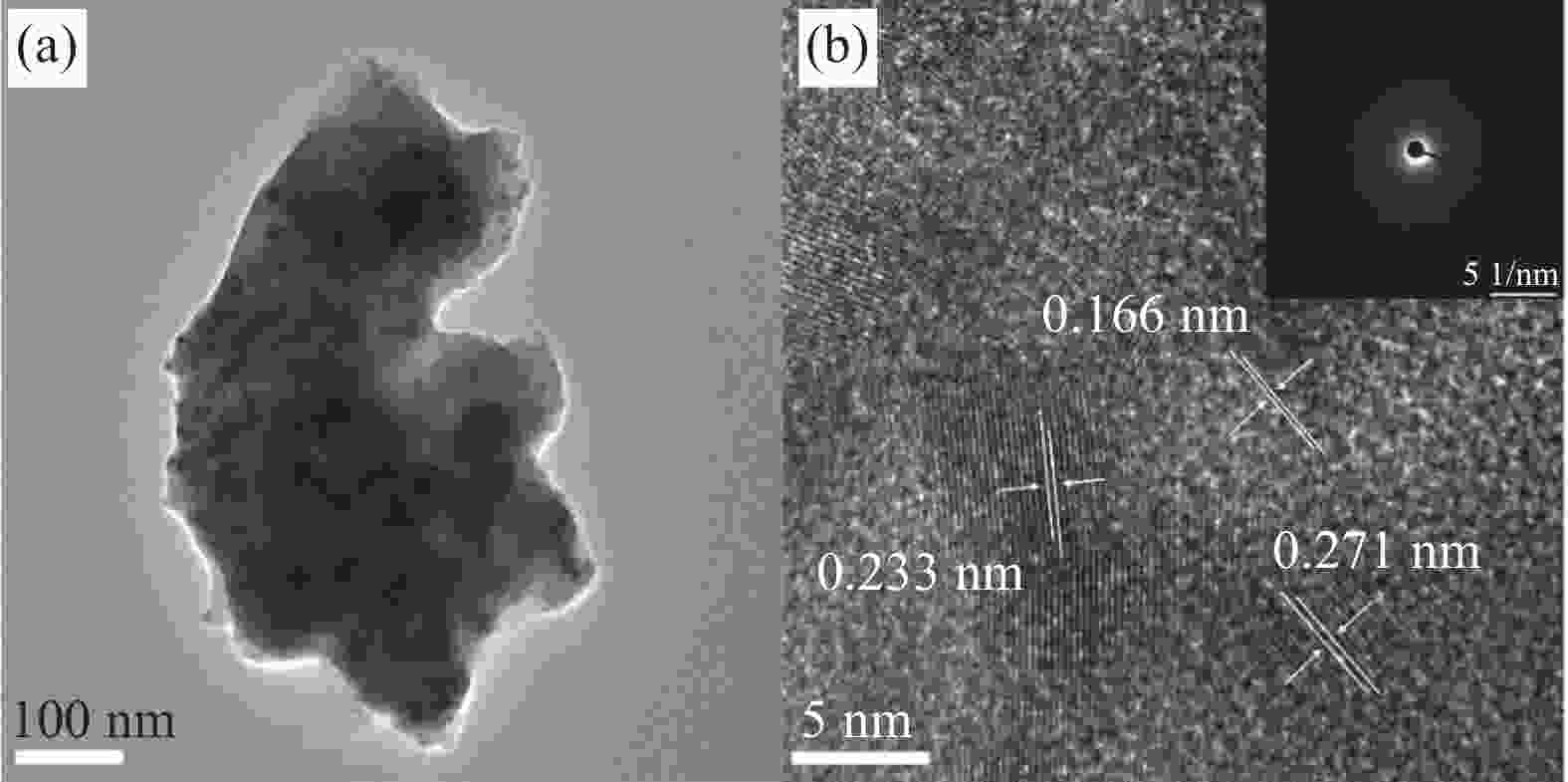
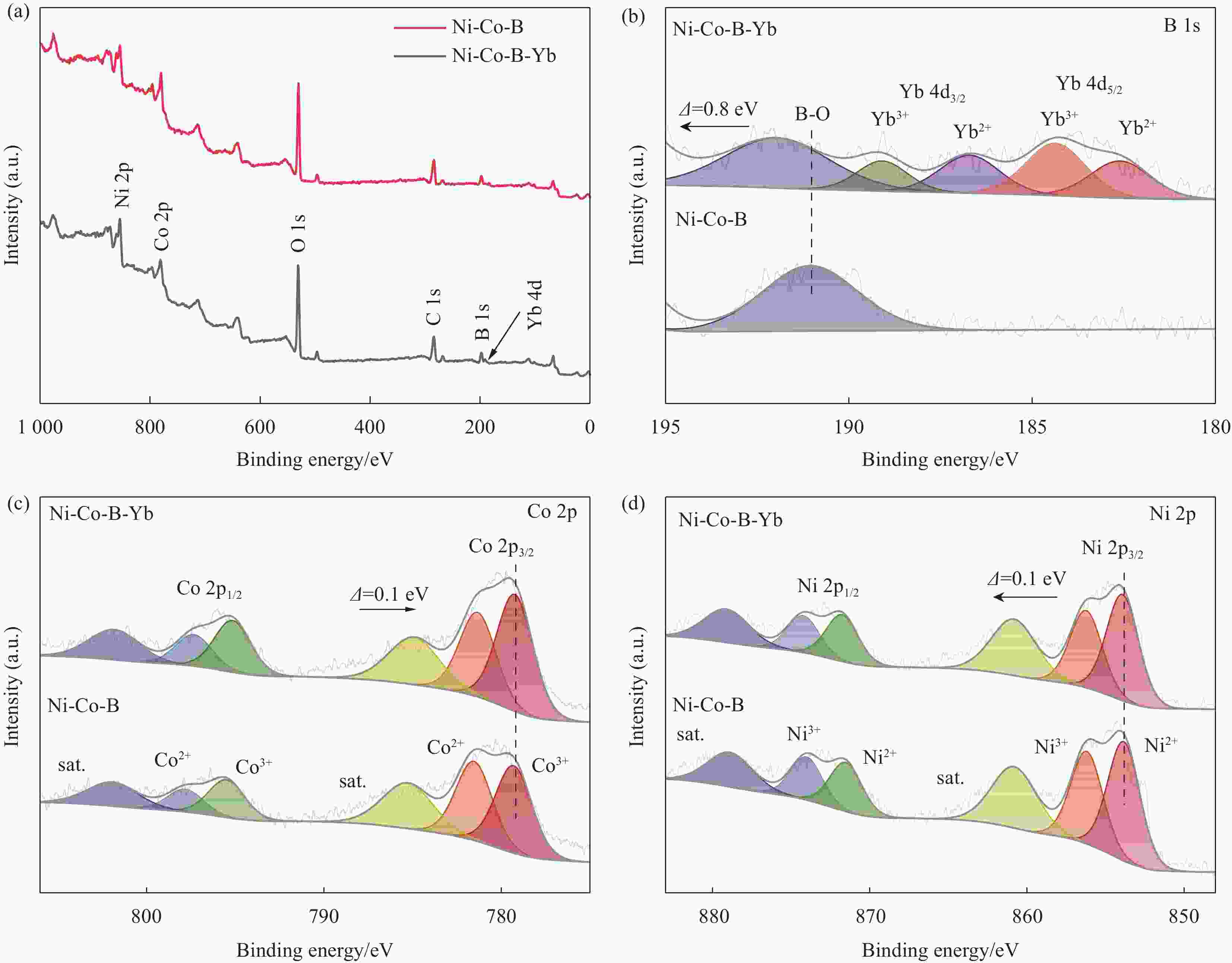
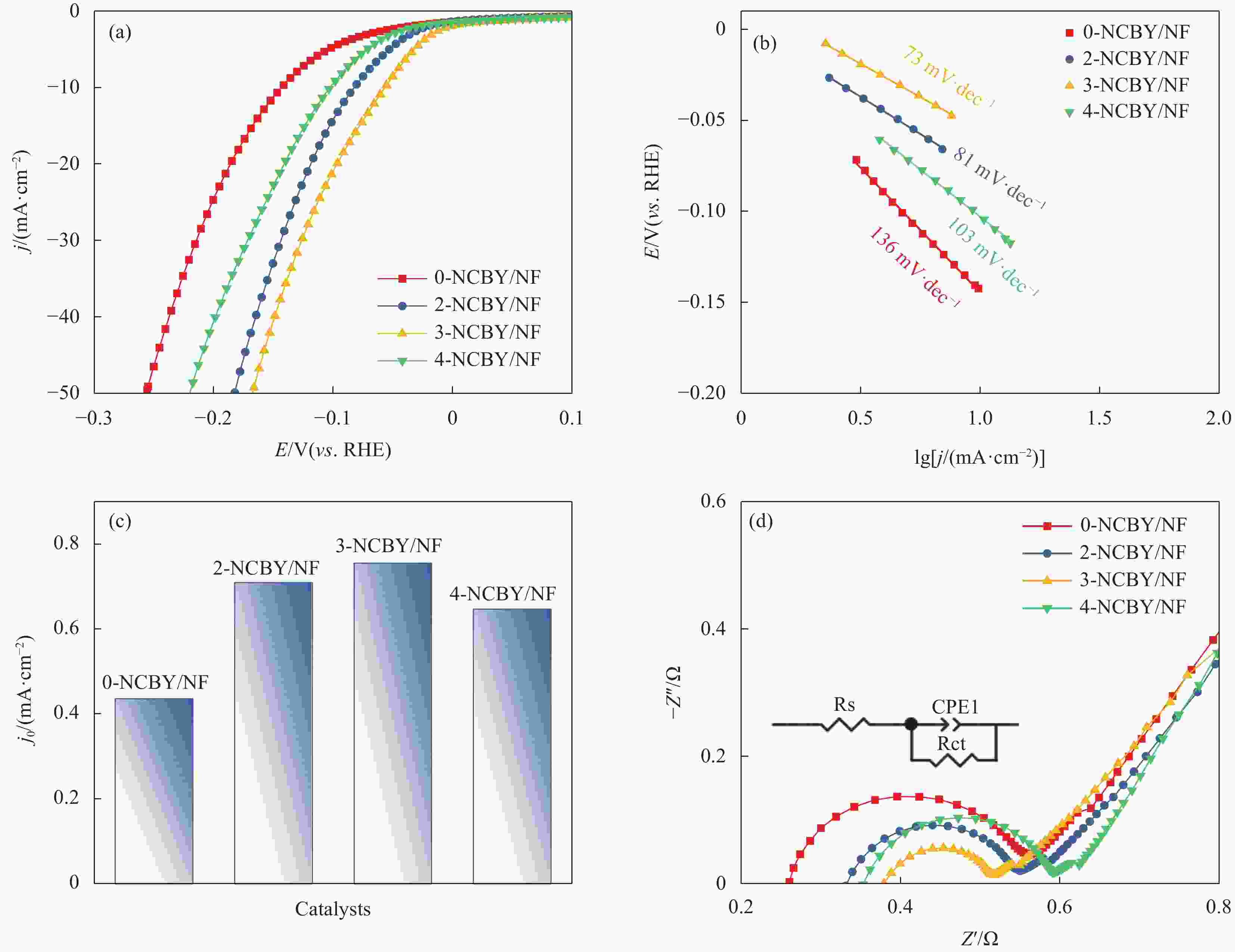
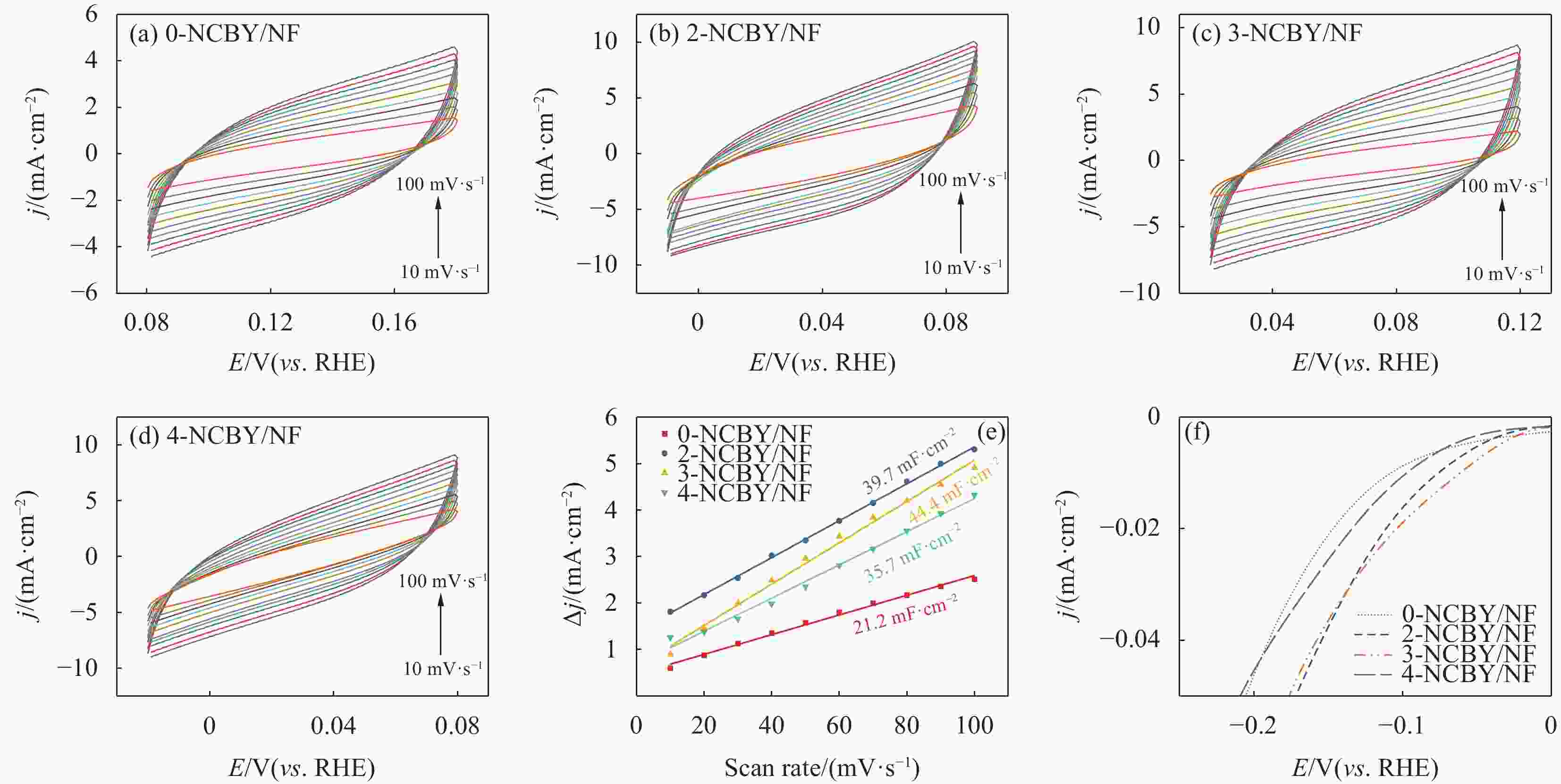
In situ growth of a Ni-Co-B-Yb rare earth composite electrode: preparation and electrocatalytic hydrogen precipitation performance
-
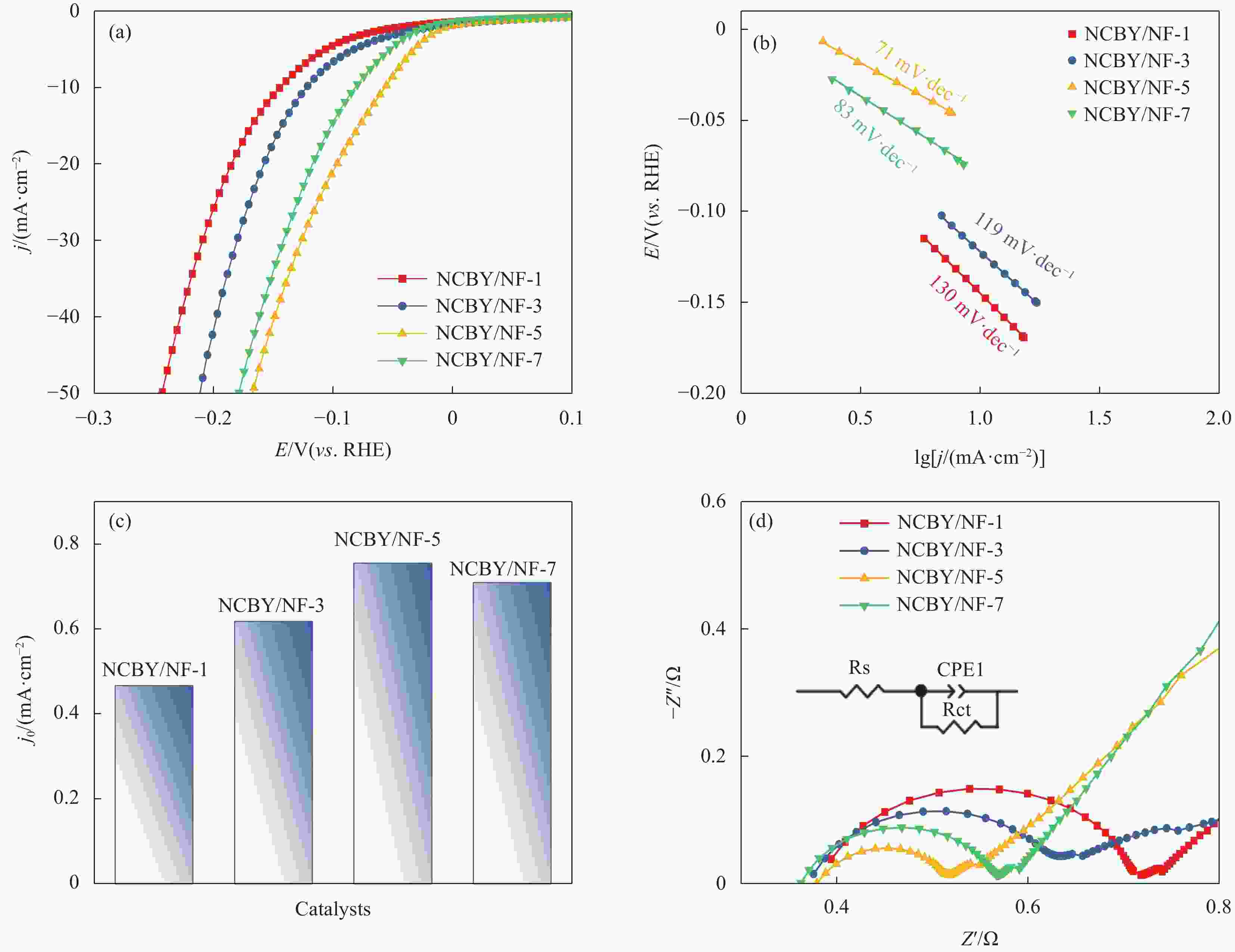
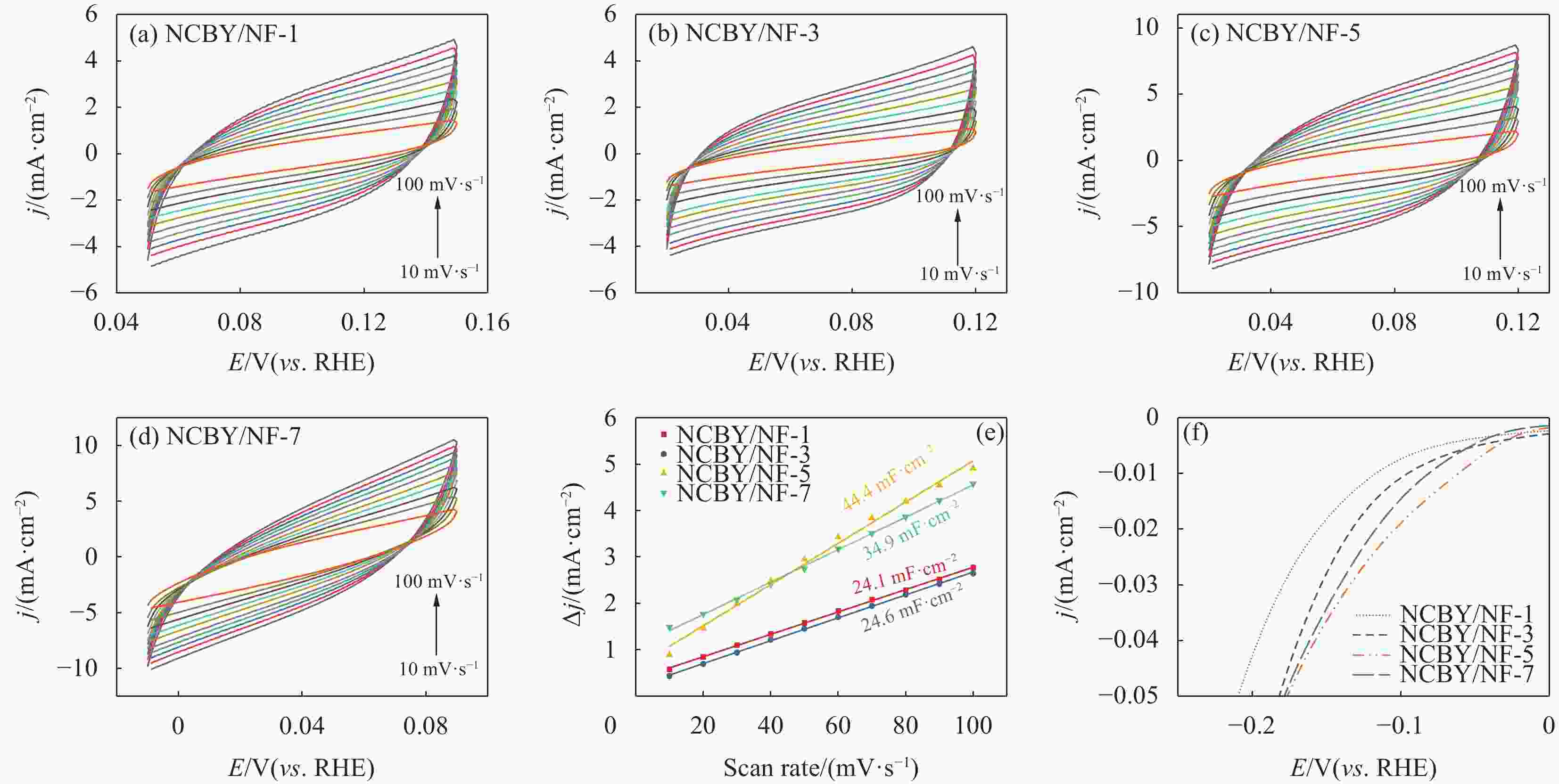
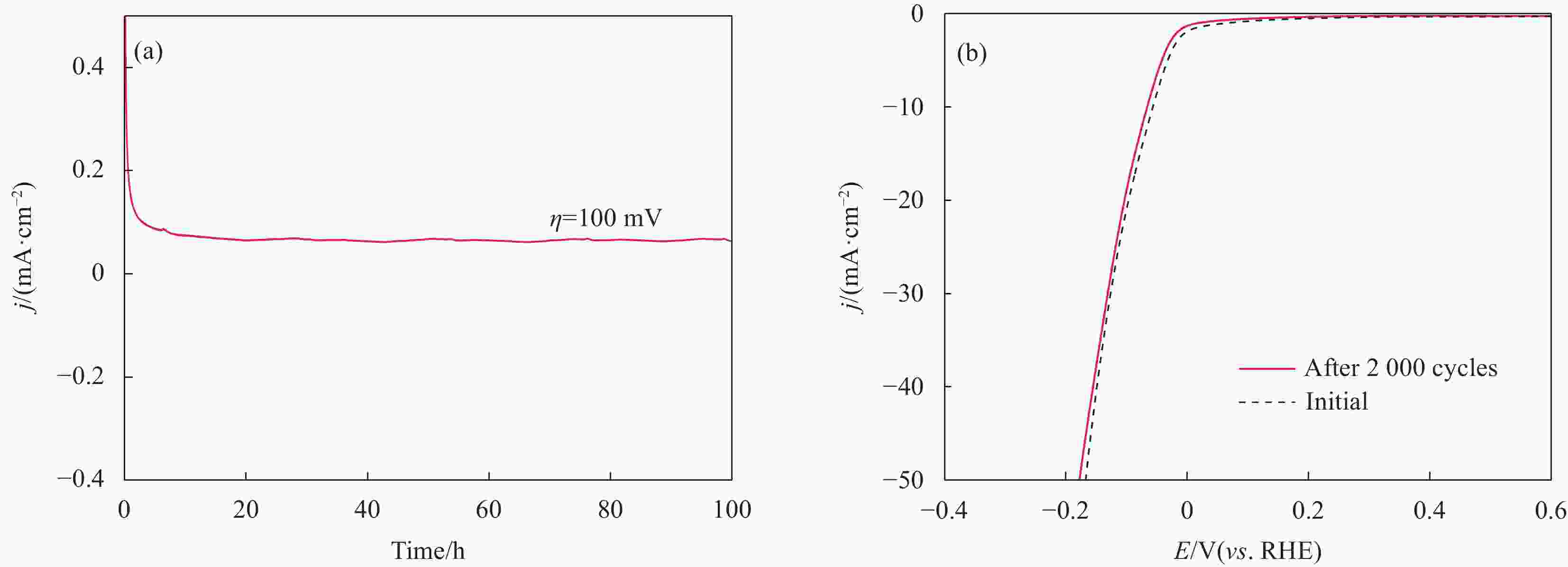
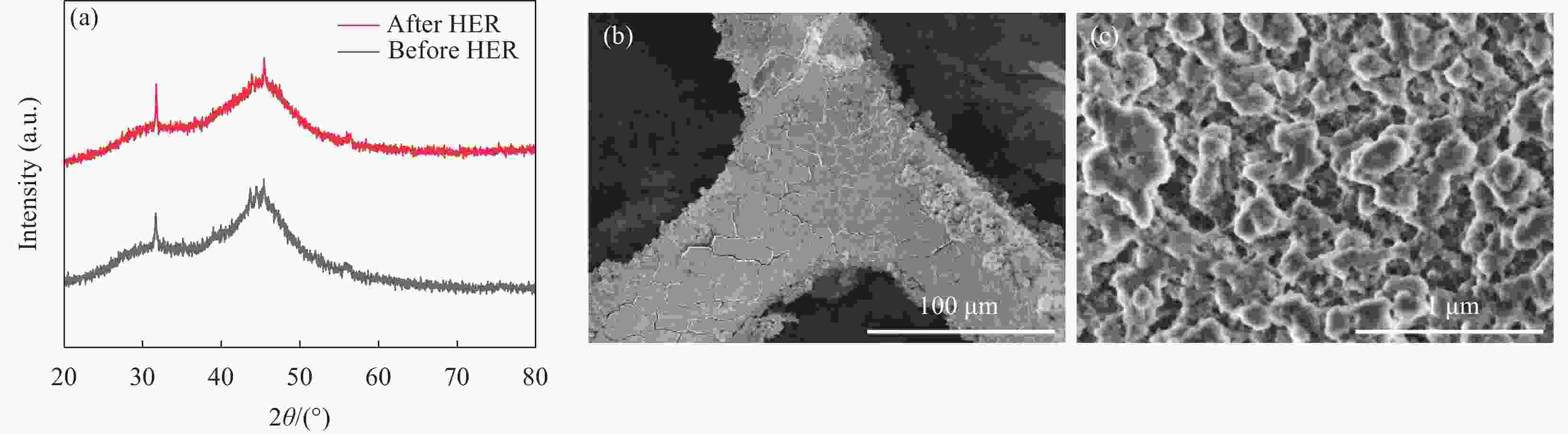
摘要: 探索和开发低成本、高活性的非贵金属析氢反应(Hydrogen Evolution Reaction, HER)电催化剂,对于电解水的实际应用具有重要意义但仍具有挑战性。本文采用化学沉积法在三维的泡沫镍基底上制备了原位生长的稀土(Rare Earth, RE)复合催化电极(Ni-Co-B-Yb/NF),对催化电极的结构和形貌进行了表征,并研究其在1 mol·L−1 KOH溶液中的析氢性能。结果表明,添加Yb可使电极的形貌及电子结构发生改变,改善催化剂材料的HER催化性能。当Yb和Co浓度分别为3 g·L−1和5 g·L−1时,Ni-Co-B-Yb /NF表现出最佳的析氢性能。当电流密度为10 mA·cm−2时,析氢过电位为57 mV,Tafel斜率仅为73 mV·dec−1,此外,经过100 h长期稳定性测试和2000次循环伏安(Cyclic voltammetry,CV)测试后,该催化剂表现出良好的电化学稳定性。实验结果表明:Yb的引入可以提升Ni-Co-B材料的HER催化性能,且Yb和Co浓度的改变对电催化性能影响较大。这项工作丰富了稀土复合催化剂在电解水催化方面的知识。Abstract: The exploration and development of low-cost and highly active non-precious metal hydrogen evolution reaction (HER) electrocatalysts are important but still challenging for practical applications in water electrolysis. In this study, in-situ-grown rare earth (RE) composite catalytic electrodes (Ni-Co-B-Yb/NF) were prepared on a three-dimensional nickel foam (NF) substrate by chemical deposition, and the structure and morphology of the catalytic electrodes were characterized and their hydrogen precipitation performance was investigated in 1 mol·L−1 KOH solution. The results show that the addition of Yb can change the morphology and electronic structure of the electrode and improve the HER catalytic performance of the catalyst material. The Ni-Co-B-Yb /NF exhibited the best hydrogen precipitation performance when the Yb and Co concentrations were 3 g·L−1 and 5 g·L−1, respectively. At a current density of 10 mA·cm−2, the hydrogen precipitation overpotential was 57 mV, and the Tafel slope was only 73 mV·dec−1. In addition, after 100 h of long-term stability test and 2000 cycles of cyclic voltammetry (CV) test, the catalyst showed good electrochemical stability. good electrochemical stability. The experimental results show that the introduction of Yb can enhance the HER catalytic performance of Ni-Co-B materials, and the changes of Yb and Co concentrations have a large effect on the electrocatalytic performance. This work enriches the knowledge of rare-earth composite catalysts for electrolytic water catalysis.
-
表 1 化学镀镀液配方
Table 1. Chemical deposition plating solution formula
Composition of plating solution Concentration/
(g·L−1)Anhydrous nickel chloride (NiCl2) 10.0 Anhydrous cobalt chloride (CoCl2) 1.0-7.0 Borane dimethylamine (C2H10BN) 1.0 Ytterbium Nitrate [Yb(NO3)3] 2.0-4.0 Adipic acid (C6H10O4) 1.5 Citric acid (C6H8O7) 1.5 Malic acid (C4H6O5) 1.5 表 2 Ni-Co-B-Yb/NF与最近报道的电催化剂的 HER 性能进行对比
Table 2. Comparison of HER performance of Ni-Co-B-Yb/NF with recently reported electrocatalysts
Notes Electrocatalysts Electrolyte η10/mV Tafel slop/(mV dec−1) Ref. 1 Ni-Co-B-Yb/Nickel foam(NF) 1.0 mol/L KOH 57 73 This work 2 Ni3N/Ni 1.0 mol/L KOH 144 107 [34] 3 Ni-MgO/ Carbon nanotube (CNT) 1.0 mol/L KOH 117 116 [35] 4 Co2NiMo-N 1.0 mol/L KOH 69 77.8 [36] 5 (Ag:Cu)/ Boron nanosheets (BNS) 1.0 mol/L KOH 101 57 [37] 6 NiCoP 1.0 mol/L KOH 141 66 [38] 7 Ni-Co/Mo2C/Co6Mo6C2@C 1.0 mol/L KOH 95 99.92 [39] 8 Ru-NiSe2/ Nickel foam(NF) 1.0 mol/L KOH 39.3 36 [40] 9 Ni-Ce-Pr-Ho/ Nickel foam(NF) 1.0 mol/L KOH 78 121.6 [41] 10 Ni2P-Pr 1.0 mol/L KOH 87 65.4 [42] 11 Ni-La 1.0 mol/L KOH 190 68 [43] 12 Ni-P-La 1.0 mol/L KOH 139 93 [44] -
[1] ZHU J, HU L S, ZHAO P X, et al. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles[J]. Chemical Reviews, 2020, 120(2): 851-918. doi: 10.1021/acs.chemrev.9b00248 [2] ZHU P, XIONG X, WANG D S. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction[J]. Nano Research, 2022, 15(7): 5792-5815. doi: 10.1007/s12274-022-4265-y [3] YANG J S, LI J, WANG Y, et al. Tailoring the Pore Structure of Porous Ni-Sn Alloys for Boosting Hydrogen Evolution Reaction in Alkali Solution[J]. Metals, 2022, 12(12): 13. [4] XU B, LIANG J, SUN X P, et al. Designing electrocatalysts for seawater splitting: surface/interface engineering toward enhanced electrocatalytic performance[J]. Green Chemistry, 2023, 25(10): 3767-3790. doi: 10.1039/D2GC03377A [5] ZHANG X, JIA F F, SONG S X. Recent advances in structural engineering of molybdenum disulfide for electrocatalytic hydrogen evolution reaction[J]. Chemical Engineering Journal, 2021, 405: 16. [6] GUO F, MACDONALD T J, SOBRIDO A J, et al. Recent Advances in Ultralow-Pt-Loading Electrocatalysts for the Efficient Hydrogen Evolution [J], 2023, 10(21): 2301098. [7] XU H, SHANG H Y, WANG C, et al. Ultrafine Pt-Based Nanowires for Advanced Catalysis[J]. Advanced Functional Materials, 2020, 30(28): 18. [8] EL-REFAEI S M, RUSSO P A, PINNA N. Recent Advances in Multimetal and Doped Transition-Metal Phosphides for the Hydrogen Evolution Reaction at Different pH values[J]. Acs Applied Materials & Interfaces, 2021, 13(19): 22077-22097. [9] FENG J X, WU J Q, TONG Y X, et al. Efficient Hydrogen Evolution on Cu Nanodots-Decorated Ni3S2 Nanotubes by Optimizing Atomic Hydrogen Adsorption and Desorption[J]. Journal of the American Chemical Society, 2018, 140(2): 610-617. doi: 10.1021/jacs.7b08521 [10] BARATI Q, HADAVI S M M. Electroless Ni-B and composite coatings: A critical review on formation mechanism, properties, applications and future trends[J]. Surfaces and Interfaces, 2020, 21: 13. [11] HAYAT A, SOHAIL M, ALI H, et al. Recent Advances and Future Perspectives of Metal-Based Electrocatalysts for Overall Electrochemical Water Splitting[J]. Chemical Record, 2023, 23(2): 64. [12] CHEN Z J, DUAN X G, WEI W, et al. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution[J]. Journal of Materials Chemistry A, 2019, 7(25): 14971-15005. doi: 10.1039/C9TA03220G [13] ZHANG H Y, WANG Y, SONG D Q, et al. Cerium-Based Electrocatalysts for Oxygen Evolution/Reduction Reactions: Progress and Perspectives[J]. Nanomaterials, 2023, 13(13): 24. [14] LI Y F, YUAN X L, WANG P, et al. Rare earth alloy nanomaterials in electrocatalysis[J]. Journal of Energy Chemistry, 2023, 83: 574-594. doi: 10.1016/j.jechem.2023.04.050 [15] WANG X, TANG Y W, LEE J M, et al. Recent advances in rare-earth-based materials for electrocatalysis[J]. Chem Catalysis, 2022, 2(5): 967-1008. doi: 10.1016/j.checat.2022.02.007 [16] CARDOSO D S P, AMARAL L, SANTOS D M F, et al. Enhancement of hydrogen evolution in alkaline water electrolysis by using nickel-rare earth alloys[J]. International Journal of Hydrogen Energy, 2015, 40(12): 4295-4302. doi: 10.1016/j.ijhydene.2015.01.174 [17] LU Y Z, TANG L L, WANG P, et al. Rare Earth-Based Alloy Nanostructure for Hydrogen Evolution Reaction[J]. Acs Catalysis, 2023, 13(20): 13804-13815. doi: 10.1021/acscatal.3c03350 [18] 景欣欣, 陈必清, 翟佳鑫, 等. Ni-Co-B-RE(Sm、Dy、Tb)复合电极: 化学沉积法制备及电催化析氢性能研究[J]. 无机材料学报, 2024, 39(5): 467-476. doi: 10.15541/jim20230491JING Xinxin, CHEN Biqing, ZHAI Jiaxin, et al. Ni-Co-B-RE (Sm, Dy, Tb) composite electrodes: preparation by chemical deposition method and electrocatalytic hydrogen evolution performance[J]. Journal of Inorganic Materials, 2024, 39(5): 467-476(in Chinese). doi: 10.15541/jim20230491 [19] R. R M, G. A E-M M, H. A H, et al. Tailor-designed bimetallic Co/Ni macroporous electrocatalyst for efficient glycerol oxidation and water electrolysis[J]. International Journal of Hydrogen Energy. 2022, 47(75): 32145-32157. [20] VESZTERGOM S, DUTTA A, RAHAMAN M, et al. Hydrogen Bubble Templated Metal Foams as Efficient Catalysts of CO2 Electroreduction[J]. Chemcatchem, 2021, 13(4): 1039-1058. doi: 10.1002/cctc.202001145 [21] ANASTASIADOU D, LIGT B, HE Y Y, et al. Carbon dioxide and nitrate co-electroreduction to urea on CuOxZnOy[J]. Communications Chemistry, 2023, 6(1): 8. doi: 10.1038/s42004-022-00803-3 [22] 邱文婕, 胡珍, 周其洪, 等. 稀土氧化铈增强的钴基电解水催化材料及其性能[J]. 复合材料学报, 2024, 41(2): 804-815.QIU Wenjie, HU Zhen, ZHU Qihong, et al. Rare earth cerium oxide reinforced cobalt based catalysts for electrolysed water and their properties[J]. Acta Materiae Compositae Sinica, 2024, 41(2): 804-815(in Chinese). [23] MAREK L, MARIA B, KAROLINA J, et al. Transition metal borides of Ni-B (Co-B) as alternative non-precious catalytic materials: Advances, potentials, and challenges. Short review[J]. Journal of Industrial and Engineering Chemistry, 2022, 116: 75-98. doi: 10.1016/j.jiec.2022.09.031 [24] 张士民, 陈必清, 高利霞, 等. 基于Eu-Ni-B稀土-复合电极电催化偏硼酸钠制备硼氢化钠的探索[J]. 功能材料, 2020, 51(4): 4207-4214. doi: 10.3969/j.issn.1001-9731.2020.04.035ZHANG Shimin, CHEN Biqing, GAO Lixia, et al. Exploration of electrocatalytic preparation of sodium borohydride with sodium metaborate based on Eu-Ni-B rare earth-composite electrode[J]. Journal of Functional Materials, 2020, 51(4): 4207-4214(in Chinese). doi: 10.3969/j.issn.1001-9731.2020.04.035 [25] LI X S, ZHOU J, SHEN L Q, et al. Exceptionally high saturation magnetic flux density and ultralow coercivity via an amorphous-nanocrystalline transitional microstructure in an FeCo-based alloy[J]. Advanced Materials, 2022, 35(50): 2205863. [26] BHATTACHARYA S, PHATAKE R S, BARNEA S N, et al. Fluorescent Self-Healing Carbon Dot/Polymer Gels[J]. Acs Nano, 2019, 13(2): 1433-1442. [27] JOKAR A, TOGHRAEI A, MALEKI M, et al. Facile electrochemical synthesis of Ni-Co-B film on Cu sheet for dual-electrocatalysis of hydrogen and oxygen evolution reactions[J]. Electrochimica Acta, 2021, 389: 10. [28] OHNO Y. XPS studies of the intermediate valence state of Yb in (YbS)1.25CrS2[J]. Journal of Electron Spectroscopy and Related Phenomena, 2008, 165(1-3): 1-4. doi: 10.1016/j.elspec.2008.05.009 [29] LI D X, GUO Z M, ZHAO R H, et al. An efficient cerium dioxide incorporated nickel cobalt phosphide complex as electrocatalyst for All-pH hydrogen evolution reaction and overall water splitting[J]. Journal of Colloid and Interface Science, 2024, 653: 1725-1742. doi: 10.1016/j.jcis.2023.09.144 [30] HAOYU L, DUODUO G, PING W, et al. Amorphization-induced reverse electron transfer in NiB cocatalyst for boosting photocatalytic H2 production[J]. Applied Catalysis B: Environmental, 2024, 340: 123270. doi: 10.1016/j.apcatb.2023.123270 [31] GAO W, WEN D, HO J C, et al. Incorporation of rare earth elements with transition metal-based materials for electrocatalysis: a review for recent progress[J]. Materials Today Chemistry, 2019, 12: 266-281. doi: 10.1016/j.mtchem.2019.02.002 [32] ASGARI M, DARBAND G B, MONIRVAGHEFI M. Electroless deposition of Ni-W-Mo-Co-P films as a binder-free, efficient and durable electrode for electrochemical hydrogen evolution[J]. Electrochimica Acta, 2023, 446: 13. [33] CHAIKA M, VOVK O, MANCARDI G, et al. Dynamics of Yb2+ to Yb3+ ion valence transformations in Yb: YAG ceramics used for high-power lasers[J]. Optical Materials, 2020, 101: 8. [34] XIONG L W, QIU Y F, DONG H, et al. Metallic Ni3N/Ni heterostructure for efficient hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2024, 59: 400-407. doi: 10.1016/j.ijhydene.2024.01.312 [35] MOHANA P, ISACFRANKLIN M, YUVAKKUMAR R, et al. Facile Synthesis of Ni-MgO/CNT Nanocomposite for Hydrogen Evolution Reaction[J]. Nanomaterials, 2024, 14(3): 14. [36] YU S S, XU J, WANG Q Y, et al. Oxygen-vacancy-enriched Co2NiMo-N hollow polymetallic nitrides for the electrocatalytic hydrogen evolution reaction[J]. Journal of Alloys and Compounds, 2024, 977: 7. [37] SINGLA A, DHIMAN R, MAHAJAN A. A Bimetallic-doped boron nanosheet electrocatalyst for efficient hydrogen evolution reaction[J]. Journal of Electronic Materials, 2024, 10.1007/s11664-024-11042-8: 9. [38] BERA C, STRECKOVA M, ORINAKOVA R, et al. NiCoP fibers as novel catalysts for hydrogen evolution in alkali and acidic environment[J]. International Journal of Hydrogen Energy, 2024, 60: 118-132. doi: 10.1016/j.ijhydene.2024.02.195 [39] GU J X, ZHU Y, ZHENG H Y, et al. Small-sized NiCo/Mo2C/Co6Mo6C2@C for efficient alkaline and acidic hydrogen evolution reaction by an anchoring calcination strategy[J]. Frontiers of Chemical Science and Engineering, 2024, 18(5): 9. [40] MA S, YANG P Y, CHANG J, et al. High-density accessible Ru-Se-Ni moieties boost the hydrogen evolution reaction by optimizing H absorption[J]. Inorganic Chemistry Frontiers, 2024, 11(6): 1733-1741. doi: 10.1039/D3QI02668J [41] LIU W, TAN W Y, HE H W, et al. One-step electrodeposition of Ni-Ce-Pr-Ho/NF as an efficient electrocatalyst for hydrogen evolution reaction in alkaline medium[J]. Energy, 2022, 250: 10. [42] WANG Q, LIU J X, YAN X C, et al. RE-doped (RE = La, Ce and Er) Ni2P porous nanostructures as promising electrocatalysts for hydrogen evolution reaction[J]. Dalton Transactions, 2023, 52(7): 1895-1901. doi: 10.1039/D2DT03850A [43] KOPCZYNSKI K, LOTA G. Ni-La composite coating obtained using deep eutectic solvent and its electrocatalytic activity[J]. Chemical Papers, 2020, 74(5): 1691-1696. doi: 10.1007/s11696-019-00993-6 [44] MADRAM A, MOHEBBI M, NASIRI M, et al. Preparation of Ni-P-La alloy as a novel electrocatalysts for hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2020, 45(7): 3940-3947. doi: 10.1016/j.ijhydene.2019.12.028 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 41
- HTML全文浏览量: 33
- 被引次数: 0




 下载:
下载: