Research progress of graphene oxide composite coatings in metal corrosion protection
-
摘要:
氧化石墨烯(GO)作为石墨烯的衍生物具有优异的综合性能,在金属的防腐蚀领域中表现出了巨大的应用潜力。GO不仅具有石墨烯的二维层状结构,还含有羟基、羰基、羧基和环氧基团等官能团可作为活性位点与其他物质进行共价/非共价性功能化改性,因此GO常被用作填料来增强涂层的综合性能。本文以GO复合涂层为中心,简要地介绍了其理化性质,以当前世界金属腐蚀的情况和腐蚀类型为切入点,针对一些常用的腐蚀防护方法进行了讨论。综述了近年来国内外关于GO与有机物和无机物的复合涂层在金属腐蚀与防护领域的研究进展并对复合涂层的防腐机制进行了简述;最后,总结了目前研究工作中存在的关键科学难题与挑战,对涂层的研究方向与应用前景进行了展望。
Abstract:As a derivative of graphene, graphene oxide (GO) has excellent comprehensive performance, showing great application potential in metal corrosion and protection. GO has not only a two-dimensional layered structure but also contains hydroxyl, carbonyl, carboxyl, epoxy groups and other functional groups that can be used as active sites with other substances for covalent/non-covalent functionalization modification, so GO is often used as a filler to enhance the comprehensive performance of coatings. This paper reviews the recent research progress on GO composite coating in metal corrosion and protection. The first part summarizes the morphology of metal corrosion and the corrosion protection methods, explains the primary forms of metal corrosion, and discusses the current main metal corrosion protection methods. The second part introduces the physicochemical properties of graphene and its derivatives. In this paper, GO is the main focus. It has tremendous application potential in metal corrosion protective coatings due to its sheet structure, excellent dispersion, and abundant oxygen-containing functional groups on the surface, which are the active point of the reaction and easy modification. The third part introduces the current GO composite coating, which compares the corrosion resistance of traditional coatings, GO organic/inorganic unit composite coatings and GO multi-composite coatings. It is found that the performance of GO multi-component composite coating is far better than that of GO organic/inorganic unit composite coating and traditional coating. This is due to the excellent dispersion of GO, which enables it to fill the pores that occur during the curing and film-forming process of organic polymer materials and improve the corrosion resistance of the coating. The photogenic cathodic protection mechanism and current situation of GO anti-corrosion coating are introduced, and due to the green environmental protection and excellent anti-corrosion effect, it is regarded as the future development trend of GO composite coating. The fourth part summarizes the key scientific issues and challenges of GO composite anti-corrosion coating in the current research. It looks forward to the research direction and application prospect of GO composite anti-corrosion coating.
-
火箭推进器的轻量化设计不仅能提高运载能力和降低制造成本,而且有效地促进了火箭推进器的循环利用。碳纤维增强树脂基复合材料(CFRP)作为一种轻质高强度材料,已被用作传统金属替代材料,实现轻量化设计[1-4]。胶接技术能有效地连接金属和CFRP复合材料,避免了CFRP复合材料的加工损伤、局部应力集中及金属-CFRP粘结界面处潜在的电化学腐蚀,该技术也被应用于制造高性能结构件的工业生产[5-7]。
火箭推进剂贮箱[8-9]常用于盛装液态燃料,如液氧。箱内温度低至−183℃, 潜在的热冷交变应力会导致应用树脂粘结的非相似基体界面失效,主要是由于胶粘剂脆性随温度的下降而继续增大[10-11],基体材料与胶粘剂之间的粘结界面较弱。通常,室温下的力学性能强化仍是非相似基体粘结性能的主要研究方向,除了航空航天工业外,关于结构强度在实际应用温度下的演变规律的研究较少。目前针对粘结界面问题,普通的金属与CFRP板的表面改性,如砂磨[12-14]、喷砂[15-17]和NaOH刻蚀[5,18-19]等,可提高环氧树脂的润湿性,增强粘结界面[20]的扩散和相容性,从而加强机械咬合。基于渗透和机械咬合理论,以刻蚀或喷射处理在基体表面形成垂直通道,能有效促进粘结[21-23]。
在机械和化学处理工艺中,阳极氧化[24-26]已被证实是一种更有效的处理方法,通过电解液、电解电压与电流、电解槽温度等[27] 多因素的联动调控,能刻蚀形成利于粘结的多孔表面。同时,将高性能界面增强粒子引入多孔氧化膜中,构建准Z方向纤维集团,可增韧环氧树脂胶体,也能强化树脂与基体的粘结界面[4,15,28]。低温环境下,纯环氧树脂的硬度和强度会增加,但其脆性也会增加[10],而添加增强粒子可以改善脆性[29-31]。此外,粘结界面也存在树脂脆性问题,且不同基体的不相容性也影响粘结界面的稳定性。基于此,准Z方向的纤维分布模型可以消除胶体和基体的潜在的弱界面结构,构建一种结构更稳定、性能更强且断裂阻抗更优的复合结构体,为低温液体燃料贮箱的应用提供了更可靠的复合材料。
本文创新性地设计了一种有效的铝合金和CFRP板表面改性处理系统,采用树脂预涂层(RPC)技术进一步提高改性表面与固化环氧树脂的相容性,引导碳纳米管嵌入或竖直分布于多孔氧化膜内。采用砂纸打磨和RPC处理CFRP板表面,形成更牢固的CFRP板/环氧树脂粘结界面。通过三点弯曲(3-P-B)试验测试了弯曲强度,确定了不同表面处理方法的破坏模式演变规律;此外,表征了铝合金多孔氧化膜的微观形貌、内部微纳米孔道形貌、化学成分及阳极氧化前后的粗糙度等性能。
1. 材料设计、制备与表征
1.1 复合材料设计概念
润湿性、相容性和机械咬合性更佳的粘接界面,有助于形成更稳定的复合材料结构。为了提高界面粘接性能,因而需对粘接基体进行表面改性。采用阳极氧化处理铝合金表面,将相对平整且不易润湿的表面刻蚀形成孔道准Z方向分布的多孔膜,该多孔膜对环氧树脂有较好润湿性,也为RPC技术负载碳纳米管提供垂直空间,利于消除孔道根部的空穴缺陷。同样,采用RPC技术优化CFRP/环氧树脂胶接界面,能使其具备更高的机械咬合性能。图1显示了经过不同的处理方法的粘结工艺设计模型,对于各种复杂服役环境下Al-CFRP复合材料的结构强化发挥了关键作用。低温环境下,该胶接界面对提高机械咬合性能的作用尤为明显。由于冷缩作用,环氧树脂与基体(包括铝合金和CFRP板)间的粘结界面会产生不可逆的潜在裂纹并沿其扩展,准Z方向分布的纤维能减少或消除潜在的界面裂纹,从而改变复合材料在室温或低温下的结构失效模式,进一步提高粘结强度。
1.2 复合材料制备
通过预处理、阳极氧化处理或磨砂处理、RPC处理及粘结等工艺制备了最终的Al-CFRP复合材料。
首先,铝合金和CFRP板的预处理阶段。阳极氧化处理前,用丙酮超声清洗6060 T5铝合金(澳大利亚Midalia Steel Pty 公司,厚度3 mm,宽度25 mm)约30 min,去除污垢、灰尘和油等一些表面污渍。放入10wt%的NaOH溶液中浸泡10 min,去除难溶性物质[26],然后用去离子水洗掉表面的NaOH。然后放入10wt%的HNO3溶液中浸泡5 min,反应残余的NaOH,防止碱性粒子带入电解槽,再用去离子水清除残留的HNO3溶液获得电解用铝合金。用P240氧化铝砂纸打磨3K斜纹编织CFRP板(深圳市领航者新材料科技有限公司,厚度2 mm,宽度25 mm),再用丙酮超声清洗已磨的CFRP板10 min,去除表面污渍获得粘结用CFRP板。
其次,铝合金阳极氧化处理阶段。称取定量的硫酸(澳大利亚Chem supply集团有限公司;H2SO4,AR,98%,密度1.84 g/cm3)和草酸(澳大利亚Merck 集团有限公司;C2H2O4·2H2O,M=126.07 g/mol),制备质量分别为20wt%和0.5wt%的最终电解质溶液。在阳极氧化槽中,铝合金为阳极,碳棒为阴极。电解液温度为室温,电解电压为12 V,阳极氧化过程为1 h[5]。
然后,铝合金和CFRP板的RPC工艺处理阶段。配置铝合金碳纳米管RPC处理液,其中树脂∶碳纳米管∶丙酮=10wt%∶1wt%∶89wt%,并对阳极氧化后的铝合金进行处理。配置CFRP板RPC处理液,其中树脂∶丙酮=10wt%∶90wt%,并对磨砂后的CFRP板进行处理。
最后,铝合金和CFRP板的粘结阶段。采用1∶1的质量比均匀混合普通树脂(澳大利亚Dulux 集团有限公司,双酚A氯丙烷环氧树脂)和固化剂(澳大利亚Dulux 集团有限公司,三乙烯四胺固化剂)制得粘结胶体,采用不同表面条件组合的铝合金和CFRP板制备了四组Al-CFRP复合材料:(1) 铝合金和CFRP板均仅采用丙酮超声清洗;(2) 铝合金阳极氧化,CFRP板丙酮超声清洗;(3) 铝合金阳极氧化,CFRP板磨砂处理和RPC处理;(4) 铝合金阳极氧化和RPC (添加碳纳米管)处理,CFRP板磨砂处理和RPC处理。
1.3 化学刻蚀原理
铝合金表面的电化学腐蚀实际上是多孔膜的生成和分解反应,取决于在电化学环境中进行的一系列阳极氧化反应[32]。图2显示了阳极区发生的多孔膜的产生和分解,铝合金失去电子变成Al3+离子,与O2−反应从而生成稳定的Al2O3薄膜。整个薄膜生长过程中,薄膜生长速度并不完全相同,因此,生成的薄膜在微观上是不均匀的。处于电解槽的酸性环境中,生成的薄膜也会发生分解反应,Al2O3与活性H+反应再次生成Al3+,并分解回溶液。该分解反应主要集中在较薄的多孔膜区域,而非整个Al2O3多孔膜。薄膜区由于电阻小、电流大,在电场作用下更易被穿透。铝合金表面Al2O3薄膜的形成和分解过程是同时发生的,并形成了硬质的Al2O3多孔薄膜层和垂直通道区。最后,达到Al2O3多孔薄膜生成与分解的平衡条件,整个薄膜结构趋于稳定。
1.4 复合材料性能表征
室温下,采用Instron 5982万能试验机(100 kN)对复合材料的进行三点弯曲试验。液氮温度下,复合材料的弯曲试验通过Instron 5982万能试验机和液氮储存装置进行,复合材料浸泡于液氮约10 min,降温到−196℃。图3展示试样的特征参数,测试样品的尺寸为110 mm×25 mm×6 mm,被放置在上压头两侧等距约48 mm处,分布于两个下支点中间。位移控制速度为2 mm/min,负载力骤降后停止试验,加载过程中未观察到滑动和整体屈曲。每组共5个样品用于三点弯曲试验。
采用扫描电子显微镜(FEI Verios XHR SEM,Thermo Fisher Scientific Inc.,USA)和Everhart-Thornley探测器在10 kV/0.4 nA下观察了阳极氧化前后铝合金的表面形貌。通过聚焦离子束(Focus ion beam,FIB)对阳极氧化后的铝合金切槽,采用Ga离子束完成深度为30 μm的孔道切割和抛光,并用扫描电子显微镜(FEI-Helios 650,Thermo Fisher Scientific Inc.,USA)在5.0 kV的高压和0.34 nA的电流下观察了内孔结构。
利用原子力显微镜(AFM,Bruker,Germany)测量三维表面粗糙度。使用纳米镜分析对数据进行分析,以获得区域粗糙度参数和三维表面形貌。
使用AlKα (hv=1846.6 eV)正常起飞角的单色辐射源的ESCALAB 250Xi(Thermo Scientific K-Alpha,Thermo Fisher Scientific Inc.,USA)获得X射线光电子能谱(XPS)光谱。记录了Al2p和C1s峰的高分辨光谱,光斑尺寸为400 μm,使用通过能量50 eV和步长0.1 eV。在曲线拟合之前,结合能标度相对于结合能为284.8 eV的不定烃进行了校准。利用XPS PEAK软件对Al2p的高分辨率扫描进行分析。
2. 结果与讨论
2.1 铝合金表面微观形貌
图4为经过丙酮清洗和阳极氧化处理后铝合金表面的SEM图像。如图4(a)所示,能观察到挤压成型过程中产生的平行沟壑结构及在搬运、储存和运输过程中产生的一些细小划痕。图4(b)~4(d)显示了铝合金独特的微孔结构,主要是由于硫酸/草酸混合物在不同区域进行不同程度的刻蚀和氧化行为,这也造成阳极氧化膜的不同生成和溶解速率。通过图4(b)的90°俯视角可以看到峰谷状结构,根据图4(c)的52°俯视角图像显示,该峰谷状结构更加立体清晰。图4(d)为FIB处理后多孔铝合金表面切口壁的正面图。据测算,该孔径宽度约为200~800 nm,孔径深度约为3~5 nm。图4(e)和图4(f)呈现了经过RPC处理后铝合金表面形貌。观察可知,碳纳米管已嵌入多孔氧化层的孔道结构。随着RPC溶液中丙酮的挥发,碳纳米管被残留的树脂溶液拽入到孔道结构,形成半嵌入式结构,该结构能促进准Z方向的纤维桥联行为的形成[5,32]。
![]() 图 4 6060 T5铝合金的SEM图像:(a) 铝合金表面丙酮超声清洗;(b) 阳极氧化后的铝合金表面具有较深的微/纳米结构通道;(c) 阳极氧化后的铝合金52°视角图像;(d) 聚焦离子束(FIB)处理后多孔铝合金表面切口壁的正面图;(e)、(f) RPC处理后CNTs嵌入到铝合金表面孔道结构[32]Figure 4. SEM images of 6060 T5 Al substrate: (a) Al substrate surface with acetone ultrasonic cleaning; (b) Al substrate surface with deep micro-/nano-structured channels after anodizing treatment; (c) 52° view image of the Al substrate after anodized corresponding to (b); (d) Frontal view of the notched wall on porous Al substrate surface after focus ion beam (FIB); (e),(f) CNTs inserted into the channel of Al substrate surface after RPC technique[32]
图 4 6060 T5铝合金的SEM图像:(a) 铝合金表面丙酮超声清洗;(b) 阳极氧化后的铝合金表面具有较深的微/纳米结构通道;(c) 阳极氧化后的铝合金52°视角图像;(d) 聚焦离子束(FIB)处理后多孔铝合金表面切口壁的正面图;(e)、(f) RPC处理后CNTs嵌入到铝合金表面孔道结构[32]Figure 4. SEM images of 6060 T5 Al substrate: (a) Al substrate surface with acetone ultrasonic cleaning; (b) Al substrate surface with deep micro-/nano-structured channels after anodizing treatment; (c) 52° view image of the Al substrate after anodized corresponding to (b); (d) Frontal view of the notched wall on porous Al substrate surface after focus ion beam (FIB); (e),(f) CNTs inserted into the channel of Al substrate surface after RPC technique[32]图5是阳极氧化处理前后铝合金表面的AFM图像。图5(a)和图5(b)显示了仅用丙酮清洗的铝合金试样在两个不同的典型区域的三维高度图案轮廓,除了挤压过程导致的不均匀表面形态外,几乎没有观察到多孔结构,与图4(a)所示的形貌吻合。图5(c)和图5(d)显示了经过阳极氧化的铝合金的表面多孔形貌,在两个区域均能观察到明显的峰谷状结构。根据表1中铝合金阳极氧化前后的表面粗糙度值,经阳极氧化后表面粗糙度主要特征值表面算术平均粗糙度Sa和表面均方根粗糙度Sq都明显增大。因此,阳极氧化处理可以通过改变表面形貌来优化表面性能。
表 1 经丙酮超声清洗和阳极氧化处理后6060 T5铝合金的表面粗糙度参数Table 1. Surface roughness measurements of the 6060 T5 Al substrate treated by acetone ultrasonic cleaning and anodizingTreatment method Sample number Sa/nm Sq/nm Rmax/nm Acetone ultrasonic cleaning Fig.5(a) 53.4 62.5 353 Fig.5(b) 36.5 42.9 238 Anodizing surface treatment Fig.5(c) 91.3 114 785 Fig.5(d) 95.4 134 1037 Notes: Sa—Surface arithmetical mean roughness; Sq—Surface root mean square roughness; Rmax—Maximum roughness depth. 2.2 铝合金表面元素组成
图6为经丙酮超声清洗和硫酸-草酸溶液阳极氧化的铝合金XPS图谱。仅丙酮清洗后的铝合金XPS图谱表明,6060 T5铝合金的基本成分为Al、O、Mg、Si和Cu[6]。然而,经阳极氧化的铝合金曲线上仅剩下Al和O,原本存在的Mg、Si、Cu消失了,这是由于阳极氧化反应生成了一种新的多孔超薄氧化膜。同时在该曲线上,某些不定烃类污染物的表面出现一个C1s峰,其强度曲线明显降低。
![]() 图 6 丙酮超声清洗和阳极氧化的铝合金表面的光谱分析 (a) ;丙酮超声清洗 (b) 和阳极氧化处理后铝合金表面 (c) 的高分辨Al2p核心级光谱Figure 6. Survey spectra of Al substrate surfaces cleaned ultrasonically by acetone and anodized (a); high resolution Al2p core-level spectra for Al substrate surfaces after acetone ultrasonic cleaning (b) andanodizing treatment (c)Aloh—Aluminium hydroxide hydroxide; Almet—Metallic aluminium; Alox—Aluminium oxide
图 6 丙酮超声清洗和阳极氧化的铝合金表面的光谱分析 (a) ;丙酮超声清洗 (b) 和阳极氧化处理后铝合金表面 (c) 的高分辨Al2p核心级光谱Figure 6. Survey spectra of Al substrate surfaces cleaned ultrasonically by acetone and anodized (a); high resolution Al2p core-level spectra for Al substrate surfaces after acetone ultrasonic cleaning (b) andanodizing treatment (c)Aloh—Aluminium hydroxide hydroxide; Almet—Metallic aluminium; Alox—Aluminium oxide通过分析两种衬底的高分辨Al2p光谱,进一步研究了阳极氧化前后铝合金表面的变化。如图7(a)所示,丙酮清洗的铝合金的Al2p光谱由3个不同的峰组成:(1) 72.8 eV[33]处的金属铝(Almet);(2) 74.4 eV[34]处的氢氧化铝和羟基氢氧化铝(Aloh);(3) 74.8 eV[35]处的氧化铝(Alox)。氢氧化铝的产生归因于大气中的水蒸气。这些颗粒结合不良,会削弱黏附力。由于氧化薄膜厚度小于XPS的取样深度,因此仍能检测到铝合金的信号。图7(b)显示了阳极氧化后的铝合金,由于经过NaOH溶液和HNO3溶液的预处理,因此表面层上没有Alox。而经过阳极氧化的铝合金表面,金属铝的峰消失了,表明该峰与基体表面的金属间化合物有关。根据表2中表面层的Almet、Alox和Aloh的原子百分比份额可以发现,取样深度内的金属铝含量下降到0%,并完全转变为Aloh。最终元素组分由Aloh组成,主要是由于刚经过阳极氧化处理的铝合金用去离子水清洗,以去除残留的硫酸-草酸溶液,导致形成的Alox再次与H2O反应生成最终的Aloh。
2.3 Al-CFRP复合材料弯曲强度
表3为4组不同处理方式下Al-CFRP复合材料的弯曲强度,随着铝合金阳极氧化、CFRP板表面磨砂及RPC技术等处理方式的叠加运用,复合材料的峰值载荷Pmax和弯曲强度均得到持续提升。图7(a)显示了仅丙酮清洗和铝合金阳极氧化+RPC(碳纳米管)与CFRP板磨砂+RPC两组试样的典型力-位移曲线,由于非相似粘结基体的接头变形,在峰值载荷前呈现出非线性行为[13]。液氮温度和室温下测得的两个峰值载荷Pmax明显处于不同的值域内,说明低温有助于提升粘结强度。对比同一温度的对照组试样和实验组试样,也能观察到峰值载荷Pmax的增强。根据图7(b)和表3的弯曲强度值,通过铝合金阳极氧化+RPC(碳纳米管)与CFRP板磨砂+RPC的联合处理,室温下强度比丙酮清洗提高14.6%,液氮温度下强度提高27.6%。液氮温度下增强27.6%,可能是由于准Z方向的碳纳米管,随着温度的降低,相较于纯环氧胶接接头,位于粘结界面的碳纳米管纤维桥联行为对于环氧接头的增强效果会更佳。
表 2 丙酮清洗和阳极氧化后铝合金上超薄表面层的表面元素组成Table 2. Surface elemental composition of the ultra-thin surface layer on both acetone-cleaned and anodized Al substratesSample Almet Alox Aloh Acetone-cleaned Al substrate 21.0% 62.7% 17.5% Anodized Al substrate − − 100.0% 表 3 室温(RT)和液氮温度(LNT)下对照组和实验组Al-CFRP复合材料的3-P-B试验结果Table 3. 3-P-B test results of control and treated Al-CFRP composites at room temperature (RT) and liquid nitrogen temperature (LNT)Sample Treatment process Test
conditionAverage
Pmax/NStandard
deviation/NShear
strength/MPaStandard
deviation/MPaControl Acetone ultrasonic cleaning for both RT 2564.21 136.87 269.97 48.14 LNT 2915.79 591.69 532.92 60.51 T1 Anodizing for Al and Acetone ultrasonic cleaning for CFRP RT 2690.45 142.53 285.76 23.42 LNT 5364.05 514.17 569.73 49.64 T2 Anodizing for Al and sanding +
RPC for CFRPRT 2760.61 121.31 290.15 17.75 LNT 5541.01 531.58 582.38 42.73 T3 Anodizing for Al and RPC (CNTs) sanding + RPC for CFRP RT 5015.57 109.55 309.31 13.05 LNT 6374.42 545.44 680.01 48.14 Note: Pmax—Maximum peak load. 2.4 Al-CFRP复合材料失效模式
图8为Al-CFRP复合材料经过弯曲测试后的失效表面,能更清晰地解释图7所示的弯曲强度变化。仅用丙酮清洗铝合金和CFRP板的Al-CFRP复合材料,无论在室温下还是在液氮温度下,铝合金与环氧胶体的胶接界面都发生了典型脱胶失效(图8(a))。当材料结构发生明显变形时,很容易导致粘结界面失效,但低温可以增强胶接接头,提高铝合金的刚度,延缓潜在的粘结失效,从而提高弯曲强度。整个负载过程中,上表面压力负载区域(即上部CFRP板)没有损坏,下部铝合金逐渐弯曲。然而,仅丙酮清洗的Al-CFRP复合材料的粘结破坏模式可以通过阳极氧化、磨砂和RPC技术等方法实现转变。如图8(b)所示,在室温和液氮温度下完成弯曲试验后,受损的Al-CFRP复合材料仍保持结构半粘合状态,铝合金和CFRP板结构未分离,即使从侧面观察也未观察到明显的粘结破坏。与仅丙酮清洗的复合材料相比,在加载过程中,除铝合金发生弯曲变形外,CFRP板也发生了弯曲断裂破坏,同时经联合处理的Al-CFRP复合材料的弯曲变形量比仅丙酮清洗的复合材料大,这也解释复合材料的弯曲强度增强。对于联合处理后的Al-CFRP复合材料,液氮温度下的抗弯强度高于室温下的抗弯强度的原因与仅丙酮清洗的复合材料相似,在液氮中,主要归因于CFRP板与铝合金更强的粘结界面和结构刚度。
![]() 图 8 Al-CFRP复合材料三点弯曲试验后的部分粘合及分离的失效表面:(a) 丙酮超声清洗后的铝合金和CFRP板;(b) 铝合金经过阳极氧化和RPC(添加碳纳米管),CFRP板经过砂纸打磨和RPCFigure 8. Failure surfaces of adhesive bonded Al-CFRP composite and the separated pieces: (a) Acetone ultrasonic cleaning for Al substrate and CFRP; (b) Anodized Al substrate with RPC + CNTs and sanding and RPC for CFRP
图 8 Al-CFRP复合材料三点弯曲试验后的部分粘合及分离的失效表面:(a) 丙酮超声清洗后的铝合金和CFRP板;(b) 铝合金经过阳极氧化和RPC(添加碳纳米管),CFRP板经过砂纸打磨和RPCFigure 8. Failure surfaces of adhesive bonded Al-CFRP composite and the separated pieces: (a) Acetone ultrasonic cleaning for Al substrate and CFRP; (b) Anodized Al substrate with RPC + CNTs and sanding and RPC for CFRP2.5 增强机制
裂纹总是产生于较弱的区域并沿粘结界面扩展,最终导致Al-CFRP复合材料的结构破坏[13]。如图9所示,阳极氧化处理前后不同的表面条件可以改变弯曲破坏模式。对于仅丙酮超声清洗的铝合金,完全脱胶失效发生在铝基体与环氧树脂的粘结界面(图9(a)),随着变形量的增加,裂纹沿铝合金/环氧树脂粘结界面扩展,这是由于铝合金与环氧树脂粘结界面较差的相容性,几乎没有机械咬合行为[5-6]。在液氮条件下,即使铝合金的刚度获得提高并减小弯曲变形量,但也不能改变这种现象。然而,良好的粘结界面有助于改变这种弱界面失效模式。垂直分布的多孔结构不仅可以优化与环氧树脂的相容性,增加接触面积,加强机械咬合,还能为碳纳米管的嵌入创造纵向孔道空间,形成准Z方向分布的纤维桥联[32]。它们的共同作用提升了胶接接头性能,促进了CFRP板的断裂失效代替胶接界面的部分脱胶失效。
![]() 9 Al-CFRP复合材料的粘接表面失效模式示意图:(a) 仅经过丙酮超声清洗的铝合金与CFRP板呈现出典型的脱胶失效;(b) 经过阳极氧化和RPC(添加碳纳米管)的铝合金与经过砂纸打磨和RPC的CFRP板呈现出碳纤维基体的断裂失效9. Schematic diagram of failure mode on Al substrate and CFRP adhesive surface: (a) Typical adhesive failure only with acetone ultrasonic cleaning for Al substrate and CFRP; (b) CFRP fracture failure with anodizing + RPC with carbon nanotubes for Al substrate and sanding + RPC for CFRPCFRP—Carbon fiber reinforced polymer; CNTs—Carbon nanotubes
9 Al-CFRP复合材料的粘接表面失效模式示意图:(a) 仅经过丙酮超声清洗的铝合金与CFRP板呈现出典型的脱胶失效;(b) 经过阳极氧化和RPC(添加碳纳米管)的铝合金与经过砂纸打磨和RPC的CFRP板呈现出碳纤维基体的断裂失效9. Schematic diagram of failure mode on Al substrate and CFRP adhesive surface: (a) Typical adhesive failure only with acetone ultrasonic cleaning for Al substrate and CFRP; (b) CFRP fracture failure with anodizing + RPC with carbon nanotubes for Al substrate and sanding + RPC for CFRPCFRP—Carbon fiber reinforced polymer; CNTs—Carbon nanotubes3. 结 论
本工作成功增强了铝合金-碳纤维增强树脂基复合材料(CFRP)复合材料在室温和液氮温度下的粘接性能。通过阳极氧化表面处理和树脂预涂层(RPC)技术有效的应用,在铝合金表面刻蚀形成一层具有垂直孔道的多孔氧化薄膜,为碳纳米管的嵌入提供了纵向空间,从而在环氧树脂/铝合金粘接界面上构筑准
Z方向的碳纳米管桥联。同时CFRP板表面磨砂与RPC处理提高了环氧树脂/CFRP界面的粘结性能。通过铝合金与CFRP板表面的联合处理,室温下复合材料的抗弯强度比仅丙酮清洗处理增强了14.6%,液氮温度下,增强效果进一步提升到27.6%。基于碳纳米管桥联对裂纹产生和扩展的抑制作用,Al-CFRP复合材料的失效模式由原本较弱的脱胶失效转变为CFRP板的断裂失效。因此,“铝合金阳极氧化+RPC(添加碳纳米管)与CFRP板磨砂+RPC”的联合处理是一种有效的Al-CFRP复合材料环氧胶接接头强化方法,为低温液体燃料箱的工业化生产提供了一项可参照的技术方案,促进了航空航天工业的发展。 -
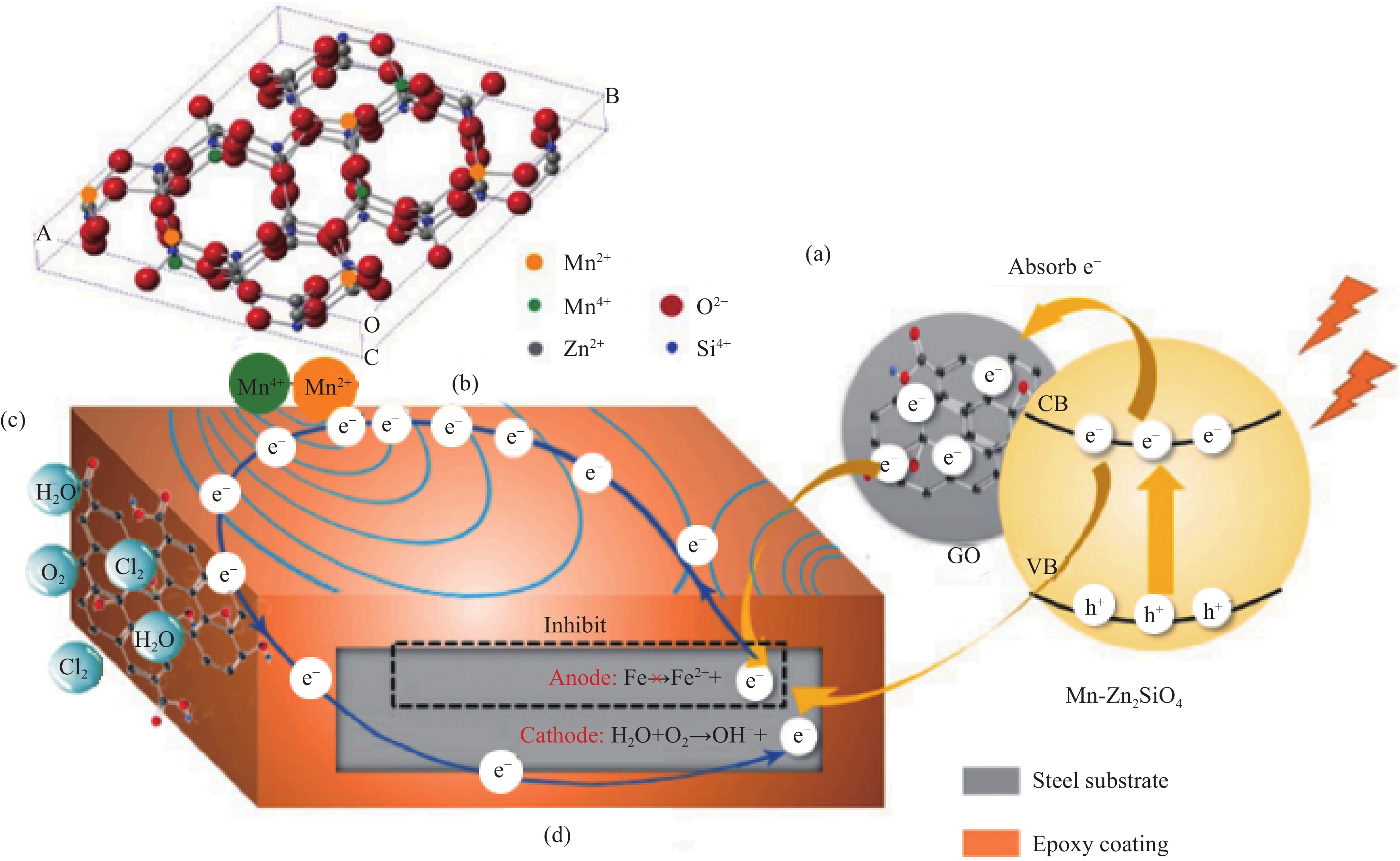
图 5 GO/Mn-Zn2SiO4材料的腐蚀防护机制图[43]:(a)光生阴极效应;(b)无机锰离子磁感应效应;(c)二维GO屏蔽效应;(d)阴极二次保护效应
Figure 5. Corrosion protection mechanism of GO/Mn-Zn2SiO4 material[43]: (a) Photogenerated cathode effect; (b) Inorganic manganese ion magnetic induction effect; (c) Two-dimensional GO shielding effect; (d) Cathode secondary protection effect
VB—Valence band; CB—Conduction band
表 1 GO作为涂层填料相对于其他常见无机填料的优缺点
Table 1 Advantages and disadvantages of GO as a coating filler over other common inorganic fillers
Type Character Advantages Disadvantages GO 1. High specific surface area, good mechanical properties, excellent barrier and shielding properties;
2. Excellent response reactivity, thermal and chemical stabilities;
3. Oxygen-containing functional group can serve as active sites for reactions; Hydrophilic groups on the surface are more easily modified by polymers or alkali metal oxides;
4. "Maze effect" can increase the diffusion path of the corrosion factor in the coating, and has a high resistance to permeability.1. Easy to agglomerate, dispersibility and stability are reduced after agglomeration;
2. Electrical conductivity, prone to galvanic coupling corrosion at locations of coating defects.Nano-ZnO 1. High melting point;
2. Good oxidation and corrosion resistance.1. High surface activity, easy to agglomerate and lose the special effect of nanoparticles after agglomeration;
2. Hydrophilic and oleophobic, poor dispersibility and stability in organic media;
3. Weak bonding with the substrate, poor interfacial compatibility, easy to produce voids, micro-cracks and other interfacial defects.Nano-Al2O3 1. High strength, thermal conductivity and wear resistance;
2. Excellent electrical insulation;
3. Stable physical and chemical properties.1. Poor compatibility with the substrate, poor dispersion, easy to agglomerate;
2. Functionalisation of the surface may lead to a reduction in filler size, resulting in defects on the surface;
3. Different shapes and sizes also have an effect on the corrosion resistance of the coating.Micro/Nano-
SiO21. High hardness, high mechanical strength;
2. Excellent thermal and chemical stability;
3. Low density, small particle size, large specific
surface area;
4. Colorless, odorless and pollution-friendly;
5. Good corrosion resistance.1. Fine particles and high hydrophilicity, easy to agglomerate;
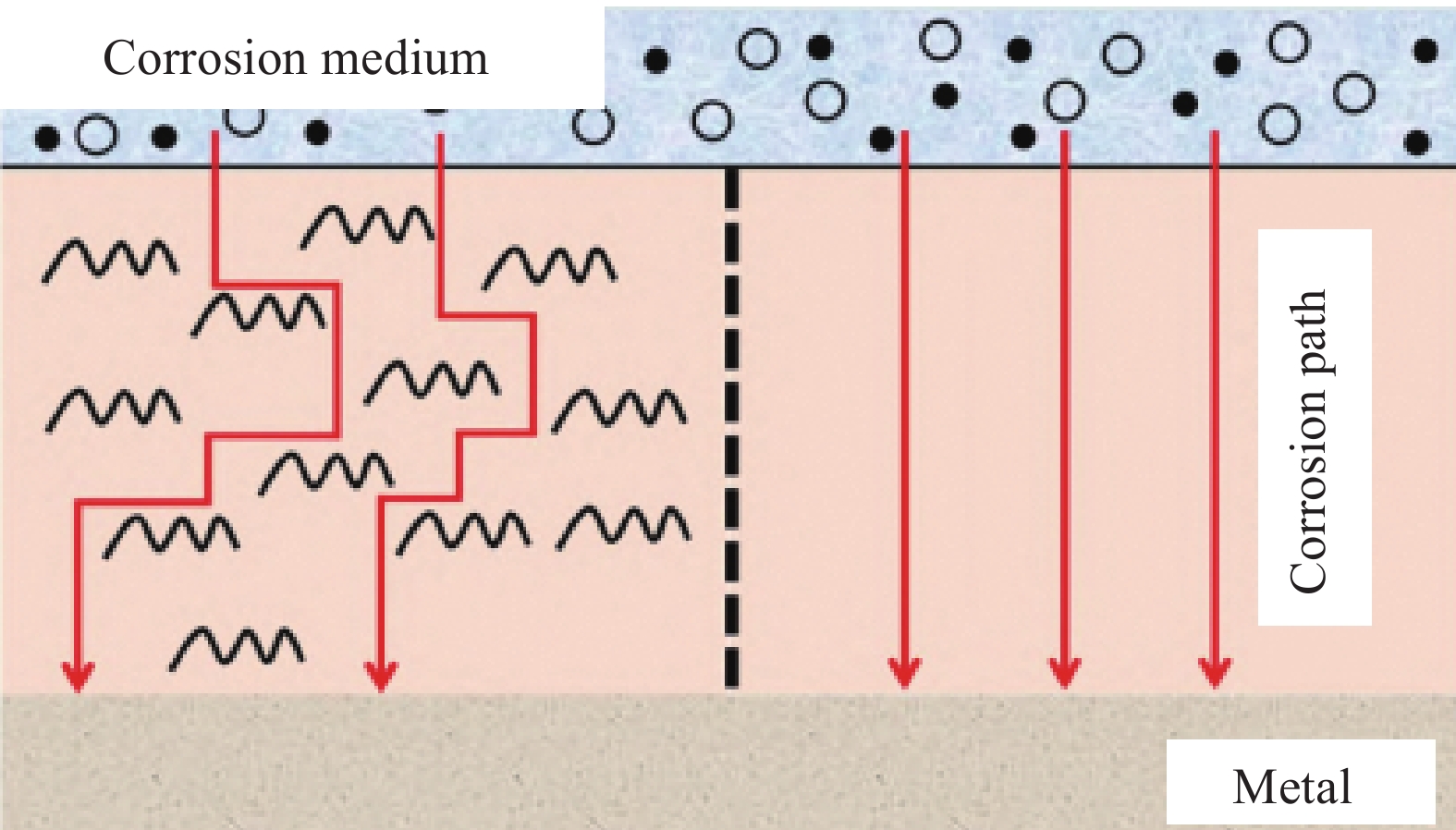
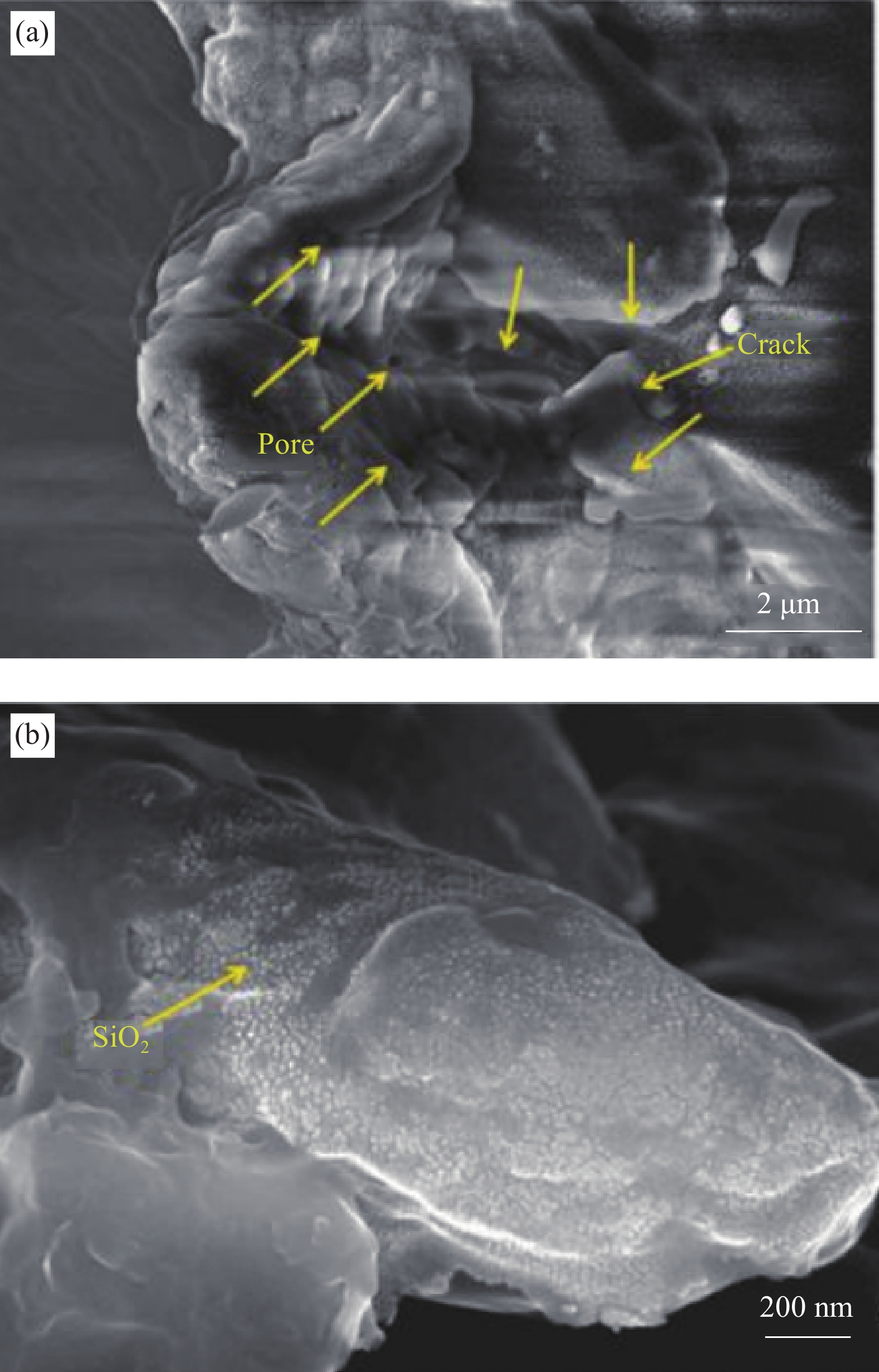
2. The coating is prone to cracking during curing and reducing the corrosion resistance of the coating. -
[1] BALANDIN A A, GHOSH S, BAO W Z, et al. Superior thermal conductivity of single-layer graphene[J]. Nano Letters, 2008, 8(3): 902-907. DOI: 10.1021/nl0731872
[2] GUO S J, DONG S J. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications[J]. Chemical Society Reviews, 2011, 40(5): 2644-2672. DOI: 10.1039/c0cs00079e
[3] LIANG X, LI X J, TANG Y, et al. Hyperbranched epoxy resin-grafted graphene oxide for efficient and all-purpose epoxy resin modification[J]. Journal of Colloid and Interface Science, 2022, 611: 105-117. DOI: 10.1016/j.jcis.2021.12.068
[4] WANG J Q, ZHANG P, LIANG B, et al. Graphene oxide as an effective barrier on a porous nanofibrous membrane for water treatment[J]. ACS Applied Materials & Interfaces, 2016, 8(9): 6211-6218. DOI: 10.1021/acsami.5b12723
[5] CHEN J H, LI W G, ZHAO Y T, et al. Application of graphene in anti-corrosive and anti-fouling coating[J]. Surface Technology, 2019, 48(6): 89-97.
[6] ZHANG Y J, SHAO Y W, LIU X L, et al. A study on corrosion protection of different polyaniline coatings for mild steel[J]. Progress in Organic Coatings, 2017, 111: 240-247. DOI: 10.1016/j.porgcoat.2017.06.015
[7] CHENG L, LIU C, HAN D, et al. Effect of graphene on corrosion resistance of waterborne inorganic zinc-rich coatings[J]. Journal of Alloys and Compounds, 2019, 774: 255-264. DOI: 10.1016/j.jallcom.2018.09.315
[8] LE H S, NGUYEN D L, JUKKA S. Enhanced mechanical and thermal properties of polyurethane functionalized graphene oxide composites by in situ polymerization[J]. Plastics, Rubber and Composites, 2019, 48(10): 466-476. DOI: 10.1080/14658011.2019.1664820
[9] SALAVAGIONE H J, MARTÍNEZ G, ELLIS G. Recent advances in the covalent modification of graphene with polymers[J]. Macromolecular Rapid Communications, 2011, 32(22): 1771-1789. DOI: 10.1002/marc.201100527
[10] SUN Y B, YANG S B, CHEN Y, et al. Adsorption and desorption of U(VI) on functionalized graphene oxides: A combined experimental and theoretical study[J]. Environmental Science & Technology, 2015, 49(7): 4255-4262.
[11] ASALDOUST S, RAMEZANZADEH B. Synthesis and characterization of a high-quality nanocontainer based on benzimidazole-zinc phosphate (ZP-BIM) tailored graphene oxides; A facile approach to fabricating a smart self-healing anti-corrosion system[J]. Journal of Colloid and Interface Science, 2020, 564: 230-244.
[12] LI Y F, LIU J L, DENG J S, et al. Fabrication of graphene oxide reinforced plasma sprayed Al2O3 coatings[J]. Ceramics International, 2023, 49: 1667-1677. DOI: 10.1016/j.ceramint.2022.09.129
[13] HAMID Z A, GOMAA M H, EL-HOUT S I, et al. α-Fe2O3/Fe3O4@GO nanosheets boost the functionality of Ni-P thin film deposited by electroless method[J]. Surface & Coatings Technology, 2023, 456: 129288.
[14] LALA S R F, SRIVASTAVA C. Correlation between texture, grain boundary constitution and corrosion behaviour of copper-chromium coatings containing graphene oxide[J]. Metallurgical and Materials Transactions A, 2023, 54: 634-645. DOI: 10.1007/s11661-022-06908-7
[15] BAGDE A, SHRIRAME S, BHANVASE B A, et al. Investigation on simultaneous enhancement in mechanical and anticorrosion performance of graphene oxide-zinc phosphate nanocomposite-based coatings[J]. Diamond & Related Materials, 2024, 144: 110960.
[16] 李智, 刘崇宇, 葛毓立, 等. 氧化石墨烯对纳米金属陶瓷复合镀层组织性能影响[J]. 表面技术, 2023, 52(10): 394-401. LI Zhi, LIU Chongyu, GE Yuli, et al. Effect of graphene oxide on microstructure and properties of nano-cermet composite coatings[J]. Surface Technology, 2023, 52(10): 394-401(in Chinese).
[17] ZHANG Y H. Application of water-soluble polymer inhibitor in metal corrosion protection: Progress and challenges[J]. Frontiers in Energy Research, 2022, 10: 3389.
[18] VAZIRINASAB E, JAFARI R, MOMEN G. Application of superhydrophobic coatings as a corrosion barrier: A review[J]. Surface & Coatings Technology, 2018, 341: 40-56.
[19] ZHANG F Y, LIU W Q, LIANG L Y, et al. Applications of hydrophobic α,ω-bis(amino)-terminated polydimethylsiloxane-graphene oxide in enhancement of anti-corrosion ability of waterborne polyurethane[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 600: 124981. DOI: 10.1016/j.colsurfa.2020.124981
[20] WANG M J, WANG A, ZHAO W J, et al. The ion diffusion-directed self-assembled graphene oxide coating and its synergistic lubrication mechanism against environmental moisture[J]. Tribology International, 2024, 191: 109182. DOI: 10.1016/j.triboint.2023.109182
[21] GUO R T, LI W, WANG X Y, et al. Antimicrobial corrosion study of the epoxy coating with the graphene oxide supported Schiff base quaternary ammonium salt additives[J]. Materials Today Communications, 2023, 35: 105517. DOI: 10.1016/j.mtcomm.2023.105517
[22] 李凤英, 鞠鹏飞, 陈磊, 等. 聚苯胺原位聚合改性氧化石墨烯制备复合涂层及其耐腐蚀性能研究[J]. 表面技术, 2021, 50(11): 287-296. LI Fengying, JU Pengfei, CHEN Lei, et al. Preparation and corrosion resistance of polyaniline/modified graphene oxide composite coating[J]. Surface Technology, 2021, 50(11): 287-296(in Chinese).
[23] ZHU X T, GAO Z S, LI F C, et al. Superlyophobic graphene oxide/polydopamine coating under liquid system for liquid/liquid separation, dye removal, and anti-corrosion[J]. Carbon, 2022, 190: 329-336. DOI: 10.1016/j.carbon.2022.01.018
[24] GAUR M S, RAGHAV R K, SAGAR R, et al. Investigation of anticorrosion properties of epoxy GO nanocomposites spin coated aluminum alloy 7075[J]. Polymers and Polymer Composites, 2022, 30: 1-10.
[25] DU X Q, LIU Y W, CHEN D C, et al. Co-electrodeposition of silane and graphene oxide on copper to enhance the corrosion protection performance[J]. Surface & Coatings Technology, 2022, 436: 128279.
[26] PANG W T, JIANG H, WANG S, et al. Graphene oxides enhanced polyurethane based composite coating with long term corrosion resistance and self-healing property[J]. European Polymer Journal, 2024, 207: 112825. DOI: 10.1016/j.eurpolymj.2024.112825
[27] 郭洪飞, 赵增祺, 朝宝, 等. 2-6二氨基吡啶改性氧化石墨烯复合涂层的制备及防腐性能[J]. 中国表面工程, 2022, 35(2): 126-139. GUO Hongfei, ZHAO Zengqi, CHAO Bao, et al. Preparation and anticorrosion performance of 2-6 diaminopyridine modified graphene oxide composite coating[J]. China Surface Engineering, 2022, 35(2): 126-139(in Chinese).
[28] XUE B, YU M, LIU J, et al. Corrosion protection of AA2024-T3 by sol-gel film modified with graphene oxide[J]. Journal of Alloys and Compounds, 2017, 725: 84-95.
[29] PARHIZKAR N, RAMEZANZADEH B, SHAHRABI T. Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets[J]. Applied Surface Science, 2018, 439: 45-59. DOI: 10.1016/j.apsusc.2017.12.240
[30] 楠顶, 李鑫, 徐宇, 等. 硅烷偶联剂改性氧化石墨烯增强环氧树脂复合涂料的防腐性能[J]. 化学工业与工程, 2023, 40(6): 130-135. NAN Ding, LI Xin, XU Yu, et al. Epoxy resin composite coating with silane coupling agent modified graphene oxide generating effective corrosion protection[J]. Chemical Industry and Engineering, 2023, 40(6): 130-135(in Chinese).
[31] LI M M, ZHOU D N, ZHAO B F, et al. Simultaneously improved corrosion/wear resistances of epoxy coating on Mg alloy via the coupled hybridization of GO and nano-SiO2[J]. Diamond and Related Materials, 2022, 128: 109224. DOI: 10.1016/j.diamond.2022.109224
[32] PRASETYA N B A, PUTRI M R, NGADIWIYANA, et al. Polyeugenol-gum arabic/graphene oxide composite coating for high performance anticorrosion material[J]. Case Studies in Chemical and Environmental Engineering, 2024, 9: 100658. DOI: 10.1016/j.cscee.2024.100658
[33] DADKHAH S, GHARIEH A. UV-cured acrylated PANI/graphene oxide nanocomposite coating with superior anticorrosive protection and self-healing abilities[J]. Progress in Organic Coatings, 2024, 189: 108346. DOI: 10.1016/j.porgcoat.2024.108346
[34] HUANG Y X, YANG S H, HU Y, et al. Construction of anticorrosive coatings with emergency response closure by introducing functionalized graphene oxide[J]. Chemical Engineering Journal, 2024, 487: 150539. DOI: 10.1016/j.cej.2024.150539
[35] XIE C, ZHANG P, XUE M S, et al. Long-lasting anti-corrosion of superhydrophobic coating by synergistic modification of graphene oxide with polydopamine and cerium oxide[J]. Construction and Building Materials, 2024, 418: 135283. DOI: 10.1016/j.conbuildmat.2024.135283
[36] WANG H H, YE M Y, WU J H, et al. Development of amine functionalized graphene oxide/fluorinated polyurethane topcoat with integrated anti-corrosion, anti-aging, and anti-bacterial performance[J]. Progress in Organic Coatings, 2024, 189: 108337. DOI: 10.1016/j.porgcoat.2024.108337
[37] XU C A, CHU Z Z, LI X C, et al. Vanillin and organosilicon functionalized graphene oxide modified ester resin composite coatings with excellent anti-corrosion properties[J]. Progress in Organic Coatings, 2023, 183: 107804. DOI: 10.1016/j.porgcoat.2023.107804
[38] SHU S S, WU L, LIU J M, et al. Synthesis and corrosion resistance of silane coupling agent modified graphene oxide/waterborne polyurethane[C]//Proceedings of 2019 5th International Conference on Applied Materials and Manufacturing Technology (ICAMMT 2019). Singapore: IOP Publishing Ltd., 2019, 631: 022058.
[39] WU H, CHENG L, LIU C B, et al. Engineering the interface in graphene oxide/epoxy composites using bio-based epoxy-graphene oxide nanomaterial to achieve superior anticorrosion performance[J]. Journal of Colloid and Interface Science, 2021, 587: 755-766. DOI: 10.1016/j.jcis.2020.11.035
[40] XU C A, LIANG W Z, NI C L, et al. Silicone and nano-diamond modified graphene oxide anticorrosive coating[J]. Surface & Coatings Technology, 2024, 479: 130584.
[41] ZHU J K, LI H, YANG Z Y, et al. CaIn2S4 nanosheets and SnO2 nanoflowers co-sensitized TiO2 nanotubes photoanode for continuous and efficient photocathodic protection of Q235 carbon steel[J]. Journal of Alloys and Compounds, 2024, 970: 172570. DOI: 10.1016/j.jallcom.2023.172570
[42] 陈凡伟, 刘斌, 蹇冬辉, 等. 光生阴极保护技术的研究进展及其存在的问题[J]. 材料工程, 2021, 49(12): 83-90. DOI: 10.11868/j.issn.1001-4381.2021.000469 CHEN Fanwei, LIU Bin, JIAN Donghui, et al. Research progress and existing problems of photocathodic protection technology[J]. Journal of Materials Engineering, 2021, 49(12): 83-90(in Chinese). DOI: 10.11868/j.issn.1001-4381.2021.000469
[43] HUANG W Q, CHAI Z L, ZHAO S R, et al. Design synthesis and excellent anti-corrosion property of GO/Mn-Zn2SiO4 composite materials[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 656: 130281. DOI: 10.1016/j.colsurfa.2022.130281
[44] 陈廷廷, 李波, 张小龙, 等. GO/PANI改性环氧树脂@TiO2/CdS涂层的光生阴极保护性能[J]. 微纳电子技术, 2023, 60(8): 1211-1223. CHEN Tingting, LI Bo, ZHANG Xiaolong, et al. Photogenerated cathodic protection performance of GO/PANI modified epoxy resin@TiO2/CdS coating[J]. Micronanoelectronic Technology, 2023, 60(8): 1211-1223(in Chinese).
[45] 李波, 何锦航, 余思伍, 等. TiO2/GO/EP复合涂层的光电化学防腐蚀性能研究[J]. 涂料工业, 2023, 53(1): 22-29, 36. DOI: 10.12020/j.issn.0253-4312.2022-194 LI Bo, HE Jinhang, YU Siwu, et al. Study on photoelectrochemical corrosion protection performance of TiO2/GO/EP composite coating[J]. Paint & Coatings Industry, 2023, 53(1): 22-29, 36(in Chinese). DOI: 10.12020/j.issn.0253-4312.2022-194
[46] MA L W, WANG J K, ZHAO F T, et al. Plasmon-mediated photothermal and superhydrophobic TiN-PTFE film for anti-icing/deicing applications[J]. Composites Science and Technology, 2019, 181: 107696. DOI: 10.1016/j.compscitech.2019.107696
[47] QIAN B, ZHENG Z L, MICHAILIDS M, et al. Mussel-inspired self-healing coatings based on polydopamine-coated nanocontainers for corrosion protection[J]. ACS Applied Materials & Interfaces, 2019, 10(11): 10283-10291.
[48] MARINETTI S, VAVILOV V. IR thermographic detection and characterization of hidden corrosion in metals: General analysis[J]. Corrosion Science, 2010, 52(3): 865-872. DOI: 10.1016/j.corsci.2009.11.005
[49] KHALILI P, CAWLEY P. The choice of ultrasonic inspection method for the detection of corrosion at inaccessible locations[J]. NDT & E International, 2018, 99: 80-92.
[50] SILVA M Z, GUOYON R, LEPOUTRE F. Hidden corrosion detection in aircraft aluminum structures using laser ultrasonics and wavelet transform signal analysis[J]. Ultrasonics, 2003, 414(4): 301-305.
[51] DU G, LI J, WANG W K, et al. Detection and characterization of stress-corrosion cracking on 304 stainless steel by electrochemical noise and acoustic emission techniques[J]. Corrosion Science, 2011, 53(9): 2918-2926. DOI: 10.1016/j.corsci.2011.05.030
[52] TSANGOURI E, AGGELIS D G, TITTELBOOM K V, et al. Detecting the activation of a self-healing mechanism in concrete by acoustic emission and digital image correlation[J]. The Scientific World Journal, 2013, 2013: 424560. DOI: 10.1155/2013/424560
[53] BUCHHEIT R G, GUAN H, MAHAJANAM S, et al. Active corrosion protection and corrosion sensing in chromate-free organic coatings[J]. Progress in Organic Coatings, 2003, 74(3-4): 174-182.
[54] ZHELUDKEVICH M L, TEDIM J, FERREIRA M G S. "Smart" coatings for active corrosion protection based on multi-functional micro and nanocontainers[J]. Electrochimica Acta, 2012, 82: 314-323. DOI: 10.1016/j.electacta.2012.04.095
[55] FUGOLIN A P P, FERRACANE J L, PFEIFER C S. "Fatigue-crack propagation behavior in microcapsule-containing self-healing polymeric networks"[J]. Materials & Design, 2022, 223: 111142.
[56] EXBRAYAT L, SALALUK S, UEBEL M, et al. Nanosensors for monitoring early stages of metallic corrosion[J]. ACS Applied Energy Materials, 2019, 2(2): 812-818.
[57] HU M H, PEIL S, XING Y W, et al. Monitoring crack appearance and healing in coatings with damage self-reporting nanocapsules[J]. Materials Horizons, 2018, 5: 51-58.
[58] MAIA F, TEDIM J, BASTOS A C, et al. Nanocontainer-based corrosion sensing coating[J]. Nanotechnology, 2013, 24(41): 415502. DOI: 10.1088/0957-4484/24/41/415502
[59] ZHENG X, WANG Q, LI Y, et al. Microcapsule-based visualization smart sensors for damage detection: Principles and applications[J]. Advanced Materials Technologies, 2019, 52: 1900832.
[60] LIU J G, HUANG W R, ZHANG K L, et al. Early warning and self-repair properties of o-phenanthroline modified graphene oxide anti-corrosion coating[J]. Progress in Organic Coatings, 2024, 189: 108274. DOI: 10.1016/j.porgcoat.2024.108274
[61] LI Y F, NING C Y. Latest research progress of marine microbiological corrosion and bio-fouling, and new approaches of marine anti-corrosion and anti-fouling[J]. Bioactive Materials, 2019, 4: 189-195. DOI: 10.1016/j.bioactmat.2019.04.003
[62] MELCHERS R E. Microbiological and abiotic processes in modelling longer-term marine corrosion of steel[J]. Bioelectrochemistry, 2014, 97: 89-96. DOI: 10.1016/j.bioelechem.2013.07.002
[63] LI Z C, LIU P, CHEN S W, et al. Bioinspired marine antifouling coatings: Antifouling mechanisms, design strategies and application feasibility studies[J]. European Polymer Journal, 2023, 190: 111997. DOI: 10.1016/j.eurpolymj.2023.111997
[64] LEONARDI A K, OBER C K. Polymer-based marine antifouling and fouling release surfaces: Strategies for synthesis and modification[J]. Annual Review of Chemical and Biomolecular Engineering, 2019, 10: 241-264. DOI: 10.1146/annurev-chembioeng-060718-030401
[65] XIE Q Y, PAN J S, MA C F, et al. Dynamic surface antifouling: Mechanism and systems[J]. Soft Matter, 2019, 15(6): 1087-1107.
[66] BEYER J, SONG Y, TOLLEFSEN K E, et al. The ecotoxicology of marine tributyltin (TBT) hotspots: A review[J]. Marine Environmental Research, 2022, 179: 105689. DOI: 10.1016/j.marenvres.2022.105689
[67] HU P, XIE Q Y, MA C F, et al. Silicone-based fouling-release coatings for marine antifouling[J]. Langmuir, 2022, 36(9): 2170-2183.
[68] LI C, YANG J J, HE W J, et al. A review on fabrication and application of tunable hybrid micro-nano array surfaces[J]. Advanced Materials Interfaces, 2023, 10(6): 2202160.
[69] ZHENG N, JIA B, LIU J, et al. Multi-strategy combined bionic anti-fouling coating for long-term and stable protection against marine biofouling[J]. Journal of Materials Science and Technology, 2025, 210: 265-277.
-
期刊类型引用(0)
其他类型引用(1)
-
目的
氧化石墨烯(GO)作为石墨烯的衍生物具有高比表面积、良好的机械性能、阻隔屏蔽性、反应活性、热稳定性及化学稳定性等优异的综合性能,在金属防腐蚀领域中表现出了巨大的应用潜力。本文综述了近年来国内外关于GO复合涂层在金属腐蚀与防护领域的研究进展,同时也对GO复合涂层未来发展的趋势进行了展望。
方法本文以GO复合涂层为中心,分别从GO-无机物复合涂层、GO-有机物复合涂层、GO多元复合涂层三个方面展开,将GO作为涂层填料相对于其他常见无机填料的优缺点进行了对比,同时对国内外现有GO复合涂层在制备方法、掺杂成分、GO掺杂浓度、涂层耐蚀性提高等方面取得的进展进行了归纳总结。
结果GO掺杂在不同的复合涂层中的作用机理、涂层优势及不足之处各不相同。(1)GO与无机物的复合机理主要是通过分子间氢键及静电力的作用,GO在其中起到的是物理阻隔增加腐蚀介质渗透路径及细化无机粒子晶粒的作用。但GO表面的含氧官能团能够提供大量的反应结合位点这一优势不能被充分的利用,而且由于范德华力及p-p键的相互作用,GO作为填料在无机物中很容易团聚,导致分散性和稳定性都降低,“迷宫效应”无法充分发挥,这些都导致涂层的耐蚀性受到一定限制。(2)相较于无机物,GO与有机物复合时,更能发挥出其自身的优异性能。GO的分散性使它能够填补有机高分子材料固化和成膜过程中出现的孔隙;GO的含氧官能团与有机物能够通过反应形成共价键,对有机物的化学性能和力学性能均有所改变,同时保持着GO原有的物理阻隔性能这一优点。但仍有一些不足之处存在其中。首先,GO易团聚依然是GO-有机物复合涂层耐蚀性提高的关键制约因素之一。其次,在涂层制备过程中,有机溶剂的挥发及不合理的固化方式都会导致传统的聚合物涂层在固化后,不仅会有微孔、微裂纹等缺陷的产生,而且残余的亲水基团或表面活性剂易形成极性孔道。这些缺陷及孔道会为腐蚀介质的入侵提供便捷途径,加速腐蚀介质的吸收和渗透,导致涂层的耐蚀性变差。最后,GO具有一定的导电性,在涂层缺陷的位置容易发生局部电偶腐蚀。(3)GO多元复合涂层不仅使GO的分散性有一定提高,而且其自身的可结合反应位点的利用率也大大提高。关于GO复合涂层的最新研究聚焦在以下几个方面:(1)改善GO团聚性;(2)GO光阴极保护;(3)自预警自修复GO复合涂层。
结论针对目前GO复合涂层存在的问题,未来的重点研究方向应集中在:(1)开发在耐高、低温领域可应用的GO复合涂层;(2)探索新的改性方法对复合涂层中的GO绝缘处理;(3)减少GO智能复合涂层自修复时间,提高自修复效率。





 下载:
下载: