Influence of chloride and sulfate on steel corrosion in simulated concrete pore solutions
-
摘要:
通过电化学测试、XRD测试和DFT计算,本研究探讨了在不同pH值(12.4、12.9和13.5)的模拟混凝土孔隙溶液中Cl−和\text{SO}_{\text{4}}^{{2-}}单独及共同作用对钢筋锈蚀行为的影响及其机制。结果表明,在pH值较低的CH溶液中,当Cl−浓度仅达到0.02mol/L时,开路电位(OCP)即由−375mV急剧下降至−575 mV,表明Cl−在低浓度情况下就会显著加速腐蚀;而在pH值较高的ST溶液中,随着腐蚀离子浓度从0.01 mol/L逐步增加至0.4 mol/L,钢筋的极化电阻(Rp)从约100 kΩ·cm2稳定下降至5 kΩ·cm2,但整体耐腐蚀性明显优于其他pH值的情况,显示了高pH值对腐蚀的有效抑制作用。此外,当Cl−和SO2−4共存时,由于竞争吸附机制的作用,整体腐蚀速率介于两者单独存在时之间,SO2−4的存在一定程度上减缓了Cl−引发的腐蚀。本研究基于上述结果提出了一个竞争吸附-催化腐蚀的两阶段反应模型,详细揭示了二者共同作用下的腐蚀行为,Cl−通过破坏钝化膜加速腐蚀进程,SO2−4则通过影响腐蚀产物的稳定性和分布参与腐蚀过程。
Abstract:Through electrochemical tests, XRD analysis, and DFT calculations, this study investigated the individual and combined effects of Cl− and SO2−4 ions on the corrosion behavior of steel reinforcement in simulated concrete pore solutions at different pH values (12.4, 12.9 and 13.5). The results reveal that in the lower pH CH solution, even at a Cl− concentration of only 0.02 mol/L, the open circuit potential (OCP) drops sharply from −375 mV to −575 mV, indicating significant acceleration of corrosion by Cl− at low concentrations. Conversely, in the higher pH ST solution, as the concentration of corrosion ions increases gradually from 0.01 mol/L to 0.4 mol/L, the steel’s polarization resistance (Rp) stabilizes and decreases from approximately 100 kΩ·cm2 to 5 kΩ·cm2, demonstrating superior overall corrosion resistance compared to lower pH conditions, highlighting the effective inhibition of corrosion at higher pH value. Furthermore, in the presence of both Cl− and SO2−4 ions, the overall corrosion rate lies between the rates observed when each ion is present individually, due to the competitive adsorption mechanism. The presence of SO2−4 mitigates to some extent the corrosion initiated by Cl−. Based on these findings, the study proposes a two-stage competitive adsorption-catalytic corrosion reaction model, elucidating in detail the corrosion behavior under their combined influence: Cl− accelerates corrosion by disrupting the passive film, while SO2−4 participates in the corrosion process by influencing the stability and distribution of corrosion products.
-
聚对苯二甲酸乙二醇酯(PET)是全球用量最大的高分子材料之一,其纤维制品俗称涤纶,是全球第一大化纤品种[1]。但PET属于易燃材料,且其燃烧时还伴有严重的熔滴现象,极易导致火灾蔓延和二次伤害,这为PET的应用带来了极大的安全隐患,也使其在军事、工业等诸多领域的应用受到限制[2]。目前PET阻燃改性的主流方法是引入含磷阻燃剂,但含磷聚酯的阻燃性主要是通过熔体滴落带走燃烧区域的热量和火焰来实现的[3-4],未能解决PET的熔滴问题。因此,同时赋予PET阻燃和抗熔滴特性是目前PET阻燃改性面临的一大难点。
相关研究表明,提高聚合物高温下的熔体黏度和炭化能力是实现阻燃和抗滴落的关键[5-6]。但由于PET分子链的线性结构,其在高温下具有较低的熔融黏度,这一特性在赋予其可纺性的同时,也导致其在燃烧过程中表现出严重的熔滴行为,并难以形成连续的炭层[7]。在PET中引入可交联结构单元是提高熔体黏度的一个有效途径[6, 8]。例如,Wu等[9]合成了一种芳香族席夫碱5-(亚苄基-氨基)-间苯二甲酸二甲酯,并将其用作PET的共聚单体;研究结果显示,芳族席夫碱可以在PET的熔融温度和分解温度之间形成稳定的交联网络,并在燃烧中进一步转变为致密的炭层,因而使得PET共聚酯显示出优异的自熄和抗滴落行为。但尽管上述共聚酯表现出较为理想的阻燃性和抗滴落性能,问题仍然存在。除了工艺复杂、成本高、工业化困难之外,共聚法往往会破坏分子链的规整性,从而损害PET的力学性能和可纺性。
近年来,碳基阻燃剂作为一种新型阻燃剂表现出巨大的发展潜力。研究表明,碳纳米管[10]、富勒烯[11]、纳米炭黑[12]、石墨烯[13]、碳微球[14]等碳基阻燃剂在改善聚合物的成炭质量、降低热释放速率、提高热稳定性等方面表现突出。与传统阻燃剂相比,往往少量碳基阻燃剂的引入即可显著提高聚合物的阻燃性,除此之外,引入碳基阻燃剂还能不同程度地改善聚合物的力学、热学以及电学等性能[15]。其中,碳纳米球(Carbon nanospheres,CNSs)具有无卤环保、粒径小、热稳定性高等优势,能满足PET高温加工和熔融纺丝的理论要求,但迄今为止,将碳基阻燃剂应用于PET的研究较少。
基于此,本文以CNSs为基体,在其表面接枝芳香席夫碱4-苯亚甲基氨基苯酚(4-phenyl-methyleneamino-phenol,BA)制备了一种新型碳纳米球基复合阻燃剂(CNSs-BA),旨在将CNSs和BA的优势有机结合,使阻燃剂同时具备“智能自交联”特性,从而在燃烧时在PET基体中形成三维交联网络,进而改善熔滴。重点研究了CNSs-BA/PET复合材料的阻燃性及其阻燃机制。
1. 实验材料及方法
1.1 原材料
蒸馏水,使用XY-ZL-20型蒸馏水器自制。碳纳米球(CNSs),纯度99.99%,宁波金雷纳米材料科技有限公司;30%过氧化氢,优级纯,上海沃凯生物技术有限公司;硫酸,分析纯,华东医药股份有限公司;二氯甲烷(DCM),分析纯,华东医药股份有限公司;4-苯亚甲基氨基苯酚(BA),纯度98%,东京化成工业株式会社;4-二甲氨基吡啶(DMAP),纯度99%,阿拉丁试剂(上海)有限公司;无水乙醇,分析纯,嘉兴市甬宏化工有限公司;PET切片,半消光型SD500,中国石化仪征化纤股份有限公司。
1.2 CNSs-BA阻燃剂的制备
首先采用酸化法[16]制备羧基化碳纳米球(CNSs-COOH);在DCM中加入适量氯化钙,常温振荡12 h以去除其中的水分,使用时用吸管吸取上层液体用。在三口烧瓶中加入一定量的无水DCM (50~100 mL)作为反应溶剂。搅拌状态下加入3 g CNSs-COOH,再加入1 g BA和DMAP (作为碱性催化剂,DMAP的用量为BA用量的1/10),升温至30℃,之后在搅拌状态下反应2 h,反应完成后抽滤除去液体。将反应所得固体依次用乙醇和蒸馏水洗涤,100℃下干燥6 h后研磨均匀,即得CNSs-BA阻燃剂,CNSs-BA的制备示意图见图1。
1.3 CNSs-BA/PET复合材料的制备
首先将纯PET切片和制备的CNSs-BA阻燃剂分别在120℃下真空干燥12 h后冷却至室温备用,然后分别按CNSs-BA占PET的质量分数为0.5wt%、1.0wt%、2.0wt%、3.0wt% 的比例将CNSs-BA与PET切片混合均匀后喂入BP-8188型转矩流变仪(东莞市宝品精密仪器有限公司)中,转矩流变仪各区的温度依次为150、255、273、275℃,转速为35~60 r/min,熔体依次经过熔融共混、挤出、切粒工序得到CNSs-BA/PET复合材料。
1.4 测试与表征
CNSs-BA阻燃剂的测试与表征:用JSM-6510LA型场发射扫描电镜(SEM,日本电子株式会社)和EM420型透射电子显微镜(赛默飞世尔科技)观察阻燃剂的微观形貌,加速电压3 kV 和20 kV。用Perkin Elmer Frontier型傅里叶变换红外光谱仪(FTIR),溴化钾压片法分析测定阻燃剂表面性质和化学结构,光谱记录范围
4000 ~400 cm−1。用Perkin Elmer TG4000型热重分析仪(TG),在N2气氛下测试阻燃剂的热稳定性,气体流速20 mL/min,程序设定为:30℃恒温1 min后以20℃/min的升温速率升温至800℃。CNSs-BA/PET复合材料的测试与表征:用TM606 数显氧指数测试仪(青岛睿新杰仪器有限公司),按照GB/T 2406.2—2009[17]测试PET及其阻燃复合材料的极限氧指数(LOI),样条尺寸为120 mm×6.5 mm×3 mm。用CZF-5水平垂直燃烧仪(沧州冀路试验仪器有限公司),按照 GB/T 2408—2008[18]判定PET及其阻燃复合材料的UL-94垂直燃烧等级,样品尺寸为130 mm×13 mm×3 mm。用C-1087型锥形量热仪(英国FTT),按照ISO 5660-1: 2015[19]测试PET及其阻燃复合材料的燃烧热释放(HRR)等参数,样品尺寸:100 mm×100 mm×3 mm,辐射照度50 kW/m2。用Perkin Elmer TG4000型热重分析仪(TG),在N2气氛下测试PET及其阻燃复合材料(阻燃剂含量2wt%)的热稳定性,气体流速20 mL/min,程序设定为:30℃恒温1 min后以20℃/min的升温速率升温至800℃。用Netzsch STA449F5型同步热分析仪(TG-DSC)研究PET及其阻燃复合材料(阻燃剂含量2.0wt%)的交联行为,氩气做保护气,空气气氛,气体流速20 mL/min,升温速率为10℃/min,测试温度范围为30~800℃。用气相Agilent 6980N色谱仪,Agilent 5975质谱仪,采用HP-5MS色谱柱对PET及其阻燃复合材料(阻燃剂含量2.0wt%)进行裂解-气相色谱-质谱联用(Py-GC-MS)测试。裂解条件:裂解温度750℃,时间20 s,升温速率200℃/s。色谱条件:柱温在50℃保持5 min,然后以10℃/min升温至260℃,在260℃保持10 min;进样温度220℃,传输温度280℃,He做载气,流量1.0 mL/min;裂解产物经色谱柱分离后进入质谱仪,电子能量为70 eV。
残炭的测试与表征:采用SEM观察PET及其阻燃复合材料(阻燃剂含量2.0wt%)燃烧后残炭的形貌,并用其配套的EDS能谱设备对残炭进行元素分析。采用TG在N2气氛下测试残炭的热稳定性,气体流速20 mL/min,程序设定为:30℃恒温1 min后以20℃/min的升温速率升温至800℃。
2. 结果与讨论
2.1 CNSs-BA的形貌结构和热稳定性
图2为原CNSs (图2(a))和CNSs-BA (图2(b))的SEM、TEM和EDS能谱图。可知:CNSs和CNSs-BA二者均呈规则的球形颗粒状。不同的是,原始CNSs表面光滑,平均粒径约45 nm。而经BA接枝后,CNSs-BA的表面变得粗糙,平均粒径增大到50 nm左右,由EDS谱图可知:纯CNSs中的主要成分为C元素,与CNSs相比,CNSs-BA的表面增加了N元素,源自其表面接枝的BA中的氨基。
图3是CNSs和CNSs-BA的红外图谱。对比CNSs和CNSs-BA的FTIR曲线可知,在CNSs-BA的红外曲线中,
3330 cm−1和3380 cm−1处对应N—H的伸缩振动峰,2925 cm−1和2850 cm−1处为亚甲基的伸缩振动峰,1450 cm−1处的特征峰是芳环骨架的伸缩振动峰,1269 cm−1处的特征峰是酯基C(O)—O的伸缩振动峰,1045 cm−1处的特征峰是芳环上1, 4位取代的振动峰,以上特征峰源自CMSs表面接枝的BA。图4为CNSs和CNSs-BA的TG曲线。可知:纯CNSs的初始分解温度(Tonset,定义为热失重5wt%时的温度)大于800℃,经BA接枝后Tonset降低到483.1℃,该温度远高于PET的加工温度和热分解温度。纯CNSs和CNSs-BA的最高热分解速率的温度(Tmax)分别为158.4℃和177.4℃,说明CNSs经BA接枝后热分解速率减慢。CNSs在30~800℃之间表现出3个较为明显的失重阶段,338.7℃之前对应CNSs中的少量结晶水和无定形碳的分解,338.7~544.1℃之间对应CNSs主体的热分解,544.1℃之后对应热分解产物的再分解。而CNSs-BA可划分为4个失重阶段:前两个阶段分别对应结晶水、无定形碳、小分子产物的分解以及CNSs-BA主体的分解,值得注意的是,544.1℃之后,CNSs-BA出现一个较为明显的失重峰,而CNSs的DTG曲线上并无该峰,该失重峰的出现证明CNSs表面接枝的BA在第二阶段(PET的熔融温度和分解温度之间)形成了一个较为稳定的交联网络结构,这将十分有助于燃烧时保护炭层的形成。
2.2 复合材料的阻燃性能
表1是CNSs-BA/PET复合材料的LOI和UL-94垂直燃烧测试结果。可知,与CNSs/PET相比,CNSs-BA/PET复合材料的LOI进一步提高,二者LOI规律变化一致,即随着阻燃剂含量的增大,LOI先提高后降低,当CNSs-BA含量为2.0wt%时,CNSs-BA/PET的LOI指数达到最大值28.1%,此时与纯PET相比,CNSs-BA/PET的LOI提高了33.8%。UL-94垂直燃烧测试结果表明,CNSs-BA/PET复合材料的抗熔滴性能较CNSs/PET也有明显提高,两次施加火焰后的余焰时间明显缩短,当CNSs-BA的添加量超过2.0wt%时,CNSs-BA/PET复合材料的阻燃等级可达到V-0级。
表 1 复合材料的极限氧指数(LOI)和UL-94垂直燃烧测试结果Table 1. Limiting oxygen index (LOI) and UL-94 vertical burning test results of compositesSample Flame retardant content/wt% LOI/% UL-94 vertical combustion test results t1/s t2/s t3/s Ignite cotton? Rate PET — 21.0 Burn out — — Yes NR CNSs/PET 0.5 23.2 2.6 2.5 0 Yes V-2 1.0 25.0 2.4 2.4 0 Yes V-2 2.0 26.2 2.4 2.8 0 Yes V-2 3.0 24.6 3.1 2.2 0 Yes V-2 CNSs-BA/PET 0.5 24.0 1.5 2.3 0 Yes V-2 1.0 26.9 1.2 2.1 0 Yes V-2 2.0 28.1 0.5 2.2 0 No V-0 3.0 27.5 0.6 1.9 0 No V-0 Notes: PET—Polyethylene terephthalate; t1—Afterglow time after the first application of flame; t2—Afterglow time after the second application of flame; t2—Afterglow time; NR—No rate. 锥形量热仪测试结果见图5和表2。热释放速率(HRR)是表征材料火灾危险性的主要依据。结合图5和表2可知,纯PET被点燃后热释放速率急剧增大,其峰值热释放速率(pk-HRR)为810.45 kW/m2,总热释放(THR)为150.27 MJ/m2。与之相比,CNSs-BA/PET的THR与之接近,但HRR曲线却明显变平缓。值得注意的是,CNSs-BA/PET的HRR曲线表现出两个明显的热释放阶段,即在热释放速率达到峰值之后又出现了一个较为平缓的放热平台(当CNSs-BA含量为0.5wt%时表现为放热峰),这意味着CNSs-BA/PET在燃烧过程中的热释放受到抑制,这是由燃烧时炭层的形成或可燃气体减少导致的[20]。此外,表2表明,与CNSs/PET相比,CNSs-BA/PET复合材料的pk-HRR进一步降低。当CNSs-BA 含量为2.0wt%时,CNSs-BA/PET的pk-HRR最小,为435 kW/m2,该值与相同阻燃剂含量的CNSs/PET相比降低了7.6%,较纯PET降低了46.3%,说明CNSs经BA接枝后对PET的燃烧抑制作用进一步增强,阻燃效果进一步提高。
表 2 复合材料的锥形量热仪测试数据Table 2. Data of cone calorimeter test of compositesSample FR content/wt% TTI/s Time to pk-HRR/s pk-HRR/(kW·m−2) THR/(MJ·m−2) PET 0 47 104 810.45 150.27 CNSs/PET 0.5 44 34 528.96 151.64 1 34 34 503.44 148.03 2 40 41 470.72 146.06 3 30 29 501.49 143.19 CNSs-BA/PET 0.5 35 39 485.54 146.54 1 31 55 469.98 156.04 2 34 39 435.00 146.54 3 30 39 466.05 156.69 Notes: TTI—Time to ignition; pk-HRR—Peak heat release rate; FR—Flame retardant. 2.3 复合材料的阻燃机制研究
2.3.1 阻燃复合材料的热重分析
为了研究阻燃剂的引入对PET的热降解行为的影响,对PET、CNSs/PET和CNSs-BA/PET在氮气气氛下的TG-DTG曲线作了对比分析,并计算了CNSs/PET和CNSs-BA/PET在500℃时残炭量的理论值,如图6和表3所示。由图6可知:在氮气气氛下,PET、CNSs/PET和CNSs-BA/PET三者的TG曲线和DTG曲线基本重合,说明加入少量(2.0wt%)的CNSs和CNSs-BA均不会对PET的无氧降解行为造成明显影响。由表4可知,PET、CNSs/PET和CNSs-BA/PET三者的Tonset和Tmax均较为接近,但三者在高温(500℃)下的残余质量有所不同。经计算发现CNSs/PET和CNSs-BA/PET二者在高温下残炭量的实际值(CR500℃,exp)均大于理论值(CR500℃,cal),这说明阻燃剂CNSs和CNSs-BA对PET均有促进成炭作用。其中,CNSs-BA/PET在500℃下残炭量的实际值与理论值的差值(∆CR500℃)大于CNSs/PET,这说明CNSs经BA接枝后对PET的促进成炭作用加强。聚合物在高温下形成的残炭越多,燃烧时发生热分解的部分就越少[21],这便是阻燃复合材料热释放速率降低的主要原因之一。
表 3 CNSs、CNSs-BA以及PET、CNSs/PET、CNSs-BA/PET在氮气气氛下的TG-DTG数据Table 3. TG-DTG data of CNSs, CNSs-BA, PET, CNSs/PET and CNSs-BA/PET under nitrogen atmosphereSample Tonset/℃ Tmax/℃ CR500℃/% ∆CR500℃/%c exp.a/cal.b CNSs >800 — 96.88/— — CNSs-BA 476.4 — 94.92/— — PET 379.1 419.1 10.09/— — CNSs/PET 380.1 421.4 13.97/11.52 2.45 CNSs-BA/PET 382.0 420.1 15.68/11.79 3.89 Notes: a CR500℃,exp. is the experimental value of char residue; b CR500℃,cal. is the calculated value of char residue; c ∆CR500℃=CR500℃,exp.−CR500℃,cal.. 表 4 PET、CNSs/PET和CNSs-BA/PET在空气气氛下的TG-DTG数据Table 4. TG-DTG data of PET, CNSs/PET and CNSs-BA/PET under air atmosphereSample Tonset/℃ Tmax-1/℃ Tmax-2/℃ PET 397.3 433.4 585.1 CNSs/PET 359.1 438.4 567.5 CNSs-BA/PET 391.0 439.7 563.3 Notes: Tmax-1—Maximum weightlessness temperature in the first stage; Tmax-2—Maximum weightlessness temperature of the second stage. 2.3.2 残炭分析
对纯PET、CNSs/PET和CNSs-BA/PET锥形量热仪测试后的残炭做了SEM和TG分析以进一步研究阻燃机制。
炭层的形貌结构和稳定性对于提高聚合物的阻燃性能至关重要,有效的炭层可通过阻止聚合物内部与可燃气体、氧气的接触来实现阻燃目的。图7为纯PET、CNSs/PET和CNSs-BA/PET炭层的SEM图像。可见,纯PET燃烧生成的炭层稀薄空且松散,表面存在大量气体逸出形成的气孔,显然这种形貌的炭层无法形成有效的屏障作用。与纯PET相比,CNSs/PET的炭层的致密性明显提高,气孔明显变小,意味着炭层有效性的提高。值得注意的是,与CNSs/PET相比,CNSs-BA/PET炭层的致密性和连续性得到了进一步改善,表面气孔也明显变少和变小,另外还存在大量鼓起的未破裂气泡,这种形貌的炭层在燃烧时一方面能有效地阻隔热量的传递,另一方面还能有效地阻隔PET燃烧降解生成的气态可燃物的逸出,起到隔热和隔氧的作用[22-23]。除此之外,CNSs-BA受热分解生成的CO2、氨气、氮气等难燃性气体能够稀释燃烧区域可燃气体的浓度,抑制燃烧的发展,这便是CNSs-BA/PET阻燃性提高的重要原因。
图8是PET、CNSs/PET和CNSs-BA/PET炭层的TG曲线。可知,纯PET炭层的Tonset较低,为215.08℃,其中100℃前失重为4.36wt%,这主要是由于纯PET的炭层结构松散、孔洞较多,容易吸收水分和储存小分子气体所致,其800℃时的残余质量为87.2wt%。在整个升温过程中,纯PET的炭层表现出3个失重阶段,第一个失重阶段发生在100℃之前,主要对应炭层中贮存的水分以及气态小分子的降解;第二个失重阶段发生在100~530℃之间,对应炭层主体部分的降解;第三个失重阶段发生在530℃之后,对应炭层热降解产物的再降解。与之相比,CNSs/PET炭层的Tonset提高到615.37℃,800℃时的残余质量提高到89.1wt%,这主要是由于CNSs/PET的炭层的致密性提高所致,其TG曲线基本保持了纯PET炭层的3个失重阶段。与PET和CNSs/PET的炭层相比,CNSs-BA/PET炭层的Tonset提高到800℃以上,意味着炭层在燃烧时能耐受更高的温度,从而更持久有效地起到凝聚相阻燃作用。值得注意的是,其炭层在热分解过程中只有一个较为明显的失重平台,并未像PET和的CNSs/PET的炭层一样经历3个失重阶段,说明阻燃剂CNSs-BA能使PET燃烧生成结构稳定的炭层,该炭层在燃烧过程中能耐受较高的火焰温度,从而对内部的基体起到持久有效的保护作用。
2.3.3 交联行为分析
聚合物的交联直接影响其热性能、流变性、成炭性、熔滴和自熄行为,并有助于聚合物的芳香化或炭化[24]。图9是PET、CNSs/PET和CNSs-BA/PET在热氧降解过程中的TG-DSC曲线,相关数据见表4。由图9可以看出,PET、CNSs/PET和CNSs-BA/PET在空气中均有两个失重阶段,说明PET及其复合材料发生的是两步降解反应[25]。第一个失重阶段是PET的主要失重阶段,发生在360~470℃之间。第二个失重阶段发生在470~590℃之间,该阶段对应第一个降解阶段生成的降解产物的进一步降解。值得注意的是,纯PET的Tonset为397.3℃,而PET的燃点通常在420℃左右,这说明PET在燃烧之前,首先会发生一定程度的降解并生成一些可燃性的气体或挥发性产物,以此来维持燃烧的进行。与纯PET相比,在第一个失重阶段,CNSs/PET和CNSs-BA/PET的热失重曲线稍向低温方向移动,但二者在第一个降解阶段结束时的剩余质量却大于PET,且该阶段的最大失重率所对应温度(Tmax-1)大于PET,说明阻燃剂的存在使PET的主体降解提前,但在该阶段却重组生成了热稳定性较高的物质。DSC曲线表明,CNSs-BA/PET在熔融峰和分解峰之间出现了明显的放热峰,该峰是PET的交联峰[26],而在PET和CNSs/PET的DSC曲线上交联峰却不明显,这说明CNSs经BA接枝改性后促进了PET的交联,这是由于阻燃剂表面接枝的芳香族席夫碱(BA)可以在PET的熔融温度和分解温度之间形成稳定的交联网络。
2.3.4 高温裂解产物分析
裂解-气相色谱-质谱联用(Py-GC-MS)是目前研究聚合物高温裂解产物的常用方法[27]。为了研究阻燃剂的引入对PET的热裂解行为及其高温裂解产物的影响,对PET、CNSs/PET和CNSs-BA/PET做了Py-GC-MS分析,三者的高温裂解产物对比见表5。可知,与纯PET的裂解产物相比,CNSs/PET和CNSs-BA/PET的裂解产物中都包含更多的杂环、稠环、共轭芳环类化合物,这些裂解产物具有较高的热稳定性,是难燃性的保护炭层形成的物质基础[28]。而CNSs-BA/PET的裂解产物中出现了诸如二甲基胺、偶氮苯等含氮产物,这是由于阻燃剂CNSs-BA表面接枝的苯亚甲基氨基苯酚所致。另外,与PET和CNSs/PET相比,CNSs-BA/PET的裂解产物中菲、萘、苊、芴等稠环芳烃类以及联苯类产物明显增多,佐证了CNSs-BA促进了PET降解过程中的交联,该交联一方面通过增大熔体黏度改善了熔滴现象,另一方面提高了炭层的致密性和热稳定性,这就是CNSs-BA/PET阻燃性和抗熔滴性提高的主要原因。
表 5 PET、CNSs/PET和CNSs-BA/PET裂解产物Table 5. Pyrolysis products of PET, CNSs/PET and CNSs-BA/PETPyrolysis products which found only in PET Tetrahydropyran; 2,2-dimethylpropanal; 4,8,12-trimethyl-tridecanoic acid methyl ester; 2,2-dimethoxybutane; 2-methyl-1,5-hexadien-3-yne; 1,6-heptadiyne; p-xylene; Decane; Methyl benzoate; Dodecylethyl ketone; 1-(3-methylphenyl)benzyl(2-methyl-1-methylenepropylidene); 4-methylphenyl-1-pentyn-3-ol phenol; Dimethyl 1,3-benzenedicarboxylate; Vinylmethyl terephthalate; Diphenylacetylene; Biphenyl-4-ylacetophenone; 1-(5,5-dimethyl-1,3-dioxocyclohexan-2-ylidene)-2-(N-ethylbenzothiazol-2-ylidene)-ethanes; Phthalic acid 4-formylphenyl ester; o-tertiaryl tricyclic [8.2.2.2(4,7)]hexadeca-2,4,6,8,10,12,13,15-octene; 4-(diethylaminomethyl)-2,5-dimethylphenol Pyrolysis products which found only in CNSs/PET Phenol; 1,2-dihydro-indene; 1-(4-methylphenyl)-ethanone; Stilbene; 1H-cyclopropyl[l]phenanthrene; Dihydro-p-terphenyl; 1-naphthol; Fluorene-9-methanol; 2-ethyl-1,1'-biphenyl; 1,1-diphenylethene; 4-(2-benzoyl-5-phenyl-3-thienyl)-1,2-dihydrophenanthrene; 2-phenylnaphthalenyl benzoate; 1,1-dihydro-2-phenylnaphthalenyl benzoate; 3-chlorobenzylnonyl; 1-(2,5-dimethylphenyl)ethanone; 1-(2,5-dimethylphenethyl) ethanone; Dimethyl-1H-indene; Diethylmalonic acid; 3-chlorobenzylnonyl ester Pyrolysis products which found only in CNSs-BA/PET 1,5-hexadiyne; Dimethylamine; Nitrous oxide; 1,1'-(1,4-phenylene)bis-acetophenone; 2-methylindene; Azobenzene; Benzene; (1-methyl-2-cyclopropen-1-yl)-2-methylindene; Stilbene; Ethylketone; 1-(3,4-dimethylphenyl); 1-(4-methylphenyl); 1-ethenyl-4-methylbenzene; Dibenzofuran; 2-naphthol; 4-hydroxy-1,2,3,4-tetrahydrophenanthrene; 9,10-dihydrophenanthrene; Benzopropiophenone; Fluorene; 4-vinylbiphenyl; 1,2,3,4-tetrahydrofil; 9,10-dihydrofil; 4-vinylbiphenyl; 1,4-vinylbiphenyl; Phenylacetone; 1,3,5-cycloheptatriene; 2-phenylnaphthalene; 1-acrylbenzene; 2-methylnaphthalene; 4-(2-benzoyl-5-phenyl-3-thienyl)-methylbenzoic acid; 1,3-dimethyl-1H-indene; Tricyclohexen-8-ol; Hexaethylcyclohexane; 9-phenyl-9-fluorenol; Ethylene oxide; Methoxyphenyltricyclohexadecen-5-ylmethanol; 4-benzylbiphenyl; Tritylbenzene; 9-phenylanthracene; 3-(1-phenylethoxy)-3H-isobenzofuran-1-one; 4-phenyl-3,4-dihydroisoquinoline; Oxetane; 2-phenyl; 3-phenylethynyl; Tetraphenyl; 1-[4-(2-phenylethenyl)phenyl]-ethanone; Acenaphthene; 1,2,3,5-tetraisopropyl-cyclohexane; 6,9-dimethoxy-phenazine-1-carboxylic acid; [1,1'-biphenyl]-4-yl-phenylmethanone; 1,1':4',1''-3'-methyltriphenylene Pyrolysis products which found both in PET and CNSs/PET Acetophenone; Benzoic acid; Biphenyl; 2-methyl-1,1'-biphenyl; 1,1'-(1,4-phenylene)bisacetophenone; p-terphenyl Pyrolysis products which found both in PET and CNSs-BA/PET Styrene; Acetophenone; Benzoic acid; Biphenyl; 2-ethyl-1,1'-biphenyl; Benzophenone; 9H-fluoren-9-one; p-terphenyl Pyrolysis products which found both in CNSs/PET and CNSs-BA/PET Benzene; Biphenyl; Acetophenone; Naphthalene; Toluene; Phenanthrene; Indene; 6,6-diphenylfulvene; p-terphenyl; Methylstyrene; Biphenylacetophenone; 4-ethylbiphenyl; Diphenylmethane Pyrolysis products found in PET, CNS/PET and CNSs-BA/PET Acetophenone; Benzoic acid; Biphenyl; p-terphenyl 2.4 复合材料的力学性能
图10为PET阻燃复合材料的抗拉强度和断裂伸长率图。可知,随着阻燃剂含量的增加,CNSs/PET和CNSs-BA/PET复合材料的抗拉强度和断裂伸长率均呈下降趋势。尤其是当阻燃剂含量超过2.0wt%时,PET复合材料的抗拉强度和断裂伸长率大幅度下降。这是由于高含量的阻燃剂在PET基体中形成了较大的团聚体,破坏了基体的连续性,阻碍了应力的传递所致,后续研究中应重点关注材料力学性能的改善。
3. 结 论
(1)为同时改善聚对苯二甲酸乙二醇酯(PET)的阻燃性和抗熔滴性,在碳纳米球表面接枝4-苯亚甲基氨基苯酚制备了一种新型碳基复合阻燃剂(CNSs-BA)。CNSs-BA为粒径约50 nm的球形颗粒,热稳定性良好。
(2) CNSs-BA的引入可显著提高PET的阻燃性和抗熔滴性。当CNSs-BA添加量为2.0wt%时,CNSs-BA/PET复合材料的极限氧指数(LOI)从PET的21.0%提高至28.1%,阻燃等级达到V-0级,热释放速率峰值降低了46.3%。
(3) CNSs-BA/PET表现出典型的凝聚相阻燃机制。CNSs-BA的引入能促进PET成炭,CNSs-BA/PET的高温残炭量(CR500℃)比PET提高了55.4%,且成炭量的实际值大于理论值。与纯PET的炭层相比,CNSs-BA/PET燃烧生成的炭层的致密性、连续性以及热稳定性都显著提高。这是由于CNSs-BA的引入促进了PET的高温交联,使其高温降解生成了更多的难燃性焦炭物质。
(4)本文为碳基阻燃剂的发展提供了重要理论补充,对开发无卤、阻燃、抗熔滴的PET材料具有一定的指导意义。
-
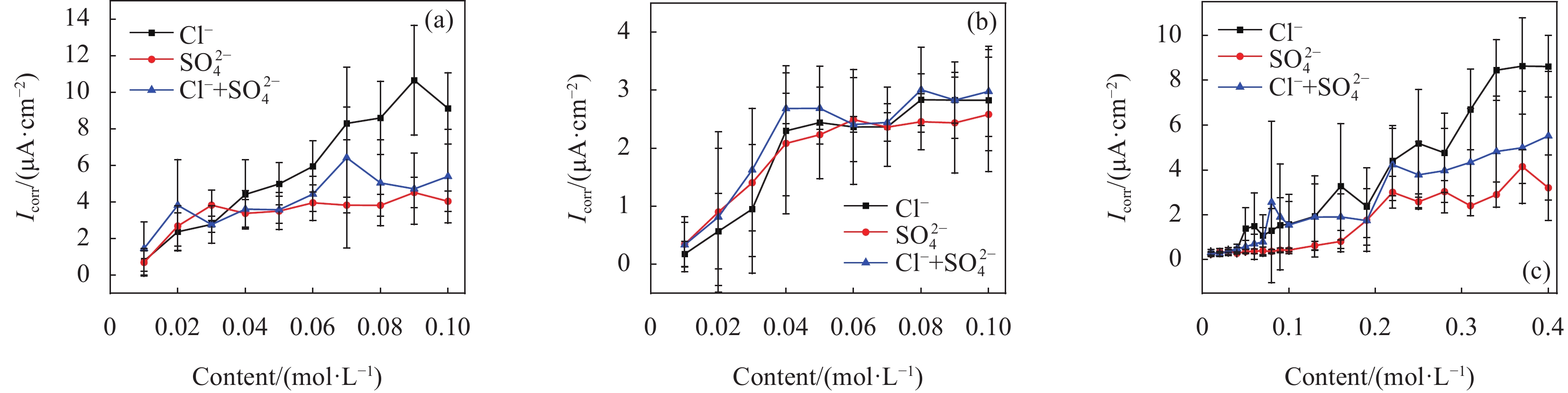
图 3 不同模拟液中腐蚀阶段钢试样的腐蚀电流密度(Icorr)曲线:(a)第一种模拟液(CH)中;(b)第二种模拟液(LC)中;(c)第三种模拟液(ST)中
Figure 3. Corrosion current density (Icorr) curves of steel specimens in corrosion stage of SCPSs: (a) in the first simulated solution (CH); (b) in the second simulated solution (LC); (c) in the third simulated solution (ST)
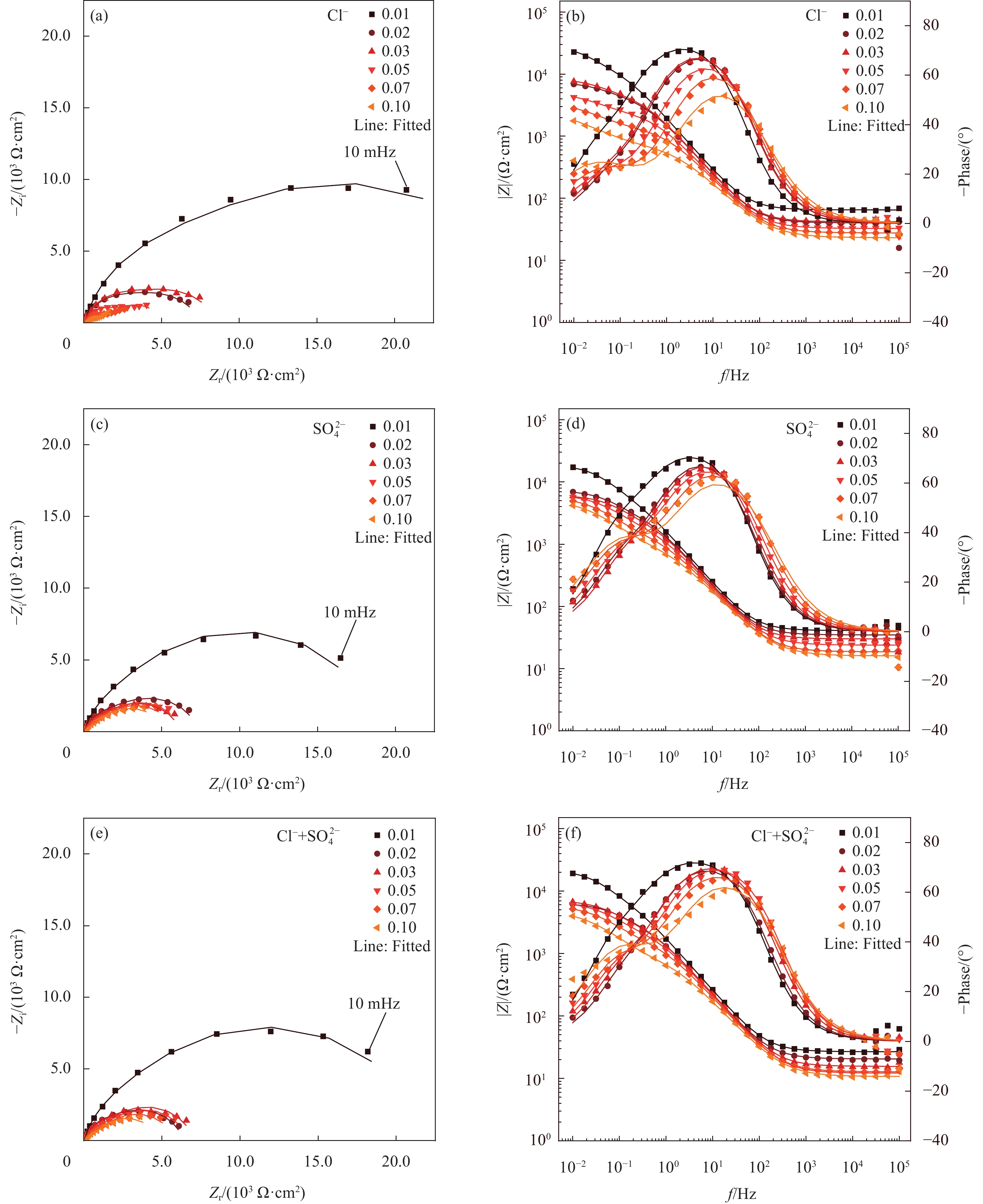
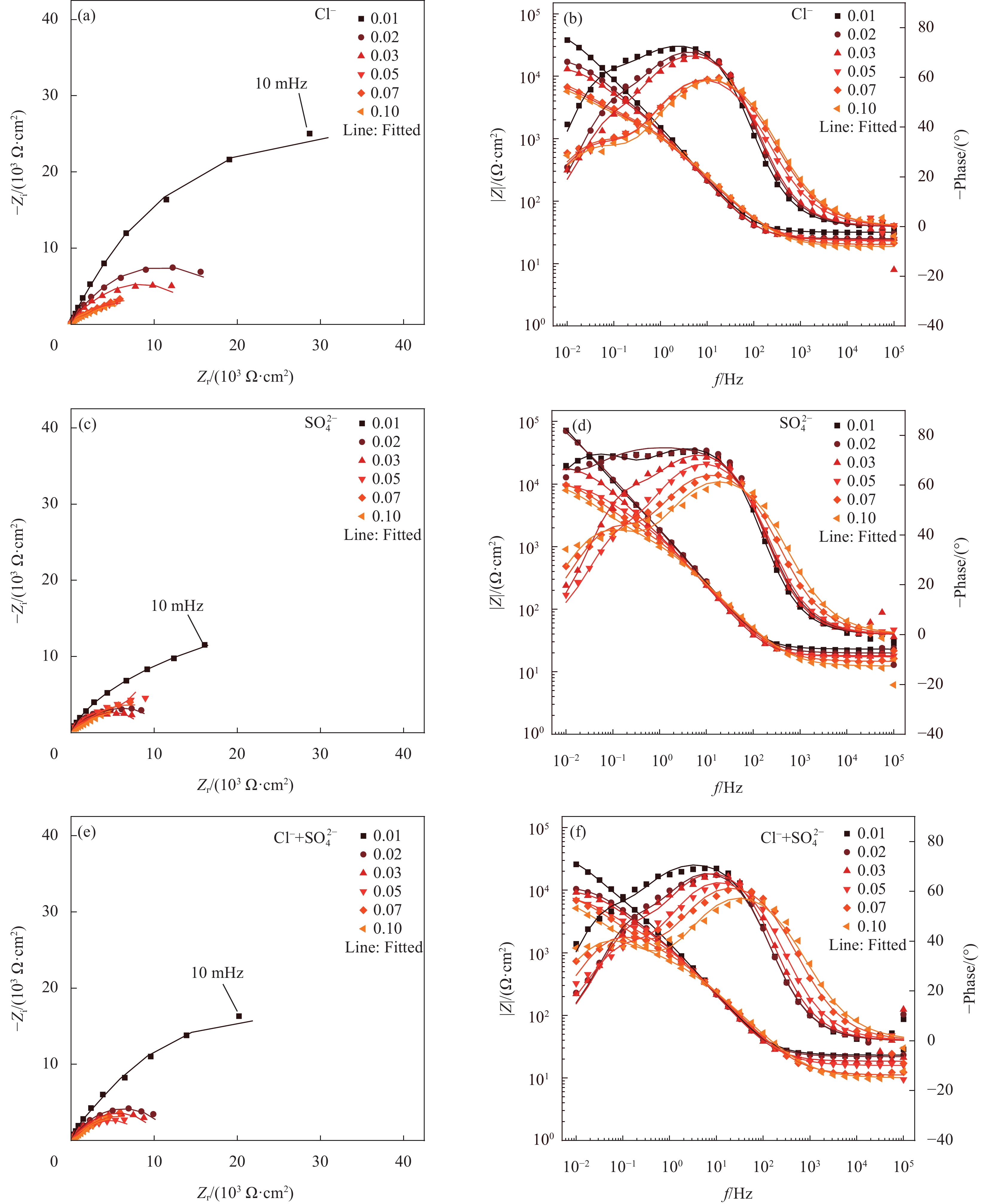
图 4 第一种模拟液(CH)中不同腐蚀离子对普通低碳钢试样腐蚀过程的电化学阻抗谱(EIS)结果及等效电路拟合结果:(a) Nyquist图;(b) Bode和Phase图
Figure 4. Electrochemical impedance spectroscopy (EIS) results and equivalent electrical circuit fitting results for the corrosion process of low-carbon steel specimens by different corrosive ions in the first simulated solution (CH): (a) Nyquist diagram; (b) Bode and Phase diagram
图 5 第二种模拟液(LC)中不同腐蚀离子对普通低碳钢试样腐蚀过程的电化学阻抗谱(EIS)结果及等效电路拟合结果:(a)Nyquist图;(b)Bode和Phase图
Figure 5. Electrochemical impedance spectroscopy (EIS) results and equivalent electrical circuit fitting results for the corrosion process of low-carbon steel specimens by different corrosive ions in the second simulated solution (LC): (a) Nyquist diagram; (b) Bode and Phase diagram
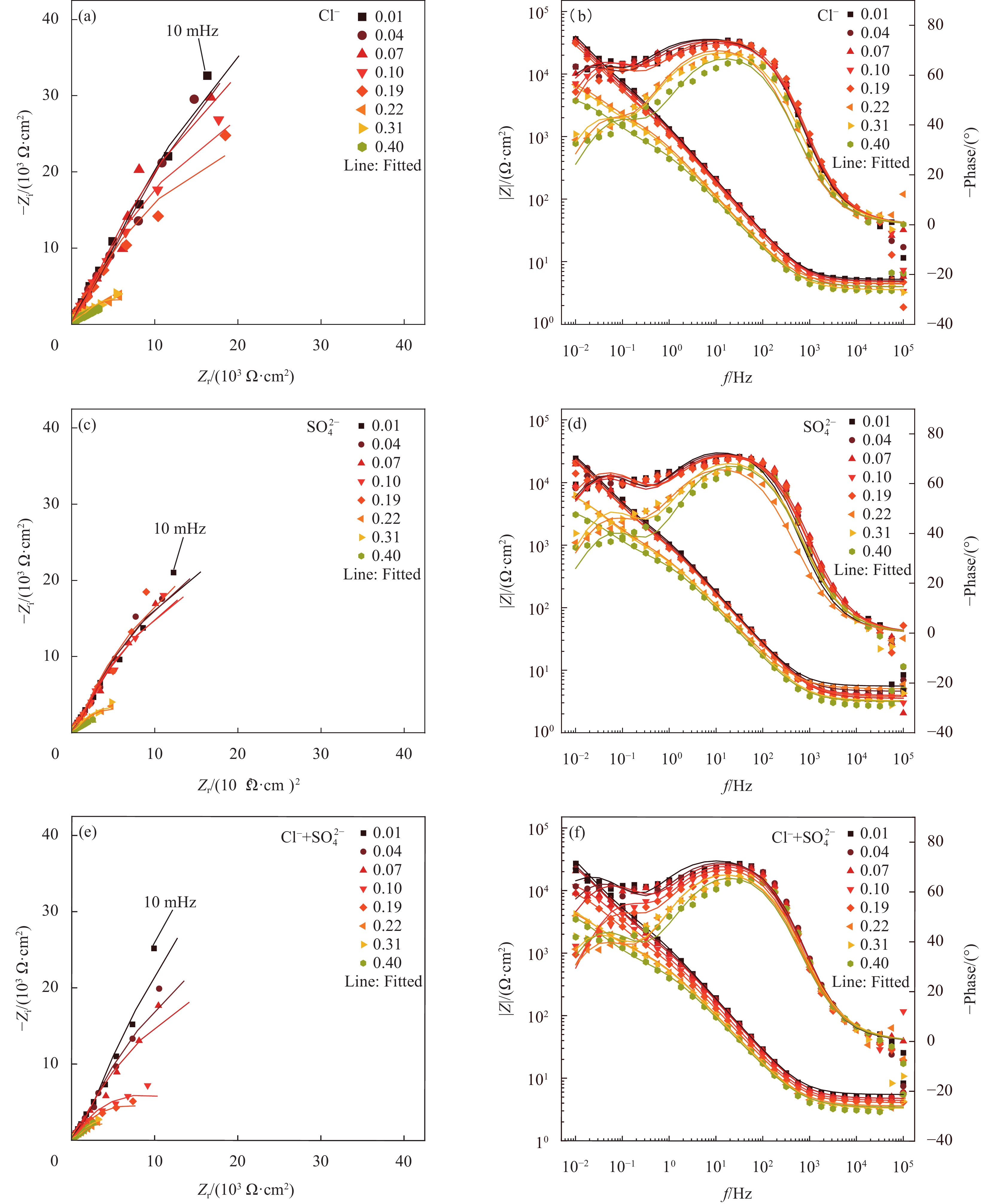
图 6 第三种模拟液(ST)中不同腐蚀离子对普通低碳钢试样腐蚀过程的电化学阻抗谱(EIS)结果及等效电路拟合结果:(a)Nyquist图;(b)Bode和Phase图
Figure 6. Electrochemical impedance spectroscopy (EIS) results and fitting results for the corrosion process of low-carbon steel specimens by different corrosive ions in the third simulated solution (ST): (a) Nyquist diagram; (b) Bode and Phase diagram
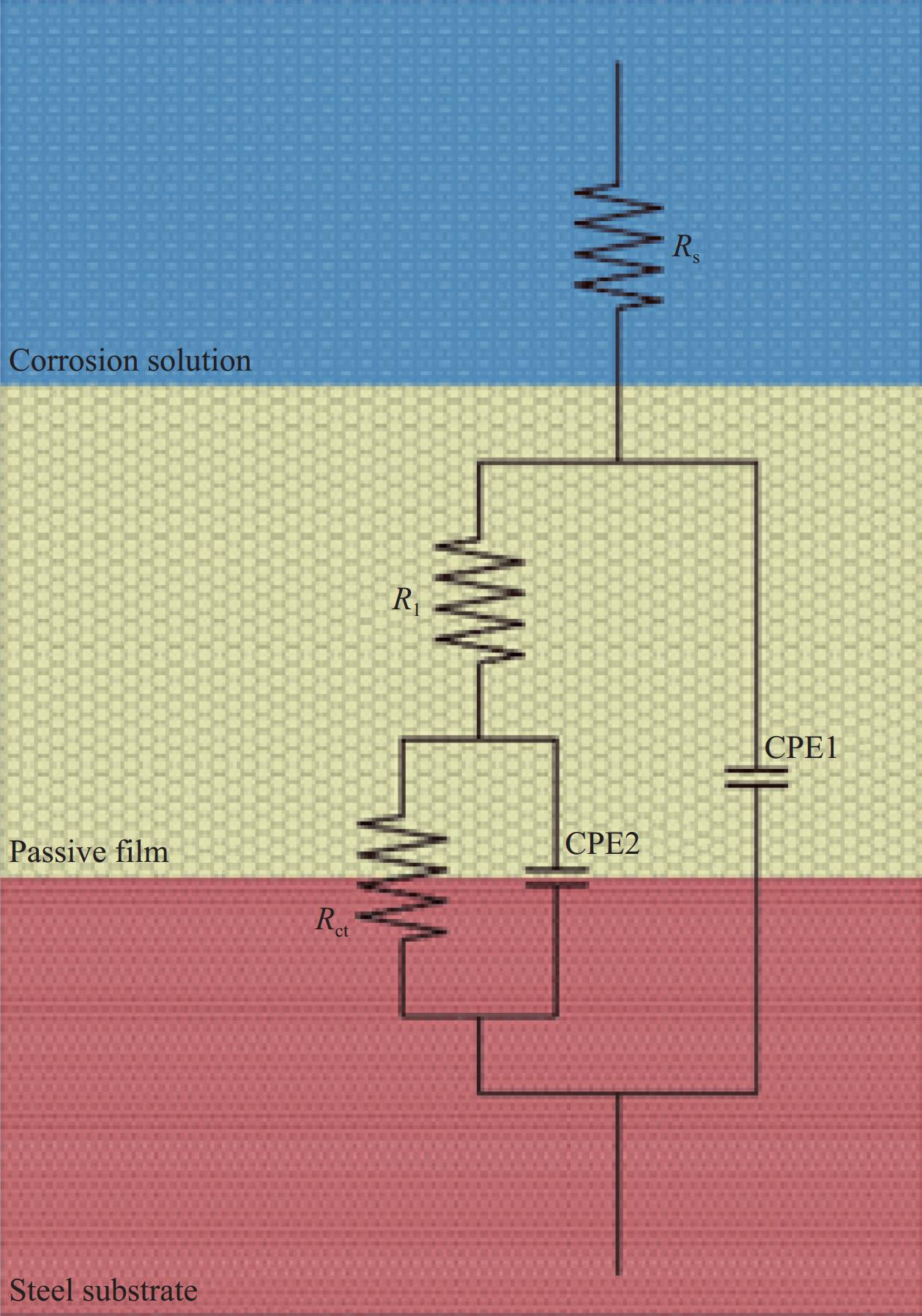
表 1 不同模拟液中腐蚀电化学阻抗谱(EIS)数据的等效电路拟合结果
Table 1 Equivalent electrical circuit fitting results of electrochemical impedance spectroscopy (EIS) data in different simulated solutions
Content/
(mol·L−1)RS/
(Ω·cm2)CPE1, Q/
(S·sn·cm−2)CPE1,a
[0<a<1]R1 /(Ω·cm2) CPE2, Q/
(S·sn·cm−2)CPE2,a
[0<a<1]Rct/
(Ω·cm2)Chi-squared CH-Cl− 0.01 65.42 0.0000917 0.8863 4164 0.0000714 0.4761 41280 5.62×10−4 0.02 40.35 0.0001129 0.8703 2597 0.0002508 0.4036 6510 1.85×10−3 0.03 42.19 0.0001079 0.8758 2195 0.0002016 0.3551 9236 7.96×10−4 0.05 33.03 0.0001247 0.8633 802.3 0.0004566 0.2367 19660 7.51×10−4 0.07 28 0.0001379 0.8521 752.2 0.0010090 0.3267 13790 6.27×10−4 0.1 23.6 0.0001617 0.83 298.7 0.0015650 0.3055 1665 6.81×10−4 CH-SO2−4 0.01 41.22 0.0001062 0.8848 4059 0.0000951 0.6181 16650 1.05×10−3 0.02 35.17 0.0001150 0.8848 1883 0.0002298 0.5316 6555 6.93×10−4 0.03 30.32 0.0001140 0.8887 1317 0.0002200 0.5174 5769 2.09×10−3 0.05 24.24 0.0001285 0.8705 1027 0.0003002 0.5019 6891 8.19×10−4 0.07 18.98 0.0001310 0.8709 605.9 0.0004202 0.4764 7953 3.39×10−3 0.1 16.46 0.0001365 0.8598 335.3 0.0005371 0.4666 8298 6.53×10−3 CH
Cl−+SO2−40.01 26.53 0.0001563 0.8879 4824 0.0000884 0.8907 26730 1.29×10−3 0.02 20.73 0.0001161 0.8732 1628 0.0001767 0.4851 5558 3.03×10−3 0.03 15.74 0.0001152 0.8735 1646 0.0002093 0.4884 6707 1.83×10−3 0.05 12.29 0.0001198 0.8693 1362 0.0002904 0.4951 7037 8.45×10−4 0.07 13.03 0.0001175 0.8771 565.2 0.0003434 0.4542 7912 1.83×10−3 0.1 11.07 0.0001478 0.8374 235 0.0005624 0.453 9741 4.18×10−3 LC-Cl− 0.01 32.04 0.0001148 0.8847 8330 0.0000563 0.6299 73860 3.93×10−4 0.02 25.3 0.0001290 0.8744 2978 0.0001182 0.6136 20830 5.60×10−4 0.03 24.19 0.0001079 0.8758 2195 0.0002016 0.3551 9236 7.96×10−4 0.05 23.1 0.0001176 0.8458 719.1 0.0002957 0.4679 12410 3.83×10−3 0.07 20.43 0.0001043 0.8391 379.3 0.0004010 0.4495 27850 3.01×10−3 0.1 18.96 0.0001411 0.7874 338.4 0.0004679 0.3499 9680 3.23×10−3 LC-SO2−4 0.01 30.1 0.0001085 0.8736 12400 0.0001089 0.4981 89150 6.82×10−4 0.02 25.22 0.0001202 0.8592 1608 0.0001916 0.4244 13390 9.59×10−4 0.03 23.07 0.0001673 0.8145 1621 0.0000909 0.4929 12360 2.51×10−3 0.05 20.76 0.0001310 0.8047 1110 0.0002258 0.4652 20660 8.88×10−4 0.07 19.5 0.0002658 0.7727 2373 0.0004917 0.4257 40920 1.52×10−3 0.1 14.82 0.0001614 0.7415 7872 0.0005338 0.3576 18148 1.16×10−3 LC
Cl−+SO2−40.01 23.06 0.0001348 0.8229 6727 0.0000550 0.5558 60790 4.27×10−3 0.02 22.16 0.0001216 0.8733 7205 0.0001543 0.5928 12610 2.06×10−3 0.03 18.79 0.0001121 0.8835 841 0.0001819 0.5644 11890 4.18×10−3 0.05 12.29 0.0001198 0.8693 1362 0.0002904 0.4951 7037 8.45×10−4 0.07 11.43 0.0001043 0.8391 379.3 0.0004010 0.4495 27850 3.01×10−3 0.1 10.37 0.0001018 0.8181 2699 0.0006246 0.4161 11730 4.13×10−3 ST-Cl− 0.01 5.391 0.0000566 0.9609 18140 0.0001331 0.6403 323900 5.83×10−3 0.04 5.112 0.0000465 0.9876 19410 0.0001591 0.6505 216900 7.50×10−3 0.07 4.982 0.0000389 0.982 9934 0.0001715 0.6763 141800 5.65×10−3 0.10 4.486 0.0001388 0.9657 1653 0.0002079 0.6374 193900 7.77×10−3 0.19 4.112 0.0001461 0.9329 1604 0.0001977 0.6628 173000 2.59×10−2 0.22 4.658 0.0001180 0.9443 2733 0.0004016 0.5089 16170 2.00×10−2 0.31 3.681 0.0001627 0.9025 2393 0.0004476 0.444 29290 2.54×10−2 0.40 4.152 0.0001806 0.9225 1345 0.0007194 0.5227 8297 2.98×10−2 ST-SO2−4 0.01 5.761 0.0000489 0.9533 45472 0.0002232 0.6262 898000 3.12×10−2 0.04 4.793 0.0000472 0.9437 32150 0.0002417 0.6331 840400 2.02×10−2 0.07 3.71 0.0000440 0.9333 12270 0.0002724 0.6245 314900 1.92×10−2 0.10 4.133 0.0000433 0.9126 11170 0.0002714 0.6367 339800 1.72×10−2 0.19 3.825 0.0002788 0.9491 1614 0.0000467 0.5169 445500 8.33×10−3 0.22 5.145 0.0002587 0.8349 2539 0.0005471 0.5214 17930 2.91×10−3 0.31 3.417 0.0000819 0.9798 836 0.0006337 0.5135 32580 1.98×10−2 0.40 3.221 0.0000762 0.9458 1027 0.0009938 0.4746 12670 3.14×10−2 ST
Cl+SO2−40.01 5.634 0.0000442 0.9522 89602 0.0000362 0.6685 262700 1.72×10−2 0.04 4.987 0.0000429 0.9726 16280 0.0002398 0.6297 267200 1.04×10−2 0.07 5.009 0.0000435 0.9695 13440 0.0002551 0.6265 15990 4.26×10−3 0.10 4.637 0.0000544 0.9868 1703 0.0003090 0.6111 26560 7.15×10−3 0.19 4.223 0.0001823 0.8697 3961 0.0003349 0.551 21510 2.00×10−3 0.22 3.682 0.0006716 0.9053 1201 0.0009439 0.514 34670 1.32×10−2 0.31 3.418 0.0007192 0.9147 913 0.0011370 0.5202 16320 1.36×10−2 0.40 3.547 0.0009536 0.9461 906 0.0010946 0.5018 8690 2.43×10−2 Notes: Rs is the electrolyte resistance; R1 is the passivation film resistance; Rct is the charge transfer resistance; Q and a are parameters in the constant phase angle elements CPE1 and CPE2; Chi-squared is the chi-squared test result. 表 2 Cl−和SO2−4在Fe(100)表面的吸附能
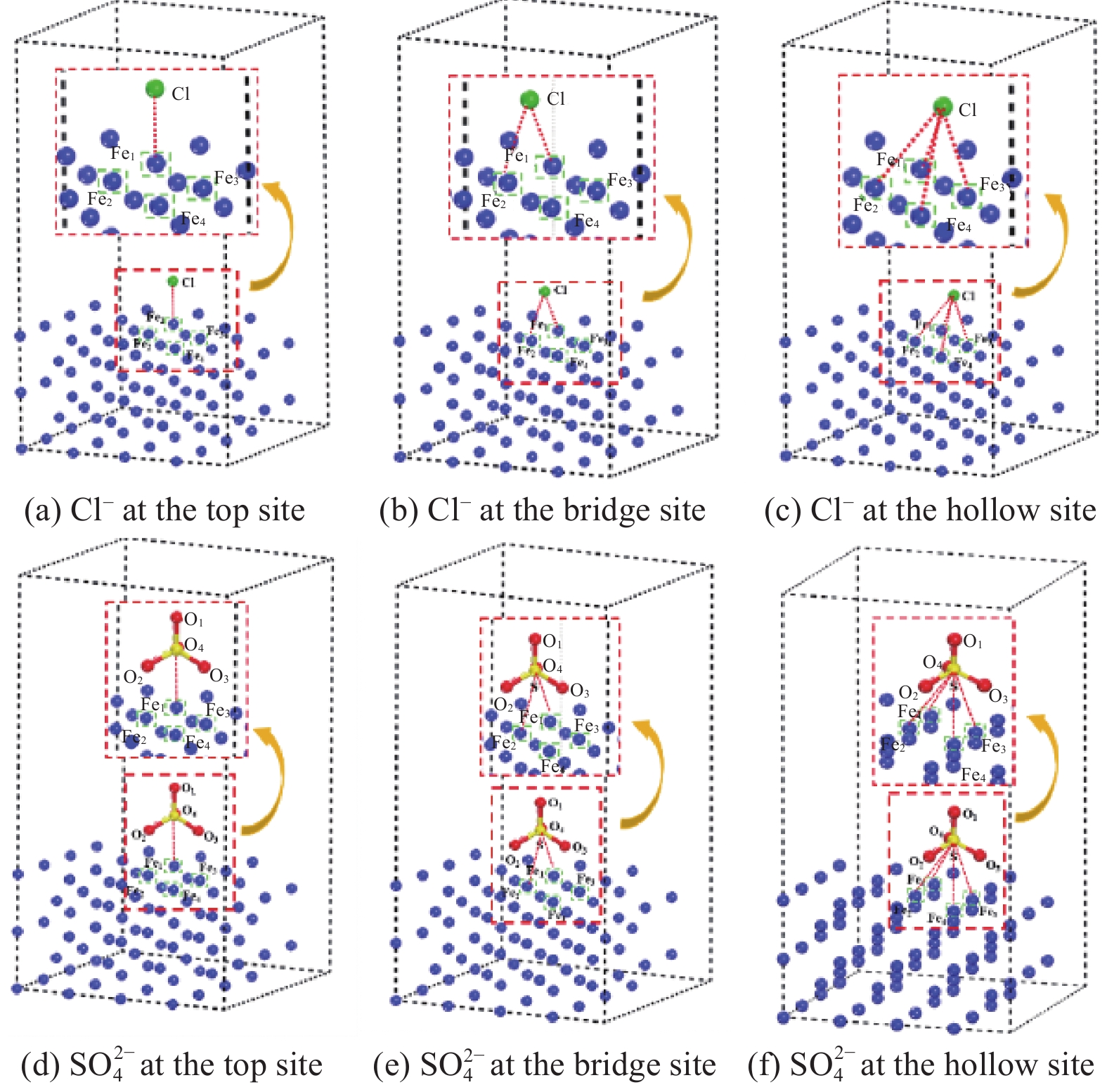
Table 2 Adsorption energy of chloride and SO2−4 adsorption on Fe (100) surface
Adsorption energy on Fe(100) /(kJ·mol−1) Top Bridge Hollow Chloride −449.896 −458.134 −363.154 SO2−4 −674.245 −691.32 −739.124 -
[1] 姚燕. 中国混凝土材料耐久性研究的新进展[J]. 中国水泥, 2002, 12: 39-42. DOI: 10.3969/j.issn.1671-8321.2002.02.019 YAO Yan. New progress of concrete material durability research in china[J]. China Cement, 2002, 12: 39-42(in Chinese). DOI: 10.3969/j.issn.1671-8321.2002.02.019
[2] CARNOT A, FRATEUR I, MARCUS P, et al. Corrosion mechanisms of steel concrete moulds in the presence of a demoulding agent[J]. Journal of Applied Electrochemistry, 2002, 32: 865-869. DOI: 10.1023/A:1020510506504
[3] 张伟平, 张誉, 刘亚芹. 混凝土中钢筋锈蚀的电化学检测方法[J]. 工业建筑, 1998, 28(12): 21-16. ZHANG Weiping, ZHANG Yu, LIU Yaqin. Electrochemical detection of corrosion of steel reinforcement in concrete[J]. Industrial Construction, 1998, 28(12): 21-16(in Chinese).
[4] DEHWAH H A F, AUSTIN S A, MASLEHUDDIN M. Chloride-induced reinforcement corrosion in blended cement concrete exposed to chloride-sulphate environments[J]. Magazine of Concrete Research, 2002, 54(4): 355-364.
[5] ALNPADU K O, TORII K. Chloride ingress and steel corrosion in cement mortars incorporating low-quality fly ashes[J]. Cement and Concrete Research, 2002, 32(6): 893-901. DOI: 10.1016/S0008-8846(02)00721-4
[6] LIU G J, SHEN F M, ZHANG Y S, et al. Reactive molecular dynamics study on carbon steel corrosion induced by chloride: Effects of applied potential and temperature[J]. Construction and Building Materials, 2024, 411: 134250. DOI: 10.1016/j.conbuildmat.2023.134250
[7] 刘国建, 张云升, 刘诚, 等. 模拟混凝土孔溶液中钢筋腐蚀与等效电路选取[J]. 材料导报, 2021, 35(14): 14072-14078. DOI: 10.11896/cldb.20040138 LIU Guojian, ZHANG Yunsheng, LIU Cheng, et al. Simulation of rebar corrosion in concrete pore solutions and equivalent circuit selection[J]. Materials Reports, 2021, 35(14): 14072-14078(in Chinese). DOI: 10.11896/cldb.20040138
[8] JIN Z Q, SUN W, ZHANG Y S, et al. Interaction between sulfate and chloride solution attack of concretes with and without fly ash[J]. Cement and Concrete Research, 2007, 37: 1223-1232. DOI: 10.1016/j.cemconres.2007.02.016
[9] TAMIMI A K, ABDALLA J A, SAKKA Z I. Prediction of long term chloride diffusion of concrete in harsh environment[J]. Construction and Building Materials, 2008, 22(5): 829-836. DOI: 10.1016/j.conbuildmat.2007.01.001
[10] EI-HAWARY M, AL-KHAIAT H, FEREIG S. Performance of epoxy-repaired concrete in a marine environment[J]. Cement and Concrete Research, 2000, 30(2): 259-266. DOI: 10.1016/S0008-8846(99)00242-2
[11] YU H F, DA B, MA H Y, et al. Durability of concrete structures in tropical Atoll environment[J]. Ocean Engineering, 2017, 135: 1-10. DOI: 10.1016/j.oceaneng.2017.02.020
[12] YUE Y F, WANG J J, BASHEER P A M, et al. Raman spectroscopic investigation of Friedel's salt[J]. Cement and Concrete Composites, 2018, 86: 306-314. DOI: 10.1016/j.cemconcomp.2017.11.023
[13] HALEEM S M A E, WANEES S A E, BAHGAT A. Environmental factors affecting the corrosion behaviour of reinforcing steel. V. Role of chloride and sulphate ions in the corrosion of reinforcing steel in saturated Ca(OH)2 solutions[J]. Corrosion Science, 2013, 75: 1-15. DOI: 10.1016/j.corsci.2013.04.049
[14] HALEEM S M A E, WANEES S A E, AAL E E A E, et al. Environmental factors affecting the corrosion behavior of reinforcing steel II. Role of some anions in the initiation and inhibition of pitting corrosion of steel in Ca(OH)2 solutions[J]. Corrosion Science, 2010, 52(2): 292-302. DOI: 10.1016/j.corsci.2009.09.004
[15] MAES M, BELIE N D. Resistance of concrete and mortar against combined attack of chloride and sodium sulphate[J]. Cement and Concrete Composites, 2014, 53: 59-72. DOI: 10.1016/j.cemconcomp.2014.06.013
[16] XU Y Z, HE L M, YANG L J, et al. Electrochemical study of steel corrosion in saturated calcium hydroxide solution with chloride ions and sulfate ions[J]. Corrosion Science Section, 2018, 74(10): 1063-1082.
[17] CAO Z L, HIBINO M, GODA H. Effect of nitrite ions on steel corrosion induced by chloride or sulfate ions[J]. International Journal of Corrosion, 2013: 853730.
[18] YANG L J, XU Y Z, ZHU Y S, et al. Evaluation of interaction effect of sulfate and chloride ions on reinforcements in simulated marine environment using electrochemical methods[J]. International Journal of Electrochemical Science, 2016, 11: 6943-6958. DOI: 10.20964/2016.08.51
[19] STROH J, MENG B, EMMERLING F. Deterioration of hardened cement paste under combined sulphate-chloride attack investigated by synchrotron XRD[J]. Solid State Sciences, 2016, 56: 29-44. DOI: 10.1016/j.solidstatesciences.2016.04.002
[20] DONG Q, ZHENG H R, ZHANG L J, et al. Numerical simulation on diffusion-reaction behavior of concrete under sulfate-chloride coupled attack[J]. Construction and Building Materials, 2023, 405: 133237. DOI: 10.1016/j.conbuildmat.2023.133237
[21] DEHWAH H A F, MASLEHUDDIN M, AUSTIN S A. Long-term effect of sulfate ions and associated cation type on chloride-induced reinforcement corrosion in Portland cement concretes[J]. Cement and Concrete Composites, 2002, 24: 17-25. DOI: 10.1016/S0958-9465(01)00023-3
[22] TUMIDAJSKI P, CHAN G W. Effect of sulfate and carbon dioxide on chloride diffusivity[J]. Cement and Concrete Research, 1996, 26: 551-556. DOI: 10.1016/0008-8846(96)00019-1
[23] 宋立康, 王曙光, 徐锋, 等. 硫酸根离子对带裂缝混凝土中氯离子扩散性能的影响研究[J]. 混凝土, 2015, (8): 26-30. DOI: 10.3969/j.issn.1002-3550.2015.08.007 SONG Likang, WANG Shuguang, XU Feng, et al. Study on the effect of sulfate ions on the diffusion properties of chloride ions in concrete with cracks[J]. Concrete, 2015, (8): 26-30(in Chinese). DOI: 10.3969/j.issn.1002-3550.2015.08.007
[24] 左晓宝, 邱林峰, 汤玉娟, 等. 氯盐和硫酸盐侵蚀下水泥浆体中钢筋锈蚀过程[J]. 建筑材料学报, 2017, 20(3): 352-358+372. DOI: 10.3969/j.issn.1007-9629.2017.03.006 ZUO Xiaobao, QIU Linfeng, Tang Yujuan, et al. Corrosion processes of steel reinforcement in cement paste under chloride and sulfate attack[J]. Journal of Building Materials, 2017, 20(3): 352-358+372(in Chinese). DOI: 10.3969/j.issn.1007-9629.2017.03.006
[25] 刘国建, 朱航, 张云升, 等. 混凝土孔溶液中不同侵蚀离子对钢筋的腐蚀行为[J]. 硅酸盐学报, 2022, 50(2): 413-419. LIU Guojian, ZHU Hang, ZHANG Yunsheng, et al. Corrosion behavior of steel reinforcement by different aggressive ions in concrete pore solutions[J]. Journal of the Chinese Ceramic Society, 2022, 50(2): 413-419(in Chinese).
[26] LIU G J, LI M H, YANG L, et al. Electrochemical dielectric response of steel corrosion induced by chloride in simulated concrete pore solution[J]. Journal of Sustainable Cement-Based Materials, 2024, 13(6): 854-864. DOI: 10.1080/21650373.2024.2333257
[27] SHEN F M, LIU G J, LIU C, et al. Corrosion and oxidation on iron surfaces in chloride contaminated electrolytes: Insights from ReaxFF molecular dynamic simulations[J]. Journal of Materials Research and Technology, 2024, 29: 1305-1312. DOI: 10.1016/j.jmrt.2024.01.194
[28] LI W X, GUAN X D, SHI J J. Electrochemical behavior of zinc in alkali-activated fly ash solution[J]. Cement and Concrete Composites, 2024, 146: 105395. DOI: 10.1016/j.cemconcomp.2023.105395
[29] LI Z D, CHEN R N, GAO Y G, et al. Function of Cu-Sb or Al microalloying on the corrosion resistance of 9Cr steel exposed to simulated concrete pore solution[J]. Corrosion Science, 2024, 231: 111983 DOI: 10.1016/j.corsci.2024.111983
[30] MONTEMOR M F, CUNHA M F. Corrosion behavior of rebar in fly ash mortar exposed to carbon dioxide and chlorides[J]. Cement and Concrete Composites, 2002, 24(8): 45-53.
[31] 唐方苗, 徐晖, 陈雯, 等. 模拟混凝土孔隙液中钢筋电化学腐蚀行为及pH值的影响作用[J]. 功能材料, 2011, 42(2): 291-293+297. TANG Fangmiao, XU hui, CHEN Wen, et al. Simulation of electrochemical corrosion behavior of steel reinforcement in concrete pore fluids and the role of pH influence[J]. Journal of Functional Materials, 2011, 42(2): 291-293+297(in Chinese).
[32] 翟海涛, 鲁道荣. 模拟混凝土孔隙液的pH值对钢筋腐蚀的Cl−质量分数的影响[J]. 合肥工业大学学报(自然科学版), 2009, 32(2): 186-189. ZHAI Haitao, LU Daorong. Effect of pH of simulated concrete pore fluid on Cl− mass fraction for rebar corrosion[J]. Journal of Hefei University of Technology(Natural Science), 2009, 32(2): 186-189(in Chinese).
[33] SHIN D M, HUR N Y, KIM W B. Study on Increasing High Temperature pH(t) to Reduce Iron Corrosion Products[J]. Corrosion Science and Technology, 2011, 10(5): 175-179.
[34] LIU L P, LI S L, GAO Z M, et al. Effects of Chloride and pH on Passivation Characteristics of Q235 Steel in Simulated Concrete Pore Solution[J]. International Journal of Electrochemical Science, 2022, 17(6): 220648. DOI: 10.20964/2022.06.51
[35] 钱如胜. 现代混凝土孔溶液离子演变规律及数值模拟[D]. 南京: 东南大学, 2018. QIAN Rusheng. Evolution law and numerical simulation of ionic evolution in modern concrete pore solution[D]. Nanjing: Southeast University, 2018(in Chinese).
[36] PAYNE M C, TETER M P, ALLAN D C, et al. Iterative minimization techniques for ab initio total-energy calculations: molecular dynamics and conjugate gradients[J]. Reviews of Modern Physics, 1992, 64: 1045-1097. DOI: 10.1103/RevModPhys.64.1045
[37] CHEVARY J A, VOSKO S H, JACKSON K A, et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation[J]. Physical Review B, 1992, 46(11): 6671-6687. DOI: 10.1103/PhysRevB.46.6671
[38] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism[J]. Physical Review B, 1990, 41(11): 7892-7895. DOI: 10.1103/PhysRevB.41.7892
[39] MONKHORST H J, PACK J D. Special points for Brillouin-zone integrations[J]. Physical Review B, 1976, 13: 5188-5192. DOI: 10.1103/PhysRevB.13.5188
[40] 宋诗哲. 腐蚀电化学研究方法[M]. 北京: 化学工业出版社, 1988. SONG Shizhe. Methods of corrosion electrochemical studies[M]. Beijing: Chemical Industry Press, 1988(in Chinese).
-
其他相关附件
-
本文图文摘要
点击下载
-
-
目的
钢筋混凝土结构因其优良的力学性能和耐久性,在现代建筑工程中得到广泛应用。然而,钢筋混凝土在使用过程中面临的主要问题之一是钢筋的腐蚀,这直接影响其使用寿命和结构安全。本文旨在揭示低碳钢在模拟混凝土孔隙溶液中,尤其是在不同pH值条件下,Cl和SO2-4单独及共同作用对其腐蚀行为的影响机制,深入探索这些离子的腐蚀效应及其相互作用。
方法本研究综合运用了电化学测试、X射线衍射(XRD)分析和密度泛函理论(DFT)计算等多种方法。通过在模拟混凝土孔隙溶液中进行腐蚀试验,分别在pH值为12.4、12.9和13.5的条件下,逐步增加Cl和SO2-4的浓度,并监测腐蚀电位、极化电阻、腐蚀电流密度和电化学阻抗谱等参数的变化,分析钢筋的腐蚀行为。同时,利用XRD分析腐蚀产物的成分,并通过DFT计算离子在铁表面的吸附能,以揭示腐蚀机制的微观细节。
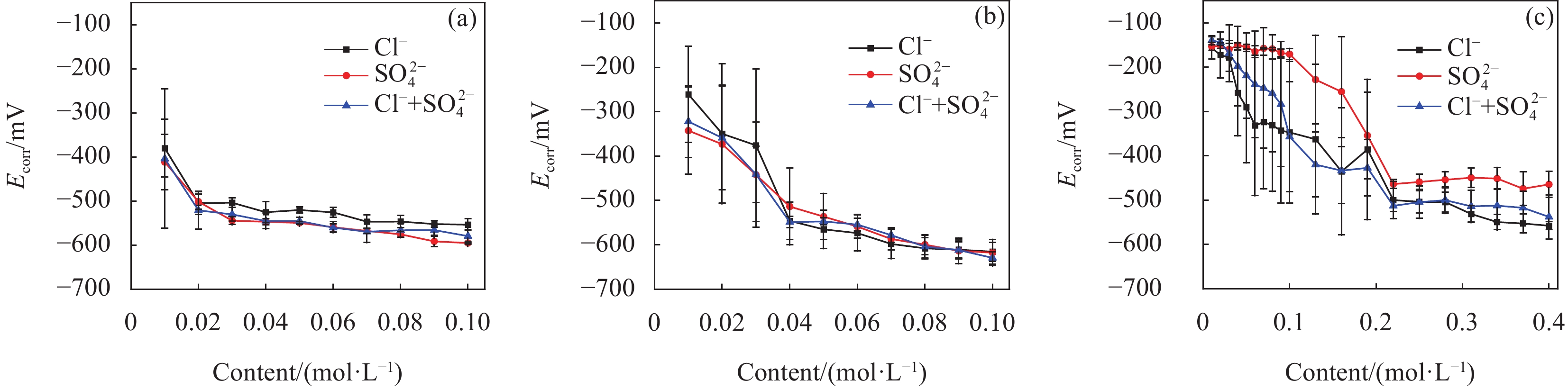
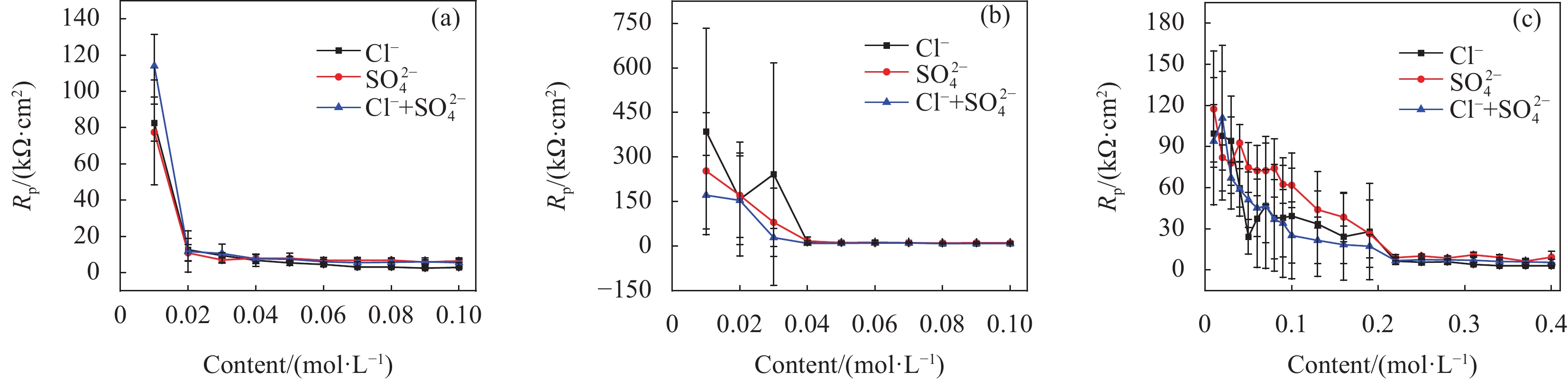
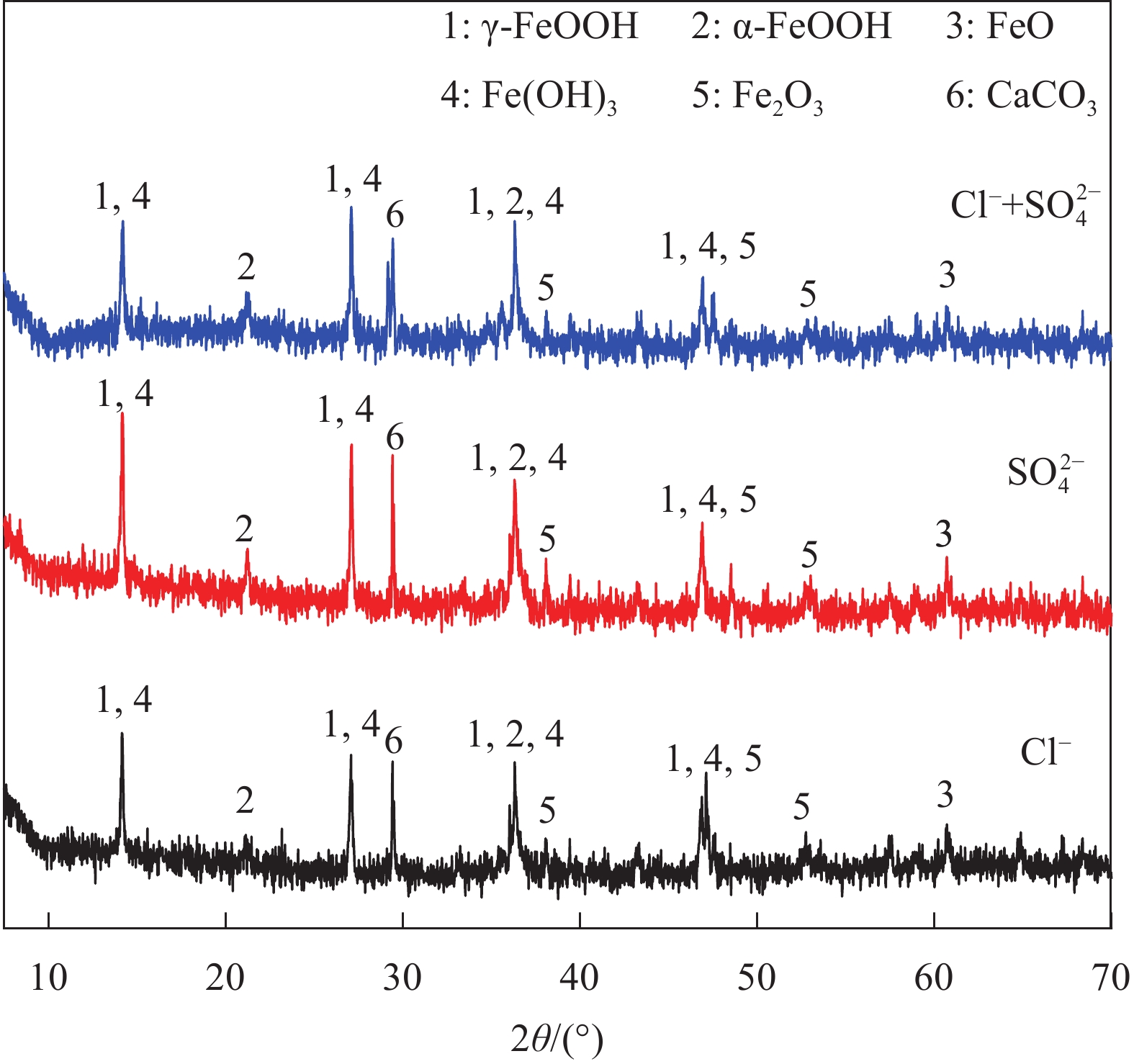
结果试验结果显示,在不同pH值的模拟溶液中,随着腐蚀离子浓度的增加,低碳钢的开路电位(OCP)普遍呈现下降趋势。特别是在pH值较低的溶液中,Cl单独存在的情况下表现出较强的腐蚀性,能够迅速破坏钢筋表面的钝化膜,加速电化学反应,导致钢筋的腐蚀速率显著增加。然而,当Cl与SO2-4共存时,SO2-4的存在一定程度上抑制了Cl的腐蚀作用,由于这种竞争吸附作用使钢筋的整体腐蚀速率介于单独存在Cl和SO2-4时的水平之间。相比之下,在pH值较高的模拟溶液中,钢筋的耐腐蚀性显著提高,腐蚀速率显著降低。线性极化电阻测试进一步验证了这一结果,钢筋在高碱性溶液中的耐腐蚀性优于相对较低碱性条件下的情况。此外,电化学阻抗谱分析表明,随着腐蚀离子浓度增加,钢筋的电化学阻抗逐渐减小,反映出腐蚀过程的加剧。此外,XRD分析结果揭示了不同离子情况下腐蚀产物的主要成分相似,包括γ-FeOOH、α-FeOOH、FeO、FeO和Fe(OH)等。通过DFT计算结果进一步确认,Cl和SO2-4在Fe(100)表面均具有负吸附能,表明它们都能有效吸附在钢筋表面,尽管SO2-4的催化腐蚀效率要低于Cl,但它具有更强的吸附能力,使其在竞争吸附中占据优势地位。
结论本研究主要得出以下
结论首先,提高混凝土孔隙溶液的pH值可以有效减少钝化膜对Cl和SO2-4的吸附,从而降低钢筋的腐蚀速率。其次,低浓度的Cl已足以引发显著腐蚀,而SO2-4则需要较高浓度才能表现出明显的腐蚀性。当两者共存时,由于竞争吸附作用的存在,总体腐蚀速率介于单独存在时之间。最后,本研究创新性地提出了竞争吸附-催化腐蚀的两阶段反应模型,深入揭示了Cl和SO2-4在钢筋腐蚀过程中的复杂交互作用机制。Cl要通过破坏钝化膜加速腐蚀进程,而SO2-4则通过影响腐蚀产物的稳定性和分布来参与腐蚀过程。
-
钢筋混凝土结构中钢筋的腐蚀严重影响了结构的耐久性,其主要为电化学腐蚀过程,在某些特定环境下,钢筋表面的钝化膜会遭到破坏,从而导致钢筋腐蚀。其中,Cl-是引发钢筋腐蚀的常见原因,它能够破坏钢筋表面的钝化膜,加速电化学腐蚀过程。同时,SO2-4也可能与钢筋发生化学反应,进一步促进腐蚀的进展,而混凝土的pH值对钢筋的腐蚀速率也有显著影响。
本文旨在阐明低碳钢在不同pH值(12.4、12.9和13.5)的模拟混凝土孔隙溶液中由Cl-和SO2-4引发的腐蚀行为。通过综合运用电化学测试方法、X射线衍射法(XRD)和密度泛函理论(DFT)计算进行分析。最终提出了一个竞争吸附-催化腐蚀的两阶段反应机制模型,揭示了Cl-和SO2-4在钢筋腐蚀过程中的交互作用机制。该模型强调了竞争吸附在初始阶段的作用,以及两种离子在催化腐蚀阶段的不同催化效率。这一研究不仅为复杂环境下的钢筋腐蚀行为提供了深入的理解,也为混凝土结构的耐久性设计和腐蚀防护措施的制定提供了科学依据。
Cl-和SO2-4的竞争吸附及腐蚀过程的示意图






 下载:
下载: