γ-Fe2O3/ZnO@CNTs catalyzes photocoupled ozone degradation of2, 4-dichlorophenol in water
-
摘要: 内分泌干扰物2,4-二氯苯酚(DCP)化学结构较稳定,传统水处理技术降解效率不高。光耦合臭氧高级氧化技术(PCO)具有高效快速处理水中有机污染物的能力,可能成为DCP有效处理技术之一。本研究将γ-Fe2O3与ZnO结合共负载于多壁碳纳米管(CNTs)之上,开发出γ-Fe2O3/ZnO@CNTs催化剂用于催化PCO降解水中DCP,结果表明,该催化剂可以有效促进DCP的降解,当DCP初始浓度为20 mg/L,O3投加量为72 mg/L/h,催化剂投加量为1 g/L,模拟太阳光光源功率密度为
5000 W/cm2时,反应90 min DCP去除率可达98.9%,降解过程遵循准一级动力学规律。研究还探究了DCP降解路径,并确定了羟基自由基(·OH)、电子空穴(h+)和超氧自由基(${\text{•}}{\rm{O}}_2^ - $)是主要氧化基团。本研究为DCP及其他难降解有机污染物的有效处理提供了一种新的处理方案。Abstract: The chemical structure of endocriuptor 2,4-dichlorophenol (DCP) is stable, rendering the degradation efficiency of traditional water treatment technology relatively low. Photocoupled ozone advanced oxidation (PCO) technology possesses the capability to treat organic pollutants in water efficiently and rapidly, and may emerge as one of the effective treatment technologies for DCP. In this study, γ-Fe2O3 and ZnO were combined and co-supported on multi-walled carbon nanotubes (CNTs), thereby developing a catalyst, γ-Fe2O3/ZnO@CNTs, to catalyze the degradation of DCP in water through PCO. The results demonstrated that the catalyst could effectively facilitate the degradation of DCP. When the initial concentration of DCP is 20 mg/L, the dosage of O3 is 72 mg/L/h, the dosage of the catalyst is 1 g/L, and the power density of the simulated sunlight source is5000 W/cm2, the removal rate of DCP within 90 minutes can reach 98.9%, and the degradation process follows the quasi-first-order kinetic law. Additionally, the degradation path of DCP was explored, and hydroxyl radical (•OH), electron hole (h+), and superoxide radical (${\text{•}}{\rm{O}}_2^ - $) were identified as the main oxidizing groups. This study offers a new treatment scheme for the effective treatment of DCP and other refractory organic pollutants.-
Key words:
- γ-Fe2O3 /
- ZnO /
- catalyst /
- photocatalyzed coupled ozone catalysis /
- degradation /
- 2 /
- 4-dichlorophenol /
- radical
-
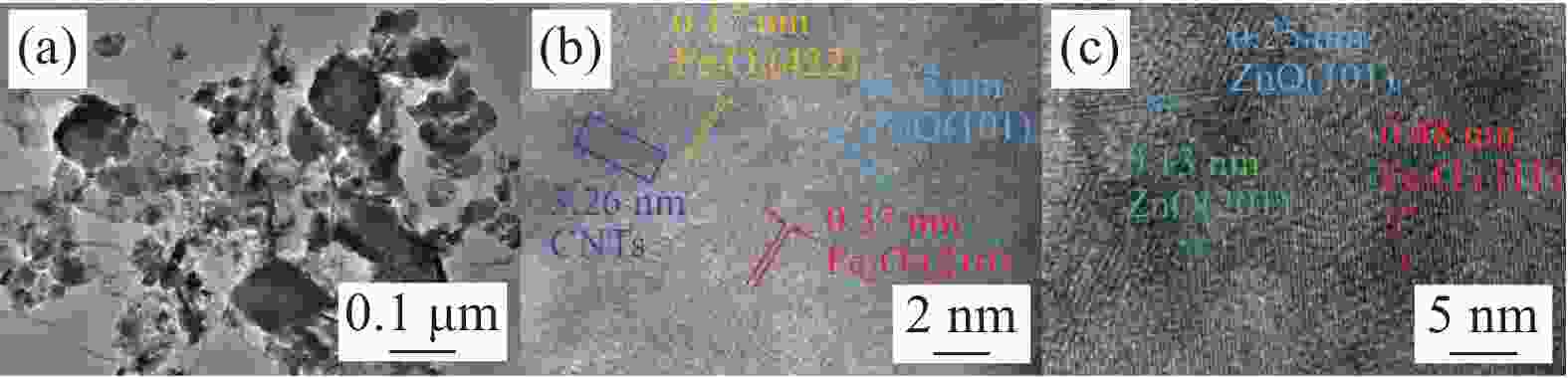
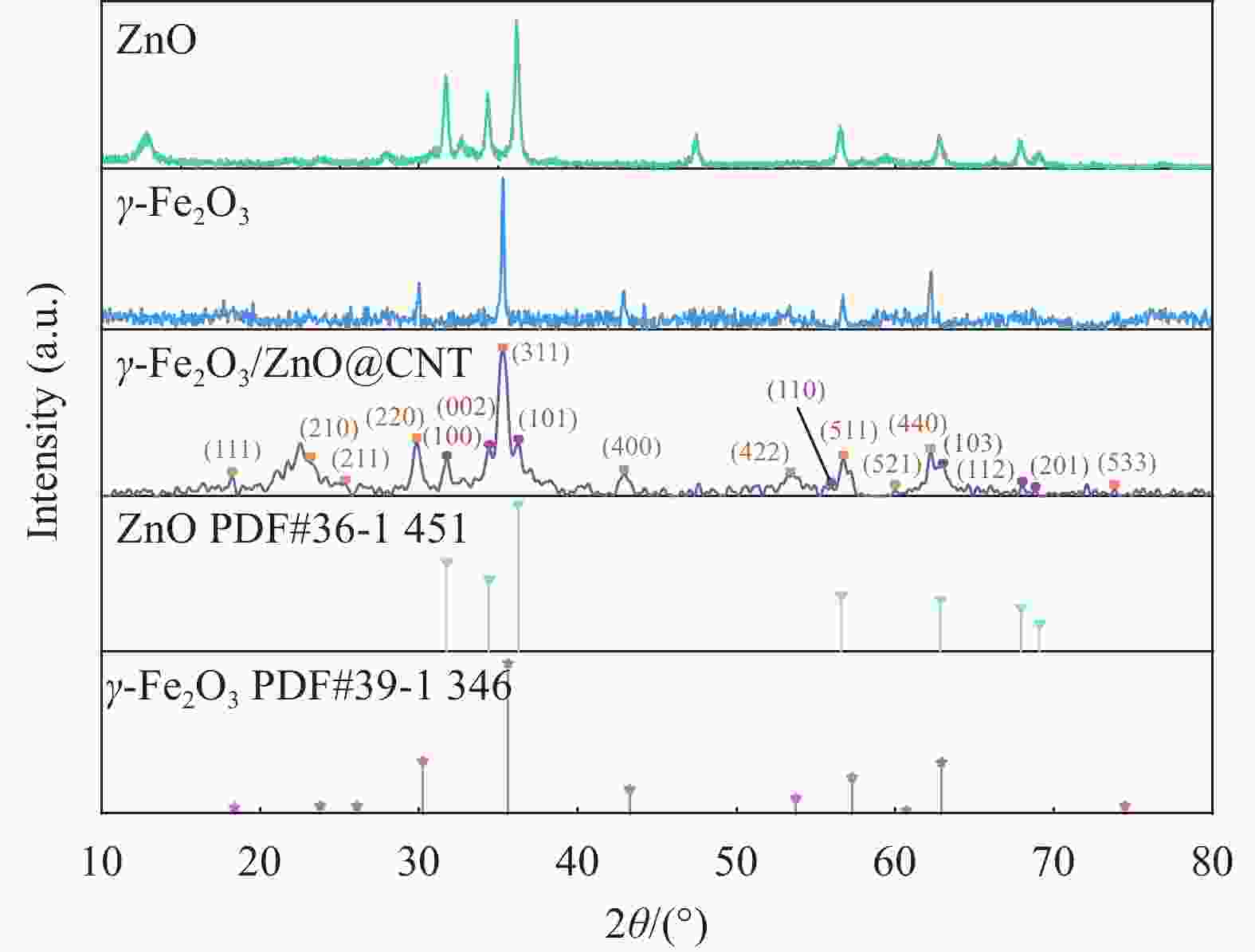
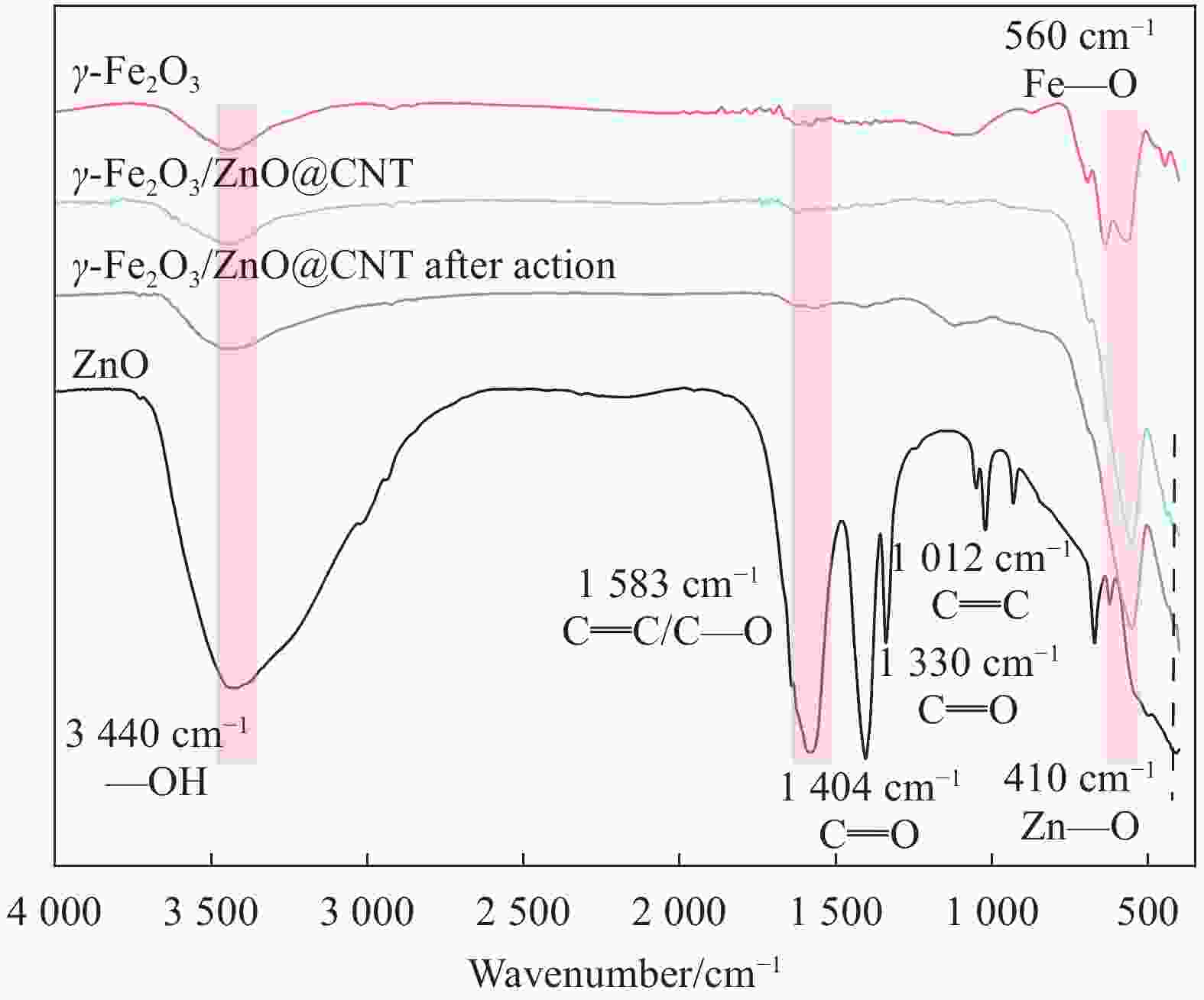
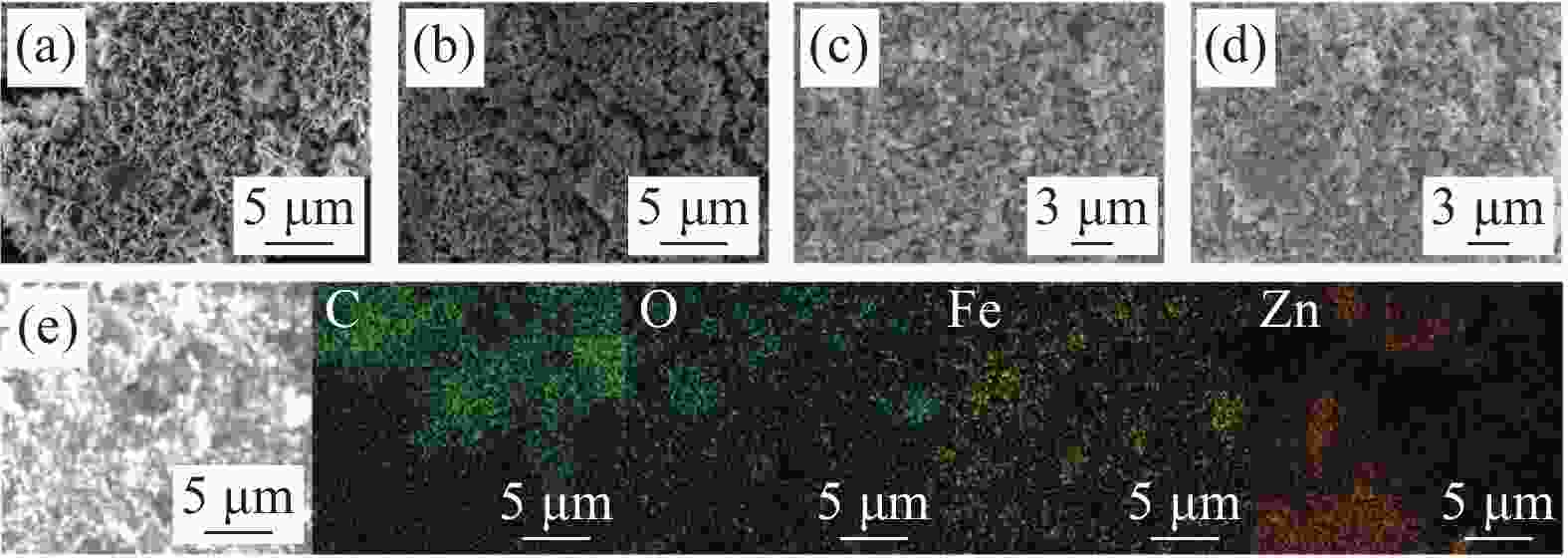
图 1 催化剂的SEM分析结果(a)ZnO;(b)γ-Fe2O3;(c)γ-Fe2O3/ZnO@多壁碳纳米管(CNTs);(d)五次循环反应后的γ-Fe2O3/ZnO@CNTs;(e)γ-Fe2O3/ZnO@CNTs的EDS分析结果
Figure 1. SEM analysis results of the catalyst: (a) ZnO; (b) γ - Fe2O3; (c) γ - Fe2O3/ZnO@ multi-walled carbon nanotubes (CNTs); (d) γ - Fe2O3/ZnO@CNTs after five cyclic reactions; (e) EDS analysis results of γ - Fe2O3/ZnO@CNTs
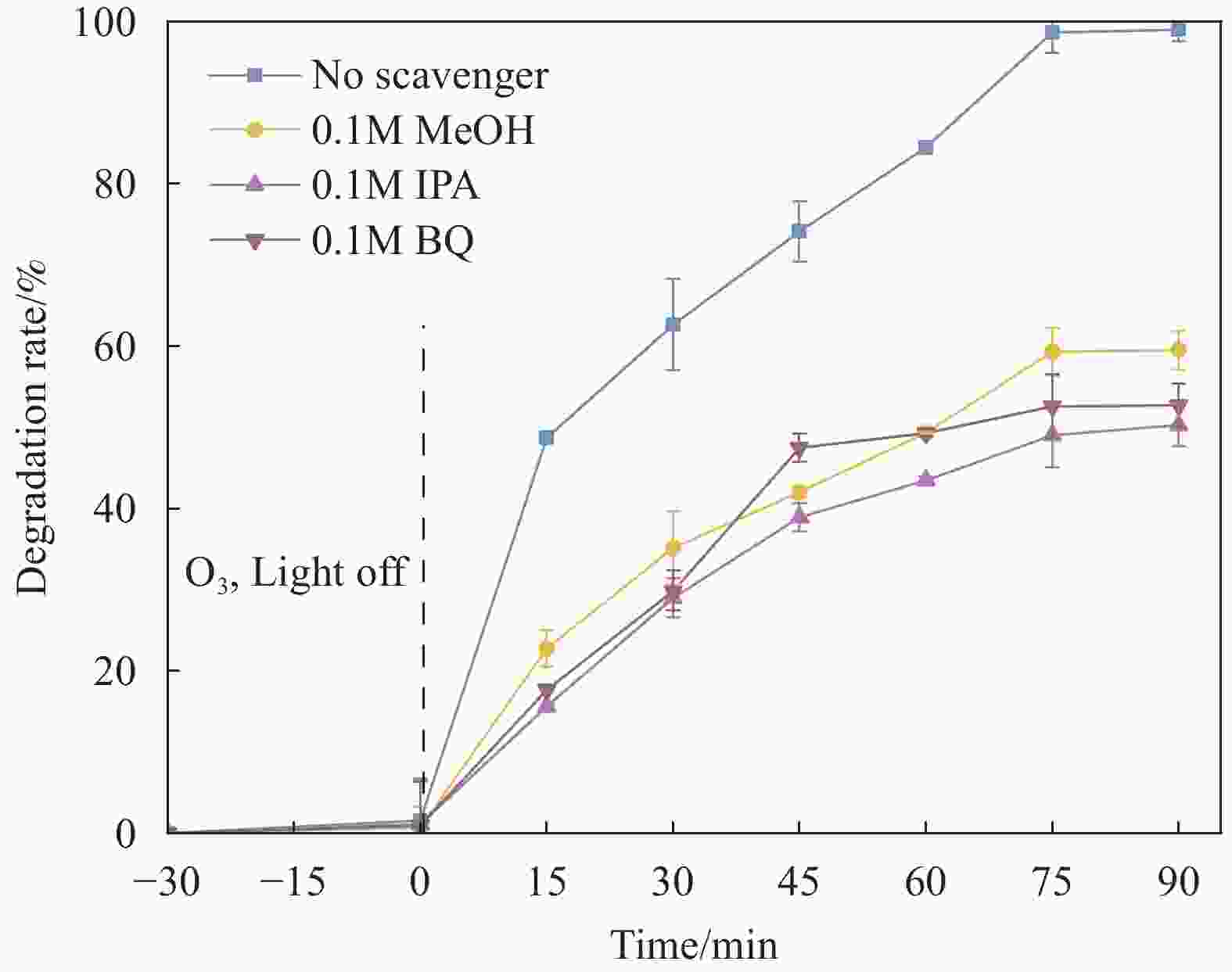
图 5 不同条件下DCP的降解效果(反应条件:曝气流速= 60 mL·min−1,氙灯光源光功率密度 =
5000 W·cm−2,DCP浓度20 mg·L−1,O3投加量为72 mg·L−1h−1,温度 = 20℃)(a) 不同γ-Fe2O3/ZnO@CNTs投加量的降解效果;(b) 不同pH的降解效果;(c)不同反应体系的降解效果;(d)不同循环次数下的降解效果Figure 5. Degradation effects of DCP under different conditions (Reaction conditions: aeration flow rate = 60 mL·min−1, light power density of xenon lamp light source =
5000 W·cm−2, DCP concentration of 20 mg·L−1, O3 dosage of 72 mg·L−1h−1, temperature = 20℃). (a) Degradation effects with different dosages of γ-Fe2O3/ZnO@CNTs; (b) Degradation effects at different pH values; (c) Degradation effects in different reaction systems; (d) Degradation effects under different cycle numbers图 6 不同猝灭剂对γ-Fe2O3/ZnO@CNTs催化光耦合臭氧降解DCP的影响(反应条件:O3投加量 = 72 mg·L−1·h−1, 曝气流速= 60 mL·min−1,DCP浓度= 20 mg·L−1,催化剂投加量= 1 g·L−1,氙灯光源光功率密度 =
5000 W·cm−2,温度 = 20℃)Figure 6. Influence of different quenchers on the degradation of DCP by catalytic photo-coupled ozone over γ-Fe₂O₃/ZnO@CNTs. (Reaction conditions: O3 dosage =72 mg·L−1·h−1, aeration flow rate = 60 mL·min−1, DCP concentration =20 mg·L−1, catalyst dosage = 1 g·L−1, light power density of the xenon lamp light source =
5000 W·cm−2,temperature = 20℃)表 1 DCP 降解的动力学拟合结果
Table 1. The kinetic fitting results of DCP degradation.
Reaction condition ln(C0/Ct)=kt 1/Ct=k2t k1/min−1 Standard error R2 k2/L·(mg−1·min−1) Standard error R2 O3 0.0020 − 0.0122 0.8867 0.0001 − 0.0505 0.8967 Light 0.0093 0.0371 0.9873 0.0007 − 0.0439 0.9451 O3+Light 0.0134 0.1194 0.9130 0.0013 − 0.0312 0.7762 O3+Light+1.0 g·L−1 γ-Fe2O3/ZnO@CNTs 0.0485 0.3023 0.8232 0.05318 1.0280 0.6801 Notes:γ-Fe2O3/ZnO@CNTs is a composite material in which γ-Fe₂O₃ and ZnO are co-loaded on carbon nanotubes;k1 is the first-order reaction rate constant; k2 is the second-order reaction rate constant; R² is the coefficient of determination. -
[1] ROSHANI M, NEMATOLLAHI D, ANSARI A, et al. Boosted electrocatalytic oxidation of organophosphorus pesticides by a novel high-efficiency CeO2-Doped PbO2 anode: An electrochemical study, parameter optimization and degradation mechanisms[J]. Chemosphere, 2024, 346. [2] 马晓雁, 高乃云, 李青松, 等. 黄浦江原水及水处理过程中内分泌干扰物状况调查[J]. 中国给水排水, 2006, 22(19): 1-4. doi: 10.3321/j.issn:1000-4602.2006.19.001MA X Y, GAO N Y, LI Q S, et al. Investigation on endocrine disruptors in raw water and water treatment in Huangpu River[J]. China Water Supply and Drainage, 2006, 22(19): 1-4(in Chinese). doi: 10.3321/j.issn:1000-4602.2006.19.001 [3] 杨廷政, 桑宇驰, 吕禾源, 等. 环境中典型内分泌干扰物(EDCs)去除技术研究进展[J]. 当代化工研究, 2024, (3): 6-8.YANG T Z, SANG Y C, LV H Y, et al. Research progress of removal technology of typical endocrine disruptors (EDCs) in environment[J]. Contemporary Chemical Research, 2024, (3): 6-8(in Chinese). [4] 江传春, 肖蓉蓉, 杨平. 高级氧化技术在水处理中的研究进展[J]. 水处理技术, 2011, 37(7): 12-16.JIANG C C, XIAO R R, YANG P. Research progress of advanced oxidation technology in water treatment[J]. Water Treatment Technology, 2011, 37(7): 12-16(in Chinese). [5] DIMITROPOULOS M, AGGELOPOULOS C A, SYGELLOU L, et al. Unveiling the photocorrosion mechanism of zinc oxide photocatalyst: Interplay between surface corrosion and regeneration[J]. Journal of Environmental Chemical Engineering, 2024, 12(2): 112102. doi: 10.1016/j.jece.2024.112102 [6] ASGARi E, SHEIKHMOHAMMADI A, NOURMORADI H, et al. Degradation of ciprofloxacin by photocatalytic ozonation process under irradiation with UVA: comparative study, performance and mechanism[J]. Process Safety and Environmental Protection, 2021, 147: 356-366. doi: 10.1016/j.psep.2020.09.041 [7] TYRPEKL V, VEJPRAVOVÁ J P, ROCA A G, et al. Magnetically separable photocatalytic composite γ-Fe2O3@ TiO2 synthesized by heterogeneous precipitation[J]. Applied Surface Science, 2011, 257(11): 4844-4848. doi: 10.1016/j.apsusc.2010.12.110 [8] MAJHI D, DAS K, MISHRA A, et al. One pot synthesis of CdS/BiOBr/Bi2O2CO3: a novel ternary double Z-scheme heterostructure photocatalyst for efficient degradation of atrazine[J]. Applied Catalysis B: Environmental, 2020, 260: 118222. doi: 10.1016/j.apcatb.2019.118222 [9] MIAO W, LI S, CAO X, et al. Wood-derived porous carbon supported γ-Fe2O3 nanoparticles as efficient catalyst for oxygen reduction reaction[J]. Applied Surface Science, 2022, 604: 154471. doi: 10.1016/j.apsusc.2022.154471 [10] JIA Z Y, YANG Y M, YANG C W, et al. Magnetic γ-Fe2O3/ZnO@ CNTs synthesized by a green precipitation method for the degradation of aniline through photocatalysis coupling catalytic ozonation[J]. Applied Surface Science, 2024, 659: 159866. doi: 10.1016/j.apsusc.2024.159866 [11] XIAO H, LIU R P, ZHAO X, et al. Effect of manganese ion on the mineralization of 2, 4-dichlorophenol by ozone[J]. Chemosphere, 2008, 72(7): 1006-1012. doi: 10.1016/j.chemosphere.2008.04.030 [12] CHU Y Y, ZHENG X L, FAN J R. Preparation of sodium and boron co-doped graphitic carbon nitride for the enhanced production of H2O2 via two-electron oxygen reduction and the degradation of 2, 4-DCP via photocatalytic oxidation coupled with Fenton oxidation[J]. Chemical Engineering Journal, 2022, 431: 134020. doi: 10.1016/j.cej.2021.134020 [13] ZHOU Z F, LU J H, ZHANG R X, et al. Construction and differential growth mechanism of uniform and controllable CNTs and CNWs by ZIF-67 precursor[J]. Applied Surface Science, 2023, 640: 158342. doi: 10.1016/j.apsusc.2023.158342 [14] WU S X, YANG Z, WANG F, et al. Effect of γ-Fe2O3 nanoparticles on the composition of montmorillonite and its sorption capacity for pyrene[J]. Science of The Total Environment, 2022, 813: 151893. doi: 10.1016/j.scitotenv.2021.151893 [15] 罗灵芝, 俞俊, 罗豪, 等. 带状 γ-Fe 2 O 3/ZnO 异质结光催化剂 光催化降解四环素[J]. Acta Materiae Compositae Sinica, 2021, 38(5): 1535-1542.LUO L Z, YU J, LUO H, et al. Photocatalytic degradation of tetracycline by banded γ - Fe₂O₃/ZnO heterojunction photocatalyst[J]. Acta Materiae Compositae Sinica, 2021, 38(5): 1535-1542 (in Chinese). [16] YU J L, ZHANG X X, ZHAO X D, et al. Heterogeneous Fenton oxidation of 2, 4-dichlorophenol catalyzed by PEGylated nanoscale zero-valent iron supported by biochar[J]. Environmental Science and Pollution Research, 2023, 30(14): 41333-41347. doi: 10.1007/s11356-023-25182-7 [17] WANG C L, KONG X W, YU Z X, et al. Construction of g-C3N4/Fe-MOFs Type-Ⅱ heterojunction promotes photo-Fenton degradation of doxycycline[J]. Separation and Purification Technology, 2023, 326: 124790. doi: 10.1016/j.seppur.2023.124790 [18] CHAI H, YANG C Y, XU P, et al. Enhanced visible-light photocatalytic activity with Fe2O3–ZnO@ C/g-C3N4 heterojunction: characterization, kinetics, and mechanisms[J]. Journal of Cleaner Production, 2022, 377: 134511. doi: 10.1016/j.jclepro.2022.134511 [19] KUZHAROV A A, GRITSAI M A, BUTOVA V V, et al. One-step electrochemical synthesis of γ-Fe2O3@ MIL-88a magnetic composite for heterogeneous Fenton-like catalysis[J]. Ceramics International, 2022, 48(23): 34864-34876. doi: 10.1016/j.ceramint.2022.08.076 [20] MECHA A C, ONYANGO M S, OCHIENG A, et al. Synergistic effect of UV–vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: A comparative study[J]. Journal of Catalysis, 2016, 341: 116-125. doi: 10.1016/j.jcat.2016.06.015 [21] HUMAYUN M, HU Z, KHAN A, et al. Highly efficient degradation of 2, 4-dichlorophenol over CeO2/g-C3N4 composites under visible-light irradiation: detailed reaction pathway and mechanism[J]. Journal of hazardous materials, 2019, 364: 635-644. doi: 10.1016/j.jhazmat.2018.10.088 [22] FERREIRO C, SANZ J, VILLOTA N, et al. Kinetic modelling for concentration and toxicity changes during the oxidation of 4-chlorophenol by UV/H2O2[J]. Scientific Reports, 2021, 11(1): 15726. doi: 10.1038/s41598-021-95083-7 [23] RAMOS-RAMÍREZ E, GUTIÉRREZ-ORTEGA N, TZOMPANTZI-MORALES F, et al. Photocatalytic Degradation of 2, 4-Dichlorophenol in Water Using MgAl Activated Hydrotalcites as Photocatalyst[J]. Topics in Catalysis, 2022, 65(13): 1469-1481. [24] VADIVEL S, HARIGANESH S, PAUL B, et al. Highly active novel CeTi2O6/g-C3N5 photocatalyst with extended spectral response towards removal of endocrine disruptor 2, 4-dichlorophenol in aqueous medium[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 592: 124583. doi: 10.1016/j.colsurfa.2020.124583 [25] LI J D, ZHANG X L, RAZIQ F, et al. Improved photocatalytic activities of g-C3N4 nanosheets by effectively trapping holes with halogen-induced surface polarization and 2, 4-dichlorophenol decomposition mechanism[J]. Applied Catalysis B: Environmental, 2017, 218: 60-67. doi: 10.1016/j.apcatb.2017.06.038 [26] YU J, ZHANG X, ZHAO X, et al. Heterogeneous Fenton oxidation of 2, 4-dichlorophenol catalyzed by PEGylated nanoscale zero-valent iron supported by biochar[J]. Environmental Science and Pollution Research, 2023, 30(14): 41333-41347. doi: 10.1007/s11356-023-25182-7 [27] PAN X X, WEI J Y, ZOU M T, et al. Products distribution and contribution of (de) chlorination, hydroxylation and coupling reactions to 2, 4-dichlorophenol removal in seven oxidation systems[J]. Water research, 2021, 194: 116916. doi: 10.1016/j.watres.2021.116916 [28] ZHANG L Y, ZHANG J J, YU H G, et al. Emerging S-scheme photocatalyst[J]. Advanced materials, 2022, 34(11): 2107668. doi: 10.1002/adma.202107668 [29] HE F, MENG A Y, CHENG B, et al. Enhanced photocatalytic H2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification[J]. Chinese Journal of Catalysis, 2020, 41(1): 9-20. doi: 10.1016/S1872-2067(19)63382-6 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 24
- HTML全文浏览量: 26
- 被引次数: 0




 下载:
下载: