 4.69 times of WO3-x/Glas and powder WO3-x systems respectively, and meanwhile methanol selectivity of triphase WO3-x/CFs system is up to 76.76%. The enhanced photocatalytic performance of WO3-x/CFs is primarily due to the gas-liquid-solid triphase interface formed by the hydrophobic catalyst. The consumed methane can be directly transferred to the catalytic interface through gas transport channel in CFs, promoting the activation and conversion of methane molecules. Additionally, the triphase photocatalytic system shows excellent cyclic stability and the methanol yield of WO3-x/CFs-0.3 can still reach 1506.98 μmol·g−1 after 6 cycles.
-
Key words:
- methane /
- methanol /
- visible-light photocatalysis /
- WO3-x /
- triphase interface /
- carbon fiber
-
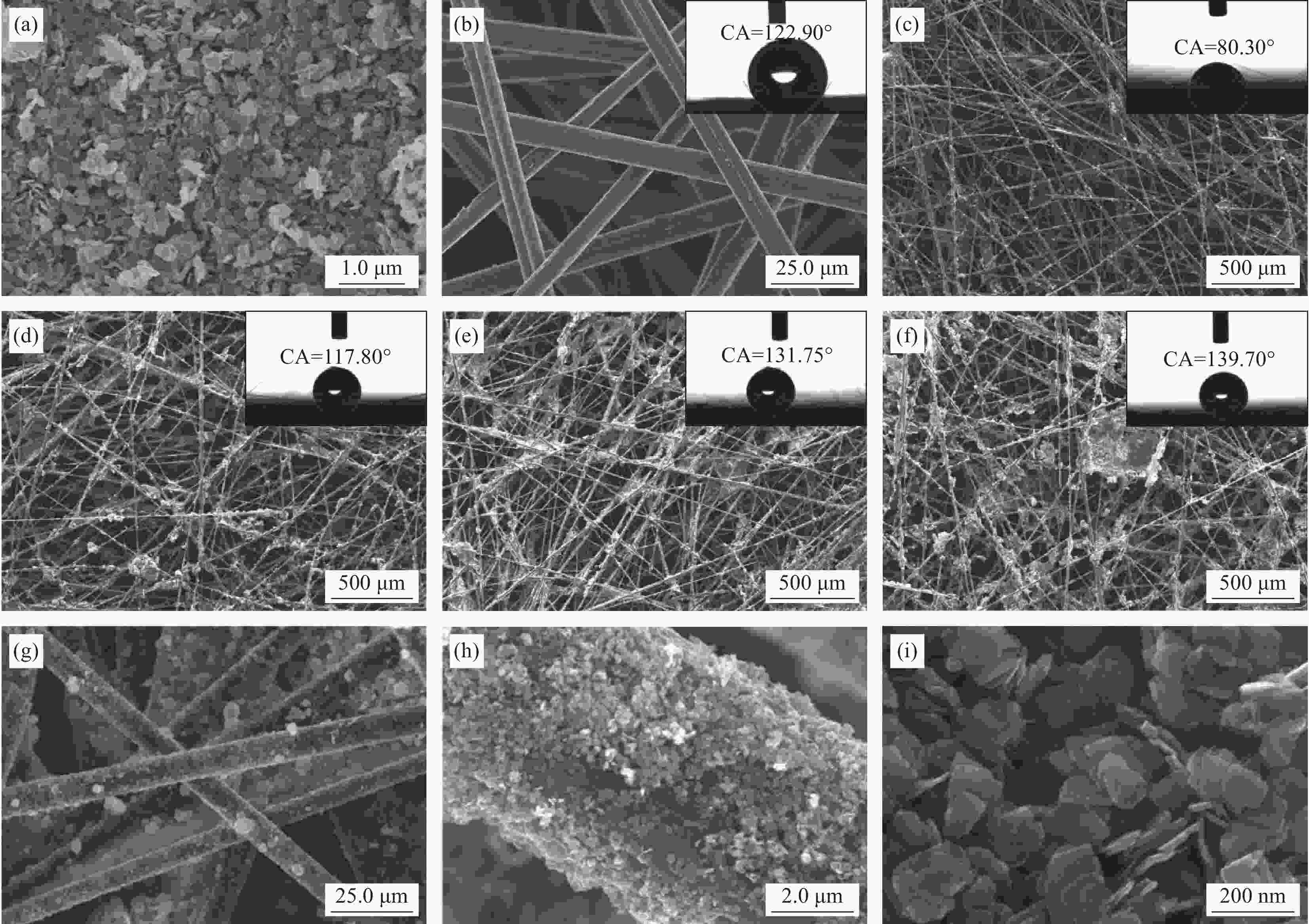
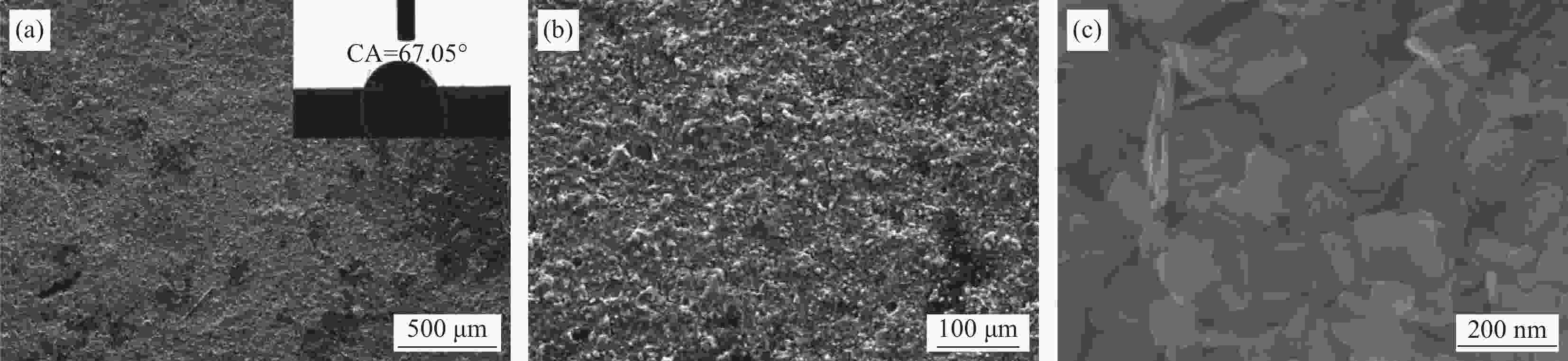
图 3 WO3-x (a)、空白CFs (b)、WO3-x/CFs-0 (c)、WO3-x/CFs-0.1 (d)、WO3-x/CFs-0.3 (e)和WO3-x/CFs-0.5 (f)的低倍SEM图像(图3(b)~3(f))的插图表示相应样品表面的水滴接触角);((g)~(i)) WO3-x/CFs-0.3的高倍SEM图像
CA—Contact angle
Figure 3. Low magnification SEM images of WO3-x (a), blank CFs (b), WO3-x/CFs-0 (c), WO3-x/CFs-0.1 (d), WO3-x/CFs-0.3 (e) and WO3-x/CFs-0.5 (f) samples respectively (The insets of Fig.3(b)-3(f) represent water contact angle of the corresponding samples surface); ((g)-(i)) High magnification SEM images of WO3-x/CFs-0.3
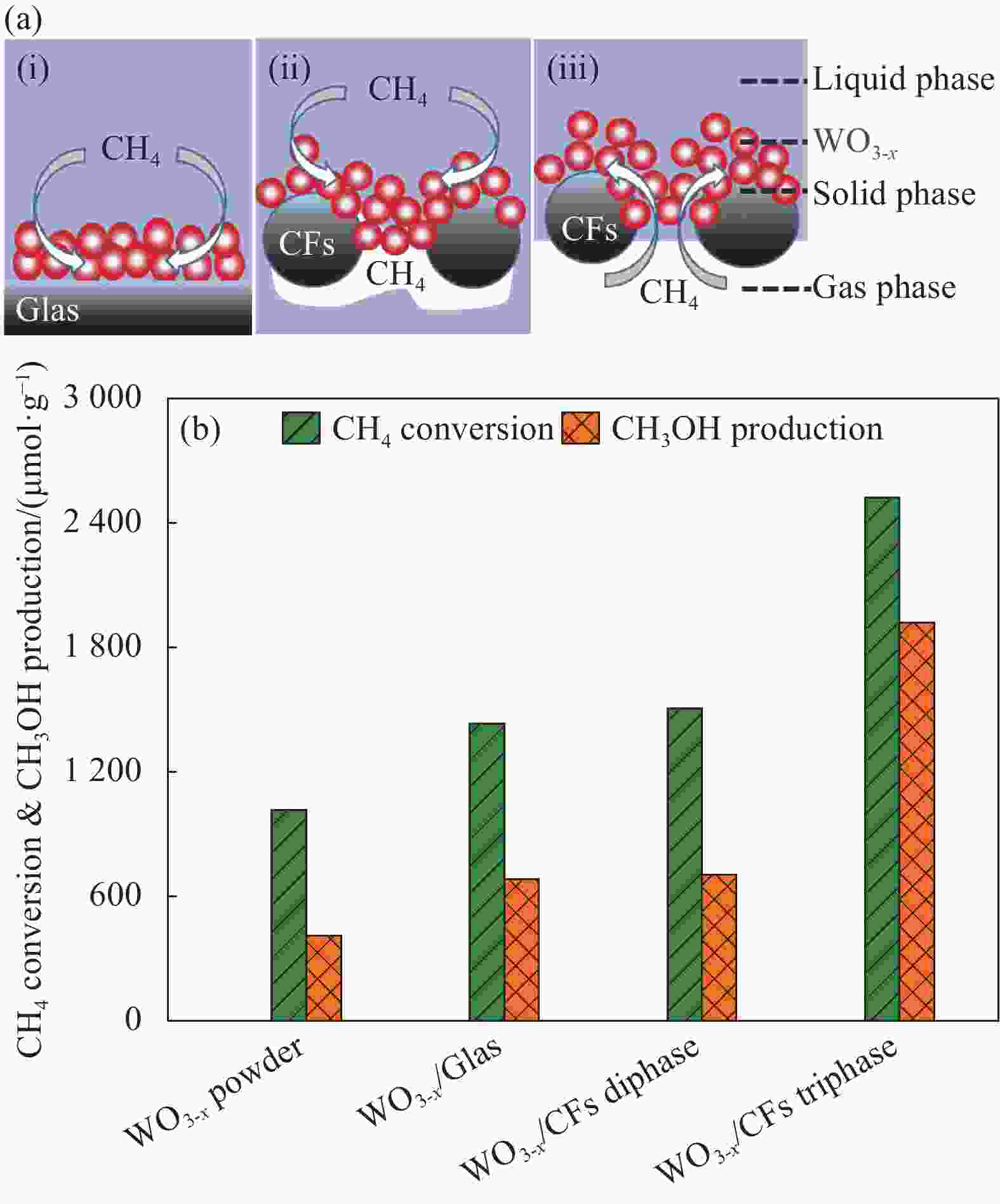
图 8 (a) WO3-x/Glas两相体系 (i)、WO3-x/CFs两相体系(通过将WO3-x/CFs完全浸入水中获得)(ii)和WO3-x/CFs三相体系示意图(iii);(b)粉末WO3-x与3种不同固载体系的甲烷转化量和甲醇产生量
Figure 8. (a) Schematic illustration of WO3-x/Glas diphase system (i), WO3-x/CFs diphase system (which was obtained by completely immersing WO3-x/CFs in the water) (ii) and WO3-x/CFs triphase system (iii); (b) Methane conversion and methanol production in WO3-x powder and three different supporting systems
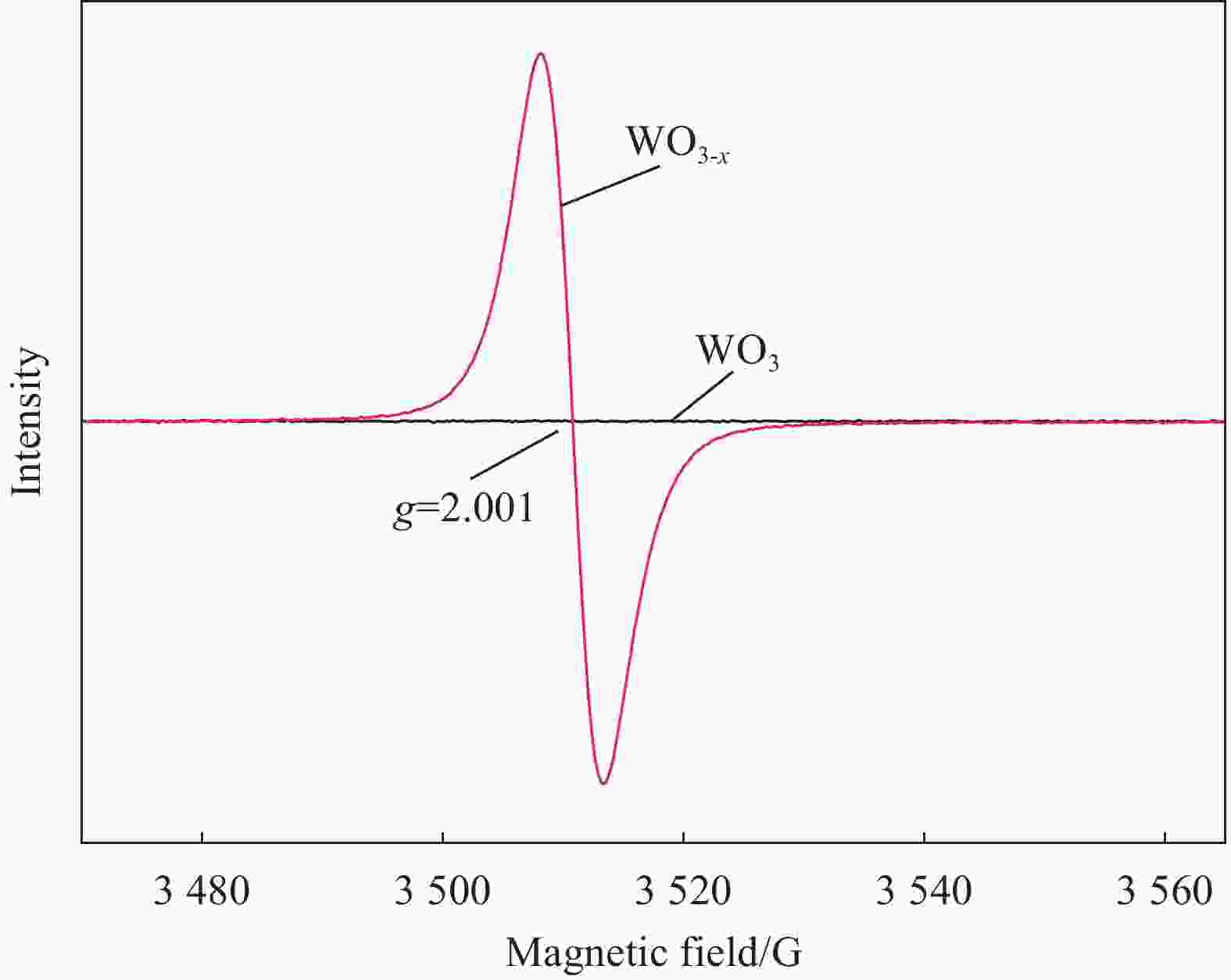
图 13 (a) WO3-x/CFs、WO3-x/Glas与粉末WO3-x体系DMPO/•CH3和DMPO/•OH加合物的EPR信号;(b) WO3-x/CFs光催化体系三相反应界面微环境示意图
DMPO—5, 5-dimethyl-1-pyrroline nitrogen oxide
Figure 13. (a) EPR signals of DMPO/•CH3 and DMPO/•OH adducts in WO3-x/CFs, WO3-x/Glas and powder WO3-x systems; (b) Schematic diagram of triphase reaction interface microenvironment in WO3-x/CFs photocatalytic system
表 1 WO3-x/CFs样品名及其成分配比
Table 1. WO3-x/CFs sample name and composition ratio
Sample PTFE dosage/µL WO3-x dosage/mg WO3-x/CFs-0 0 15 WO3-x/CFs-0.1 2.5 15 WO3-x/CFs-0.3 7.5 15 WO3-x/CFs-0.5 12.5 15 WO3-x/Glas-0.3 7.5 15 Note: Glas—Indium tin oxide conducting glass. 表 2 不同WO3-x/CFs样品的孔结构参数
Table 2. Pore structure parameters of different WO3-x/CFs samples
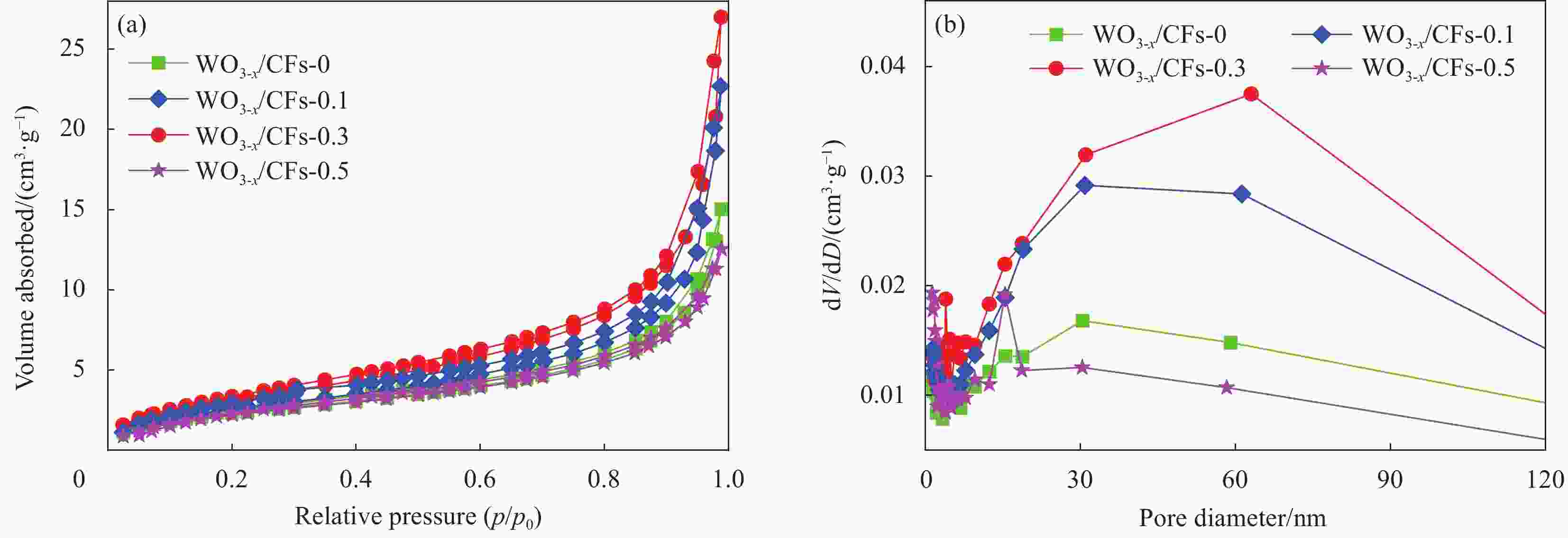
Sample SBET/(m2·g−1) Vpore/(cm3·g−1) Pore size/nm WO3-x/CFs-0 8.715 0.023 10.68 WO3-x/CFs-0.1 10.020 0.035 13.95 WO3-x/CFs-0.3 12.173 0.042 13.72 WO3-x/CFs-0.5 8.837 0.019 8.78 Notes: SBET—BET specific surface area; Vpore—Pore volume. 表 3 光催化转化甲烷制甲醇的性能比较
Table 3. Performance comparison of photocatalytic conversion of methane to methanol
Photocatalyst Light irradiation condition CH3OH yield/
(μmol·g−1)Selectivity of
CH3OH/%Ref. 0.33wt%FeOx/TiO2 300 W xenon lamp 1056 90 [7] Quantum-sized BiVO4 High-pressure mercury lamp 1100 96.6 [9] La-doped mesoporous WO3 Medium-pressure mercury lamp 63 46 [13] Mesoporous WO3 Mercury vapor lamp 111 37.4 [14] 0.1wt%Au/ZnO 300 W xenon lamp 4120 63 [18] BiVO4/NaNO2 450 W medium-pressure mercury lamp 18 90 [28] BiVO4 microcrystals 300 W xenon lamp 268 85 [29] BiVO4 450 W immersion medium-pressure mercury lamp 41.6 51 [30] WO3-x/CFs-0.3 300 W xenon lamp 1918.83 76.76 This work -
[1] SCHWACH P, PAN X, BAO X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: Challenges and prospects[J]. Chemical Reviews,2017,117(13):8497-8520. doi: 10.1021/acs.chemrev.6b00715 [2] ZHAN C G, NICHOLS J A, DIXON D A. Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: Molecular properties from density functional theory orbital energies[J]. Journal of Physical Che-mistry A,2003,107(20):4184-4195. doi: 10.1021/jp0225774 [3] AASBERG-PETERSEN K, BAK HANSEN J H, CHRISTENSEN T S, et al. Technologies for large-scale gas conversion[J]. Applied Catalysis A: General,2001,221(1):379-387. [4] RAVI M, RANOCCHIARI M, VAN BOKHOVEN J A. The direct catalytic oxidation of methane to methanol—A critical assessment[J]. Angewandte Chemie,2017,56(52):16464-16483. doi: 10.1002/anie.201702550 [5] SONG H, MENG X G, WANG Z J, et al. Solar-energy-mediated methane conversion[J]. Joule,2019,3(7):1606-1636. doi: 10.1016/j.joule.2019.06.023 [6] YULIATI L, YOSHIDA H. Photocatalytic conversion of methane[J]. Chemical Society Reviews,2008,37:1592-1602. doi: 10.1039/b710575b [7] XIE J J, JIN R X, LI A, et al. Highly selective oxidation of methane to methanol at ambient conditions by titanium dioxide-supported iron species[J]. Nature Catalysis,2018,1(11):889-896. doi: 10.1038/s41929-018-0170-x [8] ZHOU Y Y, ZHANG L, WANG W Z. Direct functionalization of methane into ethanol over copper modified polymeric carbon nitride via photocatalysis[J]. Nature Communications,2019,10(1):506-513. doi: 10.1038/s41467-019-08454-0 [9] FAN Y Y, ZHOU W C, QIU X Y, et al. Photocatalytic selec-tive oxidation of methane by quantum sized bismuth vanadate[J]. Nature Sustainability,2021,4(6):509-515. doi: 10.1038/s41893-021-00682-x [10] SHENG X, LIU Z, ZENG R S, et al. Enhanced photocatalytic reaction at air-liquid-solid joint interfaces[J]. Journal of the American Chemical Society,2017,139(36):12402-12405. doi: 10.1021/jacs.7b07187 [11] LIU J X, LI R, ZU X, et al. Photocatalytic conversion of nitrogen to ammonia with water on triphase interfaces of hydrophilic-hydrophobic composite Bi4O5Br2/ZIF-8[J]. Chemi-cal Engineering Journal,2019,371:796-803. doi: 10.1016/j.cej.2019.03.283 [12] XIA Y, XIAO K, CHENG B, et al. Improving artificial photosynthesis over carbon nitride by gas-liquid-solid interface management for full light-induced CO2 reduction to C1 and C2 fuels and O2[J]. ChemSusChem,2020,13(7):1730-1734. doi: 10.1002/cssc.201903515 [13] VILLA K, MURCIA-LÓPEZ S, MORANTE J R, et al. An insight on the role of La in mesoporous WO3 for the photocatalytic conversion of methane into methanol[J]. Applied Catalysis B: Environmental,2016,187:30-36. doi: 10.1016/j.apcatb.2016.01.032 [14] VILLA K, MURCIA-LÓPEZ S, ANDREU T, et al. Mesoporous WO3 photocatalyst for the partial oxidation of methane to methanol using electron scavengers[J]. Applied Catalysis B: Environmental,2015,163:150-155. doi: 10.1016/j.apcatb.2014.07.055 [15] WEI S L, ZHU X L, ZHANG P Y, et al. Aerobic oxidation of methane to formaldehyde mediated by crystal-O over gold modified tungsten trioxide via photocatalysis[J]. Applied Catalysis B: Environmental,2021,283:119661. doi: 10.1016/j.apcatb.2020.119661 [16] ZHANG N, LI X Y, YE H C, et al. Oxide defect engineering enables to couple solar energy into oxygen activation[J]. Journal of the American Chemical Society,2016,138(28):8928-8935. doi: 10.1021/jacs.6b04629 [17] 杨娟, 陈鹏宇, 戴俊, 等. Co3O4/WO3复合催化剂的合成及可见光催化转化甲烷制甲醇[J]. 燃料化学学报, 2022, 50(4):464-473.YANG Juan, CHEN Pengyu, DAI Jun, et al. Synthesis of Co3O4/WO3 composite catalysts for visible-light-driven conversion of methane to methanol[J]. Journal of Fuel Chemistry and Technology,2022,50(4):464-473(in Chinese). [18] SONG H, MENG X G, WANG S Y, et al. Direct and selective photocatalytic oxidation of CH4 to oxygenates with O2 on cocatalysts/ZnO at room temperature in water[J]. Journal of the American Chemical Society,2019,141(51):20507-20515. doi: 10.1021/jacs.9b11440 [19] HE H H, JIANG B, YUAN J J, et al. Cost-effective electrogeneration of H2O2 utilizing HNO3 modified graphite/polytetrafluoroethylene cathode with exterior hydrophobic film[J]. Journal of Colloid and Interface Science,2019,533:471-480. doi: 10.1016/j.jcis.2018.08.092 [20] XU A L, HE B, YU H X, et al. A facile solution to mature cathode modified by hydrophobic dimethyl silicon oil (DMS) layer for electro-Fenton processes: Water proof and enhanced oxygen transport[J]. Electrochimica Acta,2019,308:158-166. doi: 10.1016/j.electacta.2019.04.047 [21] LIU X L, XU S, CHI H B, et al. Ultrafine 1D graphene interlayer in g-C3N4/graphene/recycled carbon fiber heterostructure for enhanced photocatalytic hydrogen generation[J]. Chemical Engineering Journal,2019,359:1352-1359. doi: 10.1016/j.cej.2018.11.043 [22] THOMMES M, KANEKO K, NEIMARK A, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry,2015,87(9-10):1051-1069. doi: 10.1515/pac-2014-1117 [23] 薛超瑞, 李洋森, 黄蕊蕊, 等. BiOBr/Bi复合光热粉体的制备及其界面光热驱动水蒸发性能[J]. 复合材料学报, 2022, 39(7):3277-3286. doi: 10.13801/j.cnki.fhclxb.20210909.001XUE Chaorui, LI Yangsen, HUANG Ruirui, et al. Preparation of BiOBr/Bi composite photothermal powder and its interfacial photothermal driven water evaporation performance[J]. Acta Materiae Compositae Sinica,2022,39(7):3277-3286(in Chinese). doi: 10.13801/j.cnki.fhclxb.20210909.001 [24] DING P P, CUI L L, LI D, et al. Innovative dual-compartment flow reactor coupled with a gas diffusion electrode for in situ generation of H2O2[J]. Industrial & Engineering Chemistry Research,2019,58(16):6925-6932. [25] ZHANG Q Z, ZHOU M H, REN G B, et al. Highly efficient electrosynthesis of hydrogen peroxide on a superhydrophobic three-phase interface by natural air diffusion[J]. Nature Communications,2020,11:1731. doi: 10.1038/s41467-020-15597-y [26] MONTEVERDE VIDELA A H A, ZHANG L, KIM J, et al. Mesoporous carbons supported non-noble metal Fe-NX electrocatalysts for PEM fuel cell oxygen reduction reaction[J]. Journal of Applied Electrochemistry,2013,43(2):159-169. doi: 10.1007/s10800-012-0497-y [27] YANG J, CHEN P Y, DAI J, et al. Solar-energy-driven conversion of oxygen-bearing low-concentration coal mine methane into methanol on full-spectrum-responsive WO3-x catalysts[J]. Energy Conversion and Management,2021,247:114767. doi: 10.1016/j.enconman.2021.114767 [28] MURCIA-LÓPEZ S, VILLA K, ANDREU T, et al. Improved selectivity for partial oxidation of methane to methanol in presence of nitrite ions and BiVO4 photocatalyst[J]. Chemical Communications, 2015, 51: 7249-7252. [29] ZHU W L, SHEN M K, FAN G Z, et al. Facet-dependent enhancement in the activity of Bismuth Vanadate microcrystals for the photocatalytic conversion of methane to methanol[J]. ACS Applied Nano Materials,2018,1(12):6683-6691. doi: 10.1021/acsanm.8b01490 [30] MURCIA-LÓPEZ S, VILLA K, ANDREU T, et al. Partial oxidation of methane to methanol using Bismuth-based photocatalysts[J]. ACS Catalysis,2014,4(9):3013-3019. doi: 10.1021/cs500821r [31] 郑小强, 佟占鑫, 吴健博, 等. Bi2O3-TiO2复合氧化物光催化环己烷选择氧化[J]. 复合材料学报, 2022, 39(7):3268-3276.ZHENG Xiaoqiang, TONG Zhanxin, WU Jianbo, et al. Photocatalytic selective oxidation of cyclohexane with Bi2O3-TiO2 composite oxide[J]. Acta Materiae Compositae Sinica,2022,39(7):3268-3276(in Chinese). [32] LIN R, WAN J W, XIONG Y, et al. Quantitative study of charge carrier dynamics in well-defined WO3 nanowires and nanosheets: Insight into the crystal facet effect in photocatalysis[J]. Journal of the American Chemical Society,2018,140:9078-9082. doi: 10.1021/jacs.8b05293 [33] SONG H, MENG X G, WANG S Y, et al. Selective photo-oxidation of methane to methanol with oxygen over dual-cocatalyst-modified titanium dioxide[J]. ACS Catalysis,2020,10:14318-14326. doi: 10.1021/acscatal.0c04329 [34] YANG B C, MA S X, CUI R J, et al. Novel low-cost simultaneous removal of NO and SO2 with •OH from decomposition of H2O2 catalyzed by alkali-magnetic modified fly ash[J]. Industrial & Engineering Chemistry Research,2019,58:5339-5347. -






 下载:
下载: