In situ growth of a Ni-Co-B-Yb rare earth composite electrode: Preparation and electrocatalytic hydrogen precipitation performance
-
摘要:
探索和开发低成本、高活性的非贵金属析氢反应(Hydrogen evolution reaction,HER)电催化剂,对于电解水的实际应用具有重要意义但仍具有挑战性。本文采用化学沉积法在三维泡沫镍(NF)基底上制备了原位生长的稀土(Rare earth,RE)复合催化电极(Ni-Co-B-Yb/NF),对催化电极的结构和形貌进行了表征,并研究其在1 mol·L−1 KOH溶液中的析氢性能。结果表明:添加Yb可使电极的形貌及电子结构发生改变,改善催化剂材料的HER催化性能。当Yb和Co浓度分别为3 g·L−1和5 g·L−1时,Ni-Co-B-Yb/NF表现出最佳的析氢性能。当电流密度为10 mA·cm−2时,析氢过电位为57 mV,Tafel斜率仅为73 mV·dec−1,此外,经过100 h长期稳定性测试和
2000 次循环伏安(Cyclic voltammetry,CV)测试后,该催化剂表现出良好的电化学稳定性。实验结果表明:Yb的引入可以提升Ni-Co-B材料的HER催化性能,且Yb和Co浓度的改变对电催化性能影响较大。这项工作丰富了稀土复合催化剂在电解水催化方面的知识。Abstract:The exploration and development of low-cost and highly active non-precious metal hydrogen evolution reaction (HER) electrocatalysts are important but still challenging for practical applications in water electrolysis. In this study, in-situ-grown rare earth (RE) composite catalytic electrodes (Ni-Co-B-Yb/NF) were prepared on a three-dimensional nickel foam (NF) substrate by chemical deposition, and the structure and morphology of the catalytic electrodes were characterized and their hydrogen precipitation performance was investigated in 1 mol·L−1 KOH solution. The results show that the addition of Yb can change the morphology and electronic structure of the electrode and improve the HER catalytic performance of the catalyst material. The Ni-Co-B-Yb/NF exhibited the best hydrogen precipitation performance when the Yb and Co concentrations were 3 g·L−1 and 5 g·L−1, respectively. At a current density of 10 mA·cm−2, the hydrogen precipitation overpotential was 57 mV, and the Tafel slope was only 73 mV·dec−1. In addition, after 100 h of long-term stability test and
2000 cycles of cyclic voltammetry (CV) test, the catalyst showed good electrochemical stability, good electrochemical stability. The experimental results show that the introduction of Yb can enhance the HER catalytic performance of Ni-Co-B materials, and the changes of Yb and Co concentrations have a large effect on the electrocatalytic performance. This work enriches the knowledge of rare-earth composite catalysts for electrolytic water catalysis. -
氢气能量密度高、转化效率快、丰度高、污染低,是替代传统化石燃料的理想能源之一[1-2]。最近研究表明,制氢方法包括生物制氢法、矿物燃料制氢法、水分解制氢法等[3]。与其他制氢方法相比,电解水制氢的优点是制氢纯度高、环保可再生、来源广泛等优点,被认为是最具前途的制氢方法之一[4-5]。然而,电解水过程中阴极反应(HER)需要依赖电催化剂来提高反应速率和效率。尽管Pt和Pt基纳米材料是最高效和稳定的HER电催化剂[6-7],但存在高昂的成本、稀缺和难以实现工业化生产等问题。因此,开发一种低成本、高性能的非贵金属催化剂是当前的重要任务。
近年来,利用外来元素改性过渡金属基电催化剂的研究进展迅速。过渡金属(TM)材料与某些非金属元素复合可提高TMs固有活性,表现出较强的稳定性和电催化活性,包括过渡金属磷化物[8]、硫化物[9]、硼化物[10]和其他非贵金属,有望成为贵金属催化剂的替代品[11-12]。稀土(Rare earth,RE)元素可以调节和提升各种过渡金属基催化剂的电催化性能,21世纪以来已经成为电催化领域的热点。根据Brewer-Engel价键理论,当具有空或半空d轨道的过渡金属与拥有成对d电子的过渡金属结合时,产生的协同作用有益于析氢反应的进行,可以提高催化剂的HER活性。稀土元素有许多空或半空的d轨道和独特的4f轨道,导致与TM之间形成强烈的电子协同作用[13]。其4f轨道能与其他金属合金化时调整电子结构,从而增强电子传输并产生协同效应,可以显著提升催化活性和稳定性[14-15]。Cardoso等[16]制备了热含量为5%和10%的 Ni-Dy和Ni-Sm合金,其电催化活性均优于纯镍,这与金属间的相互作用有关。此外,稀土元素还可以通过改变金属的微观结构和组成来调整合金的电催化活性[17-18]。
催化剂载体的选择及其负载方式对催化剂析氢性能有着重要的影响。近期研究表明在导电衬底上原位生长催化剂,可以增强材料的导电性,从而提升HER性能。在这方面,泡沫镍(NF)载体作为首选,由于它具有多孔三维结构能提供大量比表面积,为催化剂的生长创造条件,同时具有高效的网状传质通道,可以促进电子传递,使活性物质分布均匀[19-20]。
本文采用化学沉积法制备了在NF上原位生长的自支撑Ni-Co-B-Yb稀土复合催化剂,并研究稀土Yb浓度和Co浓度对电催化电极的结构形貌和HER的影响。尽管在电催化剂领域对重稀土的研究较少,但采用上述方法是可行的,并为研究镀层化学成分的影响提供了可能性。
1. 实验材料及方法
1.1 电极的制备
切割尺寸为10 mm×20 mm的NF样品并进行预处理,去除表面的氧化层和油脂。将样品置于质量分数10wt%盐酸中超声10 min,然后放入无水乙醇清洗5 min,最后置于丙酮中清洗5 min。接下来在0.2 g·L−1 PdCl2的乙醇溶液中活化30 s,确保反应过程中得到连续的镀层。将活化后的泡沫镍基体用无水乙醇洗净后自然风干备用。镀液配制如表1所示,镀液中加入适量NaOH,调节镀液的pH至4并利用超声波加速其完全溶解,加入还原剂硼烷二甲胺(C2H10BN)并将备用的NF放入10 mL的镀液中。在35℃恒温条件下反应,反应完全后出现分层现象,取出催化剂样品用无水乙醇清洗并自然风干。
表 1 化学镀液配方Table 1. Chemical deposition plating solution formulaComposition of plating solution Concentration/
(g·L−1)Anhydrous nickel chloride (NiCl2) 10.0 Anhydrous cobalt chloride (CoCl2) 1.0-7.0 Borane dimethylamine (C2H10BN) 1.0 Ytterbium nitrate [Yb(NO3)3] 2.0-4.0 Adipic acid (C6H10O4) 1.5 Citric acid (C6H8O7) 1.5 Malic acid (C4H6O5) 1.5 固定镀液中氯化钴浓度为5 g·L−1,以稀土浓度为变量(0、2、3和4 g·L−1)制备的样品分别标记为0-NCBY/NF、2-NCBY/NF、3-NCBY/NF和4-NCBY/NF。固定稀土硝酸盐浓度为3 g·L−1,只改变镀液中氯化钴浓度(1、3、5、7 g·L−1)分别标记为NCBY/NF-x (x = 1、3、5、7)。
1.2 样品表征
采用日立公司SU8010型扫描电子显微镜(SEM)表征材料的微观形貌。采用X射线能谱仪(EDS)分析复合电极材料的化学组成和元素含量分布。采用日本理学公司的Uitima IV-85型X射线衍射仪(XRD)测试样品的晶体结构。采用FEI Tecnai G2 F30系统的透射电镜(TEM)表征样品的微观形态和晶格信息。通过Thermo Fisher Scientific K-Alpha X射线光电子能谱(XPS)分析样品的电子结构。
1.3 电催化性能测试
电化学实验均在1.0 mol·L−1 KOH溶液中连接CHI660 E电化学工作站的三电极系统下进行。制备的电极为工作电极,碳棒电极为对电极,汞/氧化汞电极为参比电极,形成三电极测试体系。用能斯特方程(ERHE=EHg/HgO+EHg/HgO+0.059pH)计算可逆氢电极(RHE)电位(ERHE)。在扫描速率为5 mV·s−1条件下,分别测试催化电极的线性伏安曲线(LSV),所有极化曲线均经过90%的IR补偿;在不同扫描速率(10~100 mV·s-1)下测试催化电极的循环伏安曲线(CV);在频率范围为0.1~105 Hz、扰动电位幅值为5 mV的条件下测试电化学阻抗谱(EIS);根据公式(ECSA=Cdl/Cs)计算催化剂的活性比表面积(ECSA),其中,Cdl为催化电极的双电层电容,Cs为对应实际比表面积为1 cm2样品的比电容(根据文献[21],Cs=40 μF·cm−2)。
2. 结果与讨论
2.1 形貌、结构与组成分析
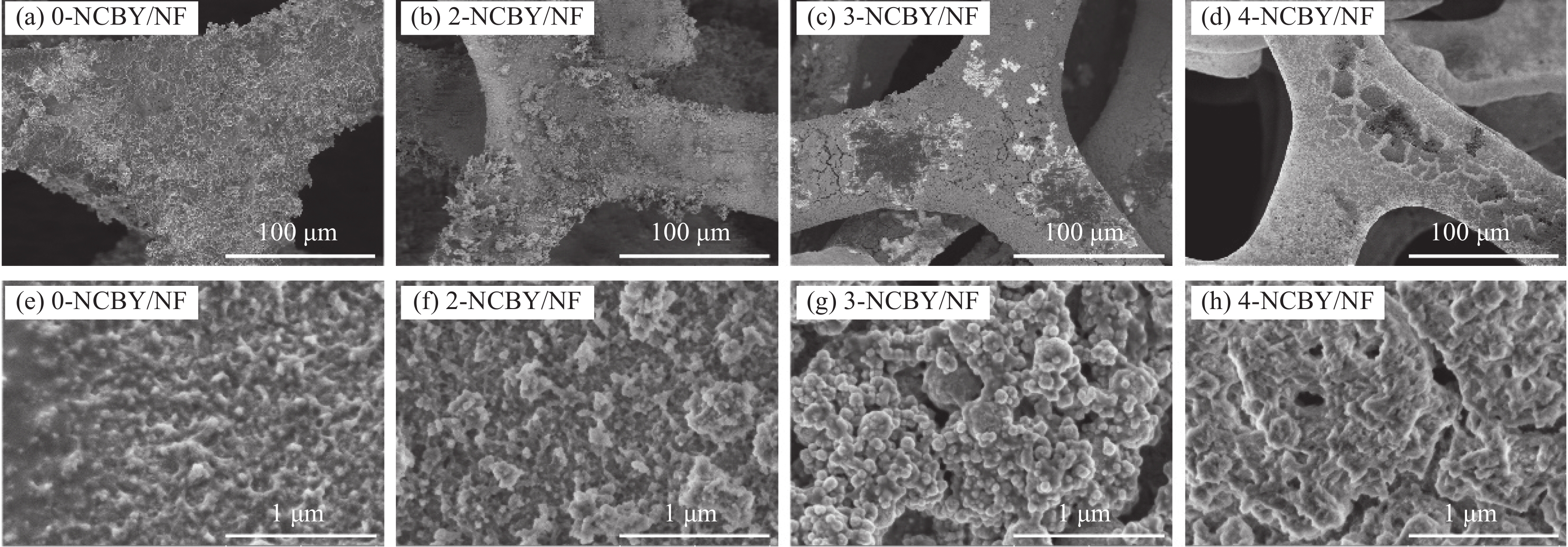
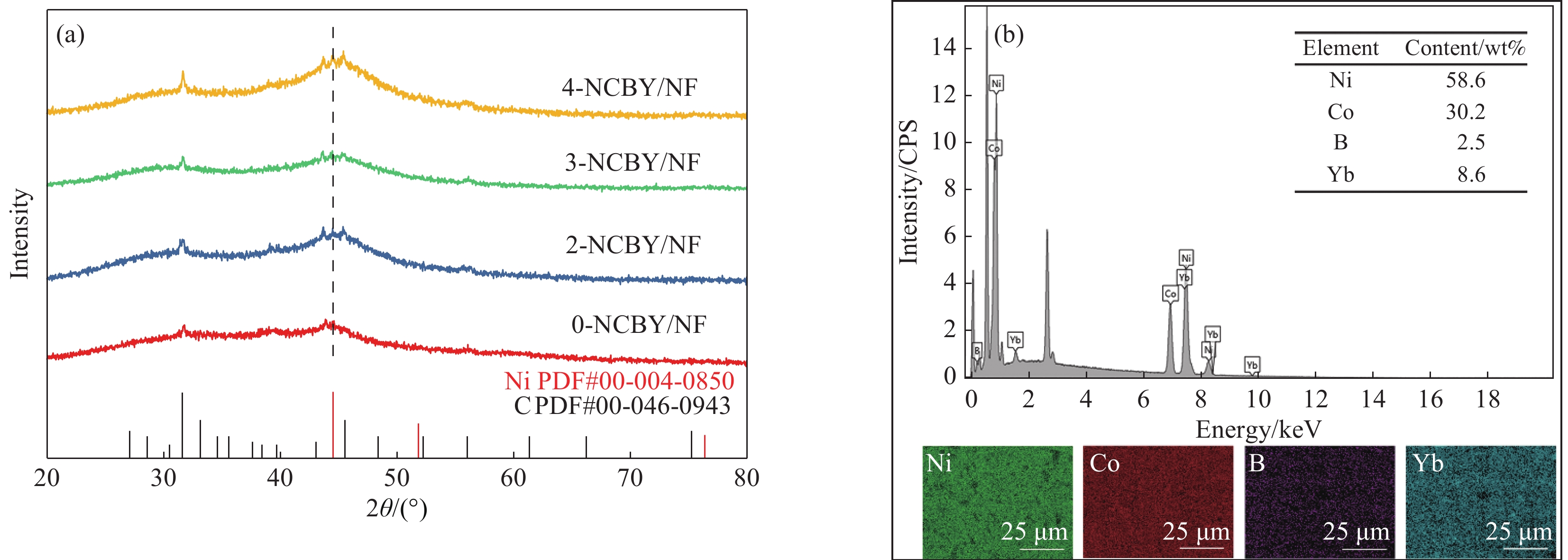
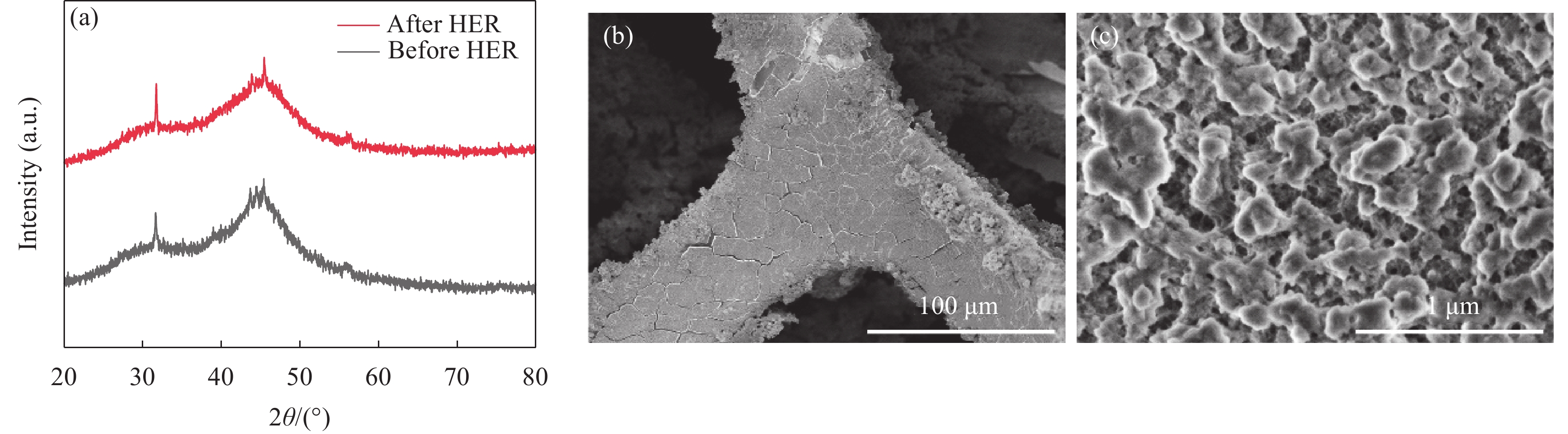
图1为0-NCBY/NF、2-NCBY/NF、3-NCBY/NF和4-NCBY/NF的SEM图像。低倍率SEM图像(图1(a)~图1(d))可知,加入稀土元素可以明显改变催化剂的表面形貌,减小催化电极表面颗粒的平均尺寸,其中3-NCBY/NF的镀层较均匀平整。高倍率SEM图像(图1(e)~图1(h))可知,随着稀土浓度的提高,镀层表面更加光滑,颗粒尺寸逐渐增大,其中3-NCBY/NF合金镀层颗粒完整,呈直径50~100 nm球状,同时形成大小不一的孔洞,较小的纳米颗粒能在电解质中捕获氢的中间体,有效提高电极表面的催化表面积,对电子在催化剂表面的电子传输有利,从而加强水分解的催化机制[22]。而随着稀土浓度继续增加,4-NCBY/NF表面颗粒开始发生团聚,催化表面积减小。图2(a)为所制催化剂粉末的XRD谱图,发现样品的衍射峰很弱,结晶度较差,这归因于含硼的过渡金属合金具有独特的非晶态结构[23]。在2θ = 44.5°的尖锐的衍射峰与Ni的(111)晶面对应,是样品粉末中夹带的泡沫镍基体峰。在2θ = 43.6°形成了尖锐的衍射峰,Ni的衍射峰向左偏移,表明杂原子成功加入导致晶格发生畸变。通过与PDF卡片(PDF#00-046-0943)进行对比发现2θ = 31°、45.5°和56°的尖锐峰为C的衍射峰,来自于无水乙醇、还原剂和镀液中的有机酸等[24]。结果表明稀土Yb浓度的改变对镀层的结构没有影响。
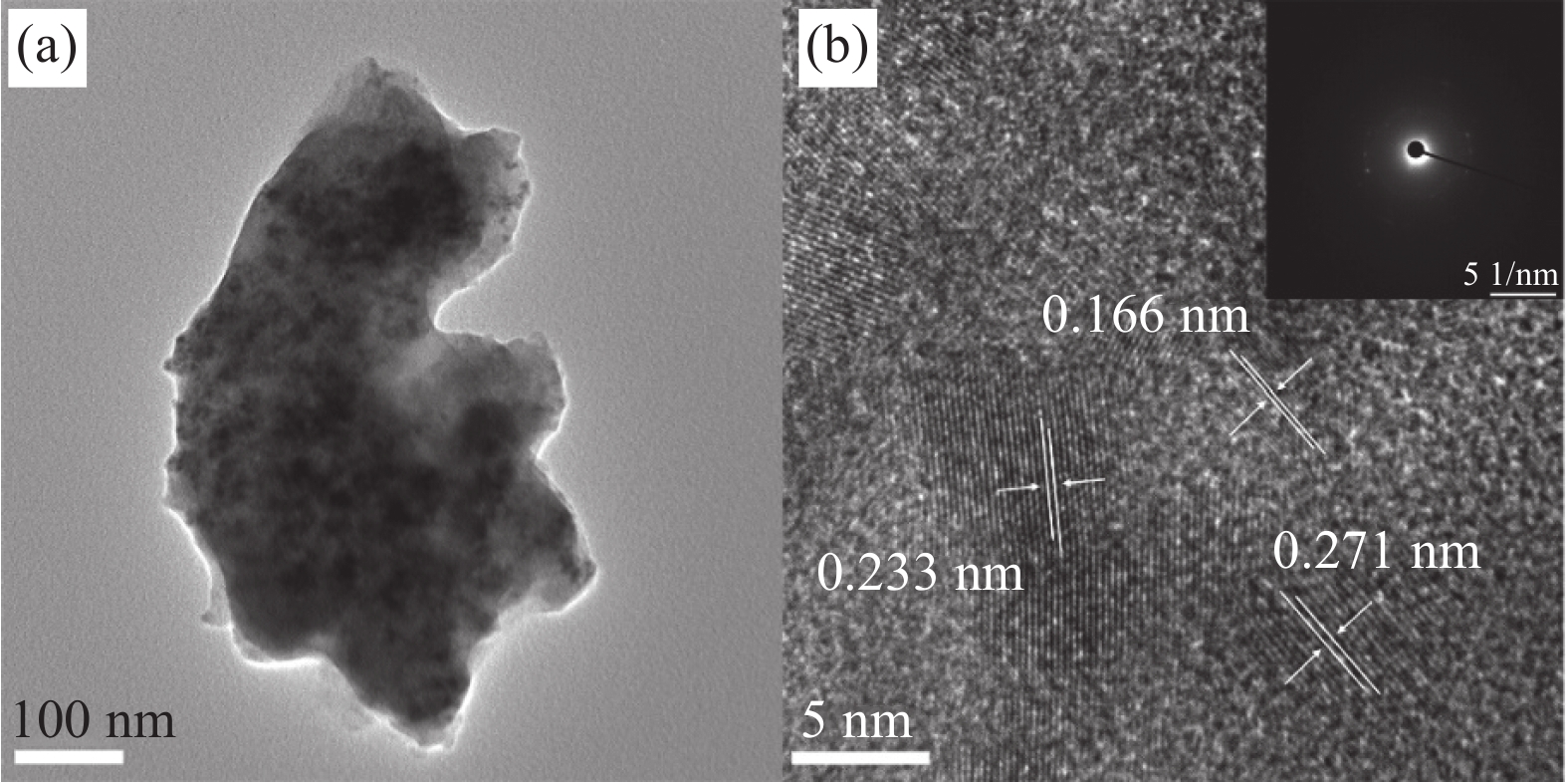
以3-NCBY/NF为例进一步获得更详细的元素分布和晶格信息。由EDS元素分布图像(图2(b))可知Yb成功加入Ni-Co-B镀层,且Ni、Co、B、Yb元素在电极表面分布均匀。图3为所制电极的透射电子显微镜(TEM)和高分辨率透射电子显微镜(HRTEM)图像,可以看出大量的非晶物质,同时观察到少量的纳米晶结构,研究表明化学镀Ni-B合金镀层的晶体结构与硼含量有关,镀层随硼含量的增加由晶态转变成非晶态[10, 25]。其中晶格间距d=0.233 nm和0.166 nm条纹归属于Ni的(111)和(200)晶面,晶格间距与PDF卡对比后发现变大,与XRD测试结果一致。晶格间距d = 0.271 nm归属于C的(020)晶面[26],来源于镀液中的有机物质。同时可以观察到不同晶体之间形成异质界面,可以降低反应能垒,促进催化中间体的电子或质子迅速转移。选区电子衍射图(SAED)图像呈现的多晶衍射环与XRD分析结果一致,说明该样品为非晶-纳米晶过渡态。
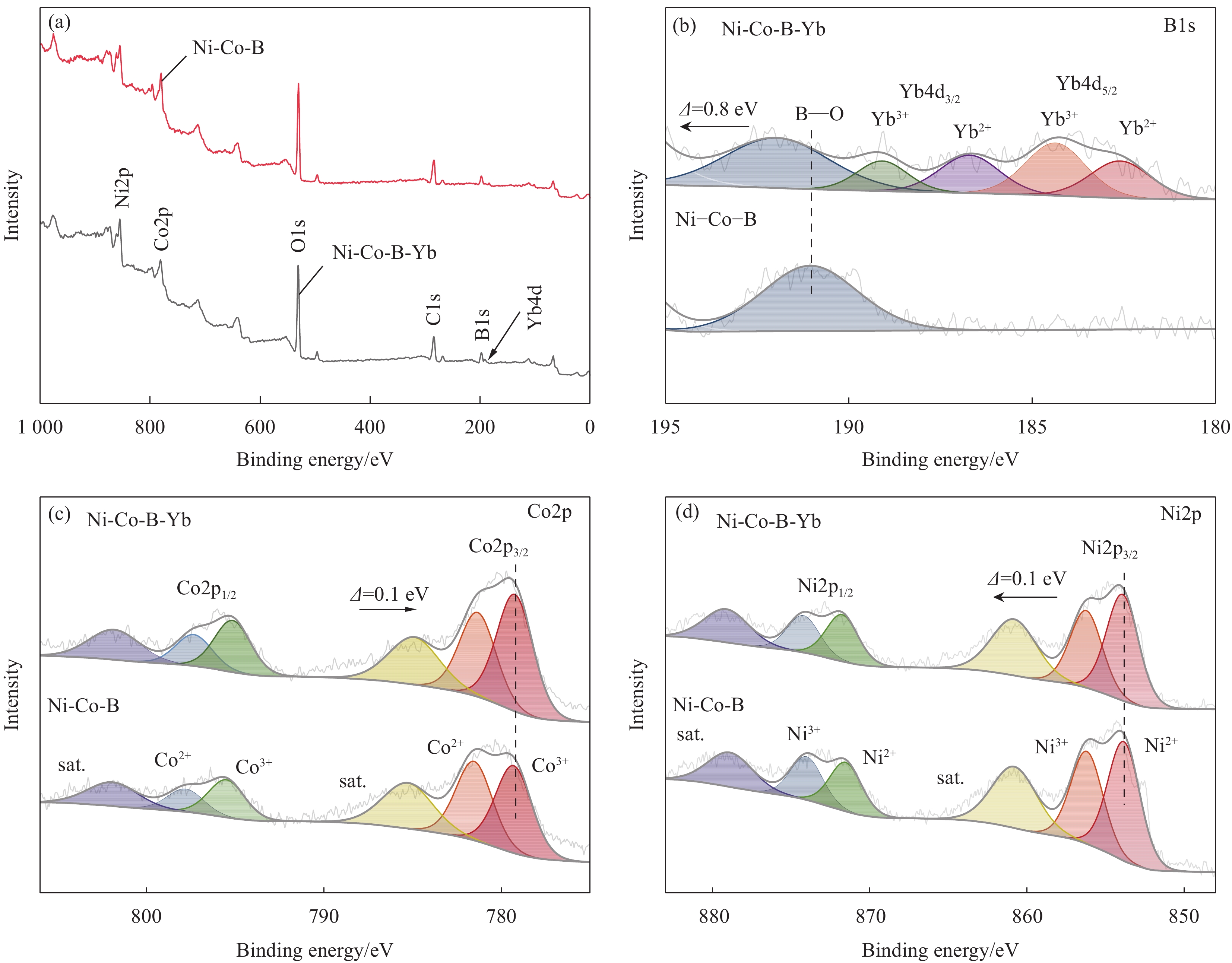
采用X射线光电子能谱研究稀土Yb对Ni-Co-B的元素组成和价态的影响。图4(a)为Ni-Co-B-Yb和Ni-Co-B的总测量光谱,可以看出Yb成功加入到Ni-Co-B催化剂中。图4(b)中191.9 eV处的结合能峰对应B元素,由于硼在空气中易被氧化,大部分以氧化物形式存在[27]。高分辨率Yb4d XPS谱图中出现了两组自旋轨道峰,182.6 eV和187.6 eV处的结合能峰为Yb2+4d5/2和Yb2+4d3/2,184.3 eV和189.0 eV处的结合能峰为Yb3+4d5/2和Yb3+4d3/2[28]。在Co2p XPS图谱中(图4(c)),779.2 eV和795.7 eV处的结合能峰为Co3+2p3/2和Co3+2p1/2,781.3 eV和797.3 eV处的结合能峰为Co2+2p3/2和Co2+2p1/2,784.9 eV和801.8 eV处为振荡卫星峰[29]。图4(d)为Ni2p XPS谱图,853.9 eV和871.7 eV处的两个结合能峰对应Ni2+2p3/2和Ni2+2p1/2,856.2 eV和874.2 eV处的两个峰对应Ni3+2p3/2和Ni3+2p1/2,860.8 eV和879.1 eV处为两个卫星峰。由于非晶金属硼化物暴露在空气中时立即被氧化,故Ni和Co主要以氧化物的形式存在。将Ni-Co-B-Yb与Ni-Co-B的Ni2p、Co2p和B1s光谱与标准结合能对照,Ni2p和Co2p的结合能均发生正位移,B1s的结合能发生负位移,这是电负性比Ni、Co高的B引起的电子迁移。Ni-Co-B-Yb与Ni-Co-B的谱图进行对比发现,Ni2p的结合能正位移大约0.1 eV,而B1s的结合能正位移大约0.8 eV。表明Yb的加入与其他金属产生了电子效应使电子密度发生改变,并诱导B的电子发生了逆向转移[30]。这是由于Yb原子通过金属键合所传导的电子改变了Ni-Co-B-Yb催化剂整体的电子结构,有助于提升催化性能[31]。
2.2 电化学性能分析
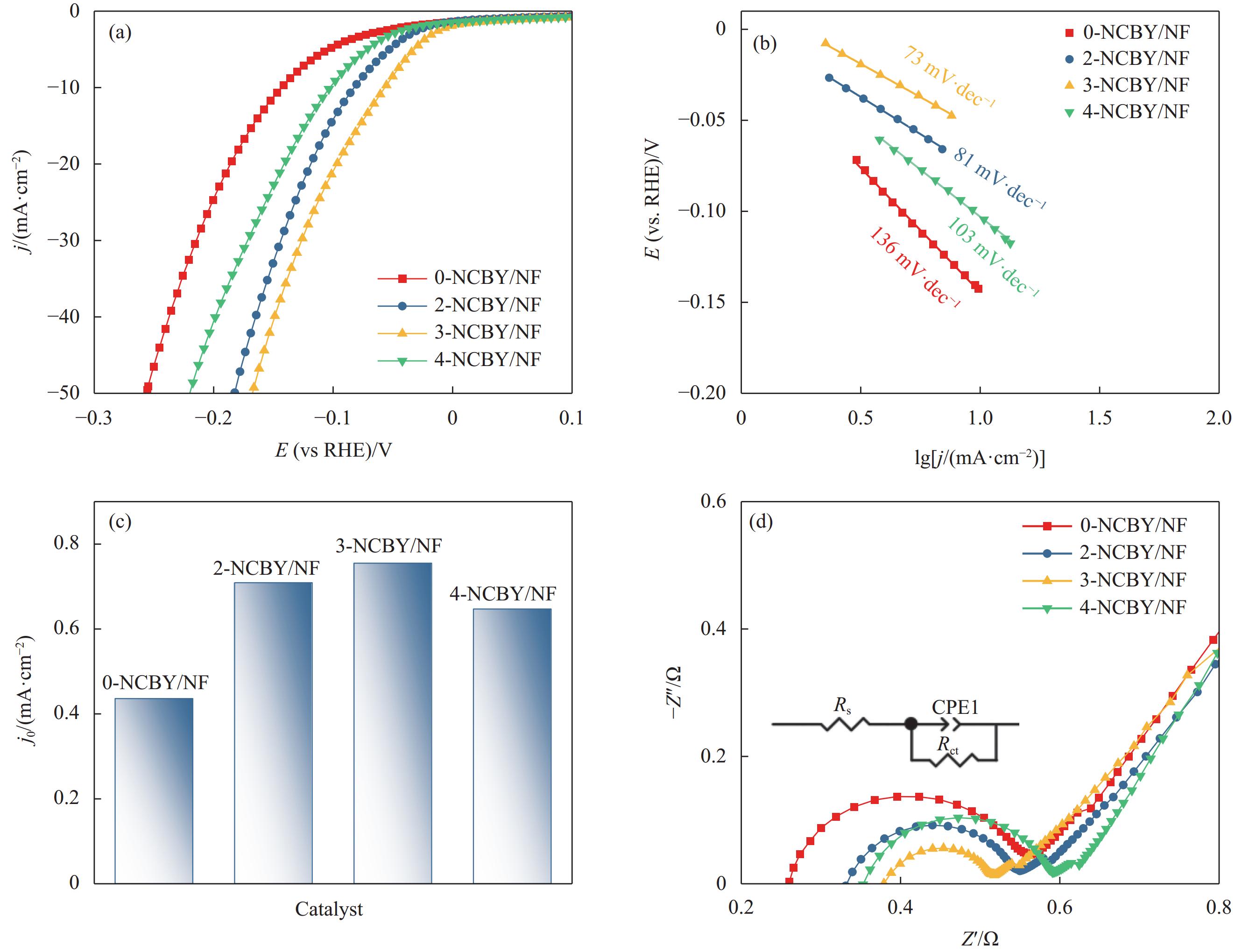
析氢过电位用作评估电极的电催化活性的关键指标,在相同的电流密度下,较低的过电位意味着催化剂具有更高的活性[32]。图5(a)为0-NCBY/NF、2-NCBY/NF、3-NCBY/NF和4-NCBY/NF在1.0 mol·L−1 KOH溶液中的析氢性能图,在10 mA·cm−2下所制电极的析氢过电位分别为145、83、57和103 mV,可知加入稀土Yb的电极的析氢过电位均变小,这是由于稀土元素与合金之间的相互作用,增加更多活性位点,从而增加反应速率[16]。此外Yb具有丰富的电子结构, Yb2+和Yb3+之间可以快速转变从而形成了丰富的氧空位[33],有利于电荷转移和吸收,从而提高催化剂的析氢性能。随着稀土浓度的增加,析氢过电位呈现先增大后减小的趋势,其中3-NCBY/NF的析氢过电位最低,析氢性能最佳。
对LSV曲线进行线性拟合得到Tafel斜率,可以进一步评价催化剂的HER反应动力学。图5(b)可知0-NCBY/NF、2-NCBY/NF、3-NCBY/NF和4-NCBY/NF的Tafel斜率分别为136 mV·dec−1、81 mV·dec−1、73 mV·dec−1和103 mV·dec−1。其中3-NCBY/NF的Tafel斜率值最小,表明具有明显的HER动力学,较好的催化活性。HER反应的催化机制包括Volmer (120 mV·dec−1)、Heyrovsky(40 mV·dec−1)和Tafel (mV·dec−1)反应。所制催化剂电极的HER过程均大于40 mV·dec−1,为Volmer-Heyrovsky反应机制。而交换电流密度(j0)是评估催化剂效率的另一关键参数,由Tafel曲线得到j0(图5(c)),3-NCBY/NF (5.62 mA·cm−2)的j0大于0-NCBY/NF (2.69 mA·cm−2)、2-NCBY/NF (5.0 mA·cm−2)和4-NCBY/NF (4.36 mA·cm−2),表明3-NCBY/NF的反应得失电子能力较强,其具有优异的电催化活性。
EIS 是评价电催化剂的重要指标,从中得到的电荷转移电阻(Rct)反映电极材料在液相反应中界面电子的传递速率。图5(d)是电极的EIS图谱,各催化剂均呈明显的半圆形曲线,弧半径越小,电荷转移阻力越小。并对曲线进行拟合得到0-NCBY/NF、2-NCBY/NF、3-NCBY/NF和4-NCBY/NF的Rct值分别为0.278 Ω·cm–2、0.190 Ω·cm–2、0.114 Ω·cm–2和0.209 Ω·cm–2,可知样品的Rct值均较小,这归因于泡沫镍基体的高导电性和三维立体结构。其中3-NCBY/NF的弧半径最小,Rct值最小,表明其具有较高导电性,较低的界面电阻。综上表明,3-NCBY/NF在HER过程中具有良好HER动力学特性。
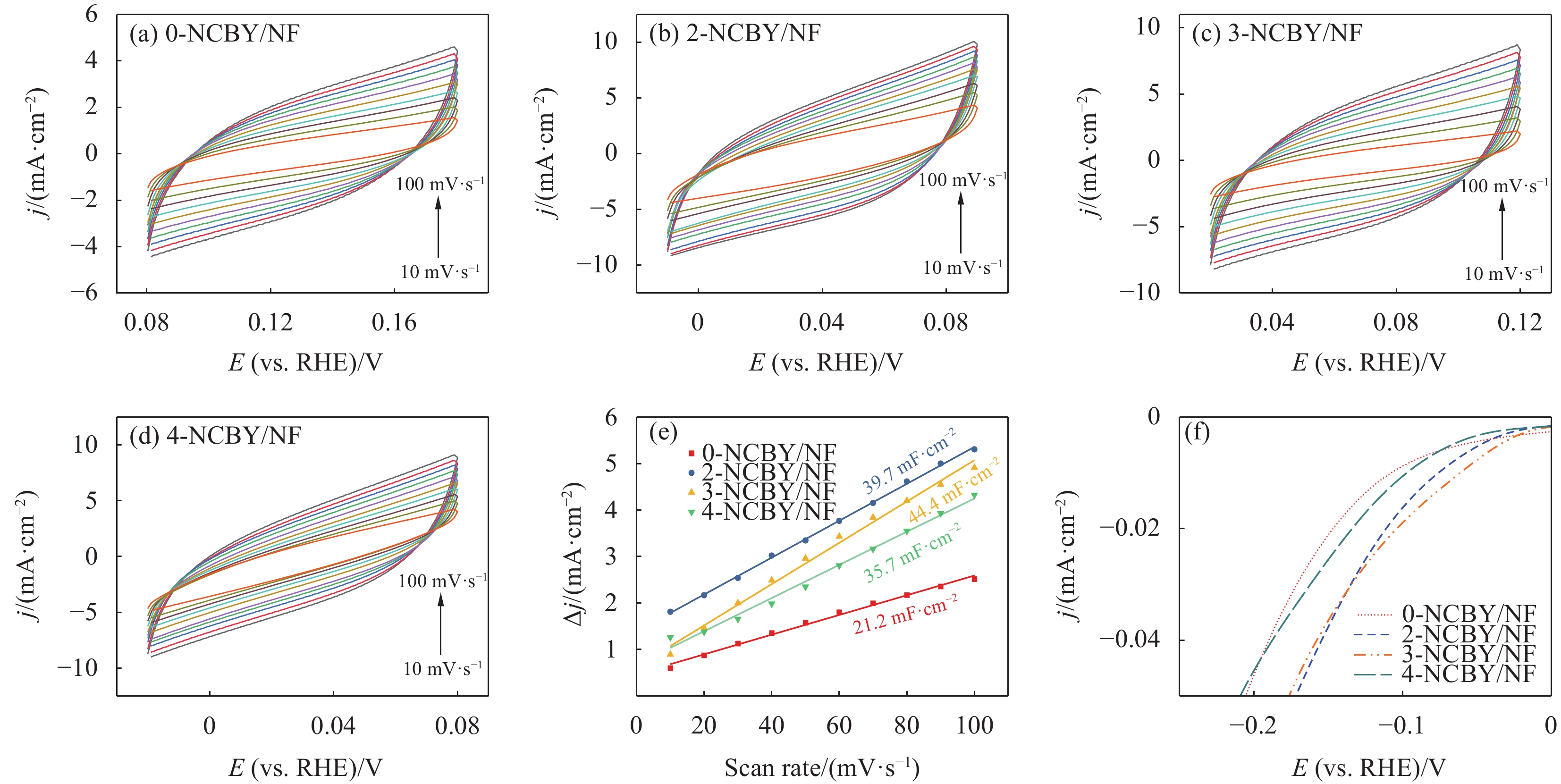
电极表面参与电化学反应的活性比表面积(ECSA)对电极的电催化性能有较大的影响。通过电化学测试估算样品的ECSA。图6(a)~图6(d)为所制电极的循环伏安曲线,根据CV测试结果绘制出催化电极的充电双电层库伦(Cdl)曲线(图6(e)),可知3-NCBY/NF的Cdl值为44.4 mF·cm−2,大于0-NCBY/NF(21.2 mF·cm−2)、2-NCBY/NF(39.7 mF·cm−2)和4-NCBY/NF(35.7 mF·cm−2)。进而计算各催化电极的ECSA值,3-NCBY/NF的ECSA值为
1110 cm2·g−1,大于0-NCBY/NF (530.0 cm2·g−1)、2-NCBY/NF (992.5 cm2·g−1)和4-NCBY/NF (892.5 cm2·g−1),说明3-NCBY/NF表面暴露较多的活性位点,具有优异的析氢活性。对极化曲线进行归一化处理,分析催化剂的本征性能。图6(f)可知3-NCBY/NF的析氢过电位较低,具有较好的内在催化活性。综上所述,稀土镱的加入可以提高催化剂的催化活性表面积和提升催化剂的本征活性。![]() 图 6 0-NCBY/NF、2-NCBY/NF、3-NCBY/NF和4-NCBY/NF电极在不同扫描速率下的CV曲线((a)~(d))、充电双电层库仑曲线(e)、电化学活性比表面积(ECSA)归一化曲线(f)Figure 6. CV curves at different scan rates ((a)-(d)), charge double-layer voltammetry (e) and electrochemcial active surface area (ECSA) normalized curves (f) of 0-NCBY/NF, 2-NCBY/NF, 3-NCBY/NF and 4-NCBY/NF electrodes
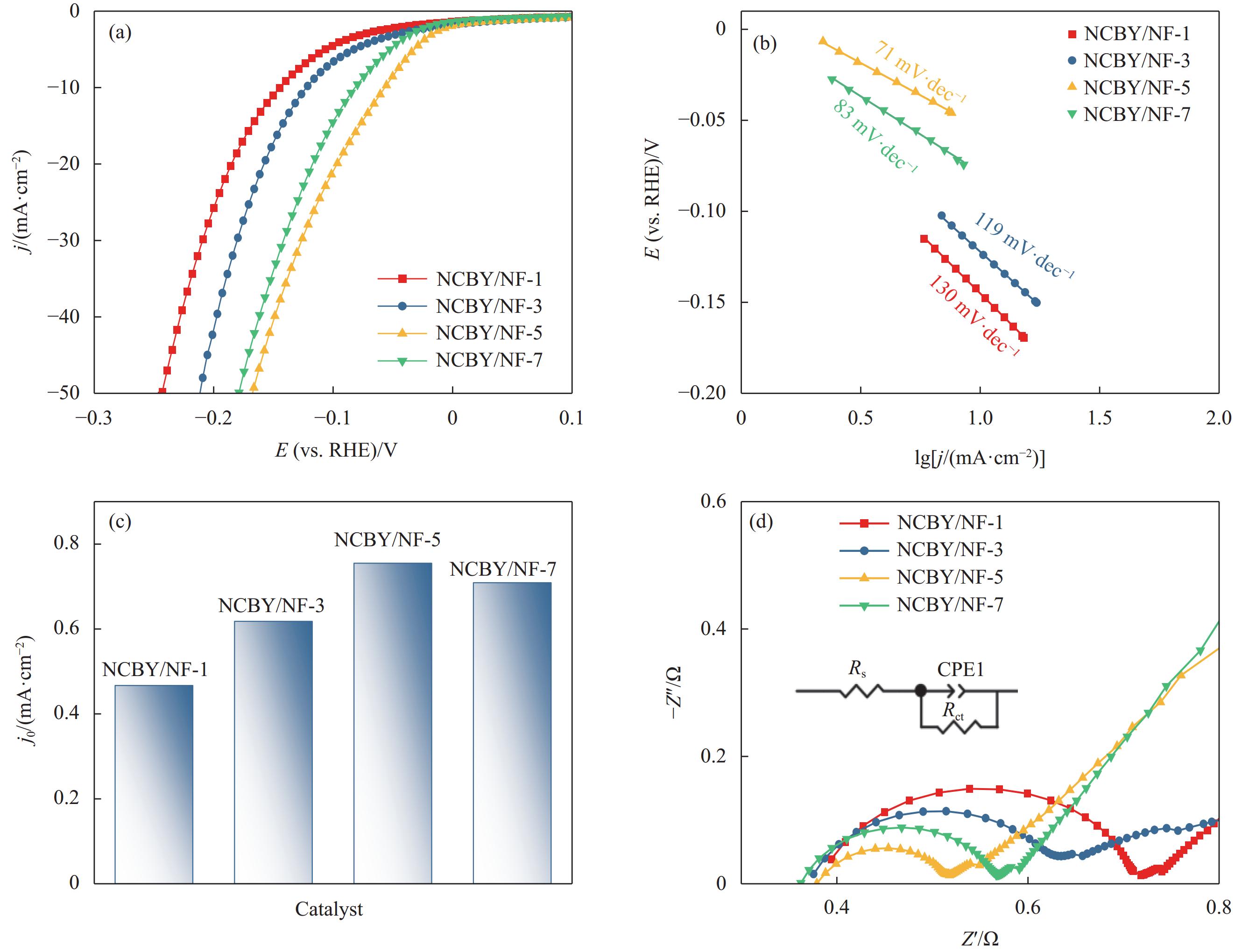
图 6 0-NCBY/NF、2-NCBY/NF、3-NCBY/NF和4-NCBY/NF电极在不同扫描速率下的CV曲线((a)~(d))、充电双电层库仑曲线(e)、电化学活性比表面积(ECSA)归一化曲线(f)Figure 6. CV curves at different scan rates ((a)-(d)), charge double-layer voltammetry (e) and electrochemcial active surface area (ECSA) normalized curves (f) of 0-NCBY/NF, 2-NCBY/NF, 3-NCBY/NF and 4-NCBY/NF electrodes比较NCBY/NF-x (x=1、3、5、7)在1.0 mol·L−1 KOH中的析氢性能,进一步研究不同钴浓度对HER性能的影响。由图7(a)可知,不同钴浓度下制备的Ni-Co-B-Yb对HER有显著差异,在10 mA·cm−2电流密度下NCBY/NF-x (x = 1、3、5、7)的过电位分别为144 mV、123 mV、57 mV和82 mV,随着Co浓度的增加呈现先增大后减小的趋势,说明钴浓度对HER 催化性能有较大影响。图7(b)为NCBY/NF-x (x = 1、3、5、7)的Tafel斜率图,NCBY/NF-5 (71 mV·dec−1)的Tafel斜率小于NCBY/NF-1 (130 mV·dec−1)、NCBY/NF-3 (119 mV·dec−1)和NCBY/NF-7 (83 mV·dec−1),表明NCBY/NF-5具有较高的催化活性,明显的HER动力学。其中各电极的反应机制均为Heyrovsky-Volmer机制。NCBY/NF-x(x = 1、3、5、7)的交换电流密度(j0)如图7(c),NCBY/NF-x(x = 1、3、5、7)的j0值分别为2.93 mA·cm−2、4.14 mA·cm−2、5.62 mA·cm−2和 5.11 mA·cm−2,可知NCBY/NF-5的j0值最大,说明NCBY/NF-5得失电子能力强,具有优异的电催化活性。通过EIS图谱,进一步评估电极的反应动力学。NCBY/NF-x (x = 1、3、5、7)的EIS图谱(图7(d))中,NCBY/NF-5的弧半径较小,表明其具有较小的电化学阻抗。并对曲线进行拟合后的Rct值分别为0.299 Ω·cm–2、0.278 Ω·cm–2、0.114 Ω·cm–2和0.181 Ω·cm–2,其中NCBY/NF-5的Rct值最小,导电性较高,具有较高的催化效率。
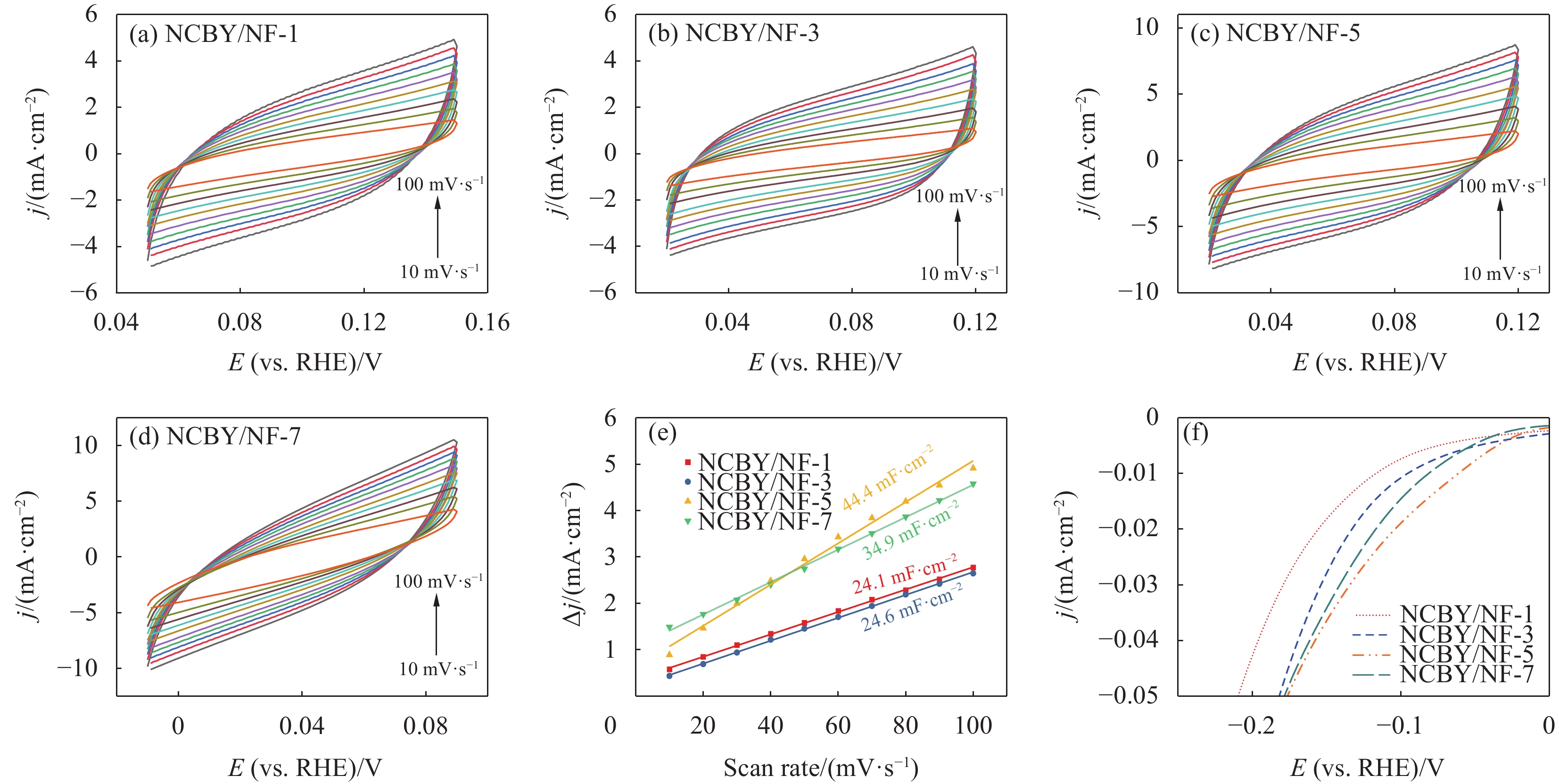
图8(a)~图8(d)为不同催化电极材料在无法拉第电流区域10~100 mV·s−1扫描速率下获得的循环伏安(CV)曲线。根据CV测试结果得到样品的Cdl曲线图(图8(e)),NCBY/NF-x(x = 1、3、5、7)的Cdl分别为24.1、24.6、44.4和34.9 mF·cm−2。计算得到的各催化电极的ECSA值分别为602.5 cm2·g−1、615.0 cm2·g−1、
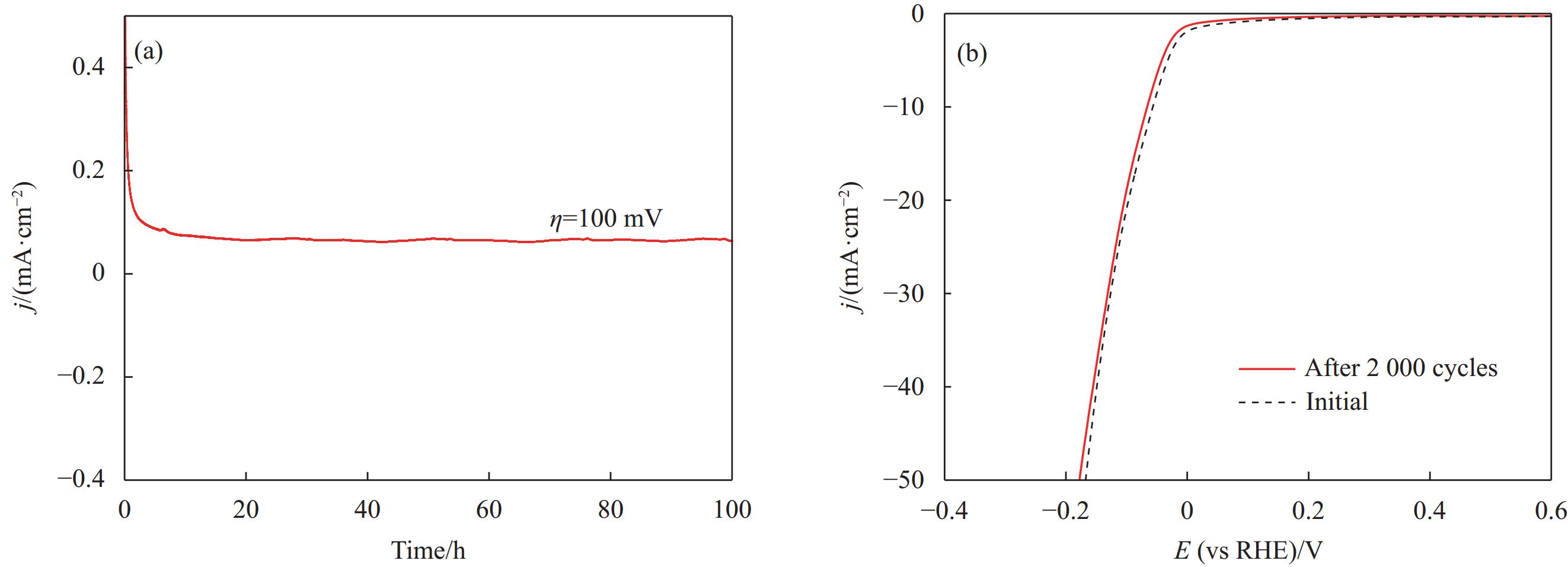
1110.0 cm2·g−1和872.5 cm2·g−1,其中NCBY/NF-5的ECSA最大,有更多的催化活性位点,加速游离的H*快速和电子结合形成H2,增大与电解质的接触面积,缩短传质路径,表现出更强的电化学性质。图8(f)是将ECSA标准化的极化曲线,分析表明NCBY/NF-5的本征催化活性更突出。综上所述,Yb和Co浓度分别为3 g·L−1和5 g·L−1条件下制备的催化电极具有优异的电催化性能。归因于在此浓度下制备的催化剂材料中各金属元素间具有较好的协同作用,高效提升材料的固有催化活性。同时改善催化剂的微观形貌,增大电极材料的比表面积,暴露更多的活性位点,从而提升材料的催化活性。电催化稳定性是对电极电催化性能的关键要求,使用计时电流法来衡量其时间与电流的变化。从图9(a)可知,Ni-Co-B-Yb在100 mV恒电位下持续电解100 h,电流密度无明显的波动,展现出杰出的稳定性。进一步采用循环伏安法,比较催化剂循环
2000 圈CV前后的LSV曲线(图9(b)),可知Ni-Co-B-Yb的LSV曲线无明显变化,基本保持恒定,表明该电极稳定性较高。用XPS和SEM表征I-t测试后Ni-Co-B-Yb/NF的形貌结构变化。图10(a)说明HER后的Ni-Co-B-Yb的晶体结构没有明显变化。从SEM图(图10(b)、图10(c))可以看出,Ni-Co-B-Yb/NF的表面出现少量裂纹,高倍照片表明形貌基本保持不变。进一步证明 Ni-Co-B-Yd/NF具有稳定的催化活性。表 2 Ni-Co-B-Yb/NF与最近报道的电催化剂的 HER 性能进行对比Table 2. Comparison of HER performance of Ni-Co-B-Yb/NF with recently reported electrocatalystsNotes Electrocatalysts Electrolyte η10/mV Tafel slop/(mV dec−1) Ref. 1 Ni-Co-B-Yb/Nickel foam(NF) 1.0 mol/L KOH 57 73 This work 2 Ni3N/Ni 1.0 mol/L KOH 144 107 [34] 3 Ni-MgO/ Carbon nanotube (CNT) 1.0 mol/L KOH 117 116 [35] 4 Co2NiMo-N 1.0 mol/L KOH 69 77.8 [36] 5 (Ag:Cu)/ Boron nanosheets (BNS) 1.0 mol/L KOH 101 57 [37] 6 NiCoP 1.0 mol/L KOH 141 66 [38] 7 Ni-Co/Mo2C/Co6Mo6C2@C 1.0 mol/L KOH 95 99.92 [39] 8 Ru-NiSe2/ Nickel foam(NF) 1.0 mol/L KOH 39.3 36 [40] 9 Ni-Ce-Pr-Ho/ Nickel foam(NF) 1.0 mol/L KOH 78 121.6 [41] 10 Ni2P-Pr 1.0 mol/L KOH 87 65.4 [42] 11 Ni-La 1.0 mol/L KOH 190 68 [43] 12 Ni-P-La 1.0 mol/L KOH 139 93 [44] 3. 结 论
(1)本文通过简单的一步化学沉积法制备出纳米球状结构的Ni-Co-B-Yb/泡沫镍(NF)稀土复合电催化剂材料,探究了稀土Yb的引入对过渡金属基材料电化学性能的影响。
(2)稀土Yb的加入可以改变催化剂的纳米结构,降低了过渡金属基电极表面颗粒的平均直径,有利于增加催化活性面积。另外Yb可以调节Ni-Co-B内部电子结构,丰富的价态增加了反应活性位点,提高其对氢离子的吸附和活化能力,增加了各金属元素之间的相互作用,进而提升析氢性能。同时稀土Yb的添加可以优化析氢动力学,降低析氢反应的过电势,提高析氢速率。
(3)原位生长的自支撑电极(Ni-Co-B-Yb/NF)的复合结构能够展现出优异的电催化性能。根据1 mol·L−1 KOH的析氢性能分析结果,表明稀土Yb元素的添加显著提高了催化剂的本征活性。当稀土含量为3 g·L−1和Co浓度为5 g·L−1时制备的Ni-Co-B-Yb/NF的催化性能最佳。在电流密度为10 mA·cm−2时,过电位仅为57 mV,Tafel斜率为73 mV·dec−1。同时,Ni-Co-B-Yb/NF也展示出优异的稳定性。这项工作表明稀土元素在电催化领域的应用,为过渡金属基高效电解水催化剂的发展提供了一种新的策略。
-
图 6 0-NCBY/NF、2-NCBY/NF、3-NCBY/NF和4-NCBY/NF电极在不同扫描速率下的CV曲线((a)~(d))、充电双电层库仑曲线(e)、电化学活性比表面积(ECSA)归一化曲线(f)
Figure 6. CV curves at different scan rates ((a)-(d)), charge double-layer voltammetry (e) and electrochemcial active surface area (ECSA) normalized curves (f) of 0-NCBY/NF, 2-NCBY/NF, 3-NCBY/NF and 4-NCBY/NF electrodes
表 1 化学镀液配方
Table 1 Chemical deposition plating solution formula
Composition of plating solution Concentration/
(g·L−1)Anhydrous nickel chloride (NiCl2) 10.0 Anhydrous cobalt chloride (CoCl2) 1.0-7.0 Borane dimethylamine (C2H10BN) 1.0 Ytterbium nitrate [Yb(NO3)3] 2.0-4.0 Adipic acid (C6H10O4) 1.5 Citric acid (C6H8O7) 1.5 Malic acid (C4H6O5) 1.5 表 2 Ni-Co-B-Yb/NF与最近报道的电催化剂的 HER 性能进行对比
Table 2 Comparison of HER performance of Ni-Co-B-Yb/NF with recently reported electrocatalysts
Notes Electrocatalysts Electrolyte η10/mV Tafel slop/(mV dec−1) Ref. 1 Ni-Co-B-Yb/Nickel foam(NF) 1.0 mol/L KOH 57 73 This work 2 Ni3N/Ni 1.0 mol/L KOH 144 107 [34] 3 Ni-MgO/ Carbon nanotube (CNT) 1.0 mol/L KOH 117 116 [35] 4 Co2NiMo-N 1.0 mol/L KOH 69 77.8 [36] 5 (Ag:Cu)/ Boron nanosheets (BNS) 1.0 mol/L KOH 101 57 [37] 6 NiCoP 1.0 mol/L KOH 141 66 [38] 7 Ni-Co/Mo2C/Co6Mo6C2@C 1.0 mol/L KOH 95 99.92 [39] 8 Ru-NiSe2/ Nickel foam(NF) 1.0 mol/L KOH 39.3 36 [40] 9 Ni-Ce-Pr-Ho/ Nickel foam(NF) 1.0 mol/L KOH 78 121.6 [41] 10 Ni2P-Pr 1.0 mol/L KOH 87 65.4 [42] 11 Ni-La 1.0 mol/L KOH 190 68 [43] 12 Ni-P-La 1.0 mol/L KOH 139 93 [44] -
[1] ZHU J, HU L S, ZHAO P X, et al. Recent advances in electrocatalytic hydrogen evolution using nanoparticles[J]. Chemical Reviews, 2020, 120(2): 851-918. DOI: 10.1021/acs.chemrev.9b00248
[2] ZHU P, XIONG X, WANG D S. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction[J]. Nano Research, 2022, 15(7): 5792-5815. DOI: 10.1007/s12274-022-4265-y
[3] YANG J S, LI J, WANG Y, et al. Tailoring the pore structure of porous Ni-Sn alloys for boosting hydrogen evolution reaction in alkali solution[J]. Metals, 2022, 12(12): 13.
[4] XU B, LIANG J, SUN X P, et al. Designing electrocatalysts for seawater splitting: Surface/interface engineering toward enhanced electrocatalytic performance[J]. Green Chemistry, 2023, 25(10): 3767-3790. DOI: 10.1039/D2GC03377A
[5] ZHANG X, JIA F F, SONG S X. Recent advances in structural engineering of molybdenum disulfide for electrocatalytic hydrogen evolution reaction[J]. Chemical Engineering Journal, 2021, 405: 16.
[6] GUO F, MACDONALD T J, SOBRIDO A J, et al. Recent advances in ultralow-Pt-loading electrocatalysts for the efficient hydrogen evolution [J]. 2023, 10(21): 2301098.
[7] XU H, SHANG H Y, WANG C, et al. Ultrafine Pt-based nanowires for advanced catalysis[J]. Advanced Functional Materials, 2020, 30(28): 18.
[8] EL-REFAEI S M, RUSSO P A, PINNA N. Recent advances in multimetal and doped transition-metal phosphides for the hydrogen evolution reaction at different pH values[J]. ACS Applied Materials & Interfaces, 2021, 13(19): 22077-22097.
[9] FENG J X, WU J Q, TONG Y X, et al. Efficient hydrogen evolution on Cu nanodots-decorated Ni3S2 nanotubes by optimizing atomic hydrogen adsorption and desorption[J]. Journal of the American Chemical Society, 2018, 140(2): 610-617. DOI: 10.1021/jacs.7b08521
[10] BARATI Q, HADAVI S M M. Electroless Ni-B and composite coatings: A critical review on formation mechanism, properties, applications and future trends[J]. Surfaces and Interfaces, 2020, 21: 13.
[11] HAYAT A, SOHAIL M, ALI H, et al. Recent advances and future perspectives of metal-based electrocatalysts for overall electrochemical water splitting[J]. Chemical Record, 2023, 23(2): 64.
[12] CHEN Z J, DUAN X G, WEI W, et al. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution[J]. Journal of Materials Chemistry A, 2019, 7(25): 14971-15005. DOI: 10.1039/C9TA03220G
[13] ZHANG H Y, WANG Y, SONG D Q, et al. Cerium-based electrocatalysts for oxygen evolution/reduction reactions: Progress and perspectives[J]. Nanomaterials, 2023, 13(13): 24.
[14] LI Y F, YUAN X L, WANG P, et al. Rare earth alloy nanomaterials in electrocatalysis[J]. Journal of Energy Chemistry, 2023, 83: 574-594. DOI: 10.1016/j.jechem.2023.04.050
[15] WANG X, TANG Y W, LEE J M, et al. Recent advances in rare-earth-based materials for electrocatalysis[J]. Chem Catalysis, 2022, 2(5): 967-1008. DOI: 10.1016/j.checat.2022.02.007
[16] CARDOSO D S P, AMARAL L, SANTOS D M F, et al. Enhancement of hydrogen evolution in alkaline water electrolysis by using nickel-rare earth alloys[J]. International Journal of Hydrogen Energy, 2015, 40(12): 4295-4302. DOI: 10.1016/j.ijhydene.2015.01.174
[17] LU Y Z, TANG L L, WANG P, et al. Rare earth-based alloy nanostructure for hydrogen evolution reaction[J]. ACS Catalysis, 2023, 13(20): 13804-13815. DOI: 10.1021/acscatal.3c03350
[18] 景欣欣, 陈必清, 翟佳鑫, 等. Ni-Co-B-RE(Sm、Dy、Tb)复合电极: 化学沉积法制备及电催化析氢性能研究[J]. 无机材料学报, 2024, 39(5): 467-476. DOI: 10.15541/jim20230491 JING Xinxin, CHEN Biqing, ZHAI Jiaxin, et al. Ni-Co-B-RE (Sm, Dy, Tb) composite electrodes: Preparation by chemical deposition method and electrocatalytic hydrogen evolution performance[J]. Journal of Inorganic Materials, 2024, 39(5): 467-476(in Chinese). DOI: 10.15541/jim20230491
[19] R. R M, G. A E-M M, H. A H, et al. Tailor-designed bimetallic Co/Ni macroporous electrocatalyst for efficient glycerol oxidation and water electrolysis[J]. International Journal of Hydrogen Energy, 2022, 47(75): 32145-32157.
[20] VESZTERGOM S, DUTTA A, RAHAMAN M, et al. Hydrogen bubble templated metal foams as efficient catalysts of CO2 electroreduction[J]. Chemcatchem, 2021, 13(4): 1039-1058. DOI: 10.1002/cctc.202001145
[21] ANASTASIADOU D, LIGT B, HE Y Y, et al. Carbon dioxide and nitrate co-electroreduction to urea on CuOx-ZnOy[J]. Communications Chemistry, 2023, 6(1): 8. DOI: 10.1038/s42004-022-00803-3
[22] 邱文婕, 胡珍, 周其洪, 等. 稀土氧化铈增强的钴基电解水催化材料及其性能[J]. 复合材料学报, 2024, 41(2): 804-815. QIU Wenjie, HU Zhen, ZHU Qihong, et al. Rare earth cerium oxide reinforced cobalt based catalysts for electrolysed water and their properties[J]. Acta Materiae Compositae Sinica, 2024, 41(2): 804-815(in Chinese).
[23] MAREK L, MARIA B, KAROLINA J, et al. Transition metal borides of Ni-B (Co-B) as alternative non-precious catalytic materials: Advances, potentials, and challenges. Short review[J]. Journal of Industrial and Engineering Chemistry, 2022, 116: 75-98. DOI: 10.1016/j.jiec.2022.09.031
[24] 张士民, 陈必清, 高利霞, 等. 基于Eu-Ni-B稀土-复合电极电催化偏硼酸钠制备硼氢化钠的探索[J]. 功能材料, 2020, 51(4): 4207-4214. DOI: 10.3969/j.issn.1001-9731.2020.04.035 ZHANG Shimin, CHEN Biqing, GAO Lixia, et al. Exploration of electrocatalytic preparation of sodium borohydride with sodium metaborate based on Eu-Ni-B rare earth-composite electrode[J]. Journal of Functional Materials, 2020, 51(4): 4207-4214(in Chinese). DOI: 10.3969/j.issn.1001-9731.2020.04.035
[25] LI X S, ZHOU J, SHEN L Q, et al. Exceptionally high saturation magnetic flux density and ultralow coercivity via an amorphous-nanocrystalline transitional microstructure in an FeCo-based alloy[J]. Advanced Materials, 2022, 35(50): 2205863.
[26] BHATTACHARYA S, PHATAKE R S, BARNEA S N, et al. Fluorescent self-healing carbon dot/polymer gels[J]. ACS Nano, 2019, 13(2): 1433-1442.
[27] JOKAR A, TOGHRAEI A, MALEKI M, et al. Facile electrochemical synthesis of Ni-Co-B film on Cu sheet for dual-electrocatalysis of hydrogen and oxygen evolution reactions[J]. Electrochimica Acta, 2021, 389: 10.
[28] OHNO Y. XPS studies of the intermediate valence state of Yb in (YbS)1.25CrS2[J]. Journal of Electron Spectroscopy and Related Phenomena, 2008, 165(1-3): 1-4. DOI: 10.1016/j.elspec.2008.05.009
[29] LI D X, GUO Z M, ZHAO R H, et al. An efficient cerium dioxide incorporated nickel cobalt phosphide complex as electrocatalyst for all-pH hydrogen evolution reaction and overall water splitting[J]. Journal of Colloid and Interface Science, 2024, 653: 1725-1742. DOI: 10.1016/j.jcis.2023.09.144
[30] HAOYU L, DUODUO G, PING W, et al. Amorphization-induced reverse electron transfer in NiB cocatalyst for boosting photocatalytic H2 production[J]. Applied Catalysis B: Environmental, 2024, 340: 123270. DOI: 10.1016/j.apcatb.2023.123270
[31] GAO W, WEN D, HO J C, et al. Incorporation of rare earth elements with transition metal-based materials for electrocatalysis: A review for recent progress[J]. Materials Today Chemistry, 2019, 12: 266-281. DOI: 10.1016/j.mtchem.2019.02.002
[32] ASGARI M, DARBAND G B, MONIRVAGHEFI M. Electroless deposition of Ni-W-Mo-Co-P films as a binder-free, efficient and durable electrode for electrochemical hydrogen evolution[J]. Electrochimica Acta, 2023, 446: 13.
[33] CHAIKA M, VOVK O, MANCARDI G, et al. Dynamics of Yb2+ to Yb3+ ion valence transformations in Yb: YAG ceramics used for high-power lasers[J]. Optical Materials, 2020, 101: 8.
[34] XIONG L W, QIU Y F, DONG H, et al. Metallic Ni3N/Ni heterostructure for efficient hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2024, 59: 400-407. DOI: 10.1016/j.ijhydene.2024.01.312
[35] MOHANA P, ISACFRANKLIN M, YUVAKKUMAR R, et al. Facile synthesis of Ni-MgO/CNT nanocomposite for hydrogen evolution reaction[J]. Nanomaterials, 2024, 14(3): 14.
[36] YU S S, XU J, WANG Q Y, et al. Oxygen-vacancy-enriched Co2NiMo-N hollow polymetallic nitrides for the electrocatalytic hydrogen evolution reaction[J]. Journal of Alloys and Compounds, 2024, 977: 7.
[37] SINGLA A, DHIMAN R, MAHAJAN A. A bimetallic-doped boron nanosheet electrocatalyst for efficient hydrogen evolution reaction[J]. Journal of Electronic Materials, 2024 : 9.
[38] BERA C, STRECKOVA M, ORINAKOVA R, et al. NiCoP fibers as novel catalysts for hydrogen evolution in alkali and acidic environment[J]. International Journal of Hydrogen Energy, 2024, 60: 118-132. DOI: 10.1016/j.ijhydene.2024.02.195
[39] GU J X, ZHU Y, ZHENG H Y, et al. Small-sized NiCo/Mo2C/Co6Mo6C2@C for efficient alkaline and acidic hydrogen evolution reaction by an anchoring calcination strategy[J]. Frontiers of Chemical Science and Engineering, 2024, 18(5): 9.
[40] MA S, YANG P Y, CHANG J, et al. High-density accessible Ru-Se-Ni moieties boost the hydrogen evolution reaction by optimizing H absorption[J]. Inorganic Chemistry Frontiers, 2024, 11(6): 1733-1741. DOI: 10.1039/D3QI02668J
[41] LIU W, TAN W Y, HE H W, et al. One-step electrodeposition of Ni-Ce-Pr-Ho/NF as an efficient electrocatalyst for hydrogen evolution reaction in alkaline medium[J]. Energy, 2022, 250: 10.
[42] WANG Q, LIU J X, YAN X C, et al. RE-doped (RE = La, Ce and Er) Ni2P porous nanostructures as promising electrocatalysts for hydrogen evolution reaction[J]. Dalton Transactions, 2023, 52(7): 1895-1901. DOI: 10.1039/D2DT03850A
[43] KOPCZYNSKI K, LOTA G. Ni-La composite coating obtained using deep eutectic solvent and its electrocatalytic activity[J]. Chemical Papers, 2020, 74(5): 1691-1696. DOI: 10.1007/s11696-019-00993-6
[44] MADRAM A, MOHEBBI M, NASIRI M, et al. Preparation of Ni-P-La alloy as a novel electrocatalysts for hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2020, 45(7): 3940-3947. DOI: 10.1016/j.ijhydene.2019.12.028
-
其他相关附件
-
目的
在碳达峰、碳中和的目标下,开发可再生能源已成为绿色低碳之路的必然选择。氢气是一种清洁无污染的二次能源,对当下能源体系发展形式的转型至关重要。电解水制氢法具有高纯度、来源广泛和可再生等优势,认为是最有前景的制氢方法。因此,开发一种低成本、高性能的非贵金属催化剂是当前的重要任务。过渡金属硼化物具有特殊的电子性能、高催化活性、高稳定性而引起人们广泛关注,特别是块状和负载型镍钴硼化物,具有耐腐蚀性、高机械强度和独特的电子性能。近年来。稀土元素因独特的4f电子结构已经成为金属催化剂改性的研究热点。但在泡沫镍(NF)基体上制备稀土复合催化剂的方法要求较高,存在成本高、工艺复杂和耗时长等问题。本文通过简单一步化学沉积方法成功制备了原位生长Ni-Co-B-Yb/NF稀土复合催化电极材料。
方法利用重稀土元素Yb掺杂的方法来改性Ni-Co-B催化剂。首先,对基体NF进行预处理,通过化学腐蚀方法来清洁基体表面,去除表面氧化膜和杂质,提高镀层薄膜的附着力。然后,在无水乙醇(99.8%)体系中配制化学镀液,加入NiCl、CoCl及缓冲剂至充分溶解。接着将100 ml镀液分别倒入10支试管中,各加入适量氢氧化钠调节溶液PH值。随后将活化后的NF放入镀液中,依次加入(CH)NH·BH还原剂。置于35℃恒温条件下,反应完全后将催化剂样品取出并风干。通过改变镀液中稀土浓度及钴浓度,得到不同浓度的催化剂材料。
结果对催化电极的结构和形貌进行表征得到,Ni-Co-B-Yb与Ni-Co-B相比,其形貌中颗粒尺寸减小,多孔结构增多,能在电解质中捕获氢的中间体,有效提高电极表面的催化表面积,对电子在催化剂表面的电子传输有利,从而加强水分解的催化机制。同时可知随着稀土浓度、钴浓度的改变,表面形貌均发生改变。通过XRD、TEM分析可知,Ni-Co-B-Yb为非晶-纳米晶的过渡态,其大部分为非晶结构。由XPS分析结果可知,Ni-Co-B-Yb与Ni-Co-B相比,Ni2p和B1s的结合能均正移,表明Yb的加入与其他金属产生了电子效应使得电子密度发生改变,并诱导B的电子发生了逆向转移。通过电化学测试,可知随着稀土浓度不断增大,析氢性能呈现先增大后减小的变化趋势,其中当稀土浓度为3 g·L表现出最佳的析氢性能。随着钴浓度不断增大,析氢性能也呈现先增大后减小的变化趋势,当钴浓度为5 g·L表析氢性能最佳。此外,经过100 h长期稳定性测试和2000次循环伏安(测后,Ni-Co-B-Yb催化剂表现出良好的电化学稳定性。
结论稀土Yb加入的可以改变催化剂的纳米结构,降低过渡金属基电极表面颗粒的平均直径,有利于增加催化活性面积。另外Yb可以调节Ni-Co-B内部电子结构,丰富的价态增加了反应活性位点,提高其对氢离子的吸附和活化能力,增加了各金属元素之间的相互作用,进而提升析氢性能。同时稀土Yb的添加可以优化析氢动力学,降低析氢反应的过电势、提高析氢速率,显著提高了催化剂的本征活性。
-
过渡金属基电催化剂的改性研究发展迅速,其中稀土元素因其独特的电子结构成为研究热点。但稀土元素具有低的还原电位和强的亲氧性,在泡沫镍基体上制备原位生长的稀土复合催化剂对方法要求较高,存在成本高、工艺复杂和耗时长等问题。
本文以三维泡沫镍为基体,通过一步化学沉积法将Ni-Co-B-Yb紧密负载于其表面,制备得到Ni-Co-B-Yb/NF复合电极材料。在一步化学沉积的作用下,Ni-Co-B-Yb牢固均匀的生长在NF表面,而且得益于稀土Yb的电子调节能力、金属元素之间的相互作用和三维NF良好的导电性,Ni-Co-B-Yb具有丰富的HER反应位点和低的电荷转移电阻。电化学测试结果表明,Ni-Co-B-Yb在1.0 mol·L–1 KOH中表现出优异的HER活性和稳定性:在10 mA·cm–2的电流密度下过电位为57 mV,Tafel斜率仅为73 mV·dec–1,并且分别在100 mV恒电位下持续电解100 h活性几乎没有衰减。
Ni-Co-B/NF与不同浓度Ni-Co-B-Yb/NF在 1.0 mol/L KOH 中 LSV 曲线 (a)和 Tafel曲线斜率(b)对比





 下载:
下载: