Preparation and properties of polycaprolactone-gelatin-bioglass-based asymmetrically infiltrated sandwich-structured composite membranes
-
摘要:
伤口愈合时敷料不仅需要提供止血作用,还需吸收过多的渗出物为伤口提供一个相对湿润又不过于干燥的环境。因此,本研究利用静电纺丝技术设计了一种三明治结构且具有不对称润湿性的Janus复合膜,外层为聚己内酯(PCL)纳米纤维膜,内层为明胶(Gel)和PCL混合的纳米纤维膜(PCL/Gel),而负载无机生物玻璃(BG)的纳米纤维膜(PCL/Gel-BG)作为中间层。对Janus复合膜形貌、结构以及力学、溶胀率、止血等性能进行了系统研究与探讨分析。结果表明:Janus复合膜力学性能、孔隙率等均符合医用敷料要求;此外,其溶胀率可高达990%能吸收大量渗出液,同时外层可以抗水和血液的渗透能防止伤口过度脱水;且在体外的血液吸收率、凝血指数、凝血时间测试中还表现出比传统医用纱布更优的止血能力,有望作为新型创面敷料应用于伤口愈合领域。
Abstract:Effective wound dressings must possess hemostatic functions and the ability to absorb excess exudate, maintaining a relatively moist but not overly dry environment for optimal healing. In this study, an asymmetric wettability Janus composite membrane with a sandwich structure was designed using electrospinning technology. The outer layer consists of a polycaprolactone (PCL) nanofibrous membrane, the inner layer is a composite nanofibrous membrane (PCL/Gel) containing a blend of gelatin (Gel) and PCL, while the intermediate layer is a composite nanofibrous membrane (PCL/Gel-BG) loaded with inorganic bioglass (BG). The morphology, structure, mechanics, swelling rate, hemostatic properties and other relevant properties of the Janus composite membranes were systematically studied and analyzed. The results show that the mechanical properties and porosity of the Janus composite membrane meet the requirements for medical dressings. Moreover, it displays a remarkable swelling rate of up to 990%, enabling efficient absorption of exudate. Additionally, its outer layer demonstrates excellent resistance to water and blood penetration, effectively preventing excessive wound dehydration. Furthermore, in vitro tests evaluating blood absorption rate, coagulation index, and clotting time reveal superior hemostatic ability compared to traditional medical gauze. Consequently, the Janus composite membrane holds great potential as an innovative wound dressing in the field of wound healing.
-
Keywords:
- electrospinning /
- Janus membranes /
- wound dressing /
- nanofibre /
- polycaprolactone /
- gelatin /
- bioglass.
-
皮肤是抵御许多病原体和化学毒素的屏障,而当皮肤受到创伤时,破损处的皮肤抵御能力会大幅度降低,导致创面极易受到病毒感染进而影响人体健康[1-3]。当破损的创面愈合时,痊愈阶段主要可划分为四个阶段:止血,炎症,增殖以及重塑[4-5]。止血属于整个愈合过程的第一阶段,因此,敷料的止血功能显得格外重要。炎症时期,组胺会增加毛细血管通透性,导致创面产生过多的渗出物[6-7]。而有渗出物的伤口处理非常困难,一般通过更换敷料解决多余渗出液问题,但频繁的更换敷料会对伤口造成二次机械性伤害[8]。所以,理想的伤口敷料应具有多个协同的功能[9-12]。首先,能屏蔽外界有害微生物;其次,需要一个优良的吸收能力用于吸收过多的组织渗出液同时为伤口提供一个相对湿润而不脱水的伤口微环境;最后,能提供凝血因子加速伤口愈合。因此,开发一个多功能的伤口敷料具有重要意义。
近年来,出现了一种新型Janus膜材料,因其双面不同的特殊结构及性能的差异而引起了广泛的关注[13-15]。Zhang[16]等人设计了一种两侧采用疏水聚乳酸纳米纤维,中间层由亲水含锌硅酸盐生物玻璃组成的Janus敷料,使得过量的渗出物被自发地清除,为伤口愈合创造了一个相对干净的环境。这种类似结构的Janus材料,会通过单向导湿将液体吸收到更亲水的一层材料中,使创面处的渗出液有效地被吸收,相较传统纱布通过不断更换来处理伤口渗出液的方法,这种高效的吸收方式更有利于伤口得愈合。因此,基于Janus特殊结构,开发具有多梯度润湿性的复合敷料也成为了目前热门的研究方向之一[17]。
聚己内酯(Poly ε-caprolactone,PCL)以其优良的可电纺特性、良好的力学特性、组织相容性、可降解性及价格低廉等诸多优点成为了目前敷料研发的首选材料[18-19]。其制备的纳米纤维膜具有强疏水特性,用作敷料屏障外界物质有极大优势。明胶(Gelatin,Gel)是一种以胶原蛋白为基础的可降解产品,可以促进血小板聚集,也可用作止血剂[20-21]。然而,它是一种脆性的生物材料,不易成丝,通过将聚己内酯与其混合纺丝,恰巧能互补这两种材料的缺陷。无机材料生物玻璃(BG)是生物医用材料的一个重要分支,主要成分为SiO2,具有良好的生物相容性、亲水性且无毒副作用[22]。基于以上生物玻璃的诸多优点,Ostomel[23]等人探讨了不同硅钙比的生物玻璃的止血效果,研究证实了在Si/Ca比值较高的MBG中观察到促凝血活性增加。
综上所述,本研究利用静电纺丝技术,以PCL纤维膜为疏水外层,PCL/Gel混合静电纺纤维膜作为亲水内层,在PCL/Gel溶液中掺入不同含量的生物玻璃所得到的PCL/Gel-BG纤维膜作为中间层,制备具有高效液体吸收、止血协同作用的Janus复合膜。对无机纳米粒子BG、单层纤维膜和Janus复合膜的形态、结构进行表征,并分析了不同纤维膜的力学性能、孔隙率、溶胀等性能,对比传统止血纱布探讨了Janus复合膜的止血性能以及不同无机纳米粒子BG含量对Janus复合膜的止血性能影响。
1. 实验材料及方法
1.1 原料及试剂
聚己内酯(PCL,相对分子质量为8万)、明胶(99%,生物技术级)、十六烷基三甲基溴化铵(CTAB,分析纯)、正硅酸四乙酯(TEOS,分析纯)、氨水(NH3·H2O),上海麦克林生化科技有限科技公司;四水合硝酸钙(CaNT,分析纯),成都科龙化工试剂厂;磷酸三乙酯(TEP,分析纯)、甲酸(FA,分析纯)、冰醋酸(AA,分析纯),上海阿拉丁生化科技股份有限公司;乙醇(EtOH,分析纯),杭州双林化工试剂有限公司。
1.2 试验设备
自组装高压静电纺丝机;S82-1型磁力搅拌器,上海志威电器有限公司;TG16-WS台式高速离心机,湖南湘仪实验室仪器开发有限公司;KQ5200E超声波清洗器,昆山超声仪器有限公司;MF-1200C马弗炉,安徽贝意克设计技术有限公司;DHG-自组装高压静电纺丝机;S82-1型磁力搅拌器,上海志威电器有限公司;TG16-WS台式高速离心机,湖南湘仪实验室仪器开发有限公司;KQ5200E超声波清洗器,昆山超声仪器有限公司;MF-1200C马弗炉,安徽贝意克设计技术有限公司;DHG-
9036 A电热恒温鼓风干燥箱,上海精宏实验设备有限公司。A电热恒温鼓风干燥箱,上海精宏实验设备有限公司。1.3 试验方法
1.3.1 生物玻璃纳米颗粒BG的制备
采用微乳液-溶胶凝胶法[24]合成BG。具体方法如下:常温条件下将0.4 g CTAB溶于78 mL无水乙醇和165 mL去离子水混合溶液中,完全溶解后,加入3 mL氨水搅拌30 min,然后每隔2 h依次加入2.79 g TEOS、0.30 g TEP、0.59 g CaNT,持续搅拌24 h,直到形成白色溶胶;将反应后得到的白色溶胶离心分离,用无水乙醇、去离子水重复离心清洗3次,之后放入60℃鼓风干燥箱中干燥24 h,得到白色粉末;最后,将白色粉末置于马氟炉中并以2℃/min的升温速率升温至650℃煅烧5 h获得BG。
1.3.2 静电纺丝液的配置
制备了五种溶液作为静电纺丝溶液。首先将PCL溶解于甲酸(FA)与乙酸(AA)混合溶液中FA∶AA (70∶30 v/v),室温下搅拌3 h得到10 wt%的PCL溶液作为敷料外层纺丝液。其次将明胶Gel∶PCL (1 : 1 w/w)加入到上述的溶液中搅拌至完全溶解,获得PCL/Gel溶液作为敷料内层纺丝液。最后分别加入相对于纺丝液中PCL质量的1%、5%、10%的BG于PCL/Gel溶液中搅拌3 h,超声30 min使纳米颗粒均匀分散在溶液中,获得对应溶液PCL/Gel-1BG、PCL/Gel-5BG、PCL/Gel-10BG作为敷料中间层纺丝液。
1.3.3 复合敷料的制备
将上述配置的纺丝液倒入10 mL注射器中,在自组装的高压静电纺丝装置上按外层、中间层、内层顺序依次叠加进行静电纺丝,即可得到Janus复合敷料。根据BG含量1%、5%和10%分别命名为Janus1、Janus2、Janus3复合膜。Janus复合膜对应的组成如表1所示。纺丝液工艺条件如表2所示,所有纺丝液均使用规格为22 G(内径0.4 mm)针头的注射器,圆筒收集器转速均为100 r/min,纺丝过程在室温下进行。
表 1 不同Janus复合膜的组成Table 1. Composition of different Janus composite membranesSample Outer layer Inter layer Inner layer Janus1 PCL PCL/Gel-1G PCL/Gel Janus2 PCL PCL/Gel-5BG PCL/Gel Janus3 PCL PCL/Gel-10BG PCL/Gel Notes: PCL—Polycaprolactone; Gel—Gelatin 表 2 不同纳米纤维膜的静电纺丝条件Table 2. Electrostatic spinning conditions for different nanofibre membranesSolution Needle-Collector
Distance/cmVoltage/
kVFlow Rate/
(mL·h−1)PCL 12 20 3.5 PCL/Gel 15 15 0.4 PCL/Gel-1BG 15 15 0.4 PCL/Gel-5BG 15 15 0.4 PCL/Gel-10BG 15 15 0.4 1.4 测试与表征
1.4.1 表面形貌及结构观察
经过镀金处理后,利用Ultra55型场发射扫描电子显微镜镜(FE-SEM)在加速电压为3 kV的真空条件下直接观察纳米颗粒和纤维膜的表面形貌,复合膜截面形貌则需要先在液氮中脆断,获得较为平整清晰的截面后,再通过FE-SEM对其截面形貌进行研究。利用ImageJ图像分析了解纤维直径和生物玻璃粒径的分布情况。
1.4.2 化学组成与结构分析
利用傅里叶红外仪(FTIR)采用ATR法模式对纤维膜进行扫描来分析材料的化学结构,结合能谱(EDS-Mapping)分析材料的化学组成及所含元素的分布情况。
1.4.3 力学性能测试
通过电子万能拉伸试验机对样品的力学性能进行研究。在所制备的静电纺丝膜上随机选取3个不同区域裁取10 mm×50 mm的长方形形状的纤维膜,在5 mm/min拉伸速度测定。
1.4.4 孔隙率测试
使用无水乙醇置换技术测量纳米纤维膜的孔隙率[25]。将样品在乙醇中浸泡1小时前后称量纤维膜重量,分别记为W1和W2,ρ为室温下乙醇的密度,V为膜浸泡乙醇后的体积,每个样品测试三次取平均值。纳米纤维膜孔隙率(P)由下式计算得到:
P/%=W2−W1ρV×100% (1) 1.4.5 润湿性和渗透性测试
利用水接触角测试仪进行静态水接触角测量研究纤维膜的润湿性,通过动态水接触角研究复合膜渗透性。随机选取纤维膜三处地方,向材料滴加0.5 μL的去离子水,用MindVision软件分析样品的接触角进行润湿性研究,将Janus复合膜亲水面和疏水面分别置于载玻片上通过动态的水接触角测量来研究其渗透性。此外,为了更有效评估材料在实际应用时的渗透性,将100 μL的抗凝兔血滴在Janus复合敷料的亲水侧,观察血液在复合膜亲水面和疏水面的浸润行为来评估材料在吸收血液时表现出的渗透性。
1.4.6 吸水性能和保湿性能测试
敷料的吸水性能和保湿性能直接影响了伤口愈合的环境条件,对伤口愈合至关重要[26]。因而可以通过溶胀率、保湿率对制备的材料进行综合评估。
(1) 溶胀率;将纤维膜先放入真空烘箱烘24 h,取出样品称量重量记为W0,然后在37℃条件下将样品完全浸没在pH=7.4的PBS缓冲液中,每间隔10 min将样品取出并用滤纸将纤维膜表面及边缘多余的溶液吸去,称量样品重量记为Wt,按下式计算得到溶胀率(SW)。
SW/%=Wt−W0W0×100% (2) (2) 保湿率;将吸收1 h水之后的样品记录质量为W,然后放入在37℃真空烘箱中,每间隔一定时间后取出记录重量记为W3,根据下式计算:
Waterloss/%=W−W3W×100% (3) 1.4.7 降解测试
将样品放入pH=7.4的PBS缓冲液中,然后置于37℃摇床,记录样品的初始重量Wi以及不同降解时间后的样品重量Wf。根据下式计算降解率:
Massloss/%=Wi−WfWi×100% (4) 1.4.8 止血性能测试
在所有止血性能测试中均将医用纱布作为对照组。
(1) 血液吸收率;将样品完全浸泡在抗凝兔血中,测定血液吸收能力。血液吸收率的计算公式如下:
Bloodabsorption/%=m1−m0m0×100% (5) 其中,m0样品的初始重量,m1为吸收血液后的重量。
(2) 体外凝血时间;测试材料促进血液凝固的能力,时间越短,其止血效果较好。称量20 mg的样品放在试管中,于37℃水浴条件下预热5 min后,加入2 mL抗凝兔血,然后加入60 μL、0.25 mol/L CaCl2溶液,记录试管倾斜时没有血液流动的时间即为凝血时间。
(3) 体外凝血指数;体外凝血指数(BCI)是衡量材料止血效果的重要指标。将样品裁剪成直径为1 cm的圆片放在培养皿中央,在37℃水浴条件下预热5 min,然后往样品滴加100 μL抗凝兔血,滴加同等血量在不含任何样品的培养皿中作为阴性对照组。随即加入10 μL、0.2 mol/L CaCl2溶液引发凝血,凝血反应5 min后,加入25 mL去离子水,在30 r/min、37℃条件下振荡,之后取适量上清液,利用可见分光光度计在540 nm下测定上清液的紫外吸光度。以对照组溶液的吸光度为参考值,假设阴性对照组为100,同等方式测量3次取其平均值。样品的体外凝血指数计算如下式:
SBCI=I1I2 (6) 式中:I1为各组样品测得的紫外吸光度;I2为阴性对照组的紫外吸光度。
(4)血细胞与膜之间的界面相互作用
将凝血后的纳米纤维膜用戊二醛将血细胞固定在样品上,采用一系列分级的乙醇-PBS溶液对血细胞脱水,然后放入45℃真空烘箱中干燥24 h,并通过SEM进行表面表征。
2. 结果与讨论
2.1 形貌及结构分析
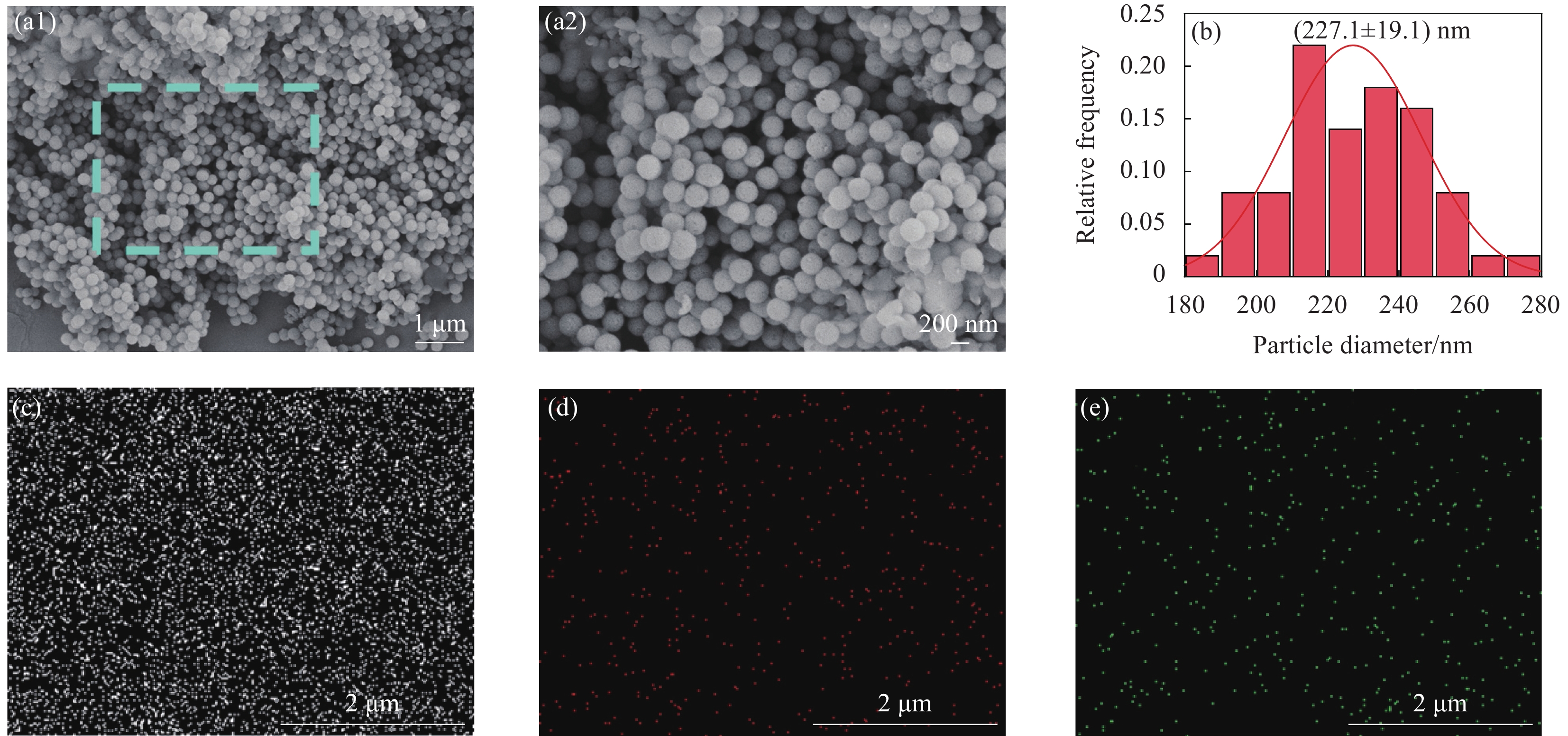
图1(a)和图1(b)示出BG的扫描电镜及粒径分布图。可见BG粒径大小均匀呈光滑的球形结构,平均粒径为(227.1 ± 19.1) nm。EDS-mapping元素映射对纳米颗粒BG的元素及其分布进行了分析,从图1(c-e)可以看出生物玻璃纳米颗粒含有Si、Ca、P三种元素,且分布均匀。
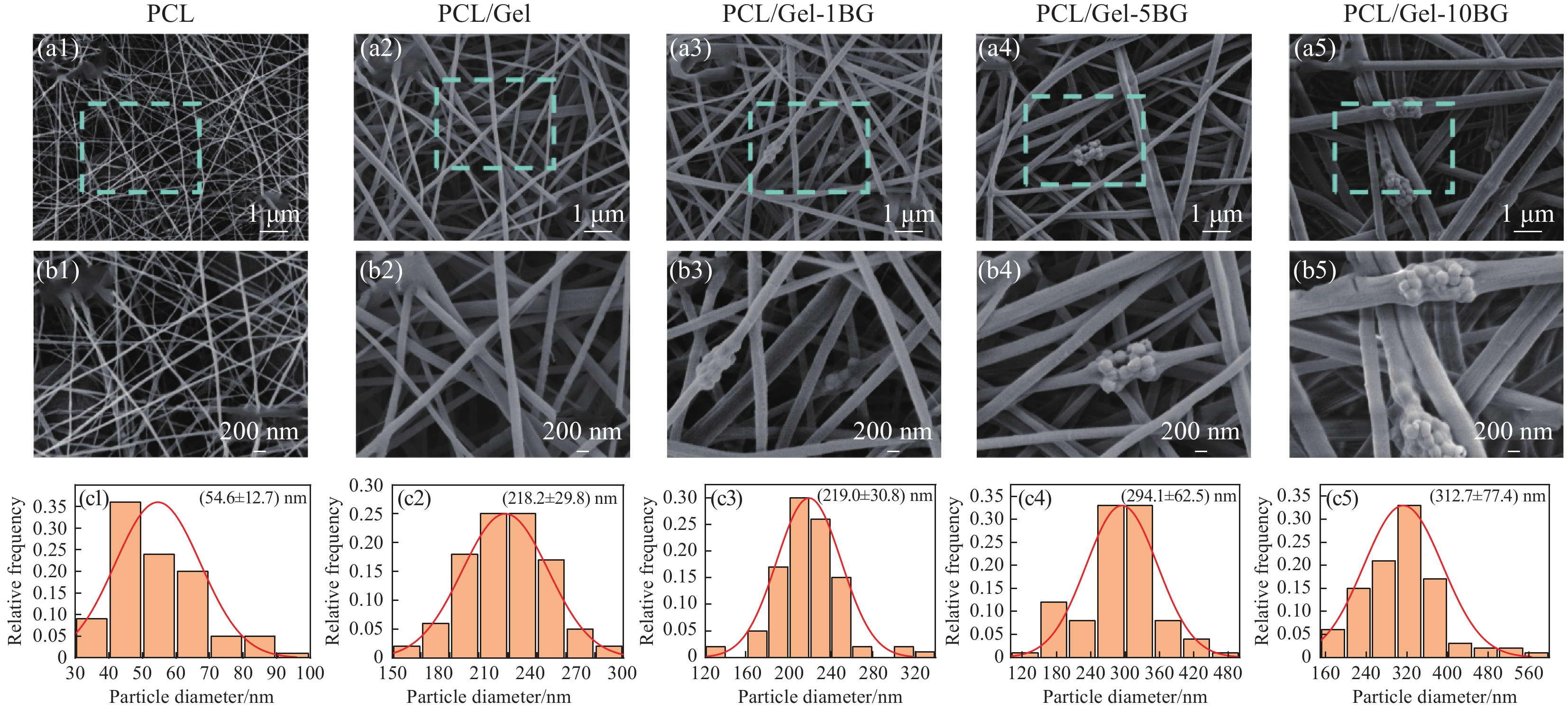
图2(a-c)为组成Janus复合膜的每层纤维膜的扫描电镜图及直径分布图。从图中可以看出纤维均无串珠,表面光滑,但不同纤维膜的纤维平均直径差异较大。其中复合膜外层PCL纤维平均直径为(54.6 ± 12.7) nm;中间层PCL/Gel-1 BG、PCL/Gel-5 BG、PCL/Gel-10 BG的平均直径分别为(219.0 ± 30.8) nm、(294.1 ± 62.5) nm、(312.7 ± 77.4) nm;内层PCL/Gel纤维平均直径为(218.2 ± 29.8) nm。结果可知纯PCL纤维的平均直径最小,加入明胶之后的纤维直径明显增加,这可能是因为PCL/Gel纺丝液相对PCL纺丝液的溶液浓度明显增大从而使其得到的纤维更粗。掺入生物玻璃后纤维直径随其掺入量增大而增加。当生物玻璃掺入量较小为1%时对纤维的直径几乎没什么影响,但当生物玻璃含量继续增加到5%、10%时直径持续增加,这可能是由于随着生物玻璃含量增加,PCL/Gel-BG纤维膜中生物玻璃纳米颗粒会发生团聚,团聚部分会进入到纤维内部,从而导致含有生物玻璃的纤维直径增大。
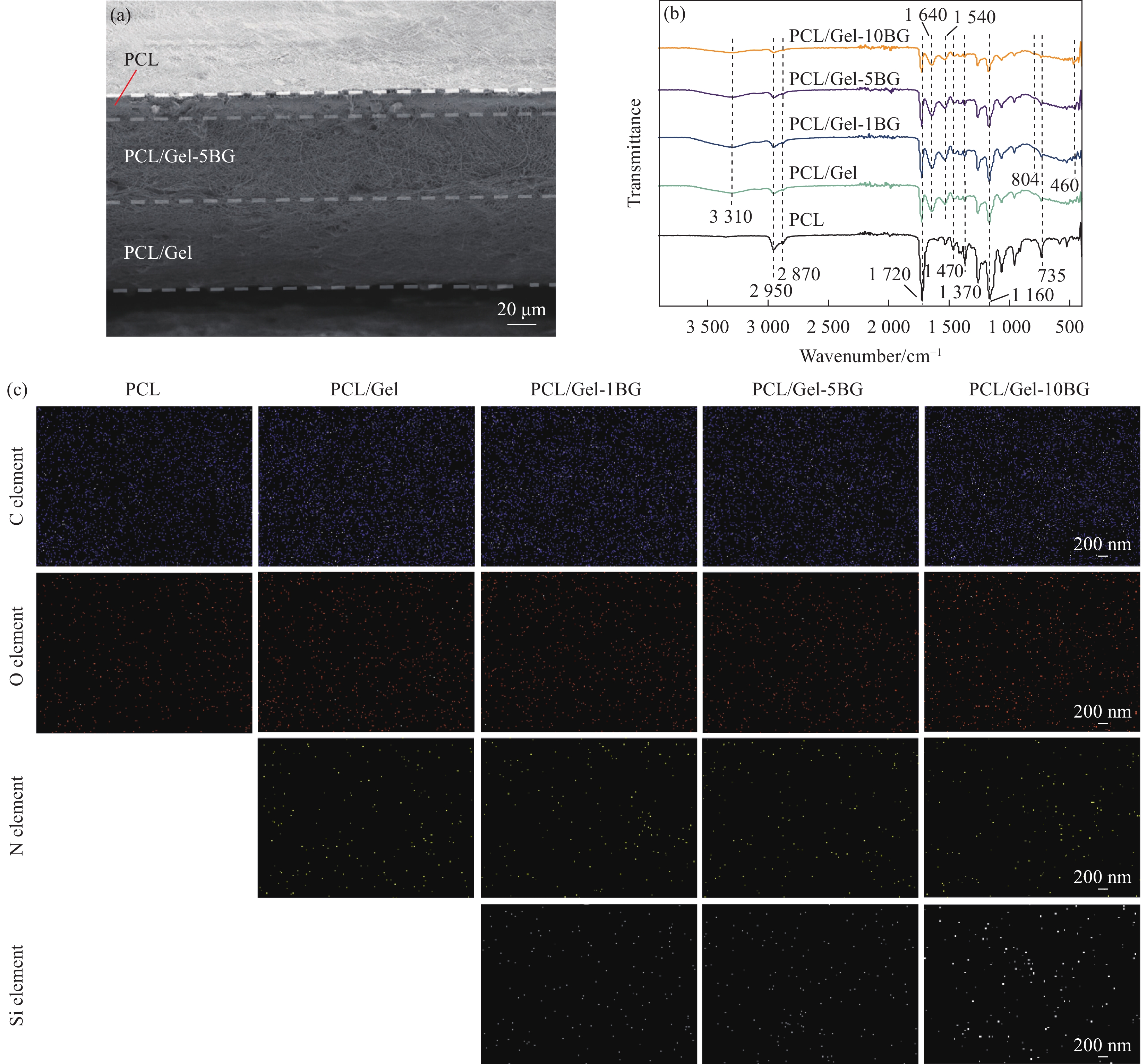
图3(a)显示了Janus复合膜横截面的扫描电镜图,可以观察到Janus复合膜中每层膜之间粘结良好,没有出现分层情况。从图中可以看出用于屏障外界物质的外层PCL纤维膜对比于中间层和内层是较为轻薄的,仅约为15 μm,用于吸收渗出液和提供凝血功能的内层纤维膜和中间层纤维膜相对较厚,均在70 μm左右,整个复合膜的厚度在150 μm左右。
图3(b)为不同纤维膜的红外光谱。纯PCL纤维膜红外光谱在
2950 cm−1和2870 cm−1左右的吸收峰,是由PCL分子中甲基和亚甲基基团的伸缩振动引起;在1720 cm−1位置吸收峰对应酯基团的伸缩振动;1470 cm−1处为C—H的面内弯曲振动;1370 cm−1处是O—H的伸缩振动峰;1160 cm−1处对应C—O—C的伸缩振动;735 cm−1为C—H吸收峰。明胶是胶原部分水解后的产物,主要特征峰为肽键,因此,将PCL与Gel混合纺丝之后,可以清晰地看到在1640 cm−1处的酰胺吸收带Ⅰ;1527 cm−1处的酰胺吸收带Ⅱ、3100 ~3655 cm−1范围内N—H伸缩振动和氢键等明胶的特征峰。掺入BG后在460 cm−1、804 cm−1出现的新吸收峰分为BG的Si—O—Si弯曲和Si—O—Si对称伸缩振动,且随着掺入量增多,特征峰越高,说明生物玻璃纳米颗粒的成功掺入,掺入生物玻璃纳米颗粒的含量为1 %时吸收峰不明显,可能是因为含量较少不能检测到。利用EDS-mapping元素映射确定纳米纤维膜的元素成分和分布情况。图3(c)显示:所有纤维膜均含有C、O元素,而在纯PCL纤维膜中仅有C、O元素均匀分布,对比之下,PCL/Gel纤维膜则多了来源于明胶的N元素。除此之外,掺杂了生物玻璃纳米颗粒的PCL/Gel-BG纤维膜中不仅有C、N、O元素分布,还均匀地分布着生物
玻璃纳米颗粒特有的Si元素,同时随着生物玻璃纳米颗粒掺入量的增多Si元素含量随之增加,进一步证明了生物玻璃均匀地掺入到静电纺丝膜中。
2.2 力学性能分析
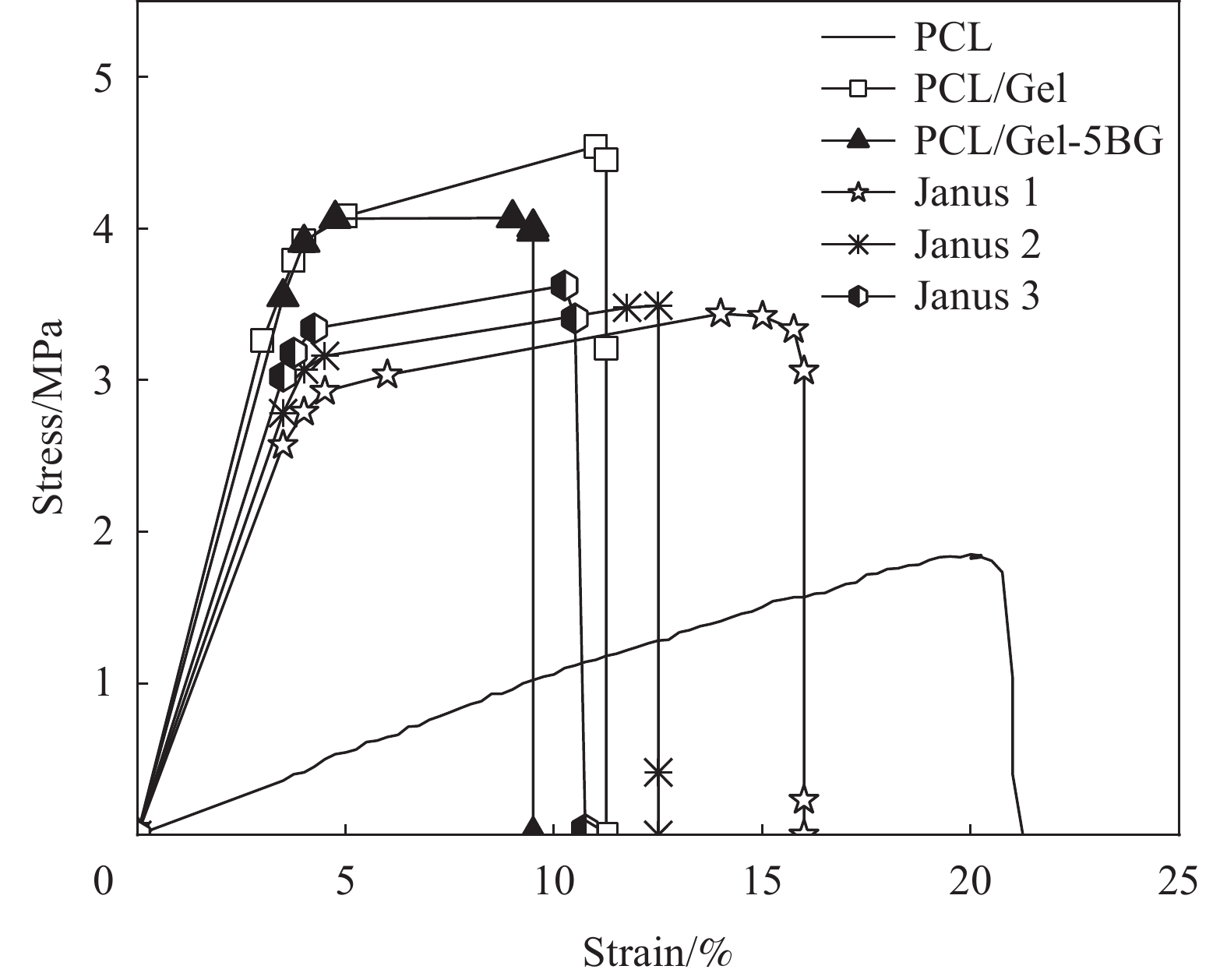
人体皮肤本身具有一定的机械强度,创面敷料可能需要用于身体的各种部位,所以为了能够更好的与伤口相贴合和覆盖,敷料就必须要有一定的力学强度。所制备的膜力学拉伸性能如图4所示,PCL纤维膜的拉伸强度较低约为1.8 MPa,加入Gel之后提高纤维膜的拉伸强度到4.5 MPa左右,而断裂伸长率有所减小;当掺入5%BG时,断裂强度降低到4 MPa,断裂伸长率减小到9%,这可能是因为添加了过多的纳米颗粒,造成了静电纺丝液的相容性变差从而影响了纤维膜的力学性能。而所制备的Janus复合膜则弥补了PCL纤维膜较小的断裂强度和PCL/Gel、PCL/Gel-BG纤维膜较低断裂伸长率的缺陷,且其拉伸强度均大于3.5 MPa,在人体正常皮肤的拉伸强度(约2.5-16 MPa)范围内。
2.3 孔隙率测试分析
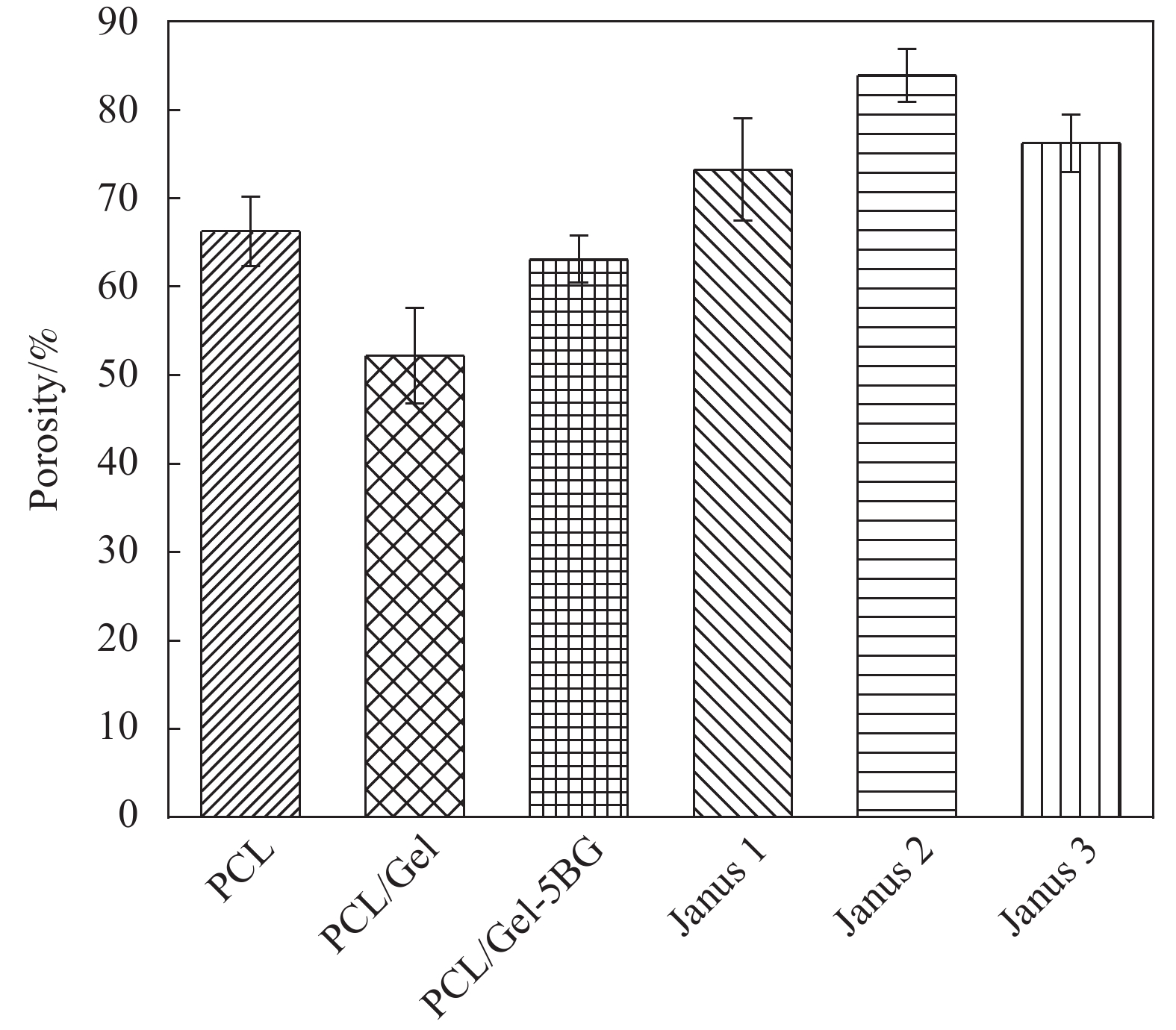
孔隙率是敷料应用的重要参数。理想的伤口敷料孔隙率应在60%-90%的理想范围内,以更好地实现细胞浸润和增殖。如图5所示单层的纤维膜PCL、PCL/Gel、PCL/Gel-5 BG平均孔隙率分别为(66.2 ± 3.9)%、(52.2 ± 5.4)%、(63.1 ± 2.6)%,而所制备的Janus1、Janus2、Janus3复合膜孔隙率分别为(73.2 ± 5.7)%、(83.8 ± 2.9)%、(76.2 ± 3.2)%高于单层的纤维膜并且在敷料应有的理想孔隙率范围内。理想孔隙率允许水和气体通过,确保伤口表面的干燥环境并促进细胞呼吸以及增加血细胞与材料充分接触,更有利于创伤的愈合。
2.4 润湿性和渗透性分析
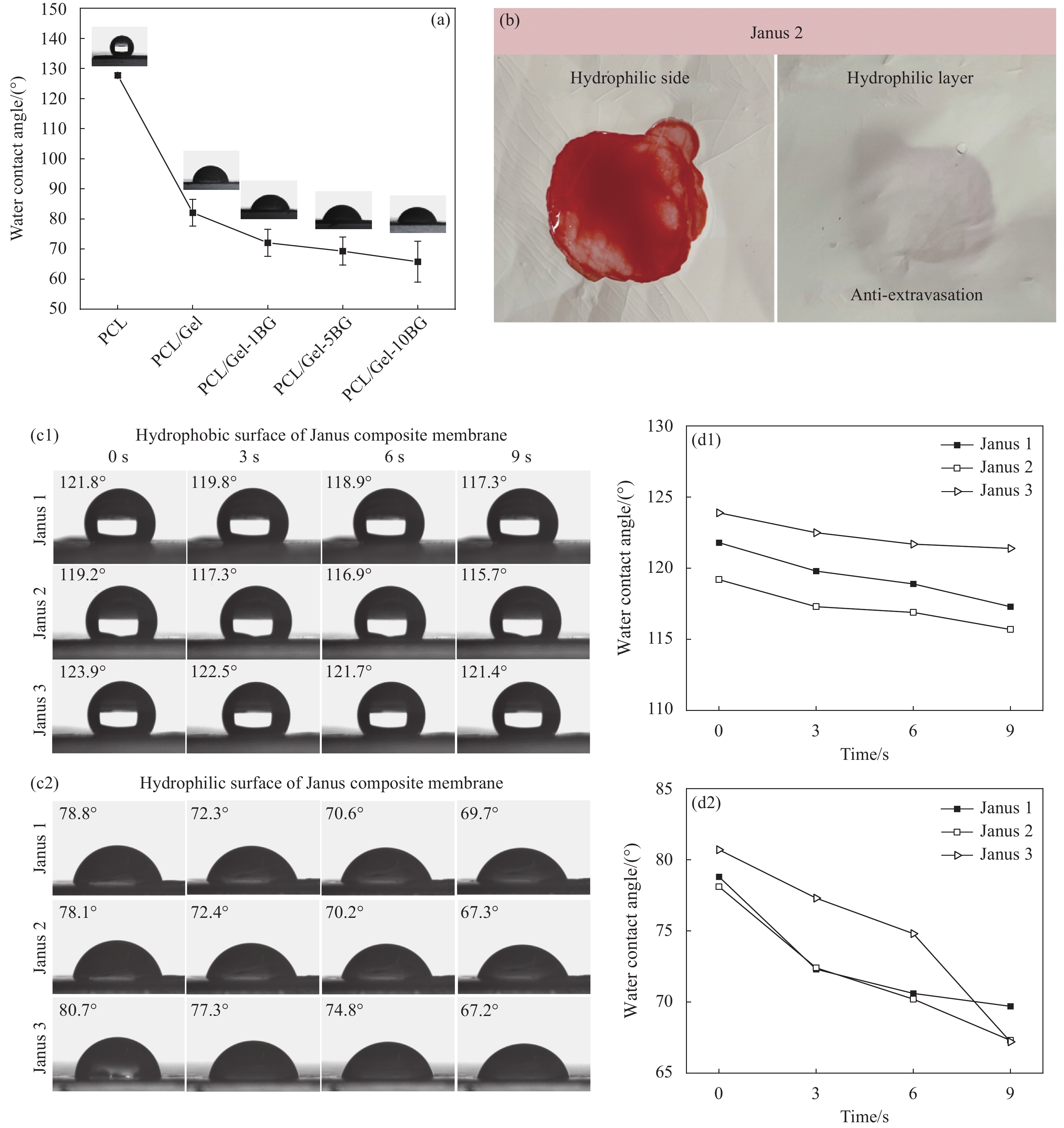
通过水接触角测试反映敷料的润湿性,水接触角愈大,敷料疏水性愈强,水接触角越小,其亲水性越好。图6(a)给出了组成Janus复合膜的所有单层纤维膜的水接触角。可以发现,敷料疏水外层纤维膜PCL水接触角大约为127.7°,明胶是一种亲水胶体,加入混合纺丝后使得作为敷料内层的PCL/Gel纤维膜水接触角减小到82.1°左右,此外,敷料中间层PCL/Gel-BG纳米纤维膜的水接触角随着生物玻璃含量增多而减小,PCL/Gel-1 BG纳米纤维膜的水接触角约为72.1°,PCL/Gel-5 BG纳米纤维膜的水接触角约为69.3°,PCL/Gel-10 BG纳米纤维膜的水接触角约为65.8°。结果说明由三层不同纤维膜组成的Janus1、Janus2、Janus3复合膜均具有不对称润湿性。不对称润湿性有利于渗出液的吸收。
![]() 图 6 不同纳米纤维膜润湿性(a); Janus2复合膜的血液渗透性(b); Janus复合膜疏水面(c)和亲水面(d)水接触角以及渗透性Figure 6. Wettability of different nanofiber membranes (a); Blood permeability of Janus2 composite membrane (b); Hydrophobic (c) and hydrophilic (d) water contact Angle and permeability behavior of different Janus composite membranes
图 6 不同纳米纤维膜润湿性(a); Janus2复合膜的血液渗透性(b); Janus复合膜疏水面(c)和亲水面(d)水接触角以及渗透性Figure 6. Wettability of different nanofiber membranes (a); Blood permeability of Janus2 composite membrane (b); Hydrophobic (c) and hydrophilic (d) water contact Angle and permeability behavior of different Janus composite membranes为了进一步探究复合膜的润湿性和渗透性,对Janus复合膜的疏水面和亲水面进行了动态水接触角的测试。图6(c1-c2)可以看出,Janus复合膜的疏水面初始水接触角在120°左右,经过几秒钟时间后水接触角始终处于疏水范围,说明复合膜的疏水面能有效为伤口提供保护屏障。观察图6(d1-d2)可知,Janus1、Janus2、Janus3亲水内层水滴渗透速率快,初始水接触角在80°左右,经过9 s之后,水接触角减小10°左右,其中Janus3复合膜的水渗透速度最快,这是因为Janus3复合膜内层纤维膜与中间层纤维膜的润湿性梯度相差最大,且都属于亲水性材料,导致复合膜亲水面接触到水滴时,水滴会朝着更亲水的中间层方向移动,从而使液体被吸收到材料内部的速度更快,这种快速的液体吸收能力更有助于血液中的细胞黏附促进伤口的愈合。通过向复合膜亲水面滴加100 μL的抗凝兔血模拟材料在真实场景应用时的血液渗透行为,如图6(b)所示,亲水面抗凝血液向四周扩散且逐渐形成团聚的血凝块,而疏水面始终无血液渗出。结果表明,Janus复合膜亲水面能吸收血液中水分,疏水面可以抗水和血液外渗,能为创口提供相对湿润而不过于干燥的环境。
2.5 吸水性和保湿性能分析
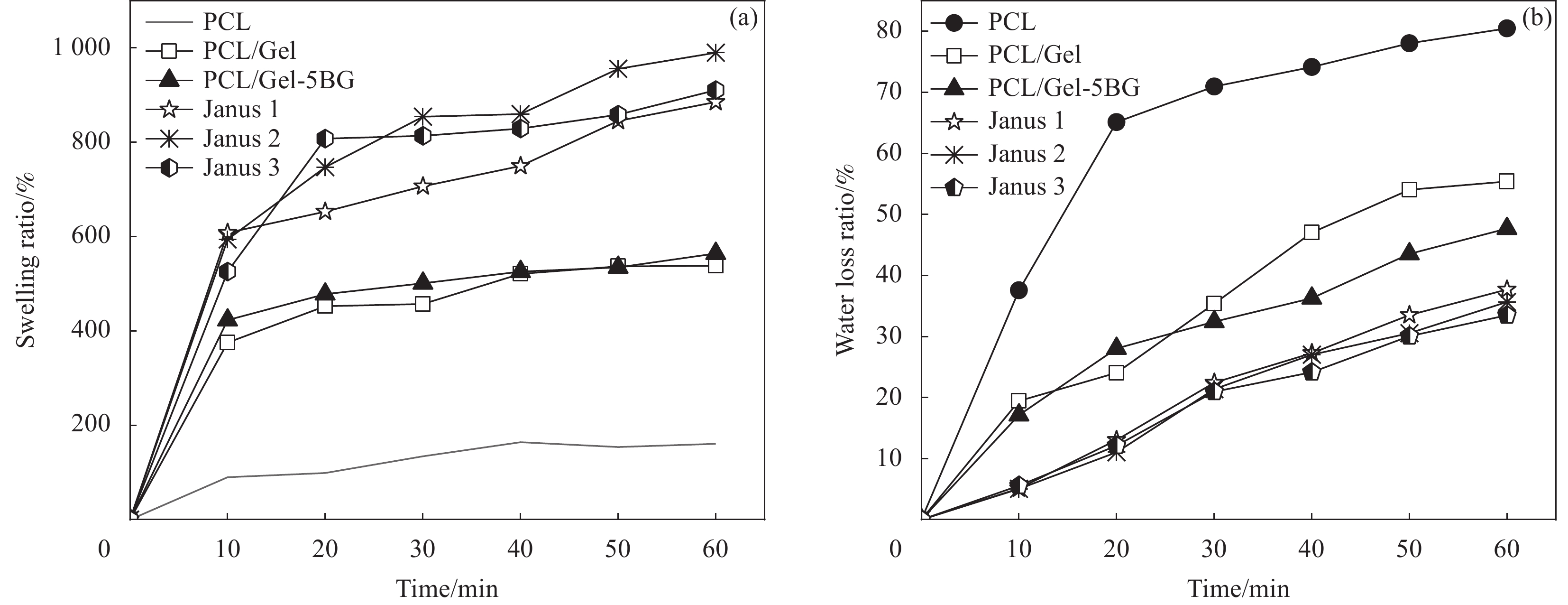
对材料液体吸收能力进行了研究,溶胀性能测试结果如图7(a)所示。纯PCL纤维膜因其疏水性能导致溶胀率较低仅约为130%,而明胶能吸收本身重量十倍左右的水,使得PCL/Gel纤维膜相较于PCL纤维膜溶胀率明显提高,吸收溶液1 h后的溶胀率增加到575.3%,掺入BG后,PCL/Gel-5 BG纤维膜溶胀率为564.2%。而对比单层纤维膜,所制备的Janus复合膜由于不对称的润湿性以及抗液体外渗的性能使得液体吸收能力得到大幅度提升。图中可以看出,当单层的纤维膜几乎达到了溶胀平衡时,Janus复合膜依旧表现出溶胀率向上增长的趋势,最终在1 h测试后,Janus1、Janus2、Janus3溶胀率分别高达885.4%、990%、910.8%。这个结果证明了所制备的Janus复合膜具有良好的吸收伤口渗出液的能力。
图7(b)示出纯PCL纤维膜失水率始终最大,对比之下,PCL/Gel纤维膜失水率显著下降,掺入生物玻璃之后,PCL/Gel-5 BG纤维膜在测试30 min之后失水率比PCL/Gel纤维膜的失水率更小。结果说明明胶以及生物玻璃的加入都有助于增加纤维膜的保湿效果。而所制备的Janus复合膜相较单层纤维膜保湿效果显著增加,在室温下放置1 h的失水率低于35%,这种持久的保湿效果满足了伤口愈合时的要求,能为伤口提供传统止血纱布敷料无法给予的愈合环境。
2.6 血液吸收能力分析
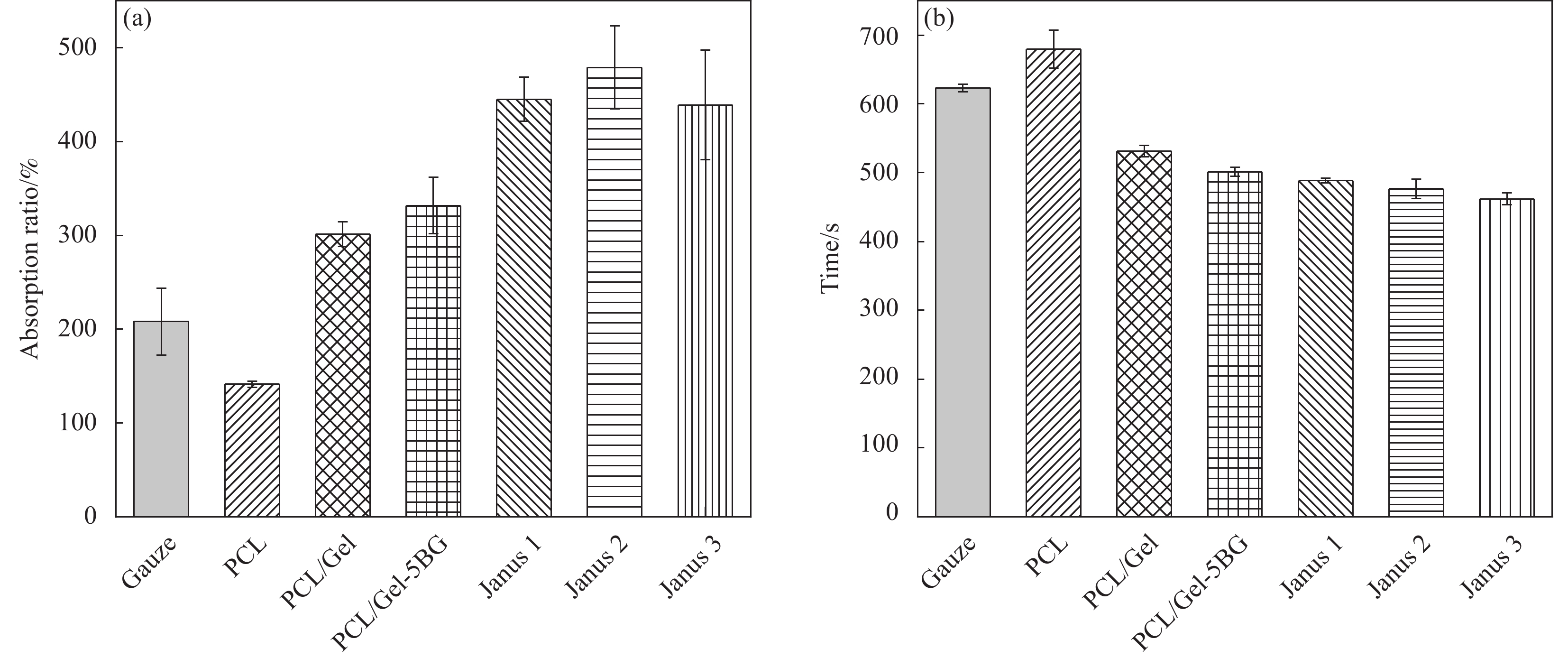
如图8(a)所示,医用止血纱布血液吸收率在208.1%左右,单层纤维膜PCL、PCL/Gel、PCL/Gel-5 BG血液吸收率分别约为141.3%,301.2%、331.7%,而所制备的Janus1、Janus2、Janus3复合膜的血液吸收能力均为自身重量的5倍以上,分别达到445.2%、479.1%、439.2%。结果表明,所制备的复合膜具有比单层纤维膜以及常用的止血纱布更优异的血液吸收能力,高血液吸收率不仅能提高材料应用于伤口时的利用率,还能减少敷料更换频率从而减小因敷料更换带来的二次机械伤害。
2.7 体外凝血时间分析
通过测定凝血时间来评估材料体外止血能力。图8(b)凝血时间结果可以看出,医用纱布凝血时间为(623 ± 5) s,PCL纤维膜凝血时间为(679.5 ± 27) s,PCL/Gel、PCL/Gel-5 BG纤维膜凝血时间分别为(531.3 ± 8) s、(501.4 ± 6) s,可知加入明胶和生物玻璃之后凝血时间相对医用纱布减少了100 s左右,这可能是因为明胶具有良好的黏附能力,使得血液中的血小板被大量聚集从而加速了血液的凝固,此外,生物玻璃能释放钙离子激活机体凝血因子,促进止血级联反应从而缩短凝血时间。Janus1、Janus2、Janus3复合膜的凝血时间分别为(488 ± 3) s、(476 ± 14) s、(462 ± 8) s,三种Janus复合膜的凝血时间相差不多,但远短于医用纱布且低于单层纤维膜的止血时间,可能是因为复合膜具有较高的溶胀率以及不对称润湿性能快速且高效的吸收血液中的水分以增加血液的黏度从而加速血液的凝固。
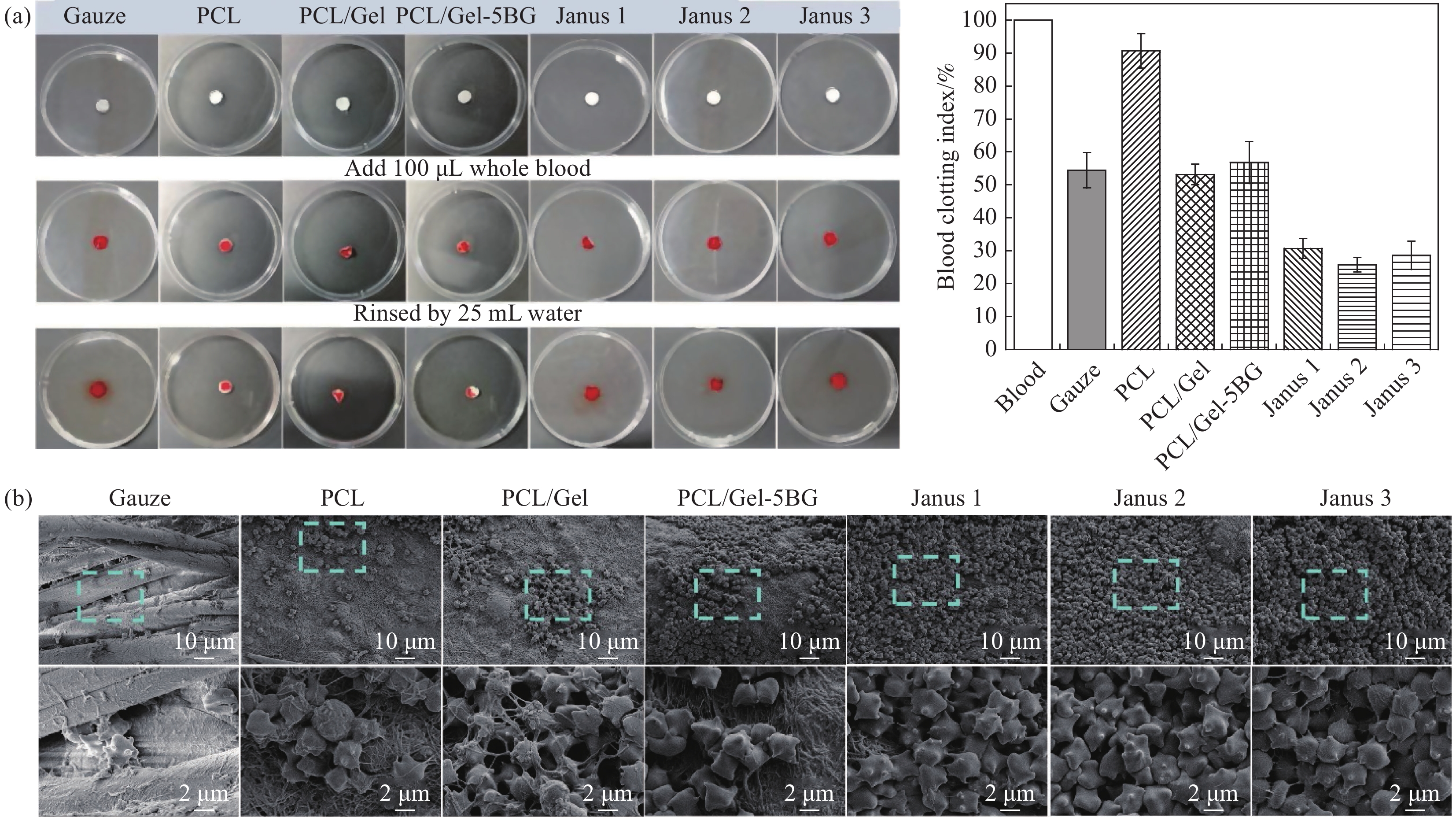
2.8 体外凝血指数分析
如图9(a)所示,在纱布对照组中,存在未凝固的血液,导致对照组的水变红了,其凝血指数约为54%,这意味着血液不易在医用纱布上凝固;PCL、PCL/Gel、PCL/Gel-5 BG纤维膜凝血指数分别约为90.7%、53.12%、56.78%,可知纯PCL纤维膜几乎没有凝血功能,加入明胶和生物玻璃之后提高了凝血效果;相比之下,Janus1复合膜组被水冲洗之后溶液颜色略带红色凝血指数为30.68%,Janus2、Janus3复合膜组的血液几乎完全凝固,所以被水冲洗之后的颜色几乎无色,凝血指数分别为25.73%、28.6%。结果表明Janus复合膜的体外凝血指数均低于纱布。材料的凝血能力越高,未凝结的血红蛋白越少,水的颜色就越浅,凝血指数越低。综上说明了所制备的Janus复合膜凝血效果明显高于纱布,Janus2复合膜凝血能力最高。这可能是由于Janus复合膜不对称润湿性和独特的动态润湿行为可以快速吸收血液中水分从而使大量的红细胞和血小板有效地积聚在复合膜表面加速凝血。此外,掺入的生物玻璃释放的生物活性离子Ca2+和SiO44-可以通过激活伤口愈合过程中的旁分泌作用,促进血管形成和细胞外基质蛋白沉积从而加快血液的凝结。
为进一步揭示其止血机制,采用扫描电镜对血细胞在细胞膜上的表面粘附和形态学进行了研究。图9(b)显示大量红细胞聚集在Janus复合膜表面并嵌入膜内,几乎观察不到纳米纤维,这一现象表明,当全血与细胞膜接触时,纳米纤维膜可以很容易地将血液成分捕获并聚集到纳米纤维网络中,形成图9(a)中不能被水溶液冲走的血蛋白凝块,凝块不仅聚集和浓缩血液成分(如血小板和红细胞)能进一步增强凝血反应,还可以作为机械堵塞以阻止伤口内的额外出血。
3. 结 论
本文通过静电纺丝制备出具有不对称润湿性的三明治结构Janus复合膜,分析了纤维膜形貌、结构等理化性能以及止血性能得出以下结论:
(1)所制备Janus复合膜的每层纤维表面光滑,无串珠结构,生物玻璃的掺入影响了纤维直径,纤维直径随其掺入量增加而增大。截面形貌看出复合膜三层之间粘结良好无分层现象。
(2)所制备的Janus复合膜断裂强度均大于3.5 MPa,断裂伸长率均在10%以上,表现出比单层纤维膜更好的力学性能;孔隙率均在70%以上,符合伤口敷料60%—90%理想孔隙率范围要求且Janus2孔隙率最高约为83.8%;亲疏水性和渗透性说明Janus复合膜能快速吸收液体并且疏水面可以抗血液外渗,Janus1、Janus2、Janus3复合膜溶胀率分别高达到885.4%、990%、910.8%,其中Janus2吸收伤口渗出液的能力最佳。
(3) Janus复合膜在止血性能测试中表现出比传统止血纱布更佳的止血效果。具体表现为在血液吸收率测试中Janus1、Janus2、Janus3复合膜高达445.2%、479.1%、439.2%远高于纱布(208.1%),体外凝血指数分别为30.7%、25.7%、28.6%低于纱布(54.4%),体外凝血时间分别约为488 s、476 s、462 s相对纱布623 s减少近2.5 min。
综上,本文制备的Janus复合膜在止血方面表现出比纱布更优异的性能。综合一系列性能评估Janus2复合膜表现更优。本文所制备的Janus复合膜作为创面敷料使用具有广阔的应用前景,有望作为新型敷料应用于伤口愈合领域。
-
图 6 不同纳米纤维膜润湿性(a); Janus2复合膜的血液渗透性(b); Janus复合膜疏水面(c)和亲水面(d)水接触角以及渗透性
Figure 6. Wettability of different nanofiber membranes (a); Blood permeability of Janus2 composite membrane (b); Hydrophobic (c) and hydrophilic (d) water contact Angle and permeability behavior of different Janus composite membranes
表 1 不同Janus复合膜的组成
Table 1 Composition of different Janus composite membranes
Sample Outer layer Inter layer Inner layer Janus1 PCL PCL/Gel-1G PCL/Gel Janus2 PCL PCL/Gel-5BG PCL/Gel Janus3 PCL PCL/Gel-10BG PCL/Gel Notes: PCL—Polycaprolactone; Gel—Gelatin 表 2 不同纳米纤维膜的静电纺丝条件
Table 2 Electrostatic spinning conditions for different nanofibre membranes
Solution Needle-Collector
Distance/cmVoltage/
kVFlow Rate/
(mL·h−1)PCL 12 20 3.5 PCL/Gel 15 15 0.4 PCL/Gel-1BG 15 15 0.4 PCL/Gel-5BG 15 15 0.4 PCL/Gel-10BG 15 15 0.4 -
[1] CHAMBERS E S, VUKMANOVIC-STEJIC M. Skin barrier immunity and ageing[J]. Immunology, 2020, 160(2): 116-125. DOI: 10.1111/imm.13152
[2] 张天蔚, 刘方, 田卫群. 促皮肤创面愈合新型敷料研究现状与进展[J]. 生物医学工程学杂志, 2019, 36(6): 1055-1059+1068. DOI: 10.7507/1001-5515.201811023 ZHANG T W, LIU F, TIAN W Q. Research Status and Progress on New Dressings for Promoting Skin Wound Healing[J]. Journal of Biomedical Engineering, 2019, 36(6): 1055-1059+1068(in Chinese). DOI: 10.7507/1001-5515.201811023
[3] KIM H J, PARK J, KIM S K, et al. Autophagy: Guardian of Skin Barrier[J]. Biomedicines, 2022, 10(8): 1817. DOI: 10.3390/biomedicines10081817
[4] CIOCE A, CAVANI A, CATTANI C, et al. Role of the Skin Immune System in Wound Healing[J]. Cells, 2024, 13(7): 624. DOI: 10.3390/cells13070624
[5] PATRICK A L, CHEN-CHARPENTIER B. Building erudition in the wound healing process: an inflammation model analysis[J]. International Journal of Computer Mathematics, 2024: 1–20.
[6] LAN J Z, SHI L X, XIAO W Y, et al. An enhanced fractal self-pumping dressing with continuous drainage for accelerated burn wound healing[J]. Frontiers in Bioengineering and Biotechnology, 2023, (11): 1188782.
[7] SAITO1 C T M H, BERNABÉ2 P F E, OKAMOTO3 T, et al. Evaluation of tissue response to periodontal dressings: histological study in tooth sockets of rats[J]. Journal of Applied Oral Science, 2008, 16(3): 219-25. DOI: 10.1590/S1678-77572008000300011
[8] NYGREN E, GEFEN A. Little news is good news? What is missing in the recently published EN 13726: 2023 test standard for wound dressings[J]. International Wound Journal, 2024, 3(21): e14787.
[9] BOATENG J, CATANZANO O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review[J]. Journal of Pharmaceutical Sciences, 2015, 104(11): 3653-3680. DOI: 10.1002/jps.24610
[10] SHEOKAND B, VATS M, KUMAR A, et al. Natural polymers used in the dressing materials for wound healing: Past, present and future[J]. Journal of Applied Polymer Science, 2023, 61(14): 1389-1414. DOI: 10.1002/pol.20220734
[11] Chen Fan, Qing Xu, Ruiqi Hao, et. al. Multifunctional wound dressings based on silicate bioactive materials[J]. Biomaterials, 2022, 287.
[12] BOATENG J, CATANZANO O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review[J]. Journal of Pharmaceutical Sciences, 2015, 104(11): 3653-3680. DOI: 10.1002/jps.24610
[13] LI X N, WANG X N, ZHENG L X, et al. Smart Janus textiles for biofluid management in wearable applications[J]. IScience, 2024, 27(3): 2589-0042.
[14] HOU L, LIU J, LI D, et al. Electrospinning Janus Nanofibrous Membrane for Unidirectional Liquid Penetration and Its Applications[J]. Chemical Research in Chinese Universities, 2021, 37: 337-354. DOI: 10.1007/s40242-021-0010-4
[15] 刘思彤, 金丹, 孙东明, 等. 静电纺丝制备Janus微纳米纤维研究进展[J]. 复合材料学报, 2024, 41(5): 2321-2332. LIU Sitong, JIN Dan, SUN Dongming, et al. Advances in the preparation of Janus micro and nanofibres by electrostatic spinning[J]. Acta Materiae Compositae Sinica, 2024, 41(5): 2321-2332(in Chinese).
[16] ZHANG Z, LI W, LIU Y, et al. Design of a biofluid-absorbing bioactive sandwich-structured Zn–Si bioceramic composite wound dressing for hair follicle regeneration and skin burn wound healing[J]. Bioactive materials, 2021, 6(7): 1910-1920. DOI: 10.1016/j.bioactmat.2020.12.006
[17] ZHANG M, CHU L, CHEN J, et al. Asymmetric wettability fibrous membranes: Preparation and biologic applications[J]. Composites Part B: Engineering, 2024, 269: 1359-8368.
[18] Woodruff M A, Hutmacher D H. The return of a forgotten polymer—Polycaprolactone in the 21st century[J]. Progress in Polymer Science, 2010, 35(10): 1217-1256. DOI: 10.1016/j.progpolymsci.2010.04.002
[19] Nair L S, Laurencin C T. Biodegradable polymers as biomaterials[J]. Progress in Polymer Science, 2007, 32(8-9): 762-798. DOI: 10.1016/j.progpolymsci.2007.05.017
[20] SASMAL P K, GANGULY S. Polymer in hemostasis and follow-up wound healing[J]. Applied Polymer, 2023, 140(9).
[21] DEMIR A U, AHI Z B, SANCAKLI A, et al. A comparative study of gelatin-based nanofiber and sponge wound dressings[J]. Journal of Bioactive and Compatible Polymers, 2024, 39(3): 145-161 DOI: 10.1177/08839115241241297
[22] ZAHID S, SHAH A T, JAMAL A, et al. Biologcal behavior of bioactive glasses and their compsites[J]. RSC Advances, 2016, 6(74): 70197-70214. DOI: 10.1039/C6RA07819B
[23] OSTOMEL T A, SHI Q, STUCKY G D. Oxide Hemostatic Activity[J]. Journal of the Am-erican Chemical Society, 2006, 128(26): 8384-8385. DOI: 10.1021/ja061717a
[24] BOKOV D, JALIL A T, CHUPRADIT S, et al. Nanomaterial by Sol-Gel Method: Synthesis and Application[J]. Advances in Materials Science and Engineering, 2021.
[25] CORREIA T R, ANTUNES B P, CASTILHO P H, et al. A bi-layer electrospun nanofiber membrane for plasmid DNA recovery from fermentation broths[J]. Separation and Purification Technology, 2013, 112: 20-25. DOI: 10.1016/j.seppur.2013.03.049
[26] LIU J, XIE X, WANG T, et al. Promotion of Wound Healing Using Nanoporous Silk Fibroin Sponges[J]. ACS Applied Materials & Interfaces, 2023, 15(10): 12696-12707.
-
其他相关附件
-
本文图文摘要
点击下载
-
-
目的
伤口愈合时敷料不仅需要提供止血作用,还需吸收过多的渗出物为伤口提供一个相对湿润又不过于干燥的环境。而传统纱布敷料的功能较为单一,无法为伤口提供良好的愈合环境。所以需要开发具有止血、能屏蔽外界有害微生物、能吸收大量渗出液等多个协同功能的敷料。
方法复合膜的制备主要由三个步骤组成:(1)采用微乳液-溶胶凝胶法合成生物玻璃纳米颗粒BG;(2)利用甲酸(FA)与乙酸(AA)混合溶液FA:AA (70:30 v/v)作为溶剂制备纯聚己内酯(PCL)、聚己内酯/明胶混合溶液(PCL/Gel)以及掺入不同生物玻璃的混合溶液(PCL/Gel-BG(1%、5%、10%))五种静电纺丝液;(3)将纺丝液通过静电纺丝技术以及逐层叠加法得到三明治结构的Janus复合膜,其中外层为PCL纳米纤维膜,中间层为负载BG的纳米纤维膜(PCL/Gel-BG),内层为PCL/Gel纳米纤维膜。通过SEM、FTIR、EDS等生物玻璃及复合膜形貌和结构进行了表征,测试了Janus复合膜力学性能、孔隙率、溶胀率以及渗透性等,通过对比传统止血纱布的血液吸收率、止血时间、凝血指数等测试探究了其止血性能。
结果从SEM可见BG粒径大小均匀呈光滑的球形结构,平均粒径为(227.1 ± 19.1) nm;Janus复合膜的每层纤维表面光滑,无串珠结构,生物玻璃的掺入影响了纤维直径,且纤维直径随其掺入量增加而增大。Janus复合膜截面的SEM图可以看出复合膜三层之间粘结良好无分层现象。力学性能测试结果表明:复合膜断裂强度均大于3.5 MPa,在人体正常皮肤的拉伸强度(约2.5—16 MPa)范围内,断裂伸长率均在10%以上,表现出比单层纤维膜更好的力学性能;孔隙率均在70%以上,符合伤口敷料(60%—90%)理想孔隙率范围要求;润湿性和渗透性证明了Janus复合膜具有不对称润湿性,使其亲水面能快速吸收液体且疏水面可以抗血液外渗;其中中间层相对含量为5%BG的Janus2复合膜吸收渗出液的能力最佳,其溶胀率可高达990%。此外,Janus复合膜在止血性能测试中表现出比传统止血纱布更佳的止血效果。具体表现为:在血液吸收率测试中Janus1、Janus2、Janus3复合膜高达445.2%、479.1%、439.2%远高于纱布(208.1%),体外凝血指数分别为30.7%、25.7%、28.6%低于纱布(54.4%),体外凝血时间分别约为488 s、476 s、462 s相对纱布623s减少近2.5 min。综上,本文制备的Janus复合膜在止血方面表现出比纱布更优异的性能。
结论本研究成功利用静电纺丝技术制备了一种具有不对称润湿性的三明治结构敷料。将生物玻璃纳米颗粒负载于敷料的中间层纤维膜,不但提高了敷料的溶液吸收,还增强了其凝血效果。此外,该敷料不仅具有与细胞质基质相似的结构,还具有较高的孔隙率,同时还兼具较好的溶液吸收、保湿能力,能为伤口提供相对湿润又不过与干燥的愈合环境加速伤口愈合。有望作为新型创面敷料应用于伤口愈合领域。
-
随着科技的发展,创面敷料的种类越来越多,尤其是多功能的敷料研究受越来越多研究者的关注。理想的创面敷料不仅需要促进伤口愈合,还应具有吸收伤口渗出液、减少细菌的病毒感染等功能,同时还能为伤口提供相对湿润而不过与干燥的环境。然而,目前应用于临床医学的敷料功能都比较单一。
本文通过利用静电纺丝技术,以PCL纤维膜为疏水外层,PCL/Gel混合静电纺纤维膜作为亲水内层,在PCL/Gel溶液中掺入不同含量的生物玻璃所得到的PCL/Gel-BG纤维膜作为中间层,制备具有高效液体吸收、止血协同作用的Janus复合膜。所制备的复合膜具有不对称润湿性,不仅使其溶胀率可高达990%,能吸收大量渗出液,同时还能提供更利于伤口愈合的相对湿润而不脱水的微环境。此外,掺入中间层自制的高硅钙比的硅基无机生物玻璃,不仅能提高吸收溶液能力,还增大了复合膜孔隙率,同时还释放凝血因子加速止血。在血液吸收率测试中Janus1、Janus2、Janus3复合膜高达445.2%、479.1%、439.2%远高于纱布(208.1%),体外凝血指数分别为30.7%、25.7%、28.6%低于纱布(54.4%),体外凝血时间分别约为488 s、476 s、462 s相对纱布623s减少近2.5 min。
单层纤维膜及Janus复合膜的润湿性(a);溶胀率(b); 血液渗透性(c); 凝血时间(d)





 下载:
下载: